Abstract
Purpose
Post-transplant lymphoproliferative disorder (PTLD) represents a spectrum of disorders associated with Epstein Barr Virus infection in up to 80% of cases in the setting of pharmacologic immunosuppression following hematopoietic stem cell or solid organ transplantation. Ocular involvement is a rare finding in PTLD.
Observation
We report the case of a 38-year-old man who presented with unilateral retinal infiltrates as first manifestation of PTLD relapse. Diagnosis relied on the presence of EBV DNA in anterior chamber fluids and vitrectomy that showed the presence of a B cell clone. Systemic relapse of PTLD was detected 12 weeks after retinal findings. Treatment of ocular disease included systemic injections of rituximab and intravitreal injections of methotrexate, halting the extension of retinal infiltrates.
Conclusion
Ocular involvement in PTLD is rare and needs to be acknowledged because it can precede a systemic relapse of the hematological condition.
Keywords: Post-transplant lymphoproliferative disorder, Epstein-Barr virus, Retinal infiltration, Masquerade syndrome
1. Case report
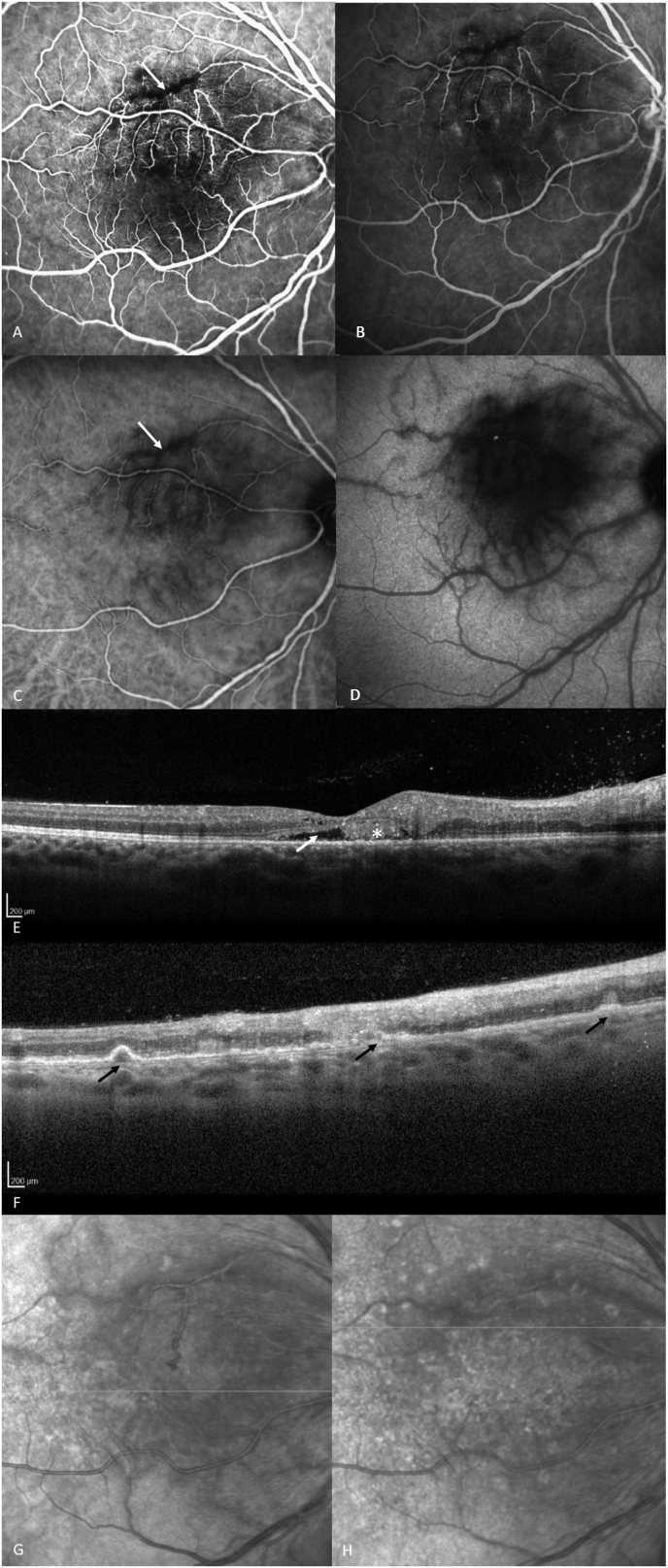
A 38-year-old man was referred to our tertiary clinic in July 2019 for an acute visual loss of his right eye (OD). His medical history consisted in a T-cell acute lymphoblastic leukemia (ALL) for which he received allogeneic hematopoietic stem cell transplantation (HSCT) in second complete remission from an HLA-mismatch unrelated donor in March 2019. Pre-transplant serological status for Epstein-Barr virus (EBV) was positive for both the donor and recipient. At 4 months of HSCT (June 2019), he presented with fever, tonsillitis and multiple swollen lymph nodes, leading to suspect an EBV-induced post-transplant lymphoproliferative disorder (PTLD). This diagnosis was confirmed virologically by positive PCR for EBV in the blood (5,4 log UI/ml) and histologically on lymph node specimens; and treated successfully with seven rituximab injections in June and July 2019 and reduction of graft-versus-host disease (GVHD) prevention (ie. decrease of cyclosporin). At referral, the best corrected visual acuity (BCVA) was 20/40 OD and 20/20 OS (left eye). Intraocular pressure was normal and slit lamp examination of anterior segments was unremarkable. Fundus examination was normal in the left eye, but disclosed the presence of