Abstract
Purpose
This study compares reduced (<27 Gy) to standard dose (≥30 Gy) radiation therapy (RT) in the treatment of gastric extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (gMALT lymphoma).
Methods and Materials
Forty-two patients with stage I or II disease were retrospectively reviewed. Response to RT was assessed with endoscopy after RT. Complete response rate (CR), freedom from treatment failure, and overall survival (OS) were calculated.
Results
All patients were stage I (n = 40) or II (n = 2). All patients had residual biopsy proven gMALT lymphoma before RT. Twenty-six patients (61.9%) were treated with standard dose RT, 30 to 36 Gy, and 16 (38.1%) with the reduced dose RT, 23.5 to 27 Gy. The median follow-up was 29.5 months (range, 6-85). Thirty-six patients (86%) achieved complete response (CR), and 6 patients (14%) achieved partial response (PR). The complete response rate (CR) at the first endoscopic assessment, median time of 3 months, was 81% (95% confidence interval, 0.61%-0.93%) for standard RT, and 94% (confidence interval, 0.69%-0.99%) for reduced RT. Among CR patients, one patient had locally relapsed disease at 50 months. The 1-year overall survival (OS) was 100% in both groups. The 1-year freedom from treatment failure (FFTF) was 100% in the reduced RT group and 92% in the standard RT group. The 2-year FFTF and OS of the whole cohort were 92% and 96%, respectively. There was no significant difference in the OS, FFTF, and CR between the 2 treatment groups (P = .38, P = .18, and P = .267, respectively). For toxicity, the mean liver dose and the mean V20 heart dose were significantly lower in the reduced RT group (P <.001 and P = .001, respectively). However, incidence and severity of reported toxicities were similar between the 2 groups.
Conclusions
Reduced dose RT (23.5-27 Gy) achieved excellent complete response rates with minimal toxicity, comparable with standard dose RT (30-36 Gy), for gMALT.
Introduction
Primary gastric lymphoma accounts for 30% to 40% of all extranodal lymphomas and represents the most common extranodal form of non-Hodgkin lymphoma.1 The most common histologic subtypes are gastric mucosa-associated lymphoid tissue (gMALT) lymphoma, and diffuse large B-cell lymphoma (DLBCL).2
MALT lymphoma is a low-grade, highly radiation-sensitive indolent non-Hodgkin lymphoma and is closely associated with H. pylori infection.3,4 For Helicobacter pylori (H. pylori) positive gMALT lymphoma, antibiotic treatment is the primary treatment modality,5,6 and radiation therapy (RT) serves as a salvage treatment for patients with incomplete response.7 For localized H. pylori negative gMALT lymphoma, RT is considered the frontline treatment modality.8,9
Historically a dose of 30 Gy or greater has been used for the treatment of localized gMALT; excellent local control rates (>90%) has been reported in previous studies.10, 11, 12, 13, 14 Lowering the dose of RT has been effective in other indolent lymphomas and may achieve similar outcomes in gMALT lymphoma.15, 16, 17 Reducing RT dose also lends toward less toxicity as reported by previous studies.18
The aim of this study is to explore the effect of reducing the dose of RT in the treatment of gMALT lymphoma. It represents the largest study to date comparing standard to reduced dose RT for the treatment of gMALT lymphoma.
Methods
After Institutional Review Board approval, records of consecutive patients who received a biopsy-proven gMALT lymphoma at our institution between 2010 and 2020 were retrospectively studied. Only patients with stage I and II gMALT treated with RT were included. Patients who lack follow-up information were excluded.
Staging and workup
All patients had an endoscopic biopsy confirming gMALT lymphoma. Presence or absence of H. Pylori infection was documented by hematoxylin and eosin (H&E) stain or immunohistochemical study. Computed tomography (CT) or positron emission tomography-computed tomography (PET-CT) imaging was performed for staging, including documentation of gastric fluorodeoxyglucose uptake. Staging was based on Ann Arbor staging criteria. The MALT International Prognostic Index (IPI) was calculated for each patient based on age ≥70 years, elevated lactate dehydrogenase level, and Ann Arbor stage III or IV.19 Multifocal disease was defined as lymphoma involving more than one anatomic location of the stomach (antrum, body, fundus).
Treatment
Patients received RT as frontline therapy in cases of negative H. pylori disease, or as salvage therapy after incomplete response to antibiotics in cases of positive H. pylori disease. Some patients were treated with Rituximab before RT. All patients had residual biopsy proven gMALT lymphoma before RT. Patients were either treated with the standard dose RT (≥30 Gy) or reduced dose RT (<27 Gy). Dose was selected based on physician preference, with reduced dose RT being implemented more recently at our institution. Treatment planning involved either CT simulation with free breathing 4-dimensional (4D) CT (n = 26), free breathing with no 4D CT (n = 6), or deep-inspiration breath hold (DIBH) CT (n = 10), with DIBH implemented more recently at our institution. Larger planning target volume margins were used for patients treated with free breathing (4DCT patients included the creation of an internal target volume or ITV to account for tumor motion) versus DIBH. Treatment technique involved either 3-dimensional (3D) conformal RT, static field intensity modulated radiation therapy (IMRT), or volumetric modulated arc therapy (VMAT). The clinical target volume included the stomach only in stage I disease, and the involved lymph nodes in stage II disease as per the International Radiation Oncology Lymphoma Group (ILROG) guidelines.20
Disease assessment/follow-up
Treatment response was assessed with endoscopies approximately 3 months after RT. Complete response (CR) or partial response (PR) was documented based on the post RT endoscopic biopsy result showing eradication or persistence of gMALT lymphoma, respectively. In case of PR, subsequent endoscopies with biopsies were repeated on 3 to 6 months intervals. Patients were followed up for RT toxicity on weekly basis during treatment. Toxicity was reported and graded based on the Common Terminology Criteria for Adverse Events version 5.0 of the National Cancer Institute.21
Statistical methods and study endpoints
Statistical analysis was performed using IBM SPSS 20.0 software. Continuous data were reported as medians, ranges, means, and standard deviations. Categorical data were reported as counts and proportions. Comparisons of different demographic and tumor characteristics between the 2 groups were done using χ2 and Fisher exact tests. Continuous variables were compared with the Student t test. Overall survival (OS) and FFTF were estimated by Kaplan-Meier survival curves. Survival differences were assessed by the log-rank test. The binary regression model was conducted for treatment response rate. Odds ratios (OR) with 95% confidence interval (CI) were calculated. All reported P values were 2 sided, and differences were considered statistically significant at P < .05.
