Abstract
Objective
In the PALOMA-2 trial, palbociclib in combination with letrozole prolonged progression-free survival (PFS) and exhibited an acceptable safety profile in patients with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ABC). This post hoc analysis of PALOMA-2 evaluated the efficacy and safety of palbociclib plus letrozole in patients with preexisting conditions grouped by Medical Dictionary for Regulatory Activities (MedDRA) System Organ Class (SOC).
Methods
Postmenopausal patients without prior treatment for ABC were randomized 2:1 to receive palbociclib (125 mg/d on a 3 weeks on/1 week off schedule) plus letrozole (2.5 mg/d, continuous) or placebo plus letrozole. Patients were grouped by the following MedDRA SOC preexisting conditions: gastrointestinal, musculoskeletal, metabolic, and vascular/cardiac. Median PFS was estimated by the Kaplan-Meier method, and treatment emergent adverse events (AEs) were compared between treatment arms within each preexisting condition subgroup.
Results
At baseline, 276 (41.4 %) patients had preexisting gastrointestinal disorders, 390 (58.6 %) had musculoskeletal disorders, 259 (38.9 %) had metabolic disorders, and 382 (57.4 %) had vascular/cardiac disorders. Baseline characteristics were similar between subgroups and between each arm within subgroups. Regardless of baseline preexisting condition, palbociclib plus letrozole prolonged PFS compared with placebo plus letrozole. Treatment-emergent AEs associated with palbociclib plus letrozole and dose modifications due to AEs were similar across preexisting condition subgroups.
Conclusion
This post hoc analysis of PALOMA-2 demonstrated a favorable effect of palbociclib on PFS and a safety profile consistent with previous observations, regardless of underlying preexisting condition. Pfizer Inc (NCT01740427).
Keywords: Advanced breast cancer, Preexisting condition, Palbociclib, Progression-free survival, Safety
Highlights
-
•
Preexisting conditions can affect the safety and efficacy of breast cancer therapies.
-
•
This is a post hoc analysis of patients with preexisting conditions from PALOMA-2.
-
•
Palbociclib prolonged median PFS, regardless of preexisting condition.
-
•
Within each treatment arm, AEs were similar regardless of preexisting condition.
1. Introduction
Preexisting conditions are common in cancer patients, particularly in those who are older, and are associated with worse survival rates [1] and higher rates of complications [2]. Studies and clinician surveys have shown that there is considerable inconsistency in decisions relating to cancer treatment in patients with preexisting conditions [2]. Because of clinician concerns that preexisting conditions may exacerbate treatment-related toxicities or affect the clinical benefit provided by a therapy, these patients are typically less likely to receive cancer therapy [2]. Because patients with preexisting conditions are often underrepresented in clinical trials, information regarding treatment effectiveness and safety is often limited [2]. To improve clinical decision making with regard to cancer therapy, an understanding of the effect of preexisting conditions on the efficacy and safety of cancer therapies is needed.
Palbociclib, a selective cyclin-dependent kinase 4/6 (CDK4/6) inhibitor, is approved in combination with endocrine therapy (ET) to treat hormone receptor–positive (HR+)/human epidermal growth factor receptor 2–negative (HER2–) advanced breast cancer (ABC) [3]. In the phase III PALOMA-2 study, palbociclib plus letrozole significantly prolonged median progression-free survival (PFS) versus placebo plus letrozole (27.6 vs 14.5 months; hazard ratio, 0.563 [95 % CI, 0.461–0.687]; P < 0.0001) in patients with estrogen receptor positive (ER+)/HER2– ABC [4]. Previous analyses also demonstrated that median PFS was longer with palbociclib plus letrozole versus placebo plus letrozole across all subgroups examined (eg, age, visceral and nonvisceral disease, bone-only and no bone-only disease) [4].
The effect of preexisting conditions on the efficacy and safety of palbociclib has not been previously evaluated. This post hoc analysis assessed the efficacy and safety of palbociclib plus letrozole in patients from PALOMA-2 with baseline preexisting conditions grouped by Medical Dictionary for Regulatory Activities (MedDRA) System Organ Class (SOC).
2. Patients and methods
2.1. Study design and patients
PALOMA-2 is a double-blind, placebo-controlled, multicenter, phase III randomized trial in women with ER+/HER2− ABC (NCT01740427). Postmenopausal women diagnosed with ER+/HER2− recurrent or metastatic tumors were eligible if they had not received prior systemic therapy for advanced disease, had adequate organ function, had an Eastern Cooperative Oncology Group performance status of 0–2 and measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, or bone-only lesions. Patients at risk for life-threatening complications due to advanced, symptomatic visceral spread were excluded. Patients (n = 666) were randomized in a 2:1 ratio to receive 125 mg/d palbociclib orally in 4-week cycles (3 weeks of treatment followed by 1 week off) or matching placebo. All patients were treated orally with 2.5 mg/d of letrozole. Additional details of the study design and eligibility criteria have been previously published [5]. The PALOMA-2 study was approved by an institutional review board/independent ethics committee and was conducted in accordance with Good Clinical Practice principles and the Declaration of Helsinki. All patients provided informed consent before the study.
