Abstract
Background
Given increasing incidence of cognitive impairment and dementia, further understanding of modifiable factors contributing to increased healthspan is crucial. Extensive literature provides evidence that physical activity (PA) delays the onset of cognitive impairment; however, it is unclear whether engaging in PA in older adulthood is sufficient to influence progression through cognitive status categories.
Method
Applying a coordinated analysis approach, this project independently analyzed 14 longitudinal studies (NTotal = 52 039; mean baseline age across studies = 69.9–81.73) from North America and Europe using multistate survival models to estimate the impact of engaging in PA on cognitive status transitions (nonimpaired, mildly impaired, severely impaired) and death. Multinomial regression models were fit to estimate life expectancy (LE) based on American PA recommendations. Meta-analyses provided the pooled effect sizes for the role of PA on each transition and estimated LEs.
Results
Controlling for baseline age, sex, education, and chronic conditions, analyses revealed that more PA is significantly associated with decreased risk of transitioning from nonimpaired to mildly impaired cognitive functioning and death, as well as substantially longer LE. Results also provided evidence for a protective effect of PA after onset of cognitive impairment (eg, decreased risk of transitioning from mild-to-severe cognitive impairment; increased likelihood of transitioning backward from severe-to-mild cognitive impairment), though between-study heterogeneity suggests a less robust association.
Conclusions
These results yield evidence for the importance of engaging in PA in older adulthood for cognitive health, and a rationale for motivating older adults to engage consistently in PA.
Keywords: Cognitive aging, Exercise, Longevity, Successful aging
Given shifting demographics worldwide, researchers and the public are concerned with factors that contribute to increased longevity and, in particular, increased healthspan. As Alzheimer’s disease is one of the leading causes of morbidity and mortality and the 12th most burdensome disease in older adults (1), further understanding of modifiable factors that protect against cognitive changes characteristic of Alzheimer’s disease and other dementias is imperative. For example, delaying the onset of dementia by as little as 1 year is projected to reduce the number of dementia cases in 2050 by over 9 million, and substantially decrease the global financial burden associated with dementia care (1–3).
A considerable body of observational and experimental research suggests that physical activity (PA) moderates declines in cognitive functioning (see reviews, 4–7) possibly by facilitating neural plasticity processes (see expert consensus report, (8)) such as increasing hippocampal volume (9). An umbrella review synthesizing systematic reviews, meta-analyses, and pooled analyses found moderate-to-strong evidence indicating that PA benefits cognitive functioning and reduces risk of developing cognitive impairment, particularly in older adulthood (7). For example, a meta-analysis synthesizing prospective studies examining the association between PA and risk of cognitive decline in healthy older adults indicated that vigorous and low-to-moderate PA was significantly associated with 38% and 35% decreased risk, respectively, of cognitive decline at follow-up occasions (10). Research also suggests that PA may be protective for individuals with mild cognitive impairment (MCI (11)) and dementia (12,13), which is consistent with autopsy research indicating that PA may assist in maintaining function despite accumulation of dementia pathology (ie, cognitive reserve) (14).
Delineating the relative importance of PA in the progression of cognitive changes due to nonpathological and pathological aging, however, is challenging. Approaches based on autopsy data and applying growth curve and Cox regression models are not typically able to determine the timing of when PA is most critical, whether the impact of engaging in PA in older adulthood is of a magnitude to impact transitions through cognitive status categories, or account for death as a competing risk factor. Further understanding of protective factors during different stages of cognitive aging is critical, as individuals do not typically transition from a state of nonimpaired cognitive functioning directly to Alzheimer’s disease. The insidious nature of the disease commonly results in a transitional phase in which cognitive decline is more substantial than that observed in normal aging but not severe enough to impact activities of daily living (ie, MCI (11)).
Research suggests that cognitive decline and associated neural degeneration observed in MCI represents an early stage of Alzheimer’s disease and other dementias (15), though heterogeneity in transitions from MCI is common. For example, not all individuals classified with MCI will develop Alzheimer’s disease (16,17). Further, individuals who revert to normal cognitive functioning at some point during follow-up remain at higher risk for progression to dementia (18,19). In addition, transitions in cognitive status can be challenging to capture given the timing of measurement occasions in longitudinal data collection. Research aiming to investigate interindividual differences in protective factors associated with heterogeneity in cognitive status transitions is timely due to recent advances in multistate modeling (MSM), which allows simultaneous estimation of transitions through cognitive states while accounting for death, as well as estimation of life expectancy (LE) based on the hazard ratios (HRs) estimated by the MSM. Additionally, MSM is desirable when the data are interval-censored, as is the case with panel data. That is, although cognitive impairment occurs as a process in continuous time, prescheduled interviews restrict the ability to measure the precise timing of changes.
The current work investigates the impact of PA on transitions between cognitive status categories (nonimpaired, mildly impaired, severely impaired) and death, applying a coordinated analysis approach to 14 longitudinal studies of aging. Coordinated analysis can protect against Type I and Type II errors by executing independent but conceptually identical analyses (to the extent possible) across multiple studies, permits a powerful basis to evaluate cross-country replicability, and facilitates accelerated accumulation of knowledge (20) (eg, see (21–23)). This project will investigate 3 research questions. First, based on the literature outlined above, to what extent does PA predict transitions between cognitive status categories? Existing literature also shows that PA decreases risk of mortality (see reviews, (24,25)); however, the dose–response relationship remains unclear and, further, existing research tends to focus on the relationship between PA and cognition or PA and mortality. Thus, to complement the MSM analyses, to provide a practical estimation of the impact of PA on mortality, and to highlight that these analyses account for death as a competing risk factor, a second research question examines whether individuals who engage in more PA over the course of the study have longer LEs. Third, given the coordinated analysis approach, we examine the extent to which a consistent pattern of results, in terms of transitions estimates and LEs, emerges across several studies of aging. Based on previous research examining PA as a protective factor for cognitive functioning and mortality in older adulthood, we predict that individuals who engage in more PA will be less likely to transition to mildly and severely impaired cognitive status categories, as well as death, and will have longer LEs, than individuals who engage in less PA.
Method
Studies
Data were drawn from longitudinal studies that are publicly available or are affiliated with the Integrative Analysis of Longitudinal Studies of Aging and Dementia (IALSA) network (20). Study selection was based on availability of repeated measurement of cognitive functioning and PA, as well as availability of an associated data analyst proficient in executing multistate modeling. One of these studies (SHARE; (26)) includes data collected independently from 28 countries; studies with 6 or more waves of data and clear delineation of cognitive status categories (N = 8; additional information regarding eligibility criteria outlined in Supplementary Text 1) were therefore analyzed independently, making a total of 14 longitudinal studies included in the current project. Baseline characteristics from each study are presented in Table 1, with additional demographic information presented in Supplementary Table 1. Given that transitioning to a cognitively impaired state during the years of study follow-up is relatively rare for individuals who are less than 60 years old at baseline, and to limit between study heterogeneity due to age, only participants who were 60 years or older at baseline were included in the analysis. Eligibility also required that individuals have complete demographic information and measurement of cognition at 2 or more occasions. Age at death was used to identify individuals who died during the study and when a known death date occurred after completion of the study. All participants provided informed consent, and ethical approval for each study was granted by governing research committees. The following is a list of contributing studies: German Study on Ageing, Cognition, and Dementia (AgeCoDe) (27) (Germany); Einstein Aging Study (EAS) (28) (United States); Rush Memory and Aging Project (MAP) (29) (United States); Longitudinal Aging Study Amsterdam (LASA) (30,31) (the Netherlands); Health and Retirement Study (HRS) (32) (United States); English Longitudinal Study of Aging (ELSA) (33) (United Kingdom); Survey of Health, Ageing and Retirement in Europe (SHARE) (26) (Austria, Belgium, Denmark, France, Germany, the Netherlands, Sweden, and Switzerland). Study details are included in Supplementary Text 1.