The median follow-up period was calculated from the end date of RT until last documented follow-up visit in our electronic health system records. Overall survival (OS) was defined from the end date of RT to the date of death from any cause. FFTF was defined from the end date of RT to any local/regional disease recurrence found on subsequent biopsies after achieving CR, or receipt of salvage treatment post RT for persistent residual disease. Complete response rate (CR) was defined based on the number of complete remissions at the first endoscopic assessment.
Results
Forty-eight patients who received RT for gastric MALT lymphoma (gMALT) were retrieved. Six patients were excluded due to lack of follow up data. The final cohort included 42 eligible patients.
Patient baseline and tumor clinicopathologic characteristics are outlined in Table 1. All patients were stage I (n = 40) or II (n = 2). The median age of this cohort was 71 years (range, 25-88). The majority of patients had a normal serum lactate dehydrogenase level (n = 35), no autoimmune disease (n = 32), and no B symptoms (n = 40). Most patients had a Marginal Zone Lymphoma International Prognostic Index (MALT-IPI) score of 0 (n = 17) or 1 (n = 18), and the disease was located in the body of the stomach (n = 14). H. pylori infection was identified in 11 patients (26.2%), half of which also had an autoimmune disease. PET-CT was performed in 30 patients (71.4%). Of these, 16 patients (53.3%) had abnormal gastric avidity. Patients’ characteristics were not significantly different between the 2 treatments groups except for sex (P = .029).
Table 1.
Patients demographics and clinicopathologic characteristics
| All patients | Value or no. (%) |
|||
|---|---|---|---|---|
| Characteristics | All patients (n = 42) | Reduced RT dose ≤27 Gy (n = 16) | Standard RT dose ≥30 Gy (n = 26) | P value |
| Age at diagnosis, mean (range) | 71 (25-88) | 67.5 (25-86) | 73 (47-88) | .34 |
| Sex | ||||
| Female | 22 (52.4) | 12 (75) | 10 (38.5) | .029 |
| Male | 20 (47.6) | 4 (25) | 16 (61.5) | |
| Ethnicity | ||||
| Hispanic or Latino | 1 (2.4) | 0 (0) | 1 (3.9) | 1.00 |
| Non-Hispanic or Latino | 41 (97.6) | 16 (100) | 25 (96.2) | |
| Stage | ||||
| IEA | 38 (90.5) | 14 (87.5) | 24 (92.3) | .132 |
| IEB | 2 (4.75) | 2 (12.5) | 0 (0) | |
| IIEA | 2 (4.75) | 0 (0) | 2 (7.7) | |
| Elevated LDH | ||||
| No | 35 (83.3) | 14 (87.5) | 21 (80.8) | .823 |
| Yes | 2 (4.8) | 0 (0) | 2 (7.7) | |
| Unknown | 5 (11.9) | 2 (12.5) | 3 (11.5) | |
| MALT-IPI | ||||
| 0 | 17 (40.5) | 8 (50) | 9 (34.6) | .710 |
| 1 | 18 (42.9) | 6 (37.5) | 12 (46.2) | |
| 2 | 2 (4.7) | 0 (0) | 2 (7.7) | |
| Unknown | 5 (11.9) | 2 (12.5) | 3 (11.5) | |
| B symptoms | ||||
| No | 40 (95.2) | 14 (87.5) | 26 (100) | .139 |
| Yes | 2 (4.8) | 2 (12.5) | 0 (0) | |
| Multifocal | ||||
| No | 27 (64.3) | 9 (56.2) | 18 (69.2) | .511 |
| Yes | 15 (35.7) | 7 (43.8) | 8 (30.8) | .793 |
| Disease location | ||||
| Antrum | 7 (16.7) | 3 (18.8) | 4 (15.4) | |
| Body | 14 (33.3) | 4 (25) | 10 (38.5) | |
| Fundus | 6 (14.3) | 2 (12.5) | 4 (15.4) | |
| Multifocal | 15 (35.7) | 7 (43.8) | 8 (30.8) | |
| H. Pylori infection | ||||
| No | 31 (73.8) | 11 (68.75) | 20 (76.9) | .720 |
| Yes | 11 (26.2) | 5 (31.25) | 6 (23.1) | |
| Autoimmune disease | ||||
| No | 32 (76.2) | 12 (75) | 20 (76.9) | 1.00 |
| Yes | 10 (23.8) | 4 (25) | 6 (23.1) | |
| Lymph nodes on CT | ||||
| No | 40 (95.2) | 16 (100) | 24 (92.3) | .517 |
| Yes | 2 (4.8) | 0 (0) | 2 (7.7) | |
| PET-CT | ||||
| No | 12 (28.6) | 2 (12.5) | 10 (38.5) | .09 |
| Yes | 30 (71.4) | 14 (87.5) | 16 (61.5) | |
| PET avid gastric lesion | ||||
| No | 14 (33.3) | 6 (37.5) | 8 (30.8) | .183 |
| Yes | 16 (38.1) | 8 (50) | 8 (30.8) | |
| PET not done | 12 (28.6) | 2 (12.5) | 10 (38.5) | |
Abbreviations: CT = computed tomography; LDH = lactate dehydrogenase; MALT-IPI = Marginal Zone Lymphoma International Prognostic Index; PET = positron emission tomography; RT = radiation therapy.
