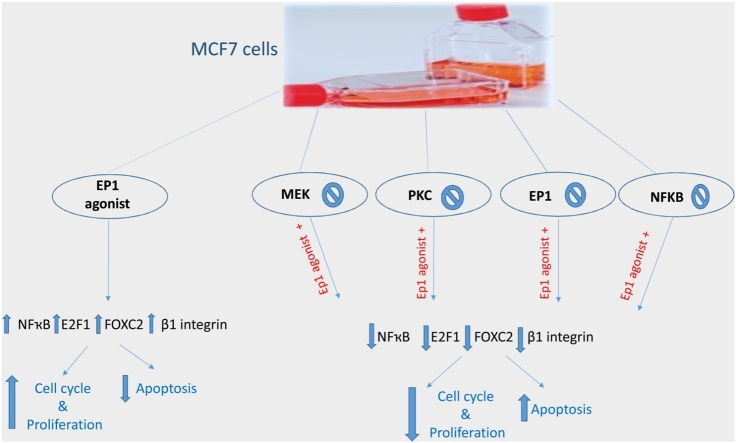
Graphical abstract
Keywords: Breast cancer, E2F1, FOXC2, NF-ҡB, Prostaglandin E2, β1-integrin
Highlights
-
•
PGE2 enhanced β1- integrin expression via EP1 receptor, PKC, MEK and NfҡB.
-
•
FOXC2, E2F1 and survivin play a role in PGE2 mediated effect in MCF7 cells.
-
•
PGE2 enhances breast cancer cell cycle through E2F1, FOXC2, survivin and β integrin.
-
•
Biochemical mediators of PKC/MEK pathway could be considered as targets for breast cancer treatment.
Abstract
Prostaglandin E2 (PGE2) and β1-integrin have been correlated with breast cancer, where both could enhance progression and metastasis. Protein kinase C (PKC) and MEK have played a vital role in breast cancer development. Our study was conducted to elucidate the effect of inhibition of E-prostanoid receptor 1 (EP1)/ PKC/ MEK/ β1-integrin pathway in mitigating breast cancer progression and to evaluate the role of the intermediate signals FOXC2, E2F1, NF-ҡB and survivin. MCF7 cells were treated with 17 -PT-PGE2, an EP1 agonist, for 24 h, and β1-integrin was measured. To MCF7 cells treated with 17-PT-PGE2, inhibitors of either EP1, MEK, PKC or NF-ҡB were added followed by measurement of β1-integrin gene expression and cell proliferation in each case. Addition of 17- PT-PGE2 to MCF7 cells showed enhancement of both cell proliferation, and cell cycle transition from G1 to S phase. In addition, activation of EP1 receptor increased β1-integrin expression. On the contrary, inhibition of EP1 receptor showed a decrease in the cell proliferation, β1-integrin expression and cells transition to S phase, but increased cell count in apoptotic phase. Selective inhibition of each of MEK, PKC, and NF-ҡB suppressed 17 -PT-PGE2-mediated β1-integrin expression as well as cell proliferation. Furthermore, FOXC2, phosphorylated NF-ҡB, E2F1, and survivin levels were upregulated with 17- PT-PGE2 and suppressed by MEK, PKC and NF-ҡB inhibitors. Targeting the biochemical mediators of PKC/MEK pathway may be of value in developing new chemical entities for cancer treatment.
1. Introduction
Over expression of cyclooxygenase 2 (COX2) was documented in different cancer tissues. Prostaglandin E2 (PGE2), is one of the most important products of COX2 and is implicated in many cancer types. In breast cancer, COX2 could modulate growth, progression, invasion and even metastasis. The effect of PGE2 is mediated by activation of multiple mediators such as endothelial growth factor receptor (EGFR), extracellular-signal-regulated kinase (ERK) and metalloproteinase-9 (MMP9) [[1], [2], [3]].
PGE2 exerts its action through coupling with four E-Prostanoid receptors (EP), among them EP1 that plays a crucial role in hepatocellular carcinoma and was also expressed in other cancerous tissues including breast cancer [4]. Targeting EP1 receptor with specific antagonist significantly inhibits the development of chemically-induced breast cancer in rats [5].
Integrins are cell surface family of receptors for extracellular matrix proteins, including collagen and fibronectin. These integrins are glycoproteins that composed of two main subunits alpha and beta which transduce the signal from extracellular matrix to inside the cell. β1-integrin is reported to play a significant role in breast cancer in vivo and in vitro by mediating proliferation, invasion and migration [6]. Moreover, PGE2 is positively correlated with β1-integrin in hepatocellular carcinoma [7]. Interestingly, β1-integrin itself was reported to increase COX2 and PGE2 levels and hence tumerogenesis and metastasis of breast cancer cell line, as noted in earlier studies [8].
Cancer cells might utilize inflammatory mediators like nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ҡB) and other tumor-propagating factors like mitogen activated protein kinase (MAPK), vascular endothelial growth factor (VEGF) and protein kinase C (PKC), to maintain growth, proliferation and migration [9].
Forkhead box protein C2 (FOXC2) is a member of the family of helix/forkhead transcription factors. It is known to be involved in the upregulation of β1-integrin expression by coupling of fox-binding element to the integrin promotor. FOXC2 is also responsible for controlling a huge range of biological processes; including cell growth, longevity and progression [10]. Furthermore, E2F transcription factor 1 (E2F1) is a transcription factor, which is involved in regulation of cell renewal, differentiation and cell cycle check points in breast cancer [11].
Another molecular protein is survivin which is overexpressed in many types of malignancies with the ability to make cells bypass the cell cycle checkpoints, resulting in uncontrolled, continuous cells replication. Survivin also inhibits apoptosis by acting on caspase 3, 7 and 9, and is involved in breast cancer development by interacting with protein kinase B (Akt) pathway [12].
Progression of cells through the cell cycle is an essential process governing genome duplication and cell division. Cells go through four phases of cell cycle; G1 phase, where cells grow properly to enter the S phase where DNA is replicated and cells get ready for another growth and preparations for mitosis in G2 phase, then the mitosis phase, where cells actually divide. Cell cycle dysregulation is one of the hallmarks in cancer through which cells can go uncontrolled and show continuous replication and proliferation [13].
Up till now, little was known about PGE2 exact effect on β1-integrin expression in breast cancer. Thus, our study aimed to elucidate the ability of PGE2 to elicit β1-integrin over-expression in MCF7 breast cancer cells through EP1 receptor and the possible underlying molecular mechanistic pathway that may be involved. In the presence of PGE2 analog 17-PT-PGE2 (EP1 agonist), we used EP1 antagonist and PKC, MEK and NF-ҡB inhibitors to investigate their effect on cell proliferation, cell cycle transition and the related biochemical intermediates FOXC2, E2F1, NF-ҡB, survivin as well as β1-integrin expression.