Table 1.
Population Characteristics at Baseline for Each Study
| Study | AgeCoDe | EAS | MAP | LASA | HRS | ELSA | Austria | Belgium | Denmark | France | Germany | Netherlands | Sweden | Switzerland |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 2706 | 821 | 1428 | 2386 | 16 135 | 5383 | 3306 | 3464 | 2370 | 3385 | 3224 | 1790 | 3525 | 2116 |
| Mean age (SD) | 81.73 (3.50) | 79.34 (5.40) | 80.52 (6.44) | 70.23 (7.53) | 71.42 (7.54) | 70.09 (7.28) | 70.51 (7.45) | 71.28 (7.84) | 70.75 (7.72) | 71.75 (8.15) | 69.92 (6.97) | 69.94 (7.36) | 71.05 (7.78) | 70.77 (7.64) |
| Female: n; % | 1758; 65.0% | 511; 62.2% | 1010; 70.7% | 1271; 53.2% | 9442; 58.5% | 2525; 46.9% | 1900; 57.5% | 1865; 53.8% | 1262; 53.2% | 1925; 56.9% | 1586; 49.2% | 898; 50.2% | 1812; 51.4% | 1094; 51.7% |
| Male: n; % | 948; 35.0% | 310; 37.8% | 418; 29.3% | 1115; 46.7% | 6693; 41.5% | 2858; 53.1% | 1406; 42.5% | 1599; 46.2% | 1108; 46.8% | 1460; 43.1% | 1638; 50.8% | 892; 49.8% | 1713; 48.6% | 1022; 48.3% |
| State 1: n; % | 2081; 77.3% | 692; 84.3% | 870; 61% | 1717; 72.0% | 14525; 90.0% | 4844; 90.0% | 3084; 93.3% | 3196; 92.3% | 2166; 91.4% | 3145; 92.9% | 2996; 92.9% | 1655; 92.5% | 3284; 92.3% | 1964; 92.8% |
| State 2: n; % | 544; 20.2% | 126; 15.4% | 458; 32.1% | 574; 24.0% | 1305; 8.1% | 326; 6.1% | 130; 3.9% | 184; 5.3% | 119; 5.0% | 146; 4.3% | 140; 4.3% | 86; 4.8% | 160; 4.5% | 87; 4.1% |
| State 3: n; % | 67; 2.5% | 3; 0.4% | 100; 7.0% | 116; 4.9% | 309; 1.9% | 139; 2.6% | 92; 2.8% | 84; 2.4% | 85; 3.6% | 94; 2.8% | 88; 2.7% | 49; 2.7% | 111; 3.1% | 65; 3.1% |
| Deaths overall | 1730 (63.9%) | 138 (16.8%) | 1050 (73.5%) | 1754 (73.5%) | 5931 (36.8%) | 1202 (22.3%) | 374 (11.31%) | 502 (14.5%) | 473 (20.0%) | 444 (13.1%) | 242 (7.5%) | 223 (12.5%) | 603 (17.1%) | 200 (9.5%) |
Note: AgeCoDe = German Study on Ageing, Cognition, and Dementia; EAS = Einstein Aging Study; ELSA = English Longitudinal Study of Aging; HRS = Health and Retirement Study; LASA = Longitudinal Aging Study Amsterdam; MAP = Memory and Aging Project. Age = mean age at baseline; State 1 = nonimpaired cognitive functioning at baseline; State 2 = mildly impaired cognitive functioning at baseline; State 3 = severely impaired cognitive functioning at baseline. Country names are all part of SHARE (Survey of Health, Ageing and Retirement in Europe).
Measures
Each study involved an extensive battery of measures. We did not coordinate based on the lowest possible denominator (ie, only using measures that were identical between studies); instead, we aimed to maximize available data to preserve the strengths of studies (eg, clinical diagnosis > MMSE > summed cognitive test scores). This results in operational definitions that are not identical between studies; however, comparison of variables at the construct level is consistent with recommendations for coordinated analysis (20).
Cognitive status categories
Detailed operational definitions of cognitive status categories are included in Supplementary Text 2. Formal clinical diagnoses of MCI and dementia were used for AgeCoDe, EAS, and MAP. Cutoff scores on the Mini-Mental State Examination (MMSE) were used for LASA. The Telephone Interview for Cognitive Status (TICS (34)) was used for HRS. For ELSA and SHARE, select cognitive tests were summed, and study specific z-scores based on −2 and −1.5 SDs below the study norm at baseline were used to operationalize severely and mildly impaired cognitive status categories, respectively. For LASA, HRS, ELSA and SHARE, operational definitions of cognitive status categories based solely on cognitive functioning (not formal criteria for clinical diagnosis) were used to determine suggestive rather than clinical diagnosis of MCI and dementia.
Physical activity
To maximize available data while still allowing comparability between studies, and to characterize PA on a single scale, 2 transformations were executed to compute continuous PA variables representing intensity and frequency of PA at each measurement occasion. The first transformation utilized the Metabolic Equivalent of Task (MET) method developed by the Compendium of Physical Activities (35,36) to assign each PA item an intensity score, in which more vigorous activities are assigned higher values (eg, walking = 3; swimming = 5; cycling = 6). The Compendium approach enhances comparability of self-report PA measures across studies by providing quantification of energy cost of common physical activities (35). The second transformation calibrated the reported frequency to represent approximate weekly engagement in PA. Thus, the PA variable represents PA intensity units per week (eg, 15 units is reflective of approximately 150 minutes of moderate PA per week). Supplementary Table 2 lists assigned MET scores and frequency transformations across studies, while Supplementary Text 3 provides detailed operational definitions. Based on the intraclass correlation coefficient (ICC; Supplementary Table 1), PA was highly heterogeneous at the within-person level over time, which justifies entering the variable into the multistate models as a time-varying covariate.
Covariates
Sex was included as a dichotomous variable (male as the reference group). Age was measured in years and centered at the baseline mean of each study. Due to the response options provided in the original surveys, education was operationalized differently between studies (see Supplementary Text 4). For 12 out of 14 studies, education was measured in years and centered at the value that indicates completion of high school. For AgeCoDe, education was dummy coded into 3 categories representing compulsory schooling, high school, and post-secondary education. For ELSA, education was dichotomized (0 = up to high school; 1 = more than high school) and entered into models with up to a high school education as the reference group. An overall chronic conditions variable, computed as a count, was included to control for reduced PA due to health. See Supplementary Text 5 and Supplementary Table 3 for differences in inclusion and ascertainment of chronic conditions between studies.
