Abstract
Background
HIV-1 infections occur following viral exposure at anogenital mucosal surfaces in the presence of semen. Semen contains immunosuppressive and pro-inflammatory factors. Semen from HIV-1-infected donors contains anti-HIV-1 antibodies. We assessed if passively infused anti-HIV-1 neutralizing antibody conferred protection from rectal SHIVSF162P3 challenge at semen exposed mucosae.
Methods
We pooled seminal plasma from HIV-1-infected donors. The pool was screened by ELISA for antibodies against HIV-1SF162 gp140. The ability of seminal plasma to inhibit macaque NK cells from responding to direct and antibody-dependent stimulation was assessed. The ability of seminal plasma to inhibit macaque granulocytes from mediating oxidative burst was also assessed. To demonstrate viral infectivity in the presence of seminal plasma, macaques (n = 4) were rectally challenged with SHIVSF162P3 following exposure to 2.5 mL of seminal plasma. To evaluate if anti-HIV-1 neutralizing antibody confers protection against rectal SHIV challenge at semen exposed mucosae, eight macaques were intravenously infused with PGT121, either wild type (n = 4) or the Fc receptor binding deficient LALA variant (n = 4), and rectally challenged with SHIVSF162P3 following exposure to 2.5 mL of seminal plasma.
Findings
Anti-HIV-1SF162 gp140 antibodies were detected in seminal plasma. Seminal plasma inhibited direct and antibody-dependent NK cell activation and granulocyte oxidative burst in vitro. Rectal SHIVSF162P3 challenge of control macaques following seminal plasma exposure resulted in infection of all animals. All macaques infused with wild type or LALA PGT121 and challenged with SHIVSF162P3 following seminal plasma exposure were protected.
Interpretation
PGT121 conferred protection against rectal SHIVSF162P3 challenge at semen exposed mucosae. Future research should investigate if semen alters protection conferred by antibodies more dependent on non-neutralizing functions.
Keywords: HIV-1, Broadly neutralizing antibodies, Semen, Fc-dependent functions
Research in context.
Evidence before this study
HIV-1 transmission primarily occurs at anogenital mucosae and in the presence of seminal fluid. A large body of evidence has demonstrated seminal fluid to exhibit immunosuppressive properties. It remains unknown if the immunosuppressive nature of seminal fluid can interfere with biomedical HIV-1 prevention strategies, such as the provision of neutralizing antibodies.
Previous work has shown that the PGT121 anti-HIV-1 broadly neutralizing antibody protects macaques from mucosal challenge with cell-free SHIVSF162P3 or intravenous challenge with cell-associated SHIVSF162P3. Protection from intravenous challenge with cell-associated SHIVSF162P3 did not require antibody Fc-dependent functions. Prior to this study it was unknown if PGT121-conferred protection against rectal challenge with cell-free virus: (I) requires Fc-dependent functions; and (II) occurs in the presence of seminal fluid.
Added value of this study
We provide robust evidence that PGT121-conferred protection from rectal cell-free SHIVSF162P3 challenge does not require Fc-dependent functions and occurs in the presence of seminal fluid. This data is highly informative for efforts to use neutralizing antibodies to prevent HIV-1 transmission.
Implications of all the available evidence
There are ongoing efforts to utilize anti-HIV-1 broadly neutralizing antibodies to prevent HIV-1 transmission. These include passive immunization strategies and attempts to design immunogens to elicit neutralizing antibodies via vaccination. The data presented here show that the PGT121 neutralizing antibody can confer protection from rectal challenge with cell-free SHIVSF162P3 despite the presence of immunosuppressive seminal fluid. Importantly, PGT121 did not require Fc-dependent functions to confer protection. Future studies need to assess if seminal fluid impacts the protection conferred by antibodies that require Fc-dependent functions to block infection.
Alt-text: Unlabelled box
1. Introduction
Novel strategies are urgently needed to prevent new HIV-1 infections. An important approach for preventing HIV-1 infection in individuals at high risk of exposure is to elicit or deliver broadly neutralizing antibodies (bNAbs). Many prototypic bNAbs have now been characterized [1]. These antibodies target several vulnerable sites within the HIV-1 envelope, including the membrane proximal external region, CD4 binding site, trimer apex, gp120-gp41 interface and the high-mannose patch. Passive administration of these antibodies to non-human primates provides robust protection against challenges with chimeric simian/human immunodeficiency viruses (SHIV) [2], [3], [4], [5], [6], [7], [8], [9], [10].
A major mechanism of bNAb-conferred protection is neutralization of free virions. The capacity of bNAbs to trigger Fc-dependent functions also contributes to protection conferred by some antibodies. Indeed, mutating the bNAb b12 in order to diminish its capacity to bind Fc receptors (i.e. LALA mutations) reduced its protective capacity in macaques vaginally challenged with SHIV [2,3]. Similar data highlighting the importance of Fc-dependent functions for the efficacy of more potent next generation bNAbs has been reported in a murine model of HIV-1 entry [11]. However, we recently reported that the LALA version of the PGT121 bNAb, which recognizes an epitope containing the V3 loop and surrounding glycans, fully protected pigtail macaques from high-dose intravenous challenge with cell-associated SHIV [8]. Whether this observation extends to rectal SHIV challenge using cell-free virus remains unknown.
Most natural mucosal exposures to HIV-1 involve the presence of semen, and this could impact the protective utility of bNAbs. Semen from HIV-1-infected men contains factors capable of enhancing (e.g. semen-derived enhancer of viral infection) or decreasing (e.g. anti-viral antibodies) viral infectivity [12]. Additionally, semen contains both pro-inflammatory and immunosuppressive factors. We and others have previously demonstrated the plasma fraction of semen to inhibit in vitro functions of human natural killer (NK) cells, T lymphocytes, monocytes and granulocytes [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]. The ability of seminal plasma to inhibit NK cells reduces their capacity to mediate Fc-dependent functions. The utility of bNAbs for protection from HIV-1 could be undermined by the immunomodulatory effects of seminal plasma, although this has not been directly studied.
In the current study, we built on our previous data showing that: (I) a version of PGT121 with diminished Fc-dependent functions protects against high-dose intravenous challenge with cell-associated SHIV [8]; and (II) seminal plasma inhibits anti-HIV-1 immune responses [16,17,[19], [20], [21]]. Here we aimed to determine if PGT121 required Fc-dependent functions to protect against rectal challenge with cell-free SHIV and if PGT121 could protect against cell-free SHIV challenge in the context of seminal fluid exposure. We assessed factors related to semen and the functionality of PGT121 that could impact bNAb-conferred protection. We found seminal plasma from HIV-1-infected men contained anti-HIV-1 antibodies capable of triggering Fc-dependent activation of macaque NK cells. We also demonstrated that immunosuppressive factors within seminal plasma inhibited functions of macaque NK cells and granulocytes. However, both wild type and LALA PGT121 fully protected macaques from a high-dose cell-free rectal SHIV challenge following exposure to seminal plasma. These results are discussed in terms of their implications for understanding HIV-1 transmission and importance for HIV-1 vaccine design.
2. Materials and methods
2.1. Seminal plasma
Seminal plasma samples from four HIV-1-uninfected donors were purchased from BioIVT. A pool of seminal plasma from aviremic HIV-1-infected donors was generated using samples obtained from the Opposites Attract cohort study [23]. Informed consent was obtained prior to collection and storage of biological samples. Ethics approval was granted by participating institutions: St Vincent's Hospital Human Research Ethics Committee, Sydney, Australia (Approval #11/SVH/170); Faculty of Medicine Institutional Review Board, Chulalongkorn University, Bangkok, Thailand (Approval #261/2014); and Evandro Chagas Institute of Clinical Research Ethics Committee, Rio de Janeiro, Brazil (Approval #490.884).
2.2. Enrichment of IgG from seminal plasma samples
IgG was derived from seminal plasma using the Protein G HP Multitrap and antibody buffer kit (Cytiva – catalog numbers: 28903135 and 28903059), as previously described [17]. Elutes were washed with PBS using 30k Amicon Ultra-4-centrifugal Units (Millipore – catalog number: UFC803024). Enriched IgG was suspended in PBS, using a volume matching the original seminal plasma sample.
