Abstract
Background
Individual case series and cohort studies have reported conflicting results in people with asthma on the vulnerability to and risk of mortality from coronavirus disease 2019 (COVID-19).
Research question
Are people with asthma at a higher risk of being infected or hospitalised or poorer clinical outcomes from COVID-19?
Methods
A systematic review and meta-analysis based on five main databases including the World Health Organization COVID-19 database between 1 December 2019 and 11 July 2021 on studies with a control (non-asthma) group was conducted. Prevalence and risk ratios were pooled using Sidik–Jonkman random-effects meta-analyses.
Findings
51 studies with an 8.08% (95% CI 6.87–9.30%) pooled prevalence of people with asthma among COVID-19 positive cases. The risk ratios were 0.83 (95% CI 0.73–0.95, p=0.01) for acquiring COVID-19; 1.18 (95% CI 0.98–1.42, p=0.08) for hospitalisation; 1.21 (95% CI 0.97–1.51, p=0.09) for intensive care unit (ICU) admission; 1.06 (95% CI 0.82–1.36, p=0.65) for ventilator use; and 0.94 (95% CI 0.76–1.17, p=0.58) for mortality for people with asthma. Subgroup analyses by continent revealed a significant difference in risk of acquiring COVID-19, ICU admission, ventilator use and death between the continents.
Interpretation
The risk of being infected with severe acute respiratory syndrome coronavirus 2 was reduced compared to the non-asthma group. No statistically significant differences in hospitalisation, ICU admission and ventilator use were found between groups. Subgroup analyses showed significant differences in outcomes from COVID-19 between America, Europe and Asia. Additional studies are required to confirm this risk profile, particularly in Africa and South America, where few studies originate.
Short abstract
The risk of being infected with SARS-CoV-2 was reduced in patients with asthma compared to the non-asthma group. No significant differences in hospitalisation, ICU admission, ventilator use and mortality were found between groups. https://bit.ly/3izKB9h
Introduction
Asthma is one of the most common chronic conditions with an estimated prevalence of >300 million people globally [1]. As coronavirus disease 2019 (COVID-19) continues to spread across the world with >4.05 million deaths as of 15 July 2021 [2], there are concerns that people with asthma are at a higher risk of acquiring the disease, or of poorer outcomes.
There are differing reports on the vulnerability of asthmatics to COVID-19 based on various local or national level case series and analyses [3]. Several meta-analyses have been conducted, but their conclusions suffer limitations from the inclusion of COVID-19 non-PCR-confirmed cases and inclusion of case series in their analyses which confer significant selection bias [4–6] (supplementary table S1). Most focus only on mortality, but not on other important considerations such as risk of being infected, hospitalised, admission to an intensive care unit (ICU) and importantly ventilator use when admitted [7–9].
A comprehensive understanding of COVID-19 risk among asthmatics globally is crucial as countries lift lockdown, and for prioritisation of vaccine allocation considering the limited supply of vaccines globally. Hence, we aimed to conduct a comprehensive systematic review and meta-analysis based only on controlled studies with reverse transcriptase (RT)-PCR-confirmed COVID-19 cases to ascertain the pooled prevalence and overall risk of infection, hospitalisation, ICU admission, ventilator use and mortality from COVID-19 among patients with asthma.
Methods
Search strategy and selection criteria
This systematic review and meta-analysis form part of a living systematic review on the risk of COVID-19 for people with asthma. Our first meta-analysis, which included studies up to 26 May 2020, has been published previously [5] and included pre-prints due to the early stage of the pandemic at that point. The protocol was prospectively registered and published in PROSPERO (www.crd.york.ac.uk/PROSPERO CRD42020222303) (appendix 1). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (www.prisma-statement.org) was used in reporting this study.
A comprehensive search of electronic databases including Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews, PubMed, MEDLINE and the World Health Organization COVID-19 database were conducted between 1 December 2019 and 11 July 2021. In addition, a hand search of references of relevant systematic reviews was conducted. In the case of missing information, we contacted the authors whenever possible. If the study identified patients with chronic respiratory conditions, we asked them to specify if this included asthma and requested these data.
We included all primary controlled studies reporting on adults with confirmed COVID-19 based on positive RT-PCR, with a pre-existing diagnosis of asthma, published in the English language. Asthma was defined according to definitions in the individual studies and included those sourced from medical records, physician-diagnosed and self-reported asthma. We excluded studies with ≤15 participants, pre-prints and those not published in English. The search strategy is available in appendix 2.
Data collection
Two reviewers (AS and SA) screened titles and abstracts and excluded irrelevant studies using Rayyan QCRI [10]. Full-text articles were subsequently reviewed independently, and disagreement resolved via consensus and referral to a third reviewer (CJ). Potential overlaps between studies were identified at full-text review to prevent double counting individual patients. A decision on inclusion was made by comparing the study country, location, setting (hospital/community), participant (adults/children), study period and sample size. Data extraction was conducted using a standard electronic form while quality assessment of included studies was performed using the Newcastle–Ottawa Scale [11]. Disagreements were resolved by discussion within the wider team (AS, SA, GL and CJ). No institutional review board approval was required as this study did not independently or prospectively collect patient data.
Outcomes
The outcomes were 1) the risk of acquiring COVID-19, expressed as the proportion of confirmed COVID-19 patients with a pre-existing diagnosis of asthma; 2) risk of hospitalisation from COVID-19 (proportion of confirmed COVID-19 patients hospitalised with asthma); 3) risk of being admitted to ICU (proportion of confirmed COVID-19 patients with asthma admitted to ICU); 4) risk of being ventilated (proportion of confirmed COVID-19 patients with asthma treated with mechanical ventilation once admitted to ICU); and 5) risk of death (proportion of confirmed COVID-19 patients with asthma who are dead or alive).
Data analysis
Descriptive statistics were utilised to summarise the details of the included studies in table 1. The Newcastle–Ottawa Scale [11] was used to assess the methodological quality of included studies based on the relevant study designs cohort or case–control. One star is allocated in the domains of selection and outcome or exposure and up to two stars are allocated to the comparability domain. A total of nine stars are allocated across all three domains. An overall score of 1–3 stars is categorised as low quality, 4–6 as medium quality and 7–9 as high quality.
TABLE 1.