2. Materials and methods
2.1. Reagents and cell line
MCF7 cell line was purchased from the Egyptian company for production of vaccines, sera, and drugs (VACSERA, Cairo, Egypt). Cells were verified by karyotyping analysis according to American Type Culture Collection (ATCC) Guidelines. MCF7 cells were cultured in Roswell Park Memorial Institute Medium (RPMI 1640) (Gibco®, USA) supplemented with 10 % heat-inactivated fetal bovine serum (FBS) (Gibco®, USA) and 1% penicillin/streptomycin (Gibco®, USA).
17-phenyl trinor-prostaglandin E2 (17-PT-PGE2) as EP1 agonist, PD98059, as MEK inhibitor, and Bisindolylymaleimide I (GF109203X) as PKC inhibitor, were all purchased from SantaCruiz Biotechnology, Inc (USA). SC-19220 as EP1 antagonist and ammonium pyrrolidinedithiocarbamate (PDTC) as NF-ҡB inhibitor were obtained from Sigma-Aldrich (USA).
Ca2+ Mg2+ free phosphate-buffered saline (PBS) was obtained from Lonza Verviers Sprl® (Belgium), phenol red-free RPMI 1640 media was purchased from Lonza® (USA), Dimethyl Sulfoxide (DMSO) was obtained from S D Fine-Chem Limited Company® (India). Trypsin-ethylenediaminetetraacetic acid (trypsin-EDTA) was obtained from Gibco® (USA) and MTT cell proliferation assay kit was obtained from (Thermo Fisher Scientifc®, USA).
2.2. Experimental design
MFC7 culture flasks were divided into 6 groups, each of 6 replicates, according to treatment, as follows:
-
-
Control group; cells were treated with DMSO for 24 h.
-
-
EP1 agonist group; cells were treated with 5 μM 17-PT-PGE2 [14] for 24 h.
-
-
EP1 antagonist + EP1 agonist group; cells were treated with 10 μM EP1 antagonist (SC-19220) [15], for one hour, followed by 17-PT-PGE2 (for 24 h).
-
-
MEK inhibitor + EP1 agonist group; cells were treated with 10 μM MEK inhibitor (PD98059) [16] for one hour, followed by 17-PT-PGE2 for 24 h.
-
-
PKC inhibitor + EP1 agonist group; cells were treated with 10 μM PKC inhibitor (GF109203X) [17], for one hour, followed by 17-PT-PGE2 for 24 h.
-
-
NF-ҡB inhibitor + EP1 agonist; cells were treated with 20 μM NF-ҡB inhibitor (PDTC) [18], for one hour, followed by 17-PT-PGE2 for 24 h.
Separately, the same experimental design was conducted using a-six-wells cell culture plates (plates were divided into 6 groups, each of 6 replicates) for measurement of total and phosphorylated NF-ҡB P65, where the inhibitors (SC-19220, PD98059, GF109203X and PDTC) were added for two hours followed by addition of 17-PT-PGE2 for one hour (as the phosphorylated form of NF-ҡB is best to be measured at 2 h after the activation).
2.3. Preparation of cell lysate
MCF7 cells were harvested and detached by trypsinization (trypsin-EDTA) then cells were washed and resuspended in PBS (pH 7.4) to get a concentration of one million cells/mL. Cells were exposed to five repeated cycles of freezing and thawing (freezing at −80 °C for 10 min then thawing at 60 °C for 5 min) to obtain the cell lysate, which was then centrifuged at 2500 rpm for 10 min to remove any cell debris. The supernatant was collected and stored at −80 °C for analysis of E2F1, FOXC2, survivin and NF-ҡB.
2.4. Biochemical analysis
2.4.1. Cell proliferation test (MTT assay)
Methylthiazolyl blue tetrazolium (MTT; Thermo Fisher, USA) spectrophotometric dye assay was used to measure the cell proliferation of MCF7 cell line. Cells were cultured in 96-well tissue culture plate at a density of 1 × 104 cells per well in a final volume of 100 μL phenol red-free RPMI 1640 media (Lonza®, USA). The cultured cells were incubated for 24 h in the incubator Shel lab® (USA) at 37 °C and 5% CO2, then treatments were added and the plate was incubated for another 24 h. After that, 10 μL MTT was added to each well, followed by incubation for 4 h at 37 °C. Then, 150 μL DMSO was added to each well in order to dissolve the crystals with gentile shaking for 10 min. The absorbance was measured at 540 nm with a microplate reader (Tecan, Austria). The rate of cell proliferation was calculated as a percentage of the control group.
2.4.2. Cell cycle analysis
According to the protocol of the kit purchased from abcam Co. Ltd (USA), MCF7 cells were trypsinised and washed with PBS. Then, cells were resuspended in ice cold PBS (pH 7.4) to obtain a concentration of 2 million cell/mL. Ice cold 70 % ethanol was added to keep the cells fixed, and then stored at 4°C. Finally, cells were labeled by Propidium Iodide/triton staining solution [19]. The flow cytometric analysis was done using BD facsCanto class II (BD Biosciences, USA) and data were analyzed using BD FACS Diva software (USA) and presented as histograms of cell count percent in each cell cycle phase.
2.4.3. Determination of FOXC2, E2F1, NF-ҡB and survivin
Cell lysate was used to measure the level of FOXC2 and E2F1 in MCF7 breast cancer cell line by using ELISA kits purchased from SunRed Co. Ltd (China) according to the manufacturer protocols. Whereas NF-ҡB p65 levels (phosphorylated and total) and survivin were analyzed using ELISA kits purchased from Bioneovan Co. Ltd (China), according to the manufacturer protocols.
2.4.4. Determination of β1-integrin gene expression by quantitative-real time PCR (qRT-PCR)
MCF7 cell lysate was used for RNA extraction which was conducted using Simply P® total RNA extraction kit (Bior Technology, China). Then, ScanDrop Nano-volume spectrophotometer (Analytik Jena®, Italy) was used to detect the purity and the concentration of extracted RNA. cDNA was prepared using HiSenScriptRH(-) cDNA synthesis kit (iNtRON Biotechnology Co., Korea) according to the manufacturer instructions. The program was set as the following: 50 °C for 60 min for cDNA synthesis followed by 85°C for 10 min for inactivation by using thermal cycler (Thermo Fisher Scientific®, USA)and samples were stored at −80 °C.