Statistical Analysis
Multistate survival modeling
A coordinated analysis approach entails independent analysis at the level of the individual study by applying the same analytic models to variables representing the same construct. MSM (37) was used to assess cognitive status transitions, aligned according to chronological age, in which more frequent occasions allow for more precise estimation of transition hazards. While Cox regression models one transition (eg, (13)), MSM provides the opportunity to simultaneously model transitions between multiple cognitive status categories, include death as a competing risk, and examine the impact of factors associated with each transition. A 4-state model was applied (State 1 = nonimpaired cognitive functioning; State 2 = mildly impaired cognitive functioning; State 3 = severely impaired cognitive functioning; State 4 = death; see Figure 1). The MSM package (38) for R was used to estimate multistate survival models; the Broyden–Fletcher–Goldfarb–Shanno (BFGS) method of algorithm was applied to optimize functioning. As PA was the main focus of these analyses, time-varying PA was included as a covariate of all forward and backward transitions. To prevent numerical problems, the PA variable was scaled in some cases (eg, the range was constrained by dividing PA values by 15); however, scaling does not impact significance of the HRs. Covariates (age, sex, education, and chronic conditions) were included as covariates on forward transitions to simplify the model estimation. For most studies (AgeCoDe, MAP, HRS, ELSA, Austria, Denmark, Germany, and Switzerland) with few individual backwards transitions, the effects of covariates were excluded from the backward transition estimates. Transitioning from clinically diagnosed dementia back to MCI did not occur in AgeCoDe or EAS; therefore, the transition was not modeled in these studies. For all other studies, models included the possible backward transition from severely to mildly impaired cognitive functioning. See Supplementary Table 1 for total individual transitions between cognitive status categories.
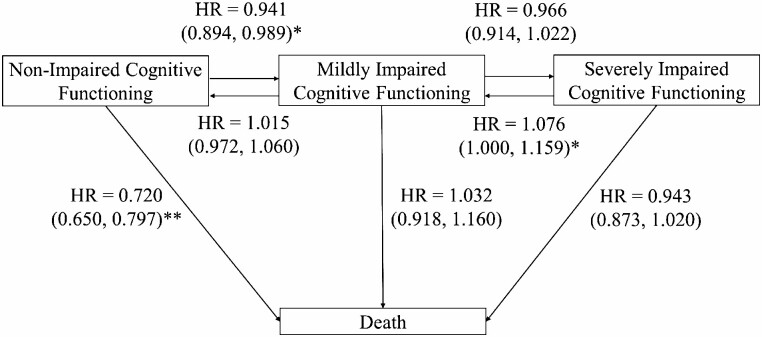
Figure 1.
Four-state model illustrating the pooled meta-analytic effect of approximately 150 min of moderate physical activity per week on transitions between cognitive states and death including pooled hazard ratios (HRs) and 95% confidence intervals. *p < .05, **p < .01.
Life expectancies
To complement the MSM analyses, to provide an estimate of the impact of PA on mortality, and to be consistent with previous research applying an MSM approach (eg, (23)), total LEs were estimated, conditional on age, using the elect package in R (39). The package fits a multinomial regression model using the transition probabilities estimated by the MSM for time-invariant covariates (besides age). For this purpose, the within-person average of the PA measure across all available occasions was computed, which is representative of an individual’s overall level of PA compared to one measurement occasion (40,41), and entered into this second set of multistate models as a time-invariant (rather than time-varying) variable for the LE analysis. Within each study, LEs were estimated for male and female participants at 70 and 80 years of age, at a high-school education or less, at no chronic conditions, and at 3 levels for PA. American guidelines for PA recommend that, at minimum, older adults should engage in 150–300 minutes of moderate PA or 75–150 minutes of vigorous PA per week (42). Therefore, we translated the “PA intensity units per week” to reflect a sedentary lifestyle (ie, 0 min/wk), as well as to approximate the lower (approx. 150 minutes of moderate PA) and upper (approx. 300 minutes of moderate PA) recommendations of minimum engagement in PA.
Meta-analysis
To provide an overall effect size for the effect of PA on each transition, as well as pooled LEs, meta-analytic techniques were executed in R using the Metafor (43) package, in which studies with more precise standard errors are assigned more weight. A random-effects approach was chosen, as the goal was to investigate the average true effect in the larger population of studies, as well as due to between-study differences. Separate meta-analyses were fit for each transition to account for the different nature of the effect sizes (eg, normal cognition to death vs. normal cognition to mild impairment) as well as differences in prevalence of each transition. In order to facilitate interpretability, all HRs were scaled to indicate approximately 150 minutes of moderate PA per week; however, given the between-study differences in PA variables, the computed meta-analysis effect size for each transition is intended to indicate direction and significance of engaging in more PA. The Hartung–Knapp (HK) method for random effects (44) was applied, which uses a refined estimator of variance and results in adequate error rates (45), particularly when there is heterogeneity in precision between studies (46).
Results
Given the emphasis of this project, we focus on the impact of PA on transitions. Results regarding the covariates (ie, age, sex, education, and chronic conditions) are reported in Supplementary Table 4. The reported HRs and confidence intervals (CIs) reflect the effect of engaging in approximately 150 minutes of moderate PA per week on transitions between cognitive states. Although the magnitude of the estimated HR would change depending on the way in which PA is scaled, the direction and significance of the impact of PA on each transition remains constant (eg, for 1 or 15 intensity units of PA per week).
Multistate Survival Models
HRs (and 95% CIs) of the effect of time-varying PA on transitions between status categories for each study are presented in Table 2. The pooled, meta-analytic HRs (95% CIs) for the effect of approximately 150 minutes of moderate PA per week on each transition are depicted in Figure 1. The meta-analytic estimates indicate that engaging in more PA was associated with a significantly decreased risk of transitioning from nonimpaired cognitive functioning to both mildly impaired cognitive functioning (HR = 0.941; CIs = 0.894, 0.989) and death (HR = 0.720; CIs = 0.650, 0.797). These results indicate a protective effect of PA, particularly for preventing death, and, to a lesser extent, prior to onset of cognitive impairment. In addition, the meta-analysis revealed that more PA was associated with a marginally significant increased likelihood of transitioning backward from severely impaired to mildly impaired cognitive functioning (HR = 1.076; CIs = 1.000, 1.159), suggesting that PA may also be protective after onset of cognitive impairment. Meta-analyses indicated that PA did not have a significant impact on the transition from mildly impaired to severely impaired cognitive functioning (HR = 0.966, CIs = 0.914, 1.022), from mildly impaired cognitive functioning to death (HR = 1.032, CIs = 0.918, 1.160), from mildly impaired to nonimpaired cognitive functioning (HR = 1.015, CIs = 0.972, 1.060), or from severely impaired cognitive functioning to death (HR = 0.943, CIs = 0.873, 1.020).
Table 2.
