Abstract
Background
Carbapenems are recommended treatment for serious infections caused by AmpC-producing gram-negative bacteria but can select for carbapenem resistance. Piperacillin-tazobactam may be a suitable alternative.
Methods
We enrolled adult patients with bloodstream infection due to chromosomal AmpC producers in a multicenter randomized controlled trial. Patients were assigned 1:1 to receive piperacillin-tazobactam 4.5 g every 6 hours or meropenem 1 g every 8 hours. The primary efficacy outcome was a composite of death, clinical failure, microbiological failure, and microbiological relapse at 30 days.
Results
Seventy-two patients underwent randomization and were included in the primary analysis population. Eleven of 38 patients (29%) randomized to piperacillin-tazobactam met the primary outcome compared with 7 of 34 patients (21%) in the meropenem group (risk difference, 8% [95% confidence interval {CI}, –12% to 28%]). Effects were consistent in an analysis of the per-protocol population. Within the subcomponents of the primary outcome, 5 of 38 (13%) experienced microbiological failure in the piperacillin-tazobactam group compared to 0 of 34 patients (0%) in the meropenem group (risk difference, 13% [95% CI, 2% to 24%]). In contrast, 0% vs 9% of microbiological relapses were seen in the piperacillin-tazobactam and meropenem arms, respectively. Susceptibility to piperacillin-tazobactam and meropenem using broth microdilution was found in 96.5% and 100% of isolates, respectively. The most common AmpC β-lactamase genes identified were blaCMY-2, blaDHA-17, blaCMH-3, and blaACT-17. No ESBL, OXA, or other carbapenemase genes were identified.
Conclusions
Among patients with bloodstream infection due to AmpC producers, piperacillin-tazobactam may lead to more microbiological failures, although fewer microbiological relapses were seen.
Clinical Trials Registration
Keywords: ampC β-lactamase, carbapenem, clinical trial, Enterobacterales, piperacillin-tazobactam
Among patients with bloodstream infection due to AmpC-producing Enterobacterales, no difference in clinical and microbiological efficacy was observed between piperacillin-tazobactam and meropenem. More microbiological failures occurred in the piperacillin-tazobactam group, although fewer relapses were seen. A larger trial is required.
AmpC β-lactamase genes are chromosomal genes found in certain bacterial species (eg, Enterobacter spp, Klebsiella [formerly Enterobacter] aerogenes, Serratia marcescens, Citrobacter freundii, Providencia stuartii, Morganella morganii) [1, 2]. AmpC expression among these organisms is inducible in response to β-lactam exposure. For example, in C freundii there was an 11-fold increase in AmpC expression in response to ampicillin [3]. On removal of β-lactam exposure, AmpC production generally decreases; however, if mutations have occurred in certain regulatory genes (eg, ampD, ampR), selection of mutants with stable AmpC de-repression can occur [4]. AmpC β-lactamases exhibit a broad substrate specificity and their expression causes resistance to a wide range of antimicrobials [1]. High levels of inducible resistance and clinical failures have been documented with these agents, particularly third-generation cephalosporins [5–8]. The hydrolysis rate for fourth-generation cephalosporins, such as cefepime, and carbapenems is low. Carbapenems have been advocated as the primary therapeutic agents used to treat these infections [9]. Rising incidence of carbapenem-resistant organisms globally has prompted a search for suitable alternative therapy to treat these infections [10]. The Meropenem versus piperacillin-tazobactam for definitive treatment of bloodstream infections due to ceftriaxone non-susceptible Escherichia coli and Klebsiella spp (MERINO) trial failed to demonstrate noninferiority, with respect to 30-day all-cause mortality, of piperacillin-tazobactam when compared to meropenem for treatment of bloodstream infection (BSI) due to ceftriaxone-resistant Escherichia coli and Klebsiella spp [11]. Both piperacillin and tazobactam are known to be a weak inducers of AmpC enzymes and when combined appear to be a viable treatment option [12]. In addition, tazobactam has demonstrated inhibitory activity against AmpC enzymes [13]. Observational studies have assessed the efficacy of piperacillin-tazobactam in treating AmpC producers [14–17]. Some of these studies suggest that piperacillin-tazobactam may be as clinically effective as carbapenems in the treatment of these infections. Following on from the MERINO trial, this study aimed to assess whether piperacillin-tazobactam, when compared to meropenem, was similar with regard to clinical efficacy in the treatment of BSI due to AmpC-producing organisms.
METHODS
Study Design and Inclusion Criteria
The trial protocol was endorsed by the Australasian Society of Infectious Diseases and was approved by the institutional review boards for each recruiting center. The trial was registered at ClinicalTrials.gov (identifier NCT02437045). The results are reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement extension for noninferiority and equivalence trials. This was a pilot international, multicenter, open-label, parallel-group, randomized controlled trial of piperacillin-tazobactam vs meropenem for the definitive treatment of BSIs caused by species of gram-negative bacilli with chromosomally encoded AmpC β-lactamases. Adult patients (aged ≥18 years, or ≥21 years in Singapore) were eligible for enrollment if they had at least 1 positive blood culture with Enterobacter spp, Klebsiella (formerly Enterobacter) aerogenes, Serratia marcescens, Providencia spp, Morganella morganii, or Citrobacter freundii (ie, likely AmpC producers) that demonstrated susceptibility to third-generation cephalosporins, piperacillin-tazobactam, and meropenem according to local laboratory protocols. Demonstrating susceptibility to third-generation cephalosporins as an inclusion criteria was added as a protocol amendment on 30 April 2018 following the results of the MERINO trial, in order to reduce the risk of enrolling patients infected with isolates harboring extended-spectrum β-lactamase (ESBL) enzymes [11]. Patients had to be randomized within 72 hours of initial positive blood culture collection. Exclusion criteria included allergy to penicillin or carbapenems, no expectation of survival >4 days, polymicrobial BSI (except likely skin contaminants), treatment without curative intent, pregnancy or breastfeeding, use of concomitant antimicrobials with known gram-negative activity within the first 4 days after randomization, and likely central nervous system source of infection.
Ethical Approvals and Patient Consent Statement
The study was conducted in accordance with ethical standards of the Helsinki Declaration. Written informed consent was obtained from all patients or their substitute decision maker. The study was approved by local ethical committees at participating sites and conforms to standards currently applied in all countries where recruitment occurred. The Royal Brisbane and Women’s Hospital Human Research Ethics Committee provided approval and oversight for the trial (HREC/14/QRBW/350).
Study Population, Stratification, and Randomization
Patients were screened for enrollment in 7 hospitals in 3 countries (Australia, Singapore, and Turkey) from July 2015 to December 2019. Patients were stratified according to infecting species (Enterobacter spp or other), and source of infection (urinary tract or non–urinary tract).
Patients were randomly assigned to either meropenem or piperacillin-tazobactam in a 1:1 ratio according to a randomization list prepared in advance for each recruiting site and stratum. The sequence was generated blinded using random permuted blocks of 2 and 4 patients, with the allocated drug revealed using an online randomization module within the REDCap data management system.
