Highlights
-
•
MDD is associated with larger right sided medial nuclei amygdala volumes.
-
•
MDD is associated with increased right:left whole and substructural volume ratios.
-
•
MDD cortisol inversely correlated with left corticoamygdaloid transition area.
-
•
The study implies the potential importance of amygdala substructure volumes in MDD.
Keywords: Major Depressive Disorder, Amygdala, Centromedial nucleus, Cortisol awakening response, Hypothalamic-pituitary-adrenal axis, Freesurfer
Abstract
The role of the amygdala in the experience of emotional states and stress is well established. Connections from the amygdala to the hypothalamus activate the hypothalamic–pituitaryadrenal (HPA) axis and the cortisol response. Previous studies have failed to find consistent whole amygdala volume changes in Major Depressive Disorder (MDD), but differences may exist at the smaller substructural level of the amygdala nuclei. High-resolution T1 and T2-weighted-fluid-attenuated inversion recovery MRIs were compared between 80 patients with MDD and 83 healthy controls (HC) using the automated amygdala substructure module in FreeSurfer 6.0. Volumetric assessments were performed for individual nuclei and three anatomico-functional composite groups of nuclei. Salivary cortisol awakening response (CAR), as a measure of HPA responsivity, was measured in a subset of patients. The right medial nucleus volume was larger in MDD compared to HC (p = 0.002). Increased right-left volume ratios were found in MDD for the whole amygdala (p = 0.004), the laterobasal composite (p = 0.009) and in the central (p = 0.003) and medial (p = 0.014) nuclei. The CAR was not significantly different between MDD and HC. Within the MDD group the left corticoamygdaloid transition area was inversely correlated with the CAR, as measured by area under the curve (AUCg) (p ≤ 0.0001). In conclusion, our study found larger right medial nuclei volumes in MDD compared to HC and relatively increased right compared to left whole and substructure volume ratios in MDD. The results suggest that amygdala substructure volumes may be involved in the pathophysiology of state depression.
1. Introduction
The defining experience in Major Depressive Disorder (MDD) is of an emotional change where the individual becomes persistently sad and/or unable to experience pleasure. The experiential aspects of MDD theoretically map onto brain limbic circuitry, in particular the subcortical temporal lobe limbic structures of the hippocampus and the amygdala (Kaiser et al., 2015, Pruessner et al., 2010). Evidence indicates that state depression is associated with reduced hippocampal volumes (Brown et al., 2014, Nolan et al., 2020, Schmaal et al., 2016, Stephanie Campbell et al., 2004, Wise et al., 2017). Hippocampal pathology is consistent with the key cognitive difficulties experienced in MDD, such as impairments in short term and autobiographical memory formation (Kohler et al., 2015) and attentional problems (Disner et al., 2011).
In a previous study we replicated the common finding of smaller hippocampal volumes in MDD, and also that the core hippocampal neuronal subfields involved in coding biographical memories – in particular, the left cornu ammonis (CA1-CA4), and the dentate gyrus were relatively reduced in an MDD (Doolin et al., 2018, Roddy et al., 2019). This implies that hippocampal substructure volumes, rather than whole hippocampal volumes, may yield more precise information on the potential brain pathology in MDD. Core subfields volume reduction at the center of the hippocampal hub increased with chronicity of MDD, with CA1 volume being a predictor of depression (Roddy and O'Keane, 2019, Roddy et al., 2019). The latter finding suggests a disease process in the hippocampus of those suffering from more severe depressive illness.
The amygdala, tightly nestled on top of and densely interconnected with the hippocampus, plays an essential role in emotional processing, affective/mood state, fear conditioning and extinction and social behaviours (Fox and Shackman, 2019, Hur et al., 2019, Janak and Tye, 2015, Lindquist et al., 2012, Putnam and Chang, 2021, Shackman et al., 2016). In contrast to the more reliable decrease in hippocampal volumes in MDD, studies investigating amygdalae volumes are inconsistent (Schmaal et al., 2020). Studies show reduced (Ancelin et al., 2019, Bora et al., 2012, Kronenberg et al., 2009, Schuhmacher et al., 2012, Tang et al., 2007), increased (Frodl et al., 2002, Frodl et al., 2003, Saleh et al., 2012, van Eijndhoven et al., 2009, van Elst et al., 2000), and unchanged amygdala volumes in MDD (Arnone et al., 2012, Brown et al., 2019, Shen et al., 2017, Stephanie Campbell et al., 2004). Inconsistencies may reflect sample heterogeneity, confounding factors and methodological differences (Hamilton et al., 2008, Saygin et al., 2017).
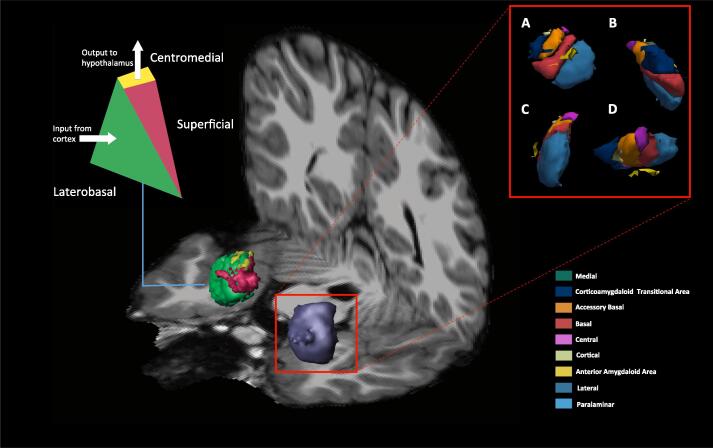
Like the hippocampus, rather than being a unitary structure, the amygdala is formed from a collection of interconnected substructures (nuclei) that relay signals from multiple brain areas (Fig. 1, Table S1). Amygdala nuclei can be clustered into three anatomico-functional groups; laterobasal, centromedial and superficial (Table 2) (Heimer et al., 1999). Broadly speaking, the main cortical and sensory inputs enter the amygdala through the laterobasal nuclei, where they are returned to their cortical areas of origin (Freese and Amaral, 2005). The superficial nuclei are important in affective, memory and social processing and have widespread connections to other limbic structures (Goossens et al., 2009). Consolidated outputs from the amygdala emerge primarily from the medial structures, i.e. the medial and central nuclei (Table S1) (Heimer et al., 1999), and this area of the amygdala is found to be particularly activated following negative emotional stimuli (Hrybouski et al., 2016).
Fig. 1.
Whole amygdala and amygdalar subfields. Representative oblique T1 image showing the left whole amygdala (purple) and right amygdala divided into the laterobasal (green), centromedial (yellow) and superficial (red) clusters of nuclei. The right amygdala is expanded out to show all nine computed nuclei from four views; A, frontal; B, medial; C, lateral; D, superior. Different colours represent specific nuclei; green, medial; dark blue, corticoamygdaloid transitional area; orange, accessory basal; red, basal; purple, central; off-white, cortical; yellow, anterior amygdaloid area; light blue, lateral; turquoise, paralaminar. The left nuclear groups are colour coded and represented diagrammatically to show amygdalar input and output pathways. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Between group differences for individual amygdalar measures and R-L amygdalar ratios (log transformed).
| Structure |
MDD (mm3) |
Controls (mm3) |
Con v MDD |
Con v MDD R-L Asymmetry |
|---|---|---|---|---|
| Nucleus/Composite measure | Mean (SEM, 95% CI) | Mean (SEM, 95% CI) | p-uncorrected | p-uncorrected |
| LEFT | ||||
| Lateral | 643.53 (5.83, 632–655) | 642.59 (5.79, 631–654) | 0.91 | 0.25 |
| Basal | 417.06 (3.38, 410–424) | 422.39 (3.40, 416–429) | 0.27 | 0.047 |
| Acc basal | 252.31 (2.21, 248–257) | 254.11 (2.12, 250–258) | 0.57 | 0.076 |
| AAA | 52.70 (0.68, 51–54) | 53.81 (0.67, 53–55) | 0.25 | 0.815 |
| Central | 43.27 (0.79, 42–45) | 45.40 (0.78, 44–47) | 0.059 | 0.003 |
| Medial | 20.68 (0.64, 19–22) | 19.70 (0.64, 18–21) | 0.28 | 0.014 |
| Cortical | 24.97 (0.44, 24–26) | 24.77 (0.44, 24–26) | 0.75 | 0.239 |
| CATA | 192.07 (1.80, 189–196) | 192 (1.8, 188–195) | 0.82 | 0.855 |
| Paralaminar | 48.77 (0.52, 48–50) | 50.52 (0.51, 50–52) | 0.018 | 0.287 |
| Whole amygdala | 1692 (13, 1666–1718) | 1699 (13, 1673–1725) | 0.7 | 0.004 |
| Laterobasal | 1358 (11, 1337–1379) | 1367 (11, 1346–1388) | 0.57 | 0.009 |
| Centromedial | 64.25 (1.3, 62–67) | 65.10 (1.29, 63–68) | 0.65 | 0.107 |
| Superficial | 269.72 (2.4, 265–274) | 269.86 (2.4, 265–275) | 0.97 | 0.164 |
| RIGHT | ||||
| Lateral | 668.18 (5.05, 658–678) | 652.81 (5.02, 643–663) | 0.033 | |
| Basal | 434.95 (3.18, 429–441) | 430.91 (3.20, 425–437) | 0.37 | |
| Acc basal | 261.46 (2.20, 257–266) | 259.69 (2.12, 255–264) | 0.57 | |
| AAA | 56.73 (0.75, 55–58) | 56.30 (0.74, 55–58) | 0.69 | |
| Central | 48.86 (0.96, 47–51) | 46.58 (0.94, 45–48) | 0.095 | |
| Medial | 23.40 (0.61, 22–25) | 20.64 (0.60, 20–22) | 0.002 | |
| Cortical | 26.87 (0.41, 26–28) | 26.96 (0.41, 26–28) | 0.87 | |
| CATA | 193.30 (1.74, 190–197) | 193.00 (1.72, 190–196) | 0.87 | |
| Paralaminar | 50.84 (0.44, 50–52) | 50.93 (0.43, 50–52) | 0.88 | |
| Whole amygdala | 1760 (12, 1736–1785) | 1734 (12, 1710–1758) | 0.13 | |
| Laterobasal | 1412 (10, 1393–1431) | 1392 (10, 1373–1412) | 0.16 | |
| Centromedial | 72.33 (1.42, 70–75) | 67.11 (1.40, 64–70) | 0.014 | |
| Superficial | 277.20 (2.4, 273–282) | 275.64 (2.4, 271–280) | 0.65 | |
Between group differences for individual amygdalar measures and R-L amygdalar ratios following ANCOVA correcting for age, sex and eTIV. Correction for multiple comparisons using the Benjamini-Hochberg procedure determined a significance value of p ≤ 0.002 for 18 nuclei/composites (9 left and right) with ‘Con v MDD’ and p ≤ 0.014 for R-L asymmetry. Bold text survives FDR correction. Italic text denotes p ≤ 0.05 but not surviving the FDR threshold. For details of nuclei and composites see Table S1. AAA, anterior amygdalar area; ANCOVA, analysis of covariance; CATA, cortical amygdalar transition area; FDR, false discovery rate; MDD, major depressive disorder; R-L, right-left; SEM, standard error of the mean.