2.2. Data analysis
Patients in this analysis were grouped by the following MedDRA SOC preexisting conditions reported at baseline: gastrointestinal disorders, musculoskeletal and connective tissue disorders, metabolic and nutrition disorders, and vascular/cardiac disorders. Preferred terms in the vascular and cardiac disorders SOC were combined into one category (vascular/cardiac) owing to interrelatedness. Twelve patients were excluded from the gastrointestinal subgroup because it was believed that their preexisting conditions would not affect the efficacy or safety of treatment: dental caries, flatulence, oral hypoesthesia, oral disorder, oral pain, periodontal disease, poor dental condition, sensitivity of teeth, tooth deposit, tooth resorption, and toothache. Patients who received ≥1 dose of the study drug were included in safety analyses. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Progression-free survival was assessed by investigators in the intent-to-treat population and defined as the time from randomization to radiologically confirmed disease progression, according to RECIST version 1.1., or death during the study. The Kaplan-Meier method was used to obtain estimates of median PFS, with corresponding two-sided 95 % CIs. A Cox proportional-hazards model was used to estimate hazard ratios. The data cutoff date for this analysis was May 31, 2017.
3. Results
3.1. Baseline demographic and disease characteristics
From February 2013 through July 2014, a total of 666 women were randomized, with 444 assigned to receive palbociclib plus letrozole and 222 assigned to receive placebo plus letrozole. At baseline, 276 patients (41.4 %) had preexisting gastrointestinal disorders, 390 (58.6 %) had musculoskeletal disorders, 259 (38.9 %) had metabolic disorders, and 382 (57.4 %) had vascular/cardiac disorders. The most common gastrointestinal, musculoskeletal, metabolic, and vascular/cardiac disorders were constipation (33.3 %), back pain (31.0 %), hypercholesterolemia (29.0 %), and hypertension (69.6 %), respectively. Among all patients, 53.2 % had preexisting vascular disorders and 14.1 % had preexisting cardiac disorders. Baseline disease characteristics and demographics were generally similar across the preexisting condition subgroups (Table 1; Supplementary Table 1) and between treatment arms within each subgroup. The median (range) age was 65 (30–88) years for patients with gastrointestinal disorders, 63 (32–88) years for those with musculoskeletal disorders, 65 (33–88) years with metabolic disorders, and 63 (30–89) years with vascular/cardiac disorders. Within each subgroup, ≥45 % of patients also had ≥1 of the other preexisting conditions.
Table 1.
Select baseline demographic and disease characteristics by preexisting condition.
| Demographics and Disease Characteristics | Preexisting Condition |
|||
|---|---|---|---|---|
| Gastrointestinal (n = 276) | Musculoskeletal (n = 390) | Metabolic (n = 259) | Vascular/Cardiac (n = 382) | |
| Age, median (range), y | 63 (30–88) | 63 (32–88) | 65 (33–88) | 63 (30–89) |
| Race, n (%) | ||||
| White | 227 (82.2) | 316 (81.0) | 204 (78.8) | 320 (83.8) |
| Black | 5 (1.8) | 6 (1.5) | 7 (2.7) | 8 (2.1) |
| Asian | 24 (8.7) | 45 (11.5) | 34 (13.1) | 34 (8.9) |
| Other | 20 (7.2) | 23 (5.9) | 14 (5.4) | 20 (5.2) |
| Disease site, n (%) | ||||
| Visceral | 132 (47.8) | 169 (43.3) | 135 (52.1) | 179 (46.9) |
| Nonvisceral | 144 (52.2) | 221 (56.7) | 124 (47.9) | 203 (53.1) |
| Disease-free interval, n (%) | ||||