Intervention and Follow-up
Piperacillin-tazobactam 4.5 g was administered every 6 hours intravenously, and meropenem 1 g was administered every 8 hours intravenously, both over 30 minutes. Trial drug was administered for a minimum of 3 days after randomization up to 14 days, with the total duration determined by the treating clinician. Treating clinicians and investigators were not blinded to treatment allocation. All patients had a blood culture collected at day 3 after randomization or on any other day if febrile (temperature >38°C) up to day 5. Patients were followed up for 30 days after randomization, by telephone call if the patient was discharged from hospital. Additional positive blood cultures were recorded up to day 30. Collected clinical and demographic data as well as trial definitions can be found in the Supplementary Materials.
Outcomes
The primary efficacy outcome was a composite defined as (1) all-cause mortality at 30 days postrandomization; (2) ongoing fever (temperature >38°C) or leukocytosis (white cell count >12 × 109/L) on day 5 postrandomization; (3) microbiological failure (growth of index organism from blood culture or other sterile site) on days 3–5 postrandomization; and (4) microbiological relapse (growth of index organism from blood culture or other sterile site) on days 5–30 postrandomization. Secondary outcomes are shown in Table 1.
Table 1.
Secondary Outcomes
| Outcome |
|---|
| Time to clinical resolution of infection (defined as number of days from randomization to resolution of fever [temperature <38°C]) |
| Clinical and microbiological success at day 5 (defined as survival plus resolution of fever and leukocytosis [white blood cell count <12 × 109/L]) |
| Length of hospital or intensive care unit stay |
| Requirement of intensive care unit admission |
| Infection with piperacillin-tazobactam or carbapenem-resistant organisms or Clostridioides difficile |
| Microbiological failure with third-generation cephalosporin-resistant isolate (same species as index blood culture isolated) in a subsequent sterile site |
| Colonization with multidrug-resistant organism(s) |
| Requirement of escalation of antibiotic therapy |
Microbiological Studies
Initial and follow-up bloodstream isolates were collected prospectively and stored at the recruiting site laboratory at –80°C and later shipped to the coordinating laboratory in Queensland, Australia. Phenotypic susceptibility testing by broth microdilution (BMD) using European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints and whole-genome sequencing analysis was performed on all collected isolates. A detailed description of methodologies used can be found in the Supplementary Materials.
Statistical Analysis and Sample Size Calculation
This was a pilot randomized clinical trial and therefore no formal sample size calculation was performed. We planned to recruit 100 participants in total (50 in the piperacillin-tazobactam arm, 50 in the meropenem arm). Primary analysis and per-protocol populations were defined (Supplementary Materials). Protocol deviations defining exclusion from the primary analysis and inclusion in the per-protocol sample were determined by the study investigators (A. G. S., P. N. A. H.). Proportions of patients, absolute risk differences, and 2-sided 95% confidence intervals (CIs) were calculated for the individual subcomponents of the composite primary outcome (death, clinical failure, microbiological failure, and microbiological relapse). An analysis of the primary outcome was undertaken in prespecified subgroups: (1) infecting species (Enterobacter spp vs other); (2) urinary tract vs non–urinary tract source; (3) health care associated vs non–health care associated; (4) appropriate vs inappropriate empiric therapy; (5) immunocompromise vs non-immunocompromise, and (6) Quick sequential Organ Failure Assessment score ≥2 vs <2.
Study Monitoring
A data and safety monitoring board was established comprising 2 independent infectious diseases physicians with support provided by an independent statistician. Interim analyses were performed after the first 25 and 50 patients completed the 30-day follow-up period.
RESULTS
Demographics and Clinical Characteristics of Patients
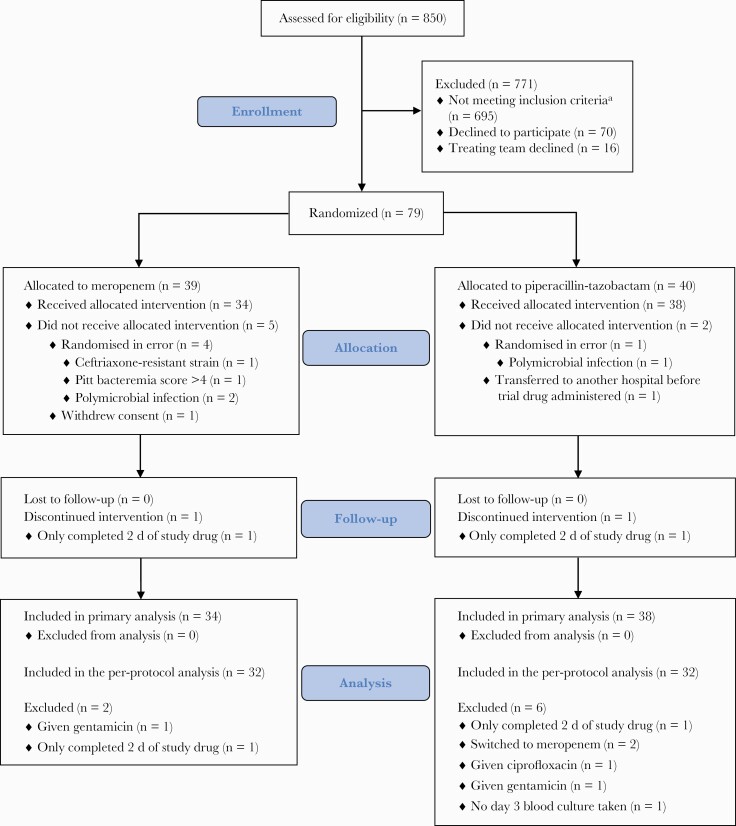
A total of 850 patients were screened during the trial period. Of these, 79 (9%) were randomized, although 7 patients (5 in the meropenem group and 2 in the piperacillin-tazobactam group) were randomized in error, met study exclusion criteria after randomization (prior to receipt of study drug), or did not receive the allocated study drug and were therefore excluded from the primary analysis population, which included 72 patients at baseline (34 received meropenem and 38 received piperacillin-tazobactam) (Figure 1). Baseline demographic and clinical details are summarized in Table 2. Overall, treatment groups were balanced with respect to baseline characteristics, although there were more patients in the piperacillin-tazobactam group undergoing surgery in the previous 14 days (14/38 [37%] vs 4/34 [12%]) and more liver disease present in the meropenem group (0/34 [0%] vs 4/34 [12%]). By the day of randomization, 30 of 72 (42%) patients had resolved objective markers of infection (as defined in the secondary outcome measure of clinical resolution of infection), (10/38 [37%] in the piperacillin-tazobactam group vs 16/34 [47%] in the meropenem group). Six of 38 patients (16%) received a third-generation cephalosporin as empiric antibiotic therapy compared with 3 of 34 (9%) in the piperacillin-tazobactam and meropenem groups, respectively.
Figure 1.