The ventral amygdalofugal pathway, anterior commissure and stria terminalis are the three main efferent pathways from the amygdala (Kamali et al., 2016). The stria terminalis and amygdalofugal pathways emerging from the centromedial nuclei travel to the emotion-making and homeostatic stress systems in the hypothalamus (Heimer et al., 1999). Projections from the bed nuclei of stria terminalis to the lateral hypothalamus and the ventral tegmental area have been shown to modulate divergent emotional/physiological states (Giardino et al., 2018, Jennings et al., 2013).
An anxiety-generating circuit from the central amygdala to CRH-secreting neurons in the dorsolateral stria terminalis has been identified in animal studies (Kalin et al., 2016, Kovner et al., 2020, Pomrenze et al., 2019) and the central amygdala is involved in social and non-social threat processing (Andraka et al., 2021). The medial amygdala is also implicated in complex social behaviors (Hu et al., 2021, Kwon et al., 2021, Shemesh et al., 2016). Similar to the hippocampus, glucocorticoid receptors are expressed in the amygdala (Wang et al., 2014) but, in contrast to the hippocampal feedback, amygdalar drive promotes hypothalamic CRH secretion (Herman et al., 2016, Myers et al., 2012), mainly mediated by centromedial nuclei (Herman et al., 2016). This is of relevance to MDD because overdrive in the hypothalamic–pituitary-adrenal (HPA) axis has been established in subgroups of MDD (Doolin et al., 2017, Stetler and Miller, 2011).
Several studies have investigated whole amygdala volumes and HPA axis reactivity in MDD (Kronenberg et al., 2009, Schuhmacher et al., 2012) and HCs (Barry et al., 2017) and the findings are mixed. Schuhmacher and colleagues (Schuhmacher et al., 2012) reported that larger left and right amygdala volumes at baseline positively correlated with normalisation of HPA response to the dexamethasone-corticotrophic releasing hormone challenge (Dex/CRH) following antidepressant treatment in a subgroup of inpatients with recurrent depressive disorder.
Elevated maternal cortisol levels during gestation were associated with larger right amygdala volumes (Buss et al., 2012) and increased amygdala functional connectivity in school-age girls, and may be related to a higher level of affective problems (Graham et al., 2019). Enlargement of the basolateral amygdala has been linked to childhood anxiety (Qin et al., 2014), whereas other studies in children have shown associations between greater cortisol stress responses and reduced amygdala volumes (Fowler et al., 2021, Pagliaccio et al., 2014). In a subgroup of young healthy adults exposed to maternal postnatal depression in early life, right hemisphere amygdala volume was reported to be negatively correlated with cortisol reactivity in response to the Trier Social Stress Test (Barry et al., 2017). In healthy adults, cortisol responses to the Dex/CRH test (Kiem et al., 2013) and acute social stress (Vaisvaser et al., 2013) were found to be inversely correlated with amygdala-hippocampal functional connectivity (FC). Greater cortisol levels have been reported to be correlated with increased amygdala-centred FC in responses to fearful faces (Hakamata et al., 2017), and in young adults with a history of depression, cortisol levels correlated with increased amygdala connectivity to cognitive control network regions (Peters et al., 2019).
Precise measurement of the amygdala and its composite nuclei is now possible using Freesurfer 6.0 software that provides objective detailed automated segmentation measures (Saygin et al., 2017). A 7 Tesla MRI pilot study using this approach did not show any differences in amygdala nuclei volumes in 24 antidepressant free MDD participants compared to 20 matched healthy controls, though MDD severity as measured by the Montgomery-Asberg Depression Rating Scale (MADRS) was negatively correlated with multiple amygdala nuclei (Brown et al., 2019). The same group reported that MDD was associated with structural hyperconnectivity between the right lateral, basal, and central nuclei, and the rest of the brain, whereas the left medial nucleus showed significantly lower connection density compared to HC (Brown et al., 2020).
To date, no study has explored the relationship between amygdala subnuclei volumes and HPA axis reactivity in MDD. The aim of this study was to assess whole and functionally grouped amygdala nuclei volumes in MDD using automated segmentation and to explore the relationship between amygdala volumetrics and HPA axis function.
2. Methods
2.1. Participants and clinical data
Eighty people with Major Depressive Disorder (MDD) were compared to eighty-three HC without MDD. All participants completed the Structured Clinical Interview for DSM IV (SCID) (American Psychiatric Association, 1994) and Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1960). Inclusion in the MDD group required a current SCID diagnosis of MDD and a HAM-D score of ≥ 17. Controls were required to have no active or previous SCID diagnosis and a HAM-D less than 8. Ethical approval was obtained from the Tallaght Hospital/St James Hospital Joint Research Ethics Committee, Dublin and fully written informed consent was obtained prior to enrolment. Full inclusion and exclusion criteria are described in Supplementary Information (SI).
2.2. MRI acquisition
All data was acquired on a Philips (Best, Netherlands) Intera Achieva 3.0 Tesla MR system (32-channel head coil) at Trinity College Institute of Neuroscience, Dublin.
-
•
T1. 180 axial high-resolution T1-weighted anatomical images (T1W-IR1150 sequence, TE = 3.8 ms, TR = 8.4 ms, FOV 230 mm, 0.898 × 0.898 mm2, in-plane resolution, slice thickness 0.9 mm, flip angle alpha = 8°).
-
•
T2-FLAIR. 60 axial T2-FLAIR images (TE = 120 ms, TR = 2800 ms, 0.49 mm × 0.49 mm in-plane resolution, slice thickness 3 mm, flip angle alpha = 8°).
2.3. Image Analyses
Cortical reconstruction and segmentation were performed using the Freesurfer 6.0 (http://surfer.nmr.mgh.harvard.edu/) with the hippocampus/amygdala module to extract amygdala measures (Fischl, 2012, Fischl and Dale, 2000). The technical details of these procedures are described elsewhere (Desikan et al., 2006, Fischl et al., 2002, Reuter et al., 2012). This module interrogates contrast differences between amygdala substructures using previously defined in-vivo and ex-vivo amygdala atlases to determine substructure characteristics. The procedure was optimized by combining T1 and T2-Flair inputs and selecting the 3 T MRI flag and multispectral segmentation in Freesurfer. Nine substructures were computed (Table 2). Full details on amygdala segmentation are described in SI.
2.4. Composite measures
Computed nuclei volumes were summed together to create composite measures based on neuroanatomical definitions. Three amygdala composites were generated to correspond with the three anatomic-functional groups: superficial, laterobasal and centromedial (Table 2) (Heimer et al., 1999, Johnston, 1923). A further whole amygdala volume was generated by summing all computed outputs.
2.5. Cortisol measures
Salivary cortisol was measured in a subset of 30 MDD patients and 25 HC. Saliva samples were collected by the study participants at three time points after wakening (0, 30, and 60 min) on the day prior to the scan, and samples were analysed by Liquid Chromatography-Mass Spectrometry (LC-MS). Participants were excluded if they did not complete all three samples. Laboratory analysis methods and subsequent CAR parameter calculations (areas under the curve with respect to increase and ground) are described in the SI.