Characteristics of included studies
| First author [reference] | Country | Setting | Design | Study period | COVID-19 positive | Age (years) | Male (n) | Current smokers (n) | COPD (n) | Diabetes (n) | Hypertension (n) | NOS score (out of 9) | ||
| Asthma (n) | Overall (n) | Mean | Median | |||||||||||
| Ahlström [ 17 ] | Sweden | Mixed | Case–control study | 6 March to 27 May 2020 | 133 | 1981 | 61 | 1465 | 75 | 522 | 982 | 9 | ||
| Almazeedi [ 41 ] | Kuwait | Hospital | Retrospective cohort study | 24 February to 20 April 2020 | 43 | 1096 | 41 | 888 | 44 | 5 | 155 | 177 | 9 | |
| Arslan [ 46 ] | Turkey | Hospital | Retrospective cohort study | 18 March to 15 May 2020 | 58 | 767 | 51.99 | 374 | 80 | 43 | 137 | 220 | 8 | |
| Ashinyo [ 47 ] | Ghana | Hospital | Retrospective cohort study | 23 March to 29 June 2020 | 24 | 307 | 37.9 | 174 | 20 | 219 | 7 | |||
| Aveyard [ 13 ] | Mexico | Hospital | Retrospective cohort study | 27 February to 21 June 2020 | 4942 | 178 306 | 44.1 | 88 083 | 8 | |||||
| Baumer [ 12 ] | UK | Hospital | Prospective cohort study | 9 March to 7 May 2020 | 12 | 52 | 54.82 | 29 | 8 | |||||
| Bergman [ 18 ] | Sweden | Mixed | Case–control study | To mid-September 2020 | 4493 | 68 575 | 46 | 26 808 | 2168 | 4897 | 16 416 | 9 | ||
| Beurnier [ 48 ] | France | Hospital | Prospective cohort study | 15 March to 15 April 2020 | 37 | 112 | 60 | 49 | 17 | 32 | 9 | |||
| Calmes [ 49 ] | Belgium | Hospital | Retrospective cohort study | 18 March to 17 April 2020 | 57 | 596 | 58.75# | 294 | 9 | |||||
| Castilla [ 19 ] | Spain | Mixed | Retrospective cohort study | July to December 2020 | 2330 | 35 387 | 38.8 | 17 172 | 6119 | 1404 | 1893 | 4543 | 9 | |
| Chhiba [ 50 ] | USA | Hospital | Retrospective cohort study | 1 March to 15 April 2020 | 220 | 1526 | 53.3# | 654 | 43 | 9 | ||||
| Choi [ 20 ] | South Korea | Mixed | Retrospective cohort study | To 15 May 2020 | 218 | 7372 | 44.5# | 3000 | 9 | |||||
| Dennis [ 30 ] | UK | Hospital | Retrospective cohort study | 1 March to 27 July 2020 | 1557 | 17 606 | 67 | 10 560 | 231 | 421 | 9 | |||
| Eggert [ 51 ] | USA | Hospital | Retrospective cohort study | 1 March to 30 September 2020 | 598 | 5596 | 38.4 | 2635 | 123 | 88 | 609 | 1021 | 9 | |
| Emami [ 52 ] | Iran | Hospital | Retrospective cohort study | 20 February to 1 March 2020 | 25 | 1239 | 51.48 | 692 | 27 | 176 | 7 | |||
| Ferastraoaru [ 53 ] | USA | Hospital | Retrospective cohort study | 14 March to 27 April 2020 | 951 | 4558 | 60.5 | 9 | ||||||
| Fong [ 54 ] | UK | Hospital | Retrospective cohort study | 1 March to 31 May 2020 | 102 | 617 | 65 | 9 | ||||||
| Garcia-Pachon [ 14 ] | Spain | Community | Retrospective cohort study | 3 March to 12 April 2020 | 10 | 376 | 54 | 192 | 8 | |||||
| Green [ 21 ] | Israel | Mixed | Retrospective cohort study | February to June 2020 | 153 | 2266 | 33.31 | 1200 | 102 | 200 | 276 | 9 | ||
| Guan [ 55 ] | China | Hospital | Retrospective cohort study | December 2019 to 6 May 2020 | 244 | 39 420 | 55.7 | 19 655 | 9 | |||||
| Gude-Sampedro [ 22 ] | Spain | Mixed | Retrospective cohort study | 6 March to 7 May 2020 | 288 | 10 454 | 58 | 4172 | 258 | 180 | 619 | 1457 | 9 | |
| Gupta [ 29 ] | USA | Hospital | Retrospective cohort study | To 4 March 2020 | 30 | 529 | 70 | 286 | 39 | 36 | 289 | 416 | 6 | |
| Hansen [ 23 ] | Denmark | Mixed | Retrospective cohort study | 1 February to 10 July 2020 | 354 | 5104 | 54.6 | 2399 | 432 | 598 | 9 | |||
| Ho [ 38 ] | USA | Hospital | Retrospective cohort study | 7 March to 7 June 2020 | 468 | 10 523 | 58.35 | 5707 | 286 | 1679 | 2662 | 9 | ||
| Je [ 56 ] | Australia | Hospital | Retrospective cohort study | March to April 2020 | 22 | 197 | 45 | 94 | 4 | 8 | 28 | 7 | ||
| Kim [ 57 ] | South Korea | Hospital | Case–control study | February to May 2020 | 66 | 2200 | 56.71 | 785 | 92 | 30 | 378 | 645 | 9 | |
| Kipourou [ 58 ] | Kuwait | Hospital | Prospective cohort study | 24 February to 27 May 2020 | 235 | 3995 | 40.4 | 2814 | 140 | 17 | 730 | 778 | 9 | |
| Lee [ 15 ] | South Korea | Community | Retrospective cohort study | January to 27 May 2020 | 686 | 7272 | 45.3 | 2927 | 1041 | 1401 | 9 | |||
| Lemus Calderon [ 59 ] | Spain | Hospital | Retrospective cohort study | To July 2020 | 577 | 6310 | 59 | 2983 | 873 | 1641 | 3239 | 9 | ||
| Liao [ 60 ] | USA | Hospital | Retrospective cohort study | 11 March to 23 June 2020 | 41 | 113 | 50 | 53 | 2 | 57 | 11 | 18 | 9 | |
| Lieberman-Cribbin [ 61 ] | USA | Hospital | Retrospective cohort study | 29 February to 24 April 2020 | 272 | 6245 | 57 | 3060 | 8 | |||||
| Lombardi [ 62 ] | Italy | Hospital | Retrospective cohort study | 20 February to 20 April 2020 | 20 | 1043 | 52.5# | 704 | 9 | |||||
| Louie [ 24 ] | Australia | Mixed | Case series | 19 March to 15 May 2020 | 10 | 99 | 54 | 51 | 2 | 8 | 14 | 8 | ||
| Lovinsky-Desir [ 63 ] | USA | Mixed | Prospective cohort study | 11 February to 7 May 2020 | 163 | 1298 | 52 | 762 | 55 | 9 | ||||
| Martos-Benítez [ 25 ] | Mexico | Mixed | Retrospective cohort study | 1 January to 12 May 2020 | 1188 | 38 324 | 46.9 | 22 362 | 3277 | 889 | 7168 | 8340 | 9 | |
| Mash [64] | South Africa | Hospital | Retrospective cohort study | March to June 2020 | 67 | 1376 | 46.3 | 571 | 95 | 50 | 364 | 564 | 8 | |
| Mather [65] | USA | Hospital | Case–control study | February to November 2020 | 88 | 1045 | 64.6 | 352 | 18 | 221 | 307 | 8 | ||
| Murillo-Zamora [66] | Mexico | Hospital | Retrospective cohort study | 4 March to 15 August 2020 | 1448 | 66 123 | 52.4 | 40 124 | 2619 | 21 840 | 26 728 | 9 | ||
| Nystad [26] | Norway | Mixed | Retrospective cohort study | 1 March to 13 May 2020 | 515 | 7632 | 33.22# | 161 | 468 | 977 | 7 | |||
| Patone [27] | UK | Mixed | Retrospective cohort study | 1 November to 26 January 2021 | 29 792 | 198 420 | 37.7 | 93 765 | 22 134 | 1873 | 10 347 | 19 636 | 9 | |
| Robinson [67] | USA | Hospital | Case–control study | 4 March to 2 July 2020 | 562 | 3248 | 51 | 911 | 131 | 321 | 107 | 8 | ||
| Rosenthal [68] | USA | Hospital | Retrospective cohort study | March to May 2020 | 105 | 727 | 49.46 | 165 | 278 | 8 | ||||
| Salacup [69] | USA | Hospital | Retrospective cohort study | 1 March to 24 April 2020 | 18 | 242 | 66 | 123 | 30 | 118 | 180 | 8 | ||
| Schönfeld [28] | Argentina | Mixed | Retrospective cohort study | 3 March to 2 October 2020 | 12 580 | 207 079 | 41 | 103 487 | 4074 | 4405 | 20 058 | 39 833 | 9 | |
| Shah [70] | USA | Hospital | Retrospective cohort study | 3 February to 31 March 2020 | 4 | 33 | 63 | 22 | 0 | 1 | 9 | 16 | 8 | |
| Tutiya [71] | Brazil | Hospital | Retrospective cohort study | 13 March to 7 June 2020 | 7 | 114 | 32.4 | 0 | 12 | 13 | 7 | |||
| Valverde-Monge [72] | Spain | Hospital | Retrospective cohort study | 31 January to 17 April 2020 | 113 | 2539 | 62.66 | 1275 | 154 | 89 | 403 | 1054 | 9 | |
| Wang [40] | China | Hospital | Retrospective cohort study | 28 January to 25 February 2020 | 68 | 562 | 47 | 265 | 8 | |||||
| Yang [37] | South Korea | Community | Retrospective cohort study | 1 January to 15 May 2020 | 725 | 7340 | 47.1 | 2970 | 350 | 951 | 1638 | 9 | ||
| Yordanov [16] | France | Mixed | Prospective cohort study | March to August 2020 | 814 | 7320 | 43 | 2301 | 790 | 87 | 402 | 978 | 6 | |
| Zhang [31] | China | Hospital | Retrospective cohort study | 29 December 2019 to 16 February 2020 | 1 | 290 | 57 | 155 | 10 | 6 | 27 | 81 | 8 | |
COVID-19: coronavirus disease 2019; NOS: Newcastle–Ottawa Scale. #: imputed values based on weighted average.