MERINO-2 patient recruitment, randomization, and flow through study. aPatients could meet >1 exclusion criteria. A total of 235 were excluded because >72 hours had elapsed since initial blood culture; 337, based on microbiology criteria; 48, allergy to trial drug; 205, polymicrobial infection; 46, not expected to survive >96 hours; 3, pregnant or breastfeeding; 12, no intent to cure; 16, <18 years old (<21 years in Singapore); and 4, previously enrolled. For 337 patients, microbiological exclusions based on susceptibility testing were as follows: 105 were nonsusceptible to third-generation cephalosporins, 120 were nonsusceptible to either meropenem or piperacillin-tazobactam and 205 were polymicrobial infections. Other exclusions included patient requiring ongoing antibiotic therapy (other than study drug) with activity against gram-negative bacilli (n = 21), Pitt bacteremia score >4 (n = 67), central nervous system source of infection (n = 25).
Table 2.
Baseline Characteristics of Patients in the Primary Analysis
| Characteristic | Meropenem (n = 34) | Piperacillin-Tazobactam (n = 38) |
|---|---|---|
| Species | ||
| Klebsiella (formerly Enterobacter) aerogenes | 2 (6) | 3 (8) |
| Enterobacter cloacae | 12 (35) | 15 (39) |
| Citrobacter freundii | 1 (3) | 1 (3) |
| Citrobacter braakii | 0 (0) | 1 (3) |
| Morganella morganii | 6 (18) | 5 (13) |
| Serratia marcescens | 12 (35) | 11 (29) |
| Providencia spp | 1 (3) | 0 (0) |
| Serratia spp | 0 (0) | 2 (5) |
| Stratum | ||
| E1 (Enterobacter spp urinary tract source) | 2 (6) | 3 (8) |
| E2 (Enterobacter spp non–urinary tract source) | 12 (35) | 15 (39) |
| C1 (Citrobacter spp, Serratia spp, Morganella spp, and Providencia spp urinary tract source) | 4 (12) | 5 (13) |
| C2 (Citrobacter spp, Serratia spp, Morganella spp, and Providencia spp non–urinary tract source) | 16 (47) | 15 (39) |
| Hospital | ||
| Istanbul Medipol Mega Hospital Complex | 4 (12) | 6 (16) |
| John Hunter Hospital | 10 (29) | 5 (13) |
| National University Hospital | 0 (0) | 4 (11) |
| Princess Alexandra Hospital | 3 (9) | 4 (11) |
| Royal Brisbane & Women’s Hospital | 3 (9) | 4 (11) |
| Singapore General Hospital | 0 (0) | 1 (3) |
| Tan Tock Seng Hospital | 14 (41) | 14 (37) |
| Country | ||
| Australia | 16 (22) | 13 (18) |
| Singapore | 14 (19) | 19 (26) |
| Turkey | 4 (12) | 6 (16) |
| Demographics | ||
| Age, mean ± SD | 67 ± 16 | 63 ± 15 |
| Female | 11 (32) | 11 (29) |
| Male | 23 (68) | 27 (71) |
| Acquisition type | ||
| Hospital-acquired | 10 (29) | 15 (39) |
| Healthcare-associated | 14 (41) | 20 (53) |
| Community-associated | 10 (29) | 3 (8) |
| Source | ||
| Urinary tract infection | 6 (18) | 8 (21) |
| Biliary tract/cholecystitis/cholangitis | 5 (15) | 7 (18) |
| Intra-abdominal infection | 3 (9) | 3 (8) |
| Line-related infection | 8 (24) | 9 (24) |
| Surgical site infection | 0 (0) | 2 (5) |
| Pneumonia (including ventilator-associated) | 1 (3) | 0 (0) |
| Septic arthritis/osteomyelitis/discitis | 1 (3) | 0 (0) |
| Skin and soft tissue including burns | 3 (9) | 2 (5) |
| Othera | 4 (12) | 3 (8) |
| Unknown or not recorded | 3 (9) | 4 (11) |
| Risk factors | ||
| Surgery within 14 d | 4 (12) | 14 (37) |
| Admitted in ICU at time of enrollment | 4 (12) | 4 (11) |
| Vascular catheter | 16 (47) | 12 (32) |
| Vascular catheter removal | 8 (24) | 9 (24) |
| Time to vascular catheter removal, d, median (range) | 2 (0–11) | 4 (0–7) |
| Urinary catheter | 13 (38) | 14 (37) |
| Immunosuppressionb | 5 (15) | 6 (16) |
| Neutropenia | 1 (3) | 0 (0) |
| Malignancy | 7 (21) | 8 (21) |
| Diabetes mellitus | 16 (47) | 14 (37) |
| Liver disease | 4 (12) | 0 (0) |
| qSOFA score ≥2 | 9 (26) | 9 (24) |
| Charlson Comorbidity Score, median (IQR) | 3 (2–5) | 2 (1–3) |
| Pitt Score, median (IQR) | 0 (0–2) | 0 (0–1) |
| Empirical antibiotics | ||
| β-lactam/β-lactamase inhibitorc | 8 (24) | 12 (32) |
| Carbapenem | 4 (12) | 8 (21) |
| Fourth-generation cephalosporin | 1 (3) | 0 (0) |
| Third-generation cephalosporin | 3 (9) | 6 (16) |
| Other | 18 (53) | 12 (32) |
| Hours to first effective antibiotic, median (IQR) | 1.5 (1.0–12.0) | 7.0 (1.0–18.0) |
| Empirical antibiotic appropriate | 33 (97) | 35 (92) |
| Trial antibiotic | ||
| Duration of study drug, d, mean (SD) | 5.79 (3.54) | 6.15 (6.65) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ICU, intensive care unit; IQR, interquartile range; qSOFA, quick Sequential Organ Failure Assessment; SD, standard deviation.
aOther sources include intravenous drug use and infective endocarditis.
bImmunosuppression defined as receiving cytotoxic chemotherapy, corticosteroids (>15 mg prednisolone daily equivalent), tumor necrosis factor alpha antagonist, azathioprine, or methotrexate.
cβ-lactam/β-lactamase inhibitors include piperacillin-tazobactam, ticarcillin-clavulanate, amoxicillin-clavulanate, and ampicillin-sulbactam.
Primary Outcome
A total of 11 of 38 patients (29%) within the primary analysis population randomized to receive piperacillin-tazobactam as definitive therapy met the primary outcome of death, microbiological failure, clinical failure, or microbiological relapse compared with 7 of 34 patients (21%) in the meropenem group (risk difference, 8%, [95% CI, –12% to 28%]). Results were consistent within the per-protocol population, with 8 of 32 patients (25%) meeting the primary outcome in the piperacillin-tazobactam group compared with 6 of 32 patients (19%) in the meropenem group (risk difference, 6% [95% CI, –14% to 24%]). Adjustment for a urinary tract source and severity of illness resulted in little change in the findings. Enterobacter spp infection, when compared to other organisms, resulted in a higher proportion of patients in the piperacillin-tazobactam group who met the primary outcome when compared to the meropenem group (28% vs 7%), although this was not significant (P = .14) (Table 3).
Table 3.
