Abstract
Study Objectives
Memory consolidation benefits from a retention period filled with sleep. Several theoretical accounts assume that slow-wave sleep (SWS) contributes functionally to processes underlying the stabilization of declarative memories during sleep. However, reports on correlations between memory retention and the amount of SWS are mixed and typically rely on between-subject correlations and small sample sizes. Here we tested for the first time whether the amount of SWS during sleep predicts the effect of sleep on memory consolidation on an intra-individual level in a large sample.
Methods
One hundred and fifty-nine healthy participants came to the lab twice and took a 90 min nap in both sessions. Sleep-mediated memory benefits were tested using the paired associates word-learning task in both sessions.
Results
In contrast to the theoretical prediction, intra-individual differences in sleep-mediated memory benefits did not significantly correlate with differences in SWS or SWA between the two naps. Also between subjects, the amount of SWS did not correlate with memory retention across the nap. However, subjective ratings of sleep quality were significantly associated with the amount of SWS.
Conclusion
Our results question the notion that the amount of SWS per se is functionally related to processes of memory consolidation during sleep. While our results do not exclude an important role of SWS for memory, they suggest that “more SWS” does not necessarily imply better memory consolidation.
Keywords: slow–wave sleep, sleep-dependent memory consolidation, declarative memory, within-subjects
Statement of Significance.
Sleep benefits memory, but it remains an open question whether more time spent in deeper sleep stages predict better memory consolidation. By showing the lack of an association between deep sleep and memory in a large sample during two daytime naps, we close an important gap in theories of memories and sleep. Importantly, our results show that even if the same person spends more time in deep sleep or has higher slow-wave activity power in nap A compared to nap B, this does not lead to a better memory retrieval after nap A. Thus, mechanisms underlying memory consolidation during sleep must be more specific, as the simple passage of time spent in deep sleep appears to not have enough explanatory power.
Introduction
More than 100 years ago, studies about forgetting showed a slower forgetting rate when the retention interval was filled with sleep compared to wakefulness [1–3]. Although early theoretical accounts explained the beneficial effect of sleep on memory formation by passive protection of memory traces against interference, current theories assume a more active role in memory consolidation [4]. The active system consolidation hypothesis [5] assumes that memory traces are actively strengthened by sleep. Slow oscillations (SO) orchestrate memory replay in a complex interplay of sleep spindles and hippocampal sharp-wave ripples and thereby promote the integration of newly learned memory traces into cortical long-term memory. According to this theory, memories are particularly stabilized during slow-wave sleep (SWS) due to repeated reactivations of memory content [6]. Also, the synaptic down-selection hypothesis [7] attributes oscillatory processes occurring mainly during SWS a key function in plastic processes occurring during sleep. Here, irrelevant and weak synaptic connections are “downscaled” during slow oscillations, thereby improving the signal-to-noise ratio and preparing the brain for new learning the upcoming day. While the assumed mechanisms differ between the two theories, a possible prediction from both theories is that more SWS should lead to higher memory benefits after sleep: The active system consolidation hypotheses would argue that the longer period of slow-wave sleep offered a prolonged window in which reactivations, and hence, consolidation could take place. The synaptic homeostasis hypothesis would explain those findings by an improved signal-to-noise-ratio in memory after more slow oscillations occurring during longer periods of SWS.
In support of this notion, several studies have reported strong associations between memory retention across sleep and the amount of SWS. For example, correlations between r = 0.50 [8] and r = 0.69 [9] between the retention of word pairs across sleep and the amount of SWS have been reported in young healthy participants. At the same time, age-related declines of memory retention across a night of sleep were correlated with a reduced amount of early nocturnal deep sleep in middle-aged adults [10]. High positive correlations were also reported for memory retention and slow-wave activity, a frequency band of 0.5–4.5 Hz associated with slow-wave sleep [11–13].
In contrast to these reports of very high and positive correlations between sleep-mediated memory benefits and SWS, findings from other studies were less consistent [14]. For example, two studies reported positive, but non-significant associations between the percentage of SWS in a nap and improvement in a paired associates task [15] or a recognition task [16]. Others found the positive correlations only in some of their subgroups, for instance, high versus low performers or subjects who expected a retrieval test after the sleep period versus those that did not [17, 18]. Some reported positive correlations in nocturnal naps of 90 min duration, but not 40 min [19] and only marginal memory improvements across a 60 min daytime nap [20]. The latter even specified further that for those 60 min naps which included rapid eye movement (REM) sleep, memory improvement did correlate with the product of SWS and REM sleep. Other studies reported correlations with performance in a paired associates learning-task or in a visuospatial task in young, but not old participants [21, 22]. Another study found a correlation between SWS and memory performance only for items challenged by retroactive interference, but not spared ones [23]. Finally, a number of studies did not find any positive associations between the amount of SWS and memory improvement across sleep with younger adults [24–27]. Interestingly, even negative correlations (r = –0.45 and r = –0.62) between sleep-mediated memory benefits and SWS were reported in a recognition test of neutral and emotional pictures in young and old adults [28].
This inconsistency in findings might be due to chance findings after testing various correlations without correcting for multiple testing. Another reason might be that usually small numbers of subjects had been tested. This is particularly critical when analyzing correlations [29]. Such methodological problems might have led to an overestimation of the association strength. To overcome this problem, we were one of the few groups testing sleep with home recordings in 929 subjects [30]. Memory for emotional and neutral pictures was tested before the nocturnal sleep in an evening short delay memory test. Subjects recalled the same pictures in the morning (long delay) after having learned and recalled a parallel set of pictures (morning short delay). No associations between recall performance in that task and time spent in SWS were significant. Thus, on an inter-individual level, it seems that differences in time spent in SWS do not predict differences in memory retention across sleep. We however pointed out how important it would be to examine intra-individual variations, which might be “much more important for memory processes occurring during sleep as compared to interindividual differences” [30] (p. 957).
One of the main issues with inter-individual variations is that both memory and the duration of SWS strongly vary between individuals [31], while the intra-individual EEG profiles in the same subjects across nights are highly stable [31, 32]. The stable trait components of the sleep EEG might be related to genetics [33], neuroanatomy [34], age [35] or other factors. Thus, specific amounts of SWS might be relatively high for one person, but low for another, and memory consolidation might require more SWS in one person compared to another to achieve the same memory score. In addition, confounding third variables like age and gender influence both the amount of SWS and memory: SWS [36, 37] and memory are higher in young as compared to older adults [38, 39], and women have typically more SWS and better verbal and episodic memory than men [40, 41]. Thus, intra-individual associations between SWS and memory might be much more informative: if the same person has more SWS during sleep A as during sleep B, then sleep-mediated memory benefits across sleep A should be higher as compared to sleep B. Therefore, the difference in SWS between sleep A and B should be positively correlated with the difference between sleep-mediated memory benefits across sleep A versus B. According to the theoretical accounts cited above, we would expect such a positive, intra-individual correlation between the amount of SWS and memory retention across sleep.
