Abstract
Background
During the first wave of the COVID-19 pandemic, shortages of ventilators and ICU beds overwhelmed health care systems. Whether early tracheostomy reduces the duration of mechanical ventilation and ICU stay is controversial.
Research Question
Can failure-free day outcomes focused on ICU resources help to decide the optimal timing of tracheostomy in overburdened health care systems during viral epidemics?
Study Design and Methods
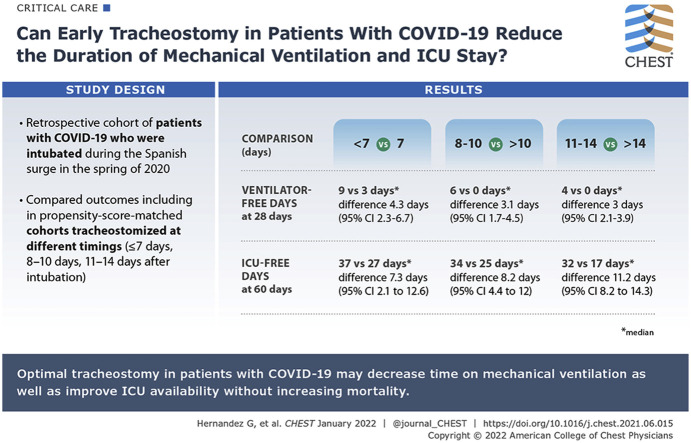
This retrospective cohort study included consecutive patients with COVID-19 pneumonia who had undergone tracheostomy in 15 Spanish ICUs during the surge, when ICU occupancy modified clinician criteria to perform tracheostomy in Patients with COVID-19. We compared ventilator-free days at 28 and 60 days and ICU- and hospital bed-free days at 28 and 60 days in propensity score-matched cohorts who underwent tracheostomy at different timings (≤ 7 days, 8-10 days, and 11-14 days after intubation).
Results
Of 1,939 patients admitted with COVID-19 pneumonia, 682 (35.2%) underwent tracheostomy, 382 (56%) within 14 days. Earlier tracheostomy was associated with more ventilator-free days at 28 days (≤ 7 days vs > 7 days [116 patients included in the analysis]: median, 9 days [interquartile range (IQR), 0-15 days] vs 3 days [IQR, 0-7 days]; difference between groups, 4.5 days; 95% CI, 2.3-6.7 days; 8-10 days vs > 10 days [222 patients analyzed]: 6 days [IQR, 0-10 days] vs 0 days [IQR, 0-6 days]; difference, 3.1 days; 95% CI, 1.7-4.5 days; 11-14 days vs > 14 days [318 patients analyzed]: 4 days [IQR, 0-9 days] vs 0 days [IQR, 0-2 days]; difference, 3 days; 95% CI, 2.1-3.9 days). Except hospital bed-free days at 28 days, all other end points were better with early tracheostomy.
Interpretation
Optimal timing of tracheostomy may improve patient outcomes and may alleviate ICU capacity strain during the COVID-19 pandemic without increasing mortality. Tracheostomy within the first work on a ventilator in particular may improve ICU availability.
Key Words: capacity, failure-free, resource, timing, tracheostomy
Abbreviations: BFD, bed-free day; IQR, interquartile range; LOS, length of stay; VFD, ventilator-free day
Graphical Abstract
FOR EDITORIAL COMMENT, SEE PAGE 8
SARS-CoV-2, the coronavirus that is responsible for the COVID-19 pandemic, overwhelmed critical care resources, making the management of ICU capacity a crucial challenge worldwide. Up to 20% of patients hospitalized with COVID-19 require ICU admission,1 more than 50% of those admitted to ICUs need invasive ventilatory support,2 and 30% of those undergoing mechanical ventilation eventually undergo a tracheostomu,3 because of the need for relatively prolonged respiratory support or airway problems (eg, laryngeal edema associated with COVID-19 complicating airway management),4 making it essential to optimize the patient’s prognosis and the use of ICU beds and ventilators. Various strategies have been suggested to overcome the shortage of these resources during the pandemic.1 , 2
Some data from studies carried out before the COVID-19 pandemic suggest that early tracheostomy reduces the length of mechanical ventilation and ICU stay,5, 6, 7, 8 reduces ventilator-associated pneumonia,8 and improves cost-effectiveness,7 without modifying the mortality rate. However, methodologic pitfalls in these studies preclude firm conclusions, and scant data are available from patients with COVID-19.9 Furthermore, performing tracheostomy and post-tracheostomy care generate aerosols, placing health care professionals at risk, making it essential to protect them too.10
General guidelines on managing critically ill patients with COVID-19 include recommendations regarding tracheostomy,11 , 12 and clinical decisions have been guided by recommendations based on expert opinion.10 , 13, 14, 15, 16, 17 Expert recommendations on timing tracheostomy during the COVID-19 pandemic vary widely. One panel concluded that no specific timing could be recommended17; other panels recommend 7 days,18 10 days,10 14 days,14 , 19 or 21 days13 , 16 , 20 after intubation. These recommendations aim to balance the benefits of earlier tracheostomy for patients and health care systems based on evidence from before the COVID-19 pandemic, while minimizing risk for health care professionals, because infectivity declines over time.10
Studies from before the COVID-19 pandemic preclude definitive conclusions on the best timing of tracheostomy because they used heterogeneous outcome measures and definitions of early tracheostomy (2-14 days); moreover, they relied on physicians’ predictions of which patients would require prolonged mechanical ventilation, limiting the ability of randomized trials21, 22, 23 and of meta-analyses5 , 6 , 8 to demonstrate a clear benefit for early tracheostomy.
