Abstract
Purpose
Stereotactic body radiation therapy (SBRT) use has increased among patients without pathologic confirmation (PC) of lung cancer. Empirical SBRT without PC raises concerns about variation in workup and patient selection, but national trends have not been well described. In this study, we assessed patterns of empirical SBRT use, workup, and causes of death among a large national non-small cell lung cancer (NSCLC) cohort.
Methods and Materials
We identified 2221 patients treated with SBRT for cT1-T2aN0M0 NSCLC in the Veterans Affairs health care system from 2008 to 2015. We reviewed their pretreatment workup and assessed associations between absence of PC and clinical and demographic factors. We compared causes of death between PC and non-PC groups and used Cox proportional hazards modeling to compare overall survival and lung cancer specific survival (LCSS) between these groups.
Results
Treatment without PC varied from 0% to 61% among Veterans Affairs medical centers, with at least 5 cases of stage I NSCLC. Overall, 14.9% of patients were treated without PC and 8.8% did not have a biopsy attempt. Ten percent of facilities were responsible for almost two-thirds (62%) of cases of treatment without PC. Of non-PC patients, 95.5% had positron emission tomography scans, 40.6% had biopsy procedures attempted, and 12.7% underwent endobronchial ultrasound. Non-PC patients were more likely to have cT1 tumors and live outside the histoplasmosis belt. Age, sex, smoking status, and Charlson comorbidity index were similar between groups. Lung cancer was the most common cause of death in both groups. Overall survival was similar between groups, whereas non-PC patients had better LCSS (hazard ratio = 0.77, P = .031).
Conclusions
Empirical SBRT use varied widely among institutions and appropriate radiographic workup was consistently used in this national cohort. Future studies should investigate determinants of variation and reasons for higher LCSS among non-PC patients.
Introduction
Stereotactic body radiation therapy (SBRT) is increasingly used for definitive treatment of early stage non-small cell lung cancer (NSCLC).1,2 It is considered the standard of care for medically inoperable patients with early stage node-negative disease,3 and studies show excellent local control with relatively low toxicity in this population.4, 5, 6 Based on these results, SBRT has also been investigated as an alternative to lobectomy in operable patients with early stage NSCLC.7, 8, 9, 10, 11, 12 Although pathologic confirmation (PC) of NSCLC before SBRT is ideal, many early stage lung tumors are difficult to biopsy owing to small size and/or unfavorable location. In these instances, biopsies may be nondiagnostic or not attempted owing to low likelihood of success. In other patients, biopsies are avoided owing to potential associated risks of major complications that are associated with medical comorbidities, including chronic obstructive pulmonary disease (COPD). In the absence of PC of NSCLC, clinical and imaging characteristics are used to assess the likelihood of malignancy and to guide empirical treatment decisions.13, 14, 15, 16
Multiple single-institution studies comparing SBRT with and without PC have been reported with percentages of non-PC cases ranging from 29% to 65%.17, 18, 19, 20, 21 This rate appears to be much lower in general practice in the United States, with reported values of 4.5% from the National Cancer Database (NCDB) and 9.2% from the Surveillance, Epidemiology, and End Results (SEER) registries.22,23 No differences in overall survival (OS) or local control between groups were observed in these studies, whereas the SEER study showed improved cancer-specific survival in the non-PC group.23 A recent systematic review also found better lung cancer specific survival (LCSS) in patients without PC relative to those with biopsy-proven disease undergoing SBRT.24 Two-year OS was better in those without PC, whereas 5-year OS was the same in both groups. Although these findings are important to consider when comparing outcomes from surgery and SBRT in trials not requiring PC, they do not provide information about the appropriate use of empirical SBRT, or how it is being implemented in practice.
The purpose of this study was to assess patterns of SBRT use without PC for patients with early stage NSCLC in a large national sample of veterans diagnosed within the Veterans Affairs (VA) integrated health care system. The subjective nature of risk-to-benefit analysis and the influence of clinician experience on the decision to offer SBRT without tissue confirmation may lead to substantial variability in the use of empirical SBRT across institutions. Little is known about this variability, as reported data on practice patterns of empirical SBRT on a large scale are scarce. This information, along with data regarding workup, survival outcomes, and specific cause of death analysis in this population could assist thoracic oncology practitioners when faced with this clinical dilemma. We hypothesized that treatment without PC would be common in this group of patients who often have comorbidities that put them at higher risk of complications from biopsies and/or surgery. We aimed to assess variability among institutional use of SBRT to identify predictors of treatment without PC and hypothesized that a small number of facilities would be responsible for the majority of cases of treatment without PC. We also aimed to ascertain typical pretreatment workup in this cohort to compare survival outcomes and specific causes of death between patients with and without PC.