Antimicrobial and systemic therapy administered before RT
Fourteen patients received antibiotics (33.3%; Table 2). All patients who were H. pylori positive converted to H. Pylori negative after receiving antibiotics, but none achieved CR for the gMALT lymphoma. Four patients received rituximab before RT (9.5%); however, none of these patients achieved a CR with systemic therapy. No patient received concurrent systemic therapy with RT. Regardless of previous antibiotics or systemic treatments, all patients (n = 42) had residual biopsy proven gMALT lymphoma before RT.
Table 2.
Treatment characteristics
| All Patients | Value or no. (%) |
|||
|---|---|---|---|---|
| Characteristics | All patients (n = 42) | Reduced RT dose ≤27 Gy (n = 16) | Standard RT dose ≥30 Gy (n = 26) | P value |
| Radiation dose and fractionation | ||||
| 2340/13 | 1 (2.4) | 1 (6.3) | 0 (0.0) | <.001 |
| 2400/12 | 10 (23.8) | 10 (62.5) | 0 (0.0) | |
| 2520/14 | 2 (4.8) | 2 (12.5) | 0 (0.0) | |
| 2550/17 | 1 (2.4) | 1 (6.3) | 0 (0.0) | |
| 2700/15 | 2 (4.8) | 2 (12.5) | 0 (0.0) | |
| 3000/15 | 1 (2.4) | 0 (0.0) | 1 (3.8) | |
| 3000/17 | 1 (2.4) | 0 (0.0) | 1 (3.8) | |
| 3000/20 | 19 (45.2) | 0 (0.0) | 19 (73.1) | |
| 3060/17 | 5 (11.9) | 0 (0.0) | 5 (19.2) | |
| RT technique | ||||
| 3D | 17 (40.5) | 5 (31.3) | 12 (46.2) | .518 |
| Static IMRT or VMAT | 25 (59.5) | 11 (68.8) | 14 (53.8) | |
| Breath technique | ||||
| DIBH | 10 (23.8) | 5 (31.3) | 5 (19.2) | .465 |
| FB +/− ITV | 32 (76.2) | 11 (68.8) | 21 (80.8) | |
| Antibiotics before RT | ||||
| Yes | 14 (33.3) | 5 (31.3) | 9 (34.6) | 1.00 |
| No | 28 (66.7) | 11 (68.8) | 17 (65.4) | |
| Rituximab before RT | ||||
| Yes | 4 (9.5) | 1 (6.3) | 3 (11.5) | 1.00 |
| No | 38 (90.5) | 15 (93.8) | 23 (88.5) | |
| Salvage treatment post RT | ||||
| Yes | 4 (9.5) | 0 (0.0) | 4 (15.4) | .28 |
| No | 38 (90.5) | 16 (100.0) | 22 (84.6) | |
| Median follow-up time, mo (range) | 29.5 (6-85) | 16 (6-85) | 43 (11-74) | .004 |
| Mean time to first follow-up endoscopy, mo (range) | 3 (1-11) | 3.5 (2-11) | 3 (1-6) | .507 |
Abbreviations: 3D = 3-dimensional; DIBH = deep-inspiration breath hold; FB = free breathing; IMRT = intensity modulated radiation therapy; RT = radiation therapy; ITV = internal target volume; VMAT = volumetric modulated arc therapy.
Radiation therapy
Twenty-six patients (61.9%) were treated with standard dose RT, 30 to 36 Gy, and 16 (38.1%) were treated with reduced dose RT, 23.5 to 27 Gy. In the standard RT group, most patients (n = 19, 73.1%) were treated with 30 Gy in 20 fractions. In the reduced dose RT group, most patients (n = 10, 62.5%) were treated with 24 Gy in 12 fractions. Two patients were treated with static field IMRT (5%), 23 with VMAT (55%), and 17 with 3D conformal RT (40%). Ten patients (23.8%) were treated with DIBH and 32 (76.2%) in free-breathing planned with an ITV from the 4D CT (n = 26, 62.9%) or without an ITV (n = 6, 14.3%; Table 2).