2.3. NK cell activation assays
Two NK cell activation assays were performed to assess direct and antibody-dependent NK cell activation. First, we assessed antibody-dependent NK cell activation following exposure to plate-bound antigen/antibody complexes. This assay has been previously employed to measure antibody-dependent NK cell responses against influenza and HIV-1 [8, 9, 24]. ELISA plates were coated with 600 ng/well of HIV-1SF162 gp140 (NIH HIV Reagent Repository – catalog number: 12026) overnight at 4 °C. Next, plates were washed and blocked for one hour at 37 °C with PBS + 5% BSA. Following an additional wash, plates were incubated for two hours with wild type PGT121 antibody (20 μg/mL) or semen-derived IgG (used at the equivalent of a 1:10 dilution). Subsequent to the incubation, the plate was again washed and 106 freshly isolated macaque Peripheral blood mononuclear cells (PBMC) were added to each well along with APC-H7 conjugated anti-CD107a antibody (clone: H4A3; BD Biosciences – catalog number: 561343; RRID: AB_10644020), brefeldin A (Sigma – catalog number: B7651) and monensin (GolgiStop; BD Biosciences – catalog number: 554724). The isolation of PBMC was accomplished by ficoll density gradient centrifugation on heparinised macaque whole blood. For some renditions of this assay, a 1:100 dilution of HIV-1-uninfected seminal plasma was included at this stage to assess inhibition of NK cell activation. Plates were incubated at 37 °C for five hours with 5% CO2. Following incubation, cells were stained with APC conjugated anti-human NKG2A (clone Z199; Beckman Coulter – catalog number: A60797), Pacific Blue conjugated anti-human CD3 (clone: SP34-2; BD Biosciences – catalog number: 558124; RRID: AB_397044) and PerCP conjugated anti-human CD8 (clone SK1; BD Biosciences – catalog number: 347314; RRID: AB_400280) antibodies. Cells were then washed, fixed with 1% formaldehyde and acquired using a LSRFortessa flow cytometry instrument (BD Bioscience). Analysis was performed using FlowJo Software, version 10.0.8.
Direct and antibody-dependent NK cell activation was also assessed following stimulation with 721.221 cells coated or not with rituximab (Roche). The 721.221 cell line was a kind gift from Dr. Andrew G Brooks (University of Melbourne). The cell line was subjected to major histocompatibility complex class I (MHC-I or HLA-I) typing and confirmed to lack genes for HLA-A and HLA-B but not HLA-C. This is consistent with a previous characterization of this cell line [25]. Briefly, 106 PBMC were incubated at a 5:1 ratio with rituximab coated or uncoated 721.221 cells for five hours at 37 °C with 5% CO2. To assess the ability of seminal plasma to inhibit NK cell activation, some conditions contained a 1:100 dilution of pooled HIV-1-infected seminal plasma. Incubations were conducted in a final volume of 100 μL, containing APC-H7 conjugated anti-CD107a antibody (clone H4A3; BD Biosciences – catalog number: 561343; RRID: AB_10644020), brefeldin A (Sigma – catalog number: B7651) and monensin (GolgiStop; BD Biosciences – catalog number: 554724). Following incubation, cells were stained with APC conjugated anti-human NKG2A (clone Z199; Beckman Coulter – catalog number: A60797), Pacific Blue conjugated anti-human CD3 (clone: SP34-2; BD Biosciences – catalog number: 558124; RRID: AB_397044) and PerCP conjugated anti-human CD8 (clone SK1; BD Biosciences – catalog number: 347314; RRID: AB_400280). Cells were then washed, fixed with 1% formaldehyde and acquired using a LSRFortessa flow cytometry instrument (BD Bioscience). Analysis was performed using FlowJo Software, version 10.0.8.
2.4. Oxidative burst assay
The oxidative burst activity of peripheral blood granulocytes was assessed using the PHAGOBURST kit (Celonic – catalog number: 10–0200), as previously described [20]. This kit allows detection of oxidative burst through measurement of conversion of a fluorogenic substrate dihydrorhodamine 123 to rhodamine 123 following phagocytosis of Escherichia coli. Briefly, 99 μL of macaque whole blood was mixed with 11 μL of the HIV-1-infected seminal plasma pool (a final seminal plasma dilution of 1:10) or RPMI 1640 (Thermo Fisher Scientific – catalog number: 11875093). Next, optimal (20 × 106) and suboptimal (2 × 106) amounts of E. coli were added and samples were incubated at 37 °C for 10 minutes. Following incubation, 20 μL of substrate was added to each tube and incubated for a further 10 minutes at 37 °C. Finally, red blood cells were lysed, and samples were acquired on a LSRFortessa flow cytometry instrument (BD Biosciences). Analysis was performed using FlowJo Software, version 10.0.8.
2.5. Animals
All macaque studies were approved by the Monash University Animal Ethics Committee and Australian Commonwealth Scientific and Industrial Research Organization Animal Ethics Committee (Approval #24539). Twelve pigtail macaques (Macaca nemestrina) were sourced from the Monash University Animal Research Platform. These animals included seven males and five females, weighing an average of 4.4 kg at the initiation of the study. Prior to experimental intervention, all animals were acclimatised for four weeks. At study initiation, all animals were SHIV uninfected. The four animals used as controls were previously intravenously exposed to cell-associated SHIVSF162P3 but protected by passively administered PGT121.
2.6. Sample collection and processing
Rectal biopsies were collected using pinch biopsy forceps placed 5 cm into the rectum. Samples were transported on ice. Biopsy tissues were washed in RPMI 1640 (Thermo Fisher Scientific – catalog number: 11875093) and incubated in digestion buffer (0.1 mg/mL collagenase [Sigma-Aldrich – catalog number: C2139] and 1.5 U/mL DNase [Sigma-Aldrich - catalog number: 4716728001] in RF10 - RPMI 1640 supplemented with 10% FCS [Thermo Fisher Scientific - catalog number: 10091-148] and penicillin-streptomycin-glutamine [Thermo Fisher Scientific - catalog number: 10378016]) at 37 °C for 2 hours. The buffer and remaining tissue samples were passed through a 70 μm filter and washed in RF10. The resulting cell pellet was resuspended in RF10 and passed through a 30 μm cell filter, then transferred immediately for antibody staining and flow cytometry. PBMC were isolated from Heparin-treated whole blood samples by Ficoll gradient.
2.7. Antibodies
Both the wild type and LALA PGT121 antibodies were purchased from the Center for Antibody Development and Production (Scripps Research Institute, La Jolla, California, USA). The ability of both the wild type and LALA PGT121 antibodies to bind HIV-1 gp120 was confirmed by ELISA. In a previous report, we implemented an ELISA to assess the ability of wild type and LALA PGT121 to bind pigtail macaque FcγRIIIa [8]. The experiment confirmed that LALA PGT121 exhibited deficient binding to FcγRIIIa, as compared to wild type PGT121. The twelve macaques were randomly divided into three groups of four on the basis of the antibody they were administered: (I) no antibody; (II) wild type PGT121; and (III) LALA PGT121. Antibodies were administered at 1 mg/kg via the intravenous route one hour prior to rectal challenge with SHIVSF162P3. No blinding of group allocation was performed. The sample size of four animals per group was chosen to facilitate confirmation of the reproducibility of observations across animals. There were no criteria for including or excluding data points resulting from the experiments assessing antibody-conferred protection from SHIVSF162P3 challenge in the context of seminal plasma exposure. Furthermore, no data points were excluded.
Pooled HIV-1 immunoglobulin (HIVIG; obtained from the NIH HIV Reagent Repository – catalog number: 3957) was used a positive control in ELISAs measuring anti-HIV-1SF162 gp140 antibodies in the HIV-1-infected seminal plasma pool.
2.8. Rectal SHIVSF162P3 challenge
One hour prior to viral challenge, and at the same time as the IV antibody infusion, all macaques were rectally administered 2.5 mL of the HIV-1-infected human seminal plasma pool. Viral challenges were performed with a previously described stock of cell-free SHIVSF162P3 [9]. Challenges were performed by atraumatic intrarectal application of 847 TCID50 of the SHIVSF162P3 stock, as previously described for intravaginal challenges [26].