Methods and Materials
Patients and institutions
We identified patients with newly diagnosed American Joint Committee on Cancer 7th edition stage I (T1-T2aN0M0) NSCLC from 2008 to 2015 in the VA Corporate Data Warehouse (CDW),25 accessed through the VA Informatics and Computing Infrastructure (VINCI). The CDW provides both clinical data and administrative claims, including diagnoses and current procedural terminology (CPT).26 VINCI incorporates tumor registry data from VA centers that are collected by trained registrars according to American College of Surgeons standards. It is estimated that 90% of incident cancers within the VA system are captured within VINCI.27 Treatment with SBRT was determined by a combination of claims data using CPT codes for SBRT and radiation dose and elapsed days consistent with SBRT (minimum 48 Gy in maximum 16 elapsed days). We included patients treated at any of the 20 VA facilities that offer SBRT28 and those treated outside the VA system. A subset of electronic health records was reviewed to confirm that SBRT was used in 100% of the identified cases. Single fraction SBRT use (eg, 34 Gy x 1) was extremely rare in this cohort.
Patients were divided into 2 groups based on the presence or absence of PC before SBRT as determined by the cancer registrar-coded field on type of diagnosis (PC: histologic or cytologic, non-PC: clinical or radiologic). The percentage of cases without PC was determined for the whole cohort and for each institution in which the diagnoses of NSCLC were made. For patients treated without PC, biopsy attempts before empirical SBRT were determined using CPT codes. Biopsy procedures were identified from CPT codes for transthoracic needle lung biopsy, bronchoscopy with brushings or biopsy, bronchoscopy with cytology and brush, and surgical lung biopsy. CPT codes were also used to determine use of positron emission tomography (PET) scans and endobronchial ultrasound in the year before treatment for all patients. VA patients who had procedures or treatment outside the VA system but paid for by the VA on a fee-basis were also included, as they are captured by the CDW with the same CPT codes.
Statistical analysis
We reported descriptive statistics for the overall cohort and separately for facilities at or above and below the 90th percentile for treatment without PC. Associations between PC and clinical and demographic variables were assessed using χ2 tests for categorical variables and Student t tests for continuous variables. Covariates included age, sex, geographic location, tumor-stage, smoking status, Charlson comorbidity index (calculated using CPT codes from the year before diagnosis), primary payer at diagnosis, and VA facility complexity score of the facility in which the diagnosis was made (not necessarily where treatment was provided).29 Complexity scores divide VA facilities into 5 levels based on patient volume, patient risk teaching, and research, with lower numbers and letters representing more complex facilities.29 For geographic location, the fraction of patients from within the “histoplasmosis belt” treated without PC was compared with that of patients outside this region.30 The number of patients with and without PC were compared by year of diagnosis, and logistic regression was used to assess for changes in this rate over time. For patients without PC, the association between T-stage and biopsy attempt was assessed using a χ2 test.
The Kaplan-Meier method was used to generate OS curves. For deceased patients, cause of death was established using the National Death Index (NDI). Deaths coded as “secondary malignant neoplasm” were included in the lung cancer group, based on published results comparing death certificates and NDI.31 Cox proportional hazards modeling was used to compare OS and LCSS between PC and non-PC groups. Competing risk analysis was performed using the Fine-Gray model, and cumulative incidences of lung cancer death were compared between the groups. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC). P values ≤ .05 from a 2-tailed test were considered statistically significant.