We tested this prediction in a large sample of healthy young participants, consisting of a merged data set of four different nap studies performed in our lab with undisturbed sleep. All studies contained a within-subjects design with two naps. One served as a control nap while for the other we used different non-pharmacological interventions presented during falling asleep to increase the amount of SWS. While we were interested in the effect of the intervention in the primary studies, we were only interested in the differences between the two naps in terms of SWS and memory performance here. This was either possible by comparing session 1 to session 2 or intervention compared to non-intervention session. We decided to perform the latter difference which for us was more interesting and avoided unspecific habituation effects. On a mathematical level, we did not expect to find any differences between this choice and the comparison between session 1 versus 2, but decided to do control analyses for the main analysis. In each study, we tested declarative memory performance before and after the sleep period with the same memory task. Thus, we had a large sample of within-subjects’ undisturbed naps including different sleep patterns and sleep-dependent memory consolidation measures to investigate. If indeed the amount of SWS is relevant for sleep-dependent memory consolidation, we should find a correlation between the amount of SWS and memory performance across the sleep period. Additionally, we compared subgroups with and without REM sleep and sleeping more and less than 40 min.
Methods
Subjects
The analyzed sample consisted of n = 159 subjects. In one of the studies, we had tested n = 39 women >60 years, thus the age range was 18–82 years with a mean of 33.91 ± 19.29 (mean ± SD). In all except one of the studies we only included females, thus gender distribution is highly unequal (n = 149 females, 93.7% versus n = 10 males). In all studies, subjects were healthy, did not consume alcohol or caffeine on the experimental days, were asked to get up before 8 am and did not have intercontinental flights or shift work within 6 weeks before participation. Except from the older sample, we tested non-habitual nappers. All subjects were previously informed about the study intent and the hypotheses. As we had however assumed that non-hypnotizable subjects counteract hypnotic suggestions, we wanted to prevent any associations to hypnosis by framing it as guided imagery. Thus, we included a cover story in the guided imagery study which was only resolved after session three [42]. All studies had been evaluated by the respective responsible Ethics Committee and subjects had signed informed consents before participation. All subjects were paid for participation.
With n = 150 subjects, we would be able to detect an effect size (i.e. positive correlation coefficient) of r = 0.2 (r = 0.3) with a probability of 80% (>95%) (performed with G*power3 [43]).
Procedure
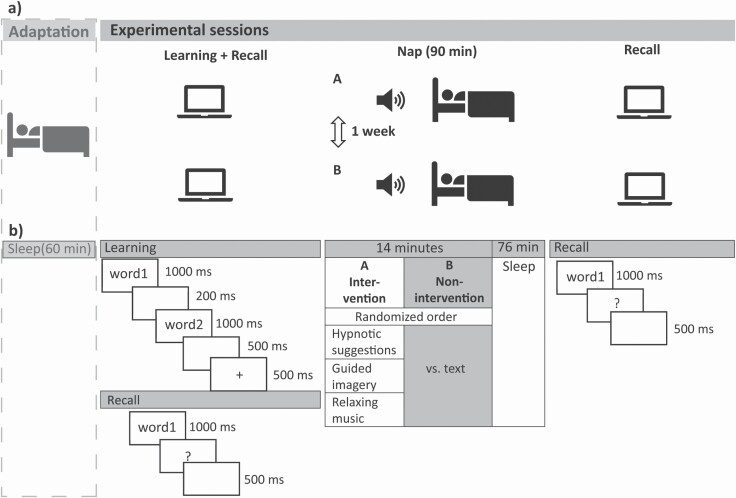
We sampled data from four different studies performed in our labs in Zurich and Fribourg, Switzerland. Their study design and session flow were comparable. Each study included one adaptation nap which took place at least one week before the two experimental naps (see Figure 1A). Only experimental naps were included in this analysis. Those took place on the same weekday within two weeks and at the same time of day. The sessions usually started at around 1 pm so that the naps took place at around 2 pm. Before each experimental nap, an intervention or a control text was presented as soon as subjects were lying in bed. The intervention was either relaxing music, guided imagery or a hypnotic suggestion. The control text was a neutral text about mineral deposits. Those texts or interventions were played in a randomized order via loudspeakers for about 14 min. During this time, subjects were allowed to fall asleep. In total, subjects were in bed for 90 min irrespective of the time they fell asleep and were offered an undisturbed sleep opportunity within this time frame. Before each nap, a declarative memory task was performed. Learning and immediate cued recall took place before sleep, a second cued recall after the nap.
Figure 1.
Study design and session flow.
(A) The study design encompassing an adaptation and two experimental sessions. In the experimental sessions, which took place on the exact weekday within two weeks, subjects learned and recalled word pairs before the 90 min nap. When lying in bed they listened to an acoustic intervention during falling asleep. After the nap, they recalled the previously learned word pairs.
(B) Exact learning sequence of one trial and the two recall phases are displayed. Please note that the times differed for older adults, see methods section. Also, the three types of intervention are listed. They were all tested against the very same non-intervention condition in a randomized order.
Material
Memory task
Paired associates learning-task (PAL). This declarative memory task had been used in previous studies and had been shown to be sleep-dependent [44]. To enable comparability, we adopted the procedure as previously used. During learning, word pairs were visually presented in black font on white ground on a screen. The words of each pair appeared consecutively for 1000 ms, each and were separated from each other by a blank interval of 200 ms (see Figure 1B). Between the pairs, a blank interval of 500 ms and a fixation cross (500 ms) were presented. 80 pairs of words were presented during learning. Subjects were asked to remember as many of them as possible for a later cued recall. In our older adults’ sample, only 40 word pairs were displayed in a slower presentation rate to adapt task difficulty (i.e. words were presented for 2 s, each, separated by a blank interval of 500 ms before the fixation cross, see Cordi et al. [45]). The first cued recall took place directly after learning. Thereby, the first word of each pair was displayed for 1000 ms, followed by a question mark. Subjects were then allowed to answer with the correct second word aloud or to say “next” if the word could not be recalled. There was no time pressure to answer. The experimenter coded correct answers with 1, wrong or no answers with 0. Only the very exact wording was accepted as the correct answer, except plural/singular. No feedback was given. A blank interval of 500 ms prepared for the next cue. The word pairs were recalled in a different order than previously learned. A second recall took place after the sleep period. The procedure hereby was the very same, including the same order of recall as before sleep. Parallel versions were used for the two sessions and used in a randomized order. We here analyzed memory performance as a change in recall performance across the nap. In absolute numbers, we subtracted the amount of correctly recalled word pairs before the nap from the post-sleep performance. Thus, negative numbers mean forgetting. For a percentage of memory consolidation, we defined pre-sleep performance as 100% and calculated the percentage of remembered word pairs after learning given pre-sleep 100%. Thus, memory consolidation of 100% means no change, while numbers above 100% mean improvement across the retention interval. In the between-subjects analyses, we further analyzed the difference between those scores for intervention minus non-intervention naps (or session 1 minus 2, respectively). In n = 5 subjects, one or both memory measures were not available due to data loss or recording difficulties. Thus, analyses including PAL only refer to n = 154 subjects before outlier exclusion.