Hazard Ratios for the Effect of Physical Activity as a Time-Varying Variable on Transitions of Older Adults Through Cognitive Functioning Status Categories and Death for Each Study
| Sample | AgeCoDe | EAS | MAP | LASA | HRS | ELSA | Austria | Belgium | Denmark | France | Germany | Netherlands | Sweden | Switzerland | Meta-analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| State 1–2 | 0.996 | 0.904* | 0.944 | 1.019 | 0.997* | 0.984* | 0.963* | 0.636* | 0.965* | 0.805 | 0.982* | 0.780 | 0.989 | 0.691* | 0.941* |
| State 1–4 | 0.974* | 0.879 | 0.398* | 0.355* | 0.908* | 0.916* | 0.940* | 0.524* | 0.962* | 0.447* | 0.902* | 0.398* | 0.953* | 0.622* | 0.720* |
| State 2–1 | 1.001 | 0.895* | 1.075 | 0.989 | 1.003* | 1.007 | 1.017 | 0.860 | 1.013 | 1.514* | 1.000 | 1.299 | 1.003 | 0.913 | 1.015 |
| State 2–3 | 0.984* | 0.903 | 0.951 | 0.910* | 0.993* | 0.998 | 1.028 | 0.695* | 0.998 | 0.958 | 1.014 | 1.097 | 0.996 | 4.754 | 0.966 |
| State 2–4 | 1.011 | 1.026 | 1.124 | 1.029 | 1.011* | 1.069* | 0.470 | 0.255 | 0.988 | 1550.077 | 0.842 | 0.301 | 0.864 | 0.782 | 1.032 |
| State 3–2 | 1.110 | 1.082* | 1.006 | 1.011 | 1.010 | 0.962 | 1.054* | 1.696* | 1.030 | 0.499 | 1.041 | 5.217 | 1.076* | ||
| State 3–4 | 0.991* | 0.946 | 0.837 | 0.879* | 0.991* | 0.991 | 1.038* | 0.867 | 0.943* | 0.711 | 1.026* | 1.386 | 0.984 | 0.055 | 0.943 |
Notes: AgeCoDe = German Study on Ageing, Cognition, and Dementia; EAS = Einstein Aging Study; ELSA = English Longitudinal Study of Aging; HRS = Health and Retirement Study; LASA = Longitudinal Aging Study Amsterdam; MAP = Memory and Aging Project. State 1 = nonimpaired cognitive functioning; State 2 = mildly impaired cognitive functioning; State 3 = severely impaired cognitive functioning; State 4 = death. Analyses controlled for age, sex, education, and chronic conditions. Meta-analysis estimates are pooled results from individual studies. Country names are all part of SHARE (Survey of Health, Ageing and Retirement in Europe); see Supplementary Table 4 for confidence intervals.
*p< .05.
The meta-analytic results were mostly consistent with the individual study results, though there was some heterogeneity between studies. The proportion of true variability of the effect of PA across studies relative to the total variability in observed effects was negligible (I2 = 0%) for the majority of transitions (6 out of 7), indicating relatively consistent estimates between studies. In contrast, for the effect of PA on the transition from nonimpaired cognitive functioning to death, the relative proportion of true variability was substantial (I2 = 99.98%), indicating considerable heterogeneity that is likely due to sample specific characteristics, such as average age at baseline and targeted sample (eg, EAS aimed to recruit healthy older adults). Further, the percentage of individuals who died was highly heterogeneous between studies (9.5% in SHARE’s Switzerland–73.5% in LASA), mostly as a function of between sample differences in year of baseline measurement, length of follow-up, and mean age.
Overall LEs
Estimated LEs are presented in Table 3. Results indicate that female participants consistently live substantially longer than male participants. Pairwise comparisons of the pooled LEs for approximately 0, 150, and 300 minutes of moderate PA per week revealed a positive linear effect of PA. Meta-analytic results indicate that, irrespective of sex or age, individuals who engage in approximately 300 minutes of PA per week live significantly longer than individuals who engage in approximately 150 minutes, and those individuals live significantly longer than individuals who do not engage in PA. Study-level results were consistent with meta-analytic results in all studies except LASA. The difference could be due to a combination of study-level and cultural differences; specifically, LASA has a very high mortality rate (73.5%) due to long-term follow-up, which provides more information for estimation of mortality. Additionally, participants in LASA were instructed to not include walking or cycling for transportation purposes, as these activities are considered common daily activities in The Netherlands (47). These activities are likely to have similar physical benefits despite the purpose, which may contribute to excess variability in LASA’s PA variable (ie, measurement error) and consequently diminish the estimate of the true effect of PA. Supplementary Table 5 presents the HRs (95% CIs) for the multistate models on which the LEs are based.
Table 3.
Overall Pooled Estimates of Life Expectancies in Years for Male and Female Participants With up to a High School Education, No Chronic Conditions, and Physical Activity Values Based on American Standards
| Life Expectancies in Years (95% CIs) | ||
|---|---|---|
| For a 70 y Old | For a 80 y Old | |
| Male, PA (0 min) | 9.82 (8.40, 11.24) | 6.50 (5.56, 7.44) |
| Male, PA (150 min) | 13.88 (12.45, 15.31) | 9.23 (8.29, 10.18) |
| Male, PA (300 min) | 15.19 (13.74, 16.64) | 10.31 (9.34, 11.28) |
| Female, PA (0 min) | 12.66 (10.95, 14.38) | 9.07 (7.79, 10.36) |
| Female, PA (150 min) | 16.63 (14.92, 18.34) | 11.46 (10.17, 12.75) |
| Female, PA (300 min) | 18.09 (16.36, 19.81) | 12.59 (11.27, 13.91) |
Note: PA = physical activity; PA (0 minutes) = approximately 0 min of moderate PA per week; PA (150 min) = approximately 150 min of moderate PA per week; PA (300 min) = approximately 300 min of moderate PA per week. Estimates are based on meta-analysis of results from 14 longitudinal studies of aging.
Discussion
Based on independent analysis of 14 longitudinal studies (NTotal = 52 039), our results indicate that engaging in more PA in older adulthood is associated with a decreased risk of mortality, adjusting for age, sex, education, and chronic conditions. Meta-analytic results also reveal that more PA was significantly associated with a decreased risk of transitioning from nonimpaired cognitive functioning to mildly impaired cognition. Together, these results provide evidence for the importance of engaging in PA throughout older adulthood, particularly prior to onset of cognitive impairment. We modeled the backward transition in studies that included individual transitions from severely impaired back to mildly impaired cognitive status. The meta-analytic summary indicated that more PA was associated with an increased likelihood of transitioning backwards, suggesting that engaging in more PA at the severely impaired stage may contribute to diminishing the symptoms that exacerbate poor cognitive performance or may assist individuals in regaining some lost function. Alternatively, these results may indicate that individuals who are still able to engage in PA may have relatively better cognitive performance, and thus are more likely to perform below the cutoff for severe impairment at follow-up visits.
More PA was also associated with a significantly reduced risk of transitioning forward through cognitive status categories in some individual studies (eg, 29% from mildly to severely impaired, and 29% from severely impaired to death). Additional studies (eg, an additional 42% from mildly to severely impaired, and 50% from severely impaired to death) suggested a trend that more PA was associated with a reduced risk of these forward transitions, though estimates did not meet statistical significance. Due to fewer individuals within impaired cognitive status (compared to nonimpaired) categories, the power to detect these effects is more limited. Further, participants are more likely to drop out of a longitudinal panel study after onset of cognitive impairment (48). Although death data were ascertained via national death records in many of the studies, observing the transition to severely impaired cognition prior to death would be impossible for individuals who drop out at the stage of mildly impaired cognitive functioning. Overall, the results point to a protective effect of PA after the onset of cognitive impairment; however, heterogeneity between studies and uncertainty in the pooled estimates indicates a less robust association.