2.9. Measurement of SHIV DNA and RNA
SHIVSF162P3 infection of macaques was confirmed through the detection of viral RNA copies in plasma and cell-associated viral DNA copies in PBMC. Nucleic acid quantification was performed via digital droplet PCR (ddPCR), as previously described [8]. The utilized assays had limits of detection of 1.3 log10 SHIV DNA copies/106 cells and 1.90 log10 copies RNA/mL plasma. Viral RNA was obtained from 140 μL of plasma using QIAamp viral RNA mini kit (Qiagen – catalog number: 52904) and 10 μL of the eluted viral RNA was subjected to reverse transcription using SuperScript III (Invitrogen – catalog number: 18080–085), according to manufacturer recommendations. To normalize plasma samples and efficiencies of reverse transcription and PCR amplification, viral RNA samples were spiked during reverse transcription with a known concentration of in vitro transcribed reference importin [8] RNA (500 copies of IPO8 RNA). Quantification was accomplished using TaqMan probes designed to recognize, in a multiplex reaction, conserved sequences within SHIV Gag (6-FAM/MGBNFQ) and IPO8 (HEX/BHQ1). The ddPCR reaction consisted of 1x ddPCR supermix for probes (no dUTP, Bio-Rad – catalog number: 1863024), 1x IPO8 primers-probes (dHsaCPE5044719, Bio-Rad – catalog number: 10031255), 1 μM forward and reverse SIV Gag primers (detailed in reference [8]), 200 nM probe, and 6 μL cDNA in a 24 μL reaction. After droplet generation (15,000–18,000 on average), thermal cycling was performed as follows: 95 °C for 10 minutes, 40 cycles of 94 °C for 30 seconds, and 60 °C for 1 minute, followed by 98 °C for 10 minutes (ramp rate 2 °C/second for each step) on a C1000 Touch Thermal Cycler (Bio-Rad). The droplets were read using a QX200 Droplet Reader (Bio-Rad), and data were analyzed using QuantaSoft 1.7.4 software (Bio-Rad – catalog number: 1864011). Positive droplets were identified using the minus reverse transcriptase, the no-template and fluorescence minus one controls (SIV–/IPO8+, SIV+/IPO8– and SIV+/IPO8+) that were present in each run. Each sample was run in duplicate, and the merged data (copies/μL) were used to determine the amount of SHIVSF162P3 RNA/mL plasma. Quantification of the cell-associated viral DNA within PBMCs was conducted using the same primers-probe set used for SHIV vRNA. Genomic DNA (gDNA) was obtained from PBMCs using the QIAamp DNA Mini Kit (QIAGEN – catalog number: 51304), per the manufacturer's protocol. To assess the amount of cellular SHIV DNA per 106 PBMCs, equal amounts of gDNA (100 ng) were consequently used in a ddPCR reaction as described above. All samples were run in duplicate and SHIV DNA copies were calculated using the following formula: [gag copies per μL]/[(IPO8 copies per μL)/2] x 106 cells.
2.10. ELISAs to detect antibodies to gp41, gp120 and gp140
We performed ELISAs to detect antibodies directed against gp41 (Prospec – catalog number: HIV-112), HIV-1BaL gp120 (NIH HIV Reagent Repository – catalog number: 4961) and HIV-1SF162 gp140 (NIH HIV Reagent Repository – catalog number: 12026). These assays were performed to assess anti-HIV-1 gp140 antibodies within seminal plasma, further validate SHIVSF162P3 infection in macaques (ELISAs measuring gp41 and HIV-1BaL gp120 binding) and measure plasma PGT121 (ELISAs measuring HIV-1BaL gp120 binding). ELISAs were conducted using a protocol that has been previously described [9].
2.11. Flow cytometry
Single cell suspensions of rectal biopsies or PBMC were washed in PBS, stained with live/dead Blue (Thermo Fisher Scientific – catalog number: L23105), and then incubated with a cocktail of the following antibodies for 30 minutes at 4 °C: CD45 BUV395 (Clone: D058-1283, BD Biosciences – catalog number: 564099; RRID: AB_2738591), CCR5 BV421 (Clone: J418F1; Biolegend – catalog number: 359118; RRID: AB_2563577), CD3 Alexa488 (Clone: SP34-2, BD Biosciences – catalog number: 557705; RRID: AB_396814), CD4 BV605 (Clone: L200, BD Biosciences – catalog number: 562843; RRID: AB_2737833), CD8 BV650 (Clone: RPA-T8, Biolegend – catalog number: 301042; RRID: AB_2563505), NKG2A APC (Clone: Z199, Beckman Coulter – catalog number: A60797), CD69 BB700 (Clone: FN50; BD Biosciences – catalog number: 747520; RRID: AB_2744097), EpCam BV711 (Clone: EBA-1; BD Biosciences – catalog number: 743544; RRID: AB_2741575), CD16 Alexa700 (Clone: 3G8; BD Biosciences – catalog number: 560713; RRID: AB_1727430), CD14 BUV737 (Clone: M5E2, BD Biosciences – catalog number: 612763; RRID: AB_2870094), HLA-DR APC-Fire750 (Clone: L243; Biolegend – catalog number: 307658; RRID: AB_2572101), CCR6 BV785 (Clone: G034E3; Biolegend – catalog number: 353422; RRID: AB_2563660), CD66abce PE-Vio770 (Clone: TET2; Miltenyi – catalog number: 130-119-936; RRID: AB_2784268) and CD20 BUV805 (Clone: 2H7; BD Biosciences – catalog number: 612905; RRID: AB_2870192). Cells were then washed in PBS + 2% fetal calf serum and fixed with 1% paraformaldehyde. Data were acquired on a BD LSR II using BD FACS Diva (both from BD Biosciences).
2.12. Statistics
GraphPad Prism software was used to perform the reported data analyses. Two-tailed Wilcoxon matched-pairs tests were used to assess differences between paired data. A Friedman test with Dunn's post-hoc tests was used to assess differences between multiple matched datasets. Data comparisons were considered statistically significant at a p value of <0.05. The (median [range]) format is used to report data throughout the text.
Effect sizes and their 95% confidence intervals were calculated using R Package statix (V0.7.0). The Effect sizes were calculated using the wilcox_effsize and friedman_effsize functions. Effect sizes and their confidence intervals are provided in Supplemental Table 1.
3. Results
3.1. Generation of seminal plasma pool and assessment of antibody responses
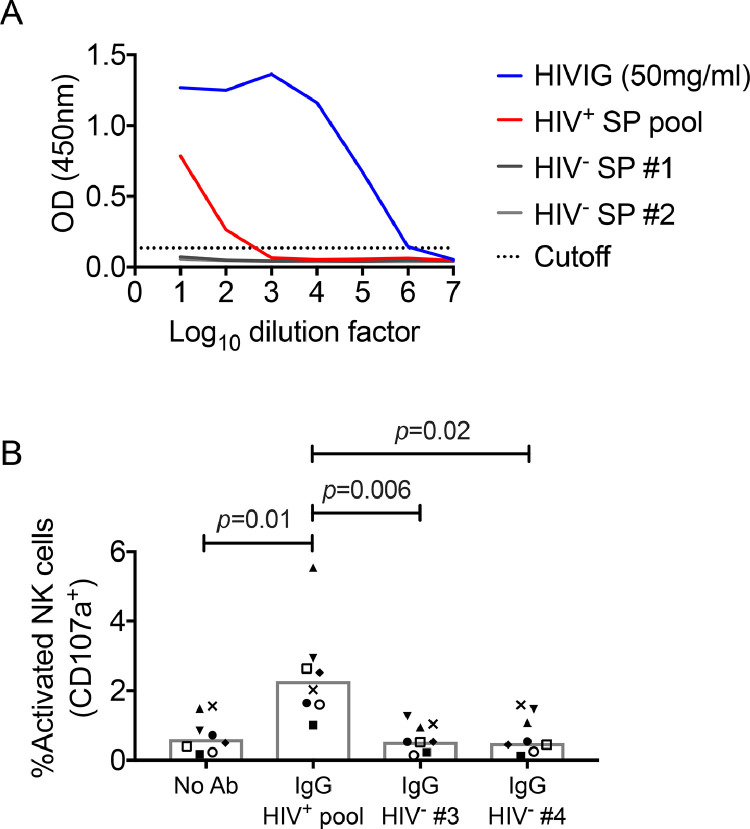
To facilitate experiments assessing if passively administered bNAbs protect macaques from rectal SHIV challenge at semen exposed mucosae, we generated a pool of seminal plasma from 70 aviremic HIV-1-infected donors participating in a recently published HIV-1 transmission study [23]. As semen from HIV-1-infected men carries anti-viral antibodies that could potentially interfere with viral transmission [17], we characterized the anti-gp140 antibody response within the seminal plasma pool by ELISA. The seminal plasma pool from HIV-1-infected donors had low titre anti-HIV-1 antibodies, detectable only at 1:10 and 1:100 dilutions (Fig. 1A).