Studies carried out after the appearance of COVID-19 have additional methodologic pitfalls. Given the difficulties in performing randomized trials under pandemic conditions, all available evidence comes from observational studies. Moreover, the time-dependent outcomes of these studies are especially prone to selection, immortal-time, and competing-risk biases.24 However, some characteristics of the COVID-19 pandemic actually favor the analysis of tracheostomy timing. COVID-19 is a more homogeneous clinical condition in which it is easier to predict whether a patient will require prolonged mechanical ventilation.3 , 25 The surge in ICU admissions resulted in a high volume of tracheostomies, and tracheostomies were performed earlier to allow patients to be discharged to wards. Finally, about 30% to 50% of patients with COVID-19 die while receiving mechanical ventilation, powering the failure-free days outcome, but making it futile for many of these patients.17 , 26
Specific measures of the impact of different treatment strategies on the availability of ICU resources under these conditions are lacking. Composite outcome measures based on the concept of failure-free days summarize the effect of an intervention on morbidity in the presence of the competing event of death.26 Thus, we used ventilator-free days (VFDs) and ICU and hospital bed-free days (BFDs) as measures of the effectiveness of tracheostomy in freeing up ICU and hospital resources during the COVID-19 outbreak to determine the best timing of tracheostomy to optimize the clinical course of patients and the use of ventilators and beds during the surge.
Methods
Study Design
This retrospective cohort study included all consecutive patients in 15 Spanish ICUs diagnosed with hypoxemic respiratory failure secondary to reverse-transcriptase polymerase chain reaction-confirmed COVID-19 pneumonia who underwent tracheostomy between February 15 and May 15, 2020. During the outbreak, attending physicians decided who underwent tracheostomy when and how based on ICU occupancy and anticipated benefit to the patient of tracheostomy. Criteria for tracheostomy included anticipated need for prolonged mechanical ventilation (≥ 10 days since tracheostomy), ventilator parameters (positive end-expiratory pressure ≤ 12 cm H2O, Fio 2 ≤ 60%), no anticipated need for future prone positioning, any patient within 24 to 36 h of being administered extracorporeal membrane oxygenation, and absence of negative prognostic indicators (ie, high probability of death, coagulopathy, extrapulmonary organ dysfunction other than acute renal failure with dialysis).
Outcomes were compared with patients who underwent early vs late tracheostomy, with the following cutoffs: ≤ 7 days, 8 to 10 days, and 11 to 14 days. The institutional review boards of the participating hospitals approved the study (the departments of health of the regional governments to which these hospitals are affiliated: Madrid, Catalonia, Mallorca, and Castilla-la Mancha), waiving the need for written informed consent because of the retrospective and observational nature of the study (CEIM Complejo Hospitalario de Toledo, 10/7/2020, no. 546). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies were followed.
Cohorts
To prevent competing-risk bias, we excluded patients with factors associated with tracheostomy: admission to the ICU with positive polymerase chain reaction results for COVID-19, but without indications for mechanical ventilation for COVID-19 pneumonia; admission after otorhinolaryngology surgery; low level of consciousness; swallowing dysfunction; neuromuscular disease other than ICU-acquired weakness; tracheostomy; advanced directives to withhold life-sustaining interventions; or being expected to die before hospital discharge.
To prevent residual selection bias resulting from the lack of randomization of the timing of tracheostomy, we matched cohorts based on propensity scores. Propensity scores were calculated using variables predictive of the timing of tracheostomy in the ICU (age, sex, comorbidities, Acute Physiology and Chronic Evaluation II score at ICU admission, extrapulmonary organ failures at ICU admission, type of ICU), additional covariates for patients with COVID-19 (date of ICU admission, time from clinical presentation to invasive mechanical ventilation, and medical treatment with corticoids or remdesivir), and variables predictive of tracheostomy or prolonged mechanical ventilation (need for reintubation before tracheostomy, neurologic failure at ICU admission, and underlying chronic respiratory disease).
We excluded post-tracheostomy factors that could lead to immortal-time bias, except the use of high-flow oxygen therapy during weaning. For matched comparisons, patients in the late tracheostomy cohort were selected according to the propensity score from among the remaining patients (≥ 8 days, ≥ 11 days, and ≥ 15 days, respectively).
Data Collection
We collected data regarding patients’ characteristics, course of COVID-19, ICU and hospital admission, severity of illness at ICU admission and at tracheostomy, respiratory and COVID-19 treatments, extubation episodes before tracheostomy (counting the time off ventilator in the calculation of VFD), weaning or decannulation failure (counting the time off ventilator before weaning failure in the calculation of VFD), ICU and hospital length of stay (LOS), ICU readmission (counting the time between admissions in the calculation of BFD), course of mechanical ventilation and tracheostomy, vital status at ICU and hospital discharge, and cause of death. We also recorded tracheostomy-related and post-tracheostomy-related ICU complications (e-Appendix 1).
Outcomes
The primary outcome was VFD at 28 days, calculated as VFD28 = 28 – x, where x represents the number of days from intubation to liberation from ventilation or death. Secondary outcomes included VFD at 60 days (VFD60 = 60 – x, where x represents the number of days from intubation to liberation from ventilation or death) and modified ICU or hospital BFD at 28 days (BFD28 = 28 – y, where y represents the number of days from ICU or hospital admission to discharge to the ward or home or death) and at 60 days (BFD60 = 60 – y, where y represents the number of days from ICU or hospital admission to discharge to the ward or home or death). Therefore, the value of these variables is 0 when the patient uses the resource (ventilator or bed) for longer than the specified period (28 or 60 days).