Investigation of modifiable factors that contribute to transitioning back to nonimpaired cognitive functioning in older adulthood are critical, as pharmacological solutions administered at the MCI stage tend to merely slow, rather than reverse, progression of cognitive impairment (49). The meta-analytic estimates do not indicate that individuals who engage in more PA are more likely to transition from mildly impaired back to nonimpaired cognitive functioning, which is consistent with previous research examining the impact of education on cognitive status transitions (23). However, study characteristics may have influenced these results. The timing of measurement occasions ranged from annually (EAS and MAP) to every 3 years (LASA). Koepsell and Monsell (18) found that 16% of individuals with MCI (N = 3020) revert back to nonimpaired cognitive functioning approximately 1 year later, but that these individuals are also more likely to retransition back to cognitive impairment at later occasions. Thus, the timing of measurement occasions may limit the ability to capture cognitive status transitions that occur between measurement intervals in some cases. Further, in addition to other lifestyle characteristics (eg, social and cognitive engagement), PA may contribute to cognitive reserve (6). An individuals’ accumulation of cognitive reserve may then differentially affect the likelihood of cognitive status transitions; individuals higher in reserve typically have higher neuropathological burden prior to the emergence of cognitive symptoms and tend to progress more quickly through the stages of cognitive decline (50). As such, engaging in more PA may no longer have the power to protect against cognitive decline once an individual with high reserve transitions to mildly impaired cognition. Thus, the intersection of PA and cognitive reserve may contribute to heterogeneity in the impact of PA on transitions from mildly impaired cognitive status.
Causal inferences cannot be made based on the observational data analyzed in the current work because we cannot exclude the possibility of a third, unmeasured variable causing changes in both cognitive functioning and PA, or reverse causation. Recent work (51,52) found no evidence for an association of PA with dementia when PA is assessed more than 10 years prior to dementia onset, leading the researchers to posit that the relationship between PA and conversion to dementia may be due to reverse causation. Namely, the prodromal phase of dementia may be characterized by a reduction in PA, such that increased PA does not cause less cognitive impairment, but less cognitive decline allows more PA. We believe that our results are not completely consistent with this reasoning. If decline in PA is a consequence of the dementia process (ie, a marker of prodromal dementia), one would expect a strong association of PA in the transition from mildly impaired to severely impaired cognitive functioning because a large proportion of these participants (though not all) are likely to eventually transition to severe cognitive functioning. In contrast, one would expect a weaker effect in the transition from nonimpaired to mildly impaired cognitive functioning because the nonimpaired state includes, proportionally, fewer participants who will eventually transition to mildly impaired cognitive functioning. However, we observe the opposite pattern of results (ie, a stronger effect of PA for the transition from nonimpaired to mildly impaired cognition compared to the transition from mildly to severely impaired cognition, based on parameter estimate and p-value). Future research applying interventional designs could explore whether changes in PA are causal for changes in cognition on time scales shorter than 10 years.
Strengths, Limitations, and Future Directions
Coordinating at the lowest possible denominator is common when coordinating analyses, but such an approach may disregard important qualities of a study. For this coordinated analysis, we aimed to maximize available data, while still allowing comparability between studies. For example, using sample-specific SD’s is not optimal for identifying cognitive status categories (eg, approach used for ELSA and SHARE), but this approach has been used in previous studies and maximized the number of studies included in the analyses. Likewise, although EAS, MAP, and AgeCoDe included administration of the MMSE and other cognitive tasks, we used the formal diagnosis of MCI and dementia available in these studies, which provided a more fine-grained and precise estimate of cognitive status. By weighting the meta-analysis based on standard error, the overall estimates are impacted most by the studies with more precise estimates (and fine-grained operational definitions tend to contribute to estimates that are more precise). For example, the transition from mildly impaired to death was estimated to be extremely high in France, likely due to very few individual transitions in this study, but the meta-analytic results were not swayed. Thus, this project represents conceptual replications rather than strict replications given between-study heterogeneity in study characteristics (eg, frequency and timing between measurement occasions, country of origin) and operational definition of constructs (eg, differences in measurement of cognitive functioning, PA, education, and chronic conditions). Between-study differences may be considered a limitation, particularly because the quality of PA variables and variables used to operationalize cognitive states varied between studies. Yet, between-study results were mainly consistent despite these differences, and heterogeneity in the key features of studies reinforces the implications of consistent results in a coordinated analysis (20).
Computation of a single PA variable for each study was important to simplify the models. This approach facilitated reporting and interpretation of results (particularly important for a coordinated analysis) and allowed greater comparability between studies. However, by characterizing PA on a single scale, our approach does not allow differentiation between PA intensity and frequency. Future research differentiating the impact of PA frequency and intensity on cognitive status transitions may provide a more fine-grained account of the benefits of PA. Additionally, given the computational complexity of MSM, limiting the covariates included in the models was necessary. Adjusting for additional covariates associated with PA and cognition, such as depressive symptoms, pain, functional limitations, and APOE status would have strengthened the current project. Indeed, reviewers made this recommendation. Though we did not have access to APOE across all studies, we executed post hoc sensitivity analyses including depressive symptoms, pain, and functional limitations in HRS and ELSA (the 2 largest samples). Within HRS, the estimated HR for PA did not meaningfully change. In ELSA, the model did not converge with sufficient optimization after 50,000 iterations. The other studies include 1858–4562 less participants than ELSA, and consequently have less power to prevent numerical problems. As such, it is unlikely that the models would have converged with the additional covariates. Future research adjusting for these covariates would improve the literature.
Our analyses adjusted for sex, though there may be further sex differences in the magnitude of the relationship between PA and cognition. Consistent with previous literature (23,53), our findings indicate that across studies, male participants are more likely to transition from nonimpaired cognitive functioning to MCI and death, and from severe cognitive functioning to death (see Supplementary Table 4). However, merely adjusting for sex assumes that the effect of PA on transitions is the same for male and female participants. Sex-stratified MSM analyses may provide an improved understanding of sex differences in the impact of PA on transitions between cognitive status categories and death. Furthermore, given response options available in the original surveys, this project considered sex as a binary construct. Future research in this area examining sex/gender according to a spectrum would improve the literature.
Computation and centering of values for the dependent and independent variables required several researcher decisions. Many of these decisions (eg, statistical plan, covariates to be included in models) were preregistered on December 4, 2018 on the Open Science Framework (https://osf.io/6t3sk/). Three specific aspects of the preregistration were slightly modified: (i) We originally planned to use cutoff scores on the MMSE, TICS and summed cognitive scores to operationalize cognitive states, but later decided to maximize available data wherever possible, such that incident formal diagnoses were used when available in order to highlight the strengths of individual studies, (ii) One study (OCTO-Twin) was not included because the available measurement of PA did not allow computation of a continuous PA score, and (iii) We originally planned to fit multistate models for the LEs analysis using baseline PA, but later decided to use the average within-person PA score, as this value is more indicative of an individual’s overall level of PA compared to one measurement occasion at an arbitrary time in the life span (ie, “baseline”). We did not, however, fit the models using MMSE in the studies that also had formal diagnoses, or in the excluded study, or using baseline PA; therefore, we were not confronted with the opportunity to select the more “appealing” results. In addition, the current analyses were quite complicated, and therefore, only the main features of the analyses were preregistered. For example, we decided to estimate LEs based on the minimum and maximum values of the American Physical Activity Standards after reading the 2018 Physical Activity Guidelines Advisory (42).