This study was approved by the VA Ann Arbor Healthcare System institutional review board.
Results
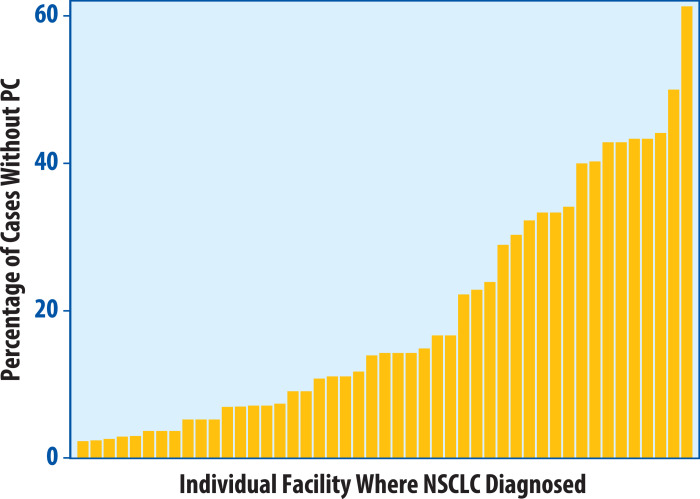
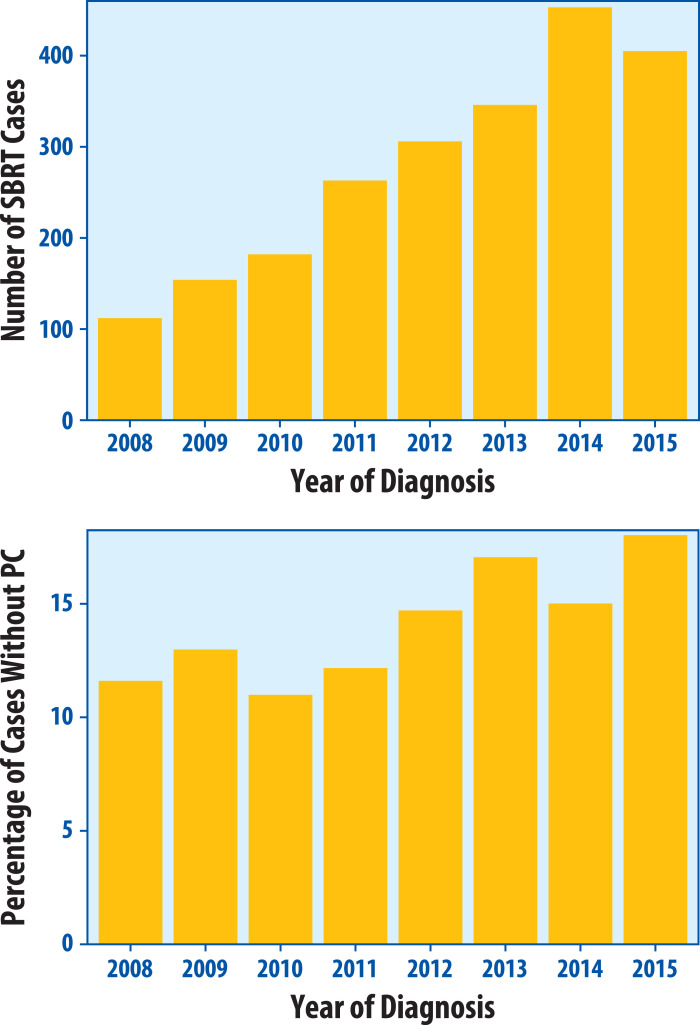
During the study period of 2008 to 2015, 2221 patients with early stage NSCLC at 117 institutions were treated with SBRT. The median number of cases diagnosed per institution was 12 (interquartile range, 3-32). Three hundred thirty patients (14.9%) received diagnoses without PC with an institutional rate of 0% to 100% (median, 0%; interquartile range, 0%-15%). When excluding 36 institutions with fewer than 5 cases diagnosed, this range was 0% to 61% (median, 4%; interquartile range, 0%-15%) (Fig 1). Of the 81 institutions with at least 5 cases diagnosed, 34 had no patients treated without PC. Biopsies were attempted in 134 (40.6%) of non-PC patients, whereas 196 (59.4%) patients were treated without an attempt at biopsy. This represents 8.8% of the total cohort who were treated without a pretreatment biopsy attempt. Almost two-thirds (62%) of patients treated without PC were treated at facilities whose rates of treatment without PC were at or above the 90th percentile (90th percentile represented a treatment without PC rate of 43.3%). When separately considering facilities at or above versus below the 90th percentile with respect to treatment without PC, a greater proportion of patients was treated without a biopsy attempt in the at or above 90th percentile group (76%) compared with the below 90th percentile group (51%). PET use was 98% versus 94% at facilities at or above versus below the 90th percentile, respectively. The number of SBRT treatments increased over time from 112 in 2008 to 453 in 2014 (Fig 2A). The percentage of non-PC cases ranged from 11.0% in 2010 to 18.0% in 2015 (Fig 2B), with a significant trend for increasing proportion over time (P = .0085).
Figure 1.
Distribution of non-pathologic confirmation (PC) cases by institution. Waterfall plot showing the range in percentage of stereotactic body radiation therapy treatments without PC among the institutions that diagnosed at least 5 patients in the study, of which at least 1 was treated without PC. An additional 34 institutions diagnosed at least 5 patients but none went on to receive SBRT without PC. PC = pathologic confirmation; NSCLC = non-small cell lung cancer.
Figure 2.
Stereotactic body radiation therapy cases by year of diagnosis. Total number of stereotactic body radiation therapy cases A, for early stage non-small cell lung cancer in the Veterans Affairs system by year of diagnosis and percentage of cases B, treated without pathologic confirmation (PC). PC = pathologic confirmation; SBRT = stereotactic body radiation therapy.
Patient characteristics for both groups are shown in Table 1. Most patients were men with a smoking history and at least 1 major comorbidity. There were no significant differences between PC and non-PC groups in terms of age, sex, smoking status, or Charlson comorbidity score. A higher percentage of patients in the PC group had T2 tumors (23.6% compared with 10.9% in the PC group, P < .0001) and lived within the histoplasmosis belt (39.1% compared with 27.0% in the non-PC group, P < .0001). Most patients in both groups had PET scans before SBRT, whereas only a small percentage had endobronchial ultrasound. Of the 330 non-PC patients, 21 of 36 patients (58.3%) with T2 tumors underwent biopsy procedures compared with 113 of 294 patients (38.4%) with T1 tumors (P = .02).
Table 1.