For qRT-PCR of β1-integrin, SensiFAST SYBR® No-ROX kit (Bioline Co., USA) was used. The relative gene expression level of β1-integrin was measured by a RT- PCR system (Thermo Fisher Scientific, PikoReal5100, Finland). The amplification program was adjusted as follows: the initial activation lasted for 2 min at 94 °C, followed by 45 cycles (94 °C for 5 s for denaturation, 60 °C for 10 s for annealing and 72 °C for 20 s for extension). The relative gene expression was normalized to housekeeping gene GAPDH and the fold difference in the expression of the target gene was calculated using Livak method (RQ = 2−ΔΔCT) [20]. Primers were obtained from invitrogen Co. (USA) and the Primer 3 plus software was used for designing the primers. β1-integrin (forward, 5′TCCAACCTGATCCTGTGTCC3′, reverse, 5′AACCATGACCTCGTTGTTCC3′). GAPDH (forward, 5′ACAGTCAGCCGCATCTTCTT3′ and reverse: 5′TGACTCCGACCTTCACCTTC3′).
2.4.5. Statistical analysis
Statistical analysis was performed by using Statistical Package for Social Science (SPSS) version 25. Differences between groups were statistically analyzed using one-way analysis of variance (ANOVA). The results were expressed as mean ± SD. p values less than 0.05 were considered statistically significant.
3. Results
3.1. Effect of different treatments on MCF7 cell proliferation
Table 1 illustrates that EP1 agonist treated group was significantly increased in cellular proliferation (p< 0.001, 1.65 fold) compared to control group. However, the cells proliferation significantly decreased (p < 0.001) when treated with EP1, MEK, PKC and NF-ҡB inhibitors before EP1 agonist by 26.56 %, 19.97 %, 21.63 % and 39.48 %, respectively, compared to control group. In addition, MCF7 cells treated with EP1, MEK, PKC and NF-ҡB inhibitors showed a significant decrease in cellular proliferation by 55.44 %, 51.43 %, 52.44 %, 63.27 %, respectively, compared with EP1 agonist treated group.
Table 1.
Effect of different treatments on cell proliferation.
| Groups | % Cell proliferation rate |
|---|---|
| Control | 100 |
| EP1 agonist | 164.8 ± 6.6a |
| EP1 antagonist + EP1 agonist | 73.44 ± 1.68 a, b |
| MEK inhibitor + EP1 agonist | 80.03 ± 2.07 a, b |
| PKC inhibitor + EP1 agonist | 78.37 ± 1.19 a, b |
| NF-ҡB inhibitor + EP1 agonist | 60.52 ± 3.7 a, b |
Data are presented as mean ± SD, n = 6 (tissue culture flask) per each group. a: Significant versus control group. b: Significant versus EP1 agonist group. n =6 replicates. EP1: E prostanoid receptor 1, MEK: Mitogen activated protein kinase kinase, PKC: Protein kinase C, NF-ҡB: Nuclear factor kappa-light-chain-enhancer of activated B cells.
3.2. Effect of treatments on MCF7 cell cycle analysis
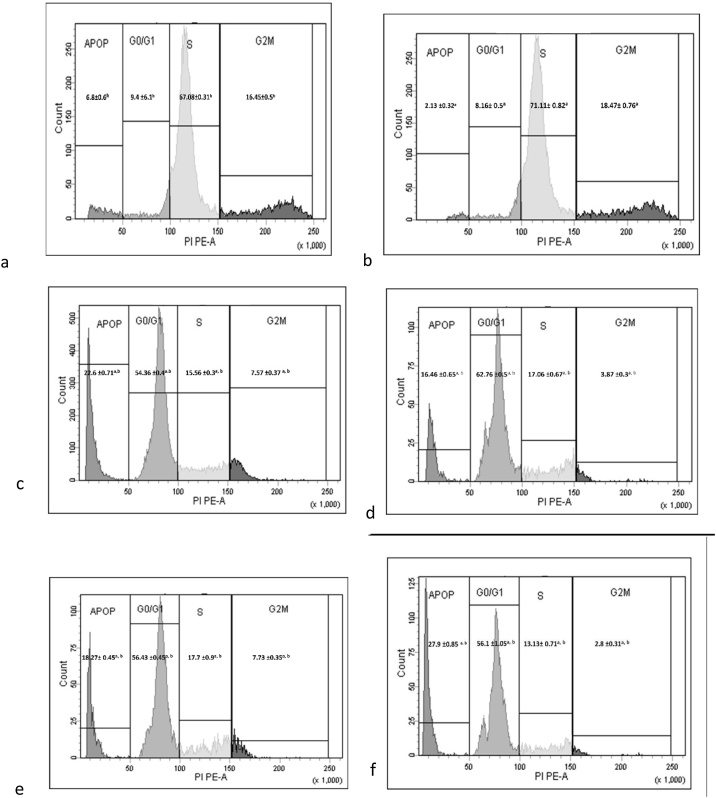
EP1 agonist group showed a significant increase in S phase (1.06 fold, p < 0.001) when compared with control group. On the other hand, groups pretreated with inhibitors EP1, MEK, PKC and NF-ҡB showed significant (p < 0.001) decrease in the S phase by 78.12 %, 76.01 %, 75.11 %, 81.5 %, respectively when compared with EP1 agonist group. Pretreatment with EP1, MEK, PKC and NF-ҡB inhibitors before EP1 agonist showed blocking in G1 phase which was reflected as a significant (p < 0.001) increase in cell count by 6.7, 7.7, 6.9 and 6.87 fold, respectively when compared with EP1 agonist group (Fig. 1 and Table 2).
Fig. 1.
Cell cycle analysis of the studied groups. a: Control group, b: EP1 agonist, c: EP1 antagonist +EP1 agonist, d: MEK inhibitor+EP1 agonist, e: PKC inhibitor +EP1 agonist and f: NF-ҡB inhibitor+EP1 agonist. EP1: E prostanoid receptor 1, MEK: Mitogen activated protein kinase kinase, PKC: Protein kinase C, NF-ҡB: Nuclear factor kappa -light-chain-enhancer of activated B cells. Apop: Apoptosis ; G0/G1: Gap phase G0 and G1 ; S: Synthesis phase; G2/M: Gap phase 2 and Mitosis phase. PIPE-A: Propidium Iodide Phycoerythrin Area.
Table 2.