Primary Analysis and Subgroup Analysis
| Analysis | Primary Outcome, No./Total No. (%) | Risk Difference, % (2-Sided 95% CI) | P Value | |
|---|---|---|---|---|
| PTZ | Meropenem | |||
| Primary analysis | 11/38 (29) | 7/34 (21) | 8.4 (–11 to 28) | .41 |
| Per-protocol analysis | 8/32 (25) | 6/32 (19) | 6.2 (–14 to 26) | .55 |
| Subcomponents of the primary outcome | ||||
| Death | 0/38 (0) | 2/34 (6%) | 5.9 (–13 to 2) | .13 |
| Clinical failure | 8/38 (21) | 4/34 (12) | 9.3 (–8 to 26) | .29 |
| Microbiological failure | 5/38 (13) | 0/34 (0) | 13.2 (2 to 24) | .03 |
| Microbiological relapse | 0/38 (0) | 3/34 (9) | 8.8 (–18 to 1) | .06 |
| Subgroup analyses | ||||
| Infecting species | ||||
| Enterobacter spp | 5/18 (28) | 1/14 (7) | 20.7 (–4 to 45) | .14 |
| Other | 6/14 (43) | 6/14 (43) | 0.0 (–28 to 28) | 1.0 |
| Urinary tract vs non–urinary tract source | ||||
| Urinary tract | 1/8 (12) | 1/6 (17) | –4.2 (–42 to 33) | .83 |
| Non–urinary tract | 10/30 (33) | 6/28 (21) | 11.9 (–11 to 35) | .31 |
| Infection | ||||
| Healthcare-associated | 11/35 (31) | 5/24 (21) | 10.6 (–12 to 33) | .37 |
| Non–health care associated | 0/3 (0) | 0/3 (0) | … | |
| Appropriate empirical antibiotic therapy | ||||
| Appropriate | 10/35 (29) | 7/33 (21) | 7.4 (–13 to 28) | .48 |
| Inappropriate | 1/3 (33) | 0/1 (0) | 33.3 (–20 to 87) | .50 |
| Immunocompromise | ||||
| Present | 1/6 (17) | 1/5 (20) | –3.3 (–49 to 43) | .89 |
| Absent | 10/32 (31) | 6/29 (21) | –10.5 (–11 to 32) | .35 |
| qSOFA ≥2 | ||||
| Yes | 2/9 (22) | 2/9 (22) | 0.0 (–38 to 38) | 1.0 |
| No | 9/29 (31) | 5/25 (20) | 11.0 (–12.0 to 34) | .36 |
| Total duration of study drug | ||||
| <5 d | 6/20 (30) | 2/17 (12) | 18 (–7 to 43) | .18 |
| ≥5 d | 5/18 (28) | 5/17 (30) | –16 (–32 to 28) | .91 |
Abbreviations: CI, confidence interval; PZT, piperacillin-tazobactam; qSOFA, quick Sequential Organ Failure Assessment.
In the primary analysis population, a total of 0 of 38 (0%) died in the piperacillin-tazobactam group compared to 2 of 34 patients (6%) in the meropenem group (risk difference, 5.9% [95% CI, –13% to 2%]). A total of 8 of 38 (21%) experienced clinical failure in the piperacillin-tazobactam group compared to 4 of 34 patients (12%) in the meropenem group (risk difference, 9% [95% CI –8% to 26%]). A total of 5 of 38 (13%) experienced microbiological failure in the piperacillin-tazobactam group compared to 0 of 34 patients (0%) in the meropenem group (risk difference, 13% [95% CI, 2%–24%]). The majority of these were line-related infections; the difference in median time to line removal was 6 days and 4 days in the piperacillin-tazobactam and meropenem arms, respectively. A total of 0 of 38 (0%) experienced microbiological relapse in the piperacillin-tazobactam group compared to 3 of 34 patients (9%) in the meropenem group (risk difference, 9% [95% CI, –18% to 1%]). Patients meeting the primary outcome, stratified by minimum inhibitory concentration (MIC) and β-lactamase type, are presented in Table 4.
Table 4.
Death, Clinical Failure, Microbiological Failure, and Relapse at 30 Days in Patients With Bloodstream Infection due to AmpC-Producing Enterobacterales by Baseline Minimum Inhibitory Concentration (Microbiologic Modified Intention-to-Treat Population, n = 57)
| Piperacillin-Tazobactam (n = 30) | Meropenem (n = 27) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No./Total (%) | No./Total (%) | ||||||||
| MIC, mg/L | Deatha | Clinical Failure (n = 7) | Microbiological Failure (n = 5) | Microbiological Relapseb | MIC, mg/L | Deathc | Clinical Failure (n = 4) | Microbiological Failured | Microbiological Relapse (n = 2) |
| 0.25 | … | … | … | … | 0.016 | … | … | … | … |
| 0.5 | … | … | … | … | 0.03 | … | 2/4 (50) | … | 1/2 (50) |
| 1 | … | 1/7 (14) | 2/5 (40) | … | 0.06 | … | … | … | … |
| 2 | … | 2/7 (29) | 1/5 (20) | … | 0.125 | … | 2/4 (50) | … | 1/2 (50) |
| 4 | … | 3/7 (43) | 2/5 (40) | … | 0.25 | … | … | … | … |
| 8 | … | … | … | … | 0.5 | … | … | … | … |
| 16 | … | 1/7 (14) | … | … | 1 | … | … | … | … |
| β-lactamase gene family | β-lactamase gene family | ||||||||
| CMY | … | 1 | … | … | CMY | … | … | … | … |
| ACT | … | 1 | 1 | … | ACT | … | … | … | … |
| CMH | … | … | 1 | … | CMH | … | … | … | … |
| DHA | … | 1 | … | … | DHA | … | … | … | … |
| MIR | … | … | … | … | MIR | … | … | … | … |
Abbreviation: MIC, minimum inhibitory concentration.
aNo deaths occurred in the piperacillin-tazobactam arm.
bNo microbiological relapses occurred in the piperacillin-tazobactam arm.
cTwo deaths occurred in the meropenem arm; however, isolates were not obtained.
dNo microbiological failures occurred in the piperacillin-tazobactam arm.
Secondary Outcomes
Time to clinical resolution of infection was similar between groups; the median day of resolution after randomization was 1 (interquartile range [IQR], 0–1) in both groups. Of those that did not show signs of clinical resolution at time of randomization, median day of resolution was also 1 (IQR, 1–2) for both groups. Clinical and microbiological success by day 5 occurred in 28 of 38 patients (74%) in the piperacillin-tazobactam group compared to 28 of 34 patients (82%) in the meropenem group (risk difference, –9% [95% CI, –28% to 10%]). Requirement of intensive care unit admission during the study period was observed in 4 of 38 patients (11%) in the piperacillin-tazobactam group compared with 6 of 34 patients (18%) in the meropenem group (risk difference, –7% [95% CI, –23% to 9%]). One patient in the piperacillin-tazobactam group had a piperacillin-tazobactam–resistant Enterobacter cloacae in blood culture identified on day 4 postrandomization, as determined by local laboratory antimicrobial susceptibility testing. The pair of bacterial isolates were sequenced which demonstrated genetic homology and presence of blaACT-49. No significant change in MIC or zone diameter was demonstrated. Of the 30 patients (42%) who had a vascular device (apart from a peripheral venous cannula), 17 of these had their device removed, 9 in the piperacillin-tazobactam group and 8 in the meropenem group. Median time to device removal was 4 days in the piperacillin-tazobactam group and 2 days in the meropenem group. No carbapenem-resistant isolates were identified in the primary analysis population. One patient in the meropenem group had Clostridioides difficile infection. Identification of a multidrug-resistant organism (ie, vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, ESBL-producing Enterobacterales) occurred in 2 of 38 patients (5%) in the piperacillin-tazobactam group and 2 of 34 (6%) in the meropenem group (risk difference, –1% [95% CI, –11% to 10%]). Four of 38 patients (11%) in the piperacillin-tazobactam group and 1 of 34 (3%) in the meropenem group required escalation of antibiotic therapy or addition of second gram-negative agent in the first 5 days postrandomization (risk difference, 8% [95% CI, –4% to 19%]). Median length of hospital stay was 9 days for the piperacillin-tazobactam group (IQR, 4–29) and 8 days for the meropenem group (IQR, 5–18).