Patient and tumor characteristics
| PC (n = 1891) | No PC (n = 330) | P value | |
|---|---|---|---|
| (n, %) | (n, %) | ||
| Mean age (SD) | 72.1 (8.6) | 72.1 (8.6) | .96 |
| Sex | .38 | ||
| Male | 1841 (97.4) | 324 (98.2) | |
| Female | 50 (2.6) | 6 (1.8) | |
| Clinical T | < .0001 | ||
| T1 | 1444 (76.4) | 294 (89.1) | |
| T2a | 447 (23.6) | 36 (10.9) | |
| Histology | NA | ||
| Adenocarcinoma | 828 (43.5) | NA | |
| Squamous cell | 21 (1.1) | NA | |
| Mixed | 784 (41.5) | NA | |
| Large cell | 16 (0.9) | NA | |
| Other | 242 (12.8) | NA | |
| Grade | NA | ||
| 1 | 95 (5.0) | NA | |
| 2 | 281 (14.9) | NA | |
| 3-4 | 344 (18.1) | NA | |
| Unknown | 1171 (61.9) | NA | |
| Charlson index | .61 | ||
| 0 | 200 (10.6) | 30 (9.1) | |
| 1 | 719 (38.0) | 131 (39.7) | |
| 2 | 230 (12.2) | 35 (10.6) | |
| 3 | 387 (21.0) | 65 (19.7) | |
| ≥4 | 345 (18.2) | 69 (20.9) | |
| Smoking status | .18 | ||
| Current | 987 (52.2) | 171 (51.8) | |
| Former | 808 (42.7) | 151 (45.8) | |
| Never | 43 (2.3) | 3 (0.9) | |
| Unknown | 53 (2.8) | 5 (1.5) | |
| Histoplasmosis belt | < .0001 | ||
| Yes | 739 (39.1) | 89 (27.0) | |
| No | 1,152 (60.9) | 241 (73.0) | |
| Workup | |||
| PET | 1773 (93.8) | 315 (95.5) | 0.23 |
| Biopsy | NA | 134 (40.6) | NA |
| EBUS | 301 (15.9) | 42 (12.7) | 0.14 |
Abbreviations: EBUS = endobronchial ultrasound; PC = pathologic confirmation; PET = positron emission tomography; SD = standard deviation.
Histoplasmosis belt includes: Alabama, Arkansas, Iowa, Illinois, Indiana, Kansas, Kentucky, Maryland, Mississippi, Nebraska, Oklahoma, Tennessee, Texas, Virginia, West Virginia, Washington DC.1
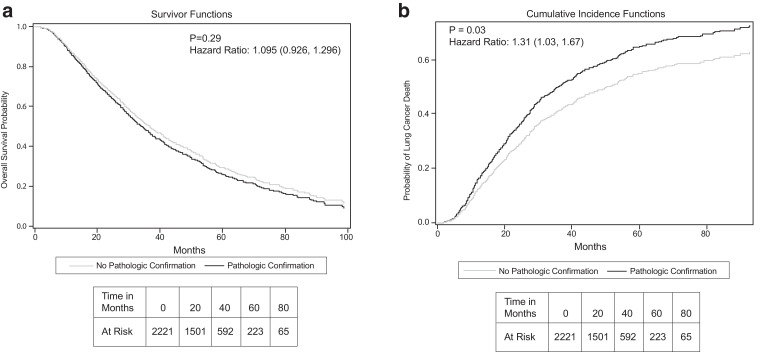
OS was not significantly different between PC and non-PC groups (Fig 3A), with medians of 34 and 37 months, respectively (P = .29). Conversely, LCSS was significantly higher in the non-PC group than in the PC group (median 49 months vs 38 months, P = .031). This is illustrated in Figure 3B, showing cumulative incidence of lung cancer death curves generated using a competing risks model. Multivariable analyses demonstrated that increasing age, T2a tumor stage, and Charlson score were negatively associated with OS, whereas sex, smoking status, PC, and PET scan use were not (Table 2). T2a tumor stage, PC, and lack of PET scan were negatively associated with LCSS, whereas age, sex, smoking status, and Charlson score were not (Table 2).
Figure 3.
Outcomes by pathologic confirmation status. Overall survival A, and cumulative incidence of lung cancer death B, using competing risks correction for patients with and without pathologic confirmation from Cox proportional hazard model adjusted for age, sex, tobacco history, Charlson comorbidity index, endobronchial ultrasound use, and geographic location (histoplasmosis belt or not).
Table 2.