Fig. 1.
Detection and functional characterization of anti-HIV-1 seminal plasma (SP) antibodies. (A) Relative amounts of anti-HIV-1SF162 gp140 binding antibodies in HIVIG, HIV-1-infected SP pool and SP from two individual HIV-1-uninfected donors, as determined by ELISA. The dotted line represents the cut-off point for a positive response, which was defined as two times the average OD obtained for the HIV-1-uninfected donors at the highest dilution. (B) Antibody-dependent activation (i.e., CD107a expression) of bulk NK cells within PBMC from eight macaques following stimulation with plate-bound HIV-1SF162 gp140 alone or in the presence of semen-derived IgG from the HIV-1-infected SP pool or two HIV-1-uninfected individual donors.
Antibodies contribute to anti-viral immune responses through neutralizing virus and non-neutralizing Fc-dependent functions, such as antibody-dependent cellular cytotoxicity (ADCC) [27]. We next assessed if anti-HIV-1 antibodies within the human seminal plasma pool could trigger degranulation of macaque NK cells. We found that macaque NK cells expressed CD107a on exposure to plate-bound conjugates of HIV-1SF162 gp140 and the HIV-1-infected seminal plasma pool-derived IgG (2.28% [1.01–5.54%]; Fig. 1B), which was significantly higher than degranulation observed following stimulation in the absence of antibody (0.61% [0.17–1.56%], p = 0.01) or IgG derived from HIV-1-uninfected donors (HIV-1- seminal plasma donor #3: 0.53% [0.14–1.27%], p = 0.006; HIV-1- seminal plasma donor #4: 0.50% [0.12–1.59%], p = 0.02).
3.2. Suppression of macaque NK cell responses by seminal plasma
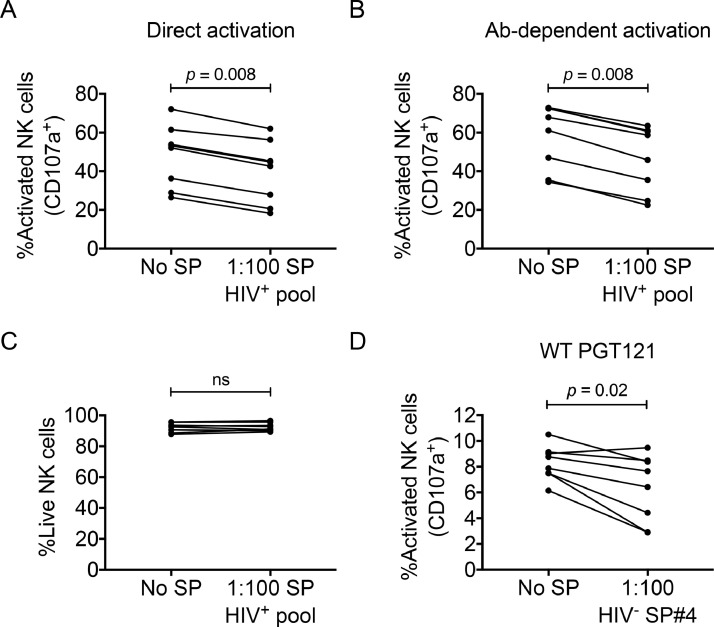
Human seminal plasma potently inhibits the responsiveness of human NK cells and T cells to activating stimuli in vitro [14,[16], [17], [18], [19],21,22]. Although the relevance of this phenomenon to HIV-1 transmission remains unknown, it has been hypothesized that the immunosuppressive nature of seminal plasma might contribute to HIV-1 transmission [28]. As non-human primate models are a means of assessing the utility of passive and active immunization for preventing HIV-1 infection, they are potentially useful models for understanding the impact of seminal plasma on the protective efficacy of vaccine-induced or passively administered immune responses. As such, we next assessed if human pooled seminal plasma from HIV-1-infected donors inhibited the responsiveness of macaque NK cells to direct and antibody-dependent stimuli. Macaque peripheral blood NK cells were stimulated with 721.221 target cells coated (antibody-dependent) or uncoated (direct) with the anti-CD20 monoclonal antibody, Rituximab. Stimulations were done in the presence or absence of a 1:100 dilution of pooled HIV-1-infected seminal plasma. As shown in Fig. 2A and 2B, the pooled seminal plasma decreased the responsiveness (measured as percentage of degranulating NK cells) of macaque NK cells to both direct (no seminal plasma: 52.65% [26.50–72.10%] Vs. seminal plasma: 43.70% [18.30–62.00%], p = 0.008) and antibody-dependent stimulation (no seminal plasma: 64.50% [34.40–72.80%] Vs. seminal plasma: 52.25% [22.50–63.50%], p = 0.008). This inhibition of macaque NK cell responses was not due to semen-induced cytotoxicity, as live/dead staining revealed no difference in the viability of untreated NK cells versus NK cells treated with seminal plasma over a five-hour incubation (no seminal plasma: 92.75% [87.80–95.60%] Vs. seminal plasma: 91.95% [89.40–96.50%], p = 0.64; Fig. 2C).
Fig. 2.
Suppression of direct and antibody-dependent activation of macaque NK cells by seminal plasma (SP). Bulk NK cells within PBMC were stimulated with rituximab coated (i.e., antibody-dependent activation) or uncoated (i.e., direct activation) 721.221 cells in the presence or absence of a 1:100 dilution of HIV-1-infected SP pool. The relative (A) direct and (B) antibody-dependent activation (i.e., CD107a expression) of NK cells following stimulation in the absence or presence of SP. To assess the impact of culture in SP on NK cell viability, PBMCs were cultured alone or in the presence of SP for five hours. (C) Relative percentage of viable NK cells following culture in the absence or presence of SP. To determine if SP suppresses anti-HIV-1 antibody-dependent NK cell activation, bulk NK cells within PBMC were stimulated with plate-bound complexes of PGT121 and HIV-1SF162 gp140 in the presence or absence of a 1:100 dilution of SP from an HIV-1-uninfected donor. (D) The graph shows the relative anti-HIV-1 antibody-dependent NK cell activation (i.e., CD107a expression) in the absence or presence of SP. Data were compared using Wilcoxon matched pairs tests. p < 0.05 was considered statistically significant.
We next assessed if seminal plasma could inhibit anti-HIV-1 antibody-dependent responses of macaque NK cells, since this could be relevant to the protective capacity of Fc-functional neutralizing antibodies. For this purpose, we utilized seminal plasma from an HIV-1-uninfected donor to avoid the function of HIV-1-specific antibodies within HIV-1-infected seminal plasma. We found that NK cell degranulation following stimulation with gp140-bound PGT121 was decreased in the presence of seminal plasma (no seminal plasma: 8.32% [6.14–10.50%] Vs. seminal plasma: 7.04% [2.89–9.47%], p = 0.02; Fig. 2D).