Sleep data
Sleep was recorded with electromyographic (EMG), electrocardiographic (ECG), and electroencephalographic (EEG) electrodes. Three out of the four studies were recorded with a 128-channel Geodesic Sensor Net, one with Brain Products cap including 32 electrodes. All data was preprocessed with Brain Vision Analyzer (Brain Products, Gilching, Germany). Data were filtered according to the settings suggested by the American Association of Sleep Manual (AASM), [46]. Based on 30 s segments, two scorers, blind to condition, had scored the data into sleep stages 1–3, REM sleep and wake after sleep onset (WASO). Again, for the within-subjects comparisons, we calculated the difference between SWS in the nap after the intervention minus SWS in the non-intervention nap (as a control we tested the difference between SWS in session 1 minus 2).
We further preprocessed the EEG data with the same software to prepare for the analyses of the power in frequency bands of slow-wave activity (SWA, 0.5–4.5 Hz) and separately slow oscillation (SO, 0.5–1 Hz) and Delta activity (1–4.5 Hz). We transformed the reference to the mean of the mastoids and low- and high-pass filtered (0.1–50 Hz) the data. Segments scored as N2 or N3 were selected and further epoched into 4,096 data point segments (ca 8 s) with an overlap of 409 data points. Artifacts were excluded semi-manually. The FFT was run with a resolution of 0.2 Hz and a Hamming window of 10%. We extracted the areas (muV*Hz) of the mentioned frequency bands as well as the total power (0.5–50 Hz) from the nap recording. We calculated the percentage of the frequency bands with the total spectrum power set to 100% to account for any unspecific differences in total power between the two nap recordings. Thus the values on which we performed the statistical tests were percent.
Questionnaire
In all mentioned studies we assessed subjective rating of sleep quality experience in each of the naps with the “Schlafqualitäts Fragebogen Revised” (S-FA R), [47]. We selected questions 9 and 11 in which subjects indicate on seven adjectives how they rated the nap (question 9) and how they now feel after the nap (question 11). We recoded adjectives where necessary and calculated the mean from the seven items for each of the questions. Higher numbers mean higher sleep quality. In n = 8 subjects, data were not available in one or both sessions due to incomplete responses. Again, the difference score was calculated (intervention minus non-intervention and session 1 minus 2).
Statistical analysis
In this analysis, we were not interested in the condition which elicited a higher or lower amount of SWS. Still, for the main analysis, we sorted the naps according to the experimental condition and computed the difference between sleep stage amounts after the intervention minus non-intervention nap, which we correlated to the respective difference in memory retention across sleep (in absolute numbers and percentage). In subgroups, we tested the correlations only for naps including REM sleep versus without REM sleep as well as short versus long naps (more/less than 40 min of total sleep time (TST)). To account for any unspecific time component of the two measures, we additionally analyzed the differences between session 1 minus session 2 for the sleep stages, memory performance across sleep and subjective sleep quality rating, although we did not expect an influence on the results of the correlation of those two different scores. As a further analysis, we correlated sleep and memory of the single sessions (intervention versus non-intervention session) separately as with two samples of between-subjects data. In an exploratory analysis and for the sake of completeness, we present a summary table including all sleep stages and their correlation to memory in percent, for the whole sample and split according to condition. As those were additional analyses for which we did not have concrete assumptions, we applied Bonferroni correction and divided the critical p value of 0.05 by 5, so the level of significance was p = 0.01 for those analyses while it was p = 0.05 (uncorrected) for the others. We additionally report the Bayes Factor for our correlations.
Data were analyzed using Pearson correlations. Outliers were defined by values that deviated more than ±3 standard deviations (SDs) from mean in each of the variables included in the respective analysis. The difference between the correlation coefficients was analyzed by Fisher’s z-transformation.
Besides averaging frequency power across all electrodes, we pre-defined three sites of the frontal, central and parietal electrodes which we averaged accordingly. In one of the studies, we had used a smaller EEG setup and could not analyze those means. We hence did additional analyses including all studies on electrodes F3 and F4. For those variables, the difference between intervention and non-intervention session was again correlated with the respective difference in memory performance across the nap.
Results
Correlations between SWS and memory consolidation
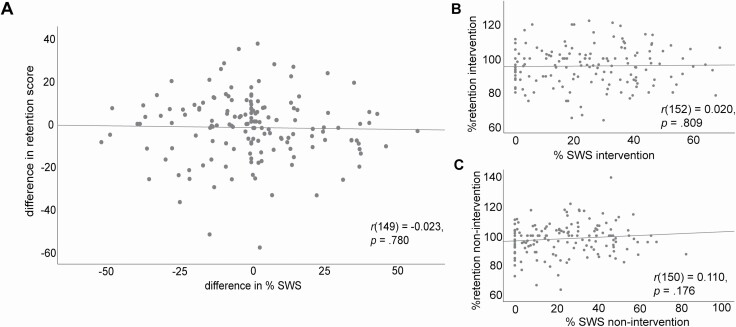
Across the whole sample, after outlier exclusion, the correlation of the difference between both sessions in % SWS and memory retention difference in % was non-significant with r(149) = –0.023, p = 0.780 (see Figure 2A). Additional analyses using the Bayes Factor (null/alternative) (BF01) revealed a factor of 14.91 for this correlation, which indicates a strong evidence for H0. The difference in % SWS and absolute memory retention was also non-significant with r(150) = –0.047, p = 0.569, BF01 = 13.20, strong evidence for H0. The pattern was the same for the amount of minutes spent in SWS and memory retention across the nap in absolute numbers of items remembered as well as in percent (r(149) = –0.057, p = 0.488, BF01 = 12.20, strong evidence for H0 and r(148) = –0.034, p = 0.679, BF01 = 14.19, strong evidence for H0, respectively).