Cox proportional hazards model for OS and LCSS
| OS |
LCSS |
|||||
|---|---|---|---|---|---|---|
| Characteristic | HR | 95% CI | P value | HR | 95% CI | P value |
| Age | 1.016 | (1.009, 1.023) | < .0001 | 1.003 | (0.994, 1.013) | .52 |
| Sex | ||||||
| Male | Ref | Ref | ||||
| Female | 0.73 | (0.49, 1.10) | .14 | 0.81 | (0.48, 1.36) | .42 |
| PC | ||||||
| No | Ref | Ref | ||||
| Yes | 1.1 | (0.93, 1.30) | .29 | 1.31 | (1.03, 1.67) | .031 |
| T stage | ||||||
| T1 | Ref | Ref | ||||
| T2a | 1.27 | (1.11, 1.45) | .0005 | 1.56 | (1.31, 1.85) | < .0001 |
| Smoking history | ||||||
| None | Ref | Ref | ||||
| Current | 0.99 | (0.68, 1.45) | .96 | 0.86 | (0.52, 1.44) | .57 |
| Previous | 0.95 | (0.65, 1.39) | .81 | 0.87 | (0.52, 1.44) | .58 |
| Charlson index | ||||||
| 0 | Ref | Ref | ||||
| 1 | 1.20 | (0.97, 1.49) | .097 | 1.19 | (0.90, 1.57) | .23 |
| 2 | 1.78 | (1.39, 2.27) | < .0001 | 1.48 | (1.07, 2.05) | .017 |
| 3 | 1.45 | (1.16, 1.81) | .0013 | 1.30 | (0.97, 1.74) | .08 |
| ≥4 | 1.76 | (1.40, 2.22) | < .0001 | 1.39 | (1.03, 1.88) | .033 |
| PET | ||||||
| No | Ref | Ref | ||||
| Yes | 0.80 | (0.64, 1.02) | .067 | 0.69 | (0.51, 0.93) | .016 |
| EBUS | ||||||
| No | Ref | Ref | ||||
| Yes | 0.94 | (0.79, 1.11) | .46 | 0.90 | (0.71, 1.13) | .36 |
| Histoplasmosis belt | ||||||
| No | Ref | Ref | ||||
| Yes | 1.00 | (0.89, 1.13) | .98 | 0.91 | (0.77, 1.06) | .22 |
| Facility complexity | ||||||
| 1a | Ref | Ref | ||||
| 1b | 0.98 | (0.84, 1.15) | .83 | 1.06 | (0.86, 1.29) | .60 |
| 1c | 0.99 | (0.85, 1.14) | .86 | 0.94 | (0.76, 1.15) | .52 |
| 2 | 1.11 | (0.89, 1.38) | .34 | 1.07 | (0.81, 1.41) | .64 |
| 3 | 1.34 | (0.88, 2.05) | .17 | 1.70 | (1.04, 2.77) | .034 |
| Primary insurance | ||||||
| VA/military/Tricare | Ref | Ref | ||||
| Medicare/Medicaid | 1.11 | (0.98, 1.26) | .10 | 1.15 | (0.97, 1.36) | .098 |
| Private | 0.97 | (0.53, 1.77) | .92 | 0.51 | (0.20, 1.30) | .16 |
| Uninsured | 1.18 | (0.49, 2.86) | .72 | 0.69 | (0.15, 3.09) | .63 |
| Treatment year | ||||||
| 2008 | Ref | Ref | ||||
| 2009 | 0.940 | (0.72, 1.23) | .6490 | 1.071 | (0.74, 1.54) | .7102 |
| 2010 | 0.962 | (0.74, 1.26) | .7760 | 1.150 | (0.81, 1.64) | .4359 |
| 2011 | 1.006 | (0.78, 1.23) | .9614 | 1.003 | (0.71, 1.41) | .9879 |
| 2012 | 0.879 | (0.68, 1.13) | .3177 | 0.880 | (0.62, 1.24) | .4690 |
| 2013 | 1.011 | (0.78, 1.31) | .9333 | 0.985 | (0.70, 1.39) | .9337 |
| 2014 | 0.879 | (0.68, 1.14) | .3382 | 0.898 | (0.63, 1.28) | .5495 |
| 2015 | 0.899 | (0.67, 1.21) | .4836 | 0.884 | (0.59, 1.32) | .5530 |
Abbreviations: CI = confidence interval; EBUS = endobronchial ultrasound; HR = hazard ratio; LCSS = lung cancer specific survival; OS = overall survival; PC = pathologic confirmation; PET = positron emission tomography; VA = Veterans Affairs.
By the time of analysis, 1220 patients (55%) died: 56% of PC patients and 49% of non-PC patients (Table 3). Lung cancer was the most common cause of death in both groups (57% PC, 47% non-PC). Deaths due to other cancers were also more common in the PC group (9% vs 4%). COPD was the most common nonlung cancer cause of death in the non-PC group at 21% compared with 9% in the PC group. Percentages of deaths due to cardiovascular disease, infection, and other causes were similar between groups.
Table 3.
Causes of death by PC status
| Category | PC (n = 1059) | No-PC (n = 161) |
|---|---|---|
| Lung cancer | 608 (57.4) | 76 (47.2) |
| COPD | 93 (8.8) | 34 (21.1) |
| Other pulmonary | 15 (1.4) | 5 (3.1) |
| Cardiovascular | 144 (13.6) | 22 (13.7) |
| Other cancer | 94 (8.9) | 7 (4.4) |
| Infection | 30 (2.8) | 6 (3.7) |
| Other cause | 75 (7.1) | 11 (6.8) |
Abbreviations: COPD = chronic obstructive pulmonary disease; PC = pathologic confirmation.
Discussion
In this study, we found that 15% of patients treated with SBRT for early stage NSCLC in a large integrated health care system were treated without PC. Among those who were treated without PC, approximately 40% had evidence of a biopsy attempt, and 96% had a fluorodeoxyglucose-PET/computed tomography scan before treatment. The use of empirical SBRT was highly variable among medical centers, and a trend toward increasing use over time was identified. The delivery of SBRT without PC was lower in the histoplasmosis belt, where benign nodules are expected to be more common. Ten percent of facilities were responsible for almost two-thirds of cases of treatment without PC. The analysis identified a higher rate of LCSS in the cohort treated with PC, although nearly half of deaths in the non-PC group were attributed to lung cancer in the NDI despite early stage disease and competing medical comorbidities.