Outcomes
The median follow-up for all patients was 29.5 months. The standard RT group had a significantly (P = .004) longer median follow-up period (43 months) compared with the reduced dose RT group (16 months; Table 2). Median time to first follow-up endoscopy was 3 months (range, 1-11). Thirty-six patients (86%) achieved CR, and 6 patients (14%) achieved PR on the first endoscopic assessment after the completion of RT. Five of the PR patients were in the standard RT group and one was in the reduced RT dose group. Four PR patients received salvage treatment with Rituximab (n = 3; 75%) or Rituximab + Cyclophosphamide (n = 1; 25%). The timing of the salvage treatment was based on the patient disease status and overall clinical picture. These 4 patients had salvage treatment at 5-, 7-, 18-, and 34-months post RT. Among those who had a PR, all but one had subsequent endoscopies to evaluate for possible conversion to CR. The median time between end of RT and last subsequent endoscopy in these patients was 26 months (range, 3-37). Only one converted to CR at 18 months post salvage therapy and belonged to the standard RT group. Among those who had CR, only one patient had locally relapsed disease at 50 months and belonged to the standard RT group. The relapse involved the stomach body and fundus without nodal involvement. This patient had a MALT-IPI score of 1, stage IE disease based on PET staging, no H. pylori infection and did not receive prior antibiotics or Rituximab before RT. There were 4 deaths in the cohort, all in the standard RT group. Only one death was attributed to gMALT lymphoma. This patient had a MALT-IPI score of 1, stage IE disease based on PET staging, no H. pylori infection and did not receive antibiotics or Rituximab before RT. The patient achieved PR to RT but never converted to CR despite receiving salvage Rituximab. Before his death, he had persistent gastrointestinal symptoms including abdominal fullness, lack of appetite, ongoing acid reflux, weight loss, and mild anemia (hemoglobin 10.9). Unfortunately, these symptoms led to failure to thrive in a 90-year-old who was not a candidate for additional therapy. The rest were either in CR at the time of death (n = 2) or had stable residual lymphoma with no significant toxicity from RT (n = 1).
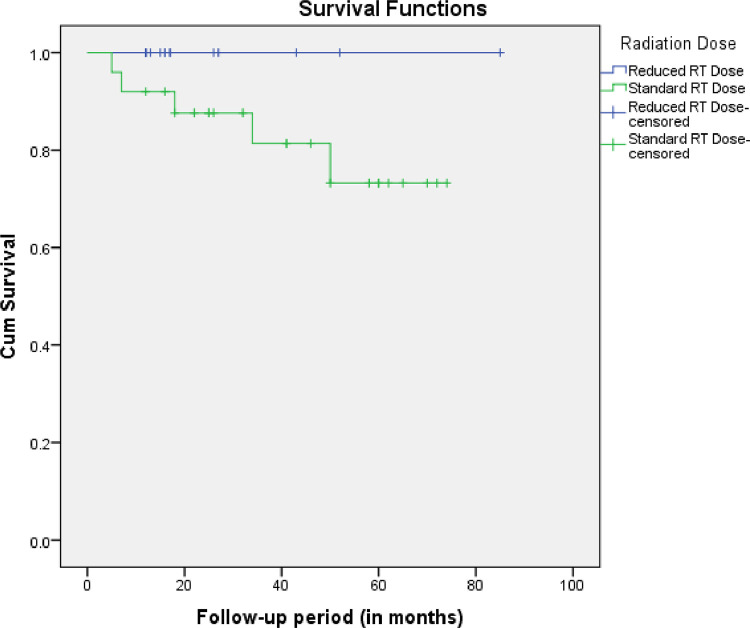
The complete response rate (CR) at a median time of 3 months was 86% (95% confidence interval [CI], 0.71%-0.95%) for the whole cohort, 81% (95% CI, 0.61%-0.93%) for standard RT, and 94% (95% CI, 0.69%-0.99%) for reduced RT group. The 1-year overall survival (OS) was 100% in both groups. The 1-year FFTF was 100% in the reduced RT group and 92% in the standard RT group. The 2-years FFTF and OS of the whole cohort were 92% and 96%, respectively. When CR was assessed by the binary logistic regression method, standard dose RT compared with reduced dose RT (statistical reference) had an odds ratio (OR) of 0.28 (95% CI, 0.03%-2.65%), not statistically significant (P = .267). Similarly, there was no significant difference in the OS and FFTF when assessed by the KM method and compared by the log-rank test (P = .38, and P = .18; Fig. 1, respectively). When the response rate was taken as an outcome, there was no significant difference in the patients’ and treatment's characteristics between complete and partial responders except for sex.
Fig. 1.
Kaplan-Meier plot showing the freedom from treatment failure of the 2 treatment groups. Abbreviation: RT = radiation therapy.
Dosimetric analysis and toxicity
Mean absolute dose to the liver, right and left kidneys, and mean V20 and absolute dose to the heart are presented in Table 3. The mean dose for all organs was lower in the reduced dose RT group. However, only the mean absolute dose to the liver, mean V20 dose and mean absolute dose to the heart were significantly lower (P <.001, P = .001, P <.001, respectively).
Table 3.
Dosimetric analysis showing the difference in the mean organ dosage between the 2 treatment groups
| All patients | Dose in Gy |
|||
|---|---|---|---|---|
| Total (n = 42) | Reduced RT dose ≤27 Gy (n = 16) | Standard RT dose ≥30 Gy (n = 26) | P value | |
| Mean absolute right kidney dose (SD) | 3.3 (2.6) | 2.6 (2.0) | 3.7 (2.9) | .177 |
| Mean absolute left kidney dose (SD) | 6.1 (4.4) | 5.1 (3.5) | 6.7 (4.9) | .256 |
| Mean absolute liver dose (SD) | 11.2 (3.5) | 8.8 (2.9) | 12.7 (3.1) | <0.001 |
| Mean V20% heart dose (SD) | 9.7% (6.6) | 5.5% (4.7) | 12.4% (6.2) | 0.001 |
| Mean absolute heart dose (SD) | 588.05 (277.86) | 406.67 (198.42) | 699.70 (262.64) | <0.001 |
Abbreviations: SD = standard deviation; V20 = percentage of an organ receiving at least 20 Gy.