diffuse yellowish infiltrations of the posterior pole associated with a perivascular cuffing (Fig. 1A) in the right eye. There was no vitreous infiltration at this time. Fluorescein (FA) and indocyanine green angiography (ICG-A) showed the perivascular cuffing (dark rim) with a mild leakage at the level of both the arteries and veins, associated with a masking effect at the level of the yellow retinal infiltrates (Fig. 2A–D). Optical coherence tomography (OCT) showed the presence of a subretinal infiltration, and a serous retinal detachment of the fovea (Fig. 2E). An anterior chamber tap (ACT) was performed and came back negative for PCR and Goldmann-Witmer coefficient of HSV 1 and 2, VZV, CMV, EBV and toxoplasmosis; and dosage of interleukin 10 (IL-10). Four weeks later, BCVA of his right eye dropped to counting fingers corresponding to an enlargement of the yellowish dot shaped retinal infiltrations (Fig. 1B–C) appearing this time as drusen like deposits on OCT (Fig. 2F). At this point, the two main hypotheses were an ocular PTLD or an ocular relapse of his ALL (since there was no systemic sign of disease). Iterative ACTs were persistently negative for infectious diseases and IL-10 except in August 2019 when a PCR performed on the aqueous humor came back positive for EBV DNA (4.77 Log UI/mL). Considering the poor visual outcome, and despite the absence of vitritis or vitreous cells, a diagnostic vitrectomy was performed in November 2019, showing the presence of a B cell clone suggestive of an ocular PTLD. The B cell clone presence in the vitreous was revealed by PCR amplification. At the same time, EBV DNA was detected in several blood samples and in a liver biopsy confirming a subsequent relapse of the systemic disease while the patient was treated for gut and skin steroid-refractory acute GVHD. Systemic treatments including rituximab, brentuximab-vedotin and chemotherapy were administered with partial control of the disease initially, followed by progression despite successive lines of treatment until the death of the patient in July 2019. Locally he was treated with three intravitreal injections of methotrexate halting the extension of PTLD infiltrates without relapsing until the death of the patient (Fig. 1E). Despite the good anatomic response to treatments, no visual improvement in his best corrected visual acuity was noted overtime.
Fig. 1.