Adverse Events
There were no nonfatal serious adverse events recorded for either study group. Two deaths occurred in the meropenem group. One death occurred 28 days postrandomization and was thought to be secondary to multiorgan failure and sepsis due to S marcescens BSI. The other death occurred 8 days postrandomization and was secondary to ischemic heart disease.
Microbiological Analysis
Fifty-seven of 72 patients (79%) in the in the primary analysis population had their index blood culture isolate sent to the coordinating laboratory in Brisbane, Australia. The isolates available from all enrolled patients grouped by country were Australia, 24/29; Singapore, 23/33; and Turkey, 10/10. Of the 57 isolates, species included Serratia marcescens (19), Enterobacter cloacae (13), Morganella morganii (9), Enterobacter hormaechei (7), Enterobacter aerogenes (5), Citrobacter freundii (2), Citrobacter braakii (1), and Providencia stuartii (1).
Antibiotic Susceptibility Testing
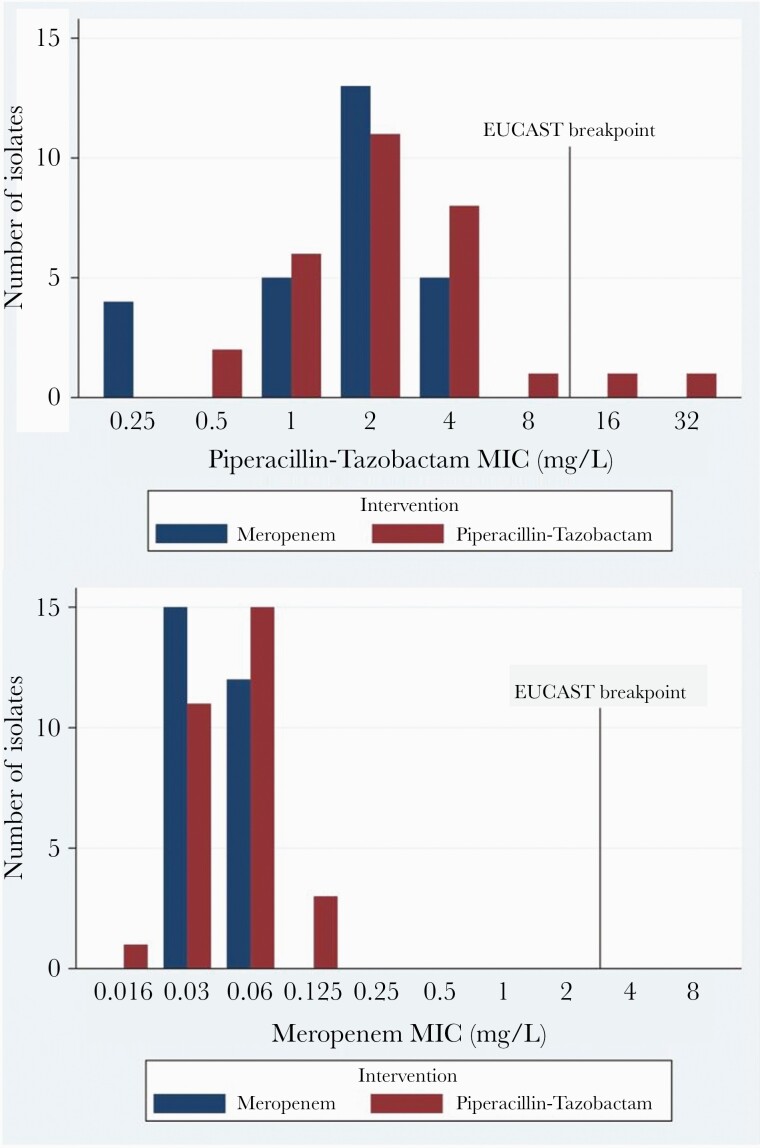
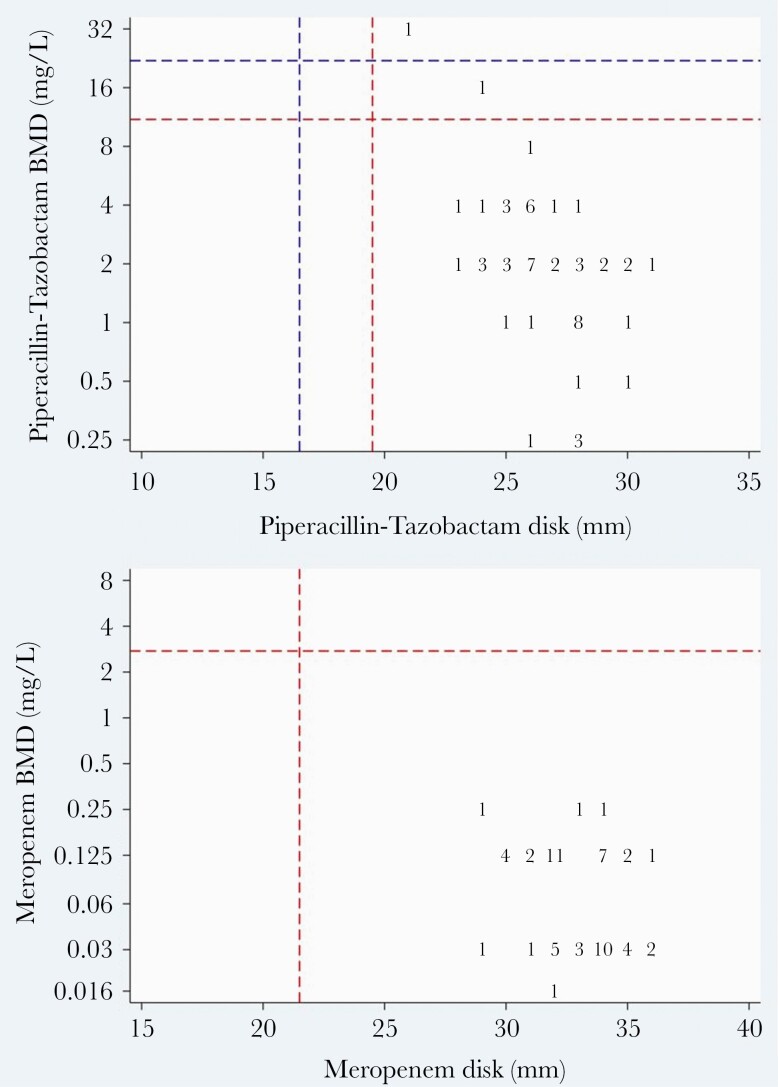
Of the available index blood culture isolates (n = 57), overall susceptibility using EUCAST breakpoints to piperacillin-tazobactam and meropenem by BMD was 96% and 100%, respectively. Two isolates were resistant to piperacillin-tazobactam, C freundii (MIC 32 mg/L) and M morganii (MIC 16 mg/L); both were randomized to piperacillin-tazobactam (Figure 2). All isolates were susceptible to meropenem. On disk diffusion testing, all isolates were susceptible to piperacillin-tazobactam and meropenem. Twenty-two (39%) and 54 (94.7%) index isolates demonstrated susceptibility to cefoxitin and cefotaxime, respectively, on disk diffusion, respectively. The very major error (VME) and major error (ME) rates for piperacillin-tazobactam by disk diffusion by EUCAST breakpoints were 4% and 0%, respectively (Figure 3). There were no VME or MEs for meropenem.
