Abstract
Background
Invasive lobular carcinoma (ILC) comprises 8–15 % of all invasive breast cancers and large population-based studies with >10 years of follow-up are rare. Whether ILC has a long-time prognosis different from that of invasive ductal carcinoma, (IDC) remains controversial.
Purpose
To investigate the excess mortality rate ratio (EMRR) of patients with ILC and IDC and to correlate survival with clinical parameters in a large population-based cohort.
Material and methods
From 1989 through 2006, we identified 17,481 patients diagnosed with IDC (n = 14,583) or ILC (n = 2898), younger than 76 years from two Swedish Regional Cancer Registries. Relative survival (RS) during 20 years of follow up was analysed.
Results
ILC was significantly associated with older age, larger tumours, ER positivity and well differentiated tumours. We noticed an improved survival for patients with ILC during the first five years, excess mortality rate ratio (EMRR) 0.64 (CI 95 % 0.53–0.77). This was shifted to a significant decreased survival 10–15 years after diagnosis (EMRR 1.49, CI 95 % 1.16–1.93). After 20 years the relative survival rates were similar, 0.72 for ILC and 0.73 for IDC.
Conclusions
During the first five years after surgery, the EMRR was lower for patients with ILC as compared to patients with IDC, but during the years 10–15 after surgery, we observed an increased EMRR for patients with ILC as compared to IDC. These EMRR between ILC and IDC were statistically significant but the absolute difference in excess mortality between the two groups was small.
Keywords: Relative survival rate, Excess mortality rate ratio, Lobular breast cancer, Ductal breast cancer
Highlights
-
•
This is a large population-based study with more than 17,000 patients with a follow up exciding 20 years.
-
•
There is clinically important differences between invasive lobular and ductal carcinoma of the breast.
-
•
Lobular carcinoma shows better survival during the first period but significantly worse at the late period of observation.
1. Introduction
Breast cancer is a heterogeneous disease divided into subgroups according to histopathology [1,2] and more recently gene expression profiles [1,3]. Despite the gene expression based BC subgroups and gene array based testing of prognosis [4], high quality pathology is a cornerstone in the clinical work-up of BC. Invasive breast carcinoma of no special type (IBC-NST) in the past known as invasive carcinoma or invasive ductal carcinoma (IDC) and invasive Lobular carcinoma (ILC) are the most common types of breast cancer comprising 70–80 % and 8–15 % of the cases respectively [5,6].
ILC is characterised by growth in single cell files forming thin long masses often not detected by mammography in 30 % of cases [7]. As compared to IDC, ILC tends to be larger in size and more often multifocal [8]. ILC has a peculiar metastases pattern e.g. to the gastrointestinal tract, peritoneum, ovaries, orbital cavity or cerebral meninges [9]. The majority of ILCs are of histological intermediate grade explained by limited number of mitoses and low frequency of tubule formation but the inclusion of genomic grade has added prognostic information [10]. Survival of ILC patients is reported to be increased, equal, or decreased as compared with IDC patients [11]. Factors contributing to this conflicting results may be variation in the histologic criteria used to define ILC, differences in therapy, as well as sample size limitations [6,12,13]. The majority of published studies have shown a better survival during early follow-up for patients with ILC compared to IDC [14]. There are few large population based studies of ILC with long follow up [15,16], the latter is of importance as the majority of ILC are hormone receptor (HR) positive and may relapse late. International Breast Cancer Study Group (IBCSG) analysed data on more than 13,000 patients with early BC, demonstrating a favourable outcome for ILC patients during the first decade of follow up, but this was not seen beyond 10 years. A more recent comparison of the survival of lobular and ductal carcinoma patients confirmed this pattern [15,17].
Here long-term survival patterns in terms of excess mortality rate ratio (EMRR) are determined in patients with ILC and IDC in a population-based cohort of 17,481 patients and survival is correlated to clinical and tumour characteristics.
2. Patients and methods
2.1. Cancer registration
In Sweden, each region has a regional cancer centre that is responsible for cancer registration and management programs. Since 1958, it has been compulsory for the treating physician and the pathologist to independently report all new cases of cancer to the Swedish cancer registry (SCR). The SCR receives reports containing ICD code of the malignancy, histological systematized nomenclature of medicine (SNOMED) code, TNM stage, date of diagnosis, date of birth including a personal identification number unique to each individual in Sweden. The completeness of the SCR is about 96 % [18]. Most of the patients are additionally registered in regional quality-management registers with information of incident tumour characteristics and primary treatment. For BC, >95 % of the patients are treated according to the management programs and registered in the regional quality-management register databases [19].