Figure 2.
Correlation within-subjects and between-subjects.
Figure displays the correlation between (A) the difference in percent SWS between both naps (intervention – non-intervention nap) and the difference between memory performance across the nap in percent (memory performance before sleep set to 100%). Figures (B) (intervention) and (C) (non-intervention) display the two naps separately and show the correlation between the percentage of SWS in the respective nap and memory retention across sleep (100% indicates same amount of recalled words before and after the nap).
Significances did not change when excluding the remaining nine male subjects from the analysis (e.g. r(140) = –0.003, p = 0.974 for % SWS and % memory retention, BF01 = 15.03, strong evidence for H0).
Subgroup analyses
We split the sample into naps in which REM sleep was included in both sessions and those in which in at least one of both sleep periods REM sleep was lacking. After outlier exclusion, in n = 116 naps did not contain REM sleep. In those, percentage of SWS did not correlate with % PAL performance (r(114) = –0.046, p = 0.624, BF01 = 12.08, strong evidence for H0). We measured n = 35 naps in which REM sleep appeared in both conditions. Here, the correlation was non-significant as well (r(33) = 0.088, p = 0.617, BF01 = 6.73, moderate evidence for H0), Fisher’s z = –0.67, p = 0.251.
To investigate whether the amount of deep sleep is correlated to memory consolidation depending on the duration of the nap, as Diekelmann et al. [19] have shown, we analyzed naps that contained more and less than 40 min of sleep separately. This resulted in n = 128 naps longer than 40 min. For those, the correlation between percent SWS and memory consolidation was r(126) = –0.056, p = 0.532, BF01 = 11.77, strong evidence for H0. The correlation in the short naps (n = 23) was as well non-significant (r(21) = 0.206, p = 0.345, BF01 = 4.01, moderate evidence for H0). Also when splitting both of those groups again into whether or not REM sleep was present, none of the correlations was significant: long naps with REM sleep r(33) = 0.088, p = 0.617, BF01 = 6.73, moderate evidence for H0, without REM sleep r(91) = –0.093, p = 0.375, BF01 = 8.25, moderate evidence for H0. As none of the short naps included REM sleep, the correlation was as reported above.
Control analysis
Analyzing the SWS difference (in % and minutes) and memory across the nap by subtracting session 2 from session 1 independent from the intervention condition resulted in the same data pattern. The correlation between the difference in percentage of SWS and percent memory retention was r(149) = –0.03, p = 0.70, BF01 = 14.42, strong evidence for H0, for absolute memory retention r(149) = –0.08, p = 0.32, BF01 = 9.53, moderate evidence for H0. The relation between the difference in SWS measured in minutes and memory performance in percent was r(149) = –0.05, p = 0.55, BF01 = 13.00, strong evidence for H0 and absolute memory change r(149) = –0.10, p = 0.23, BF01 = 7.55, moderate evidence for H0.
Exploratory analysis
For completeness, we here present the within-subjects correlations for the difference across all sleep stages in percent with the difference in memory retention across the naps in percent(see Table 1). After Bonferroni correction, the critical p value is p = 0.01.
Table 1.
Within-subjects correlations to all sleep stages
| Sleep stage in % | Correlation with % memory retention difference |
|---|---|
| N1 | r(147) = –0.056, p = 0.498, BF01 = 12.26 |
| N2 | r(149) = 0.109, p = 0.183, BF01 = 6.41 |
| SWS | r(149) = –0.23, p = 0.780, BF01 = 14.91 |
| REM | r(147) = –0.075, p = 0.365, BF01 = 10.22 |
| WASO | r(147) = 0.016, p = 0.848, BF01 = 15.12 |
| TST (in minutes) | r(146) = –0.003, p = 0.968, BF01 = 15.34 |
Note. The table completes the results for within-subject correlations between the difference in each sleep stage in percent between the intervention and non-intervention condition and the difference in percentage of memory retention across the both naps. BF < 10: moderate evidence for H0, BF > 10: strong evidence for H0.
Between-subjects analysis separate for condition
Regarding both sessions as two samples of between-subjects naps, we correlated percent SWS with percent memory retention separately for the intervention and non-intervention naps. In the intervention naps, the correlation between percentage of SWS and percent memory retention was r(152) = 0.020, p = 0.809, BF01 = 15.20, strong evidence for H0 see Figure 2B and Table 2. For the non-intervention naps the correlation between both variables was r(150) = 0.110, p = 0.176, BF01 = 6.24, moderate evidence for H0 see Figure 2C.
Table 2.
Between-subjects correlations with all sleep stages
| Sleep stage in % | Correlation with % memory retention | ||
|---|---|---|---|
| Intervention condition | Non-intervention condition | Difference between correlation coefficients | |
| N1 | r(150) = –0.143, p = 0.078, BF01 = 3.32 | r(148) = –0.059, p = 0.472, BF01 = 11.94 | z = –0.73, p = 0.233 |
| N2 | r(152) = 0.183, p = 0.023, BF01 = 1.19 | r(151) = –0.057, p = 0.485, BF01 = 12.23 | z = 2.101, p = 0.018 |
| SWS | r(152) = 0.020, p = 0.809, BF01 = 15.20 | r(150) = 0.110, p = 0.176, BF01 = 6.24 | z = –0.783, p = 0.217 |
| REM | r(149) = 0.155, p = 0.058, BF01 = 2.59 | r(149) = 0.230, p = 0.005*, BF01 = 0.28 | z = –0.67, p = 0.251 |
| WASO | r(150) = –0.211, p = 0.009*, BF01 = 0.52 | r(148) = –0.132, p = 0.107, BF01 = 4.24 | z = –0.701, p = 0.242 |
| TST (in minutes) | r(148) = 0.142, p = 0.083, BF01 = 3.45 | r(148) = 0.039, p = 0.635, BF01 = 13.81 | z = 0.891, p = 0.186 |
Note. The table summarizes the results for the exploratory analysis on the correlation between the single sleep stages in % and memory retention across the nap separately for the two conditions. The difference between the correlation coefficients was analyzed by Fisher’s z-transformation. Bonferroni corrected p value is p = 0.01 (p = 0.05 divided by 5, see statistical analysis). BF < 0.3: moderate evidence for H1, BF < 1: anecdotal evidence for H1, BF < 3: anecdotal evidence for H0, BF < 10: moderate evidence for H0, BF < 30: strong evidence for H0.