| De novo metastatic | 116 (42.0) | 135 (34.6) | 111 (42.9) | 166 (43.5) |
| ≤12 mo | 50 (18.1) | 89 (22.8) | 30 (11.6) | 73 (19.1) |
| >12 mo | 110 (39.9) | 166 (42.6) | 118 (45.6) | 143 (37.4) |
| Preexisting condition,a n (%) | ||||
| Vascular/cardiac disorders | 183 (66.3) | 238 (61.0) | 192 (74.1) | 382 (100) |
| Hot flush | 37 (13.4) | 54 (13.8) | 24 (9.3) | 69 (18.1) |
| Hypertension | 126 (45.7) | 168 (43.1) | 161 (62.2) | 266 (69.6) |
| Metabolic disorders | 135 (48.9) | 177 (45.4) | 259 (100) | 192 (50.3) |
| Diabetes mellitus | 26 (9.4) | 35 (9.0) | 55 (21.2) | 43 (11.3) |
| Hypercholesterolemia | 38 (13.8) | 55 (14.1) | 75 (29.0) | 56 (14.7) |
| Body mass index >30 kg/m2 | 96 (34.8) | 113 (29.0) | 98 (37.8) | 134 (35.1) |
| Psychiatric disorders | 132 (47.8) | 178 (45.6) | 120 (46.3) | 148 (38.7) |
| Anxiety | 78 (28.3) | 103 (26.4) | 66 (25.5) | 85 (22.3) |
| Depression | 53 (19.2) | 69 (17.7) | 51 (19.7) | 52 (13.6) |
| Insomnia | 72 (26.1) | 84 (21.5) | 53 (20.5) | 62 (16.2) |
| Musculoskeletal disorders | 186 (67.4) | 390 (100) | 177 (68.3) | 238 (62.3) |
| Arthralgia | 47 (17.0) | 87 (22.3) | 47 (18.1) | 60 (15.7) |
| Back pain | 63 (22.8) | 121 (31.0) | 56 (21.6) | 75 (19.6) |
| Bone pain | 44 (15.9) | 71 (18.2) | 34 (13.1) | 40 (10.5) |
| Osteoarthritis | 33 (12.0) | 61 (15.6) | 35 (13.5) | 41 (10.7) |
| General disorders | 108 (39.1) | 125 (32.1) | 88 (34.0) | 114 (29.8) |
| Fatigue | 81 (29.3) | 87 (22.3) | 61 (23.6) | 76 (19.9) |
| Endocrine disorders | 62 (22.5) | 88 (22.6) | 60 (23.2) | 83 (21.7) |
| Hypothyroidism | 45 (16.3) | 64 (16.4) | 45 (17.4) | 59 (15.4) |
| Gastrointestinal disorders | 276 (100) | 193 (49.5) | 137 (52.9) | 186 (48.7) |
| Constipation | 92 (33.3) | 71 (18.2) | 46 (17.8) | 60 (15.7) |
| GERD | 72 (26.1) | 58 (14.9) | 47 (18.1) | 51 (13.4) |
| Nausea | 51 (18.5) | 40 (10.3) | 34 (13.1) | 33 (8.6) |
GERD = gastroesophageal reflux disease.
>15 % in any group.
3.2. Efficacy by preexisting condition
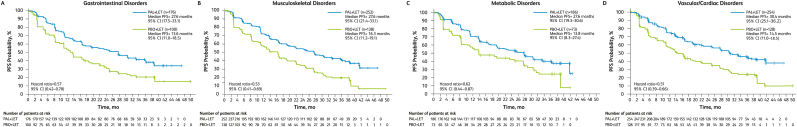
The addition of palbociclib to letrozole significantly prolonged median PFS compared with letrozole alone, regardless of preexisting condition (Fig. 1). Among patients with gastrointestinal disorders, median (95 % CI) PFS was 27.6 (17.5–33.1) months in the palbociclib arm (n = 176) versus 13.6 (11.0–18.5) months in the placebo arm (n = 100; hazard ratio [95 % CI], 0.57 [0.42–0.78]). In the musculoskeletal disorders subgroup, median PFS was 27.6 (21.4–33.1) months in the palbociclib arm (n = 252) versus 16.3 (11.2–19.1) months in the placebo arm (n = 138; hazard ratio, 0.53 [0.41–0.69]). In the metabolic disorders subgroup, median PFS was 27.6 (19.3–30.6) months in the palbociclib arm (n = 186) versus 13.8 (8.3–27.4) months in the placebo arm (n = 73; hazard ratio, 0.62 [0.44–0.87]). In the vascular/cardiac disorders subgroup, median PFS was 30.4 (25.1–36.2) months in the palbociclib arm (n = 254) versus 14.5 (11.0–18.5) months in the placebo arm (n = 128; hazard ratio, 0.51 [0.39–0.66]).
Fig. 1.
Progression-Free Survival by Preexisting Condition. LET = letrozole; PAL = palbociclib; PBO = placebo; PFS = progression-free survival.
To determine if vascular or cardiac preexisting conditions alone or a combination of four or more preexisting conditions may affect outcomes, we evaluated the effect of each of these conditions separately on PFS. The benefit of palbociclib in prolonging PFS was maintained in patients with preexisting vascular or cardiac conditions and in patients with four or more preexisting conditions (Supplemental Figure 1).