2.2. Patient cohort
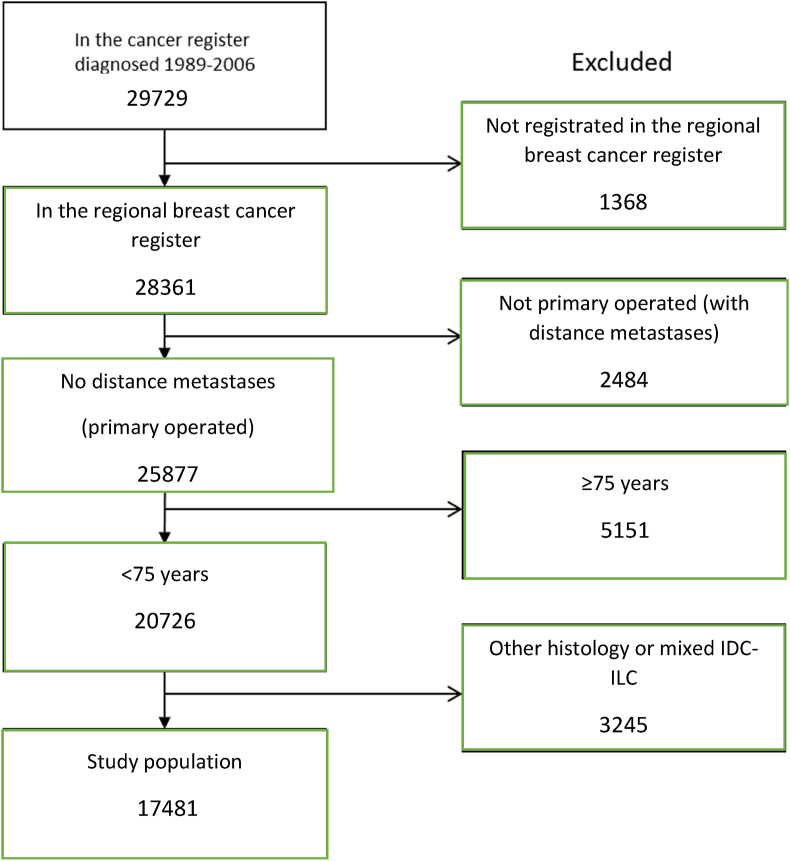
Between 1989 and 2006, 29,729 new BC cases were reported to the Swedish Cancer Register from the west (W) and southeast (SE) regions. Of these, 28,361 were also reported to the more detailed clinical quality registries. We excluded patients who at diagnosis were age ≥75 years, did not undergo primary surgery, had distant metastasis, or with histology other than lobular or ductal (Fig. 1). The remaining 17,481 patients were followed up for vital status until June 2020 through record linkage to national population registers.
Fig. 1.
Flowchart describing the identification process of the study population. Patients diagnosed and registered in regional cancer databases from western and southeast Sweden from 1989 through 2006 were used.
From the quality-management registers, we retrieved information regarding age at diagnosis; tumour size; number of examined and positive nodes; hormone receptor status and Nottingham histology grade (NHG). Tumour and patient characteristics are shown in Table 1.
Table 1.
Clinical and pathological characteristics among 17,481 invasive breast cancers of lobular or ductal histology from a population-based cohort from western and southeast Sweden.
| Lobular | Ductal | Total | P valuea | |
|---|---|---|---|---|
| Total no. of patients (%) | 2898 | 14,583 | 17,481 | |
| Age | ||||
| < 50 | 583 (20.1) | 3788 (26.0) | 4371 (25.0) | <0.001 |
| 50–74 | 2315 (79.9) | 10,795 (74.0) | 13,110 (75.0) | |
| ER | ||||
| Neg. | 201 (8.7) | 2719 (22.7) | 2920 (20.4) | <0.001 |
| Pos. | 2117 (91.3) | 9263 (77.3) | 11,380 (79.6) | |
| Unknown | 580 | 2601 | 3181 | |
| PR | ||||
| Neg. | 573 (24.8) | 4307 (36.1) | 4880 (34.3) | <0.001 |
| Pos. | 1734 (75.2) | 7636 (63.9) | 9370 (65.7) | |
| Unknown | 591 | 2640 | 3231 | |
| Type of surgery | ||||
| Breast conserving | 1359 (47.1) | 8080 (55.6) | 9439 (54.2) | <0.001 |
| Mastectomy | 1524 (52.9) | 6444 (44.4) | 7968 (45.8) | |
| Unknown | 15 | 59 | 74 | |
| Tumour size (mm) | ||||
| ≤ 10 | 500 (17.8) | 3434 (24.1) | 3934 (23.1) | <0.001 |
| 11–20 | 1196 (42.5) | 6199 (43.5) | 7395 (43.3) | |
| > 20 | 1118 (39.7) | 4623 (32.4) | 5741 (33.6) | |
| Unknown | 84 | 327 | 411 | |
| Node status | ||||
| 0 | 1856 (64.0) | 9560 (65.6) | 11,416 (65.3) | <0.001 |
| 1–3 | 619 (21.4) | 3275 (22.5) | 3894 (22.3) | |
| >3 | 423 (14.6) | 1748 (12.0) | 2171 (12.4) | |
| NHG | ||||
| 1 | 362 (20.2) | 2185 (27.0) | 2547 (25.8) | <0.001 |
| 2 | 1295 (72.4) | 3366 (41.5) | 4661 (47.1) | |
| 3 | 133 (7.4) | 2552 (31.5) | 2685 (27.1) | |
Pearson chi-squared test.