Ultra-wide field color imaging of the right eye fundus showing the presence of yellowish dot shaped infiltrations of the macula associated with a perivascular cuffing (July 2019, A), extending in size and number and reaching the vascular arcades (August 2019, B; September 2019, C), and spreading peripherally outside the vascular arcades (October 2019, D). Retinal scars are observed in February 2020 after intravitreal methotrexate and intravenous rituximab (E). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Fluorescein angiograms of the right eye, showing a masking effect of the retina and perivascular infiltrates (white arrow) during the early phase (July 2019, A) and a mild vascular leakage during the late phase (July 2019, B). Early phase indocyanine green angiogram showing a heterogeneous filling and a perivascular cuffing (white arrow) (July 2019, C); Late phase indocyanine green angiogram showing a diffuse macular hypo fluorescent spot corresponding to the masking effect of the infiltrates and a possible associated RPE dysfunction (July 2019, D). OCT B-scan imaging of the right eye showing the presence of a subretinal detachment of the fovea (white arrow), along with subretinal deposits (white asterisk) (July 2019, E); OCT B-scan imaging of the right eye showing drusen like sub RPE deposits (black arrows) matching the yellowish dot shaped infiltration found in the fundus (September 2019, F). Infrared fundus images corresponding respectively to SD-OCT image E (G) and F (H).
2. Discussion
PTLD represents a spectrum of lymphoproliferative disorders associated with EBV infection in the setting of pharmacologic immunosuppression1,2 that usually occurs within 1 year of hematopoietic stem cell or solid organ transplantation.2 Although PTLD is a common condition after hematopoietic stem cell transplantation with a reported incidence ranging from 1 to 17%,2,3 ocular involvement remains a rare finding in this context.4
Most reported cases of ocular PTLD follow solid organ transplantation and present as a masquerade syndrome with iris nodules, anterior chamber cells, and keratic precipitates.4,5 A few case reports also described the possibility of an orbital involvement.6,7 In the literature, posterior involvement includes vitritis,8,9 subretinal masses5,10 and optic disc swelling.5,10,11
In our case, the retina was the only affected organ and preceded the systemic relapse. Retinal involvement was strictly unilateral, and included perivascular, subretinal and sub retinal pigment epithelium (RPE) drusen like infiltrates along with a serous detachment of the fovea. The insidious evolution of the infiltrates eventually led to a complete loss of the photoreceptor layer. It is worthwhile to note that despite multiple ACTs with dosage of IL10, the ISOLD score remained negative. The ISOLD score was primarily designed for the detection of primary intraocular lymphoma,12 and might not be as sensitive with PTLD. Besides, it is usually contributive12 in case of vitreous infiltration, which the patient never displayed during the follow-up period. Last, the presence of EBV was documented only weeks after the first symptoms, once the infiltrates had reached the vascular arcades. Therefore, multiple ACTs with targeted PCRs and prompt vitrectomy should not be postponed in case of suspected PTLD, in order to obtain cytologic or PCR proof of malignancy and enable the use of chemotherapy. In rare cases, especially when the visual function has been compromised by retinal infiltrations, chorioretinal biopsies can be proposed in case of negativity of anterior chamber taps and/or vitrectomy. Mastropasqua R et al. showed that chorioretinal biopsy yielded a 59% diagnostic rate for lymphoma despite an increased risk of cataract, vitreous hemorrhage and retinal detachment.13 They suggested that the level of vitritis was a good predictor of the likelihood in achieving a definitive histologic diagnosis using this technique. In our patient's case, the level of vitritis was null throughout the follow-up.
To our knowledge, this is the first report of unilateral retinal and subretinal ocular PTLD preceding a systemic disease relapse.
3. Conclusion
This case shows a new presentation of ocular PTLD with retinal involvement and highlights the diagnostic difficulty of this rare entity.
Patient consent
Informed consent was obtained from the patient for case description.
Funding
No funding was received for this work.
Intellectual property
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Research ethics
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
IRB approval was obtained (required for studies and series of 3 or more cases).