2.6. Statistical Analyses
All extracted subfield volumes were systematically inspected visually and measures exported to SPSS26 (https://www.ibm.com/analytics/us/en/technology/spss/). Mixed-method-Analyses of Variance (ANOVA) were used to investigate group-wise differences in substructure/composite volumes and across all substructures/composites and hemisphere (left/right) of the amygdalae. To clarify any driving effects identified, additional post-hoc Analyses of Covariance (ANCOVA) were used to compare between-group differences (Controls vs MDD) for each substructure/composite and hemisphere independently. Age, sex and estimated total intracranial volume (eTIV) were entered as covariates throughout. The Benjamini-Hochberg procedure was used to correct for multiple comparisons (Benjamini, 2010).
Partial correlations were performed to examine the relationship between nuclei and composite amygdalar measures, and HAM-D scores within the MDD. The right: left volume (R-L) ratios were non-normally distributed and were assessed using log transformed delta volume differences via a series of nuclei/composite independent ANCOVA’s. Partial correlations were used to explore the relationship between the CAR and amygdala nuclei and composites in a subset of participants.
3. Results
3.1. Demographics
There were no differences between controls and MDD patients for age, sex, or handedness (Table 1). 87% of MDD patients were taking antidepressants. There were no demographic differences between the CAR subsamples or between these subsets and the total group (Table S7).
Table 1.
Demographic data for complete sample.
| Con N = 83 | MDD N = 80 | Con v MDD p-value | |
|---|---|---|---|
| Age: Mean (SEM) | 31.5 (1.4) | 34.5 (1.4) | 0.13 |
| Range (years) | 16–64 | 17–64 | – |
| Male/Female (%M) | 34/49, 41% | 23/57, 29% | X2: 0.14 |
| Handedness (R/L) | 75/8 | 73/7 | X2: 1.0 |
| HAMD (SEM) | 1.3 (0.4) | 22.2 (0.4) | 1.10E-75 |
| MDD duration months | – | 29 (4) | – |
Con, controls; Dep, depressed; HAM-D, Hamilton Depression scale; M, male; MDD, major depressive disorder; R/L, right or left handed; SEM, standard error of the mean. Age and HAMD analysed using t-tests, male/female and handedness analysed by chi square tests.
3.2. Amygdala volumetrics
The initial global ANOVA generated no significant main effect for group. There was no main effect for hemisphere, but a significant group × hemisphere interaction (Greenhouse-Geisser) [F(1dof, 7.11), p = 0.009, partial eta2 = 0.05, power = 0.75] was found. A main effect for substructure [F(8dof, 14.42), p = 0.00001, partial eta2 = 0.05, power = 0.75] and group × hemisphere × substructure interaction [F(2.318dof, 4.37), p = 0.01, partial eta2 = 0.03, power = 0.75] was identified (Table S2).
Post-hoc ANCOVAs following FDR correction for each independent nucleus revealed a larger right medial nucleus (p-uncorrected = 0.002) in MDD (Table 2). No correlations between HAM-D scores and amygdalar volumes survived FDR correction in the MDD group (Table S4a, b).
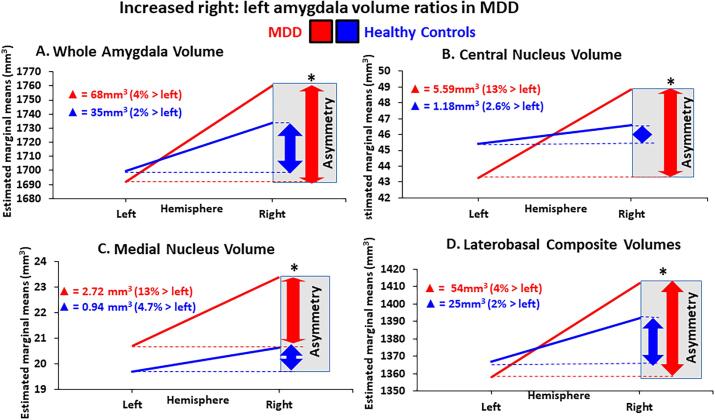
Hemisphere asymmetry ANCOVAs revealed significantly increased R:L ratios in the whole amygdala (p-uncorrected = 0.004), laterobasal composite (p-uncorrected = 0.009), central (p-uncorrected = 0.003) and medial nucleus (p-uncorrected = 0.014) volumes in MDD compared to controls (Table 2).
3.3. Cortisol awakening response
There were no significant differences in cortisol measures between the MDD and control groups (Table S8). Within the MDD group, the CAR, as measured by the AUCg was negatively correlated with the left cortical amygdalar transition area (Table 3, Fig. S1a). No correlations were found between any CAR and amygdala volumes in the HC group (Table S10).
Table 3.
Cortisol partial correlations in the MDD group.
| Structure |
T0 min |
T30 min |
T60 min |
AUCg |
|---|---|---|---|---|
| Nucleus/Composite measure | p-uncorrected, value (r-statistic) | |||
| LEFT | ||||
| Lateral | 0.507 (−0.136) | 0.024 (−0.441) | 0.025 (−0.437) | 0.015 (−0.474) |
| Basal | 0.953 (−0.012) | 0.198 (−0.261) | 0.127 (−0.307) | 0.104 (−0.326) |
| Acc basal | 0.656 (−0.092) | 0.936 (0.017) | 0.459 (−0.152) | 0.29 (−0.216) |
| AAA | 0.407 (−0.173) | 0.072 (−0.366) | 0.076 (−0.362) | 0.069 (−0.37) |
| Central | 0.897 (−0.027) | 0.899 (0.027) | 0.611 (0.107) | 0.536 (0.13) |
| Medial | 0.767 (−0.064) | 0.476 (0.153) | 0.571 (0.122) | 0.91 (−0.024) |
| Cortical | 0.999 (0) | 0.434 (0.164) | 0.992 (0.002) | 0.857 (−0.038) |
| CATA | 0.006 (−0.524) | 0.03 (−0.426) | 0.000130 (−0.681) | 0.00003 (−0.72) |
| Paralaminar | 0.958 (0.011) | 0.415 (−0.167) | 0.346 (−0.193) | 0.336 (−0.197) |
| Whole amygdala | 0.469 (−0.148) | 0.115 (−0.317) | 0.04 (−0.405) | 0.022 (−0.446) |
| Laterobasal | 0.645 (−0.095) | 0.096 (−0.333) | 0.055 (−0.38) | 0.033 (−0.419) |
| Centromedial | 0.854 (−0.039) | 0.456 (0.156) | 0.515 (0.136) | 0.675 (0.088) |
| Superficial | 0.045 (−0.396) | 0.07 (−0.361) | 0.002 (−0.579) | 0.001 (−0.624) |
| RIGHT | ||||
| Lateral | 0.388 (−0.18) | 0.353 (−0.194) | 0.321 (−0.207) | 0.356 (−0.193) |
| Basal | 0.259 (−0.23) | 0.055 (−0.381) | 0.093 (−0.336) | 0.1 (−0.33) |
| Acc basal | 0.141 (−0.297) | 0.168 (−0.279) | 0.328 (−0.2) | 0.145 (−0.294) |
| AAA | 0.828 (−0.045) | 0.398 (−0.173) | 0.984 (−0.004) | 0.671 (0.088) |
| Central | 0.848 (0.04) | 0.755 (0.064) | 0.601 (0.108) | 0.484 (0.144) |
| Medial | 0.21 (0.254) | 0.132 (0.304) | 0.014 (0.477) | 0.083 (0.347) |
| Cortical | 0.994 (−0.001) | 0.896 (−0.027) | 0.874 (−0.033) | 0.624 (−0.101) |
| CATA | 0.031 (−0.424) | 0.026 (−0.437) | 0.025 (−0.44) | 0.005 (−0.538) |
| Paralaminar | 0.318 (−0.204) | 0.098 (−0.332) | 0.097 (−0.333) | 0.169 (−0.278) |
| Whole amygdala | 0.374 (−0.186) | 0.336 (−0.201) | 0.395 (−0.178) | 0.357 (−0.192) |
| Laterobasal | 0.336 (−0.201) | 0.266 (−0.231) | 0.281 (−0.224) | 0.271 (−0.229) |
| Centromedial | 0.49 (0.142) | 0.36 (0.187) | 0.14 (0.297) | 0.196 (0.262) |
| Superficial | 0.116 (−0.316) | 0.054 (−0.383) | 0.116 (−0.316) | 0.067 (−0.364) |
Salivary cortisol was measured at 0, 30 and 60 min after waking. The cortisol awakening response was calculated using the area under the curve with respect to ground (AUCg). The FDR based threshold for significance was p ≤ 0.0003, marked in bold text. Italic text denotes p-values ≤ 0.05 but not surviving the FDR threshold. AAA, anterior amygdalar area; AUCg, area under the curve with respect to ground; CATA, cortical amygdalar transition area; FDR, false discovery rate; MDD, major depressive disorder.
4. Discussion
Using advanced automated segmentation, we compared the volumes of individual nuclei and composite groups of the amygdalae in patients with MDD and HC. The main finding was that the right medial nucleus was larger in the MDD group. Laterality differences were uncovered in whole amygdala volumes between the MDD and the HC groups, with the depressed group having an increased right-left ratio. This right amygdalar dominance in MDD was noticeably driven by increased volumes in the main amygdalar output structures on the right side, the central and medial nuclei, and the laterobasal group. Although cortisol stress responses were not statistically different between MDD and controls, there was an inverse relationship between the CAR as measured by the AUCg and the left corticoamygdaloid transition area (CATA) volume.