Statistical Analyses
To compare groups of patients who underwent tracheostomy in different timeframes (< 7 days, 8-10 days, or 11-14 days after intubation) within the entire cohort (unmatched patients), we used the χ 2 test or Fisher exact test for categorical variables and the analysis of variance or Kruskal-Wallis test for continuous variables, as appropriate. We used Kaplan-Meier plots to determine the probability of being mechanically ventilated in each tracheostomy-timing group, and we used the log-rank test to compare this probability among groups. To analyze the relationship among the timing of tracheostomy, duration of mechanical ventilation, ICU LOS, and hospital LOS, we used locally estimated scatterplot smoothing.
To determine the effect of timing of tracheostomy on outcomes (VFD28, VFD60, BFD28, and BFD60) we compared propensity score-matched cohorts of patients who underwent tracheostomy at different time points after intubation (≤ 7 days, 8-10 days, and 11-14 days). e-Appendix 1 presents detailed information about the variables included in the propensity score matching. In constituting all propensity score-matched cohorts to be compared, we used 1:1 nearest-neighbor matching without replacement and a caliper (maximum permitted difference between matched subjects) of 0.2 SD of the logit of the propensity score. An exploratory analysis also compared outcomes between two additional matched cohorts to assess differences among different timings of early tracheostomy (≤ 7 days vs 8-10 days and ≤ 7 days vs 11-14 days).
We used Stata version 14 software (StataCorp LLC) and R version 3.6.3 software (R Foundation for Statistical Computing) for all analyses, using the MatchIt package from R for propensity score matching. Two-tailed P values of ≤ .05 were considered statistically significant.
Results
Participating ICUs admitted a total of 1,939 patients with COVID-19 pneumonia during the study period; 682 patients (35.2%) underwent tracheostomy during the ICU stay, 382 patients (56%) within 14 days of intubation. The centers where and dates when tracheostomies were performed are presented in e-Table 1 and e-Figure 1.
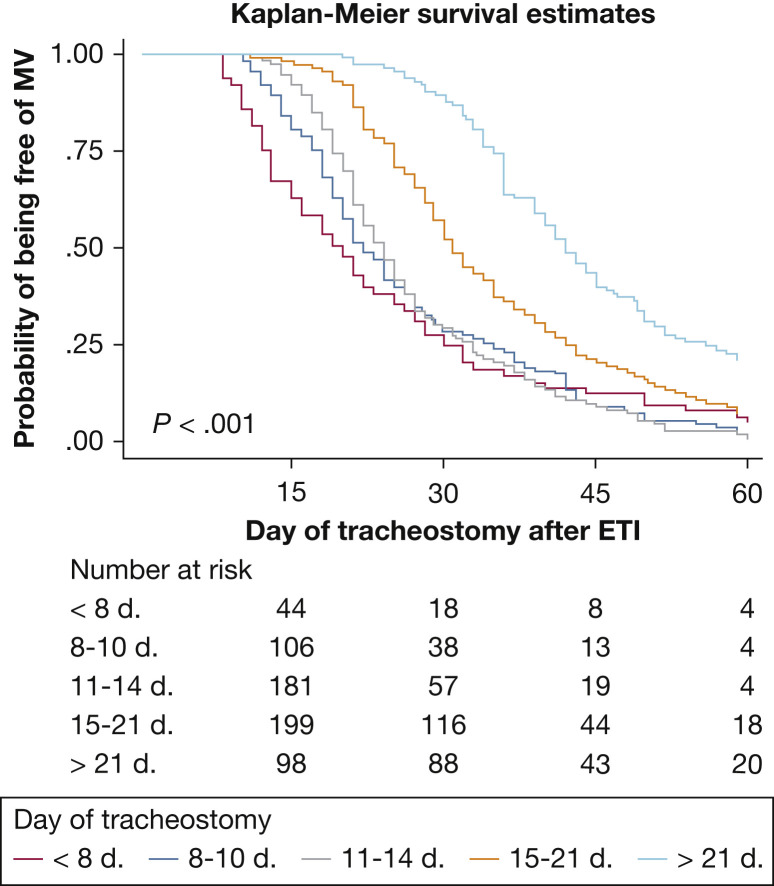
Table 1 summarizes the baseline characteristics of the entire population classified according to the timing of tracheostomy (≤ 7 days, 8-10 days, 11-14 days, 15-20 days, and ≥ 21 days) (e-Table 2). Figure 1 shows the probability of continuing mechanical ventilation for the groups of patients who underwent tracheostomy according to the timing of tracheostomy, as a surrogate for total time receiving mechanical ventilation.
Table 1.