Written consent to publish potentially identifying information, such as details or the case and photographs, was obtained from the patient(s) or their legal guardian(s).
Declaration of competing interest
No conflict of interest exists.
Acknowledgments and disclosures
None.
References
- 1.Loren A.W., Porter D.L., Stadtmauer E.A., Tsai D.E. Post-transplant lymphoproliferative disorder: a review. Bone Marrow Transplant. 2003;31(3):145–155. doi: 10.1038/sj.bmt.1703806. [DOI] [PubMed] [Google Scholar]
- 2.Fujimoto A., Suzuki R. Epstein-barr virus-associated post-transplant lymphoproliferative disorders after hematopoietic stem cell transplantation: pathogenesis, risk factors and clinical outcomes. Cancers. 2020;12(2) doi: 10.3390/cancers12020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pagliuca S., Bommier C., Michonneau D. Epstein-barr virus-associated post-transplantation lymphoproliferative disease in patients who received anti-CD20 after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2019;25(12):2490–2500. doi: 10.1016/j.bbmt.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Iu L.P., Yeung J.C., Loong F., Chiang A.K. Successful treatment of intraocular post-transplant lymphoproliferative disorder with intravenous rituximab. Pediatr Blood Canc. 2015;62(1):169–172. doi: 10.1002/pbc.25223. [DOI] [PubMed] [Google Scholar]
- 5.Cho A.S., Holland G.N., Glasgow B.J., Isenberg S.J., George B.L., McDiarmid S.V. Ocular involvement in patients with posttransplant lymphoproliferative disorder. Arch Ophthalmol Chic Ill 1960. 2001;119(2):183–189. [PubMed] [Google Scholar]
- 6.Walton R.C., Onciu M.M., Irshad F.A., Wilson T.D. Conjunctival posttransplantation lymphoproliferative disorder. Am J Ophthalmol. 2007;143(6):1050–1051. doi: 10.1016/j.ajo.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 7.Koenig K., Briese S., Wiemann D., Haffner D., Querfeld U. Post-transplantation swelling of the lower eyelid. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2004;19(4):1001–1003. doi: 10.1093/ndt/gfg597. [DOI] [PubMed] [Google Scholar]
- 8.Kheterpal S., Kirkby G.R., Neuberger J.M., Rosenthal A.R., Murray P.I. Intraocular lymphoma after liver transplantation. Am J Ophthalmol. 1993;116(4):507–508. doi: 10.1016/s0002-9394(14)71416-3. [DOI] [PubMed] [Google Scholar]
- 9.Demols P., Cochaux P., Velu T., Caspers-Velu L. Chorioretinal post-transplant lymphoproliferative disorder induced by the Epstein-Barr virus. Br J Ophthalmol. 2001;85(1):93–95. doi: 10.1136/bjo.85.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Attar L., Berrocal A., Warman R. Diagnosis by polymerase chain reaction of ocular posttransplant lymphoproliferative disorder after pediatric renal transplantation. Am J Ophthalmol. 2004;137(3):569–571. doi: 10.1016/j.ajo.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Traum A.Z., Rodig N.M., Pilichowska M.E., Somers M.J.G. Central nervous system lymphoproliferative disorder in pediatric kidney transplant recipients. Pediatr Transplant. 2006;10(4):505–512. doi: 10.1111/j.1399-3046.2006.00497.x. [DOI] [PubMed] [Google Scholar]
- 12.Costopoulos M., Touitou V., Golmard J.-L. ISOLD: a new highly sensitive interleukin score for intraocular lymphoma diagnosis. Ophthalmology. 2016;123(7):1626–1628. doi: 10.1016/j.ophtha.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 13.Mastropasqua R., Thaung C., Pavesio C. The role of chorioretinal biopsy in the diagnosis of intraocular lymphoma. Am J Ophthalmol. 2015;160(6):1127–1132. doi: 10.1016/j.ajo.2015.08.033. e1. [DOI] [PubMed] [Google Scholar]