The absence of whole amygdala volume differences in MDD in this study is consistent with neuroimaging studies that have reported no overall changes in whole amygdala volumes (Arnone et al., 2012, Brown et al., 2019, Stephanie Campbell et al., 2004). Deeper substructural analysis investigating the constituent parts of the amygdala, i.e., the nine amygdalar nuclei and three composite clusters (laterobasal, centromedial and superficial groups), demonstrated a clear increase in the right medial nuclei volumes in MDD (Table 2). The centromedial group consists of the principal output nuclei to the hypothalamus (Barbier et al., 2018, Van de Kar and Blair, 1999) and have been shown to be particularly sensitive to negative emotional stimuli in HC (Hrybouski et al., 2016).
Although structural amygdala asymmetry is recognized in healthy humans (Pedraza et al., 2004), we found that there was a marked and significant increase in right compared to left volume measures in the MDD group that was not present in the HC. In MDD the right whole amygdala and right laterobasal composite were significantly increased relative to the left (Table 2). At the level of the nuclei, greater right-left asymmetry was found exclusively in the central and medial nuclei in MDD (Fig. 2).
Fig. 2.
Increased right: left amygdala ratios in MDD compared to controls. The major depressive disorder (MDD) group (red) exhibited a greater right:left ratio compared to healthy controls (blue) in (A) whole amygdala volumes (p = 0.004); at the level of the (B) right central nucleus (p = 0.003), and the (C) right medial nucleus (p = 0.014); and at the composite level of the (D) laterobasal group (p = 0.009) (log transformed▲delta (%) = difference between right and left volume/left volume *100, *survives FDR significance threshold). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In contrast to our finding of larger right medial nucleus volumes in MDD compared to HCs, a recent pilot study by Brown and colleagues did not show any differences in amygdala nuclei volumes in MDD (Brown et al., 2019). There are several differences between the Brown et al study and our study which could account for the discrepancy in findings. Importantly, our study included 80 patients with MDD and 83 HC in contrast to 24 MDD patients and 20 HC. It is therefore possible that differences between the groups were not detected in the Brown et al study due to the small sample size. Unlike the Brown et al study where patients were antidepressant free, most of our sample of MDD participants were prescribed antidepressants, though all fulfilled criteria for moderate or severe MDD at the time of scanning. We acknowledge the potential confounding influence of antidepressant medication, however, differences between amygdala subnuclei volumes according to antidepressant treatment status did not survive correction for multiple comparisons in our MDD group (Table S6). Another difference between the studies relates to field-strength. The advantages of increased spatial resolution of the 7 T field-strength in Brown et al’s study may have limited their sample size. Moreover, there is precedence in using 3 T and Freesurfer 6.0 to investigate amygdala substructural volumes in clinical cohorts diagnosed with dementia (Bocchetta et al., 2019) and PTSD (Morey et al., 2020).
It is interesting to note that Brown and colleagues, while finding no differences in nuclei volumes between MDD and HC groups, demonstrated lateralization of amygdala white matter tracts in MDD in the form of structural hyperconnectivity between the right lateral, basal, and central nuclei and the rest of the brain, whereas the left medial nucleus showed significantly lower connection density (Brown et al., 2020). The increased connection density in the right lateral and basal nuclei was driven by the stria terminalis, whereas the right central nucleus was driven by the uncinate fasciculus (Brown et al., 2020). These results when considered in the context of our primary finding of a larger right medial nucleus and of relative increases in right compared to left amygdalar volumes in MDD, indicate the potential importance of right amygdala substructure volumes in the pathophysiology of MDD.
Both preclinical (Baker and Kim, 2004, Guadagno et al., 2020) and clinical data (Baas et al., 2004, Bruder et al., 2017) suggests lateralization of amygdala function. Electrical stimulation of the right hemisphere may produce more dysphoric/negative responses compared to the left (Lanteaume et al., 2006, Smith et al., 2006) and is involved in the rapid appraisal of aversive stimuli (Gläscher and Adolphs, 2003, Sergerie et al., 2008). The left amygdala, conversely, has been shown to be involved in generally slower emotional appraisals and with more positive emotion generation (Gläscher and Adolphs, 2003, Sergerie et al., 2008). In addition to modulating neuroendocrine responses, the amygdala modulates autonomic nervous system activity in the context of emotionally salient stimuli processing (Beissner et al., 2013, Gläscher and Adolphs, 2003). Interestingly, an electrical stimulation study of nine people with epilepsy showed that amygdala stimulation can modulate autonomic activity without eliciting concurrent subjective emotional responses, with the exception of one patient who reported marked fear and anxiety when the right amygdala was stimulated, in an area speculated to be close to the right central nucleus (Inman et al., 2018). Unilateral right sided Electroconvulsive therapy in a group of 14 antidepressant treated patients diagnosed with treatment resistant depression increased right corticoamygdaloid transition area (CATA), basal, and lateral amygdala nuclei volumes (Gryglewski et al., 2019).
It is well established that MDD is strongly associated with stressful life events. The mechanistic mediators underlying the interaction between stress and the structural development of the amygdala, include but are not exclusive to cortisol, Brain-derived neurotrophic factor (BDNF), glutamate and epigenetic mechanisms (McEwen et al., 2016). Preclinical data suggests that chronic stress may induce divergent changes in amygdala and hippocampal volumes, with enlargement of amygdala regions and reduction of hippocampal regions, largely through alterations in dendritic remodelling (Blugeot et al., 2011, Lakshminarasimhan and Chattarji, 2012, Mitra et al., 2005, Roozendaal et al., 2009, Vyas et al., 2002, Vyas et al., 2004). In our cohort of MDD participants, we also found divergent changes in amygdala and hippocampal volumes, as evidenced by smaller hippocampal substructures bilaterally, but more pronounced on the left (Roddy et al., 2019) and the present finding of larger right medial amygdala nuclei, known to modulate complex social behaviours in rodents (Hu et al., 2021, Kwon et al., 2021, Shemesh et al., 2016). We acknowledge the association between amygdala substructural volumes and phase and duration of illness, however in our MDD group these associations did not survive correction for multiple comparisons (Table S5a, b).
The reciprocal shaping of the HPA axis and limbic brain regions, under the influence of environmental stressors, especially during neurodevelopmental windows, is of particular importance for the individualized sensitivity to the development and trajectory of stress related disorders such as depression. Exposure to early stressful events may lead to increases in amygdala volumes (Tottenham et al., 2010). However, the process is dynamic and dependent on developmental stage, as highlighted by a recent longitudinal study showing a transition from blunted morning cortisol in childhood to heightened levels in late adolescence, associated with smaller amygdala volumes by adolescence in those that experienced early stressful events (VanTieghem et al., 2021). This may in part explain some of the discrepancies in other studies in younger cohorts that show that elevations in cortisol are associated with larger amygdala volumes (Buss et al., 2012), while others show smaller amygdala volumes are associated with greater cortisol stress responses (Fowler et al., 2021, Pagliaccio et al., 2014).
Our study extends the existing findings by exploring, for the first time, the relationship between HPA axis activity and amygdala subnuclei volumes in MDD. The CAR measures the reactivity of the HPA axis in response to the natural stress of waking (Farrell et al., 2018). Chronic HPA overactivity as demonstrated by a raised waking (baseline) cortisol and a reduced CAR is commonly found in depression (Doolin et al., 2017, Frodl and O'Keane, 2013, Huber et al., 2006, Juruena et al., 2018, O'Keane et al., 2012). In our sample, there were no statistically significant differences in the CAR between the MDD and HC groups (Table S8) which may be related to the limited sample size of the cortisol subgroup analysis. However, within the MDD group, while there were mostly negative CAR associations with multiple amygdala nuclei bilaterally (Table 3), only the inverse relationship between the AUCg and the left CATA survived FDR correction (Fig. S1a), which was not found in the HC group. The lack of CAR differences between MDD and HC, together with the inverse relationship between the CAR and the left CATA in the MDD group suggests that the disruption in the reciprocal relationship between the HPA axis and the amygdala in MDD is non-uniform across the amygdala nuclei.
The CATA is a zone of confluence of the medial basal, paralaminar and periamygdaloid areas. In primates, the CATA provides the main modulatory input to the lateral subdivision of the central nucleus and is a specific target for hippocampal inputs (Fudge et al., 2012, Fudge and Tucker, 2009), and together with hippocampal inputs to the adjacent paralaminar nucleus are implicated in contextual fear learning (deCampo and Fudge, 2012). Consistent with this, in rodents the CATA is indicated in the processing of olfactory information related to reproductive and defensive behaviors (Cádiz-Moretti et al., 2016, Kondoh et al., 2016). While our finding of an inverse relationship between the CAR and the left CATA in MDD is an important exploratory step to further elucidate the intricate bidirectional relationship between the HPA axis and amygdala subnuclei volumes, it should be interpreted with caution due to the limited sample size of the CAR subgroups. Nonetheless, it suggests that further exploration of this relationship in larger longitudinal samples could be helpful in understanding the neurophysiology of pathological emotional states.
There are preliminary indicators that a deeper understanding of the pathophysiology of MDD can lead to translational benefits. Decreased right basolateral amygdala gray matter density was positively correlated with reductions in perceived stress after an 8-week mindfulness-based stress reduction intervention in stressed healthy individuals (Hölzel et al., 2010). More recently, there is growing interest in real-time fMRI amygdala neurofeedback as a clinical tool (Tsuchiyagaito et al., 2021, Young et al., 2017, Zotev and Bodurka, 2020), which has been combined with substructural volume analyses. For example, left amygdala real-time fMRI neurofeedback training in Post-Traumatic Stress disorder (PTSD) resulted in volume increases in the left hippocampal CA1 head region (Misaki et al., 2021). Our study showing that MDD is associated with specific alterations in amygdala nuclei, together with exploratory CAR associations, may open up avenues for the further advance of precise-personalized treatment paradigms.