Baseline Patient Characteristics in the Entire Population (Unmatched Samples), According to Time From Intubation to Tracheostomya
| Characteristic | Time to Tracheostomy (Days From Intubation) |
P Value | ||||
|---|---|---|---|---|---|---|
| ≤ 7 (n = 65) | 8-10 (n = 126) | 11-14 (n = 191) | 15-20 (n = 197) | ≥ 21 (n = 103) | ||
| Age, y | 62 (55-70) | 65 (56-69) | 64 (57-71) | 64 (57-69) | 65 (56-72) | .863 |
| Male sex | 42 (64.6) | 88 (69.8) | 136 (71.2) | 149 (73.8) | 74 (75.5) | .563 |
| Comorbiditiesb | ||||||
| BMI > 30 kg/m2 | 28 (43.1) | 52 (41.3) | 88 (46.1) | 74 (36.6) | 41 (41.8) | .450 |
| Heart disease | 6 (9.2) | 10 (7.9) | 16 (8.4) | 15 (7.4) | 20 (20.4) | .005 |
| COPD | 2 (3.1) | 2 (2.4) | 11 (5.8) | 8 (4) | 4 (4.1) | .651 |
| Other respiratory disease | 6 (9.2) | 6 (4.8) | 24 (12.6) | 31 (15.3) | 15 (15.3) | .042 |
| COVID-19 course | ||||||
| Time from symptom onset to ICU admission, d | 9 (6-12) | 8 (6-12) | 9 (7-12) | 10 (7-14) | 9 (6-14) | .217 |
| Time from intubation to tracheostomy, d | 6 (5-7) | 9 (8-10) | 13 (12-13) | 17 (16-19) | 24 (22-29) | < .001 |
| Time from tracheostomy to weaning, dc | 7 (1-19) | 7 (0-17) | 6 (0-12) | 8 (0-22) | 11 (0-19) | .213 |
| Treatments | ||||||
| HFOT during weaning | 23 (35.4) | 45 (35.7) | 53 (27.8) | 36 (17.8) | 25 (25.5) | .003 |
| Remdesivir | 2 (3.1) | 13 (10.3) | 13 (6.8) | 10 (5) | 9 (9.2) | .217 |
| Steroids | 51 (78.5) | 107 (84.9) | 153 (80.1) | 149 (73.8) | 79 (80.6) | .177 |
| Rescue ARDS therapyd | 58 (89.2) | 120 (95.2) | 172 (90.1) | 179 (88.6) | 90 (91.8) | .336 |
| Severity at ICU admission | ||||||
| Hemodynamic failure | 21 (33.9) | 51 (42.2) | 73 (41) | 95 (48) | 49 (56.3) | .043 |
| Renal failure | 22 (33.9) | 36 (28.6) | 73 (38.2) | 59 (29.2) | 35 (35.7) | .274 |
| No. of failed organs | 2 (1-3) | 2 (1-3) | 2 (1-3) | 2 (1-3) | 2 (1-3) | .687 |
| APACHE II scored | 13 (8-16) | 13 (9-18) | 15 (10-18) | 15 (11-18) | 15 (11-17) | .212 |
| Complications during ICU stay | ||||||
| Weaning failure | 5 (7.7) | 12 (9.5) | 26 (13.6) | 14 (7.1) | 27 (26.2) | < .001 |
| VAP | 22 (33.9) | 52 (41.3) | 68 (35.6) | 86 (42.6) | 55 (56.1) | .012 |
| Sepsis | 13 (20) | 34 (27) | 53 (27.8) | 58 (28.7) | 52 (53.1) | < .001 |
| Hematologic | 15 (23.1) | 40 (31.8) | 47 (24.6) | 66 (32.7) | 43 (43.9) | .009 |
| Death | 18 (27.7) | 47 (37.3) | 71 (37.2) | 76 (37.6) | 30 (30.6) | .468 |
Data are presented as No. (%) or median (interquartile range). APACHE = Acute Physiology and Chronic Evaluation; HFOT = high-flow oxygen therapy; VAP = ventilator-associated pneumonia.
Detailed information in e-Table 2.
Coexisting conditions were assessed according to the Charlson Comorbidity Index, in which 22 clinical conditions are scored regarding the risk of death; scores range from 0 to 37, with higher scores indicating a higher risk of death.
Weaning was defined as consecutive 24 h disconnected from mechanical ventilation.
The APACHE II score was calculated from 17 variables recorded on the day of admission to the ICU; scores range from 0 to 71 points, with higher scores indicating more severe disease.
Figure 1.
Kaplan-Meier curves for groups of patients who underwent tracheostomy according to the timing of tracheostomy (≤ 7 days, 8-10 days, 11-14 days, 15-21 days, and > 21 days after intubation) related to the probability of continuing on MV in the entire population. ETI = endotracheal intubation.
Primary and Secondary Outcomes in Nonmatched and Matched Cohorts
Primary and all the secondary outcomes except hospital BFD28 differed significantly depending on the timing of tracheostomy. Locally estimated scatterplot smoothing showed that time receiving mechanical ventilation, ICU LOS, and hospital LOS increased with the time from intubation to tracheostomy (e-Figs 2, 3, 4).
e-Table 3 summarizes the outcomes for the entire population broken down by time frames when tracheostomy was performed after intubation (unmatched cohorts). Tables 2, 3, and 4 report the results of the comparisons between the matched cohorts (≤7 days vs > 7 days, 8-10 days vs > 10 days, and 11-14 days vs > 14 days, respectively); the detailed characteristics of the patients in these cohorts are presented in e-Tables 4, 5, and 6. No significant differences in mortality were found between cohorts.
Table 2.
Results for the Primary and Secondary Outcomes in the Propensity-Matched Cohorts of Patients With Tracheostomy Performed ≤ 7 Days vs > 7 Days After Intubation
| Outcome | ≤ 7 d From Intubation to Tracheostomy (n = 58) | > 7 d From Intubation to Tracheostomy (n = 58) | Difference Between Groups (95% CI) |
|---|---|---|---|
| Ventilator use, d | 20 (13-32) | 26 (21-36) | –5.8 (–10 to –0.6) |
| ICU length of stay, d | 23 (16-39) | 33 (24-47) | –6.9 (–13.4 to –0.4) |
| Hospital length of stay, d | 40 (26-60) | 55 (32-66) | –8 (–15.2 to –0.7) |
| VFD at 28 d | 9 (0-15) | 3 (0-7) | 4.5 (2.3-6.7) |
| ICU BFD at 28 d | 5 (0-12) | 0 (0-4) | 3.9 (2-5.8) |
| Hospital BFD at 28 d | 0 (0-2) | 0 (0-0) | 0.3 (–1.3 to 1.8) |
| VFD at 60 d | 41 (28-47) | 35 (24-39) | 5.4 (0.6-10.2) |
| ICU BFD at 60 d | 37 (21-44) | 27 (12-36) | 7.3 (2.1-12.6) |
| Hospital BFD at 60 d | 20 (0-34) | 5 (0-28) | 5.9 (0.8-11) |
Data are presented as median (interquartile range). BFD = bed-free day; VFD = ventilator-free day.