Toxicities among the 2 treatment groups were documented and compared (Table 4). The most common toxicity was nausea (n = 24, 57.1%). This is followed by fatigue (n = 22, 52.4%), anorexia (n = 6, 14.2%), pain (n = 4, 9.5%), diarrhea (n = 3, 7.2%), dyspnea (n = 2, 4.8%), vomiting (n = 1, 2.4%), dehydration (n = 1, 2.4%), anemia (n = 1, 2.4%), dermatitis (n = 1, 2.4%), and pericarditis (n = 1, 2.4%). Table 4 details the toxicity grade. There was no significant difference between the 2 treatment groups in terms of toxicity (P = .27). Overall toxicity taken as an outcome was not significantly different among those who had autoimmune disease (P = .404) or were treated with IMRT versus 3D (P = .490), or DIBH versus ITV (P = .213).
Table 4.
Grade of various toxicities among the 2 treatment groups
| All patients | Value or no. (%) |
|||
|---|---|---|---|---|
| Characteristics | All patients (n = 42) | Reduced RT dose ≤27 Gy (n = 16) | Standard RT dose ≥30 Gy (n = 26) | P value |
| Nausea | ||||
| G1 | 21 (50.0) | 9 (56.3) | 12 (46.2) | .559 |
| G2 | 3 (7.1) | 0 (0.0) | 3 (11.5) | |
| None | 18 (42.9) | 7 (43.8) | 11 (42.3) | |
| Vomiting | ||||
| G3 | 1 (2.4) | 1 (6.3) | 0 (0.0) | .381 |
| None | 41 (97.6) | 15 (93.8) | 26 (100.0) | |
| Diarrhea | ||||
| G1 | 2 (4.8) | 2 (12.5) | 0 (0.0) | .139 |
| G2 | 1 (2.4) | 0 (0.0) | 1 (3.8) | |
| None | 39 (92.9) | 14 (87.5) | 25 (96.2) | |
| Fatigue | ||||
| G1 | 17 (40.5) | 7 (43.8) | 10 (38.5) | .906 |
| G2 | 5 (11.9) | 2 (12.5) | 3 (11.5) | |
| None | 20 (47.6) | 7 (43.8) | 13 (50.0) | |
| Dehydration | ||||
| G1 | 1 (2.4) | 0 (0.0) | 1 (3.8) | 1.00 |
| None | 41 (97.6) | 16 (100.0) | 25 (96.2) | |
| Pain | ||||
| G1 | 3 (7.1) | 2 (12.5) | 1 (3.8) | .239 |
| G2 | 1 (2.4) | 1 (6.3) | 0 (0.0) | |
| None | 38 (90.5) | 13 (81.3) | 25 (96.2) | |
| Anorexia | ||||
| G1 | 3 (7.1) | 1 (6.3) | 2 (7.7) | 1.00 |
| G2 | 3 (7.1) | 1 (6.3) | 2 (7.7) | |
| None | 36 (85.7) | 14 (87.5) | 22 (84.6) | |
| Dyspnea | ||||
| G1 | 1 (2.4) | 1 (6.3) | 0 (0.0) | .139 |
| G2 | 1 (2.4) | 1 (6.3) | 0 (0.0) | |
| None | 40 (95.2) | 14 (87.5) | 26 (100.0) | |
| Anemia | ||||
| G1 | 1 (2.4) | 1 (6.3) | 0 (0.0) | .381 |
| None | 41 (97.6) | 15 (93.8) | 26 (100.0) | |
| Dermatitis | ||||
| G1 | 1 (2.4) | 0 (0.0) | 1 (3.8) | 1.00 |
| None | 41 (97.6) | 16 (100.0) | 25 (96.2) | |
| Pericarditis | ||||
| G2 | 1 (2.4) | 0 (0.0) | 1 (3.8) | 1.00 |
| None | 41 (97.6) | 16 (100.0) | 25 (96.2) | |
| Toxicity of any type and grade | ||||
| No | 10 (23.8) | 2 (12.5) | 8 (30.8) | .270 |
| Yes | 32 (76.2) | 14 (87.5) | 18 (69.2) | |
Abbreviations: G1 = grade 1 toxicity; G2 = grade 2 toxicity; G3 = grade 3 toxicity.
Discussion
This is the largest reported study to date evaluating the outcome of reduced dose RT in the treatment of gMALT lymphoma. In this cohort, patients treated with reduced dose RT (approximately 24 Gy) had a similar complete response rate and freedom from treatment failure to patients treated with standard dose RT (approximately 30 Gy). Reduced dose RT was recently implemented at our institution accounting for the shorter follow-up period in this subgroup. As such, binary logistic regression analysis for complete response rate, a time insensitive statistical measure, was performed, eliminating the effect of the follow-up period as the outcome is based on the first endoscopy. Despite that, there was no significant difference in the CR, FFTF, or OS between the 2 groups.