Effect of treatments on breast cancer cell counts in different cell cycle phases.
| Groups | Apoptosis | G0/G1 phase | S phase | G2/M phase |
|---|---|---|---|---|
| Control | 6.8 ± 0.6b | 9.4 ± 0.61b | 67.08 ± 0.31 b | 16.45 ± 0.5 b |
| EP1 agonist | 2.13 ± 0.32a | 8.16 ± 0.5a | 71.11 ± 0.82 a | 18.47 ± 0.76a |
| EP1 antagonist + EP1 agonist | 22.6 ± 0.71 a,b | 54.36 ± 0.4 a, b | 15.56 ± 0.3 a, b | 7.57 ± 0.37 a, b |
| MEK inhibitor + EP1 agonist | 16.46 ± 0.65 a, b | 62.76 ± 0.5 a, b | 17.06 ± 0.67 a, b | 3.87 ± 0.3 a, b |
| PKC inhibitor + EP1 agonist | 18.27 ± 0.45 a, b | 56.43 ± 0.45 a, b | 17.7 ± 0.9 a, b | 7.73 ± 0.35 a, b |
| NF-ҡB inhibitor + EP1 agonist | 27.9 ± 0.85 a, b | 56.1 ± 1.05 a, b | 13.13 ± 0.71 a, b | 2.8 ± 0.31 a, b |
Data are presented as mean ± SD. a: Significant versus control group. b: Significant versus EP1 agonist group. n = 3 replicates. EP1: E prostanoid receptor 1, MEK: Mitogen activated protein kinase kinase, PKC: Protein kinase C, NF-ҡB: Nuclear factor kappa-light-chain-enhancer of activated B cells. G0/G1: Gap phase G0 and G1, S: Synthesis phase, G2/M: Gap phase 2 and mitosis phase.
Treatment with EP1 agonist showed a significant (p < 0.001) decrease in apoptosis by 68.7 % when compared with control group. However, applying EP1, MEK, PKC and NF-ҡB inhibitors before EP1 agonist showed a significant (p < 0.001) increase in cell count by 10.6, 7.73, 8.58, 13.1 fold, respectively when compared with EP1 agonist group. Moreover, pretreatment with EP1, MEK, PKC and NF-ҡB inhibitors followed by EP1 agonist showed a significant (p < 0.001) decrease in G2 phase cell count by 59.1 %, 79.05 %, 58.15 % and 84.8 %, respectively, when compared with EP1 agonist group (Fig. 1 and Table 2).
3.3. Effect of treatments on FOXC2, E2F1, NF-ҡB and survivin levels in MCF7 cells
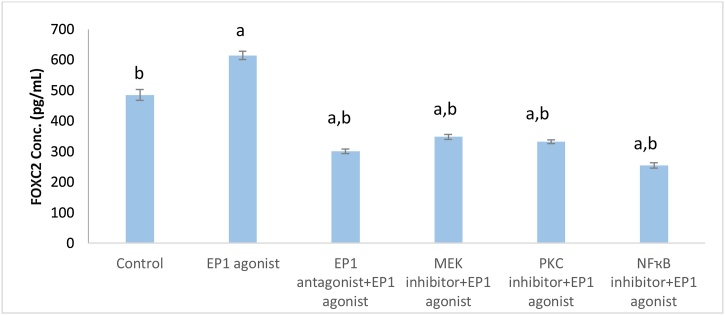
Treatment of MCF7 cell line with EP1 agonist showed significant increase in FOXC2 level (1.27 fold, p < 0.001) when compared with control group. Comparing with EP1 agonist treated group, pretreatment with inhibitors of MEK, PKC, EP1 and NF-ҡB showed a significant (p < 0.001) decrease in FOXC2 level by 43.36 %, 45.96 %, 51.07 %, 58.57 %, respectively (Fig. 2).
Fig. 2.
Effect of treatments on FOXC2 level in breast cancer cell line.
Data are presented as mean ± SD. a: Significant versus control group. b: Significant versus EP1 agonist group. n = 6 replicates. EP1: E prostanoid receptor 1, MEK: Mitogen activated protein kinase kinase, PKC: Protein kinase C, NF-ҡB: Nuclear factor kappa-light-chain-enhancer of activated B cells, FOXC2: Forkhead box protein C2.
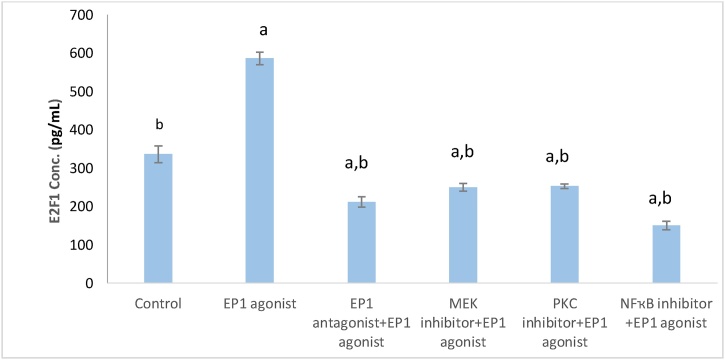
Fig. 3 illustrates that EP1 agonist group showed a significant increase in E2F1 level when compared to control group by 1.74 fold, (p < 0.001). However, cell line groups pretreated with the inhibitors of EP1, MEK, PKC and NF-ҡB, showed significant decrease in E2F1 level (p < 0.001) by 63.88 %, 56.84 %, 57.36 % and 74.3 %, respectively, compared with EP1 agonist group.
Fig. 3.
Effect of treatments on E2F1 level in breast cancer cell line.
Data are presented as mean ± SD. a: Significant versus control group. b: Significant versus EP1 agonist group. n = 6 replicates. EP1: E prostanoid receptor 1, MEK: Mitogen activated protein kinase kinase, Protein kinase C, NF-ҡB: Nuclear factor kappa-light-chain-enhancer of activated B cells, E2F1: E2F Transcription Factor 1.
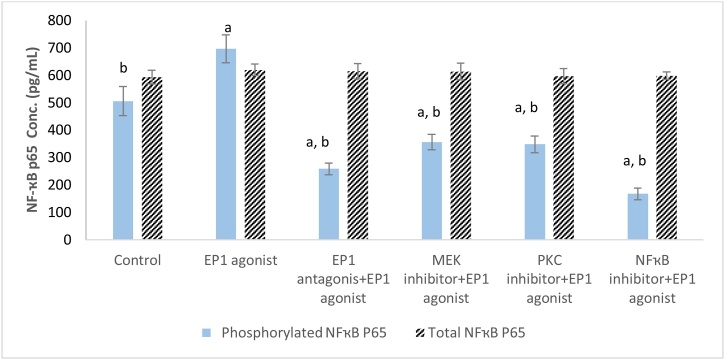
MCF7 cell line treated with EP1 agonist showed a significant increase in level of phosphorylated NF-ҡB p65 by 1.37 fold (p < 0.001) compared with control group. On the other hand, cells treated with antagonist of EP1 and inhibitors of PKC, MEK and NF-ҡB showed a significant (p < 0.001) decrease in the phosphorylated NF-ҡB p65 by 48.8 %, 31.1 %, 29.5 % and 66.8 %, respectively compared with control group. On the other hand, different treatments didn’t show significant difference in the total NF-ҡB p65 concentration (Fig. 4).