3.3. Safety by preexisting condition
Generally, more AEs were reported in the palbociclib plus letrozole arm in all subgroups; however, within each treatment arm, AEs were similar across the preexisting condition subgroups (Table 2). In the palbociclib plus letrozole arm, all-grade neutropenia was reported in 80 %–84 % of patients across the preexisting condition subgroups; in the placebo plus letrozole arm, the incidence was between 3 % and 9 % of patients. Grade 3/4 neutropenia was reported in 69 %–71 % of patients receiving palbociclib plus letrozole across the preexisting condition in the gastrointestinal, musculoskeletal, metabolic and vascular/cardiac subgroups, respectively. In the placebo plus letrozole arm, the incidence of grade 3/4 neutropenia was ≤2 % across subgroups. All-grade infection (all preferred terms in the Infections and Infestations SOC) was reported in 63 %–69 % of patients in the palbociclib plus letrozole arm in each of the preexisting condition subgroups compared with 46 %–51 % with placebo plus letrozole. Grade 3/4 infections in the palbociclib plus letrozole arm were between 8 % and 13 % in each of the preexisting condition groups and between 2 % and 6 % in the placebo plus letrozole arm. In the palbociclib plus letrozole arm, febrile neutropenia occurred in 2 %–3 % of patients in each of the preexisting condition subgroups; febrile neutropenia was not reported with placebo plus letrozole in any subgroup. The incidence of AEs was also similar in subgroups of patients with vascular or cardiac preexisting conditions alone or a combination of four or more preexisting conditions (Supplementary Table 2).
Table 2.
Treatment-emergent adverse events by preexisting condition.
| Adverse Event, % | Gastrointestinal |
Musculoskeletal |
Metabolic |
Vascular/Cardiac |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAL + LET (n = 176) |
PBO + LET (n = 100) |
PAL + LET (n = 252) |
PBO + LET (n = 138) |
PAL + LET (n = 186) |
PBO + LET (n = 73) |
PAL + LET (n = 254) |
PBO + LET (n = 128) |
||||||||||
| All Grades | Grade 3/4 |
All Grades | Grade 3/4 |
All Grades | Grade 3/4 |
All Grades | Grade 3/4 |
All Grades | Grade 3/4 |
All Grades | Grade 3/4 |
All Grades | Grade 3/4 |
All Grades | Grade 3/4 |
||
| AE in ≥20 % of any group | |||||||||||||||||
| Neutropenia | 83.5 | 57.4/13.1 | 8.0 | 1.0/1.0 | 80.6 | 61.1/10.3 | 8.7 | 1.4/0.7 | 79.6 | 58.6/10.2 | 2.7 | 1.4/0 | 79.9 | 58.3/10.2 | 3.1 | 0.8/0 | |
| Infections | 65.9 | 10.8/1.7 | 47.0 | 3.0/0 | 68.3 | 7.1/0.8 | 48.6 | 2.2/0 | 67.7 | 8.1/1.6 | 50.7 | 5.5/0 | 63.8 | 8.7/1.2 | 46.1 | 3.9/0 | |
| Alopecia | 36.9 | N/A | 19.0 | N/A | 34.5 | N/A | 18.1 | N/A | 34.4 | N/A | 16.4 | N/A | 33.9 | N/A | 16.4 | N/A | |
| Arthralgia | 36.9 | 2.3/0 | 30.0 | 1.0/0 | 44.8 | 1.6/0 | 36.2 | 1.4/0 | 37.1 | 1.1/0 | 38.4 | 1.4/0 | 36.6 | 1.2/0 | 33.6 | 1.6/0 | |
| Leukopenia | 44.9 | 27.3/1.1 | 4.0 | 0/0 | 37.7 | 23.0/0.8 | 1.4 | 0/0 | 39.2 | 23.1/0.5 | 0 | 0 | 40.9 | 25.2/0.8 | 0.8 | 0/0 | |
| Fatigue | 44.3 | 4.0/0 | 29.0 | 1.0/0 | 50.4 | 3.2/0 | 28.3 | 2.2/0 | 39.8 | 2.7/0 | 31.5 | 2.7/0 | 41.7 | 3.1/0 | 29.7 | 2.3/0 | |