5. Limitations
Automated segmentation in neuroimaging analysis has been argued to have interpretation and validity issues compared to expert manual segmentation (Dill et al., 2015). On the other hand, manual measurements are costly and time consuming whereas automated segmentation allows large volumes of data, in this case 163 subjects yielded almost 3,000 individual nuclei, to be analyzed accurately. One could argue that an automated proforma is necessary to translate research findings into routine clinical tests. While 3 T MRI is effective, we acknowledge that access to 7 T would have delivered enhanced spatial resolution.
Only a subset of participants had cortisol samples that were suitable for inclusion in the analysis, due to non– or partial compliance with the salivary collection schedule. The study would have been strengthened by having more subjects in the HPA axis arm. We acknowledge the potential confounding influence of antidepressant medication (Hamilton et al., 2008), however, differences between amygdala subnuclei volumes according to antidepressant treatment status did not survive correction for multiple comparisons in our MDD group. We did not record antidepressant type or dose. We acknowledge the mediating influence of traumatic early life events (Gerritsen et al., 2017, VanTieghem et al., 2021) but we didn’t capture this data. Nor did we precisely capture the age of onset for the entire MDD group. We did not complete the SCID for axis 2 personality assessments.
Mood and anxiety are intrinsically linked. As discussed above, enlargement of the basolateral amygdala has been linked to childhood anxiety (Qin et al., 2014). Our study focussed on MDD and while participants may have exhibited anxiety symptoms, they did not meet the threshold for DSM IV diagnosis. In keeping with the overlap of mood, anxiety, and stress, it is interesting to note that a recent well-powered 3 T MRI study of another stress related disorder, PTSD in military veterans, showed larger left and right central, medial, and cortical amygdala nuclei compared to HC (Morey et al., 2020). This study also demonstrated smaller bilateral paralaminar and lateral nuclei in PTSD compared to HC (Morey et al., 2020). Indeed, in our study, the left paralaminar nuclei were smaller in the MDD group in the initial analysis, but this significance did not survive FDR correction. Limited longitudinal inferences can be drawn from our cross-sectional data. Ultimately, large-scale longitudinal studies that incorporate a dimensional approach will be required to definitively resolve these issues (McLean et al., 2020).
6. Conclusion
This study using automated segmentation to perform amygdalar substructural volumetric analysis, found larger medial subnuclei volumes on the right in MDD compared to HC, as well as relatively increased right compared to left whole and substructural volume ratios in MDD. This implies the potential importance of right amygdala substructure volumes in the pathophysiology of state depression. CAR responses were inversely related to the left corticoamygdaloid transition area volume in MDD.
Author contributions
V.O’K, T.F, A.F, S.O’M conceived the study and edited the manuscript. D.R, E.K, A.N, C.F, K.D, and E.R collected and analysed the data and edited the manuscript. J.K analysed the data and edited the manuscript.
Funding
This project was funded under by the Irish Health Research Board as part of the REDEEM (Research in Depression, Endocrinology, Epigenetics and neuroiMaging) study at Trinity College Institute of Neuroscience and the Department of Psychiatry, Trinity College Dublin. Grant code: 201651.12553. We also thank the Meath Foundation, Tallaght University Hospital, for the initial project funding. We acknowledge use of the facilities of the Clinical Research Centre in the RCSI Education and Research Centre and Trinity College High Performance Computing resources and infrastructure funded by Science Foundation Ireland.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We sincerely thank our participants, and especially our patients who, in spite of the burden of their depression, participated in a generous spirit in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102781.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- American Psychiatric Association, 1994. Diagnostic and Statistical Manual of Mental Disorder, 4th ed, Washington, DC.
- Ancelin M.-L., Carrière I., Artero S., Maller J., Meslin C., Ritchie K., Ryan J., Chaudieu I. Lifetime major depression and grey-matter volume. J. Psychiatry Neurosci.: JPN. 2019;44:45. doi: 10.1503/jpn.180026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andraka K., Kondrakiewicz K., Rojek-Sito K., Ziegart-Sadowska K., Meyza K., Nikolaev T., Hamed A., Kursa M., Wójcik M., Danielewski K., Wiatrowska M., Kublik E., Bekisz M., Lebitko T., Duque D., Jaworski T., Madej H., Konopka W., Boguszewski P.M., Knapska E. Distinct circuits in rat central amygdala for defensive behaviors evoked by socially signaled imminent versus remote danger. Curr. Biol. 2021 doi: 10.1016/j.cub.2021.03.047. [DOI] [PubMed] [Google Scholar]
- Arnone D., McIntosh A.M., Ebmeier K.P., Munafo M.R., Anderson I.M. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. Eur. Neuropsychopharmacol. 2012;22:1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Baas D., Aleman A., Kahn R.S. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res. Brain Res. Rev. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Baker K.B., Kim J.J. Amygdalar lateralization in fear conditioning: evidence for greater involvement of the right amygdala. Behav. Neurosci. 2004;118:15–23. doi: 10.1037/0735-7044.118.1.15. [DOI] [PubMed] [Google Scholar]
- Barbier M., Fellmann D., Risold P.Y. Morphofunctional Organization of the Connections From the Medial and Intermediate Parts of the Central Nucleus of the Amygdala Into Distinct Divisions of the Lateral Hypothalamic Area in the Rat. Front. Neurol. 2018;9:688. doi: 10.3389/fneur.2018.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry T.J., Murray L., Fearon P., Moutsiana C., Johnstone T., Halligan S.L. Amygdala volume and hypothalamic-pituitary-adrenal axis reactivity to social stress. Psychoneuroendocrinology. 2017;85:96–99. doi: 10.1016/j.psyneuen.2017.07.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F., Meissner K., Bar K.J., Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J. Neurosci. 2013;33:10503–10511. doi: 10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y. Discovering the false discovery rate. J. R. Stat. Soc.: Ser. B (Stat. Methodol.) 2010;72:405–416. [Google Scholar]
- Blugeot A., Rivat C., Bouvier E., Molet J., Mouchard A., Zeau B., Bernard C., Benoliel J.-J., Becker C. Vulnerability to Depression: From Brain Neuroplasticity to Identification of Biomarkers. J. Neurosci. 2011;31:12889–12899. doi: 10.1523/JNEUROSCI.1309-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchetta M., Iglesias J.E., Cash D.M., Warren J.D., Rohrer J.D. Amygdala subnuclei are differentially affected in the different genetic and pathological forms of frontotemporal dementia. Alzheimers Dement (Amst) 2019;11:136–141. doi: 10.1016/j.dadm.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E., Fornito A., Pantelis C., Yücel M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J. Affect. Disord. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Brown E.S., Hughes C.W., McColl R., Peshock R., King K.S., Rush A.J. Association of depressive symptoms with hippocampal volume in 1936 adults. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 2014;39:770–779. doi: 10.1038/npp.2013.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.S.G., Rutland J.W., Verma G., Feldman R.E., Alper J., Schneider M., Delman B.N., Murrough J.M., Balchandani P. Structural MRI at 7T reveals amygdala nuclei and hippocampal subfield volumetric association with Major Depressive Disorder symptom severity. Sci. Rep. 2019;9:10166. doi: 10.1038/s41598-019-46687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.S.G., Rutland J.W., Verma G., Feldman R.E., Schneider M., Delman B.N., Murrough J.M., Balchandani P. Ultra-High-Resolution Imaging of Amygdala Subnuclei Structural Connectivity in Major Depressive Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2020;5:184–193. doi: 10.1016/j.bpsc.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder G.E., Stewart J.W., McGrath P.J. Right brain, left brain in depressive disorders: Clinical and theoretical implications of behavioral, electrophysiological and neuroimaging findings. Neurosci. Biobehav. Rev. 2017;78:178–191. doi: 10.1016/j.neubiorev.2017.04.021. [DOI] [PubMed] [Google Scholar]