Fig. 4.
Effect of treatments on total and phosphorylated NF-ҡB p65 level in breast cancer cell line.
Data are presented as meanSD. a: Significant versus control group. b: Significant versus EP1 agonist group. n = 6 replicates. EP1: E prostanoid receptor 1, MEK: Mitogen activated protein kinase kinase, PKC: Protein kinase C, NF-ҡB: Nuclear factor kappa-light-chain-enhancer of activated B cells.
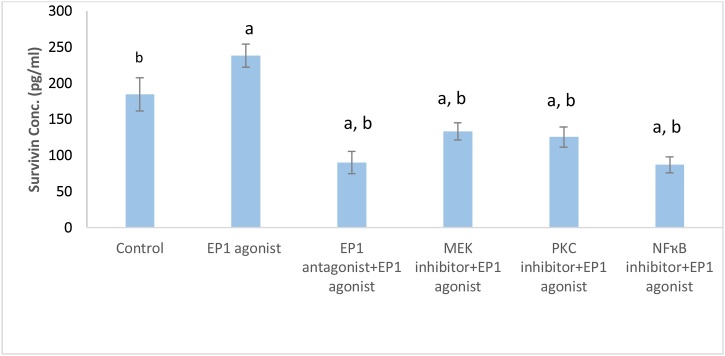
Treatment of MCF7 cells with EP1 agonist significantly increased survivin level compared to control group (1.3fold, p < 0.001). On the other hand, cells pretreated with EP1 antagonist, MEK, PKC and NF-ҡB inhibitor exhibited a significant decrease in survivin level by 62.24 %, 44.05 %, 47.33 % and 63.4 %, respectively compared to EP1 agonist group (Fig. 5).
Fig. 5.
Effect of treatments on Survivin level in breast cancer cell line.
Data are presented as mean ± SD. a: Significant versus control group. b: Significant versus EP1 agonist group. n = 6 replicates. EP1: E prostanoid receptor 1, MEK: Mitogen activated protein kinase kinase, PKC: Protein kinase C, NF-ҡB: Nuclear factor kappa-light-chain-enhancer of activated B cells.
3.4. Effect of treatments on β1-integrin gene expression
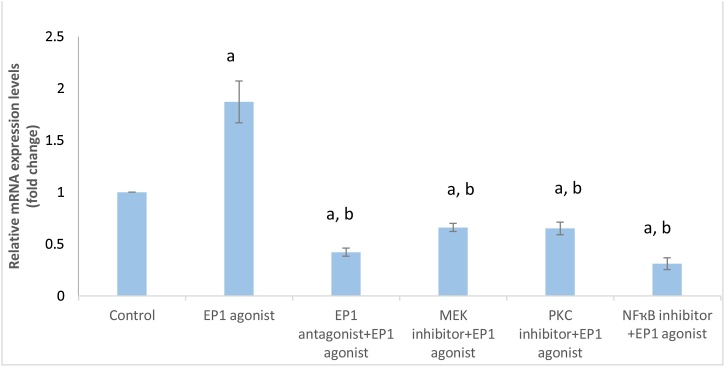
As shown in Fig. (6): Treatment of MCF-7 cells with EP1 agonist showed a significant increase in β1-integrin gene expression by 1.87 fold (p < 0.001) compared with control group. However, pretreatment with EP1, MEK, PKC and NF-ҡB inhibitors significantly (p < 0.001) decreased β1-integrin expression by 58 %, 34 %, 35 % and 69 %, respectively compared to control group. Moreover, EP1 antagonist, MEK, PKC and NF-ҡB inhibitors exhibited significant (p < 0.001) downregulation in β1-integrin gene expression by 77.5 %, 64.7 %, 65.2 % and 83.4 %, respectively, compared to EP1 agonist treated group.
Fig. 6.
Effect of treatments on β1- integrin gene expression in breast cancer cell line.
Data are presented as mean ± SD. a: Significant versus control group. b: Significant versus EP1 agonist group. n = 6 replicates. EP1: E prostanoid receptor 1, MEK: Mitogen activated protein kinase kinase, PKC: Protein kinase C, NF-ҡB: nuclear factor kappa-light-chain-enhancer of activated B cells.
4. Discussion
Cyclooxygenase-2 mediates the production of PGE2 which is involved in proliferation and growth of cancer cells. β1-integrin is highly expressed in breast cancer, where it mediates tumor initiation, proliferation and metastasis [15,21]. While previous reports discussed the importance of PGE2 and β1-integrin in cancer cells growth and proliferation, the mechanism through which PGE2 may interplay with β1-integrin in breast cancer still not revealed clearly. PGE2 exerts its tumor-related activity through EP1 receptor, which plays a vital role in cancer progression [22]. However, little is known about the role of EP1 receptor in mediating PGE2 effect on β1-integrin in breast cancer cells. Therefore, the present study aimed to examine the effect of EP1 agonist (17-PT-PGE2) along with EP1, MEK, PKC and NF-ҡB inhibitors on breast cancer cell line to explore the crosslink between PGE2 and β1-integrin expression.
Our study revealed that treating MCF7 cells with EP1 agonist showed enhancement of both cell proliferation, cell cycle transition to S phase and increased β1-integrin expression. Furthermore, FOXC2, phosphorylated NF-ҡB p65, E2F1, and survivin levels were upregulated with EP1 agonist treatment.
On the contrary, inhibition of EP1 receptor showed suppression of the cell proliferation, β1-integrin expression and cells transition to S phase, but increased cell count in the apoptotic phase. Moreover, selective inhibition of each of MEK, PKC, and NF-ҡB suppressed EP1 agonist-mediated β1-integrin over-expression, cell proliferation, cell cycle transition and downregulated FOXC2, phosphorylated NF-ҡB p65, E2F1 and survivin levels. These results were in line with Liu et al. [23], who reported the ability of PGE2 to upregulate β1-integrin expression in chondrosarcoma cells.
EP1 receptor activates PKC, focal adhesion kinase phosphorylation and promote tumor growth [24]. PKC mediates the connection between external signals and cell response. PKC expression is directly correlated with tumor size and aggressiveness and can be used as prognostic marker for malignant breast cancer tissues [24,25]. However, PKC inhibition resulted in a decrease of both cell proliferation and migration [25]. Herein, pretreated MCF7 cell line with PKC inhibitor showed downregulation of EP1-mediated β1- integrin expression together with cell proliferation, cells population in S phase and enhanced cell apoptosis when compared to EP1 agonist treated group. Our results were in consistence with Lin et al. [26], who reported that PKC inhibition decreased proliferation in triple negative breast cancer cells.