Table 3.
Results for the Primary and Secondary Outcomes in the Propensity-Matched Cohorts of Patients With Tracheostomy Performed 8-10 Days vs > 10 Days After Intubation
| Outcome | 8-10 d From Intubation to Tracheostomy (n = 111) | > 10 d From Intubation to Tracheostomy (n = 111) | Difference Between Groups (95% CI) |
|---|---|---|---|
| Ventilator use, d | 22 (18-34) | 31 (22-41) | –6.8 (–11.2 to –2.3) |
| ICU length of stay, d | 26 (19-37) | 35 (25-47) | –7.9 (–12.5 to –3.2) |
| Hospital length of stay, d | 39 (28-57) | 49 (34-69) | –9.1 (–15.2 to –3.1) |
| VFD at 28 d | 6 (0-10) | 0 (0-6) | 3.1 (1.7-4.5) |
| ICU BFD at 28 d | 2 (0-9) | 0 (0-3) | 2.4 (1.2-3.7) |
| Hospital BFD at 28 d | 0 (0-1) | 0 (0-0) | 0.8 (–0.9 to 2.5) |
| VFD at 60 d | 38 (26-42) | 29 (18-38) | 6.2 (2.7-9.7) |
| ICU BFD at 60 d | 34 (22-40) | 25 (6-35) | 8.2 (4.4-12) |
| Hospital BFD at 60 d | 21 (3-32) | 11 (0-26) | 6.4 (2.8-10) |
Data are presented as median (interquartile range). BFD = bed-free day; VFD = ventilator-free day.
Table 4.
Results for the Primary and Secondary Outcomes in the Propensity-Matched Cohort for Patients With Tracheostomy Performed 11-14 Days vs > 14 Days After Initiation of Mechanical Ventilation
| Outcome | Timing of Tracheostomy, d |
Difference Between Groups (95% CI) | |
|---|---|---|---|
| 11-14 (n = 159) | > 14 (n = 159) | ||
| Ventilator use, d | 24 (20-33) | 35 (26-46) | –10.9 (–14.1 to –7.7) |
| ICU length of stay, d | 28 (22-40) | 41 (30-57) | –12.6 (–16.2 to –9) |
| Hospital length of stay, d | 46 (32-61) | 61 (42-76) | –14.2 (–19.4 to –9.1) |
| VFD at 28 d | 4 (0-9) | 0 (0-2) | 3 (2.1-3.9) |
| ICU BFD at 28 d | 0 (0-6) | 0 (0-0) | 1.9 (1.2-2.6) |
| Hospital BFD at 28 d | 0 (0-0) | 0 (0-0) | –0.3 (–1.5 to 0.9) |
| VFD at 60 d | 36 (27-41) | 25 (15-33) | 9 (6.4-11.6) |
| ICU BFD at 60 d | 32 (18-38) | 17 (10-29) | 11.2 (8.2-14.3) |
| Hospital BFD at 60 d | 13 (0-28) | 0 (0-18) | 7.1 (4.2-9.9) |
Data are presented as median (interquartile range). BFD = bed-free day; VFD = ventilator-free day.
Exploratory Outcomes
In the exploratory analysis to assess differences among the three early timings analyzed, the comparison between ≤ 7 days and 8 to 10 days (matching cohorts of 88 patients) did not find a significant difference only for VFD28 (6 days [interquartile range (IQR), 0-13 days] in the group that underwent tracheostomy ≤ 7 days after intubation vs 8 days [IQR, 1-13 days] in the group that underwent tracheostomy 8-10 days after intubation; mean difference between groups, –0.5 day; 95% CI, –3.0 to 2.0 days), whereas the comparison between ≤ 7 days and 11 to 14 days (matching cohorts of 106 patients) found significant differences in VFD28 (8 days [IQR, 0-15 days] in the group that underwent tracheostomy ≤ 7 days after intubation vs 2 days [IQR, 0-6 days] in the group that underwent tracheostomy 11-14 days after intubation; mean difference between groups, 4.2 days; 95% CI, 2-6.4 days) and in ICU BFD28 (3 days [IQR, 0-10 days] vs 0 days [IQR, 0-3 days], respectively; mean difference between groups, 3.8 days; 95% CI, 2.1-5.5 days).
Discussion
To our knowledge, this is the largest multicenter study to examine the timing of tracheostomy in patients with COVID-19 with a propensity-matched score. We found that early tracheostomy increased VFD and BDF mainly can be attributed to a reduction in the time receiving mechanical ventilation because no differences in mortality between the groups that underwent tracheostomy at different timings were observed.
Our early tracheostomy group is similar to that reported in the Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure study. Those authors found that 13% of patients with ARDS underwent tracheostomy in the ICU; these patients had been receiving mechanical ventilation for a median of 21.5 days (IQR, 13-33 days), and 29.5% died within 60 days.27 In our study, the surge conditions meant that physicians decided who underwent tracheotomy, when to perform the procedure, and what technique to use based on weekly burden in ICUs (e-Fig 1). Thus, the COVID-19 outbreak represents a unique opportunity to advance our knowledge about the effectiveness of tracheostomy in managing ICU resources.