Correlation between SWS and subjective sleep quality
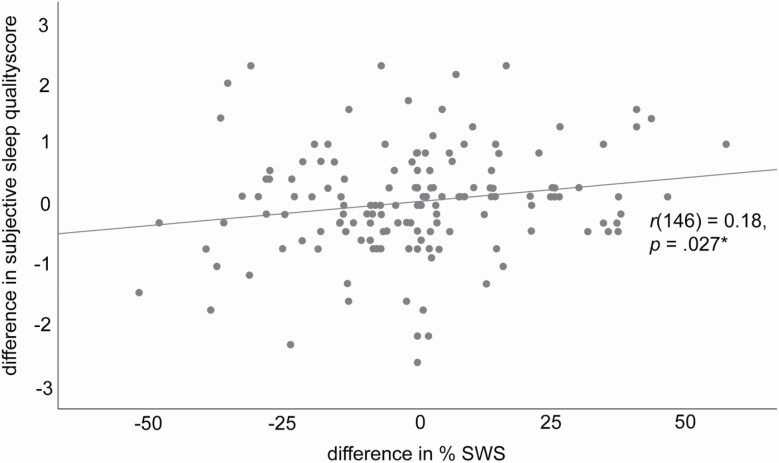
As an additional post hoc analysis, we correlated the difference in percent SWS between both conditions with the difference in subjective sleep quality rating. Their relationship was positive and significant with r(146) = 0.182, p = 0.027, BF01 = 1.34, anecdotal evidence for H0 (for % SWS, see Figure 3). Thus, if subjects increased their SWS amount, the subjective sleep quality rating also increased. When extracting the item on how deep subjects rated their sleep during the nap, this correlation was still positive and significant at the same level with r(142) = 0.181, p = 0.030, BF01 = 1.43, anecdotal evidence for H0 (in four subjects data in one of both sessions was missing on this item). The correlation between the amount of SWS and the rating of next day’s wellbeing, however, was non-significant (r(146) = –0.077, p = 0.352, BF01 = 9.97, moderate evidence for H0).
Figure 3.
Correlation between %SWS and subjective sleep quality rating.
Figure displays the relationship of the difference in percent SWS between both conditions and the difference in the subjective sleep quality rating.
In the naps that contained REM sleep in both sessions, the correlation between percent of SWS and subjective sleep quality rating was non-significant (r(30) = 0.015, p = 0.935, BF01 = 7.28, moderate evidence for H0), while it was significant in the naps in which in one or both of the sessions REM sleep was missing (r(114) = 0.212, p = 0.022, BF01 = 1.01, anecdotal evidence for H0). Thus, experiencing complete sleep cycles in both naps is not necessary for the relationship between SWS increase and subjective sleep quality rating. This might however also be due to the difference in the number of included cases.
When subtracting session 2 from session 1, the relationship between percentage of SWS was as well related to subjective sleep quality ratings (r(147) = 0.167, p = 0.042, BF01 = 1.97, anecdotal evidence for H0), but not to the feeling of being rested the next day (r(146) = –0.079, p = 0.341, BF01 = 9.77, moderate evidence for H0).
Exploratory analysis
For completeness, we here present the within-subjects correlations for the difference across all sleep stages in percent with the difference in memory retention across the naps in percent (see Table 1). After Bonferroni correction, the critical p value is p = 0.01.
Correlation between slow-wave activity and memory consolidation
Correlating the difference in overall SWA power (0.5–4.5 Hz) to the difference in % PAL resulted in a nonsignificant relationship of r(125) = 0.040, p = 0.652, Bayes Factor (null/alternative) BF01 = 12.86, indicating strong evidence for H0. We obtained a similar non-significant finding when correlating with the difference in the absolute number of remembered words in PAL r(125) = 0.046, p = 0.608, BF01 = 12.49, strong evidence for H0. This pattern remained when correlating the frontal (r(125) = 0.039, p = 9.667, BF01 = 12.98, strong evidence for H0), central (r(125) = 0.050, p = 0.578, BF01 = 12.20, strong evidence for H0) and parietal electrodes (r(125) = 0.047, p = 0.601, BF01 = 12.42, strong evidence for H0) separately with % PAL.
Including the data from the guided imagery study in which we had used another EEG setup with less electrodes, we here present the correlations in the single electrodes F3 and F4 with % PAL, which were r(148) = 0.051, p = 0.533, BF01 = 12.74, strong evidence for H0 and r(148) = 0.057, p = 0.490, BF01 = 12.18, strong evidence for H0.
We also separated SWA power into its components of SO (0.5–1 Hz) and Delta activity (1–4.5 Hz). Here, we neither found any significant correlations with memory performance. The difference in SO and % PAL was r(125) = 0.062, p = 0.487, BF01 = 11.19, strong evidence for H0; with absolute difference in PAL r(124) = 0.064, p = 0.474, BF01 = 11.02, strong evidence for H0. Separate for frontal, central and parietal electrodes, the correlations with difference in relative SO were respectively r(125) = 0.086, p = 0.338, BF01 = 9.01, moderate evidence for H0; r(125) = 0.063, p = 0.482, BF01 = 11.12, strong evidence for H0 and r(125) = 0.039, p = 0.660, BF01 = 12.93, strong evidence for H0. The correlations of SO difference measured in F3 and F4 with % PAL were: r(148) = 0.072, p = 0.383, BF01 = 10.57, strong evidence for H0 and r(148) = 0.055, p = 0.506, BF01 = 12.39, strong evidence for H0.
The correlation between difference in Delta and % PAL was r(125) = –0.029, p = 0.748, BF01 = 13.52, strong evidence for H0; with absolute difference in PAL r(125) = –0.001, p = 0.995, BF01 = 14.23, strong evidence for H0. Separate for frontal, central and parietal electrodes, the correlations with difference in relative Delta were respectively r(125) = –0.045, p = 0.613, BF01 = 12.53, strong evidence for H0; r(125) = –0.015, p = 0.864, BF01 = 14.03, strong evidence for H0 and r(125) = –0.015, p = 0.868, BF01 = 14.04, strong evidence for H0. The correlations of Delta difference measured in F3 and F4 with PAL % were r(148) = –0.007, p = 0.935, BF01 = 15.40, strong evidence for H0 and r(148) = –0.028, p = 0.732, BF01 = 15.18, strong evidence for H0.