2.3. Therapy
Patients included in this investigation were diagnosed with primary BC from 1989 through 2006. Surgery, radiotherapy, adjuvant endocrine therapy, and chemotherapy were delivered according to guidelines based on St. Gallen recommendations [20]. Nearly all patients were treated before sentinel node surgery, adjuvant taxanes and adjuvant trastuzumab were included in the guide-lines. Neoadjuvant treatment was only delivered if inoperable tumour. During the 80-ties and the beginning of the 90-ties a very small proportion of ER + patients received adjuvant chemotherapy. Between 1989 and 1995, patients in the SE region with oestrogen receptor (ER) positive, stage 1–3 BC participated in study comparing 2 and 5 years of adjuvant tamoxifen. After publication of the results in 1996, 5 years of adjuvant tamoxifen became standard [21]. The recommendation on adjuvant treatment for postmenopausal patients was further changed to 5 years of an AI following presentation of the ATAC trial in 2002 [22]. We estimate that 20 % of the whole patient cohort received adjuvant AI and 80 % tamoxifen for two or five years.
From the year 2000, adjuvant cyclophosphamide, methotrexate, fluorouracil (CMF) therapy was replaced by anthracycline-based chemotherapy and from 2002 sentinel node surgery was introduced in clinical practice.
In both regions, after breast-conserving surgery, 50 Gy in 25 fractions without a break were delivered to the remaining breast tissue. In the SE region, women with node-positive disease (i.e. ≥1 positive node) generally received radiotherapy including loco regional lymph nodes. In the W region, at least four metastatic lymph nodes were required for radiation of regional lymph nodes.
2.4. Pathology
The original pathology reports on the surgical specimen were used and no reinvestigation was performed. Data on grade were limited to pathology reports obtained after the introduction of the Nottingham grading system. ER and PgR receptor levels were determined by the Abbot enzyme immunoassay and after 1999 by immunohistochemistry.
2.5. Statistical methods
To compare the association between tumour morphology and clinical characteristics, the Pearson chi-squared test was applied. Relative survival was computed using the Ederer II method [23]. Mortality data for the general population in Sweden were used to estimate expected survival rates for the study populations. The mortality data comprised the probability of death for single-year age groups in 1-year calendar periods. Stata statistical software was used to calculate relative survival. Survival time was calculated from date of diagnosis to June 30, 2020 or to date of death if it occurred before this date. Excess mortality rate ratio (EMRR) between different groups was estimated by univariable Poisson regression [24]. A p value of <0.05 was considered to be statistically significant. All statistical analyses were performed with Stata/SE 13.1 [25].
3. Results
3.1. Clinical characterisation
Most patients (83.4 %) were diagnosed with IDC, whereas 16.6 % were diagnosed with ILC. Clinical and pathological features are summarized in Table 1. There was a statistically significant association between morphology and age. Among patients with ILC, 79.9% were 50–74 years at the time of diagnosis compared with 74.0% among patients with IDC (p < 0.001). Hormone receptor positivity was more common in ILC, where ER positivity was found in 91.3% and PgR positivity in 75.2% of the cases as compared with 77.3% and 63.9%, respectively in IDC (p < 0.001). Further, ILC was associated with larger tumours >20 mm; ILC, 39.7% and IDC 32.4% (p < 0.001).
There was only a slightly higher proportion of patients with lymph node metastases in ILC, though, due to the large number of patients in this cohort, this achieves statistical significance (p < 0.001). Low-differentiated tumours were more common in IDC. Most lobular cancers were of grade 2 (72.4 %) and only 133 (7.4 %) were of grade 3. The corresponding numbers for IDC were 41.5 % and 31.5 %, respectively. Determination of HER2 status was introduced during the last years of the study and available in only 1857 patients. The frequency of HER2 positivity was higher in IDC, 15.9 % compared with 6.9 % in ILC.
3.2. Comparison of excess mortality rate ratio in ILC and IDC
The median follow-up for the patient-cohort was 19 years with interquartile range (IQR) between 16 and 24 years for alive patients of the cohort.
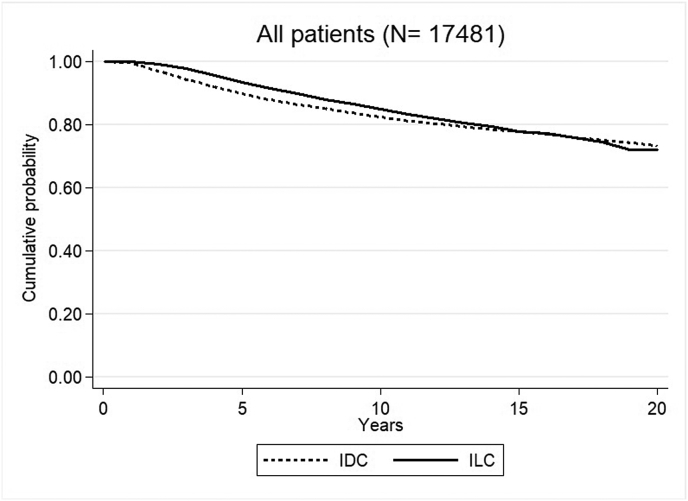
We noticed an improved RS for the patients with ILC during the first years of observation. Five years after diagnosis the RS rate was 0.93 (95%CI 0.92–0.94) for ILC and 0.90 (95%CI 0.89–0.90) for IDC, EMRR 0.64 (95 % CI 0.53–0.77, p < 0.001). This was shifted to a significantly higher EMRR for ILC compared to IDC in the interval 10–15 years after diagnosis, EMRR 1.49 (95%CI 1.16–1.93, p = 0.002). Twenty years after diagnosis, the RS rate was almost equal for patients with ILC, 0.72 (95 % CI 0.69–0.75) and 0.73 (95 % CI 0.72–0.75, p = 0.17) for patients with IDC (Fig. 2, Table 2).