The propensity score took into account all variables that were associated with the duration of mechanical ventilation, mortality, or both in previous studies in general critically ill populations28, 29, 30 or patients with COVID-19.31, 32, 33, 34, 35, 36, 37, 38 Moreover, the date of ICU admission reflects the strain on ICU resources during the surge, thus strengthening the model by improving its ability to elucidate the relationships between the timing of tracheostomy and the availability of ICU resources.39
Although a previous single-center study also found that early tracheostomy reduced the duration of mechanical ventilation,9 the reduction was achieved by shortening the duration of ventilation in a patient before tracheostomy without shortening the time from tracheostomy to successful weaning from mechanical ventilation. By contrast, in our study, early tracheostomy also reduced weaning time. This discrepancy can be explained by differences in patients’ baseline characteristics as suggested by the short time to definitive weaning achieved in that study. Our results are in line with those of a national study in Spain,40 where 52.1% of patients were liberated from mechanical ventilation within 30 days. Additional reasons for the reduced weaning time with early tracheostomy include our failure to take into account previously reported benefits of early tracheostomy (eg, reduced sedative administration and respiratory infection rate).41
Our results confirm that the increases in VFD and BFD with early tracheostomy were not related to differences in mortality rates. Possible explanations for the lack of associations with mortality include the higher complications rates during the ICU stay in the matched cohorts of patients who underwent tracheostomy later. These differences reached significance in the delayed cohort (> 14 days) (e-Tables 4, 5, 6), suggesting that some prevalent COVID-19 complications become more common as time receiving mechanical ventilation increases, leading to increases in ICU LOS and hospital LOS.39 The locally estimated scatterplot smoothing analysis showed that ICU LOS and hospital LOS increased with increased duration of mechanical ventilation according to the timing of tracheostomy, reinforcing the idea that the duration of mechanical ventilation hampers recovery in patients with COVID-19. Furthermore, very early tracheostomy probably was performed in response to emergency situations, which could explain the U-shaped curves suggesting that both very early and delayed tracheostomies may be associated with the development of clinical complications.
Regardless of the timing, tracheostomy showed a positive impact on the availability of ICU resources. The earlier the tracheostomy, the higher the improvement. The greatest benefits for ICU resources were found in the group that underwent tracheostomy within 7 days after intubation, suggesting that the mechanisms involved are time dependent. Some aspects related to very early tracheostomy deserve mention. It can be argued that this timing selects less severe patients, given that tracheostomy usually is delayed until the needs for increased Fio 2 and positive end-expiratory pressure are reduced and that prone positioning is a relative contraindication for tracheostomy. However, to avoid this bias, the propensity matching took into account rescue therapies (prone positioning and extracorporeal membrane oxygenation) applied before tracheostomy; most patients requiring prone positioning were turned definitively to the supine position before tracheostomy, and some patients who underwent tracheostomy were in the prone position. No consensus exists about the respiratory settings that compromise the safety of patients and health care professionals performing tracheostomy, but patients with COVID-19 seldom require high positive end-expiratory pressure, so this not a valid reason to delay tracheostomy in patients with persistent hypoxemia.10 , 18 , 39
Limitations of the Study
The most important limitation is the retrospective design, which precludes definite conclusions about the causality of the associations observed and cannot totally exclude selection bias. Because the conditions during the outbreak precluded carrying out a prospective randomized study, we opted for a retrospective study based on propensity matching. Furthermore, the cohort of patients who underwent tracheostomy ≤ 7 days after intubation is relatively small, limiting the ability of the analyses to extract definitive conclusions, and included one patient receiving mechanical ventilation for < 10 days. Nevertheless, VFB and ICU BFD were higher in patients who underwent tracheostomy within < 7 days than in those patients who underwent tracheostomy 11 to 14 days after intubation. These results correspond to the time ranges reported for general critically ill patients by Chorath et al.8 Given the lack of larger studies on the timing of tracheostomy in patients with COVID-19, the information from the present study may be crucial for managing ICU resources in future surges.
The high percentage of patients who underwent tracheostomy compared with other cohorts (Réseau européen de recherche en Ventilation Artificielle [REVA] Network41 and Martin-Villares et al40) can be explained by specific time frames during the first wave as learning about COVID-19 evolved rapidly and previous experience in high-volume and high-complexity recruiting centers for this study. However, recent evidence for early tracheostomy supports our results.8
The only post-tracheostomy variable that differed significantly between the cohorts that underwent tracheostomy at different timings was the use of high-flow oxygen therapy during weaning. Despite the risk of introducing an immortal time bias by including this variable in the matching because this therapy shortens the time to weaning,37 we decided to include it because its use depended only on its availability and did not modify the indication for early tracheostomy.
The results cannot be extrapolated to settings other than overwhelming periods. Life support measures were withheld in many patients, making time to death highly dependent on local practices during this first wave in an outbreak of a poorly understood disease, thereby increasing the heterogeneity of the results. However, the large number of patients included from 15 ICUs improves the external validity of the results. Finally, the overwhelming conditions in ICUs during the first wave may have limited professionals’ ability to apply standard care protocols.
Interpretation
Optimal timing of tracheostomy may improve patient outcomes and may alleviate ICU capacity strain during the COVID-19 pandemic without increasing mortality. Tracheostomy within the first week receiving ventilation in particular may improve ICU availability.
Take-home Points.
StudyQuestion: What is the best timing for tracheostomy in patients with COVID-19 pneumonia with regard to patient prognosis and ICU capacity maintenance?
Results: Early tracheostomy was associated with a significantly higher number of ventilator-free days in the first 28 and 60 days after intubation and a higher number of ICU and hospital bed-free days in the first 28 and 60 days after ICU or hospital admission. Moreover, the results suggest that the earlier the tracheostomy, the better the patient’s prognosis and the higher the maintenance of ICU resource capacity.
Interpretation: Early tracheostomy can help to optimize clinical course of patients and critical care resources during future viral pandemic and probably other overwhelming situations in ICUs.