The analysis found a higher rate of SBRT without PC than previous SEER and NCDB studies.22,23 This may have been because of a higher rate of comorbidities in the VA health system, as 90% were found to have at least 1 major comorbidity. Higher rates of treatment without PC may be appropriate among patients with greater comorbidities, as their risk of dying of untreated lung cancer may remain high despite their comorbidities, but the risks of sedation and pneumothorax may be substantially elevated compared with patients with fewer comorbidities. This may explain the higher rates of treatment without PC within the VA system as a whole and may also contribute to appropriate variation across facilities.
Our study found an increasing rate of utilization for SBRT without PC over time, which was also shown in an NCDB study.22 This may be due to experience with longer follow-up showing relatively little toxicity from SBRT, especially for smaller peripheral tumors that are challenging to biopsy or for patients with comorbidities making them poor candidates for invasive procedures but who are likely to tolerate SBRT without difficulty. To our knowledge, institutional variability in the use of empirical SBRT has not previously been reported in a national cohort, although the NCDB study showed that geographic location and institution type were significantly associated with treatment without PC. Reasons for this variability should be further investigated, as should the potential effect of this variability on patient outcomes.
An additional finding that was unique in this study regards information on radiographic workup and pretreatment procedures that was not reported in prior national database studies. The nearly universal employment of PET scans before empirical SBRT is encouraging. PET scans are important for limiting treatment of nonmalignant nodules and can also help detect involved mediastinal lymph nodes and/or distant metastatic disease that may not be ideal to treat with SBRT.32 We did note, however, that 10% of facilities were responsible for almost two-thirds of the cases of treatment without PC. At these facilities, biopsies were less likely to be attempted among patients treated without PC. Whether care is more or less appropriate at these facilities compared with others cannot be discerned from our analysis. On one hand, these facilities may be less likely to biopsy patients who are acceptable candidates for biopsy, which could be driven by resources or lack of availability of advanced technologies, like navigational bronchoscopy. On the other hand, these facilities may be appropriately forgoing biopsies among patients who are a high risk for complications and instead treating them empirically before their tumors progress to later stages. This distinction may be better understood through the construction and analysis of prospective registries that comprehensively collect data on all patients with nodules that are suspicious for lung cancer, regardless of whether they ultimately undergo biopsy or cancer-directed treatment. Ultimately, efforts should be made to increase access to newer biopsy technologies. However, when immediate technology and staffing limitations substantially increase the risks of biopsy, treatment without PC may still be the most preferable strategy to mitigate risk compared with performing high-risk biopsies or forgoing treatment altogether in patients with high-risk nodules.
The improved LCSS rate in non-PC patients observed in our study (hazard ratio [HR] = 0.78) is similar to the findings in the systematic review by IJsseldijk et al24 (HR = 0.83) and the SEER study by Shaikh et al23 (HR = 0.81). The latter study similarly did not find an OS difference between patients with or without PC on multivariate analysis, whereas the former found improved 2-year OS in the non-PC group but no difference in 5-year OS. Compared with the aforementioned SEER study, the study cohort in the current analysis had a higher rate of T1 tumors (89%) in the non-PC group than the SEER study (78%); the percentage of T1 tumors was not reported in the systematic review. Comparisons between our study population and others are difficult given lack of data on comorbidity scores, percent PET scan use, and biopsy attempts. Specific causes of death were not provided in the other studies. A potential explanation for our survival results is that there was less lung cancer death in the non-PC group owing to a component of benign lesions and more death owing to lung disease (particularly COPD) in this group, accounting for the lack of OS difference. However, other unmeasured confounders may also contribute to this difference.
The decision of whether to pursue a biopsy or employ SBRT without PC is complex, and the many factors involved likely contributed to the wide range in institutional rate of empirical SBRT observed in this study. Patient and tumor characteristics in these generally nonoperative candidates affect the likelihood of successful biopsies and procedure risks. The potential of treating a nodule that is benign or not NSCLC (eg, small cell lung cancer) must also be weighed against the known poor survival rates for untreated NSCLC.33 Facility level factors should also be considered, including availability of in-house biopsy, navigational biopsy, dedicated thoracic radiologist, multidisciplinary thoracic tumor board, geographic location, and physician experience with empirical SBRT. Specific expertise in biopsy of lung nodules that are small or in difficult locations to access can also affect the decision to forego a biopsy.
The Empiric Radiotherapy for Lung Cancer Collaborative Group published a review article on consideration of empirical SBRT for noncentral tumors,34 including recommendations based on size, risk of malignancy from the Mayo Clinic calculator, PET avidity, and/or Lung-Reporting and Data System.35 Comparison of the cumulative incidence curves in our study suggests that approximately 85% of patients in the non-PC group had a natural history similar to those with biopsy-proven NSCLC, despite the likelihood of additional unmeasured confounders. Although treatment in our study occurred before the publication of the review article, this rate is consistent with their recommended pretest probabilities of malignancy for empirical SBRT of small nodules (>85% with positive PET scan).34 Our findings suggest that cases are being carefully evaluated before empirical treatment in this integrated health care system and provide information for other clinicians to consider when faced with this common clinical dilemma.