Fig. 2.
Relative survival (RS) rate for patients with ILC and IDC in the whole study population.
Table 2.
Relative survival (RS) rate and Excess mortality rate ratio (EMRR) with 95 % CI for ILC compared with IDC estimated from univariable Poisson regression.
| Years after diagnosis | Number at risk at start of interval |
RS (%) and (95% CI) at the end of interval |
EMRR ILC vs IDC (95% CI) | P value | |||
|---|---|---|---|---|---|---|---|
| ILC | IDC | ILC | IDC | ||||
| All | 0–5 | 2898 | 14,583 | 93 (92–94) | 90 (89–90) | 0.64 (0.53–0.77) | <0.001 |
| 5–10 | 2591 | 12,595 | 85 (83–87) | 82 (82–83) | 1.09 (0.91–1.32) | 0.35 | |
| 10–15 | 2207 | 10,892 | 78 (76–80) | 78 (77–79) | 1.49 (1.16–1.93) | 0.002 | |
| 15–20 | 1607 | 8413 | 72 (69–75) | 73 (72–75) | 1.35 (0.88–2.07) | 0.17 | |
| ER+ | 0–5 | 2117 | 9263 | 93 (92–95) | 93 (92–94) | 0.97 (0.78–1.21) | 0.78 |
| 5–10 | 1894 | 8273 | 85 (83–87) | 85 (84–86) | 1.07 (0.86–1.33) | 0.57 | |
| 10–15 | 1617 | 7126 | 78 (75–80) | 79 (78–81) | 1.39 (1.05–1.84) | 0.02 | |
| ER- | 0–5 | 201 | 2719 | 87 (81–92) | 75 (74–77) | 0.46 (0.29–0.73) | 0.001 |
| 5–10 | 169 | 1982 | 74 (67–81) | 68 (66–69) | 1.27 (0.75–2.17) | 0.38 | |
| 10–15 | 136 | 1692 | 69 (60–77) | 64 (62–67) | 1.47 (0.51–4.21) | 0.48 | |
| ER+/PR+ | 0–5 | 1654 | 7226 | 93 (92–95) | 94 (94–95) | 1.21 (0.94–1.57) | 0.14 |
| 5–10 | 1485 | 6562 | 85 (83–88) | 87 (86–88) | 1.09 (0.85–1.40) | 0.49 | |
| 10–15 | 1277 | 5691 | 78 (76–81) | 81 (80–82) | 1.26 (0.91–1.75) | 0.16 | |
| ER+/PR- | 0–5 | 451 | 1985 | 93 (90–96) | 87 (86–89) | 0.55 (0.35–0.88) | 0.01 |
| 5–10 | 399 | 1662 | 83 (79–88) | 78 (76–81) | 0.99 (0.63–1.55) | 0.96 | |
| 10–15 | 330 | 1392 | 74 (68–80) | 73 (70–76) | 1.70 (0.95–3.05) | 0.08 | |
| NHG I | 0–5 | 362 | 2185 | 96 (92–98) | 99 (98–99) | 2.57 (1.02–6.44) | 0.04 |
| 5–10 | 332 | 2076 | 91 (86–95) | 96 (95–98) | 2.47 (1.01–6.03) | 0.05 | |
| 10–15 | 295 | 1917 | 83 (76–88) | 93 (91–95) | 3.82 (1.78–8.20) | 0.001 | |
| NHG II | 0–5 | 1295 | 3366 | 96 (94–97) | 94 (93–95) | 0.77 (0.54–1.12) | 0.18 |
| 5–10 | 1190 | 3047 | 89 (86–91) | 87 (85–88) | 0.90 (0.64–1.26) | 0.54 | |
| 10–15 | 1039 | 2654 | 83 (80–86) | 82 (80–84) | 1.16 (0.71–1.91) | 0.55 | |
| NHG III | 0–5 | 133 | 2552 | 78 (69–84) | 82 (80–83) | 1.22 (0.82–1.81) | 0.33 |
| 5–10 | 100 | 2019 | 65 (55–73) | 71 (69–73) | 1.32 (0.74–2.36) | 0.35 | |
| 10–15 | 79 | 1691 | 61 (50–70) | 66 (63–68) | 0.80 (0.20–3.16) | 0.75 | |
| Age | 0–5 | 583 | 3788 | 92 (90–94) | 87 (86–88) | 0.58 (0.42–0.80) | 0.001 |