| Stomatitis | 37.5 | 1.1/0 | 19.0 | 0/0 | 32.5 | 1.6/0 | 18.8 | 0/0 | 28.0 | 1.1/0 | 15.1 | 0/0 | 30.7 | 1.2/0 | 13.3 | 0/0 | |
| Nausea | 40.9 | 0.6/0 | 38.0 | 3.0/0 | 45.6 | 0.4/0 | 30.4 | 1.4/0 | 43.0 | 0/0 | 34.2 | 2.7/0 | 36.6 | 0/0 | 25.8 | 1.6/0 | |
| Diarrhea | 36.9 | 2.3/0 | 26.0 | 2.0/0 | 35.3 | 2.0/0 | 26.8 | 1.4/0 | 34.4 | 1.6/0 | 26.0 | 2.7/0 | 31.1 | 2.0/0 | 20.3 | 0.8/0 | |
| Back pain | 31.3 | 3.4/0 | 22.0 | 0/0 | 30.6 | 2.8/0 | 27.5 | 0/0 | 23.7 | 1.1/0 | 26.0 | 0/0 | 24.8 | 2.0/0 | 22.7 | 0/0 | |
| Anemia | 34.7 | 6.8/0 | 10.0 | 1.0/0 | 25.8 | 7.5/0 | 8.7 | 0.7/0 | 30.1 | 8.6/0 | 8.2 | 0/0 | 29.5 | 6.7/0.4 | 5.5 | 0.8/0 | |
| Headache | 29.5 | 0/0 | 30.0 | 3.0/0 | 29.0 | 0.4/0 | 31.9 | 2.9/0 | 25.8 | 0/0 | 34.2 | 1.4/0 | 24.4 | 0.8/0 | 28.1 | 1.6/0 | |
| Hot flush | 28.4 | 0/0 | 38.0 | 0/0 | 26.6 | 0/0 | 36.2 | 0/0 | 25.3 | 0/0 | 35.6 | 0/0 | 22.4 | 0/0 | 30.5 | 0/0 | |
| Cough | 27.8 | 0.6/0 | 20.0 | 0/0 | 29.4 | 0.4/0 | 22.5 | 0/0 | 26.3 | 0/0 | 21.9 | 0/0 | 28.7 | 0/0 | 18.0 | 0/0 | |
| Abdominal pain | 19.9 | 3.4/0 | 7.0 | 0/0 | 16.7 | 2.0/10 | 8.0 | 0/0 | 14.0 | 0.5/0 | 9.6 | 0/0 | 15.7 | 1.2/0 | 4.7 | 0/0 | |
| Dizziness | 19.9 | 0.6/0 | 22.0 | 0/0 | 19.8 | 0.8/0 | 17.4 | 0/0 | 17.7 | 0/0 | 21.9 | 0/0 | 16.9 | 0.4/0 | 17.2 | 0/0 | |
| Viral upper RTI | 15.3 | 0/0 | 10.0 | 0/0 | 16.7 | 0/0 | 13.0 | 0/0 | 22.0 | 0/0 | 13.7 | 0/0 | 17.7 | 0/0 | 10.2 | 0/0 | |
| Asthenia | 23.3 | 5.1/0 | 10.0 | 0/0 | 14.3 | 2.4/0 | 11.6 | 0/0 | 17.2 | 1.6/0 | 13.7 | 0/0 | 16.9 | 3.9/0 | 11.7 | 0/0 | |
| Rash | 23.3 | 1.1/0 | 6.0 | 0/0 | 22.6 | 0.8/0 | 13.0 | 0.7/0 | 21.5 | 1.6/0 | 13.7 | 1.4/0 | 19.7 | 0.8/0 | 11.7 | 0.8/0 | |
| Constipation | 30.1 | 1.1/0 | 19.0 | 1.0/0 | 27.4 | 0.4/0 | 18.8 | 0.7/0 | 24.7 | 0/0 | 20.5 | 1.4/0 | 22.8 | 0/0 | 18.8 | 0.8/0 | |
| Vomiting | 25.6 | 2.3/0 | 24.0 | 2.0/0 | 22.6 | 0.8/0 | 19.6 | 1.4/0 | 22.6 | 0.5/0 | 20.5 | 2.7/0 | 17.7 | 0.4/0 | 18.0 | 1.6/0 | |
| Decreased appetite | 23.3 | 1.7/0 | 10.0 | 0/0 | 23.4 | 1.2/0 | 10.1 | 0/0 | 20.4 | 1.6/0 | 6.8 | 0/0 | 17.7 | 0.8/0 | 7.0 | 0/0 | |
| Dyspnea | 23.3 | 1.7/0 | 18.0 | 1.0/0 | 15.5 | 0.8/0 | 17.4 | 1.4/0 | 18.3 | 1.6/0 | 19.2 | 2.7/0 | 16.5 | 0.4/0 | 16.4 | 3.1/0 | |
| Pain in extremity | 23.9 | 0.6/0 | 21.0 | 2.0/0 | 23.8 | 0.4/0 | 22.5 | 0.7/0 | 18.8 | 0/0 | 17.8 | 1.4/0 | 20.1 | 0.4/0 | 21.1 | 1.6/0 | |
| AEs of special interest | |||||||||||||||||
| Febrile neutropenia | 2.8 | 2.3/0.6 | 0 | 0/0 | 2.8 | 2.4/0.4 | 0 | 0/0 | 2.2 | 1.6/0.5 | 0 | 0 | 3.1 | 2.4/0.8 | 0 | 0/0 | |
| Pulmonary embolism | 1.7 | 0.6/0.6 | 3.0 | 1.0/1.0 | 2.8 | 2.0/0.4 | 2.2 | 0.7/0.7 | 2.2 | 2.2/0 | 2.7 | 0/1.4 | 2.0 | 1.2/0.4 | 3.1 | 1.6/0.8 | |
| Hypertension | 12.5 | 8.0/0 | 8.0 | 7.0/0 | 9.5 | 5.6/0 | 10.1 | 7.2/0 | 10.8 | 7.0/0 | 11.0 | 9.6/0 | 10.2 | 5.5/0 | 12.5 | 10.2/0 | |
| Hyperglycemia | 5.1 | 0.6/0 | 9.0 | 1.0/0 | 2.8 | 0.8/0 | 6.5 | 1.4/0 | 2.7 | 1.6/0 | 9.6 | 2.7/0 | 5.5 | 1.2/0 | 7.8 | 0.8/0 | |
| Hypercholesterolemia | 0.6 | 0/0 | 3.0 | 0/0 | 1.2 | 0/0 | 1.4 | 0/0 | 1.6 | 0/0 | 1.4 | 0/0 | 1.6 | 0/0 | 2.3 | 0/0 | |
AE = adverse event; LET = letrozole; N/A = not applicable; PAL = palbociclib; PBO = placebo; RTI = respiratory tract infection.