Previous studies have shown the excellent response RT has on the treatment of gMALT lymphoma with standard dose RT, 30 Gy. Memorial Sloan Kettering Cancer Center reported a 5-years relapse-free survival of 60% and OS of 89% for combined various sites of MALT lymphoma, including 155 gMALT patients treated mostly to 30 Gy.22 Princess Margaret Hospital reported a 10-years recurrence-free rate (RFR) of 92% among 25 patients with gMALT lymphoma treated to 20 to 35 Gy.23 The International Extranodal Lymphoma Study Group reported an 88% 10-year FFTF, 70% 10-year OS, and 96% CR in 102 stage I or II gMALT lymphoma patients.14 This study involved patients treated between 1981 and 2004. The median total RT dose was 40Gy (range, 26-46 Gy) administered through 3D techniques. There was no association between treatment dose and treatment failure. Patients treated to the stomach and perigastric lymph nodes had similar excellent outcomes with less toxicity compared with those treated to the whole abdomen, leading to the abandonment of whole abdomen treatment. Therefore, this study recommended a de-escalation of treatment in terms of radiation fields, but not doses.
In the same token, dose de-escalation was observed in several studies for other (nongMALT) indolent, low-grade B-cell lymphomas. A randomized clinical trial15 and several retrospective studies16,17 suggested that 24 to 25 Gy is effective for low-grade B-cell lymphoma. In some cases, 4 Gy might be effective as well.24,25 These studies in conjunction with the indolent nature and excellent prognosis of gMALT lymphoma, calls for lower RT doses in the treatment of gMALT lymphoma.
HELYX II trial randomized stage I or II gMALT patients to either receive 25.2 Gy (n = 10) or 36 Gy (n = 12). No recurrence was observed at 79 months follow-up in both arms. Moreover, the CR at a median time of 1.85 months was 100%.26 Similarly, Pinnix et al recently reported similar outcomes between gMALT lymphoma patients treated with 24 Gy (n = 11) and 30 Gy.21 The 2-years FFTF and OS were 100% and 97%, respectively, and the CR at a median time of 3.5 months was 94%.18 Our study reinforces the results of these 2 studies as 15/16 (94%) patients treated with reduced dose RT had CR at the first endoscopic assessment. The 1-year FFTF and OS were 100% in the reduced RT group. Table 5 outlines and compares the different studies that evaluated the role of RT in the treatment of gMALT lymphoma.
Table 5.
Studies that evaluated the role of radiation therapy in the treatment of gastric MALT lymphoma
| Study | No. of gMALT patients | RT dose range in Gy | Disease control rate of the whole cohort | OS of the whole cohort | CR of the whole cohort |
|---|---|---|---|---|---|
| Memorial Sloan Kettering Cancer Center21 | 155 | 30 (no specific upper and lower ranges) | N/A for gMALT alone | N/A for gMALT alone | N/A for gMALT alone |
| Princess Margaret Hospital22 | 25 | 20-35 | 92% RFR at 10 y | N/A for gMALT alone | N/A for gMALT alone |
| The International Extranodal Lymphoma Study Group14 | 102 | 26-46 | 88% FFTF at 10 and 15 y | 70% at 10 y | 96% (median time N/A) |
| HELYX II trial25 | 22 | 25.2-36 | 100% RFR at 6.5 y | N/A | 100% at a median time of 1.85 months |
| MD Anderson18 | 32 | 24-36 | 100% FFTF at 2 y | 97% at 2 y | 94% at a median time of 3.5 mo |
| Present study | 42 | 23.5-36 | 92% FFTF at 2 y | 96% at 2 y | 86% at a median time of 3 mo |
Abbreviations: FFTF = freedom from treatment failure; gMALT = of mucosa-associated lymphoid tissue; RFR = recurrence-free rate; RT = radiation therapy; N/A = not available; OS = overall survival.
In our cohort, patients treated with lower dose RT had significantly lower radiation dose to the heart and liver, consistent with prior results.18 Although this is true, patients treated more recently also underwent treatment with DIBH, which also could contribute to more favorable dosimetry.27 A larger proportion of reduced dose RT patients were treated with DIBH compared with standard dose (31% vs 19%). V20 heart dose, in addition to mean dose, was used to assess for cardiotoxicity to evaluate exposure of the inferior margin of the heart during gMALT treatment.28, 29, 30 Cardiovascular toxicity is a substantial concern in lymphoma patients.31,32 Given the linear, no-threshold radiation dose response relationship between mean heart dose and risk of cardiovascular,33, 34, 35 any possible reduction of heart dose may benefit lymphoma patients.36 Therefore, the significantly lower heart dose in the reduced RT group may be clinically valuable. Furthermore, recent literature has suggested radiation therapy may have a role in the risk of developing diabetes mellitus (DM). In a recent publication by van Nimwegen et al, higher doses of RT (≥36 Gy) increased the risk of developing DM in patients with Hodgkin lymphoma. Lower doses (10-35 Gy) did not.37 Other studies showed that even lower doses of RT (≥10 Gy38 or ≤26 Gy39) are associated with increased risk of developing DM. Although we did not assess radiation dose to the pancreas in our study, lower RT doses will likely reduce exposure of the pancreas and risk of developing DM. Overall, acute or early toxicity was not significantly different between the 2 groups, likely secondary to the low relative dose used for the treatment of lymphomas. The use of IMRT and DIBH also minimizes radiation exposure to organs at risk and therefore, lowers toxicity. Moreover, higher percentage of reduced RT patients received treatment planning with IMRT or VMAT that could have further contributed to the lower radiation dose to the organs at risk. The success of dose reduction as shown in this study raises the question of whether ultralow dose radiation therapy can obtain similar results. A prospective clinical trial is currently evaluating the 1 year response rate for 4 Gy in 2 fractions.40
This study has several limitations. The RT dose was not randomized and was given based on physician preference. Moreover, the sample size was small, and number of events was low which underpowered the statistical analysis and the generalizability of our conclusions. Furthermore, the follow-up period was limited especially for the reduced RT group. Thus, continued follow-up is warranted to confirm disease control.