Figure 2.
Piperacillin-tazobactam and meropenem minimum inhibitory concentration by intervention arm. Abbreviations: EUCAST, European Committee on Antimicrobial Susceptibility Testing; MIC, minimum inhibitory concentration.
Figure 3.
Scatterplot comparison of piperacillin-tazobactam and meropenem susceptibility testing by disk diffusion testing (x-axis; zone diameter) and broth microdilution (y-axis; minimum inhibitory concentration). The red dashed line represents the European Committee on Antimicrobial Susceptibility Testing susceptible breakpoint and the blue dashed line represents the Clinical and Laboratory Standards Institute susceptible breakpoint. Abbreviation: BMD, broth microdilution.
β-Lactamase Genes
The predominant chromosomal ampC genes identified by whole-genome sequencing were those from the CMY gene family (4 blaCMY-2, 2 blaCMY-101, 1 blaCMY-51), ACT gene family (2 blaACT-17, 1 blaACT-12, 1 blaACT-15, 1 blaACT-16, 1 blaACT-2, 1 blaACT-27, 1 blaACT-38, 1 blaACT-40, 1 blaACT-43, 1 blaACT-49, 1 blaACT-55), CMH gene family (2 blaCMH-3, 1 blaCMH-1), DHA gene family (3 blaDHA-17, 2 blaDHA-20, 1 blaDHA-12, 1 blaDHA-16, 1 blaDHA-18), and MIR gene family (1 blaMIR-18, 1 blaMIR-19, 1 blaMIR-9). There were no ESBL, OXA β-lactamase, or carbapenemase genes identified.
Isolates From Microbiological Failure and Relapse
Five of 8 patients (63%) who experienced either microbiological failure or microbiological relapse had their subsequent bacterial isolates sent to the coordinating laboratory. This included 3 S marcescens and 2 E cloacae isolates. Four of the 5 patients (80%) who experienced microbiological failure had vascular access devices in situ, which were removed 4–7 days after their index blood culture was taken (median, 6; mean, 5). This was considerably longer than the average time for device removal (Table 2). Chromosomal ampC genes identified included 1 blaACT-49 and 2 blaCMH-3. Core genome single-nucleotide polymorphism differences between index and subsequent blood culture isolates ranged from 0 to 2 (Table 5). Index and repeat isolates were susceptible to cefotaxime, piperacillin-tazobactam, and meropenem. Three isolates were cefoxitin susceptible (67%), including the blaACT-19–producing E. cloacae isolate that was identified on day 4 as piperacillin-tazobactam resistant by a local automated method.
Table 5.
Patients in Microbiologic Modified Intention-to-Treat Population (n = 5) Experiencing Microbiological Failure or Relapse
| Isolate | Organism | Intervention | Age | Acquisition | Line (Removed) | Charlson Comorbidity Index | Immunosuppression | Time From Randomization to Follow-up Positive BC, h | β-Lactamase Genes | MLST | Core Genome SNP Difference in Follow-up BC Isolate | Cefotaxime Disk Diffusion Test | PTZ MIC | MER MIC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MER2-27 | Serratia marcescens | Piperacillin-tazobactam | 64 | Healthcare-associated | Dialysis line (yes) | 2 | None | 41 | blaSRT | NA | … | 28 mm | 1 mg/L | 0.125 mg/L |
| MER2-28 | blaSRT | NA | 0 | 28 mm | 2 mg/L | 0.125 mg/L | ||||||||
| MER2-37 | Enterobacter cloacae | Piperacillin-tazobactam | 73 | Healthcare-associated | Dialysis line (yes) | 11 | Metastatic solid tumor | 16 | blaACT-49 | NA | … | 26 mm | 4 mg/L | 0.125 mg/L |
| MER2-40 | blaACT-49 | NA | 2 | 25 mm | 2 mg/L | 0.03 mg/L | ||||||||
| MER2-65 | Enterobacter cloacae | Piperacillin-tazobactam | 60 | Healthcare-associated | Dialysis line (yes) | 4 | None | 53 | blaCMH-3 | 462 | … | 29 mm | 4 mg/L | 0.125 mg/L |
| MER2-66 | blaCMH-3 | 462 | 0 | 28 mm | 4 mg/L | 0.03 mg/L | ||||||||
| MER2-82 | Serratia marcescens | Piperacillin-tazobactam | 27 | Hospital-acquired | None | 1 | Corticosteroids | 42 | blaSST-1 | NA | … | 26 mm | 1 mg/L | 0.125 mg/L |
| MER2-84 | blaSST-1 | NA | 2 | 28 mm | 1 mg/L | 0.03 mg/L | ||||||||
| MER2-86 | Serratia marcescens | Meropenem | 46 | Hospital-acquired | Central venous line (yes) | 1 | None | 46 | blaSST-1 | NA | … | 26 mm | 1 mg/L | 0.125 mg/L |
| MER2-87 | blaSST-1 | NA | 0 | 28 mm | 1 mg/L | 0.125 mg/L |
Repeat isolates are shown in bold.
Abbreviations: BC, blood culture; MER, meropenem; MIC, minimum inhibitory concentration; MLST, multilocus sequence type; NA, not available; PTZ, piperacillin-tazobactam; SNP, single-nucleotide polymorphism.