Relatedly, all PA measures were self-reported and based on typical PA engagement, which may result in social influence bias or retrospection bias. These analyses also did not differentiate within dementia (eg, Alzheimer’s disease vs vascular) and MCI (eg, amnestic MCI vs non-amnestic MCI) diagnoses. Future research using objective measures of PA (eg, accelerometers), examining the impact of PA on transitions to different types of dementia and MCI, and applying experimental or longitudinal measurement burst designs would improve our understanding of the impact of PA on cognition. Specifically, given that individuals who are exercising in late life may be continuing an exercise habit initiated earlier in the life span, parallel process latent growth modeling (LGM) examining trajectories of PA and cognitive functioning from midlife into older adulthood may provide the opportunity to examine individual differences in the importance of PA onset and maintaining PA. Alternatively, if PA was specified as a time-varying covariate in models jointly modeling mortality and trajectories of cognitive functioning, the analysis would also be able to estimate the momentaneous effects of PA on the trajectory at each occasion. MSM, however, provides a powerful analytic approach for discriminating the effect of PA at different stages of cognitive impairment, accounts for death as a competing risk factor, and allows flexibility in transitions between cognitive states (eg, backward transitions, skipping specific stages).
Additionally, with effects drawn from only 12–14 independent studies, power to detect an effect may have been limited. More individual studies included in this coordinated analysis may have provided more power to meta-analyze study-level moderators (eg, differences between operational definitions of cognitive status). Some of the effect sizes and associated CIs, particularly in the studies from SHARE, were imprecise, which is likely a function of the small number of individual transitions between cognitive status categories. For example, across studies, the most recent baseline interviews were conducted in SHARE and AgeCoDe in 2003, and baseline age in SHARE is approximately 10 years younger than AgeCoDe, resulting in fewer instances of impairment and mortality. However, study effect sizes were weighted based on standard error (ie, more precise studies were given more weight) for the meta-analysis; therefore, the pooled results were not strongly impacted by imprecise estimates. Furthermore, consideration of the context is important. Given the prevalence of cognitive decline in older adulthood, identification and further understanding of factors that may protect against cognitive aging are imperative. The existing literature documents a limited number of these factors, and although this synthesis does not make any causal determinations, PA is a modifiable lifestyle factor. Together, these considerations imply that the reduced risk associated with more PA should be seen as meaningful, despite being somewhat small. Future research examining PA across the entire life span (rather than > 60 years) may reveal critical periods of when PA may be most protective.
Conclusions
The consistency of results from 14 longitudinal studies provides strong evidence for the importance of engaging in PA throughout older adulthood. In addition to improving our understanding of the relationship between PA and transitions between cognitive status categories and death, as well as the feasibility and strengths of the coordinated analysis approach, this research provides evidence and a basis for motivating individuals to engage in PA in older adulthood, and also for physicians to consider recommending PA to their older patients.
Funding
Research reported in this publication was supported by the Integrative Analysis of Longitudinal Studies of Aging and Dementia research network under the National Institute on Aging of the National Institutes of Health (P01 AG043362 to A.M.P.); Alzheimer Society Research Program (ASRP; to T.Y. and N.A.L); Social Sciences and Humanities Research Council of Canada (SSHRC to T.Y. and J.R.); Canadian Institutes of Health Research (CIHR; GSD164243 to J.E.K); and AGE-WELL NCE Inc., a member of the Centers for Excellence Program (to J.E.K. and R.V.). German Study on Ageing, Cognition and Dementia in Primary Care Patients has been funded by the German Federal Ministry of Education and Research (under German Research Network on Dementia grants: 01GI0102, 01GI0420, 01GI0422, 01GI0423, 01GI0429, 01GI0431, 01GI0433, 01GI0434; German Research Network on Degenerative Dementia grants: 01GI0710, 01GI0711, 01GI0712, 01GI0713, 01GI0714, 01GI0715, 01GI0716; Health Service Research Initiative grants: 01GY1322A, 01GY1322B, 01GY1322C, 01GY1322D, 01GY1322E, 01GY1322F, 01GY1322G). Einstein Aging Study is supported by the National Institutes of Health (grant number P01 AG03949), Sylvia and Leonard Marx Foundation, and Czap Foundation. The Longitudinal Aging Study Amsterdam is supported by a grant from the Netherlands Ministry of Health Welfare and Sports, Directorate of Long-Term Care. Memory and Aging Project is supported by the National Institute on Aging of the National Institutes of Health (R01 AG15819 and R01 AG17917 to D.A.B). English Longitudinal Study of Aging is supported by the National Institute of Aging (grant numbers 2RO1AG7644-01A1, 2RO1AG017644) and a consortium of UK government departments coordinated by the Office for National Statistics. Health and Retirement Study is supported by National Institute on Aging (U01 AG009740) and the Social Security Administration in the United States. Survey of Health, Ageing and Retirement in Europe is supported by the European Commission Framework Programme 5 (FP5): QLK6-CT-2001-00360, FP6 (SHARE-I3: RII-CT-2006-062193, COMPARE: CIT5-CT-2005-028857), FP7 (SHARE-PREP: GA N°211909, SHARE-LEAP: GA N°227822, SHARE M4: GA N°261982), Horizon 2020 (SHARE-DEV3: GA N°676536, SERISS: GA N°654221), DG Employment, Social Affairs & Inclusion, the German Ministry of Education and Research, the Max Planck Society for the Advancement of Science, the U.S. National Institute on Aging (U01_AG09740-13S2, P01_AG005842, P01_AG08291, P30_AG12815, R21_AG025169, Y1-AG-4553-01, IAG_BSR06-11, OGHA_04-064, HHSN271201300071C), and from various national funding sources (gratefully acknowledged at www.share-project.org). This article is solely the responsibility of the authors and does not necessarily represent the official views of the funding bodies.