Mitogen activated protein kinase kinase (MEK) plays a vital role in breast cancer progression mainly through activating of extracellular signal regulated kinase (ERK) pathway. ERK in turn, phosphorylates and inhibits caspase-9 and activates B-cell lymphoma-2 (Bcl-2) proteins, resulting in enhancing cancer cells proliferation. Also, MEK activated cyclin D in cell cycle progression [27].
In the present work, pretreatment with MEK inhibitor markedly increased apoptosis and decreased β1-integrin gene expression and suppressed cell proliferation when compared to the EP1 agonist treated group. Our data were in accordance with Gong et al. [28], who reported that MEK inhibition has the ability to decrease the cell proliferation of colorectal cancer cell line HCT 116.
Nuclear factor kappa light-chain-enhancer of activated B cells is defined as a transcription factor that is mainly activated in many cancer types and its activation is vital in progression, invasion and migration [29]. It has been reported to be implicated in breast cancer progression by upregulating cyclin D1 and down-regulating programmed cell death protein 4 [29]. Herein, pre-treating MCF7 cell line with NF-ҡB inhibitor (PDTC) impaired EP1 agonist-mediated β1-integrin over-expression, cell proliferation, cell cycle transition and increased apoptosis. These results were in accordance with previous study which showed that NFκB is required for β1-integrin transactivation in T4-2 breast cancer cells and inhibition of NFκB reduced cells survival, induced apoptosis in tumor colonies [30].
Protein kinases; MEK and ERK are implicated in PKC pathway [31] and NF-ҡB is involved in PKC mediated effect in cultured MCF7 cells [32]. Our data revealed a connection between EP1, MEK, PKC with NF-ҡB; as pretreatment with their inhibitors, decreased phosphorylated NF-ҡB p65 level. Previous studies may explain our findings regarding the role of PKC/MEK pathway as TNF-α was implicated in MEK-induced NF-ҡB over-expression, which mediated invasion in oral squamous cell carcinoma [33,34].
FOXC2 is a member of the family of forkhead transcription factors and involved in many developmental processes. FOXC2 is highly expressed in breast and colon cancers [35]. NF-ҡB was shown to upregulate FOXC2 expression [36]. In the present study, EP1 agonist increased FOXC2 protein level together with increasing β1-integrin expression and cell proliferation, an effect that was reversed by EP1 antagonist pretreatment. Similarly, inhibitors of MEK, NF-ҡB and PKC inhibited FOXC2 level when compared with EP1 agonist group. Yu et al. [37] supported our findings and reported that NF-ҡB worked as positive effector of FOXC2 that upregulated signals involved in lung cancer progression.
E2F1 is an important transcription factor involved in carcinogenesis and plays a major role in G1-S phase transition in various cancers [11]. The present research showed increased E2F1 protein level along with increased S phase cell population, cell proliferation and β1 -integrin expression in EP1 agonist treated group, in reference to untreated control group. However, pretreatment with EP1, PKC, MEK and NF-ҡB inhibitors decreased E2F1 level, cell proliferation and β1-integrin expression.
Interestingly, E2F1 was found to act on specific site on the promoter of FOXC2 [11]. Herein, pretreating MCF7 cells with PD98059, GF109203X and PDTC downregulated the levels of FOXC2 and E2F1. Thus, both E2F1 and FOXC2 could be suggested as downstream mediators for PKC, MEK and NF-ҡB pathways that mediated EP1-dependent β1-integrin upregulation. Furthermore, both E2F1 and FOXC2 have the ability to control cell cycle by upregulating cyclin E, resulting in enhancing the cell transition in G1-S phase in preadipocyte cells as mentioned by Bertoli et al. [37] and Gan et al. [38].
Survivin retained the ability to interact with cyclin dependent kinase, which, in turn, phosphorylates and deactivates the cell cycle inhibitory protein retinoblastoma and permits cells transition through the cell cycle [39]. In breast cancer; survivin is correlated with aggressiveness by enhancing VEGF expression and affords chemotherapy drug resistance [40].
Herein, EP1 agonist increased survivin level compared to control cells. On the other hand, pretreatment with SC-19220 greatly decreased survivin level compared to EP1 agonist treated cells. Similarly, pretreatment with PKC, MEK and NFҡB inhibitors decreased survivin level parallel with decreased β1-integrin expression, compared to the EP1 agonist group. Previous report supported our findings, where galectin-1 activated β1-integrin enhances MDA-MB-231 cells' progression and survival by increasing survivin expression [41]. Interestingly, Jiang et al. [42] reported that E2F1 binds to survivin promotor and aggravates rat embryonic fibroblast growth. Also, FOXC2 contributed to multidrug resistance by activating survivin in gastric cancer [43] and survivin can activate Akt pathway which increases the integrin expression [44,45].
5. Limitation
Further in vivo experiments and clinical studies are needed to put hands on the exact role of PGE2/EP1/ PKC/ MEK/NF-ҡB/ β1- integrin in different grades and subtypes of breast cancer.
6. Conclusion
Altogether, in MCF7 cancer cells, EP1 agonist increased β1-integrin expression, an effect reflected by enhanced cell proliferation, cell cycle G1/S phases transition and survivin level. Furthermore, EP1, PKC, MEK and NF-ҡB inhibitors, partially, mitigated EP1 agonist mediated effect on β1- integrin through increasing apoptosis and inhibiting FOXC2, phosphorylated NF-ҡB p65 and E2F1 levels, thus, inhibited cell cycle transition, survival and proliferation. Targeting these biochemical mediators may afford a new breast cancer therapy strategy.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
CRediT authorship contribution statement
Nahla El Ashmawy: designed the work, analyzed data and reviewed the article.
Enas El zamarany: supervised on the work and provided the laboratory needed.
Eman Gouda: analyzed data, reviewed the article.
Naglaa Fathi: reviewed the article and analyzed data.
Hend Selim: suggested the idea, conducted work, data analysis, drafting the manuscript.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgment
Deep appreciation to Prof. Dr. Eman Gouda and Prof. Dr. Naglaa Khedr for the time they devoted in revising the manuscript and their valuable guidance.