Discussion
Research has focused in the past years on the role of SWS in sleep-dependent memory consolidation. Findings mainly support two current theories about the underlying mechanism, which both attribute a special role to SWS. While first studies reported high and significant correlations between those two measures, more and more following studies could not replicate this relationship or found it only under certain conditions. As many of them included low numbers of subjects or between-subjects measures, we here sampled four nap studies in which we manipulated SWS with pre-sleep cognitive interventions in a within-subjects design. Constantly using the same word pair associate task with cued recall across those studies, we were able to analyze the correlation between the two measures in about 150 data pairs. Our data revealed a non-significant, close to zero correlation between the difference in SWS (percent or minutes) between both naps and the difference in memory performance change across the naps. As expected, this was independent of the way we calculated the difference scores (depending on the condition or on session order). Also when splitting the sample of within-subjects measures in two separate measures of n = 150 between-subjects samples, the correlation with SWS amounts was non-significant and close to zero in both samples. In three of the four studies we had tested only female subjects, therefore our sample here contained 94% females. However, excluding the nine men did not change results. Moreover, as there are gender differences in sleep and memory performance, there is no hint at a difference in the specific relationship between SWS and memory retention between men and women (although there might be slow oscillation/spindle coincidences and sleep-dependent memory consolidation [see 41]). One could argue that the 90-min nap opportunity we offered the subjects is not sufficient for the benefit of sleep to occur. However, our memory task was taken from previous studies which had shown the effect across night halves [48, 49] and daytime[15] as well as nocturnal naps [50]. Further, even an ultra-short nap of 6 min was more beneficial for free recall performance in list learning than the same amount of wakefulness [24]. Contrary, a nap study including an object-location task showed that while 40 min of sleep was indeed insufficient to strengthen memory compared to wakefulness, a 90 min nap was [19]. Moreover, the nap duration was relevant for the SWS memory correlation: while in the ultra-short and the 40 min naps, SWS was uncorrelated to memory performance, it was in the 90 min nap. As some of our subjects did not fall asleep immediately and hence did not fill the 90 min with sleep, not all naps were actually longer than 40 min. We hence analyzed the naps shorter and longer than 40 min separately, which however did not result in a difference for the SWS memory correlation.
Our focus was on intra-individual variations in sleep pattern which are assumed to be usually highly stable [32] and we hence assumed that deviations might be more functionally relevant. However, also within-subjects, sleep is determined by external factors like sleep restriction in prior sleep episodes [51] or learning intensity [12]. Thus, spontaneous variations in sleep patterns might be relevant for memory consolidation. Naturally, they, however, appear only to a small degree. Currently, Tamminen et al. [52] have not found differences in any sleep stages between two within-subject recorded nights and none of the correlations with memory performance were significant. To increase the variation between sleep patterns, previous studies investigating the within-subjects correlation between SWS differences and memory consolidation had thus manipulated at least one of the recorded sleep episodes. Thereby, some either stated that consolidation was not affected even if marked differences in REM or SWS amounts appear [53], that both, REM and SWS affect memory consolidation [54] or that only REM fragmentation is predictive for the retention of neutral declarative information [55]. However, most of those studies had either included a low number of participants (e.g. n = 12) and/or manipulated sleep by disturbing particular sleep stages (REM or SWS), to increase the within-subjects’ differences in sleep patterns. This can cause methodological problems. For instance, in Casey et al. [54], more disruptions were necessary for SWS than REM deprivation. Consequently, total sleep time was lower in SWS deprivation than REM deprivation nights. Moreover, although subjective ratings of stress or sleepiness in the morning did not vary depending on the condition in Genzel et al. [53], the method is generally criticized for such potential, non-specific effects [56].
It is possible that night-time sleep is more relevant for memory consolidation than naps as investigated here. Strongly diverging neurochemical concentrations (of neurotransmitters and hormones for instance) and processes and factors regulating the circadian rhythm or sleep homeostasis inevitably dominate during both time points and could therefore play a mediator role in the relationship. Mednick et al. [20] reported that for sleep-dependent learning of a texture discrimination task, a full night was as good as a nap if this contained SWS and REM sleep. While NREM sleep restored deteriorated learning to baseline levels, performance improvements above baseline were detected in naps including SWS and REM sleep [20]. Following up on this, we split our sample according to whether REM sleep appeared in the nap or not. In both cases, the correlation between the amount of SWS difference and memory change across the nap was however non-significant. It must be noted, however, that in only 35 subjects, REM sleep appeared in both sessions. This result is in line with those of Diekelmann et al. [19] where memory consolidation benefitted from a 90 min nap, independent of whether this sleep contained REM sleep or not. Still, while SWS might be a critical component of sleep for memory, it might need the interplay with other variables to effectively influence memory consolidation. Thus, the amount of deep sleep might still be relevant if other sleep parameters also change and interact with SWS to the advantage of memory processes. One could argue as well, that explanations on a sleep stage level are too simple and less coarse physiological processes during sleep or their interplay should be taken more into account. We hence analyzed the correlation between frequency power values in SWA, SO and Delta which all showed non-significant results. However, it was previously shown that learning increases SWA locally [12]. Thus, a general change in power might be too rough to see the relationship to changes in memory consolidation. Correlating frontal, central and parietal electrodes separately with memory consolidation did not hint at local differences, but we did not analyze more fine-grained aspects such as the amplitudes of the oscillations, spindles or spindle-SO couplings, which are all directly referenced by the active systems consolidation theory and supported by other findings see [57]. This would have gone beyond the focus of this paper, but we are currently analyzing these aspects in a separate study.
Besides physiology, nap behavior could play a role in sleep-dependent memory consolidation. McDevitt et al. [58] showed that nap-dependent memory improvement in a perceptual learning task was higher in subjects habitually napping at least once a week compared to non-nappers. They further reported that memory improvement was associated with different sleep parameters between groups. Still, interestingly, the nap’s sleep structures did not differ between both groups in any sleep stage (all p > 0.29), neither was the prior night’s sleep, measured with actigraphy. Genetics and developmental habits were discussed as possible factors fostering habitual napping or not. As we had only included non-nappers (except the elderly sample where we did not consider this as exclusion criterion), we might have recorded a non-representative subsample. This limits the generalizability of our results.
Another obvious methodological issue of the current paper is it being a re-analysis of published data. Secondary analyses often limit the preciseness of operationalization to answer the research question. As described in our recent review [14], if research resources were unlimited, the ideal design would be to test an a priori defined sample size in a pre-registered study. Pre- and post-sleep memory should be tested across 3 or 4 undisturbed, natural nights without any intervention on sleep or memory following the same experimental setting (time frame of data collection, EEG settings, etc.). This would measure natural fluctuations intra-individually. Due to the stability of intra-individual sleep patterns [31], those would probably be smaller than observed here. Increasing the differences by intervention as in our data could hence be interpreted as an advantage.