Supplementary Material
Acknowledgments
In recent years, the importance of knowledge mobilization and research translation has been gaining momentum. Researchers are encouraged to advocate for strategies that promote healthy aging by providing clearly defined recommendations (eg, (42,54)), especially in the form of specific health behaviors recommended for older adults (eg, 150 minutes of moderate PA per week) (55). Based on previous literature, medical professionals are encouraged to consider promoting and even prescribing PA to their patients as a cost-effective approach to improve longevity and well-being (56). Though the current project is based solely on longitudinal survey data, our findings contribute to the evidence for mechanisms of action through which improved cognitive outcomes and increased healthspan may occur. Supplementary Figure 1, developed in collaboration with the Alzheimer Society of British Columbia, Canada, is an infographic summarizing the current research using graphics and easy-to-understand language. Medical professionals and interested readers are encouraged to print and post the poster in their offices in an effort to mobilize knowledge. We gratefully acknowledge and thank all members of the AgeCoDe & AgeQualiDe Study Group Principal Investigators*: Wolfgang Maier, Martin Scherer, Steffi G. Riedel-Heller Heinz-Harald Abholz, Christian Brettschneider, Cadja Bachmann, Horst Bickel, Wolfgang Blank, Hendrik van den Bussche, Sandra Eifflaender-Gorfer, Marion Eisele, Annette Ernst, Angela Fuchs, André Hajek, Kathrin Heser, Frank Jessen, Hanna Kaduszkiewicz, Teresa Kaufeler, Mirjam Köhler, Hans-Helmut König, Alexander Koppara, Diana Lubisch, Tobias Luck, Dagmar Lühmann, Melanie Luppa, Tina Mallon, Manfred Mayer, Edelgard Mösch, Michael Pentzek, Jana Prokein, Alfredo Ramirez, Susanne Röhr, Anna Schumacher, Janine Stein, Susanne Steinmann, Franziska Tebarth, Carolin van der Leeden, Michael Wagner, Klaus Weckbecker, Dagmar Weeg, Jochen Werle, Siegfried Weyerer, Birgitt Wiese, Steffen Wolfsgruber, and Thomas Zimmermann. We also wish to thank all participants who volunteered their time, as well as all patients and their general practitioners, for participating in AgeCoDe, EAS, MAP, LASA, HRS, ELSA, and SHARE studies. *Hendrik van den Bussche (2002–2011).
Conflict of Interest
None declared.
Author Contributions
T.Y. conceptualized the research questions, wrote the manuscript, coordinated analysis across studies, conducted analyses for SHARE studies and ELSA, and conducted the meta-analyses; N.A.L. assisted with manuscript editing, consulted on statistical analyses, conducted statistical analyses for HRS, and assisted with analyses for SHARE studies; A.M.P., J.R., S.M.H., and G.M.-T. assisted with manuscript editing and consulted on statistical analyses; J.E.K., R.V., L.K., and J.H. assisted with manuscript editing and conducted statistical analyses for LASA, MAP, AgeCoDe, and EAS, respectively; D.A.B., E.O.H., C.A.D., M.S., S.R.-H., and M.W. are principal investigators for MAP, LASA, EAS, and AgeCoDe, respectively, and assisted with manuscript editing; and A.H. and W.W. consulted on statistical analyses and assisted with manuscript editing.
References
- 1.Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement J Alzheimers Assoc. 2018;14(3):367–429. doi: 10.1016/j.jalz.2018.02.001 [DOI] [Google Scholar]
- 2.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007;3(3):186–191. doi: 10.1016/j.jalz.2007.04.381 [DOI] [PubMed] [Google Scholar]
- 3.Zissimopoulos J, Crimmins E, St Clair P. The value of delaying Alzheimer’s disease onset. Forum Health Econ Policy. 2014;18(1):25–39. doi: 10.1515/fhep-2014-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11(6):718–726. doi: 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 5.Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res. 2013;2013:657508. doi: 10.1155/2013/657508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng ST. Cognitive reserve and the prevention of dementia: the role of physical and cognitive activities. Curr Psychiatry Rep. 2016;18(9):85. doi: 10.1007/s11920-016-0721-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erickson KI, Hillman C, Stillman CM, et al. . Physical activity, cognition, and brain outcomes: a review of the 2018 Physical Activity Guidelines. Med Sci Sports Exerc. 2019;51(6):1242–51. doi: 10.1249/MSS.0000000000001936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dishman RK, Berthoud HR, Booth FW, et al. . Neurobiology of exercise. Obesity (Silver Spring). 2006;14(3):345–356. doi: 10.1038/oby.2006.46 [DOI] [PubMed] [Google Scholar]
- 9.Firth J, Stubbs B, Vancampfort D, et al. . Effect of aerobic exercise on hippocampal volume in humans: a systematic review and meta-analysis. NeuroImage. 2018;166:230–238. doi: 10.1016/j.neuroimage.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 10.Sofi F, Valecchi D, Bacci D, et al. . Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med. 2011;269(1):107–117. doi: 10.1111/j.1365-2796.2010.02281.x [DOI] [PubMed] [Google Scholar]
- 11.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303 [DOI] [PubMed] [Google Scholar]
- 12.Henskens M, Nauta IM, van Eekeren MCA, Scherder EJA. Effects of physical activity in nursing home residents with dementia: a randomized controlled trial. Dement Geriatr Cogn Disord. 2018;46(1–2):60–80. doi: 10.1159/000491818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llamas-Velasco S, Contador I, Villarejo-Galende A, Lora-Pablos D, Bermejo-Pareja F. Physical activity as protective factor against dementia: a prospective population-based study (NEDICES). J Int Neuropsychol Soc. 2015;21(10):861–867. doi: 10.1017/S1355617715000831 [DOI] [PubMed] [Google Scholar]
- 14.Buchman AS, Yu L, Wilson RS, et al. . Physical activity, common brain pathologies, and cognition in community-dwelling older adults. Neurology. 2019;92(8):e811–22. doi: 10.1212/WNL.0000000000006954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris JC, Cummings J. Mild cognitive impairment (MCI) represents early-stage Alzheimer’s disease. J Alzheimers Dis. 2005;7(3):235–239; discussion 255. doi: 10.3233/jad-2005-7306 [DOI] [PubMed] [Google Scholar]
- 16.Kaduszkiewicz H, Eisele M, Wiese B, et al. ; Study on Aging, Cognition, and Dementia in Primary Care Patients (AgeCoDe) Study Group . Prognosis of mild cognitive impairment in general practice: results of the German AgeCoDe study. Ann Fam Med. 2014;12(2):158–165. doi: 10.1370/afm.1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visser PJ, Kester A, Jolles J, Verhey F. Ten-year risk of dementia in subjects with mild cognitive impairment. Neurology. 2006;67(7):1201–1207. doi: 10.1212/01.wnl.0000238517.59286.c5 [DOI] [PubMed] [Google Scholar]