DISCUSSION
In patients with BSI caused by AmpC-producing organisms, we were unable to quantify precisely the difference of piperacillin-tazobactam, when compared to meropenem, in the primary analysis population with respect to a composite endpoint of death, clinical cure, microbiological failure, and microbiological relapse. This trial has generated preliminary data directed toward a definitive randomized clinical trial. Overall, 18 of 72 (25%) individuals in the primary analysis population reached the primary endpoint during the 30-day follow-up period. Moreover, 29% vs 21% met the primary endpoint in the piperacillin-tazobactam and meropenem arms, respectively (risk difference, 8% [95% CI, –11% to 28%]), which was not statistically significant. Interestingly, microbiological failure was more common in the piperacillin-tazobactam arm (13% vs 0%; P = .03), although index and repeat blood culture isolates remained susceptible to piperacillin-tazobactam with MIC range 1–4 mg/L on BMD testing. This result most likely represented delayed removal of vascular devices in the piperacillin-tazobactam group (median time to removal, 6 vs 4 days). The increased empirical use of third-generation cephalosporins in the piperacillin-tazobactam arm (16%) compared with meropenem (9%) may have influenced this result, although none of the recovered isolates from patients with microbiological failure demonstrated a de-repressed ampC phenotype. Microbiological relapse was more common in the meropenem arm (9% vs 0%; P = .06), although antibiotic susceptibility remained similar between index and repeat blood culture isolates, suggesting that this was a chance finding and mechanisms outside of antimicrobial resistance were likely responsible (eg, source control). Of the 3 patients who experienced microbiological relapse, none had evidence of immunosuppression. Only 2 of 57 (4%) index blood culture isolates recovered were nonsusceptible to piperacillin-tazobactam on BMD testing with MIC values of 16 and 32 mg/L, also indicating that treatment failure due to resistance would be rare in this cohort. Although there was a minor difference observed in the mean duration of study drug between groups, no significant difference in primary outcome was noted in a subgroup analysis (<5 days vs ≥5 days) in Table 3. Of those who had Enterobacter spp infection, more patients met the primary composite outcome in the piperacillin-tazobactam group (28% vs 7%). Among chromosomal AmpC producers, there exist species-specific mutation rates for ampC de-repression, which may approximate the extent to which resistant mutants are present in infection and may correlate with risk of treatment failure [18]. Enterobacter spp are known to demonstrate high mutation rates for ampC de-repression when compared to other chromosomal AmpC producers [18]. Two deaths occurred in the meropenem arm with zero occurring in the piperacillin-tazobactam arm, likely representing a chance finding. One death was caused by severe heart failure secondary to sepsis where infection contributed but was not the major cause. The other death also occurred due to severe heart failure, although infection was not a substantial contributor.
In the MERINO trial, 36 of 320 (11%) of isolates possessed an ampC gene (mainly blaCMY-2) [11, 19]. Many isolates that co-harbored an AmpC β-lactamase (including de-repressed ampC gene) demonstrated a piperacillin-tazobactam MIC bordering the EUCAST clinical breakpoint (≤8 mg/L) [19]. The importance of accurately determining the piperacillin-tazobactam MIC and its positive correlation with percentage mortality was demonstrated. In our trial, of the patients experiencing clinical failure in the piperacillin-tazobactam arm, only 1 index blood culture isolate demonstrated resistance to piperacillin-tazobactam on BMD testing (16 mg/L).
A recently published case-control study of 165 adult patients with BSIs due to AmpC producers compared clinical outcomes between piperacillin-tazobactam (n = 88) vs meropenem or cefepime (n = 77) [12]. They carried out propensity score matching and demonstrated no significant difference between groups with respect to 30-day mortality, 7-day mortality, and microbiological failure; although this was a single-center retrospective study. Similarly, a meta-analysis of 11 observational studies comparing treatment effects of different antibiotics in patients with BSI due to AmpC producers found no significant difference in mortality between β-lactam/β-lactamase inhibitors vs carbapenems (odds ratio, 0.9 [95% CI, .3–2.4]) [17]. More contemporary clinical data tell a similar story. A retrospective cohort study of 241 patients with BSI due to Enterobacter spp., Serratia spp., Citrobacter spp., Providencia spp., Morganella spp. (ESCPM) organisms demonstrated no difference in 30-day mortality in patients receiving empirical piperacillin-tazobactam and definitive cefepime, compared with carbapenems [14]. Another retrospective study of 277 patients with BSI due to Enterobacter spp again demonstrated no difference in 30-day all-cause mortality between carbapenem and piperacillin-tazobactam definitive therapy [20]. Given the many limitations of observational studies and the presence of microbiological and case report data demonstrating the selection of ampC de-repressed mutants by piperacillin-tazobactam, questions regarding the true clinical significance remain unanswered [21, 22].
This study has several limitations. First, this was a small trial. One hundred patients were planned to be included in the primary analysis population; however, due to a slow accrual, recruitment was suspended after 72 were enrolled. Recruiting patients within the 72-hour window is problematic given the time limitations of standard microbiological techniques for speciation and antibiotic susceptibility testing. Indeed, the majority of patients (235/771 [31%]) were excluded for this reason. In addition, during the recruitment phase of the trial, the results of the MERINO trial were made public, which caused a temporary pause in recruitment and interim data analysis. Second, the inclusion criteria were changed a number of times early during the trial due to poor recruitment. This consisted of changing the required duration of trial antibiotic, exclusion of patients with third-generation cephalosporin–resistant isolates, and including neutropenic patients and solid organ transplant recipients. Third, empiric therapy was not under control of the study team and many patients in the piperacillin-tazobactam group received meropenem as empiric therapy and vice versa. In addition, many patients had evidence of resolved infection at the time or shortly after randomization. Fourth, not all index and follow-up blood culture isolates were sent to our reference laboratory for further analysis, thereby limiting the interpretation of microbiological data. In addition, not all isolates had ampC-type genes identified, which could reflect inadequate coverage level or nucleotide sequence identity. Fifth, we did not define or document whether adequate source control was achieved, and possible imbalance between groups with respect to this measure would bias outcomes. Sixth, trial participants did not have their β-lactam therapy administered via an extended or continuous infusion. Many nonrandomized studies have demonstrated improved clinical outcomes in patients receiving carbapenems or piperacillin-tazobactam via extended or continuous infusions [23–25].
CONCLUSIONS
Among patients with BSI due to AmpC producers, our pilot randomized trial was unable to show a difference in primary outcome between the piperacillin-tazobactam and meropenem treatment groups; we found that piperacillin-tazobactam may lead to more microbiological failures, albeit fewer microbiological relapses. Of the components in our composite outcome, it is unclear what weighting should be attached to each to achieve optimum clinical relevance. A larger clinical trial is required to answer these questions more definitively.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. P. N. A. H. and D. L. P. designed the study. M. C. and P. N. A. H. wrote the statistical analysis plan. P. N. A. H. designed the data collection tools. A. G. S., M. B., and A. H. conducted laboratory testing. B. M. F. and A. G. S. undertook bioinformatic analysis. A. G. S. cleaned and analyzed the data. P. N. A. H., T. H. B., and A. G. S. monitored the data collection for the whole trial. P. N. A. H. and A. G. S. interpreted the data. A. G. S. drafted the manuscript, and all authors revised the manuscript.
Acknowledgments. The authors thank the following study staff for project implementation: Andrew Walczak, Neil Underwood, Chwee Fang Bok, Christina Titin, Elda Righi, Janaye Fish, Jonathan Lim, Jose Cuenca, Melissa Finney, Penny Lorenc, Samia Shawkat, Shilpa Mukherjee, Siok Ching Cha, Tan Chien Hua, Timothy Chia. The MERINO 2 Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN): Po Ying Chia (Tan Tock Seng Hospital); Gail Cross, Jyoti Somani, Gabriel Yan (National University Hospital).