3.3. Suppression of macaque granulocyte responses by seminal plasma
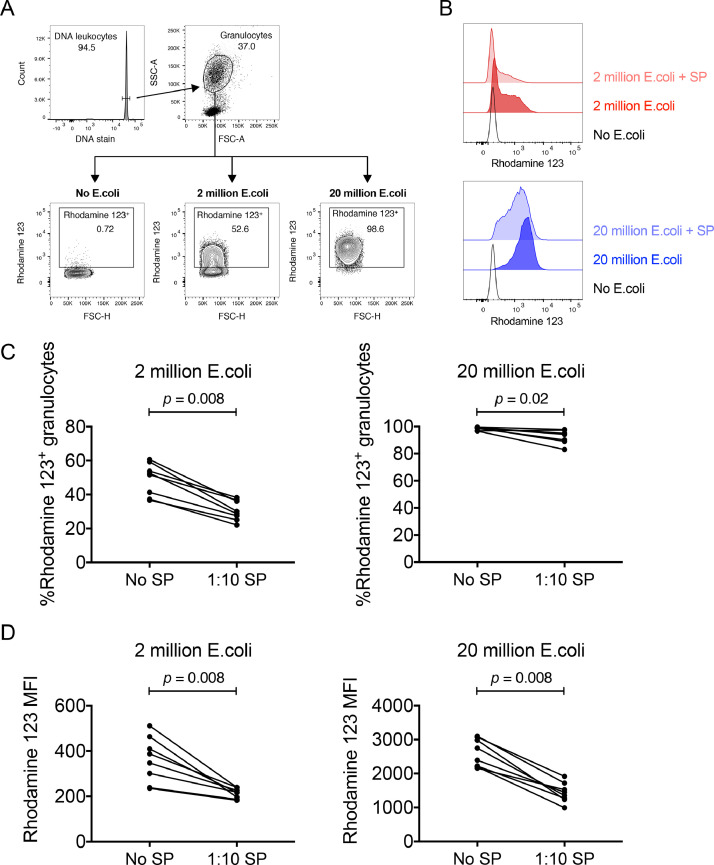
Neutrophils are present in anogenital tissues and antibody-dependent neutrophil functions could be relevant for antibody-conferred protection from HIV-1 infection [29,30]. In addition to NK cell and T cell responses, human seminal plasma can inhibit the responsiveness of human granulocytes to antibody-opsonized targets [13,15,20], but whether human seminal plasma can inhibit macaque granulocyte functions is unknown. A robust means of assessing granulocyte function is measuring oxidative burst following stimulation with opsonized E. coli [20]. We measured macaque granulocyte oxidative burst to E. coli in the presence or absence of 1:10 dilutions of the HIV-1-infected seminal plasma pool. The 1:10 dilution used here differs from the 1:100 dilution used for the NK cell experiments shown in Fig. 2, as our previous work with human cells demonstrated that higher dilutions of seminal plasma are required to inhibit granulocytes [20]. While a 1:10 dilution of seminal plasma significantly inhibited oxidative burst mediated by human granulocytes, further dilution of seminal plasma eliminated its inhibitory capacity. Fig. 3A depicts the gating procedure implemented to identify granulocytes undergoing an oxidative burst. Inclusion of seminal plasma in assay incubations containing E. coli decreased granulocyte oxidative burst (Fig. 3B), when measured either as the percentage of granulocytes positive for an oxidative burst (2 × 106 E. coli: no seminal plasma: 52.10% [36.60–60.60%] Vs. seminal plasma: 29.40% [22.10–38.20%], p = 0.008; 20 × 106 E. coli: no seminal plasma: 99.10% [96.70–99.60%] Vs. seminal plasma: 94.40% [83.00–97.80%], p = 0.02; Fig. 3C) or as the MFI of the oxidative burst (2 × 106 E. coli: no seminal plasma: 367 [235–511] Vs. seminal plasma: 217 [182–239], p = 0.008; 20 × 106 E. coli: no seminal plasma: 2570 [2156–3101] Vs. seminal plasma: 1400 [992–1916], p = 0.008; Fig. 3D). Importantly, significant decreases in oxidative burst activity were noted when either optimal (20 × 106) or suboptimal (2 × 106) amounts of E. coli were used during the stimulation.
Fig. 3.
Suppression of macaque granulocyte oxidative burst activity by seminal plasma (SP). Oxidative burst activity of granulocytes within macaque blood following exposure to optimal (20 × 106) and suboptimal (2 × 106) amounts of Escherichia coli was measured using the PHAGOBURST kit. Oxidative burst was measured as the conversion of the dihydrorhodamine 123 substrate to fluorogenic rhodamine 123. Experiments were conducted in the absence or presence of a 1:10 final dilution of HIV-1-infected SP pool. (A) The FACS plots depict the gating procedure implemented to identify leukocytes, granulocytes and assess oxidative burst activity following incubation in the absence of E. coli or the presence of suboptimal or optimal amounts of E. coli.(B) The histograms show the relative levels of rhodamine 123 expressed by granulocytes following incubation in the absence of E. coli or stimulation with 2 × 106 (Red histogram; Top) or 20 × 106 (Blue histogram; Bottom) E. coli in the absence or presence of SP. (C) The graphs show the relative percentage of rhodamine 123 positive granulocytes within heparinised blood from eight macaques following stimulation with 2 × 106 or 20 × 106E. coli in the absence or presence of SP. (D) The graphs show the rhodamine 123 MFI of granulocytes within heparinised blood from eight macaques following stimulation with 2 × 106 or 20 × 106E. coli in the absence or presence of SP. Data were compared using Wilcoxon matched pairs tests. p < 0.05 was considered statistically significant.
3.4. Changes in macaque rectal immune cell phenotype and frequency following human seminal plasma exposure
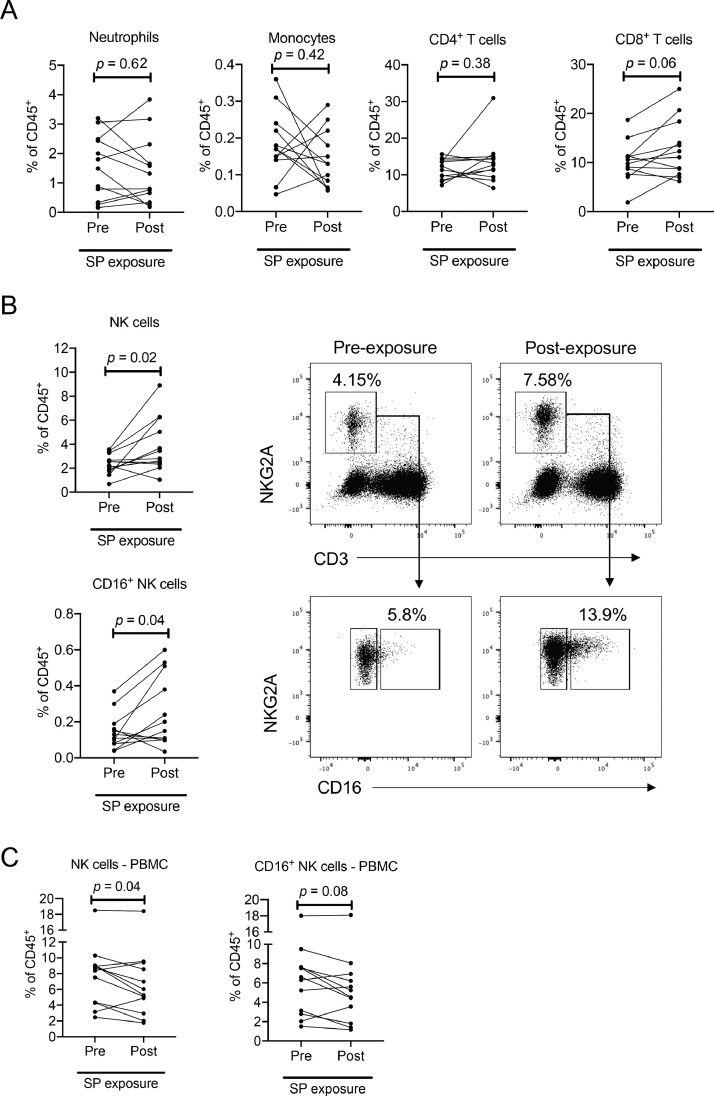
There have been limited in vivo studies on the influence of seminal plasma on the phenotype and frequency of anogenital mucosal immune cells. In humans, vaginal exposure to semen during coitus results in the recruitment of CD45+ leukocytes, including macrophages, T-cells and NK cells [31]. We and others have shown human seminal plasma can alter the frequency of immune cells in the female reproductive tract of macaques [16, 32]. However, there have been no studies, to our knowledge, of the impact of HIV-1+ human seminal plasma on immune cells within the macaque rectum. To address this issue, we isolated mononuclear cells from rectal biopsies two weeks prior and one day after the atraumatic instillation of 2.5 mL of the human HIV-1+ seminal plasma pool in 12 pigtail macaques. Supplemental Fig. 1 shows the gating procedure used to assess rectal leukocytes. We found no significant changes in the frequencies of CD4+ T-cells (pre-exposure: 11.85% [7.17–15.60%] Vs. post-exposure: 12.95% [6.39–30.90%] of total CD45+ cells, p = 0.38), monocytes (pre-exposure: 0.17% [0.05–0.36%] Vs. post-exposure: 0.13% [0.06–0.29%] of total CD45+ cells, p = 0.42) or neutrophils (pre-exposure: 1.65% [0.16-3.20%] Vs. post-exposure: 1.06% [0.19–3.84%] of total CD45+ cells, p = 0.62) following seminal plasma exposure, although there was a trend toward an increase in CD8+ T-cell (pre-exposure: 10.18% [1.89–18.70%] Vs. post-exposure: 11.70% [6.20–25.00%] of total CD45+ cells, p = 0.06) frequency (Fig. 4A). However, we did find a significant increase in NK cell frequency among the total CD45+ cell population (pre-exposure: 2.38% [0.68–3.56%] Vs. post-exposure: 3.13% [1.04–8.90%], p = 0.02; Fig. 4B). This was at least partially attributable to an increase in CD16+ NK cells at the rectal mucosa, which were also significantly increased in frequency at the rectum following seminal plasma exposure (pre-exposure: 0.12% [0.04–0.37%] Vs. post-exposure: 0.18% [0.03–0.60%] of total CD45+ cells, p = 0.04; Fig. 4B). Interestingly, the frequency of NK cells in PBMC significantly declined after seminal plasma exposure (pre-exposure: 8.55% [2.46–18.50%] Vs. post-exposure: 5.66% [1.77–18.40%] of CD45+ cells, p = 0.04 Fig. 4C). This included a trend toward lower CD16+ NK cells in the circulation (pre-exposure: 6.45% [1.50–18.00%] Vs. post-exposure: 4.88% [1.17–18.10%] of CD45+ cells, p = 0.08; Fig. 4C), suggesting possible recruitment of NK cells from the periphery to the rectal mucosa following seminal plasma exposure.