- Buss C., Davis E.P., Shahbaba B., Pruessner J.C., Head K., Sandman C.A. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc. Natl. Acad. Sci. 2012;109:E1312–E1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cádiz-Moretti B., Abellán-Álvaro M., Pardo-Bellver C., Martínez-García F., Lanuza E. Afferent and Efferent Connections of the Cortex-Amygdala Transition Zone in Mice. Front. Neuroanatomy. 2016;10 doi: 10.3389/fnana.2016.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCampo D.M., Fudge J.L. Where and what is the paralaminar nucleus? A review on a unique and frequently overlooked area of the primate amygdala. Neurosci. Biobehav. Rev. 2012;36:520–535. doi: 10.1016/j.neubiorev.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dill V., Franco A.R., Pinho M.S. Automated methods for hippocampus segmentation: the evolution and a review of the state of the art. Neuroinformatics. 2015;13:133–150. doi: 10.1007/s12021-014-9243-4. [DOI] [PubMed] [Google Scholar]
- Disner S.G., Beevers C.G., Haigh E.A., Beck A.T. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. England. 2011:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Doolin K., Allers K.A., Pleiner S., Liesener A., Farrell C., Tozzi L., O'Hanlon E., Roddy D., Frodl T., Harkin A., O'Keane V. Altered tryptophan catabolite concentrations in major depressive disorder and associated changes in hippocampal subfield volumes. Psychoneuroendocrinology. 2018;95:8–17. doi: 10.1016/j.psyneuen.2018.05.019. [DOI] [PubMed] [Google Scholar]
- Doolin K., Farrell C., Tozzi L., Harkin A., Frodl T., O’Keane V. Diurnal hypothalamic-pituitary-adrenal axis measures and inflammatory marker correlates in major depressive disorder. Int. J. Mol. Sci. 2017;18:2226. doi: 10.3390/ijms18102226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell C., Doolin K., O' Leary N., Jairaj C., Roddy D., Tozzi L., Morris D., Harkin A., Frodl T., Nemoda Z., Szyf M., Booij L., O'Keane V. DNA methylation differences at the glucocorticoid receptor gene in depression are related to functional alterations in hypothalamic-pituitary-adrenal axis activity and to early life emotional abuse. Psychiatry Res. 2018;265:341–348. doi: 10.1016/j.psychres.2018.04.064. [DOI] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fowler C.H., Bogdan R., Gaffrey M.S. Stress-induced cortisol response is associated with right amygdala volume in early childhood. Neurobiol. Stress. 2021;14 doi: 10.1016/j.ynstr.2021.100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A.S., Shackman A.J. The central extended amygdala in fear and anxiety: Closing the gap between mechanistic and neuroimaging research. Neurosci. Lett. 2019;693:58–67. doi: 10.1016/j.neulet.2017.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese J.L., Amaral D.G. The organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey. J. Comp. Neurol. 2005;486:295–317. doi: 10.1002/cne.20520. [DOI] [PubMed] [Google Scholar]
- Frodl T., Meisenzahl E., Zetzsche T., Bottlender R., Born C., Groll C., Jäger M., Leinsinger G., Hahn K., Möller H.-J. Enlargement of the amygdala in patients with a first episode of major depression. Biol. Psychiatry. 2002;51:708–714. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- Frodl T., Meisenzahl E.M., Zetzsche T., Born C., Jager M., Groll C., Bottlender R., Leinsinger G., Moller H.J. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biol. Psychiatry. 2003;53:338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- Frodl T., O'Keane V. How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans. Neurobiol. Dis. 2013;52:24–37. doi: 10.1016/j.nbd.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Fudge J.L., deCampo D.M., Becoats K.T. Revisiting the hippocampal-amygdala pathway in primates: association with immature-appearing neurons. Neuroscience. 2012;212:104–119. doi: 10.1016/j.neuroscience.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge J.L., Tucker T. Amygdala projections to central amygdaloid nucleus subdivisions and transition zones in the primate. Neuroscience. 2009;159:819–841. doi: 10.1016/j.neuroscience.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritsen L., Milaneschi Y., Vinkers C.H., van Hemert A.M., van Velzen L., Schmaal L., Penninx B.W.J.H. HPA Axis Genes, and Their Interaction with Childhood Maltreatment, are Related to Cortisol Levels and Stress-Related Phenotypes. Neuropsychopharmacology. 2017;42:2446–2455. doi: 10.1038/npp.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino W.J., Eban-Rothschild A., Christoffel D.J., Li S.B., Malenka R.C., de Lecea L. Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nat. Neurosci. 2018;21:1084–1095. doi: 10.1038/s41593-018-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J., Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J. Neurosci. 2003;23:10274–10282. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens L., Kukolja J., Onur O.A., Fink G.R., Maier W., Griez E., Schruers K., Hurlemann R. Selective processing of social stimuli in the superficial amygdala. Hum. Brain Mapp. 2009;30:3332–3338. doi: 10.1002/hbm.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A.M., Rasmussen J.M., Entringer S., Ben Ward E., Rudolph M.D., Gilmore J.H., Styner M., Wadhwa P.D., Fair D.A., Buss C. Maternal Cortisol Concentrations During Pregnancy and Sex-Specific Associations With Neonatal Amygdala Connectivity and Emerging Internalizing Behaviors. Biol. Psychiatry. 2019;85:172–181. doi: 10.1016/j.biopsych.2018.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski G., Baldinger-Melich P., Seiger R., Godbersen G.M., Michenthaler P., Klöbl M., Spurny B., Kautzky A., Vanicek T., Kasper S., Frey R., Lanzenberger R. Structural changes in amygdala nuclei, hippocampal subfields and cortical thickness following electroconvulsive therapy in treatment-resistant depression: longitudinal analysis. Br. J. Psychiatry: J. Mental Sci. 2019;214:159–167. doi: 10.1192/bjp.2018.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno A., Verlezza S., Long H., Wong T.P., Walker C.-D. It Is All in the Right Amygdala: Increased Synaptic Plasticity and Perineuronal Nets in Male, But Not Female, Juvenile Rat Pups after Exposure to Early-Life Stress. J. Neurosci. 2020;40:8276–8291. doi: 10.1523/JNEUROSCI.1029-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakamata Y., Komi S., Moriguchi Y., Izawa S., Motomura Y., Sato E., Mizukami S., Kim Y., Hanakawa T., Inoue Y., Tagaya H. Amygdala-centred functional connectivity affects daily cortisol concentrations: a putative link with anxiety. Sci. Rep. 2017;7:8313. doi: 10.1038/s41598-017-08918-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Siemer M., Gotlib I.H. Amygdala volume in Major Depressive Disorder: A meta-analysis of magnetic resonance imaging studies. Mol. Psychiatry. 2008;13:993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L., De Olmos J., Alheid G., Pearson J., Sakamoto N., Shinoda K., Marksteiner J., Switzer R. The human basal forebrain. Part II. The primate nervous system. Part III. Handbook Chem. Neuroanatomy. 1999;15:57–226. [Google Scholar]
- Herman J.P., McKlveen J.M., Ghosal S., Kopp B., Wulsin A., Makinson R., Scheimann J., Myers B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Comprehensive Physiol. 2016;6:603–621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel B.K., Carmody J., Evans K.C., Hoge E.A., Dusek J.A., Morgan L., Pitman R.K., Lazar S.W. Stress reduction correlates with structural changes in the amygdala. Soc. Cognit. Affect. Neurosci. 2010;5:11–17. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrybouski S., Aghamohammadi-Sereshki A., Madan C.R., Shafer A.T., Baron C.A., Seres P., Beaulieu C., Olsen F., Malykhin N.V. Amygdala subnuclei response and connectivity during emotional processing. Neuroimage. 2016;133:98–110. doi: 10.1016/j.neuroimage.2016.02.056. [DOI] [PubMed] [Google Scholar]
- Hu R.K., Zuo Y., Ly T., Wang J., Meera P., Wu Y.E., Hong W. An amygdala-to-hypothalamus circuit for social reward. Nat. Neurosci. 2021;24:831–842. doi: 10.1038/s41593-021-00828-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber T.J., Issa K., Schik G., Wolf O.T. The cortisol awakening response is blunted in psychotherapy inpatients suffering from depression. Psychoneuroendocrinology. 2006;31:900–904. doi: 10.1016/j.psyneuen.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Hur J., Stockbridge M.D., Fox A.S., Shackman A.J. Dispositional negativity, cognition, and anxiety disorders: An integrative translational neuroscience framework. Prog. Brain Res. 2019;247:375–436. doi: 10.1016/bs.pbr.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman C.S., Bijanki K.R., Bass D.I., Gross R.E., Hamann S., Willie J.T. Human amygdala stimulation effects on emotion physiology and emotional experience. Neuropsychologia. 2018 doi: 10.1016/j.neuropsychologia.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak P.H., Tye K.M. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings J.H., Sparta D.R., Stamatakis A.M., Ung R.L., Pleil K.E., Kash T.L., Stuber G.D. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J.B. Further contributions to the study of the evolution of the forebrain. J. Compar. Neurol. 1923;35:337–481. [Google Scholar]
- Juruena M.F., Bocharova M., Agustini B., Young A.H. Atypical depression and non-atypical depression: Is HPA axis function a biomarker? A systematic review. J. Affect. Disord. 2018;233:45–67. doi: 10.1016/j.jad.2017.09.052. [DOI] [PubMed] [Google Scholar]
- Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin N.H., Fox A.S., Kovner R., Riedel M.K., Fekete E.M., Roseboom P.H., Trompdo P.M., Grabow B.P., Olsen M.E., Brodsky E.K., McFarlin D.R., Alexander A.L., Emborg M.E., Block W.F., Fudge J.L., Oler J.A. Overexpressing corticotropin-releasing factor in the primate amygdala increases anxious temperament and alters its neural circuit. Biol. Psychiatry. 2016;80:345–355. doi: 10.1016/j.biopsych.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamali A., Sair H.I., Blitz A.M., Riascos R.F., Mirbagheri S., Keser Z., Hasan K.M. Revealing the ventral amygdalofugal pathway of the human limbic system using high spatial resolution diffusion tensor tractography. Brain Struct. Funct. 2016;221:3561–3569. doi: 10.1007/s00429-015-1119-3. [DOI] [PubMed] [Google Scholar]
- Kiem S.A., Andrade K.C., Spoormaker V.I., Holsboer F., Czisch M., Sämann P.G. Resting state functional MRI connectivity predicts hypothalamus-pituitary-axis status in healthy males. Psychoneuroendocrinology. 2013;38:1338–1348. doi: 10.1016/j.psyneuen.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Kohler C.A., Carvalho A.F., Alves G.S., McIntyre R.S., Hyphantis T.N., Cammarota M. Autobiographical Memory Disturbances in Depression: A Novel Therapeutic Target? Neural Plast. 2015;2015 doi: 10.1155/2015/759139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh K., Lu Z., Ye X., Olson D.P., Lowell B.B., Buck L.B. A specific area of olfactory cortex involved in stress hormone responses to predator odours. Nature. 2016;532:103–106. doi: 10.1038/nature17156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovner R., Souaiaia T., Fox A.S., French D.A., Goss C.E., Roseboom P.H., Oler J.A., Riedel M.K., Fekete E.M., Fudge J.L., Knowles J.A., Kalin N.H. Transcriptional Profiling of Primate Central Nucleus of the Amygdala Neurons to Understand the Molecular Underpinnings of Early-Life Anxious Temperament. Biol. Psychiatry. 2020 doi: 10.1016/j.biopsych.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G., Tebartz van Elst L., Regen F., Deuschle M., Heuser I., Colla M. Reduced amygdala volume in newly admitted psychiatric in-patients with unipolar major depression. J. Psychiatr. Res. 2009;43:1112–1117. doi: 10.1016/j.jpsychires.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Kwon J.T., Ryu C., Lee H., Sheffield A., Fan J., Cho D.H., Bigler S., Sullivan H.A., Choe H.K., Wickersham I.R., Heiman M., Choi G.B. An amygdala circuit that suppresses social engagement. Nature. 2021;593:114–118. doi: 10.1038/s41586-021-03413-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarasimhan H., Chattarji S. Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PLoS One. 2012;7:e30481. doi: 10.1371/journal.pone.0030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanteaume L., Khalfa S., Régis J., Marquis P., Chauvel P., Bartolomei F. Emotion induction after direct intracerebral stimulations of human amygdala. Cereb. Cortex. 2006;17:1307–1313. doi: 10.1093/cercor/bhl041. [DOI] [PubMed] [Google Scholar]
- Lindquist K.A., Wager T.D., Kober H., Bliss-Moreau E., Barrett L.F. The brain basis of emotion: a meta-analytic review. Behav Brain Sci. 2012;35:121–143. doi: 10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Nasca C., Gray J.D. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean S.A., Ressler K., Koenen K.C., Neylan T., Germine L., Jovanovic T., Clifford G.D., Zeng D., An X., Linnstaedt S., Beaudoin F., House S., Bollen K.A., Musey P., Hendry P., Jones C.W., Lewandowski C., Swor R., Datner E., Mohiuddin K., Stevens J.S., Storrow A., Kurz M.C., McGrath M.E., Fermann G.J., Hudak L.A., Gentile N., Chang A.M., Peak D.A., Pascual J.L., Seamon M.J., Sergot P., Peacock W.F., Diercks D., Sanchez L.D., Rathlev N., Domeier R., Haran J.P., Pearson C., Murty V.P., Insel T.R., Dagum P., Onnela J.P., Bruce S.E., Gaynes B.N., Joormann J., Miller M.W., Pietrzak R.H., Buysse D.J., Pizzagalli D.A., Rauch S.L., Harte S.E., Young L.J., Barch D.M., Lebois L.A.M., van Rooij S.J.H., Luna B., Smoller J.W., Dougherty R.F., Pace T.W.W., Binder E., Sheridan J.F., Elliott J.M., Basu A., Fromer M., Parlikar T., Zaslavsky A.M., Kessler R. The AURORA Study: a longitudinal, multimodal library of brain biology and function after traumatic stress exposure. Mol. Psychiatry. 2020;25:283–296. doi: 10.1038/s41380-019-0581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki M., Mulyana B., Zotev V., Wurfel B.E., Krueger F., Feldner M., Bodurka J. Hippocampal volume recovery with real-time functional MRI amygdala neurofeedback emotional training for posttraumatic stress disorder. J. Affect. Disord. 2021;283:229–235. doi: 10.1016/j.jad.2021.01.058. [DOI] [PubMed] [Google Scholar]
- Mitra R., Jadhav S., McEwen B.S., Vyas A., Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R.A., Clarke E.K., Haswell C.C., Phillips R.D., Clausen A.N., Mufford M.S., Saygin Z., Brancu M., Beckham J.C., Calhoun P.S., Dedert E., Elbogen E.B., Fairbank J.A., Hurley R.A., Kilts J.D., Kimbrel N.A., Kirby A., Marx C.E., McDonald S.D., Moore S.D., Naylor J.C., Rowland J., Swinkels C., Szabo S.T., Taber K.H., Tupler L.A., van Voorhees E.E., Yoash-Gantz R.E., Wagner H.R., LaBar K.S. Amygdala Nuclei Volume and Shape in Military Veterans With Posttraumatic Stress Disorder. Biol.: Cognit. Neurosci. Neuroimaging. 2020;5:281–290. doi: 10.1016/j.bpsc.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B., McKlveen J.M., Herman J.P. Neural regulation of the stress response: the many faces of feedback. Cell. Mol. Neurobiol. 2012;32:683–694. doi: 10.1007/s10571-012-9801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan M., Roman E., Nasa A., Levins K.J., O’Hanlon E., O’Keane V., Willian Roddy D. Hippocampal and Amygdalar Volume Changes in Major Depressive Disorder: A Targeted Review and Focus on Stress. Chronic Stress. 2020;4 doi: 10.1177/2470547020944553. 2470547020944553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keane V., Frodl T., Dinan T.G. A review of Atypical depression in relation to the course of depression and changes in HPA axis organization. Psychoneuroendocrinology. 2012;37:1589–1599. doi: 10.1016/j.psyneuen.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Pagliaccio D., Luby J.L., Bogdan R., Agrawal A., Gaffrey M.S., Belden A.C., Botteron K.N., Harms M.P., Barch D.M. Stress-system genes and life stress predict cortisol levels and amygdala and hippocampal volumes in children. Neuropsychopharmacology. 2014;39:1245–1253. doi: 10.1038/npp.2013.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza O., Bowers D., Gilmore R. Asymmetry of the hippocampus and amygdala in MRI volumetric measurements of normal adults. Journal of the International Neuropsychological Society. 2004;10:664–678. doi: 10.1017/S1355617704105080. [DOI] [PubMed] [Google Scholar]
- Peters A.T., Jenkins L.M., Stange J.P., Bessette K.L., Skerrett K.A., Kling L.R., Welsh R.C., Milad M.R., Phan K.L., Langenecker S.A. Pre-scan cortisol is differentially associated with enhanced connectivity to the cognitive control network in young adults with a history of depression. Psychoneuroendocrinology. 2019;104:219–227. doi: 10.1016/j.psyneuen.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomrenze M.B., Tovar-Diaz J., Blasio A., Maiya R., Giovanetti S.M., Lei K., Morikawa H., Hopf F.W., Messing R.O. A Corticotropin Releasing Factor Network in the Extended Amygdala for Anxiety. J. Neurosci. 2019;39:1030–1043. doi: 10.1523/JNEUROSCI.2143-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner J.C., Dedovic K., Pruessner M., Lord C., Buss C., Collins L., Dagher A., Lupien S.J. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35:179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Putnam P.T., Chang S.W.C. Toward a holistic view of value and social processing in the amygdala: Insights from primate behavioral neurophysiology. Behav. Brain Res. 2021;411 doi: 10.1016/j.bbr.2021.113356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S., Young C.B., Duan X., Chen T., Supekar K., Menon V. Amygdala Subregional Structure and Intrinsic Functional Connectivity Predicts Individual Differences in Anxiety During Early Childhood. Biol. Psychiatry. 2014;75:892–900. doi: 10.1016/j.biopsych.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddy D., O'Keane V. Cornu Ammonis Changes Are at the Core of Hippocampal Pathology in Depression. Chronic Stress (Thousand Oaks) 2019;3 doi: 10.1177/2470547019849376. 2470547019849376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddy D.W., Farrell C., Doolin K., Roman E., Tozzi L., Frodl T., O'Keane V., O'Hanlon E. The Hippocampus in Depression: More Than the Sum of Its Parts? Advanced Hippocampal Substructure Segmentation in Depression. Biol. Psychiatry. 2019;85:487–497. doi: 10.1016/j.biopsych.2018.08.021. [DOI] [PubMed] [Google Scholar]