Conclusions
This study further illustrates the excellent outcome of RT as frontline and salvage treatment for gMALT lymphoma. Reduced dose of RT (23.5-27 Gy) achieves excellent first posttreatment response rate, comparable to standard dose radiation therapy, yielding low toxicity and a shorter treatment course for patients. The reduced dose RT group will continue to be followed to confirm these results and long-term outcomes.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: Dr Tun reports grants from Celgene, grants from Mundipharma, grants from Acrotech, grants from Bristol Meyers Squibb, grants from Curis, grants from Spectrum pharmaceutical, grants from TG therapeutics, outside the submitted work; Dr Sher reports other from Janssen, other from InCyte, other from Akcea, other from Alnylam, outside the submitted work.
Data Sharing Statement: Research data are not available at this time.
References
- 1.Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. 2011;17:697–707. doi: 10.3748/wjg.v17.i6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang YG, Zhao L-Y, Liu C-Q. Clinical characteristics and prognostic factors of primary gastric lymphoma: A retrospective study with 165 cases. Medicine (Baltimore) 2016;95:e4250. doi: 10.1097/MD.0000000000004250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stolte M. Helicobacter pylori gastritis and gastric MALT-lymphoma. Lancet. 1992;339:745–746. doi: 10.1016/0140-6736(92)90645-j. [DOI] [PubMed] [Google Scholar]
- 4.Du MQ, Isaccson PQ. Gastric MALT lymphoma: From aetiology to treatment. Lancet Oncol. 2002;3:97–104. doi: 10.1016/s1470-2045(02)00651-4. [DOI] [PubMed] [Google Scholar]
- 5.Bayerdörffer E, Neubauer A, Rudolph B. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet. 1995;345:1591–1594. doi: 10.1016/s0140-6736(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 6.Savio A, Franzin G, Wotherspoon AC. Diagnosis and posttreatment follow-up of Helicobacter pylori-positive gastric lymphoma of mucosa-associated lymphoid tissue: Histology, polymerase chain reaction, or both? Blood. 1996;87:1255–1260. [PubMed] [Google Scholar]
- 7.Tsang RW, Gospodarowicz MK, Pintilie M. Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol. 2003;21:4157–4164. doi: 10.1200/JCO.2003.06.085. [DOI] [PubMed] [Google Scholar]
- 8.Schechter NR, Portlock CS, Yahalom J. Treatment of mucosa-associated lymphoid tissue lymphoma of the stomach with radiation alone. J Clin Oncol. 1998;16:1916–1921. doi: 10.1200/JCO.1998.16.5.1916. [DOI] [PubMed] [Google Scholar]
- 9.Wündisch T, Thiede C, Morgner A. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol. 2005;23:8018–8024. doi: 10.1200/JCO.2005.02.3903. [DOI] [PubMed] [Google Scholar]
- 10.Teckie S, Qi S, Lovie S. Long-term outcomes and patterns of relapse of early-stage extranodal marginal zone lymphoma treated with radiation therapy with curative intent. Int J Radiat Oncol Biol Phys. 2015;92:130–137. doi: 10.1016/j.ijrobp.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 11.Tsang RW, Gospodarowicz MK, Pintilie M. Stage I and II MALT lymphoma: Results of treatment with radiotherapy. Int J Radiat Oncol Biol Phys. 2001;50:1258–1264. doi: 10.1016/s0360-3016(01)01549-8. [DOI] [PubMed] [Google Scholar]
- 12.Ohkubo Y, Saito Y, Ushijima H. Radiotherapy for localized gastric mucosa-associated lymphoid tissue lymphoma: Long-term outcomes over 10 years. J Radiat Res. 2017;58:537–542. doi: 10.1093/jrr/rrw044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruskoné-Fourmestraux A, Matysiak-Budnik T, Fabiani B. Exclusive moderate-dose radiotherapy in gastric marginal zone B-cell MALT lymphoma: Results of a prospective study with a long term follow-up. Radiother Oncol. 2015;117:178–182. doi: 10.1016/j.radonc.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 14.Wirth A, Gospodarowicz M, Aleman BMP. Long-term outcome for gastric marginal zone lymphoma treated with radiotherapy: A retrospective, multi-centre, International Extranodal Lymphoma Study Group study. Ann Oncol. 2013;24:1344–1351. doi: 10.1093/annonc/mds623. [DOI] [PubMed] [Google Scholar]
- 15.Lowry L, Smith P, Qian W. Reduced dose radiotherapy for local control in non-Hodgkin lymphoma: A randomised phase III trial. Radiother Oncol. 2011;100:86–92. doi: 10.1016/j.radonc.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Goda JS, Le LW, Lapperriere NJ. Localized orbital mucosa-associated lymphoma tissue lymphoma managed with primary radiation therapy: Efficacy and toxicity. Int J Radiat Oncol Biol Phys. 2011;81:e659–e666. doi: 10.1016/j.ijrobp.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 17.Tran KH, Campbell BA, Fua T. Efficacy of low dose radiotherapy for primary orbital marginal zone lymphoma. Leuk Lymphoma. 2013;54:491–496. doi: 10.3109/10428194.2012.717279. [DOI] [PubMed] [Google Scholar]
- 18.Pinnix CC, Gunther JR, Milgrom SA. Outcomes after reduced-dose intensity modulated radiation therapy for gastric mucosa-associated lymphoid tissue (MALT) lymphoma. Int J Radiat Oncol Biol Phys. 2019;104:447–455. doi: 10.1016/j.ijrobp.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thieblemont C, Cascione L, Conconni A. A MALT lymphoma prognostic index. Blood. 2017;130:1409–1417. doi: 10.1182/blood-2017-03-771915. [DOI] [PubMed] [Google Scholar]
- 20.Illidge T, Specht L, Yahalom J. Modern radiation therapy for nodal non-Hodgkin lymphoma-target definition and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2014;89:49–58. doi: 10.1016/j.ijrobp.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf. Accessed March 23, 2021.