Two main sets of meta analyses were performed. To pool the prevalence of asthmatics among those with COVID-19, we used the binomial distribution to model the within-study variability and calculated Wilson score 95% confidence intervals.
For all the binary outcomes, we performed Sidik–Jonkman random-effects meta-analysis (assuming that there is not only one true effect size, but a distribution of true effect sizes). We assessed the quantitative heterogeneity by conducting a formal test of homogeneity and evaluating the proportion of variability due to heterogeneity (I2). Pre-specified subgroup analyses were conducted by continent and by the quality of the studies (low, medium, high) and univariable meta-regressions using age and proportions of current and former smokers as covariates.
The assessment of small-study effects has been done by regression-based Egger test and eyeball evaluation of the contour-enhanced funnel plots.
Along with the pooled effect sizes and 95% confidence intervals, we also reported the prediction intervals. All pooled results are presented in the form of forest plots. All statistical analyses were performed using Stata 16 (StataCorp LLC, College Station, TX, USA).
Results
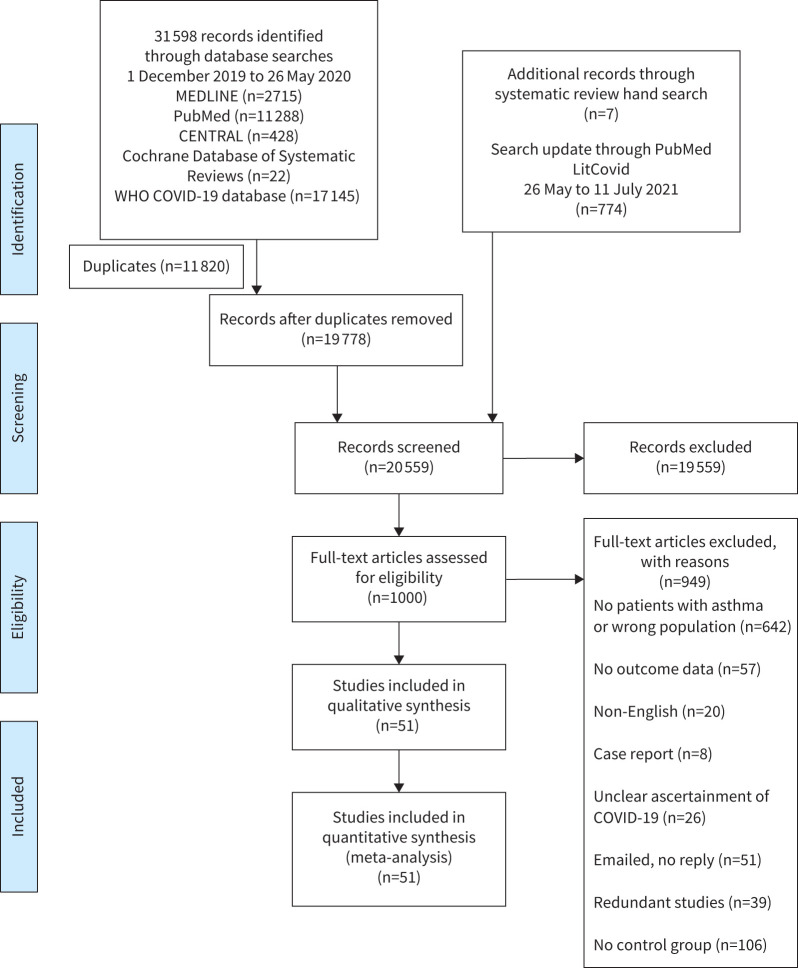
The searches resulted in 32 379 citations. After duplicates were removed, 20 559 titles and abstracts were screened, 19 559 articles were excluded. Of the remaining 1000 articles, 949 were excluded after full-text review. A total of 51 studies were included in the review. Studies with overlapping patient populations were excluded if they reported the same outcome (figure 1).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram [73]. CENTRAL: Cochrane Central Register of Controlled Trials; WHO: World Health Organization; COVID-19: coronavirus disease 2019.
Descriptive characteristics
This review is based on a pooled sample of 1 471 643 COVID-19-tested patients, of whom 965 551 were COVID-19 positive with reported information related to asthma. The sample sizes ranged from 52 [12] to 417 366 [13]. Most of the studies were hospital-based (34 studies) while three were studies [14–16] in the community and 14 had a mixed setting [16–28]. Studies originate from 21 countries spread on all five continents: Europe (n=17), North America (n=13), Asia (n=12), South America (n=5), Africa (n=2) and Australia (n=2). The summary of included studies is presented in table 1.
Among COVID-19 positive patients, based on RT-PCR assay results, the mean±sd age of participants was 52.0±12.9 years, 42.64% were male (n=459 640 from 47 out of 51 studies), 5.4% were current smokers (n=38 672 from 23 out of 51 studies) and 9.8% were former smokers (n=43 622 from 10 out of 51 studies). The prevalence of asthma among those infected with COVID-19 was 8.08% (95% CI 6.87–9.30%; test of homogeneity p<0.001). ∼25% had hypertension (n=135 274 from 35 out of 51 studies), 14.3% had diabetes (n=78 923 from 38 out of 51 studies) and 3% had COPD (n=15 636 from 29 out of 51 studies).
Risk-of-bias results
Scores on the Newcastle–Ottawa Scale ranged between 6 and 9 (maximum 9) [16, 29], with a higher score indicating a higher quality. All studies scored ≥7 and were of high quality. A full assessment is presented in supplementary table S3.
Meta-analysis of the risk of acquiring COVID-19
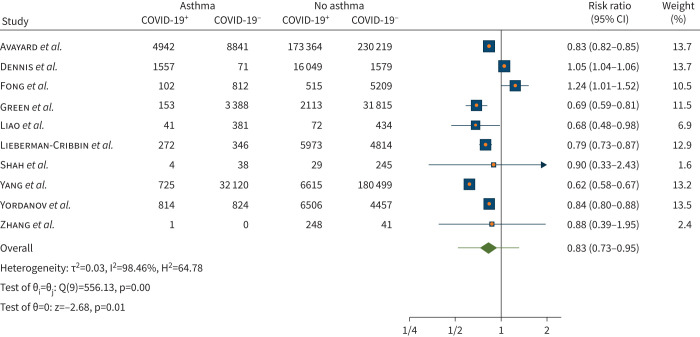
The pooled analysis of 10 studies (n=785 151) showed a risk ratio reduction in acquiring COVID-19 of 17% for people with asthma compared to those without asthma (risk ratio 0.83, 95% CI 0.73–0.95; p=0.01; figure 2). There was considerable heterogeneity (I2=98.46%) across the studies. Meta-regression by age revealed that older age was associated with increased risk of acquiring COVID-19 in individuals with asthma (meta-regression coefficient 0.014, 95% CI 0.004–0.025; p=0.006). Furthermore, R2 showed that 45.51% of the variance between studies can be explained by age. Heterogeneity remains high when age is included as a moderator in the meta-regression (I2=92.03%) meaning that it is not a main factor in the difference between studies. No statistically significant association for current smoker (five out of 10 studies; p=0.09) and former smoker were found (two out of 10 studies; p=0.94).