Fig. 4.
Impact of seminal plasma (SP) on macaque rectal leucocytes. (A) Frequency of neutrophil (CD20−CD3−CD14−HLA-DR−CD66abce+), monocyte (CD20−CD3−NKG2A−CD66abce−CD14+HLA-DR+), and T cell (CD20−CD14−CD3+) populations among rectal biopsy samples (expressed as % of CD45+ cells) prior to and following SP exposure. (B) Frequency of total NK cells (CD14−CD20−CD3−NKG2A+) or CD16+ NK cells among rectal biopsy samples prior to and following SP exposure. Plots show representative gating for NK cells and CD16 expression among matched samples from one macaque. (C) NK cell and CD16+ NK cell frequencies among PBMC prior to and following SP exposure. Statistics assessed by Wilcoxon tests.
3.5. PGT121-conferred protection from SHIV infection in the context of seminal plasma
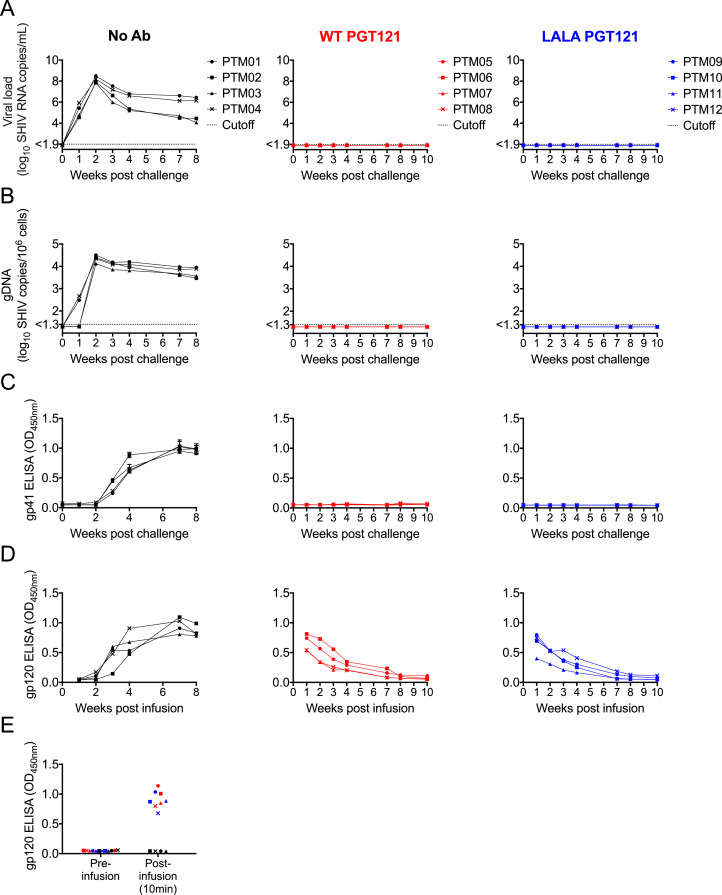
Human HIV-1 infections commonly occur in the presence of HIV-1-infected seminal plasma, but the influence of seminal plasma on immune mechanisms that protect against viral challenge in animal models has not been well studied. bNAbs protect against mucosal SHIV challenges in macaques [[2], [3], [4], [5], [6], [7],10]. As such, macaques provide a model to explore the influence of seminal plasma on protective immunity. We studied 12 macaques divided into three groups: (i) Four controls that received no bNAbs; (ii) Four animals infused with 1mg/kg of wild type human IgG1 bNAb PGT121 one hour prior to challenge; and (iii) Four animals infused with 1mg/kg of PGT121 containing the Fc LALA mutations one hour prior to challenge. The Fc LALA mutations diminish the ability of antibodies to bind Fcγ receptor and trigger ADCC in pigtail macaques [8]. All macaques were rectally administered 2.5 mL of seminal plasma one hour prior to rectal challenge with 847 TCID50 of SHIVSF162P3. All four control macaques were readily infected and exhibited viral RNA in plasma by one week post-challenge (Fig. 5A), viral DNA in PBMC within 1-2 week(s) post-challenge (Fig. 5B) and anti-gp41 and anti-gp120 antibodies in plasma within 3 weeks of challenge (Fig. 5C-D). The uniform infection and kinetics of SHIV infection in the presence of seminal plasma were similar to our previously published studies on SHIV infection following vaginal challenges in the absence of seminal plasma [26, 33].
Fig. 5.
Protection from rectal SHIVSF162P3 challenge by wild type and LALA PGT121. Twelve pigtail macaques were divided into three groups of four. One hour prior to rectal viral challenge, the three groups were either intravenously infused with wild type PGT121 (Red graphs, Middle panel), intravenously infused with LALA PGT121 (Blue graphs, Right panel) or given no antibody (Black graphs, Left panel). One hour prior to challenge, all animals were rectally exposed to 2.5 mL of HIV-1-infected seminal plasma pool. Graphs show (A) plasma viral load and (B) cell-associated viral DNA within PBMC in the weeks post challenge. The dotted lines on the graphs represent the sensitivity cutoffs for the implemented assays. (C) Seroconversion to the gp41 envelope glycoprotein following SHIVSF162P3 challenge was assessed by ELISA using a 1:1000 dilution of plasma. (D) Infused wild type and LALA PGT121 were detected by ELISA designed to detect anti-gp120 antibodies. The graphs show the relative ODs for 1:50 plasma dilutions in the weeks post-infusion. The increased OD in the no antibody group reflects seroconversion to gp120 following SHIVSF162P3 infection. (E) The graph depicts the detection of infused wild type and LALA PGT121 in blood plasma of animals 10 minutes after antibody administration.
All four animals infused with wild type PGT121 were protected from the cell-free SHIV infection despite the presence of seminal plasma, which contains immunosuppressive factors. At no time post-challenge did the macaques exhibit either viral RNA in plasma (Fig. 5A), viral DNA in PBMC (Fig. 5B) or anti-gp41 antibodies in plasma (Fig. 5C). The infused PGT121 antibody was detectable in plasma samples from all four animals at a sampling time 10 minutes post-infusion (Fig. 5E) and decayed over the weeks post-infusion (Fig. 5D), as expected. These results are similar to previous studies by our group and others showing PGT121 protects against challenges with SHIVSF162P3 [5, 8, 9].
Similar to macaques passively immunized with wild type PGT121, all four macaques infused with LALA PGT121 were also completely protected from infection. No viral RNA or DNA was detected at any time post-challenge (Fig. 5A-B) and the animals did not develop anti-gp41 antibodies (Fig. 5C). The infused PGT121 LALA antibody was detectable in plasma at a 10 minute post-infusion sampling (Fig. 5E) and waned over the weeks post-infusion (Fig. 5D).
4. Discussion
Improving macaque models of HIV-1 challenge to more accurately reflect natural exposure is important for assessing preventative approaches. Most human HIV-1 infections occur following mucosal exposure in the presence of semen. Macaque models of SIV or SHIV challenge, however, have only rarely incorporated seminal fluid [6]. Human seminal plasma is known to strongly inhibit human anti-HIV-1 immune responses in vitro [16,17,[19], [20], [21]]. We now demonstrate that seminal plasma from HIV-1-infected humans also inhibits the in vitro functions of macaque NK cells and granulocytes. Despite the suppression of the functions of macaque effector cells by seminal plasma, we observed the PGT121 bNAb to prevent infection following rectal SHIV challenge at semen exposed mucosae. Importantly, PGT121-conferred protection was independent of Fc-dependent functions, as all animals infused with PGT121 LALA were also protected.