| < 50 | 5–10 | 534 | 3285 | 82 (79–85) | 78 (76–79) | 0.96 (0.71–1.28) | 0.77 |
| 10–15 | 470 | 2882 | 73 (69–76) | 72 (71–74) | 1.78 (1.28–2.48) | 0.001 | |
| Age | 0–5 | 1336 | 6712 | 94 (92–95) | 91 (90–91) | 0.66 (0.51–0.87) | 0.002 |
| 50–64 | 5–10 | 1217 | 5915 | 87 (84–89) | 84 (83–85) | 1.07 (0.80–1.43) | 0.64 |
| 10–15 | 1084 | 5293 | 80 (77–83) | 81 (80–82) | 1.87 (1.32–2.66) | <0.001 | |
| Age | 0–5 | 979 | 4083 | 94 (91–96) | 91 (89–92) | 0.72 (0.49–1.06) | 0.09 |
| ≥ 65 | 5–10 | 840 | 3395 | 84 (80–88) | 84 (82–86) | 1.37 (0.92–2.03) | 0.12 |
| 10–15 | 653 | 2717 | 78 (73–83) | 78 (75–80) | 0.93 (0.46–1.86) | 0.83 | |
| T size | 0–5 | 500 | 3434 | 98 (95–100) | 98 (97–99) | 1.02 (0.38–2.77) | 0.97 |
| ≤ 10 mm | 5–10 | 467 | 3224 | 96 (92–99) | 95 (94–97) | 1.17 (0.37–3.69) | 0.78 |
| 10–15 | 425 | 2941 | 93 (87–97) | 93 (91–95) | 1.12 (0.25–5.14) | 0.88 | |
| T size | 0–5 | 1196 | 6199 | 97 (95–98) | 93 (92–94) | 0.42 (0.25–0.70) | 0.001 |
| 11–20 | 5–10 | 1111 | 5550 | 90 (88–93) | 87 (85–88) | 0.96 (0.68–1.36) | 0.84 |
| Mm | 10–15 | 967 | 4861 | 84 (81–87) | 82 (80–83) | 1.40 (0.92–2.13) | 0.12 |
| T size | 0–5 | 1118 | 4623 | 87 (85–90) | 79 (78–80) | 0.57 (0.46–0.69) | <0.001 |
| >20 mm | 5–10 | 939 | 3530 | 75 (72–78) | 67 (65–69) | 0.93 (0.74–1.16) | 0.52 |
| 10–15 | 757 | 2833 | 65 (62–69) | 61 (59–62) | 1.26 (0.91–1.74) | 0.16 | |
CI: Confidence interval, EMRR: Excess mortality rate ratio, ER: Estrogen receptor, IDC: Invasive ductal carcinoma, ILC: Invasive lobular carcinoma, NHG: Nottingham histologic grade, PR: Progesterone receptor, RS: Relative survival, T size: Tumour size.
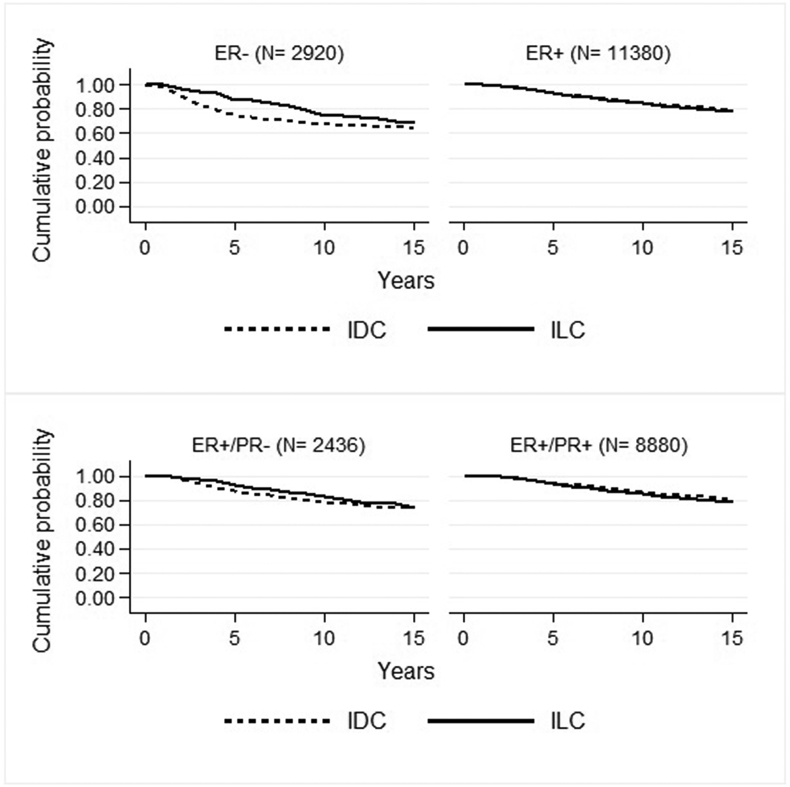
We further investigated differences in RS rate (Fig. 3) and EMRR (Table 2) between patients with ILC and IDC according to HR status. During the early phase of follow-up, the RS rates in ER positive disease was similar for ILC and IDC group, but 10–15 years after diagnosis, we found a significantly increased EMRR for patients with ILC as compared to those with IDC (EMRR 1.39; 95 % CI 1.05–1.84, p = 0.02) (Table 2). During the first five years after diagnosis but not later, patients with ER negative ILC had a superior outcome compared to those with ER negative IDC (EMRR 0.46, 95 % CI 0.29–0.73, p = 0.001) (Fig. 3, Table 2). Similarly, during the first five years of follow up but not later, also patients with ER positive but PgR negative ILC had a significantly increased RS rate as compared to those with ER positive but PgR negative IDC, EMRR 0.55 (95 % CI: 0.35–0.88, p = 0.01) (Table 2).