FIGURE 2.
Risk of acquiring coronavirus disease 2019 (COVID-19) in individuals with asthma compared with no asthma.
Meta-analysis of the risk of hospitalisation
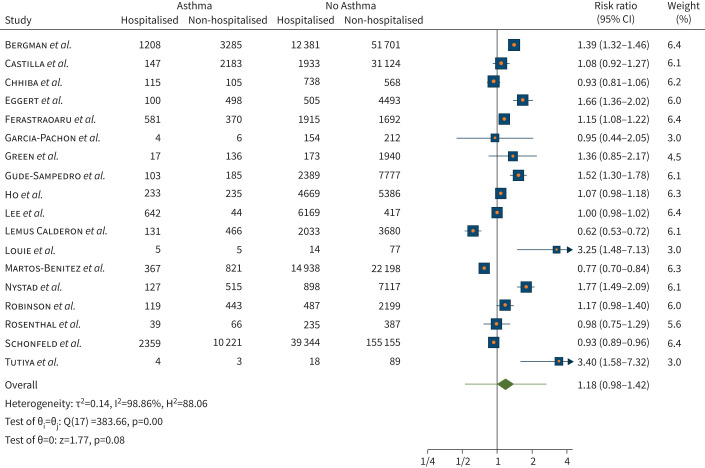
We observed a non-statistically significant different risk for hospitalisation from COVID-19 for people with asthma compared to no asthma (risk ratio 1.18, 95% CI 0.98–1.42; p=0.08), in the 18 studies (n=411 093) included in this analysis. There was considerable heterogeneity observed (I2=98.86%) across the studies (figure 3). Meta-regression by age, current smoker (only from nine out of 18 studies) and former smoker (only from six out of 18 studies) revealed no relevant association in risk of being hospitalised with COVID-19 in individuals with asthma.
FIGURE 3.
Risk of hospitalisation when infected with coronavirus disease 2019 in individuals with asthma compared with no asthma.
Meta-analysis of the risk of ICU admission
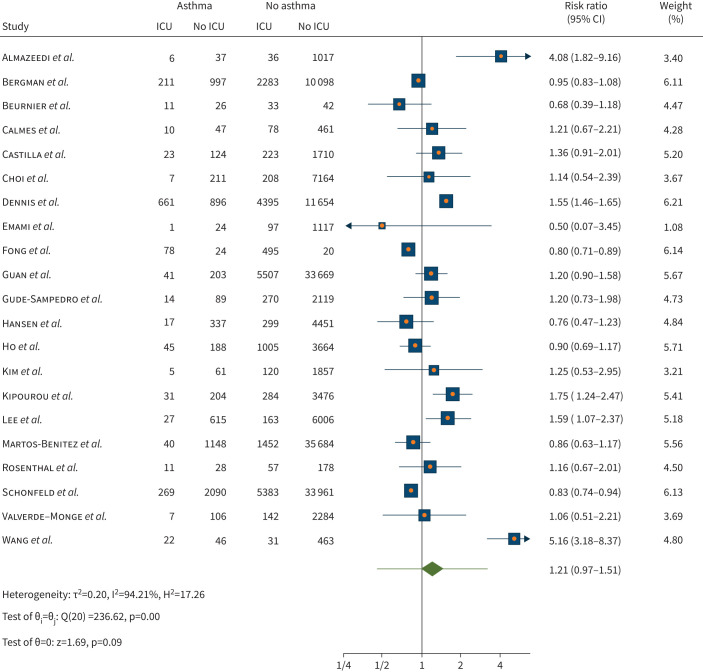
There was a non-statistically significant different risk of ICU admission (risk ratio 1.21, 95% CI 0.97–1.51; p=0.09) for people with asthma compared to those without asthma in a pooled analysis of 21 studies (n=192 694). Substantial heterogeneity was observed (I2=94.21%) across the studies (figure 4). Meta-regression with former smoker (four out of 21 studies) as moderator found a statistically significant decrease in risk of ICU admission (meta-regression coefficient −0.00009, 95% CI −0.0002– −2.65×106; p=0.043). Meta-regression with age and current smoker (nine out of 21 studies) as moderator did not reveal statistically significant results (p=0.15 and p=0.37, respectively).
FIGURE 4.
Risk of intensive care unit (ICU) admission when infected with coronavirus disease 2019 in individuals with asthma compared with no asthma.
Meta-analysis of the risk of ventilator use when admitted into the ICU
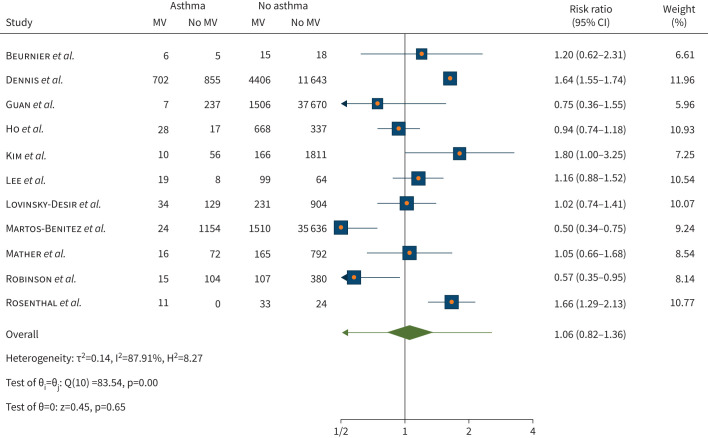
In relation to probability of mechanical ventilation, of the 11 studies (n=101 694) pooled for this analysis, there was no statistically significant difference in risk of being treated with ventilator once admitted to ICU for people with asthma compared to those without asthma (risk ratio 1.06, 95% 0.82–1.36; p=0.65). Considerable heterogeneity was observed (I2=87.91%) across the studies (figure 5). Meta-regression with age and current smoker (four out of 11 studies) did not reveal statistically significant results (p=0.276 and p=0.260, respectively). Whereas meta-regression with former smoker as a moderator (two out of 11 studies) found a reduction in risk of ventilator use (meta-regression coefficient −0.0022, 95% CI −0.0037– −0.0007; p=0.004).
FIGURE 5.
Risk of mechanical ventilator (MV) use upon admission to intensive care unit with coronavirus disease 2019 in individuals with asthma compared with no asthma.