- Roozendaal B., McEwen B.S., Chattarji S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Saleh K., Carballedo A., Lisiecka D., Fagan A.J., Connolly G., Boyle G., Frodl T. Impact of family history and depression on amygdala volume. Psychiatry Res. 2012;203:24–30. doi: 10.1016/j.pscychresns.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Saygin Z., Kliemann D., Iglesias J., van der Kouwe A.J., Boyd E., Reuter M., Stevens A., Van Leemput K., McKee A., Frosch M.P. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. NeuroImage. 2017;155:370–382. doi: 10.1016/j.neuroimage.2017.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L., Pozzi E., Ho C.T., van Velzen L.S., Veer I.M., Opel N., Van Someren E.J.W., Han L.K.M., Aftanas L., Aleman A., Baune B.T., Berger K., Blanken T.F., Capitão L., Couvy-Duchesne B., Cullen R.K., Dannlowski U., Davey C., Erwin-Grabner T., Evans J., Frodl T., Fu C.H.Y., Godlewska B., Gotlib I.H., Goya-Maldonado R., Grabe H.J., Groenewold N.A., Grotegerd D., Gruber O., Gutman B.A., Hall G.B., Harrison B.J., Hatton S.N., Hermesdorf M., Hickie I.B., Hilland E., Irungu B., Jonassen R., Kelly S., Kircher T., Klimes-Dougan B., Krug A., Landrø N.I., Lagopoulos J., Leerssen J., Li M., Linden D.E.J., MacMaster F.P., McIntosh M.A., Mehler D.M.A., Nenadić I., Penninx B.W.J.H., Portella M.J., Reneman L., Rentería M.E., Sacchet M.D., Sämann G.P., Schrantee A., Sim K., Soares J.C., Stein D.J., Tozzi L., van Der Wee N.J.A., van Tol M.-J., Vermeiren R., Vives-Gilabert Y., Walter H., Walter M., Whalley H.C., Wittfeld K., Whittle S., Wright M.J., Yang T.T., Zarate C., Thomopoulos S.I., Jahanshad N., Thompson P.M., Veltman D.J. ENIGMA MDD: seven years of global neuroimaging studies of major depression through worldwide data sharing. Transl. Psychiatry. 2020;10:172. doi: 10.1038/s41398-020-0842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L., Veltman D.J., van Erp T.G.M., Sämann P.G., Frodl T., Jahanshad N., Loehrer E., Tiemeier H., Hofman A., Niessen W.J., Vernooij M.W., Ikram M.A., Wittfeld K., Grabe H.J., Block A., Hegenscheid K., Völzke H., Hoehn D., Czisch M., Lagopoulos J., Hatton S.N., Hickie I.B., Goya-Maldonado R., Krämer B., Gruber O., Couvy-Duchesne B., Rentería M.E., Strike L.T., Mills N.T., de Zubicaray G.I., McMahon K.L., Medland S.E., Martin N.G., Gillespie N.A., Wright M.J., Hall G.B., MacQueen G.M., Frey E.M., Carballedo A., van Velzen L.S., van Tol M.J., van der Wee N.J., Veer I.M., Walter H., Schnell K., Schramm E., Normann C., Schoepf D., Konrad C., Zurowski B., Nickson T., McIntosh A.M., Papmeyer M., Whalley H.C., Sussmann J.E., Godlewska B.R., Cowen P.J., Fischer F.H., Rose M., Penninx B.W.J.H., Thompson P.M., Hibar D.P., for the, E.-M.D.D.W.G. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol. Psychiatry. 2016;21:806–812. doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmacher A., Mössner R., Jessen F., Scheef L., Block W., Belloche A.C., Lennertz L., Welper H., Höfels S., Pfeiffer U., Wagner M., Maier W., Schwab S., Zobel A. Association of amygdala volumes with cortisol secretion in unipolar depressed patients. Psychiatry Res. 2012;202:96–103. doi: 10.1016/j.pscychresns.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Sergerie K., Chochol C., Armony J.L. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Shackman A.J., Tromp D.P.M., Stockbridge M.D., Kaplan C.M., Tillman R.M., Fox A.S. Dispositional negativity: An integrative psychological and neurobiological perspective. Psychol. Bull. 2016;142:1275–1314. doi: 10.1037/bul0000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh Y., Forkosh O., Mahn M., Anpilov S., Sztainberg Y., Manashirov S., Shlapobersky T., Elliott E., Tabouy L., Ezra G., Adler E.S., Ben-Efraim Y.J., Gil S., Kuperman Y., Haramati S., Dine J., Eder M., Deussing J.M., Schneidman E., Yizhar O., Chen A. Ucn3 and CRF-R2 in the medial amygdala regulate complex social dynamics. Nat. Neurosci. 2016;19:1489–1496. doi: 10.1038/nn.4346. [DOI] [PubMed] [Google Scholar]
- Shen X., Reus L.M., Cox S.R., Adams M.J., Liewald D.C., Bastin M.E., Smith D.J., Deary I.J., Whalley H.C., McIntosh A.M. Subcortical volume and white matter integrity abnormalities in major depressive disorder: findings from UK Biobank imaging data. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-017-05507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.R., Lee G.P., Fountas K., King D.W., Jenkins P.D. Intracranial stimulation study of lateralization of affect. Epilepsy Behav. 2006;8:534–541. doi: 10.1016/j.yebeh.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Stephanie Campbell, Michael Marriott, Claude Nahmias, MacQueen Glenda M. Lower Hippocampal Volume in Patients Suffering From Depression: A Meta-Analysis. Am. J. Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Stetler C., Miller G.E. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom. Med. 2011;73:114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Tang Y., Wang F., Xie G., Liu J., Li L., Su L., Liu Y., Hu X., He Z., Blumberg H.P. Reduced ventral anterior cingulate and amygdala volumes in medication-naïve females with major depressive disorder: A voxel-based morphometric magnetic resonance imaging study. Psychiatry Res.: Neuroimaging. 2007;156:83–86. doi: 10.1016/j.pscychresns.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Hare T.A., Quinn B.T., McCarry T.W., Nurse M., Gilhooly T., Millner A., Galvan A., Davidson M.C., Eigsti I.-M., Thomas K.M., Freed P.J., Booma E.S., Gunnar M.R., Altemus M., Aronson J., Casey B.J. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev. Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiyagaito A., Smith J.L., El-Sabbagh N., Zotev V., Misaki M., Al Zoubi O., Kent Teague T., Paulus M.P., Bodurka J., Savitz J. Real-time fMRI neurofeedback amygdala training may influence kynurenine pathway metabolism in major depressive disorder. NeuroImage: Clin. 2021;29 doi: 10.1016/j.nicl.2021.102559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisvaser S., Lin T., Admon R., Podlipsky I., Greenman Y., Stern N., Fruchter E., Wald I., Pine D.S., Tarrasch R., Bar-Haim Y., Hendler T. Neural traces of stress: cortisol related sustained enhancement of amygdala-hippocampal functional connectivity. Front. Hum. Neurosci. 2013;7:313. doi: 10.3389/fnhum.2013.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Kar L.D., Blair M.L. Forebrain pathways mediating stress-induced hormone secretion. Front. Neuroendocrinol. 1999;20:1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- van Eijndhoven P., van Wingen G., van Oijen K., Rijpkema M., Goraj B., Jan Verkes R., Oude Voshaar R., Fernández G., Buitelaar J., Tendolkar I. Amygdala Volume Marks the Acute State in the Early Course of Depression. Biol. Psychiatry. 2009;65:812–818. doi: 10.1016/j.biopsych.2008.10.027. [DOI] [PubMed] [Google Scholar]
- van Elst L.T., Woermann F., Lemieux L., Trimble M. Increased amygdala volumes in female and depressed humans. A quantitative magnetic resonance imaging study. Neurosci. Lett. 2000;281:103–106. doi: 10.1016/s0304-3940(00)00815-6. [DOI] [PubMed] [Google Scholar]
- VanTieghem M., Korom M., Flannery J., Choy T., Caldera C., Humphreys K.L., Gabard-Durnam L., Goff B., Gee D.G., Telzer E.H., Shapiro M., Louie J.Y., Fareri D.S., Bolger N., Tottenham N. Longitudinal changes in amygdala, hippocampus and cortisol development following early caregiving adversity. Dev. Cognit. Neurosci. 2021;48 doi: 10.1016/j.dcn.2021.100916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A., Mitra R., Shankaranarayana Rao B.S., Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A., Pillai A.G., Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Wang Q., Verweij E.W., Krugers H.J., Joels M., Swaab D.F., Lucassen P.J. Distribution of the glucocorticoid receptor in the human amygdala; changes in mood disorder patients. Brain Struct. Funct. 2014;219:1615–1626. doi: 10.1007/s00429-013-0589-4. [DOI] [PubMed] [Google Scholar]
- Wise T., Radua J., Via E., Cardoner N., Abe O., Adams T.M., Amico F., Cheng Y., Cole J.H., de Azevedo Marques C., Dickstein D.P., Farrow T.F.D., Frodl T., Wagner G., Gotlib I.H., Gruber O., Ham B.J., Job D.E., Kempton M.J., Kim M.J., Koolschijn P.C.M.P., Malhi G.S., Mataix-Cols D., McIntosh A.M., Nugent A.C., O'Brien J.T., Pezzoli S., Phillips M.L., Sachdev P.S., Salvadore G., Selvaraj S., Stanfield A.C., Thomas A.J., van Tol M.J., van der Wee N.J.A., Veltman D.J., Young A.H., Fu C.H., Cleare A.J., Arnone D. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol. Psychiatry. 2017;22:1455–1463. doi: 10.1038/mp.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K.D., Siegle G.J., Zotev V., Phillips R., Misaki M., Yuan H., Drevets W.C., Bodurka J. Randomized Clinical Trial of Real-Time fMRI Amygdala Neurofeedback for Major Depressive Disorder: Effects on Symptoms and Autobiographical Memory Recall. Am. J. Psychiatry. 2017;174:748–755. doi: 10.1176/appi.ajp.2017.16060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V., Bodurka J. Effects of simultaneous real-time fMRI and EEG neurofeedback in major depressive disorder evaluated with brain electromagnetic tomography. NeuroImage: Clin. 2020;28 doi: 10.1016/j.nicl.2020.102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.