Handling Editor: Dr. Aristidis Tsatsakis
References
- 1.Bazzani L., Donnini S., Finetti F., Christofori G., Ziche M. PGE2/EP3/SRC signaling induces EGFR nuclear translocation and growth through EGFR ligands release in lung adenocarcinoma cells. Oncotarget. 2017;8(19):31270–31287. doi: 10.18632/oncotarget.16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Almeida V., Santos Guimarães I., Almendra L.R., Rondon A.M.R., Tilli T.M., de Melo A.C., Sternberg C., Monteiro R.Q. Positive crosstalk between EGFR and the TF-PAR2 pathway mediates resistance to cisplatin and poor survival in cervical cancer. Oncotarget. 2018;9:30594–30609. doi: 10.18632/oncotarget.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen E.P., Smyth E.M. COX-2 and PGE2-dependent immunomodulation in breast cancer. Prostaglandins Other Lipid Mediat. 2011;96:14–20. doi: 10.1016/j.prostaglandins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma J., Chen M., Xia S., Shu W., Guo Y., Wang Y., Xu Y., Bai X., Zhang L., Zhang H., Zhang M., Wang Y., Leng J. Prostaglandin E2 promotes liver cancer cell growth by the upregulation of FUSE-binding protein 1 expression. Int. J. Oncol. 2013;42(3):1093–1104. doi: 10.3892/ijo.2013.1782. [DOI] [PubMed] [Google Scholar]
- 5.Reader J., Holt D., Fulton A. Prostaglandin E2 EP receptors as therapeutic targets in breast cancer. Cancer Metastasis Rev. 2011;30(3-4):449–463. doi: 10.1007/s10555-011-9303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J., Springer T.A. Integrin extension enables ultrasensitive regulation by cytoskeletal force. Proc. Natl. Acad. Sci. U. S. A. 2017;114(18):4685–4690. doi: 10.1073/pnas.1704171114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Callaghan G., Houston A. Prostaglandin E2 and the EP receptors in malignancy: possible therapeutic targets? Br. J. Pharmacol. 2015;172(22):5239–5250. doi: 10.1111/bph.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subbaram S., Lyons S.P., Svenson K.B., Hammond S.L., McCabe L.G., Chittur S.V., DiPersio C.M. Integrin α3β1 controls mRNA splicing that determines Cox-2 mRNA stability in breast cancer cells. J. Cell. Sci. 2014;127(Pt 6):1179–1189. doi: 10.1242/jcs.131227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J., Hao Q., Cao W., Vadgama J.V., Wu Y. Celecoxib in breast cancer prevention and therapy. Cancer Manag. Res. 2018;10:4653–4667. doi: 10.2147/CMAR.S178567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park S.J., Gadi J., Cho K.W., Kim K.J., Kim S.H., Jung H.S., Lim S.K. The forkhead transcription factor Foxc2 promotes osteoblastogenesis via up-regulation of integrin beta1 expression. Bone. 2011;49(3):428–438. doi: 10.1016/j.bone.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Meng P., Ghosh R. Transcription addiction: can we garner the Yin and Yang functions of E2F1 for cancer therapy? Cell Death Dis. 2014;5:e1360. doi: 10.1038/cddis.2014.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lv Y.G., Yu F., Yao Q., Chen J.H., Wang L. The role of survivin in diagnosis, prognosis and treatment of breast cancer. J. Thorac. Dis. 2010;2(2):100–110. [PMC free article] [PubMed] [Google Scholar]
- 13.Thu K.L., Soria-Bretones I., Mak T.W., Cescon D.W. Targeting the cell cycle in breast cancer: towards the next phase. Cell Cycle. 2018;17(15):1871–1885. doi: 10.1080/15384101.2018.1502567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H., Cheng S., Zhang M., Ma X., Zhang L., Wang Y., Rong R., Ma J., Xia S., Du M., Shi F., Wang J., Yang Q., Bai X., Leng J. Prostaglandin E2 promotes hepatocellular carcinoma cell invasion through upregulation of YB-1 protein expression. Int. J. Oncol. 2014;44(3):769–780. doi: 10.3892/ijo.2013.2234. [DOI] [PubMed] [Google Scholar]
- 15.Bai X., Wang J., Zhang L., Ma J., Zhang H., Xia S., Zhang M., Ma X., Guo Y., Rong R., Cheng S., Shu W., Wang Y., Leng J. Prostaglandin E2 receptor EP1-mediated phosphorylation of focal adhesion kinase enhances cell adhesion and migration in hepatocellular carcinoma cells. Int. J. Oncol. 2013;42(5):1833–1841. doi: 10.3892/ijo.2013.1859. [DOI] [PubMed] [Google Scholar]
- 16.Chadet S., Jelassi B., Wannous R., Angoulvant D., Chevalier S., Besson P., Roger S. The activation of P2Y2 receptors increases MCF-7 breast cancer cells migration through the MEK-ERK1/2 signalling pathway. Carcinogenesis. 2014;35(6):1238–1247. doi: 10.1093/carcin/bgt493. [DOI] [PubMed] [Google Scholar]
- 17.Kim J.A., Tan Y., Wang X., Cao X., Veeraraghavan J., Liang Y., Edwards D.P., Huang S. Comprehensive functional analysis of the tousled-like kinase 2 frequently amplified in aggressive luminal breast cancers. Nat. Commun. 2016;7:12991. doi: 10.1038/ncomms12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao L., Zhang X., Cao F., Wang Y., Shen Y., Yang C., Uzan G., Peng B., Zhang D. Inhibiting inducible miR-223 further reduces viable cells in human cancer cell lines MCF-7 and PC3 treated by celastrol. BMC Cancer. 2015;15:873. doi: 10.1186/s12885-015-1909-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanzyl E.J., Rick K.R.C., Blackmore A.B., MacFarlane E.M., McKay B.C. Flow cytometric analysis identifies changes in S and M phases as novel cell cycle alterations induced by the splicing inhibitor isoginkgetin. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0191178. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Toscani A.M., Sampayo R.G., Barabas F.M., Fuentes F., Simian M., Coluccio Leskow F. Distinct ErbB2 receptor populations differentially interact with beta1 integrin in breast cancer cell models. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0174230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye Y., Wang X., Jeschke U., von Schönfeldt V. COX-2-PGE2-EPs in gynecological cancers. Arch. Gynecol. Obstet. 2020;301:1365–1375. doi: 10.1007/s00404-020-05559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J.F., Fong Y.C., Chang C.S., Huang C.Y., Chen H.T., Yang W.H., Hsu C.J., Jeng L.B., Chen C.Y., Tang C.H. Cyclooxygenase-2 enhances alpha2beta1 integrin expression and cell migration via EP1 dependent signaling pathway in human chondrosarcoma cells. Mol. Cancer. 2010;9:43. doi: 10.1186/1476-4598-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urtreger A.J., Kazanietz M.G., Bal de Kier Joffé E.D. Contribution of individual PKC isoforms to breast cancer progression. IUBMB Life. 2012;(1):18–26. doi: 10.1002/iub.574. [DOI] [PubMed] [Google Scholar]