Financial support. This work was supported by a Royal Brisbane and Women’s Hospital Foundation Grant; Pathology Queensland–Study, Education and Research Committee (SERC-6086). P. N. A. H. was supported by a National Health and Medical Research Council Early Career Fellowship (award number GNT1157530).
Potential conflicts of interest. D. L. P. has received funding from AstraZeneca, Pfizer, Shionogi, Leo Pharmaceuticals, Bayer, GlaxoSmithKline (GSK), Cubist, Entasis, Sumitomo, QPex, Venatorx, bioMérieux and Accelerate; reports board membership from Entasis, Qpex, Merck, Shionogi, Achaogen, AstraZeneca, Leo Pharmaceuticals, Bayer, GSK, Cubist, Venatorx, and Accelerate; reports grants/grants pending from Shionogi and Merck; and has received payment for lectures including service on speaker’s bureaus from Pfizer and Merck, outside the submitted work. P. N. A. H. has received research grants from MSD, Sandoz, and Shionogi, as well as speaker’s fees from Pfizer, and has served on an advisory board for Sandoz outside the submitted work. B. Y. has received honoraria from Sanofi and Roche. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN):
References
- 1.Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev 2009; 22:161–82, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meini S, Tascini C, Cei M, et al. AmpC β-lactamase-producing Enterobacterales: what a clinician should know. Infection 2019; 47:363–75. [DOI] [PubMed] [Google Scholar]
- 3.Lindberg F, Westman L, Normark S. Regulatory components in Citrobacter freundii ampC beta-lactamase induction. Proc Natl Acad Sci U S A 1985; 82:4620–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamma PD, Doi Y, Bonomo RA, et al. ; Antibacterial Resistance Leadership Group. A primer on AmpC β-lactamases: necessary knowledge for an increasingly multidrug-resistant world. Clin Infect Dis 2019; 69:1446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizrahi A, Delerue T, Morel H, et al. ; Saint-Joseph/Avicenna Study Group. Infections caused by naturally AmpC-producing Enterobacteriaceae: can we use third-generation cephalosporins? A narrative review. Int J Antimicrob Agents 2020; 55:105834. [DOI] [PubMed] [Google Scholar]
- 6.Choi SH, Lee JE, Park SJ, et al. Emergence of antibiotic resistance during therapy for infections caused by Enterobacteriaceae producing AmpC beta-lactamase: implications for antibiotic use. Antimicrob Agents Chemother 2008; 52:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow JW, Fine MJ, Shlaes DM, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann Intern Med 1991; 115:585–90. [DOI] [PubMed] [Google Scholar]
- 8.Siebert JD, Thomson RB Jr, Tan JS, Gerson LW. Emergence of antimicrobial resistance in gram-negative bacilli causing bacteremia during therapy. Am J Clin Pathol 1993; 100:47–51. [DOI] [PubMed] [Google Scholar]
- 9.Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother 2012; 67:2793–803. [DOI] [PubMed] [Google Scholar]
- 10.Elshamy AA, Aboshanab KM. A review on bacterial resistance to carbapenems: epidemiology, detection and treatment options. Future Sci OA 2020; 6:FSO438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris PNA, Tambyah PA, Lye DC, et al. ; MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN). Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA 2018; 320:984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng L, Nelson BC, Mehta M, et al. Piperacillin-tazobactam versus other antibacterial agents for treatment of bloodstream infections due to AmpC beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 2017; 61:e00276-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bush K, Macalintal C, Rasmussen BA, et al. Kinetic interactions of tazobactam with beta-lactamases from all major structural classes. Antimicrob Agents Chemother 1993; 37:851–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan SH, Ng TM, Chew KL, et al. Outcomes of treating AmpC-producing Enterobacterales bacteraemia with carbapenems vs. non-carbapenems. Int J Antimicrob Agents 2020; 55:105860. [DOI] [PubMed] [Google Scholar]
- 15.McKamey L, Venugopalan V, Cherabuddi K, et al. Assessing antimicrobial stewardship initiatives: clinical evaluation of cefepime or piperacillin/tazobactam in patients with bloodstream infections secondary to AmpC-producing organisms. Int J Antimicrob Agents 2018; 52:719–23. [DOI] [PubMed] [Google Scholar]
- 16.Holsen MR, Wardlow LC, Bazan JA, et al. Clinical outcomes following treatment of Enterobacter species pneumonia with piperacillin/tazobactam compared to cefepime or ertapenem. Int J Antimicrob Agents 2019; 54:824–8. [DOI] [PubMed] [Google Scholar]
- 17.Harris PN, Wei JY, Shen AW, et al. Carbapenems versus alternative antibiotics for the treatment of bloodstream infections caused by Enterobacter, Citrobacter or Serratia species: a systematic review with meta-analysis. J Antimicrob Chemother 2016; 71:296–306. [DOI] [PubMed] [Google Scholar]
- 18.Kohlmann R, Bähr T, Gatermann SG. Species-specific mutation rates for ampC derepression in Enterobacterales with chromosomally encoded inducible AmpC β-lactamase. J Antimicrob Chemother 2018; 73:1530–6. [DOI] [PubMed] [Google Scholar]
- 19.Henderson A, Paterson DL, Chatfield MD, et al. Association between minimum inhibitory concentration, beta-lactamase genes and mortality for patients treated with piperacillin/tazobactam or meropenem from the MERINO study [manuscript published online ahead of print 27 October 2020]. Clin Infect Dis 2020. doi:10.1093/cid/ciaa1479. [DOI] [PubMed] [Google Scholar]
- 20.Drozdinsky G, Neuberger A, Rakedzon S, et al. Treatment of bacteremia caused by Enterobacter spp.: should the potential for ampC induction dictate therapy? A retrospective study. Microb Drug Resist 2021; 27:410–4. [DOI] [PubMed] [Google Scholar]
- 21.Moraz M, Bertelli C, Prod’hom G, et al. Piperacillin/tazobactam selects an ampC derepressed E. cloacae complex mutant in a diabetic osteoarticular infection. Clin Microbiol Infect 2021; 27:475–7. [DOI] [PubMed] [Google Scholar]
- 22.Akata K, Muratani T, Yatera K, et al. Induction of plasmid-mediated AmpC β-lactamase DHA-1 by piperacillin/tazobactam and other β-lactams in Enterobacteriaceae. PLoS One 2019; 14:e0218589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richter DC, Frey O, Röhr A, et al. Therapeutic drug monitoring-guided continuous infusion of piperacillin/tazobactam significantly improves pharmacokinetic target attainment in critically ill patients: a retrospective analysis of four years of clinical experience. Infection 2019; 47:1001–11. [DOI] [PubMed] [Google Scholar]
- 24.Falagas ME, Tansarli GS, Ikawa K, Vardakas KZ. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: a systematic review and meta-analysis. Clin Infect Dis 2013; 56:272–82. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes NJ, Liu J, O’Donnell JN, et al. Prolonged infusion piperacillin-tazobactam decreases mortality and improves outcomes in severely ill patients: results of a systematic review and meta-analysis. Crit Care Med 2018; 46:236–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.