- 18.Koepsell TD, Monsell SE. Reversion from mild cognitive impairment to normal or near-normal cognition: risk factors and prognosis. Neurology. 2012;79(15):1591–1598. doi: 10.1212/WNL.0b013e31826e26b7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts RO, Cha RH, Mielke MM, et al. . Risk and protective factors for cognitive impairment in persons aged 85 years and older. Neurology. 2015;84(18):1854–1861. doi: 10.1212/WNL.0000000000001537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hofer SM, Piccinin AM. Integrative data analysis through coordination of measurement and analysis protocol across independent longitudinal studies. Psychol Methods. 2009;14(2):150–164. doi: 10.1037/a0015566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham EK, Weston SJ, Gerstorf Det al. . Trajectories of Big Five personality traits: a coordinated analysis of 16 longitudinal samples. Eur J Personal. 2020;34(3):301–321. doi: 10.1002/per.2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccinin AM, Muniz-Terrera G, Clouston S, et al. . Coordinated analysis of age, sex, and education effects on change in MMSE scores. J Gerontol B Psychol Sci Soc Sci. 2013;68(3):374–390. doi: 10.1093/geronb/gbs077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robitaille A, van den Hout A, Machado RJM, et al. . Transitions across cognitive states and death among older adults in relation to education: a multistate survival model using data from six longitudinal studies. Alzheimers Dement. 2018;14(4):462–472. doi: 10.1016/j.jalz.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y, Chen Y, Tseng Y, Tsai S, Tseng Y. Physical activity and successful aging among middle-aged and older adults: a systematic review and meta-analysis of cohort studies. AGING-US. 2020;12(9):7704–7716. doi: 10.18632/aging.103057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodcock J, Franco OH, Orsini N, Roberts I. Non-vigorous physical activity and all-cause mortality: systematic review and meta-analysis of cohort studies. Int J Epidemiol. 2011;40(1):121–138. doi: 10.1093/ije/dyq104 [DOI] [PubMed] [Google Scholar]
- 26.Börsch-Supan A, Brandt M, Hunkler C, et al. ; SHARE Central Coordination Team . Data resource profile: the Survey of Health, Ageing and Retirement in Europe (SHARE). Int J Epidemiol. 2013;42(4):992–1001. doi: 10.1093/ije/dyt088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luck T, Riedel-Heller SG, Kaduszkiewicz H, et al. ; AgeCoDe Group . Mild cognitive impairment in general practice: age-specific prevalence and correlate results from the German study on Ageing, Cognition and Dementia in primary care patients (AgeCoDe). Dement Geriatr Cogn Disord. 2007;24(4):307–316. doi: 10.1159/000108099 [DOI] [PubMed] [Google Scholar]
- 28.Katz MJ, Lipton RB, Hall CB, et al. . Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord. 2012;26(4):335–343. doi: 10.1097/WAD.0b013e31823dbcfc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett D, Schneider J, Buchman A, Barnes L, Boyle P, Wilson R. Overview and findings from the rush memory and aging project. Curr Alzheimer Res. 2012;9(6):646–663. doi: 10.2174/156720512801322663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoogendijk EO, Deeg DJ, Poppelaars J, et al. . The Longitudinal Aging Study Amsterdam: cohort update 2016 and major findings. Eur J Epidemiol. 2016;31(9):927–945. doi: 10.1007/s10654-016-0192-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huisman M, Poppelaars J, van der Horst M, et al. . Cohort profile: the Longitudinal Aging Study Amsterdam. Int J Epidemiol. 2011;40(4):868–876. doi: 10.1093/ije/dyq219 [DOI] [PubMed] [Google Scholar]
- 32.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort profile: the Health and Retirement Study (HRS). Int J Epidemiol. 2014;43(2):576–585. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clemens S, Phelps A, Oldfield Z, et al. . English Longitudinal Study of Ageing: Waves 0–8, 1998–2017 [Internet]. 2019 [cited Oct 30, 2019]. Accessed January 18, 2018. https://beta.ukdataservice.ac.uk/datacatalogue/studies/study?id=5050. doi: 10.5255/UKDA-SN-5050-17 [DOI]
- 34.Manly JJ, Schupf N, Stern Y, Brickman AM, Tang MX, Mayeux R. Telephone-based identification of mild cognitive impairment and dementia in a multicultural cohort. Arch Neurol. 2011;68(5):607–614. doi: 10.1001/archneurol.2011.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ainsworth BE, Haskell WL, Herrmann SD, et al. . 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 36.Caspersen C, Bloemberg B, Saris W, Merritt R, Kromhout D. The prevalence of selected physical activities and their relation with coronary heart-disease risk-factors in elderly men—the Zutphen study, 1985. Am J Epidemiol. 1991;133(11):1078–1092. doi: 10.1093/oxfordjournals.aje.a115821 [DOI] [PubMed] [Google Scholar]
- 37.Van den Hout A. Multi-state survival models for interval-censored data. CRC Press. 2017;2017(10):238. doi: 10.1002/sim.4459 [DOI] [Google Scholar]
- 38.Jackson C. Multi-state models for panel data: The msm package for R. J Stat Softw. 2011;38(8):1–28. [Google Scholar]
- 39.van den Hout A, Sum Chan M, Matthews F. Estimation of life expectancies using continuous-time multi-state models. Comput Methods Programs Biomed. 2019;178:11–18. doi: 10.1016/j.cmpb.2019.06.004 [DOI] [PubMed] [Google Scholar]
- 40.Albert PS, Follmann DA. Modeling repeated count data subject to informative dropout. Biometrics. 2000;56(3):667–677. doi: 10.1111/j.0006-341x.2000.00667.x [DOI] [PubMed] [Google Scholar]
- 41.Little RJA. Modeling the drop-out mechanism in repeated-measures studies. J Am Stat Assoc. 1995;90(431):1112–1121. doi: 10.1080/01621459.1995.10476615 [DOI] [Google Scholar]
- 42.Scientific Report—2018 Physical Activity Guidelines [Internet] [cited June 17, 2019]. Accessed December 17, 2018. https://health.gov/paguidelines/second-edition/report/
- 43.Viechtbauer W. Conducting meta-analyses in R with the Metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 44.Hartung J. An alternative method for meta-analysis. Biom J. 1999;41(8):901–16. doi: [DOI] [Google Scholar]
- 45.Hout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14(1):25. doi: 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Röver C, Knapp G, Friede T. Hartung-Knapp-Sidik-Jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med Res Methodol. 2015;15(1):99. doi: 10.1186/s12874-015-0091-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stel VS, Smit JH, Pluijm SM, Visser M, Deeg DJ, Lips P. Comparison of the LASA Physical Activity Questionnaire with a 7-day diary and pedometer. J Clin Epidemiol. 2004;57(3):252–258. doi: 10.1016/j.jclinepi.2003.07.008 [DOI] [PubMed] [Google Scholar]
- 48.Sliwinski MJ, Hofer SM, Hall C, Buschke H, Lipton RB. Modeling memory decline in older adults: the importance of preclinical dementia, attrition, and chronological age. Psychol Aging. 2003;18(4):658–71. doi: 10.1037/0882-7974.18.4.658 [DOI] [PubMed] [Google Scholar]
- 49.Tan CC, Yu JT, Wang HF, et al. . Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;41(2):615–631. doi: 10.3233/JAD-132690 [DOI] [PubMed] [Google Scholar]
- 50.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kivimäki M, Singh-Manoux A, Pentti J, et al. ; IPD-Work Consortium . Physical inactivity, cardiometabolic disease, and risk of dementia: an individual-participant meta-analysis. Br Med J. 2019;365:l1495. doi: 10.1136/bmj.l1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabia S, Dugravot A, Dartigues JF, et al. . Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II cohort study. Br Med J. 2017;357:j2709. doi: 10.1136/bmj.j2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tschanz JT, Corcoran CD, Schwartz S, et al. . Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer dementia: the Cache County Dementia Progression study. Am J Geriatr Psychiatry. 2011;19(6):532–542. doi: 10.1097/JGP.0b013e3181faec23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harmell AL, Jeste D, Depp C. Strategies for successful aging: a research update. Curr Psychiatry Rep. 2014;16(10):476. doi: 10.1007/s11920-014-0476-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Golinowska S, Groot W, Baji P, Pavlova M. Health promotion targeting older people. BMC Health Serv Res [Internet]. 2016;16(Suppl. 5):345. doi: 10.1186/s12913-016-1514-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jordan C, Butler J, Myers J, Albert MA. Exercise prescription for a healthy heart. Curr Cardiovasc Risk Rep. 2018;12(7):1–6. doi: 10.1007/s12170-018-0581-x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.