- 25.Garg R., Benedetti L.G., Abera M.B., Wang H., Abba M., Kazanietz M.G. Protein kinase C and cancer: what we know and what we do not. Oncogene. 2014;33(45):5225–5237. doi: 10.1038/onc.2013.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin W., Huang J., Yuan Z., Feng S., Xie Y., Ma W. Protein kinase C inhibitor chelerythrine selectively inhibits proliferation of triple-negative breast cancer cells. Sci. Rep. 2017;7(1):2022. doi: 10.1038/s41598-017-02222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y., Hu H.Y., Meng W., Jiang L., Zhang X., Sha J.J., Lu Z., Yao Y. MEK inhibitor effective against proliferation in breast cancer cell. Tumour Biol. 2014;35(9):9269–9279. doi: 10.1007/s13277-014-1901-5. [DOI] [PubMed] [Google Scholar]
- 28.Gong S., Xu D., Zhu J., Zou F., Peng R. Efficacy of the MEK inhibitor cobimetinib and its potential application to colorectal cancer cells. Cell. Physiol. Biochem. 2018;47(2):680–693. doi: 10.1159/000490022. [DOI] [PubMed] [Google Scholar]
- 29.Wang W., Nag S.A., Zhang R. Targeting the NFκB signaling pathways for breast cancer prevention and therapy. Curr. Med. Chem. 2015;22(2):264–289. doi: 10.2174/0929867321666141106124315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed K.M., Zhang H., Park C.C. NF-κB regulates radioresistance mediated by β1-integrin in three-dimensional culture of breast cancer cells. Cancer Res. 2013;73(12):3737–3748. doi: 10.1158/0008-5472.CAN-12-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato T., Takahashi H., Hatakeyama S., Iguchi A., Ariga T. The TRIM-FLMN protein TRIM45 directly interacts with RACK1 and negatively regulates PKC-mediated signaling pathway. Oncogene. 2014;34:1280–1291. doi: 10.1038/onc.2014.68. [DOI] [PubMed] [Google Scholar]
- 32.Noh E.M., Lee Y.R., Hong O.Y., Jung S.H., Youn H.J., Kim J.S. Aurora kinases are essential for PKC-induced invasion and matrix metalloproteinase-9 expression in MCF-7 breast cancer cells. Oncol. Rep. 2015;34(2):803–810. doi: 10.3892/or.2015.4027. [DOI] [PubMed] [Google Scholar]
- 33.Maddahi A., Kruse L.S., Chen Q.W., Edvinsson L. The role of tumor necrosis factor-α and TNF-α receptors in cerebral arteries following cerebral ischemia in rat. J. Neuroinflammation. 2011;8:107. doi: 10.1186/1742-2094-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang D., Tao D., Fang Y., Deng C., Xu Q., Zhou J. TNF-alpha promotes invasion and metastasis via NF-Kappa B pathway in oral squamous cell carcinoma. Med. Sci. Monit. Basic Res. 2017;23:141–149. doi: 10.12659/MSMBR.903910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimoda Y., Ubukata Y., Handa T., Yokobori T., Watanabe T. High expression of forkhead box protein C2 is associated with aggressive phenotypes and poor prognosis in clinical hepatocellular carcinoma. BMC Cancer. 2018;18(1):597. doi: 10.1186/s12885-018-4503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu Y.H., Chen H.A., Chen P.S., Cheng Y.J., Hsu W.H., Chang Y.W., Chen Y.H., Jan Y., Hsiao M., Chang T.Y., Liu Y.H., Jeng Y.M., Wu C.H., Huang M.T., Su Y.H., Hung M.C., Chien M.H., Chen C.Y., Kuo M.L., Su J.L. MiR-520h-mediated FOXC2 regulation is critical for inhibition of lung cancer progression by resveratrol. Oncogene. 2013;32(4):431–443. doi: 10.1038/onc.2012.74. [DOI] [PubMed] [Google Scholar]
- 37.Bertoli C., Skotheim J.M., de Bruin R.A. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013;14(8):518–528. doi: 10.1038/nrm3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gan L., Liu Z., Jin W., Zhou Z., Sun C. Foxc2 enhances proliferation and inhibits apoptosis through activating Akt/mTORC1 signaling pathway in mouse preadipocytes. J. Lipid Res. 2015;56(8):1471–1480. doi: 10.1194/jlr.M057679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X., Duan N., Zhang C., Zhang W. Survivin and tumorigenesis: molecular mechanisms and therapeutic strategies. J. Cancer. 2016;7(3):314–323. doi: 10.7150/jca.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veiga G.L.D., Silva R.D.M.D., Pereira E.C., Azzalis L.A., Alves B.D.C.A., Gehrke F.S., Gascón T.M., Fonseca F.L.A. The role of Survivin as a biomarker and potential prognostic factor for breast cancer. Rev. Assoc. Med. Bras. 2019;65(6):893–901. doi: 10.1590/1806-9282.65.6.893. [DOI] [PubMed] [Google Scholar]
- 41.Nam K., Son S.H., Oh S., Jeon D., Kim H., Noh D.Y., Kim S., Shin I. Binding of galectin-1 to integrin β1 potentiates drug resistance by promoting survivin expression in breast cancer cells. Oncotarget. 2017;8(22):35804–35823. doi: 10.18632/oncotarget.16208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang Y., Saavedra H.I., Holloway M.P., Leone G., Altura R.A. Aberrant regulation of survivin by the RB/E2F family of proteins. J. Biol. Chem. 2004;279(39):40511–40520. doi: 10.1074/jbc.M404496200. [DOI] [PubMed] [Google Scholar]
- 43.Liu H., Zhang Z., Han Y., Fan A., Liu H., Zhang X., Liu Y., Zhang R., Liu W., Lu Y., Fan D., Zhao X., Nie Y. The FENDRR/FOXC2 axis contributes to multidrug resistance in gastric cancer and correlates with poor prognosis. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.634579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernández Jaime, Rodríguez, Valenzuela, Calderon, Urzúa, Munroe, Rosas, Lemus, Díaz, Wright, Leyton, Tapia, Quest Survivin expression promotes VEGF-induced tumor angiogenesis via PI3K/Akt enhanced β-catenin/Tcf-Lef dependent transcription. Mol Cancer. 2014;13:209. doi: 10.1186/1476-4598-13-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Virtakoivu R., Pellinen T., Rantala J.K., Perälä M., Ivaska J. Distinct roles of AKT isoforms in regulating β1-integrin activity, migration, and invasion in prostate cancer. Mol Biol Cell. 2012;23(17):3357–3369. doi: 10.1091/mbc.E12-03-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]