Dose modifications were similar across all subgroups within each treatment arm (Table 3). In the palbociclib plus letrozole arm, 40 %–45 % of patients in each of the preexisting condition subgroups experienced dose reductions due to AEs. When patients with preexisting vascular or cardiac conditions were analyzed separately, the rates of dose reductions in the palbociclib plus letrozole arm (41.4 % and 36.9 %, respectively) were similar to the rate observed in patients with combined vascular/cardiac disorders (41.3 %; Table 3). Patients in the palbociclib plus letrozole arm with a least four preexisting conditions also experienced a similar level of dose reductions (41.2 %; Table 3). In the placebo plus letrozole arm, <3 % of patients in any of the preexisting condition subgroups experienced a dose reduction because of an AE (Table 3). Treatment discontinuation rates were also similar between the subgroups (Table 3). In the palbociclib plus letrozole arm, 12 %–13 % of patients in each of the preexisting condition subgroups discontinued treatment due to an AE. Similar rates of treatment discontinuation were observed with palbociclib plus letrozole in patients with either vascular or cardiac preexisting conditions (Table 3). However, in patients with at least four preexisting conditions, the discontinuation rate was 19.6 % (Table 3), although the number of patients in this subgroup was small. Among patients who received placebo plus letrozole, 4 %–6 % of patients in each of the preexisting condition subgroups discontinued treatment due to an AE. Similarly low rates of treatment discontinuation due to AEs were observed in patients with preexisting vascular or cardiac conditions or four or more preexisting conditions who received placebo plus letrozole (Table 3).
Table 3.
Dose modifications by preexisting condition.
| Dose Modification, % | Gastrointestinal |
Musculoskeletal |
Metabolic |
Vascular/Cardiac |
Vascular |
Cardiac |
≥4 Preexisting Conditions |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAL + LET (n = 176) | PBO + LET (n = 100) | PAL + LET (n = 252) | PBO + LET (n = 138) | PAL + LET (n = 254) | PBO + LET (n = 128) | PAL + LET (n = 254) | PBO + LET (n = 128) | PAL + LET (n = 232) | PBO + LET (n = 122) | PAL + LET (n = 65) | PBO + LET (n = 29) | PAL + LET (n = 51) | PBO + LET (n = 34) | |
| Dose reduction | ||||||||||||||
| No dose reduction | 55.1 | 98.0 | 59.5 | 97.1 | 59.7 | 98.6 | 58.7 | 99.2 | 58.6 | 99.2 | 63.1 | 100 | 58.8 | 100 |
| ≥1 dose reduction | 44.9 | 2.0 | 40.5 | 2.9 | 40.3 | 1.4 | 41.3 | 0.8 | 41.4 | 0.8 | 36.9 | 0 | 41.2 | 0 |
| Median time to first dose reduction, d | 99.0 | – | 97.5 | – | 91.0 | – | 99.0 | 1136 | 98.5 | 1136 | 157 | – | 179 | – |
| 1 dose reduction | 23.9 | 2.0 | 22.2 | 2.9 | 22.6 | 1.4 | 26.0 | 0.8 | 25.4 | 0.8 | 26.2 | 0 | 21.6 | 0 |
| 2 dose reductions | 21.0 | 0 | 18.3 | 0 | 17.7 | 0 | 15.4 | 0 | 15.9 | 0 | 10.8 | 0 | 19.6 | 0 |
| Discontinuations and dose modifications due to AEs | ||||||||||||||
| Discontinuation due to an AE | 12.5 | 5.0 | 13.1 | 4.4 | 12.9 | 4.1 | 12.2 | 5.5 | 12.5 | 5.7 | 12.3 | 10.3 | 19.6 | 2.9 |
| Dose reduction | ||||||||||||||
| Due to AE | 44.9 | 2 | 40.1 | 2.9 | 40.3 | 1.4 | 40.9 | 0.8 | 41.0 | 0.8 | 36.9 | 0 | 41.2 | 0 |
| Discontinued due to treatment-related AE | 6.8 | 0 | 6.8 | 0 | 4.3 | 0 | 5.5 | 0 | 5.6 | 0 | 4.6 | 0 | 7.8 | 0 |
| Dose interruption or delay | ||||||||||||||
| Due to AE | 81.3 | 17.0 | 79.4 | 19.6 | 79 | 23.3 | 78.4 | 19.5 | 78.5 | 19.7 | 72.3 | 10.3 | 78.4 | 14.7 |
| Discontinued due to treatment-related AE | 8.5 | 1.0 | 9.5 | 1.5 | 8.6 | 2.7 | 7.5 | 1.6 | 7.8 | 1.6 | 6.2 | 0 | 9.8 | 0 |
AE = adverse event; LET = letrozole; PAL = palbociclib; PBO = placebo.