Fig. 3.
Relative survival (RS) rate for patients with ILC and IDC according to hormone receptor status. Upper part; ER negative (left box) and ER positive (right box); lower part; ER positive, PgR negative (left box) and ER and PgR positive (right box).
Large tumour size negatively influenced survival. In the group of patients with tumour size 11–20 mm, we noticed an improved RS for those with ILC compared with IDC (97 % vs 93 %) after the first 5 years. The RS rate between patients with ILC and IDC converged over time and after 15 years of follow up the difference was small (84 % vs 82 %) (Table 2). A similar survival pattern was noticed for patients with tumour size larger than 20 mm, though with a larger difference between ILC and IDC. We did not notice any influence of histological subtype in tumour size smaller than 11 mm (Table 2).
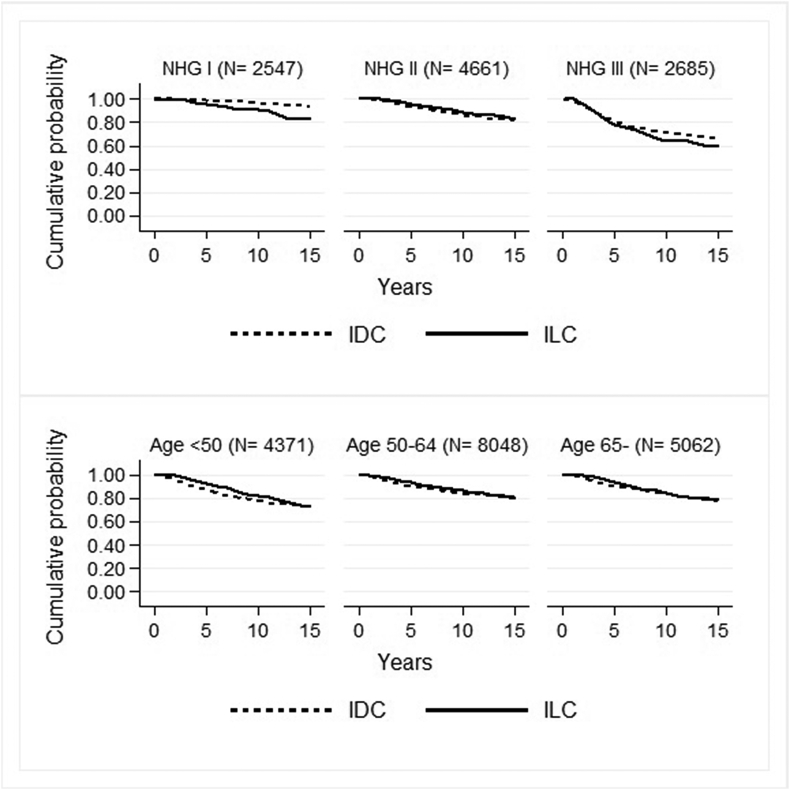
In low-grade tumours, patients with ILC demonstrated a lower RS rate, during all time intervals (Table 2, Fig. 4). Survival of patients with ILC of all ages tended to be increased during the first five years of follow up but the increase in RS rate compared to IDC was most marked for patients aged less than 50 (Table 2, Fig. 4).
Fig. 4.
Relative survival (RS) rate for patients with IDC and ILC according to grade in the upper section; NHG I (left box), NHG II (middle box) and NHG III (right box) and age in the lower section; <50 years (left box), 50–64 years (middle box) and 65 years and older (right box).
4. Discussion
Conflicting results have been reported on survival rates of ILC patients. One reason for this is the lack of large population-based studies with long follow up as the one we present here. Our results confirm that during the first five years of follow-up, there is favourable relative survival rate for patients with ILC compared to IDC patients, but after 10–15 years of follow up the survival rate is decreased for ILC patients compared to IDC patients [17].
During the first years of follow up of ER positive disease, the RS rates were almost equal for ILC and IDC patients but in the interval 10–15 years after diagnosis marginally decreased for ILC patients. We conclude that for patients with ER positive disease, there seems to be no clinically meaningful difference in outcome between ILC and IDC disease. On the contrary, during the first five years of follow up of ERnegative disease, ILC patients had an increased relative survival rate as compared to IDC patients, but with no significant difference during later periods. In the subgroup of ER positive patients with PR negativity, we also noticed that the ILC had a favourable survival compared to IDC group 0–5 years after diagnosis which resembles the hormone receptor negative pattern. This should be interpreted with caution but may be linked to reduced sensitivity to endocrine treatment, which is the main treatment for ILC that does not have the same sensitivity to chemotherapy as IDC [2].