The current data are consistent with previous experiments showing PGT121 to protect against mucosal and intravenous SHIV challenges [5,8,9]. Furthermore, the demonstration that PGT121 does not require Fc-dependent functions to protect against rectal cell-free SHIV challenge is consistent with our previous observation that LALA PGT121 protected against intravenous challenge with cell-associated SHIV [8], as well as a recent study by Hangartner et al. (2021) showing Fc-dependent functions were not required for PGT121-conferred protection from vaginal challenge with cell-free SHIV [34].
The current data also build on previous observations regarding the capacity of human seminal plasma to inhibit immune responses potentially relevant to HIV-1 vaccine design [16,17,[19], [20], [21]]. Here, we show that human seminal plasma from HIV-1-infected donors can inhibit macaque NK cell and granulocyte responses. Despite the ability of human seminal plasma to inhibit macaque immune cell functions, we observed PGT121-conferred protection following rectal SHIV challenge at semen exposed mucosae. A caveat to this observation is that PGT121 does not require engagement of FcR to protect against SHIV challenge. It remains possible that the immunosuppressive capacity of seminal plasma would be detrimental to protection conferred by other bNAbs that require Fc-dependent functions to provide optimal protection. Future studies could test this possibility by incorporating semen into SHIV challenge systems that use the b12 bNAb to prevent infection, as b12 depends on Fc-dependent functions to provide optimal protection [2,3]. However, the translational value of such a study using b12 is questionable, given that it has lower potency and breadth compared to more recently isolated bNAbs such as PGT121. Recent studies have suggested additional bNAbs, with mutations that diminish Fc-dependent functions, are less potent at controlling established SHIV infection in macaques, although whether these bNAbs require Fc function to protect from de novo infection is not known [35,36].
The potential for semen to interfere with immune responses involved in protecting against HIV-1 raises several important points for discussion. First, although experiments have repeatedly shown seminal plasma to inhibit in vitro functions of immune cells [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], these experiments have been performed with peripheral blood cells. Semen is known to contain both pro- and anti-inflammatory factors [12]. Thus, it is foreseeable that in the correct environment semen could enhance the responsiveness of immune cells. Indeed, a recent study in non-human primates highlighted that exposure to human seminal plasma enhanced mucosal responsiveness to vaccination [37]. As such, it remains unknown if semen is immunosuppressive within anogenital tissues. If semen is not immunosuppressive at anogenital tissues, it is theoretically possible that antibodies within seminal fluid could contribute to the prevention of viral infection. However, given that anti-HIV-1 antibodies within seminal fluid primarily recognize the gp120 inner domain, they are unlikely to recognize native trimeric envelope spikes or contribute to viral clearance [19]. The capacity for semen to modulate anogenital immune responses would likely be dependent on the dilution of semen present at the site of exposure following receptive intercourse. This would be impacted by the presence of mucosal fluids and lubricants. The impact of mucosal fluids and lubricants on the dilution of seminal fluid present at anogenital sites of HIV-1 exposure, as well as the potential for these factors to interfere with the immunomodulatory effects of seminal fluid, are important areas for future study in both non-human primate models and HIV-1 exposed humans. Secondly, several studies have shown that semen exposure can recruit immune cells to anogenital tissues [16,31,32]. These recruited cells include anti-viral effector cells and target cells for HIV-1 infection. In the current study, we observed evidence of recruitment of total NK cells, and NK cells expressing CD16, in seminal plasma exposed macaques. Additional studies are needed to refine our understanding of the impact of semen exposure on the immunological architecture of HIV-1-exposed tissues. This information will be highly instructive for the design of HIV-1 vaccines and immune-based prophylactics.
The current study has a series of potential caveats that require discussion. First, the study did not include control groups assessing the outcome of viral challenge, in the presence or absence of passively infused PGT121, in animals not treated with seminal fluid. Although this could technically impair our ability to assess the impact of semen on PGT121 protective efficacy, we observed uniform infection and viral kinetics similar to our previous studies of mucosal SHIV challenge performed in the absence of seminal fluid [26,33]. Additionally, both the observed antibody-conferred protection from infection, as well as the redundancy of Fc-dependent functions for protection, are consistent with other studies assessing PGT121-conferred protection from mucosal SHIV challenge in the absence of seminal fluid [5,34]. Second, we did not perform dose response analyses for passively infused PGT121 or challenge virus. While it is possible that this could restrict us to observing only an “all or nothing” effect, we note that our previous work using high-dose challenge in animals infused with high-dose antibody allowed us to observe diverse outcomes in distinct animals [9]. Third, it is possible that seminal fluid could act to enhance, not suppress, immune responses at the mucosal site of exposure and improve antibody Fc-dependent effector functions. Although this is a possibility for the wild type PGT121 antibody, we feel it is unlikely to have a physiologically significant impact on the protection conferred by the LALA PGT121 antibody that has diminished capacity to trigger Fc-dependent functions. Lastly, we biopsied all animals twice prior to the viral challenge study. It is possible that this could have enhanced susceptibility to infection following viral challenge. Importantly, we allowed two weeks between the last biopsy and viral challenge to diminish the likelihood that biopsy collection impacted virus infectivity.
The provision of antibodies capable of blocking infection with a wide range of circulating viruses is an important strategy for reducing the number of new HIV-1 infections. The data presented here further highlight the protective potential and versatility of bNAbs. Unlike previous studies showing bNAbs to require Fc-dependent functions to provide optimal protection [2,3,11], we now show that PGT121-conferred protection does not require Fc-dependent functions. Furthermore, we show that PGT121, either with or without Fc functions, is fully protective despite viral challenge occurring in the context of seminal plasma, which contains immunosuppressive factors. Together, these observations are promising for efforts to advance bNAbs for human application as prophylactics.
Conflict of Interest Statements
Matthew S. Parsons: Reports grants from the NHMRC.
Anne B. Kristensen: Has nothing to disclose.
Kevin J. Selva: Has nothing to disclose.
Wen Shi Lee: Has nothing to disclose.
Thakshila Amarasena: Has nothing to disclose.
Robyn Esterbauer: Has nothing to disclose.
Adam K. Wheatley: Has nothing to disclose.
Benjamin R. Bavinton: Reports grants from Gilead Sciences and ViiV Healthcare, as well as personal fees from Gilead Sciences.
Anthony D. Kelleher: Reports grants from the NHMRC, MRFF and NIH, personal fees from the New South Wales Government, and other support from Gilead Sciences, CSL and Janssen.
Andrew E. Grulich: Reports grants from the NHMRC, ViiV and Gilead Sciences.
Georges Khoury: Has nothing to disclose.
Jennifer A. Juno: Has nothing to disclose.
Stephen J. Kent: Has nothing to disclose.
Funding
This work was supported by a grant from the Australian National Health and Medical Research Council (APP1124680).
Declaration of Competing Interest
None.
Acknowledgements
We thank Dr. Andrew G Brooks (Peter Doherty Institute for Infection and Immunity, University of Melbourne) for providing the 721.221 cell line. We also thank Dr. Miles Davenport and Dr. Arnold Reynaldi (Kirby Institute, UNSW Sydney) for assistance with statistical analysis.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103518.
Contributor Information
Matthew S. Parsons, Email: matthew.s.parsons@emory.edu.
Stephen J. Kent, Email: skent@unimelb.edu.au.