4. Discussion
Preexisting conditions in patients with breast cancer are common, particularly in those who are older. A study of 51,950 patients ≥66 years old diagnosed with breast cancer found that 18 % of patients had coronary artery disease, 14 % had diabetes mellitus, and 50 % were hypertensive [6]. Patients with breast cancer with preexisting conditions have reduced survival, experience greater treatment-related toxicity, and diminished quality of cancer-related treatment [[7], [8], [9]]. Such findings demonstrate the importance of evaluating the effect of preexisting conditions on drug efficacy and toxicity in patients with cancer. CDK4/6 inhibitors have become the standard of care for patients with HR+/HER2− ABC and have been shown to be generally well tolerated [10]. However, there is limited information on the effect of these drugs in patients with preexisting conditions. PALOMA-2, a large phase 3 study in which 39 % of patients were ≥65 years old provides an opportunity to study the effects of preexisting conditions on the efficacy and safety of palbociclib [5].
In this analysis, palbociclib plus letrozole significantly prolonged median PFS compared with placebo plus letrozole in each of the preexisting condition subgroups examined (gastrointestinal, 27.6 vs 13.6 months; musculoskeletal, 27.6 vs 16.3 months; metabolic, 27.6 vs 13.8 months; vascular/cardiac, 30.4 vs 14.5 months; vascular only, 30.6 vs 15.9 months). Palbociclib plus letrozole prolonged median PFS compared with placebo plus letrozole to a similar extent in subgroups of patients with preexisting cardiac conditions and four or more preexisting conditions; however, there were relatively few patients within each of these subgroups, and the results should be interpreted with caution. The median PFS observed with palbociclib in these subgroups was consistent with results from the overall study population from PALOMA-2 (27.6 months in the palbociclib plus letrozole arm vs 14.5 months in the placebo plus letrozole arm) [4]. Our findings are also consistent with a recent study evaluating the efficacy and safety of palbociclib plus ET in older patients (≥65 years) using pooled data from the three PALOMA clinical trials [11]. Although older patients in that analysis had a higher incidence of preexisting conditions at baseline, including hypertension, renal disease, metabolism and nutritional disorders, and vascular disorders, the addition of palbociclib to ET prolonged median PFS compared with ET regardless of age.
Neutropenia and leukopenia are commonly reported AEs of CDK4/6 inhibitors, including palbociclib [5]. Compromised immune function as a result of leukopenia could be of particular concern in patients with preexisting conditions, potentially compounding complications associated with underlying conditions. For example, diabetes is associated with a higher risk of infection, which may be related to diminished T cell–associated immune responses [12]. However, the incidence of neutropenia, leukopenia, and infection with palbociclib treatment in this study was similar regardless of preexisting condition. Incidence of neutropenia and leukopenia was also similar to those reported in patients who received palbociclib in the overall study population of PALOMA-2. Febrile neutropenia was reported in 2.2 %–3.1 % of patients who received palbociclib in the preexisting condition subgroups, a rate that is similar to that observed with palbociclib in PALOMA-2 (2.0 %) [4].
Given that there were no new safety signals observed in patients with preexisting conditions, it is perhaps unsurprising that palbociclib dose modifications were similar across preexisting condition subgroups. In the overall PALOMA-2 population, 39.4 % of patients receiving palbociclib plus letrozole experienced a dose reduction [13]. This is similar to the rates of palbociclib dose reduction in each of the preexisting condition subgroups reported here. Rates of palbociclib discontinuation due to AEs were generally similar between the preexisting condition subgroups and were comparable to the rate of discontinuation of palbociclib (12.2 %) due to AEs in the overall PALOMA-2 population [4]. Although the palbociclib discontinuation rate due to AEs was slightly higher (19.6 %) in patients with four or more preexisting conditions, this result should be interpreted with caution because of the relatively small number of patients in this subgroup. Thus, in this post hoc analysis of PALOMA-2, there is no indication that underlying conditions affect the safety or tolerability of palbociclib treatment.
Several limitations of this study should be noted. The PALOMA-2 trial was not designed to evaluate the effect of preexisting conditions on palbociclib efficacy and safety; therefore, the results of this post hoc analysis should be interpreted with caution. Additionally, because patients with underlying conditions are typically underrepresented in randomized controlled trials, the findings in this study cannot be generalized to the wider population. However, it is reassuring that at least the patients with preexisting conditions in this trial had a positive benefit:risk ratio of palbociclib plus letrozole.