Patients with HR negative tumours are heterogeneous, some with aggressive triple negative IDC. This may partly explain the decreased survival rate for patients with IDC compared to ILC patients during the first five years of follow up. Also, receptor negative ILC may include a subset of luminal androgen receptor positive triple negative BC that has a less aggressive disease compared to basal like TNBC [26]. In addition, most patients were diagnosed before HER2 testing and adjuvant trastuzumab were introduced in 2005. HER2 positivity is twice as common in IDC compared with ILC [27] also shown in our population. Therefore, the larger proportion of HER2 positive disease without HER2 directed therapy in IDC probably contributed to the lower survival rate for patients with HR negative IDC compared to the corresponding ILC patients.
Nottingham histologic grading is based on three parameters, number of mitosis, nuclear variation and formation of tubulus structures. ILC forms tubuli-like structures less frequently than IDC and ILC grade 1 is rare. There is special variants of ILC called ILC with tubular variants according to the current WHO guidelines [5] Our data report similar outcome for patients with grade 1 and grade 2 ILC and worse outcome for grade 1 ILC patients compared to grade 1 IDC patients confirming that the previous versions of grading is less informative for ILC than for IDC (Table 2 Fig. 4) [10]. Unfortunately, risk classification of ILC with gene expression profiles is less informative. For example OncotypeDx used to select patients for whom chemotherapy can be dispensed without affecting survival, classifies approximately 20 % of IDC as high risk tumours as compared to 1.5 % of the ILC tumours [28,29].
The important differences between ILC and IDC is confirmed by trials recruiting both ILC and IDC patients showing that ILC is less responsive to chemotherapy [2,30,31]. The BIG 98-01 trial, comparing tamoxifen with letrozole as adjuvant therapy concluded that the aromatase inhibitor, letrozole, was more effective in the treatment of ILC [32,33] probably due to differential ER activity at the protein level in ILC tumours compared to IDC. Only a smaller proportion of our patients received Aromatase Inhibitors and these drugs might also improve the long-time survival of patients with ILC.
A large study revealed that more than half of ILC tumours harbour alterations within the key pathway of PIK3/PTEN/Akt/mTOR which were higher compared with ER positive IDC [34]. Subgroup analyses of the BOLERO-2 trial showed that the addition of the m-TOR inhibitor Afinitor, to endocrine treatment improves progression free survival for patients with ILC and raise the hypothesis that a group of patients with ILC also may gain from treatment with the PIK3CA inhibitor Alpelisib [35,36].
Our study has a very long follow up but this also leads to limitations. There are gradual changes of diagnostic procedures and therapy that complicates interpretation. The lack of information of HER2 status and central pathology review, different techniques for HR assays and treatment recommendations that are currently not considered adequate are important shortcomings but in spite of this, important differences between ILC and IDC were documented.
5. Conclusions
In a large population-based cohort with long follow-up we showed that ILC is an independent disease with a specific biology that affects survival. It presents with a significantly better relative survival during the first years after diagnosis and a significantly worse relative survival 10–15 years after diagnosis, thus with a controversial significance in the clinical perspective. In addition, ER-status seems to affect the survival pattern differently for ILC and IDC. We encourage subgroup analyses of ILC in BC trials and suggest international collaboration to recruit patients with ILC for separate trials.
Compliance with ethical standards
Ethical approval: The study has been conducted in accordance with the Declaration of Helsinki and the Sahlgrenska University Hospital Ethical Review Board, Gothenburg, Sweden approved the study (248-17, T01726-21). The need for informed consent was waived under the approval of the Ethical Review Board due to the retrospective design.
Funding
This study was supported by grants from the king Gustav the Vth Jubilee Clinic Cancer Foundation in Gothenburg, the Swedish Cancer Society, the Swedish Breast Cancer Association (Bröstcancerförbundet), the Swedish State under the LUA-agreement (Sahlgrenska University Hospital), Gothenburg and from Swedish governmental grants to scientist working in health care (ALF).