In a post hoc analysis, we correlated the amount of SWS with subjective ratings of sleep quality for which we found a positive and significant relationship. Exploratively isolating the item on subjectively rated sleep depth correlated positively and significantly with the percentage of SWS. Although this was not the scope of our analysis and should hence be not over-interpreted it is in line with previous reports on the influence of SWS on subjective sleep quality ratings [59, 60]. Possibly, the subjective rating of sleep quality is, at least in part, based on a bodily feeling which is associated with the amount of deep sleep. In further exploratory analyses and for completeness, we also provided correlative data for all sleep stages, wake after sleep onset and TST. After Bonferroni correction for multiple testing, only WASO or REM sleep correlated significantly with memory performance across the nap in one of the conditions. In the respective other condition, the correlation showed the same direction, but was not significant. The size of the correlation coefficients did however not differ from each other. The negative correlation with time spent awake speaks in favor of a general role of sleep for memory consolidation: Less wake times during the sleep period might support declarative memory consolidation independently of the time spent in a specific sleep stage. In addition, the positive correlation between time spent in REM sleep and memory consolidation suggests that processes during REM sleep might also support declarative memory consolidation during sleep see also [61]. For sleep studies in animals [62], abundant evidence on the involvement of REM sleep in consolidation processes exists, while the role of REM sleep in declarative memory consolidation in humans is less clear [see [63] for an overview]. According to the sequential hypothesis of sleep and memory, both SWS and REM sleep are involved in consolidation processes during sleep. Also, the active system consolidation hypotheses assume that while memory representations are reactivated and strengthened during SWS, REM sleep might be involved in global synaptic downscaling of connections, simultaneously protecting reactivated memories from this downscaling [57]. Thus, time spent in REM sleep might be important for supporting declarative memory consolidation also in humans. Interestingly, we also observed small correlations with REM sleep in our previous large-scale study [30]. However, these correlations occurred only for encoding/short-term recall processes, and not for memory consolidation across the sleep period. Still, REM sleep might be an interesting candidate for inter-individual correlations between sleep and memory, although the strength of correlation remains rather low.
Conclusions
In this data re-analysis, we could sample about 150 within-subjects naps from studies of our lab with a similar design to investigate the relationship between the amount of SWS and memory retention across the nap. Non-significant and close to zero results favor the conclusion that the extent to which memory benefits from a nap does not solely and isolatedly depend on the amount of deep sleep in the nap. Diverse task- and memory system-specific characteristics can however make the relationship more or less likely, limiting generalization of this result on other designs. Further, we cannot conclude that the amount of deep sleep is not relevant if other sleep parameters are manipulated in parallel and interact beneficially. Still, regarding sample size estimations based on effect sizes, we should stay aware of those limitations in further studies in the field.
Acknowledgments
The data were collected at the University of Fribourg (see above) and the University of Zurich, Binzmühlestrasse 14, 8050 Zürich, Switzerland.
Funding
This study was funded by a grant of the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant agreement number 667875) and the Swiss National Fonds (SNF) (PP00P1_133685 and 100014_162388).
Disclosure Statements
Financial Disclosure: none.
Non-financial Disclosure: none.
References
- 1. Jenkins JG, et al. Obliviscence during sleep and waking. Am J Psychol. 1924;35(4):605–612. [Google Scholar]
- 2. Ebbinghaus H. Über Das Gedächtnis. Untersuchungen Zur Experimentellen Psychologie. Darmstadt: Dunker & Humblot; 1885. [Google Scholar]
- 3. Heine R. Über Wiedererkennen und rückwirkende Hemmung. Zeitschrift für Psychol. 1914;(68):161–236. [Google Scholar]
- 4. Ellenbogen JM, et al. The role of sleep in declarative memory consolidation: passive, permissive, active or none? Curr Opin Neurobiol. 2006;16(6):716–722. [DOI] [PubMed] [Google Scholar]
- 5. Diekelmann S, et al. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. [DOI] [PubMed] [Google Scholar]
- 6. Born J, et al. System consolidation of memory during sleep. Psychol Res. 2012;76(2):192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tononi G, et al. Sleep and synaptic down-selection. Eur J Neurosci. 2020;51(1):413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scullin MK. Sleep, memory, and aging: the link between slow-wave sleep and episodic memory changes from younger to older adults. Psychol Aging. 2013;28(1):105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Backhaus J, et al. Impaired declarative memory consolidation during sleep in patients with primary insomnia: influence of sleep architecture and nocturnal cortisol release. Biol Psychiatry. 2006;60(12):1324–1330. [DOI] [PubMed] [Google Scholar]
- 10. Backhaus J, et al. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learn Mem. 2007;14(5):336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mander BA, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16(3):357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huber R, et al. Local sleep and learning. Nature. 2004;430(6995):78–81. [DOI] [PubMed] [Google Scholar]
- 13. Varga AW, et al. Effects of aging on slow-wave sleep dynamics and human spatial navigational memory consolidation. Neurobiol Aging. 2016;42:142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cordi MJ, et al. How robust are sleep-mediated memory benefits? Curr Opin Neurobiol. 2021;67:1–7. [DOI] [PubMed] [Google Scholar]
- 15. Tucker MA, et al. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006;86(2):241–247. [DOI] [PubMed] [Google Scholar]
- 16. Scullin MK, et al. The effects of an afternoon nap on episodic memory in young and older adults. Sleep. 2017;40(5):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tucker MA, et al. Enhancement of declarative memory performance following a daytime nap is contingent on strength of initial task acquisition. Sleep. 2008;31(2):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilhelm I, et al. Sleep selectively enhances memory expected to be of future relevance. J Neurosci. 2011;31(5):1563–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diekelmann S, et al. Offline consolidation of memory varies with time in slow wave sleep and can be accelerated by cuing memory reactivations. Neurobiol Learn Mem. 2012;98(2):103–111. [DOI] [PubMed] [Google Scholar]