Meta-analysis of the risk of death
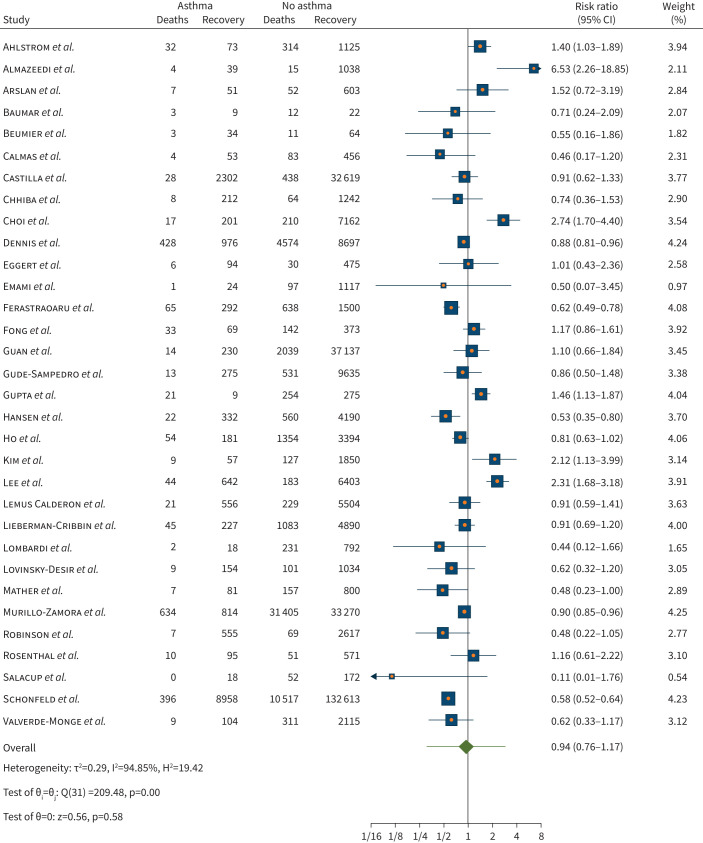
There was a non-statistically significant different risk of death from COVID-19 for people with asthma compared to those without asthma in the 32 studies (n=379 381) pooled for this analysis (risk ratio 0.94, 95% CI 0.76–1.17; p= 0.58). Considerable heterogeneity was observed (I2=94.85%) across the studies (figure 6). When age was included as moderator for meta-regression, there was no statistically significant reduction in risk of death by age (p=0.219). No statistically significant association was also found for current smoker (14 out of 21 studies) and former smoker (seven out of 21 studies) as a moderator (p=0.458 and p=0.288, respectively).
FIGURE 6.
Risk of death when infected with coronavirus disease 2019 in individuals with asthma compared with no asthma.
Subgroup analyses
Subgroup analyses by continent revealed substantial differences in risk of acquiring COVID-19 between the continents (statistically significant at p=0.001) during the period up to 11 July 2021. It showed the lowest risk in Asia (risk ratio 0.66, 95% CI 0.57–0.75) followed by North America (risk ratio 0.78, 95% CI 0.69–0.89), South America (risk ratio 0.84, 95% CI 0.82–0.85) and Europe (risk ratio 1.01, 95% CI 0.82–1.26). No major differences were found between continents in hospitalisation (p=0.128). However, relevant differences in ICU admission were found between continents (statistically significant at p=0.007). The highest risk was found to be in Asia (risk ratio 1.81, 95% CI 1.12–2.91) followed by Europe (risk ratio 1.04, 95% CI 0.86–1.27), North America (risk ratio 0.96, 95% CI 0.72–1.27) and lowest in South America (risk ratio 0.84, 95% CI 0.75–0.93).
In addition, risk of ventilator use was statistically significant different across the continents (p<0.001). The highest risk was found to be in Europe (risk ratio 1.59, 95% CI 1.26–2.00), followed by Asia (risk ratio 1.19, 95% CI 0.74–1.91), North America (risk ratio 1.02, 95% CI 0.74–1.42) and South America (risk ratio 0.50, 95% CI 0.82–1.36). Similarly, risk of death was quite different across the continents (p=0.011). The highest risk was found to be in Asia (risk ratio 2.01, 95% CI 1.19–3.39), followed by Europe (risk ratio 0.85, 95% CI 0.68–1.05), North America (risk ratio 0.79, 95% CI 0.58–1.06) and South America (risk ratio 0.72, 95% CI 0.47–1.12).
Subgroup analyses by study quality for risk for death showed significantly higher risk in the one study of medium quality compared to the 30 higher-quality ones (risk ratio 1.45, 95% CI 1.14–1.87 versus risk ratio 0.92, 95% CI 0.74–1.15; p=0.007).
Publication bias
Egger's test showed evidence of small-study effects for the pooled proportion of COVID-19-positive (RT-PCR) individuals (p<0.0001) and risk of hospitalisation (p=0.0199), but not for all other outcomes (supplementary table S3). Eyeball assessment of the contour enhanced funnel plots revealed asymmetry only for the risk of hospitalisation, but not other outcomes (supplementary figures S1–S6).
Discussion
This meta-analysis aimed to rigorously assess the vulnerability of patients with asthma to COVID-19 based on controlled studies. It revealed an 8.08% prevalence of asthma among those who tested COVID-19 positive based on RT-PCR. This pooled prevalence is higher than the 7.46% prevalence in our previous meta-analysis [30] which analysed studies including pre-prints until May 2020. Only one study [31] from the previous meta-analysis was included in this meta-analysis. This is due to the tighter inclusion criteria of including only published studies with a non-asthma control group, and excluding case series and single-arm cohort studies. Furthermore, both these prevalence rates were lower than the global prevalence of self-reported asthma symptoms of 8.6% [32].
While the proportion estimated in this meta-analysis is lower than in two recent studies in the UK [27] which reported a prevalence of ∼15% in those infected with the B.1.1.7 variant; lower prevalence rates have been reported in other studies in Italy [33] and in Turkey [34] (2.1% among 2000 patients and 3.7% among 565 patients, respectively).
In the studies that report them, we found a high pooled proportion of hypertension (25.7%) and diabetes (14.3%) as comorbidities. These were mostly contributed by hospital studies (22 of the 35 studies reporting hypertension and 24 of the 38 studies reporting diabetes).
This study found a statistically significant risk reduction of 17% (95% CI 5–27%) for acquiring COVID-19, similar to the 14% reduction reported in our previous study [5]. This result is similar to a study from Missouri, USA which reported lower COVID-19 test positivity rates in asthmatics versus non-asthmatics (69.2% versus 81.9%) [35]. Furthermore, a community study in Mexico showed a lower proportion of asthmatics in a COVID-19 positive group compared to a negative group (2.8% versus 3.7%; OR 0.74, 95% CI 0.71–0.77) [21, 36].
Subgroup analyses by continent revealed significant differences in risk of acquiring COVID-19 between the continents, the lowest risk being in Asia (risk ratio 0.66, 95% CI 0.57–0.75) followed by North America (risk ratio 0.78, 95% CI 0.69–0.89), South America (risk ratio 0.84, 95% CI 0.82–0.85) and Europe (risk ratio 1.01, 95% CI 0.82–1.26). Additionally, we noted the consistent nature of the risk reduction in three out of the four regions where data are available. The risk reduction in Asia was found to be consistent in the three studies pooled from China [31], Israel [21] and South Korea [37]; all countries with a high testing regime which might account for this variance between regions. However, analysis of community studies such as this could better reflect the true nature of the risk compared to analysis of hospital-based studies. In addition, it is important to note that this result may not reflect other countries in Asia such as India and Southeast Asia where testing regimes have not been as extensive.
Several possible mechanisms might contribute to a lower risk of acquiring COVID-19 in people with asthma compared to a non-asthmatic population. A retrospective study by Ho et al. [38] showed that not only is asthma associated with lower risk of poor outcomes, but the presence of eosinophilia (≥200 cells·μL−1) both in those with and without asthma was also reported to be associated with reduced mortality risk. While not statistically significant, a higher proportion of those with asthma in this study had eosinophilia compared to non-asthmatics (38.2% versus 32.3%) [38].
Furthermore, a lower risk of acquiring COVID-19 may be attributed to the expression of the angiotensin-converting enzyme (ACE)2 receptor, which is significantly lower in asthma patients compared to those with COPD and healthy controls, as reported in another study [6] which showed that ACE2 expression is increased with older age (at p=0.03). This supports the result of our analysis, which showed strong evidence of increasing age being associated with increased risk of acquiring COVID-19. Finally, people with asthma have been advised by health authorities to practise social distancing and be particularly careful to avoid contracting COVID-19. This was especially the case early in the pandemic when the added risks of having an underlying lung condition were assumed to be substantial. To the extent that these messages [39] were taken seriously by people with asthma, their risk of acquiring infection could have been commensurately reduced.