Appendix. Supplementary materials
References
- 1.McCoy LE, Burton DR. Identification and specificity of broadly neutralizing antibodies against HIV. Immunol Rev. 2017;275(1):11–20. doi: 10.1111/imr.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449(7158):101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 3.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15(8):951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5(5) doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci USA. 2012;109(46):18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moog C, Dereuddre-Bosquet N, Teillaud JL, Biedma ME, Holl V, Van Ham G. Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol. 2014;7(1):46–56. doi: 10.1038/mi.2013.23. [DOI] [PubMed] [Google Scholar]
- 7.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75(17):8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsons MS, Lee WS, Kristensen AB, Amarasena T, Khoury G, Wheatley AK. Fc-dependent functions are redundant to efficacy of anti-HIV antibody PGT121 in macaques. J Clin Invest. 2019;129(1):182–191. doi: 10.1172/JCI122466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parsons MS, Lloyd SB, Lee WS, Kristensen AB, Amarasena T, Center RJ. Partial efficacy of a broadly neutralizing antibody against cell-associated SHIV infection. Sci Transl Med. 2017;9(402):eaaf1483. doi: 10.1126/scitranslmed.aaf1483. [DOI] [PubMed] [Google Scholar]
- 10.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9(3):343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 11.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158(6):1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doncel GF, Anderson S, Zalenskaya I. Role of semen in modulating the female genital tract microenvironment–implications for HIV transmission. Am J Reprod Immunol. 2014;71(6):564–574. doi: 10.1111/aji.12231. [DOI] [PubMed] [Google Scholar]
- 13.Binks S, Pockley AG. Modulation of leukocyte phagocytic and oxidative burst responses by human seminal plasma. Immunol Invest. 1999;28(5-6):353–364. doi: 10.3109/08820139909062269. [DOI] [PubMed] [Google Scholar]
- 14.James K, Hargreave TB. Immunosuppression by seminal plasma and its possible clinical significance. Immunol Today. 1984;5(12):357–363. doi: 10.1016/0167-5699(84)90079-3. [DOI] [PubMed] [Google Scholar]
- 15.James K, Harvey J, Bradbury AW, Hargreave TB, Cullen RT. The effect of seminal plasma on macrophage function–a possible contributory factor in sexually transmitted disease. AIDS Res. 1983;1(1):45–57. doi: 10.1089/aid.1.1983.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Juno JA, Wragg KM, Kristensen AB, Lee WS, Selva KJ, van der Sluis RM. Modulation of the CCR5 receptor/ligand axis by seminal plasma and the utility of in vitro versus in vivo models. J Virol. 2019;93(11) doi: 10.1128/JVI.00242-19. e00242-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsons MS, Madhavi V, Ana-Sosa-Batiz F, Center RJ, Wilson KM, Bunupuradah T. Brief report: seminal plasma anti-HIV antibodies trigger antibody-dependent cellular cytotoxicity: implications for HIV transmission. J Acquir Immune Defic Syndr. 2016;71(1):17–23. doi: 10.1097/QAI.0000000000000804. [DOI] [PubMed] [Google Scholar]
- 18.Sakin-Kaindl F, Wagenknecht DR, Strowitzki T, McIntyre JA, Thaler CJ. Decreased suppression of antibody-dependent cellular cytotoxicity by seminal plasma in unexplained infertility. Fertil Steril. 2001;75(3):581–587. doi: 10.1016/s0015-0282(00)01750-7. [DOI] [PubMed] [Google Scholar]
- 19.Selva KJ, Bavinton BR, Grulich AE, Pazgier M, Kelleher AD, Kent SJ. Impact of HIV-1 viremia or sexually transmitted infection on semen-derived anti-HIV-1 antibodies and the immunosuppressive capacity of seminal plasma. Eur J Immunol. 2019;49(12):2255–2258. doi: 10.1002/eji.201848055. [DOI] [PubMed] [Google Scholar]
- 20.Selva KJ, Juno JA, Worley MJ, Chung AW, Tachedjian G, Kent SJ. Short communication: effect of seminal plasma on functions of monocytes and granulocytes. AIDS Res Hum Retroviruses. 2019;35(6):553–556. doi: 10.1089/AID.2018.0219. [DOI] [PubMed] [Google Scholar]
- 21.Selva KJ, Kent SJ, Parsons MS. Modulation of innate and adaptive cellular immunity relevant to HIV-1 vaccine design by seminal plasma. AIDS. 2017;31(3):333–342. doi: 10.1097/QAD.0000000000001319. [DOI] [PubMed] [Google Scholar]
- 22.Thaler CJ, McConnachie PR, McIntyre JA. Inhibition of immunoglobulin (Ig)G-Fc-mediated cytotoxicity by seminal plasma IgG-Fc receptor III antigens. Fertil Steril. 1992;57(1):187–192. [PubMed] [Google Scholar]
- 23.Bavinton BR, Pinto AN, Phanuphak N, Grinsztejn B, Prestage GP, Zablotska-Manos IB. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV. 2018;5(8) doi: 10.1016/S2352-3018(18)30132-2. e438-47. [DOI] [PubMed] [Google Scholar]
- 24.Jegaskanda S, Amarasena TH, Laurie KL, Tan HX, Butler J, Parsons MS. Standard trivalent influenza virus protein vaccination does not prime antibody-dependent cellular cytotoxicity in macaques. J Virol. 2013;87(24):13706–13718. doi: 10.1128/JVI.01666-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu Y, DeMars R. Production of human cells expressing individual transferred HLA-A,-B,-C genes using an HLA-A,-B,-C null human cell line. J Immunol. 1989;142(9):3320–3328. [PubMed] [Google Scholar]
- 26.Kent SJ, Dale CJ, Ranasinghe C, Stratov I, De Rose R, Chea S. Mucosally-administered human-simian immunodeficiency virus DNA and fowlpoxvirus-based recombinant vaccines reduce acute phase viral replication in macaques following vaginal challenge with CCR5-tropic SHIVSF162P3. Vaccine. 2005;23(42):5009–5021. doi: 10.1016/j.vaccine.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 27.Parsons MS, Chung AW, Kent SJ. Importance of Fc-mediated functions of anti-HIV-1 broadly neutralizing antibodies. Retrovirology. 2018;15(1):58. doi: 10.1186/s12977-018-0438-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly RW. Prostaglandins in primate semen: biasing the immune system to benefit spermatozoa and virus? Prostaglandins Leukot Essent Fatty Acids. 1997;57(2):113–118. doi: 10.1016/s0952-3278(97)90000-4. [DOI] [PubMed] [Google Scholar]
- 29.Sips M, Krykbaeva M, Diefenbach TJ, Ghebremichael M, Bowman BA, Dugast AS. Fc receptor-mediated phagocytosis in tissues as a potent mechanism for preventive and therapeutic HIV vaccine strategies. Mucosal Immunol. 2016;9(6):1584–1595. doi: 10.1038/mi.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Worley MJ, Fei K, Lopez-Denman AJ, Kelleher AD, Kent SJ, Chung AW. Neutrophils mediate HIV-specific antibody-dependent phagocytosis and ADCC. J Immunol Methods. 2018;457:41–52. doi: 10.1016/j.jim.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell-Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188(5):2445–2454. doi: 10.4049/jimmunol.1102736. [DOI] [PubMed] [Google Scholar]
- 32.Abdulhaqq SA, Martinez M, Kang G, Rodriguez IV, Nichols SM, Beaumont D. Repeated semen exposure decreases cervicovaginal SIVmac251 infection in rhesus macaques. Nat Commun. 2019;10(1):3753. doi: 10.1038/s41467-019-11814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batten CJ, De Rose R, Wilson KM, Agy MB, Chea S, Stratov I. Comparative evaluation of simian, simian-human, and human immunodeficiency virus infections in the pigtail macaque (Macaca nemestrina) model. AIDS Res Hum Retroviruses. 2006;22(6):580–588. doi: 10.1089/aid.2006.22.580. [DOI] [PubMed] [Google Scholar]
- 34.Hangartner L, Beauparlant D, Rakasz E, Nedellec R, Hoze N, McKenney K. Effector function does not contribute to protection from virus challenge by a highly potent HIV broadly neutralizing antibody in nonhuman primates. Sci Transl Med. 2021;13(585):eabe3349. doi: 10.1126/scitranslmed.abe3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asokan M, Dias J, Liu C, Maximova A, Ernste K, Pegu A. Fc-mediated effector function contributes to the in vivo antiviral effect of an HIV neutralizing antibody. Proc Natl Acad Sci USA. 2020;117(31):18754–18763. doi: 10.1073/pnas.2008236117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang P, Gajjar MR, Yu J, Padte NN, Gettie A, Blanchard JL. Quantifying the contribution of Fc-mediated effector functions to the antiviral activity of anti-HIV-1 IgG1 antibodies in vivo. Proc Natl Acad Sci USA. 2020;117(30):18002–18009. doi: 10.1073/pnas.2008190117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marlin R, Nugeyre MT, Tchitchek N, Parenti M, Lefebvre C, Hocini H. Seminal plasma exposures strengthen vaccine responses in the female reproductive tract mucosae. Front Immunol. 2019;10:430. doi: 10.3389/fimmu.2019.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.