- 20. Mednick S, et al. Sleep-dependent learning: a nap is as good as a night. Nat Neurosci. 2003;6(7):697–698. [DOI] [PubMed] [Google Scholar]
- 21. Sonni A, et al. Sleep protects memories from interference in older adults. Neurobiol Aging. 2015;36(7):2272–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baran B, et al. Age-related changes in the sleep-dependent reorganization of declarative memories. J Cogn Neurosci. 2016;28(6):792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deliens G, et al. Rapid eye movement and non-rapid eye movement sleep contributions in memory consolidation and resistance to retroactive interference for verbal material. Sleep. 2013;36(12):1875–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lahl O, et al. An ultra short episode of sleep is sufficient to promote declarative memory performance. J Sleep Res. 2008;17(1):3–10. [DOI] [PubMed] [Google Scholar]
- 25. Dresler M, et al. A double dissociation of memory impairments in major depression. J Psychiatr Res. 2011;45(12):1593–1599. [DOI] [PubMed] [Google Scholar]
- 26. Mazzoni G, et al. Word recall correlates with sleep cycles in elderly subjects. J Sleep Res. 1999;8(3):185–188. [DOI] [PubMed] [Google Scholar]
- 27. Cherdieu M, et al. Does age worsen sleep-dependent memory consolidation? J Sleep Res. 2014;23(1):53–60. [DOI] [PubMed] [Google Scholar]
- 28. Jones BJ, et al. Emotional bias of sleep-dependent processing shifts from negative to positive with aging. Neurobiol Aging. 2016;45:178–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schönbrodt FD, et al. At what sample size do correlations stabilize? J Res Pers. 2013;47(5):609–612. [Google Scholar]
- 30. Ackermann S, et al. No associations between interindividual differences in sleep parameters and episodic memory consolidation. Sleep. 2015;38(6):951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buckelmüller J, et al. Trait-like individual differences in the human sleep electroencephalogram. Neuroscience. 2006;138(1):351–356. [DOI] [PubMed] [Google Scholar]
- 32. Landolt HP. Genetic determination of sleep EEG profiles in healthy humans. Prog Brain Res. 2011;193:51–61. [DOI] [PubMed] [Google Scholar]
- 33. Ambrosius U, et al. Heritability of sleep electroencephalogram. Biol Psychiatry. 2008;64(4):344–348. [DOI] [PubMed] [Google Scholar]
- 34. Goldstone A, et al. The mediating role of cortical thickness and gray matter volume on sleep slow-wave activity during adolescence. Brain Struct Funct. 2018;223(2):669–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ohayon MM, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. [DOI] [PubMed] [Google Scholar]
- 36. Mander BA, et al. Sleep and human aging. Neuron. 2017;94(1):19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scullin MK, et al. Sleep, cognition, and normal aging: integrating a half century of multidisciplinary research. Perspect Psychol Sci. 2015;10(1):97–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Old SR, et al. Differential effects of age on item and associative measures of memory: a meta-analysis. Psychol Aging. 2008;23(1):104–118. [DOI] [PubMed] [Google Scholar]
- 39. Nyberg L, et al. Successful memory aging. Annu Rev Psychol. 2019;70:219–243. [DOI] [PubMed] [Google Scholar]
- 40. Herlitz A, et al. Gender differences in episodic memory. Mem Cognit. 1997;25(6):801–811. [DOI] [PubMed] [Google Scholar]
- 41. Sattari N, et al. The effect of sex and menstrual phase on memory formation during a nap. Neurobiol Learn Mem. 2017;145:119–128. [DOI] [PubMed] [Google Scholar]
- 42. Cordi MJ, et al. Systematic decrease of slow‐wave sleep after a guided imagery designed to deepen sleep in low hypnotizable subjects. J Sleep Res. 2020;e13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Faul, F, et al. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. [DOI] [PubMed] [Google Scholar]
- 44. Rasch BH, et al. Combined blockade of cholinergic receptors shifts the brain from stimulus encoding to memory consolidation. J Cogn Neurosci. 2006;18(5):793–802. [DOI] [PubMed] [Google Scholar]
- 45. Cordi MJ, et al. Improving sleep and cognition by hypnotic suggestion in the elderly. Neuropsychologia. 2015;69:176–182. [DOI] [PubMed] [Google Scholar]
- 46. Iber C, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, Illinois: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 47. Görtelmeyer R. SF-A/R Und SF-B/R - Schlaffragebogen A Und B - Revidierte Fassung. Göttingen: Hogrefe; 2011. [Google Scholar]
- 48. Plihal W, et al. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9(4):534–547. [DOI] [PubMed] [Google Scholar]
- 49. Plihal W, et al. Effects of early and late nocturnal sleep on priming and spatial memory. Psychophysiology. 1999;36(5):571–582. [PubMed] [Google Scholar]
- 50. Marshall L, et al. Transcranial direct current stimulation during sleep improves declarative memory. J Neurosci. 2004;24(44):9985–9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cousins JN, et al. A split sleep schedule rescues short-term topographical memory after multiple nights of sleep restriction. Sleep. 2019;42(4):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tamminen J, et al. The role of sleep spindles and slow-wave activity in integrating new information in semantic memory. J Neurosci. 2013;33(39):15376–15381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Genzel L, et al. Slow wave sleep and REM sleep awakenings do not affect sleep dependent memory consolidation. Sleep. 2009;32(3):302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Casey SJ, et al. Slow wave and REM sleep deprivation effects on explicit and implicit memory during sleep. Neuropsychology. 2016;30(8):931–945. [DOI] [PubMed] [Google Scholar]
- 55. Lipinska G, et al. Rem fragmentation, not slow-wave sleep, predicts neutral declarative memory consolidation in posttraumatic stress disorder. Sleep. 2018;28(6):e12846. [DOI] [PubMed] [Google Scholar]
- 56. Born J, et al. REM sleep deprivation: the wrong paradigm leading to wrong conclusions. Behav Brain Sci. 2000;23(6):912–913. [Google Scholar]
- 57. Klinzing JG, et al. Mechanisms of systems memory consolidation during sleep. Nat Neurosci. 2019;22(10):1598–1610. [DOI] [PubMed] [Google Scholar]
- 58. McDevitt EA, et al. The impact of frequent napping and nap practice on sleep-dependent memory in humans. Sci Rep. 2018;8(1):15053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Westerlund A, et al. Relationships between questionnaire ratings of sleep quality and polysomnography in healthy adults. Behav Sleep Med. 2016;14(2):185–199. [DOI] [PubMed] [Google Scholar]
- 60. Keklund G, et al. Objective components of individual differences in subjective sleep quality. J Sleep Res. 1997;6(4):217–220. [DOI] [PubMed] [Google Scholar]
- 61. Scullin MK, et al. Is cognitive aging associated with levels of REM sleep or slow wave sleep? Sleep. 2015;38(3):335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Poe GR. Sleep is for forgetting. J Neurosci. 2017;37(3):464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rasch B, et al. About sleep’s role in memory. Physiol Rev. 2013;93(2):681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]