We also found similar risks for hospitalisation, ICU admission when hospitalised and ventilator use in this study. Even so, we note that for hospitalisation, while not statistically significant, the pooled point estimate suggests a possible 18% increased risk of hospitalisation from COVID-19 for people with asthma, with a wide confidence interval (95% CI −2–42%).
Similarly, for ICU admission, while not statistically significant, the pooled point estimate suggests a possible 21% increased risk of ICU admission from COVID-19 for people with asthma (95% CI −3–51%). One study from China [40] and another from Kuwait [41] reported risk ratios of 5.16 and 4.08, respectively, far greater than in other studies. These differences in risk may be linked to resource allocation and availability or difference in vulnerability due to ethnicity or other environmental factors.
An important finding of this current study and our previous meta-analysis is the similar risk of death between asthmatics and non-asthmatics from COVID-19. While this may be due to a variety of factors, two recent randomised controlled trials of budesonide (an inhaled corticosteroid frequently prescribed to patients with asthma) [42, 43] have raised the possibility that this is an effect of the inhaled corticosteroid. They reported that early administration of inhaled budesonide reduced the likelihood of urgent medical care and reduced time to recovery from COVID-19. One of these studies, the STOIC open-label trial in 146 participants showed a number needed to treat of eight with budesonide to reduce COVID-19 deterioration, and that clinical recovery occurred a day faster in the budesonide group compared to usual care (7 days, 95% CI 6–9 days versus 8 days, 7–11 days; log-rank test p=0.007) [42]. The other study is an interim analysis of the PRINCIPLE trial published as a pre-print, which randomised 751 participants to budesonide compared with 1028 usual care and 643 on other interventions showed a faster recovery in the budesonide group compared to usual care (hazard ratio 1.208, 95% Bayesian credible interval (BCI) 1.076–1.356; probability of superiority 0.999, estimated benefit of 3.011 days, 95% BCI 1.134–5.41 days) [43].
A limitation of this study is the inclusion of very few studies originating from Africa and South America. Additionally, most of the studies were hospital-based, which is likely to be a consequence of including only COVID-19 cases confirmed by RT-PCR. We chose RT-PCR positivity to give more certainty to our estimation of the association between asthma and several important COVID-19 outcomes. As PCR testing regimens show substantial variation between countries, our results might not be generalisable to regions which are poorer and marginalised or to groups that might be less likely to seek testing. In these regions, it is likely that due to under-testing the true proportion of asthmatics as well as the general public with COVID-19 is substantially higher than official reports, by a magnitude of multiple folds [44, 45].
Potential selection bias to those more unwell may also be present due to the large number of hospital-based studies included in this review. Even so, 10 of the studies found to calculate the risk of getting the infection were community based (n=726 269), which we hope provides a better representation of risk for the general community.
There was minimal information provided on smoking (only 23 out of 51 studies indicated the proportion of current smokers, and 10 out of 51 indicated the proportion of former smokers). Hence, based on the 10 studies, we found that being a former smoker was associated with a lower risk of ICU admission; however, this minimal information limits the generalisability of our assessment of the impact of smoking. Despite these limitations, the majority of studies we included were of high quality with minimal selection bias due to their large sample sizes, data sourcing through electronic health records or data linkages which resulted in minimal loss to follow-up. Additionally, we used hard outcome measures such as COVID-19 infection (PCR positivity), hospitalisation, ICU admission and death, which are generally well-defined globally, limiting the risk of classification bias. Funnel plots and Egger's test for small-study effects were also conducted to explore the presence of publication bias and we found that most outcomes do not show signs of publication bias.
Furthermore, our conclusions are based on studies which report details of both asthma and non-asthma patients where COVID-19 infection status was confirmed by RT-PCR results and not only by symptoms or suspected cases in the context of the pandemic. We did not have access to information that would enable us to determine if people with asthma were over-represented among mild or asymptomatic cases that did not receive testing.
In conclusion, the findings from this analysis indicate the prevalence of asthma was 8.08% among people who were RT-PCR-positive for COVID-19 in these controlled studies. The overall findings suggest that people with asthma are at lower risk of being infected with COVID-19 compared to those without asthma, but have a similar risk of hospitalisation, ICU admission, ventilator use and mortality when RT-PCR-positive. With the fast evolution of the severe acute respiratory syndrome coronavirus 2 virus and the emergence of variants globally, caution must be maintained for people with asthma. There remains a need for higher-quality community studies as well as regular risk assessments and review of new data throughout the pandemic. Furthermore, additional studies are required to confirm this risk profile, particularly in Africa and South America, where none of the eligible studies originated.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary tables and figures ERJ-01209-2021.Supplement (662.1KB, pdf)
Appendix 1 ERJ-01209-2021.Appendix_1 (1.9MB, pdf)
Appendix 2 ERJ-01209-2021.Appendix_2 (38KB, pdf)
Shareable PDF
Acknowledgements
This study was self-funded. A.P. Sunjaya is supported by a Scientia PhD scholarship from UNSW Sydney. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
This article has supplementary material available from erj.ersjournals.com
Author contributions: A.P. Sunjaya and S.M. Allida completed the search of the literature, title/abstract screening and inclusion/exclusion review, data extraction and quality assessment and contributed to writing the review. G.L. Di Tanna performed the statistical analysis and contributed to writing the review. C.R. Jenkins initiated this review, engaged co-authors, was third independent reviewer, and oversaw the study from inception to completion. She contributed to writing and reviewing all draft manuscripts.
Conflict of interest: A.P. Sunjaya has nothing to disclose.
Conflict of interest: S.M. Allida has nothing to disclose.
Conflict of interest: G.L. Di Tanna has nothing to disclose.
Conflict of interest: C.R. Jenkins has nothing to disclose.
References
- 1.Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr 2019; 7: 246. doi: 10.3389/fped.2019.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . WHO Coronavirus (COVID-19) Dashboard. 2021. https://covid19.who.int/
- 3.Eger K, Bel EH. Asthma and COVID-19: do we finally have answers? Eur Respir J 2021; 57: 2004451. doi: 10.1183/13993003.04451-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S, Cao Y, Du T, et al. Prevalence of comorbid asthma and related outcomes in COVID-19: a systematic review and meta-analysis. J Allergy Clin Immunol Pract 2021; 9: 693–701. doi: 10.1016/j.jaip.2020.11.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sunjaya AP, Allida SM, Di Tanna GL et al.. Asthma and risk of infection, hospitalisation, ICU admission and mortality from COVID-19: systematic review and meta-analysis. J Asthma 2021; in press [doi: 10.1080/02770903.2021.1888116]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wark PAB, Pathinayake PS, Kaiko G, et al. ACE2 expression is elevated in airway epithelial cells from older and male healthy individuals but reduced in asthma. Respirology 2021; 26: 442–451. doi: 10.1111/resp.14003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Ao G, Qi X, et al. The relationship between severe or dead COVID-19 and asthma: a meta-analysis. Clin Exp Allergy 2021; 51: 354–359. doi: 10.1111/cea.13773 [DOI] [PubMed] [Google Scholar]
- 8.Shi L, Xu J, Xiao W, et al. Asthma in patients with coronavirus disease 2019: a systematic review and meta-analysis. Ann Allergy Asthma Immunol 2021; 126: 524–534. doi: 10.1016/j.anai.2021.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendes NF, Jara CP, Mansour E, et al. Asthma and COVID-19: a systematic review. Allergy Asthma Clin Immunol 2021; 17: 5. doi: 10.1186/s13223-020-00509-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan – a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. 2020. www.ohri.ca/programs/clinical_epidemiology/oxford.asp/ Date last accessed: 9 June 2020.