Acknowledgments
Author contributions: G. H. contributed to the conception, design, analysis, and interpretation of the data, as well as to drafting, critical revision, reading, and approval of the manuscript. F. J. R., J. M. A., R. O., L. C., J. R. M., C. De Haro, A. O., O. Peñuelas, M. M. C.-D., A. Canabal, O. Plans, C. V., G. R., F. G., A. L., M. M., J. C. F., A. G.-C., R. C., A. Castellvi, B. C., F. F.-V., J. P., R. D., A. N., J. C. M., C. Diaz, J. A. S.-P., R. P., J. M.-C., C. R.-S., J. A. S.-G., J. J., and R. C. contributed to interpretation of the data, drafting of the article, as well as to critical revision, reading, and approval of the manuscript. S. P.-H. and O. R. contributed to statistical analyses and interpretation of the data, as well as to critical revision, reading, and approval of the manuscript. G. H. and O. R. take responsibility for the integrity of the work as a whole.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: G. H. and J. R. M. report personal fees and travel expenses from Fisher & Paykel, J. R. M. reports an unrestricted grant from Fisher & Paykel, and O. R. reports speaker fees from Air Liquide and consultancy for Hamilton Medical. None declared (F. J. R., J. M. A., R. O., L. C., C. De Haro, A. O., O. Peñuelas, M. M. C.-D., A. Canabal, O. Plans, C. V., G. R., F. G., A. L., M. M., J. C. F., A. G.-C., R. C., A. Castellvi, B. C., F. F.-V., J. P., R. D., A. N., J. C. M., C. Diaz, J. A. S.-P., R. P., J. M.-C., C. R.-S., J. A. S.-G., J. J., R. C., S. P.-H.).
Other contributions: The authors thank all the patients and medical and nursing staff for their cooperation. John Giba, BSc, received financial compensation for editing the English in the manuscript.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
Supplementary Data
References
- 1.Phua J., Weng L., Ling L., et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aziz S., Arabi Y.M., Alhazzani W., et al. Managing ICU surge during the COVID-19 crisis: rapid guidelines. Intensive Care Med. 2020;46:1303–1325. doi: 10.1007/s00134-020-06092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karagiannidis C., Mostert C., Hentschker C., et al. Case characteristics, resource use, and outcomes of 10021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet. 2020;8(9):853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGrath B.A., Wallace S., Goswamy J. Laryngeal oedema associated with COVID-19 complicating airway management. Anaesthesia. 2020;75:962–977. doi: 10.1111/anae.15092. [DOI] [PubMed] [Google Scholar]
- 5.Wang R., Pan C., Wang X., Xu F., Jiang S., Li M. The impact of tracheostomy in critically ill patients undergoing mechanical ventilation: a meta-analysis of randomized controlled clinical trials with trial sequential analysis. Heart Lung. 2019;48:46–54. doi: 10.1016/j.hrtlng.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Hosokawa K., Nishimura M., Egi M., Vincent J.L. Timing of tracheostomy in ICU patients: a systematic review of randomized controlled trials. Crit Care. 2015;19:424. doi: 10.1186/s13054-015-1138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C., Rudmik L. A cost-effectiveness analysis of early vs late tracheostomy. Otolaryngol Head Neck Surg. 2016;142(10):981–987. doi: 10.1001/jamaoto.2016.1829. [DOI] [PubMed] [Google Scholar]
- 8.Chorath K., Hoang A., Rajasekaran K., et al. Association of early vs late tracheostomy placement with pneumonia and ventilator days in critically ill patients. A meta-analysis. JAMA Otolaryngol Head Neck Surg. 2021;147(5):450–459. doi: 10.1001/jamaoto.2021.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aviles-Jurado F.X., Prieto-Alhambra D., González-Sánchez N., et al. Timing, complications, and safety of tracheostomy in critically ill patients with COVID-19. JAMA Otolaryngol Head Neck Surg. 2020;147(1):1–8. doi: 10.1001/jamaoto.2020.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGrath B.A., Brenner M., Warrillow S., et al. Tracheostomy in the COVID-19 era: global and multidisciplinary guidance. Lancet Respir Med. 2020;8(7):717–725. doi: 10.1016/S2213-2600(20)30230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alhazzani W., Møller M.H., Arabi Y.M., et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46(5):854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. N Engl J Med. 2020;383(25):2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 13.Chao T.N., Braslow B.M., Martin N.D., et al. Tracheostomy in ventilated patients with COVID-19. Ann Surg. 2020;272(1):e30–e32. doi: 10.1097/SLA.0000000000003956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiesa-Estomba C.M., Lechien J.R., Calvo-Henriquez C., et al. Systematic review of international guidelines for tracheostomy in COVID-19 patients. Oral Oncol. 2020;108:104844. doi: 10.1016/j.oraloncology.2020.104844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delides A., Maragoudakis P., Nikolopoulos T. Timing of tracheostomy in intubated patients with COVID-19. Otolaryngol Head Neck Surg. 2020;163(2):328–329. doi: 10.1177/0194599820930668. [DOI] [PubMed] [Google Scholar]