5. Conclusions
This post hoc analysis of PALOMA-2 demonstrated a favorable effect of palbociclib on PFS and a safety profile consistent with previous observations, regardless of underlying preexisting condition [4]. These findings will aid in clinical decision making and highlight that palbociclib is beneficial in patients with HR+/HER2– ABC, including those with preexisting conditions.
Declaration of competing interest
K Gelmon has received consulting/advisory fees from Pfizer Inc, Novartis, AstraZeneca, NanoString Technologies, and Merck. JM Walshe has received consulting/advisory fees and fees for non-CME services from Roche, Genomic Health, and Pfizer Inc. R Mahtani has received research funding from Agendia, Amgen, AstraZeneca, Biotheranostics, Daiichi, Eisai, Genentech, Immunomedics, Lilly, Merck, Novartis, Pfizer, Puma, Sanofi, and SeaGen. AA Joy has received consulting/advisory fees from Mylan, Teva, Purdue, BMX, BI, Genomic Health, PUMA, Pfizer Inc, Roche, AstraZeneca, Novartis, and Lilly. M Karuturi has received consulting/advisory fees from Pfizer Inc. DR Lu, S Kim, P Schnell, and E Bananis are employees of and own stock in Pfizer Inc.
Acknowledgments
This study was funded by Pfizer Inc. Editorial support was provided by John Teiber, PhD, of ICON (North Wales, PA, USA) and was funded by Pfizer Inc.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2021.07.017.
Data sharing statement
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e., development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Ethics approval and informed consent
The PALOMA-2 study was approved by an institutional review board/independent ethics committee and was conducted in accordance with Good Clinical Practice principles and the Declaration of Helsinki. All patients provided informed consent before the study.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ording A.G., Garne J.P., Nystrom P.M., Froslev T., Sorensen H.T., Lash T.L. Comorbid diseases interact with breast cancer to affect mortality in the first year after diagnosis–a Danish nationwide matched cohort study. PloS One. 2013;8:e76013. doi: 10.1371/journal.pone.0076013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarfati D., Koczwara B., Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin. 2016;66:337–350. doi: 10.3322/caac.21342. [DOI] [PubMed] [Google Scholar]
- 3.IBRANCE® capsules (palbociclib) Pfizer Inc; New York, NY: 2019. Full prescribing information. [Google Scholar]
- 4.Rugo H.S., Finn R.S., Dieras V., Ettl J., Lipatov O., Joy A.A. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Canc Res Treat. 2019;174:719–729. doi: 10.1007/s10549-018-05125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finn R.S., Martin M., Rugo H.S., Jones S., Im S.A., Gelmon K. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 6.Danese M.D., O'Malley C., Lindquist K., Gleeson M., Griffiths R.I. An observational study of the prevalence and incidence of comorbid conditions in older women with breast cancer. Ann Oncol. 2012;23:1756–1765. doi: 10.1093/annonc/mdr486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louwman W.J., Janssen-Heijnen M.L., Houterman S., Voogd A.C., van der Sangen M.J., Nieuwenhuijzen G.A. Less extensive treatment and inferior prognosis for breast cancer patient with comorbidity: a population-based study. Eur J Canc. 2005;41:779–785. doi: 10.1016/j.ejca.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 8.Edwards M.J., Campbell I.D., Lawrenson R.A., Kuper-Hommel M.J. Influence of comorbidity on chemotherapy use for early breast cancer: systematic review and meta-analysis. Breast Canc Res Treat. 2017;165:17–39. doi: 10.1007/s10549-017-4295-4. [DOI] [PubMed] [Google Scholar]
- 9.Sogaard M., Thomsen R.W., Bossen K.S., Sorensen H.T., Norgaard M. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013;5:3–29. doi: 10.2147/CLEP.S47150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ettl J. Management of adverse events due to cyclin-dependent kinase 4/6 inhibitors. Breast Care (Basel) 2019;14:86–92. doi: 10.1159/000499534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rugo H.S., Turner N.C., Finn R.S., Joy A.A., Verma S., Harbeck N. Palbociclib plus endocrine therapy in older women with HR+/HER2- advanced breast cancer: a pooled analysis of randomised PALOMA clinical studies. Eur J Canc. 2018;101:123–133. doi: 10.1016/j.ejca.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Muller L.M., Gorter K.J., Hak E., Goudzwaard W.L., Schellevis F.G., Hoepelman A.I. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41:281–288. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 13.Finn R.S., Rugo H.S., Gelmon K.A., Cristofanilli M., Colleoni M., Loi S. Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: updated analysis with up to 5 years of follow-up. Oncologist. 2021;26:e749–e755. doi: 10.1002/onco.13684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.