- 12.Baumer T, Phillips E, Dhadda A, et al. Epidemiology of the first wave of COVID-19 ICU admissions in South Wales – the interplay between ethnicity and deprivation. Front Med 2020; 7: 569714. doi: 10.3389/fmed.2020.569714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aveyard P, Gao M, Lindson N, et al. Association between pre-existing respiratory disease and its treatment, and severe COVID-19: a population cohort study. Lancet Respir Med 2021; 9: 909–923. doi: 10.1016/S2213-2600(21)00095-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Pachon E, Zamora-Molina L, Soler-Sempere MJ, et al. Asthma prevalence in patients with SARS-CoV-2 infection detected by RT-PCR not requiring hospitalization. Respir Med 2020; 171: 106084. doi: 10.1016/j.rmed.2020.106084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SC, Son KJ, Han CH, et al. Impact of comorbid asthma on severity of coronavirus disease (COVID-19). Sci Rep 2020; 10: 21805. doi: 10.1038/s41598-020-77791-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yordanov Y, Dinh A, Bleibtreu A, et al. Clinical characteristics and factors associated with hospital admission or death in 43 103 adult outpatients with coronavirus disease 2019 managed with the Covidom telesurveillance solution: a prospective cohort study. Clin Microbiol Infect 2021; 27; 1158–1166. doi: 10.1016/j.cmi.2021.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahlström B, Frithiof R, Hultström M, et al. The Swedish COVID-19 intensive care cohort: risk factors of ICU admission and ICU mortality. Acta Anaesthesiol Scand 2021; 65: 525–533. doi: 10.1111/aas.13781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergman J, Ballin M, Nordström A, et al. Risk factors for COVID-19 diagnosis, hospitalization, and subsequent all-cause mortality in Sweden: a nationwide study. Eur J Epidemiol 2021; 36: 287–298. doi: 10.1007/s10654-021-00732-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castilla J, Guevara M, Miqueleiz A, et al. Risk factors of infection, hospitalization and death from SARS-CoV-2: a population-based cohort study. J Clin Med 2021; 10: 2608. doi: 10.3390/jcm10122608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi YJ, Park J-Y, Lee HS, et al. Effect of asthma and asthma medication on the prognosis of patients with COVID-19. Eur Respir J 2021; 57: 2002226. doi: 10.1183/13993003.02226-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green I, Merzon E, Vinker S, et al. COVID-19 susceptibility in bronchial asthma. J Allergy Clin Immunol Pract 2021; 9: 684–692. doi: 10.1016/j.jaip.2020.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gude-Sampedro F, Fernández-Merino C, Ferreiro L, et al. Development and validation of a prognostic model based on comorbidities to predict COVID-19 severity: a population-based study. Int J Epidemiol 2021; 50: 64–74. doi: 10.1093/ije/dyaa209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen ESH, Moeller AL, Backer V, et al. Severe outcomes of COVID-19 among patients with COPD and asthma. ERJ Open Res 2021; 7: 00594-2020. doi: 10.1183/23120541.00594-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louie T, Kwan B, Susanto C, et al. Respiratory failure, clinical course and community management of COVID-19 patients in a large Australian cohort. Intern Med J 2021; 51: 334–340. doi: 10.1111/imj.15206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martos-Benítez FD, Soler-Morejón CD, García-Del Barco D. Chronic comorbidities and clinical outcomes in patients with and without COVID-19: a large population-based study using national administrative healthcare open data of Mexico. Intern Emerg Med 2021; 16: 1507–1517. doi: 10.1007/s11739-020-02597-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nystad W, Hjellvik V, Larsen IK, et al. Underlying conditions in adults with COVID-19. Tidsskr Nor Laegeforen 2020; 140: doi:10.4045/tidsskr.20.0512. doi: 10.4045/tidsskr.20.0512 [DOI] [PubMed] [Google Scholar]
- 27.Patone M, Thomas K, Hatch R, et al. Mortality and critical care unit admission associated with the SARS-CoV-2 lineage B.1.1.7 in England: an observational cohort study. Lancet Infect Dis 2021; 21: 1518–1528. doi: 10.1016/S1473-3099(21)00318-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schönfeld D, Arias S, Bossio JC, et al. Clinical presentation and outcomes of the first patients with COVID-19 in Argentina: results of 207079 cases from a national database. PLoS One 2021; 16: e0246793. doi: 10.1371/journal.pone.0246793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta R, Agrawal R, Bukhari Z, et al. Higher comorbidities and early death in hospitalized African-American patients with Covid-19. BMC Infect Dis 2021; 21: 78. doi: 10.1186/s12879-021-05782-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dennis JM, Mateen BA, Sonabend R, et al. Type 2 diabetes and COVID-19-related mortality in the critical care setting: a national cohort study in England, March–July 2020. Diabetes Care 2021; 44: 50–57. doi: 10.2337/dc20-1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang JJ, Cao YY, Dong X, et al. Distinct characteristics of COVID-19 patients with initial rRT-PCR-positive and rRT-PCR-negative results for SARS-CoV-2. Allergy 2020; 75: 1809–1812. doi: 10.1111/all.14316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Global Asthma Network . The Global Asthma Report, 2018. Available from: www.globalasthmanetwork.org
- 33.Caminati M, Vultaggio A, Matucci A, et al. Asthma in a large COVID-19 cohort: prevalence, features, and determinants of COVID-19 disease severity. Respir Med 2021; 176: 106261. doi: 10.1016/j.rmed.2020.106261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caliskan T, Saylan B. Smoking and comorbidities are associated with COVID-19 severity and mortality in 565 patients treated in Turkey: a retrospective observational study. Rev Assoc Med Bras 2020; 66: 1679–1684. doi: 10.1590/1806-9282.66.12.1679 [DOI] [PubMed] [Google Scholar]
- 35.Cao L, Lee S, Krings JG, et al. Asthma in patients with suspected and diagnosed coronavirus disease 2019. Annal Allergy Asthma Immunol 2021; 126: 535–541. doi: 10.1016/j.anai.2021.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bedolla-Barajas M, Morales-Romero J, Bedolla-Pulido TR, et al. Low prevalence of asthma in Mexican children and adults with a positive rtRT-PCR test for SARS-CoV-2: a cross-sectional study during the 2020 pandemic. Allergol Immunopathol 2021; 49: 1–7. doi: 10.15586/aei.v49i3.7 [DOI] [PubMed] [Google Scholar]
- 37.Yang JM, Koh HY, Moon SY, et al. Allergic disorders and susceptibility to and severity of COVID-19: a nationwide cohort study. J Allergy Clin Immunol 2020; 146: 790–798. doi: 10.1016/j.jaci.2020.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho KS, Howell D, Rogers L, et al. The relationship between asthma, eosinophilia, and outcomes in coronavirus disease 2019 infection. Ann Allergy Asthma Immunol 2021; 127: 42–48. doi: 10.1016/j.anai.2021.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.British Thoracic Society . COVID-19: Identifying Patients for Shielding. 2021. www.brit-thoracic.org.uk/covid-19/covid-19-identifying-patients-for-shielding/ Date last updated: 1 April 2021.