- 16.Ferri E., Nata F.B., Pedruzzi B., et al. Indications and timing for tracheostomy in patients with SARS CoV2-related. Eur Arch Otorhinolaryngol. 2020;277(8):2403–2404. doi: 10.1007/s00405-020-06068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamb C.R., Desai N.R., Angel L., et al. Use of Tracheostomy During the COVID-19 Pandemic: American College of Chest Physicians/American Association for Bronchology and Interventional Pulmonology/Association of Interventional Pulmonology Program Directors Expert Panel Report. Chest. 2020;158(4):1499–1514. doi: 10.1016/j.chest.2020.05.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattioli F., Fermi M., Ghirelli M., et al. Tracheostomy in the COVID-19 pandemic. Eur Arch Otorhinolaryngol. 2020;277(7):2133–2135. doi: 10.1007/s00405-020-05982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volo T., Stritoni P., Battel I., et al. Elective tracheostomy during COVID-19 outbreak: to whom, when, how? Early experience from Venice, Italy. Eur Arch Otorhinolaryngol. 2021;278(3):781–789. doi: 10.1007/s00405-020-06190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michetti C.P., Burlew C.C., Bulger E.M., Davis K.A., Spain D.A. Performing tracheostomy during the COVID-19 pandemic: guidance and recommendations from the Critical Care and Acute Care Surgery Committees of the American Association for the Surgery of Trauma. Trauma Surg Acute Care Open. 2020;5(1) doi: 10.1136/tsaco-2020-000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young D., Harrison D.A., Cuthbertson B.H., Rowan K., TracMan Collaborators Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation. The TracMan randomized trial. JAMA. 2013;309(20):2121–2129. doi: 10.1001/jama.2013.5154. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Prieto A., Mateu A., Gorriz M., et al. A randomized clinical trial for the timing of tracheostomy in critically ill patients: factors precluding inclusion in a single center study. Crit Care. 2014;18(5):585. doi: 10.1186/s13054-014-0585-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terragni P.P., Antonelli M., Fumagalli R., et al. Early vs late tracheostomy for prevention of pneumonia in mechanically ventilated adult ICU patients. A randomized controlled trial. JAMA. 2010;303(15):1483–1489. doi: 10.1001/jama.2010.447. [DOI] [PubMed] [Google Scholar]
- 24.Wolkewitz M., Puljak L. Methodological challenges of analysing COVID-19 data during the pandemic. BMC Med Res Methodol. 2020;20(1):81. doi: 10.1186/s12874-020-00972-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wunsch H. Mechanical ventilation in COVID-19: interpreting the current epidemiology. Am J Respir Crit Care Med. 2020;202(1):1–4. doi: 10.1164/rccm.202004-1385ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yehya N., Harhay M.O., Curley M.A.Q., Schoenfeld D.A., Reeder R.W. Reappraisal of ventilator-free days in critical care research. Am J Respir Crit Care Med. 2019;200(7):828–836. doi: 10.1164/rccm.201810-2050CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abe T., Madotto F., Pham T., et al. Epidemiology and patterns of tracheostomy practice in patients with acute respiratory distress syndrome in ICUs across 50 countries. Crit Care. 2018;22(1):195. doi: 10.1186/s13054-018-2126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang Y.C., Huang K.T., Chen Y.M., et al. Ventilator dependence risk score for the prediction of prolonged mechanical ventilation in patients who survive sepsis/septic shock with respiratory failure. Sci Rep. 2018;8(1):5650. doi: 10.1038/s41598-018-24028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark P.A., Lettieri C.J. Clinical model for predicting prolonged mechanical ventilation. J Crit Care. 2013;28(5):e1–e7. doi: 10.1016/j.jcrc.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Légaré J.F., Hirsch G.M., Buth K.J., MacDougall C., Sullivan J.A. Preoperative prediction of prolonged mechanical ventilation following coronary artery bypass grafting. Eur J Cardiothorac Surg. 2001;20(5):930–936. doi: 10.1016/s1010-7940(01)00940-x. [DOI] [PubMed] [Google Scholar]
- 31.Kang B.H., Cho J., Cook-Jong Lee J., Jung K. Early versus late tracheostomy in trauma patients: a propensity-matched cohort study of 5 years’ data at a single institution in Korea. World J Surg. 2018;42(6):1742–1747. doi: 10.1007/s00268-018-4474-4. [DOI] [PubMed] [Google Scholar]
- 32.Alali A.S., Scales D.C., Fowler R.A., et al. Tracheostomy timing in traumatic brain injury: a propensity-matched cohort study. J Trauma Acute Care Surg. 2013;76(1):70–78. doi: 10.1097/TA.0b013e3182a8fd6a. [DOI] [PubMed] [Google Scholar]
- 33.Vollam S., Harrison D.A., Young J.D., Watkinson P.J. Does delaying discharge from intensive care until after tracheostomy removal affect 30-day mortality? Propensity score matched cohort study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-037762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of COVID-19—final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenbaum P.R., Rubin D.B. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 36.Thomas L.E., Li F., Pencina M.J. Overlap weighting. A propensity score method that mimics attributes of a randomized clinical trial. JAMA. 2020;323(23):2417–2418. doi: 10.1001/jama.2020.7819. [DOI] [PubMed] [Google Scholar]
- 37.Hernandez G., Rodriguez M.L., Vaquero M.C., et al. High-flow oxygen with capping or suctioning for tracheostomy decannulation. N Engl J Med. 2020;383:1009–1017. doi: 10.1056/NEJMoa2010834. [DOI] [PubMed] [Google Scholar]
- 38.Gamberini L., Tonetti T., Spadaro S., et al. Factors influencing liberation from mechanical ventilation in coronavirus disease 2019: multicentre observational study in fifteen Italian ICUs. J Intensive Care. 2020;8:80. doi: 10.1186/s40560-020-00499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz M.J., Teng M.S., Brenner M.J. Timing of tracheostomy for patients with COVID-19 in the ICU-setting precedent in unprecedented times. JAMA Otolaryngol Head Neck Surg. 2020;146(10):887–888. doi: 10.1001/jamaoto.2020.2630. [DOI] [PubMed] [Google Scholar]
- 40.Martin-Villares C., Perez C., Bartolome-Benito M., Bernal-Sprekelsen, COVID ORL ESP Collaborative Group Outcome of 1890 tracheostomies for critical COVID-19 patients: a national cohort study in Spain. Eur Arch Otorhinolaryngol. 2021;278(5):1605–1612. doi: 10.1007/s00405-020-06220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.COVID-19-ICU Group on behalf of the REVA Network and the COVID-19-ICU Investigators Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.