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Perou C.M. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Cristofanilli M. Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. J Clin Oncol. 2005;23(1):41–48. doi: 10.1200/JCO.2005.03.111. [DOI] [PubMed] [Google Scholar]
- 3.Mersin H. Is invasive lobular carcinoma different from invasive ductal carcinoma? Eur J Surg Oncol. 2003;29(4):390–395. doi: 10.1053/ejso.2002.1423. [DOI] [PubMed] [Google Scholar]
- 4.van de Vijver M.J. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 5.Cserni G. Histological type and typing of breast carcinomas and the WHO classification changes over time. Pathologica. 2020;112(1):25–41. doi: 10.32074/1591-951X-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arpino G. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6(3):R149–R156. doi: 10.1186/bcr767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter A.J. Mammographic and ultrasound features of invasive lobular carcinoma of the breast. J Med Imaging Radiat Oncol. 2014;58(1):1–10. doi: 10.1111/1754-9485.12080. [DOI] [PubMed] [Google Scholar]
- 8.Silverstein M.J. Infiltrating lobular carcinoma. Is it different from infiltrating duct carcinoma? Cancer. 1994;73(6):1673–1677. doi: 10.1002/1097-0142(19940315)73:6<1673::aid-cncr2820730620>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 9.Raap M. High frequency of lobular breast cancer in distant metastases to the orbit. Cancer Med. 2015;4(1):104–111. doi: 10.1002/cam4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metzger-Filho O. Genomic grade adds prognostic value in invasive lobular carcinoma. Ann Oncol. 2013;24(2):377–384. doi: 10.1093/annonc/mds280. [DOI] [PubMed] [Google Scholar]
- 11.Fortunato L. Lobular breast cancer: same survival and local control compared with ductal cancer, but should both be treated the same way? analysis of an institutional database over a 10-year period. Ann Surg Oncol. 2012;19(4):1107–1114. doi: 10.1245/s10434-011-1907-9. [DOI] [PubMed] [Google Scholar]
- 12.Allemani C. Prognostic value of morphology and hormone receptor status in breast cancer - a population-based study. Br J Canc. 2004;91(7):1263–1268. doi: 10.1038/sj.bjc.6602153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pestalozzi B.C. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol. 2008;26(18):3006–3014. doi: 10.1200/JCO.2007.14.9336. [DOI] [PubMed] [Google Scholar]
- 14.García-Fernández A. Comparative long-term study of a large series of patients with invasive ductal carcinoma and invasive lobular carcinoma. Loco-regional recurrence, metastasis, and survival. Breast J. 2015;21(5):533–537. doi: 10.1111/tbj.12455. [DOI] [PubMed] [Google Scholar]
- 15.Mathew A. Distinct pattern of metastases in patients with invasive lobular carcinoma of the breast. Geburtshilfe Frauenheilkd. 2017;77(6):660–666. doi: 10.1055/s-0043-109374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toikkanen S., Pylkkanen L., Joensuu H. Invasive lobular carcinoma of the breast has better short- and long-term survival than invasive ductal carcinoma. Br J Canc. 1997;76(9):1234–1240. doi: 10.1038/bjc.1997.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z. Invasive lobular carcinoma of the breast: a special histological type compared with invasive ductal carcinoma. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0182397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barlow L. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48(1):27–33. doi: 10.1080/02841860802247664. [DOI] [PubMed] [Google Scholar]
- 19.Tejler G. Survival after treatment for breast cancer in a geographically defined population. Br J Surg. 2004;91(10):1307–1312. doi: 10.1002/bjs.4697. [DOI] [PubMed] [Google Scholar]
- 20.Goldhirsch A. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22(8):1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer. Swedish Breast Cancer Cooperative Group. J Natl Cancer Inst. 1996;88(21):1543–1549. doi: 10.1093/jnci/88.21.1543. [DOI] [PubMed] [Google Scholar]
- 22.Howell A. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 23.Ederer F., Axtell L.M., Cutler S.J. The relative survival rate: a statistical methodology. Natl Canc Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- 24.Dickman P.W. Regression models for relative survival. 2004;23(1):51–64. doi: 10.1002/sim.1597. [DOI] [PubMed] [Google Scholar]
- 25.StataCorp . vol. 13. StataCorp LP; College Station, TX: 2013. (Stata statistical software: release). [Google Scholar]
- 26.Collins L.C. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses' Health Study. Mod Pathol. 2011;24(7):924–931. doi: 10.1038/modpathol.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zengel B. Comparison of the clinicopathological features of invasive ductal, invasive lobular, and mixed (invasive ductal + invasive lobular) carcinoma of the breast. Breast Cancer. 2015;22(4):374–381. doi: 10.1007/s12282-013-0489-8. [DOI] [PubMed] [Google Scholar]
- 28.Conlon N. Is there a role for oncotype dx testing in invasive lobular carcinoma? Breast J. 2015;21(5):514–519. doi: 10.1111/tbj.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bomeisl P.E. Comparison of oncotype DX recurrence score by histologic types of breast carcinoma. Arch Pathol Lab Med. 2015;139(12):1546–1549. doi: 10.5858/arpa.2014-0557-OA. [DOI] [PubMed] [Google Scholar]
- 30.Tubiana-Hulin M. Response to neoadjuvant chemotherapy in lobular and ductal breast carcinomas: a retrospective study on 860 patients from one institution. Ann Oncol. 2006;17(8):1228–1233. doi: 10.1093/annonc/mdl114. [DOI] [PubMed] [Google Scholar]
- 31.Mathieu M.C. The poor responsiveness of infiltrating lobular breast carcinomas to neoadjuvant chemotherapy can be explained by their biological profile. Eur J Canc. 2004;40(3):342–351. doi: 10.1016/j.ejca.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Metzger Filho O. Relative effectiveness of letrozole compared with tamoxifen for patients with lobular carcinoma in the BIG 1-98 trial. J Clin Oncol. 2015;33(25):2772–2779. doi: 10.1200/JCO.2015.60.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knauer S.K. The inducible E3 ubiquitin ligases SIAH1 and SIAH2 perform critical roles in breast and prostate cancers. Cytokine Growth Factor Rev. 2015;26(4):405–413. doi: 10.1016/j.cytogfr.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Desmedt C. Genomic characterization of primary invasive lobular breast cancer. J Clin Oncol. 2016;34(16):1872–1881. doi: 10.1200/JCO.2015.64.0334. [DOI] [PubMed] [Google Scholar]
- 35.André F. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380(20):1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 36.Baselga J. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]