- 40.Wang J, Guo S, Zhang Y, et al. Clinical features and risk factors for severe inpatients with COVID-19: a retrospective study in China. PLoS One 2020; 15: e0244125. doi: 10.1371/journal.pone.0244125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Almazeedi S, Al-Youha S, Jamal MH, et al. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine 2020; 24: 100448. doi: 10.1016/j.eclinm.2020.100448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramakrishnan S, Nicolau DV, Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med 2021; 9: 763–772. doi: 10.1016/S2213-2600(21)00160-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu L-M, Bafadhel M, Dorward J, et al. Inhaled budesonide for COVID-19 in people at higher risk of adverse outcomes in the community: interim analyses from the PRINCIPLE trial. medRxiv 2021; preprint [ 10.1101/2021.04.10.21254672]. [DOI] [Google Scholar]
- 44.Murhekar MV, Bhatnagar T, Selvaraju S, et al. SARS-CoV-2 antibody seroprevalence in India, August–September, 2020: findings from the second nationwide household serosurvey. Lancet Glob Health 2021; 9: e257-e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nkuba AN, Makiala SM, Guichet E, et al. High prevalence of anti-SARS-CoV-2 antibodies after the first wave of COVID-19 in Kinshasa, Democratic Republic of the Congo: results of a cross-sectional household-based survey. Clin Infect Dis 2021; in press [ 10.1093/cid/ciab515]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arslan Y, Dogan D, Ocal N, et al. The boundaries between survival and non-survival at COVID-19: experience of tertiary care pandemic hospital. Int J Clin Pract 2021; 9: e257-e266. doi: 10.1111/ijcp.14461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashinyo ME, Duti V, Dubik SD, et al. Clinical characteristics, treatment regimen and duration of hospitalization among COVID-19 patients in Ghana: a retrospective cohort study. Pan Afr Med J 2020; 37: 9. doi: 10.11604/pamj.supp.2020.37.1.25718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beurnier A, Jutant EM, Jevnikar M, et al. Characteristics and outcomes of asthmatic patients with COVID-19 pneumonia who require hospitalisation. Eur Respir J 2020; 56: 2001875. doi: 10.1183/13993003.01875-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calmes D, Graff S, Maes N, et al. Asthma and COPD are not risk factors for ICU stay and death in case of SARS-CoV2 infection. J Allergy Clin Immunol Pract 2021; 9: 160–169. doi: 10.1016/j.jaip.2020.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chhiba KD, Patel GB, Vu THT, et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol 2020; 146: 307–314. doi: 10.1016/j.jaci.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eggert L, He Z, Collins W, et al. Asthma phenotypes, associated comorbidities, and long-term symptoms in COVID-19. Allergy 2022; 77: 173–185. doi: 10.22541/au.161661725.56823358/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emami A, Javanmardi F, Akbari A, et al. Survival rate in hypertensive patients with COVID-19. Clin Exp Hypertens 2021; 43: 77–80. doi: 10.1080/10641963.2020.1812624 [DOI] [PubMed] [Google Scholar]
- 53.Ferastraoaru D, Hudes G, Jerschow E, et al. Eosinophilia in asthma patients is protective against severe COVID-19 illness. J Allergy Clin Immunol Pract 2021; 9: 1152–1162. doi: 10.1016/j.jaip.2020.12.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fong WCG, Borca F, Phan H, et al. Asthma did not increase in-hospital COVID-19-related mortality in a tertiary UK hospital. Clin Exp Allergy 2021; 51: 939–941. doi: 10.1111/cea.13855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guan W-J, Liang W-H, Shi Y, et al. Chronic respiratory diseases and the outcomes of COVID-19: a nationwide retrospective cohort study of 39,420 cases. J Allergy Clin Immunol Pract 2021; 9: 2645–2655. doi: 10.1016/j.jaip.2021.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Je D, O'Brolchain A, Ulett KB, et al. Demographics, clinical characteristics and outcomes among 197 patients with COVID-19 in the Gold Coast area. Intern Med J 2021; 51: 666–672. doi: 10.1111/imj.15260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim S-J, Jung C-G, Lee JY, et al. Characterization of asthma and risk factors for delayed SARS-CoV-2 clearance in adult COVID-19 inpatients in Daegu. Allergy 2021; 76: 918–921. doi: 10.22541/au.159672611.14210962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kipourou DK, Leyrat C, Alsheridah N, et al. Probabilities of ICU admission and hospital discharge according to patient characteristics in the designated COVID-19 hospital of Kuwait. BMC Public Health 2021; 21: 799. doi: 10.1186/s12889-021-10759-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lemus Calderon JA, Beneyto Martin P, Guzmán Rodriguez G, et al. Differentiating characteristics of patients with asthma in the severe acute respiratory syndrome coronavirus 2 infection. Ann Allergy Asthma Immunol 2020; 126: 92–93. doi: 10.1016/j.anai.2020.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liao S-Y, Petrache I, Fingerlin TE, et al. Association of inhaled and systemic corticosteroid use with coronavirus disease 2019 (COVID-19) test positivity in patients with chronic pulmonary diseases. Respir Med 2021; 176: 106275. doi: 10.1016/j.rmed.2020.106275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lieberman-Cribbin W, Rapp J, Alpert N, et al. The impact of asthma on mortality in patients with COVID-19. Chest 2020; 158: 2290–2291. doi: 10.1016/j.chest.2020.05.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lombardi C, Roca E, Bigni B, et al. Clinical course and outcomes of patients with asthma hospitalized for severe acute respiratory syndrome coronavirus 2 pneumonia: a single-center, retrospective study. Ann Allergy Asthma Immunol 2020. 125: 707–709. doi: 10.1016/j.anai.2020.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lovinsky-Desir S, Deshpande DR, De A, et al. Asthma among hospitalized patients with COVID-19 and related outcomes. J Allergy Clin Immunol 2020; 146: 1027–1034. doi: 10.1016/j.jaci.2020.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mash RJ, Presence-Vollenhoven M, Adeniji A, et al. Evaluation of patient characteristics, management and outcomes for COVID-19 at district hospitals in the Western Cape, South Africa: descriptive observational study. BMJ Open 2021; 11: e04701. doi: 10.1136/bmjopen-2020-047016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mather JF, Mosleh W, McKay RG. The impact of asthma on in-hospital outcomes of COVID-19 patients. J Asthma 2021; in press [https://doi.org10.1080/02770903.2021.1944187]. doi: 10.1080/02770903.2021.1944187 [DOI] [PubMed] [Google Scholar]
- 66.Murillo-Zamora E, Hernandez-Suarez CM. Survival in adult inpatients with COVID-19. Public Health 2021; 190: 1–3. 10.1016/j.puhe.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Robinson LB, Wang L, Fu X, et al. COVID-19 severity in asthma patients: a multi-center matched cohort study. J Asthma 2021; in press [ 10.1080/02770903.2020.1857396]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenthal JA, et al. Asthma is associated with increased risk of intubation but not hospitalization or death in coronavirus disease 2019. Ann Allergy Asthma Immunol 2020; 126: 93–95. doi: 10.1016/j.anai.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salacup G, Lo KB, Gul F, et al. Characteristics and clinical outcomes of COVID-19 patients in an underserved-inner city population: a single tertiary center cohort. J Med Virol 2021; 93: 416–423. doi: 10.1002/jmv.26252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shah SJ, Barish PN, Prasad PA, et al. Clinical features, diagnostics, and outcomes of patients presenting with acute respiratory illness: A retrospective cohort study of patients with and without COVID-19. EClinicalMedicine 2020; 27: 100518. doi: 10.1016/j.eclinm.2020.100518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tutiya C, Mello F, Chaccur G, et al. Risk factors for severe and critical Covid-19 in pregnant women in a single center in Brazil. J Matern Fetal Neonatal Med 2021; in press [ 10.1080/14767058.2021.1880561]. doi: 10.1080/14767058.2021.1880561 [DOI] [PubMed] [Google Scholar]
- 72.Valverde-Monge M, Cañas JA, Barroso B, et al. Eosinophils and chronic respiratory diseases in hospitalized COVID-19 patients. Front Immunol 2021; 12: 668074. doi: 10.3389/fimmu.2021.668074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097 [ 10.1371/journal.pmed.1000097]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary tables and figures ERJ-01209-2021.Supplement (662.1KB, pdf)
Appendix 1 ERJ-01209-2021.Appendix_1 (1.9MB, pdf)
Appendix 2 ERJ-01209-2021.Appendix_2 (38KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01209-2021.Shareable (381.9KB, pdf)