- 22.Teckie S, Qi S, Chelius M. Long-term outcome of 487 patients with early-stage extra-nodal marginal zone lymphoma. Ann Oncol. 2017;28:1064–1069. doi: 10.1093/annonc/mdx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goda JS, Gospodarowicz M, Pintilie M. Long-term outcome in localized extranodal mucosa-associated lymphoid tissue lymphomas treated with radiotherapy. Cancer. 2010;116:3815–3824. doi: 10.1002/cncr.25226. [DOI] [PubMed] [Google Scholar]
- 24.Fasola CE, Jones JC, Huang DD. Low-dose radiation therapy (2 Gy × 2) in the treatment of orbital lymphoma. Int J Radiat Oncol Biol Phys. 2013;86:930–935. doi: 10.1016/j.ijrobp.2013.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinnix CC, Dabaja BS, Milgrom SA. Ultra-low-dose radiotherapy for definitive management of ocular adnexal B-cell lymphoma. Head Neck. 2017;39:1095–1100. doi: 10.1002/hed.24717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmelz R, Miehlke S, Thiede C. Sequential H. pylori eradication and radiation therapy with reduced dose compared to standard dose for gastric MALT lymphoma stages IE & II1E: A prospective randomized trial. J Gastroenterol. 2019;54:388–395. doi: 10.1007/s00535-018-1517-4. [DOI] [PubMed] [Google Scholar]
- 27.Choi SH, Park SH, Lee JJB. Combining deep-inspiration breath hold and intensity-modulated radiotherapy for gastric mucosa-associated lymphoid tissue lymphoma: Dosimetric evaluation using comprehensive plan quality indices. Radiat Oncol. 2019;14:59. doi: 10.1186/s13014-019-1263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hahn E, Jiang H, Ng A. Late cardiac toxicity after mediastinal radiation therapy for hodgkin lymphoma: Contributions of coronary artery and whole heart dose-volume variables to risk prediction. Int J Radiat Oncol Biol Phys. 2017;98:1116–1123. doi: 10.1016/j.ijrobp.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 29.Bates JE, Howell RM, Liu Q. Therapy-related cardiac risk in childhood cancer survivors: An analysis of the childhood cancer survivor study. J Clin Oncol. 2019;37:1090–1101. doi: 10.1200/JCO.18.01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Nimwegen FA, Ntentas G, Darby SC. Risk of heart failure in survivors of Hodgkin lymphoma: Effects of cardiac exposure to radiation and anthracyclines. Blood. 2017;129:2257–2265. doi: 10.1182/blood-2016-09-740332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Nimwegen FA, Schaapveld M, Janus CP. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175:1007–1017. doi: 10.1001/jamainternmed.2015.1180. [DOI] [PubMed] [Google Scholar]
- 32.Bhakta N, Liu Q, Yeo F. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin's lymphoma: An analysis from the St Jude Lifetime Cohort Study. Lancet Oncol. 2016;17:1325–1334. doi: 10.1016/S1470-2045(16)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Nimwegen FA, Levra NG, Alongi F. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol. 2016;34:235–243. doi: 10.1200/JCO.2015.63.4444. [DOI] [PubMed] [Google Scholar]
- 34.Darby SC, Ewertz M, McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 35.Maraldo MV, Giusti F, Vogelius IR. Cardiovascular disease after treatment for Hodgkin's lymphoma: An analysis of nine collaborative EORTC-LYSA trials. Lancet Haematol. 2015;2:e492–e502. doi: 10.1016/S2352-3026(15)00153-2. [DOI] [PubMed] [Google Scholar]
- 36.Tseng YD, Cutter DJ, Plastaras JP. Evidence-based Review on the Use of Proton Therapy in Lymphoma From the Particle Therapy Cooperative Group (PTCOG) Lymphoma Subcommittee. Int J Radiat Oncol Biol Phys. 2017;99:825–842. doi: 10.1016/j.ijrobp.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 37.van Nimwegen F, Schaapveld M, Janus CPM. Risk of diabetes mellitus in long-term survivors of Hodgkin lymphoma. J Clin Oncol. 2014;32:3257–3263. doi: 10.1200/JCO.2013.54.4379. [DOI] [PubMed] [Google Scholar]
- 38.de Vathaire F, El-Fayech C, Ben Ayed FF. Radiation dose to the pancreas and risk of diabetes mellitus in childhood cancer survivors: A retrospective cohort study. Lancet Oncol. 2012;13:1002–1010. doi: 10.1016/S1470-2045(12)70323-6. [DOI] [PubMed] [Google Scholar]
- 39.Groot HJ, Gietema JA, Aleman BMP. Risk of diabetes after para-aortic radiation for testicular cancer. Br J Cancer. 2018;119:901–907. doi: 10.1038/s41416-018-0248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.US National Library of Medicine. ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT03680586?cond=lymphoma+gastric&draw=2&rank=8. Accessed March 23, 2021.