Our study has several limitations that deserve mention. These include a reliance on large cancer registries, a reliance on retrospective data that may be misclassified owing to coding errors, and potential confounding factors not captured in the registry or the Charlson score. Fortunately, the VA CDW allows access to the individual patient medical records for verification purposes, and we were able to validate the accurate coding of SBRT and PC status for a subset of randomly selected cases. We were unable to obtain results from pulmonary function tests in our cohort of patients; however, prior studies have shown better lung function in patients with PC.17,20,21 Given the higher rate of death due to COPD in our non-PC patients, it is certainly possible that this group had worse lung function, making them higher risk for biopsy procedures. An advantage of the VA registry is that it comes from an integrated health care system in which nearly all patients treated inside and outside the VA are captured. This allows for a more accurate determination of institutional variability and of changes in use of SBRT without PC over time among this patient population.
In summary, there is substantial variation across institutions in the use of empirical SBRT for patients with early stage NSCLC in the VA central cancer registry, and an overall increasing rate during our study period. Appropriate radiographic workup was used in this national cohort, and a substantial minority of patients ultimately treated without PC had biopsy attempts before SBRT. Lung cancer death rates after SBRT are only slightly lower in patients without PC, suggesting a low rate of benign disease in these patients, which is in keeping with current guidelines. Future studies should investigate determinants of variation in the use of empirical SBRT, including assessment of how availability of staging procedures and multidisciplinary tumor boards influence decision making.
Footnotes
Sources of support: This research was supported by the National Cancer Institute of the National Institutes of Health under award number P30CA046592.
Disclosures: Dr Moghanaki reports travel support and speaking honoraria from Varian Medical Systems. Dr Chapman has grants from the National Cancer Institute. All other authors have no disclosures to declare.
Research data are not publicly available at this time.
References
- 1.Corso CD, Park HS, Moreno AC. Stage I lung SBRT clinical practice patterns. Am J Clin Oncol. 2017;40:358–361. doi: 10.1097/COC.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 2.Stahl JM, Corso CD, Verma V. Trends in stereotactic body radiation therapy for stage I small cell lung cancer. Lung Cancer. 2017;103:11–16. doi: 10.1016/j.lungcan.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger DS, Wood DE, Aggarwal C. August 31, 2019. NCCN clinical practice guidelines in oncology: Non-small cell lung cancer. Version 7.2019. Available at: NCCN.org. Accessed. [Google Scholar]
- 4.Timmerman R, Paulus R, Galvin J. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onishi H, Shirato H, Nagata Y. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(7 Suppl 3):S94–100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 6.Lagerwaard FJ, Haasbeek CJA, Smit EF, Slotman BJ, Senan S. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non–small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;70:685–692. doi: 10.1016/j.ijrobp.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 7.Chang JY, Senan S, Paul MA. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630–637. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryant AK, Mundt RC, Sandhu AP. Stereotactic body radiation therapy versus surgery for early lung cancer among US veterans. Annal Thorac Surg. 2018;105:425–431. doi: 10.1016/j.athoracsur.2017.07.048. [DOI] [PubMed] [Google Scholar]
- 9.Scotti V, Bruni A, Francolini G. Stereotactic ablative radiotherapy as an alternative to lobectomy in patients with medically operable stage I NSCLC: A retrospective, multicenter analysis. Clin Lung Cancer. 2019;20:e53–e61. doi: 10.1016/j.cllc.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Nagata Y, Hiraoka M, Shibata T. Prospective trial of stereotactic body radiation therapy for both operable and inoperable T1N0M0 non-small cell lung cancer: Japan clinical oncology group study JCOG0403. Int J Radiat Oncol Biol Phys. 2015;93:989–996. doi: 10.1016/j.ijrobp.2015.07.2278. [DOI] [PubMed] [Google Scholar]
- 11.Timmerman RD, Paulus R, Pass HI. Stereotactic body radiation therapy for operable early-stage lung cancer: Findings from the NRG Oncology RTOG 0618 trial. JAMA Oncol. 2018;4:1263–1266. doi: 10.1001/jamaoncol.2018.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman B, Senan S. Treatment of stage I NSCLC in elderly patients: A population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol. 2011;101:240–244. doi: 10.1016/j.radonc.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 13.Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Int Med. 1997;157:849–855. [PubMed] [Google Scholar]
- 14.Herder GJ, van Tinteren H, Golding RP. Clinical prediction model to characterize pulmonary nodules: Validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest. 2005;128:2490–2496. doi: 10.1378/chest.128.4.2490. [DOI] [PubMed] [Google Scholar]
- 15.Hasan S, Colonias A, Mickus T, VanDeusen M, Wegner RE. Image-based management of empiric lung stereotactic body radiotherapy (SBRT) without biopsy: Predictors from a 10-year single institution experience. Thorac Cancer. 2018;9:699–706. doi: 10.1111/1759-7714.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sim YT, Goh YG, Dempsey MF, Han S, Poon FW. PET–CT evaluation of solitary pulmonary nodules: Correlation with maximum standardized uptake value and pathology. Lung. 2013;191:625–632. doi: 10.1007/s00408-013-9500-6. [DOI] [PubMed] [Google Scholar]
- 17.Verstegen NE, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy following a clinical diagnosis of stage I NSCLC: Comparison with a contemporaneous cohort with pathologically proven disease. Radiother Oncol. 2011;101:250–254. doi: 10.1016/j.radonc.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Taremi M, Hope A, Dahele M. Stereotactic body radiotherapy for medically inoperable lung cancer: Prospective, single-center study of 108 consecutive patients. Int J Radiat Oncol Biol Phys. 2012;82:967–973. doi: 10.1016/j.ijrobp.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 19.Takeda A, Kunieda E, Sanuki N, Aoki Y, Oku Y, Handa H. Stereotactic body radiotherapy (SBRT) for solitary pulmonary nodules clinically diagnosed as lung cancer with no pathological confirmation: Comparison with non-small-cell lung cancer. Lung Cancer. 2012;77:77–82. doi: 10.1016/j.lungcan.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Haidar YM, Rahn DA, 3rd, Nath S. Comparison of outcomes following stereotactic body radiotherapy for non-small cell lung cancer in patients with and without pathological confirmation. Ther Adv Respir Dis. 2014;8:3–12. doi: 10.1177/1753465813512545. [DOI] [PubMed] [Google Scholar]
- 21.Wegner RE, Ahmed N, Hasan S, Schumacher LY, Deusen MV, Colonias A. SBRT for early stage lung cancer: Outcomes from biopsy-proven and empirically treated lesions. Lung Cancer Manag. 2018;7:LMT01. doi: 10.2217/lmt-2018-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutter CE, Corso CD, Park HS. Increase in the use of lung stereotactic body radiotherapy without a preceding biopsy in the United States. Lung Cancer. 2014;85:390–394. doi: 10.1016/j.lungcan.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 23.Shaikh T, Churilla TM, Murphy CT. Absence of pathological proof of cancer associated with improved outcomes in early-stage lung cancer. J Thorac Oncol. 2016;11:1112–1120. doi: 10.1016/j.jtho.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.IJsseldijk M, Shoni M, Siegert C. Survival after stereotactic body radiation therapy for clinically diagnosed or biopsy-proven early-stage NSCLC: A systematic review and meta-analysis. J Thorac Oncol. 2019;14:583–595. doi: 10.1016/j.jtho.2018.12.035. [DOI] [PubMed] [Google Scholar]
- 25.Gaur S, Turkbey B. Prostate MR imaging for posttreatment evaluation and recurrence. Radiol Clin North Am. 2018;56:263–275. doi: 10.1016/j.rcl.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Earles A, Liu L, Bustamante R. Structured approach for evaluating strategies for cancer ascertainment using large-scale electronic health record data. JCO Clin Cancer Inform. 2018;2:1–12. doi: 10.1200/CCI.17.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zullig LL, Jackson GL, Dorn RA. Cancer incidence among patients of the U.S. Veterans Affairs health care system. Military Med. 2012;177:693–701. doi: 10.7205/milmed-d-11-00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawson GA, Cheuk AV, Lutz S, et al. The availability of advanced radiation oncology technology within the Veterans Health Administration radiation oncology centers. Fed Pract. [PMC free article] [PubMed]
- 29.Site Facility Name and Complexity of VHA Facilities. Available at:https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwij3Ke31PHsAhVDQ80KHd9qA9IQFjAAegQIBRAC&url=https%3A%2F%2Fwww.vendorportal.ecms.va.gov%2FFBODocumentServer%2FDocumentServer.aspx%3FDocumentId%3D2793591%26FileName%3DVA118-16-R-1059-A00002002.docx&usg=AOvVaw2aFYhwkpzOXkCaMov5Dnl-. Accessed May 19, 2019.
- 30.Pinsky PF, Gierada DS, Nath PH, Kazerooni E, Amorosa J. National lung screening trial: Variability in nodule detection rates in chest CT studies. Radiology. 2013;268:865–873. doi: 10.1148/radiol.13121530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcus PM, VP Doria-Rose, Gareen IF. Did death certificates and a death review process agree on lung cancer cause of death in the National Lung Screening Trial? Clin Trials. 2016;13:434–438. doi: 10.1177/1740774516638345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang CF, Rashtian A, Gould MK. The use and misuse of positron emission tomography in lung cancer evaluation. Clin Chest Med. 2011;32:749–762. doi: 10.1016/j.ccm.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Detterbeck FC, Gibson CJ. Turning gray: The natural history of lung cancer over time. J Thorac Oncol. 2008;3:781–792. doi: 10.1097/JTO.0b013e31817c9230. [DOI] [PubMed] [Google Scholar]
- 34.Berman AT, Jabbour SK, Vachani A. Empiric Radiotherapy for Lung Cancer Collaborative Group multiinstitutional evidence-based guidelines for the use of empiric stereotactic body radiation therapy for non-small cell lung cancer without pathologic confirmation. Transl Lung Cancer Res. 2019;8:5–14. doi: 10.21037/tlcr.2018.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lung CT Screening Reporting and Data System (Lung-RADS) [computer program]. Available at:https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads. Accessed May 19, 2019.