Abstract
Study Objectives
The brain appears to use internal models to successfully interact with its environment via active predictions of future events. Both internal models and the predictions derived from them are based on previous experience. However, it remains unclear how previously encoded information is maintained to support this function, especially in the visual domain. In the present study, we hypothesized that sleep consolidates newly encoded spatio-temporal regularities to improve predictions afterwards.
Methods
We tested this hypothesis using a novel sequence-learning paradigm that aimed to dissociate perceptual from motor learning. We recorded behavioral performance and high-density electroencephalography (EEG) in male human participants during initial training and during testing two days later, following an experimental night of sleep (n = 16, including high-density EEG recordings) or wakefulness (n = 17).
Results
Our results show sleep-dependent behavioral improvements correlated with sleep-spindle activity specifically over occipital cortices. Moreover, event-related potential (ERP) responses indicate a shift of attention away from predictable to unpredictable sequences after sleep, consistent with enhanced automaticity in the processing of predictable sequences.
Conclusions
These findings suggest a sleep-dependent improvement in the prediction of visual sequences, likely related to visual cortex reactivation during sleep spindles. Considering that controls in our experiments did not fully exclude oculomotor contributions, future studies will need to address the extent to which these effects depend on purely perceptual versus oculomotor sequence learning.
Keywords: sleep, memory, sequence learning, prediction, visual perception
Statement of Significance.
Our brains predict sensory inputs based on previous experience. However, it is unclear how previously acquired visual knowledge is used to predict future inputs. Because sleep is important for memory consolidation, we investigated whether humans are better at predicting a visuo-spatial sequence when allowed to sleep after learning. We found that a higher number of sleep spindles specifically over visual brain areas is associated with better predictions of visual inputs afterwards. This was accompanied by a reduced need for attention for predictable information. Sleep spindles are a hallmark of non-rapid eye-movement sleep and are associated with offline reactivation of wake experience. Our results suggest that active consolidation of previous experience during sleep spindles supports accurate predictions of future visual inputs.
Introduction
Our environment contains a multitude of statistical regularities which we exploit during perception and action [1]. Theories of efficient coding originally proposed that regularities are discarded during perception to reduce the amount of information transmitted along sensory hierarchies [2]. More recent theories of hierarchical predictive coding extended this notion to claim that, instead of being discarded, regularities are represented at different levels of a hierarchical system, and are used to predict future perceptions as well as the consequences of behavior [3–9]. An important aspect of such theories is the question of how regularities are extracted and stored in order to ensure adaptive behavior across extended periods of time. Sleep, as a pivotal factor in memory consolidation [10, 11], may support the extraction and consolidation of regularities. However, the literature is inconclusive in this respect: whereas some studies found beneficial effects of sleep on implicit regularity extraction [12–24], others found no effect [25–31] (for reviews, see [32, 33]). In particular, it remains unclear whether sleep plays a role in extracting and distributing predictive templates [34].
In a behavioral study, we previously demonstrated that sleep improves predictive processing of spatio-temporal sequences, as well as the transfer of learning to a different temporal context [35]. However, first, the sleep-associated neural events underlying this selective consolidation and transformation remain to be determined [36], and second, it is unclear how sleep affects subsequent neural responses to predicted vs. unpredicted stimuli.
Regarding the first question, thalamocortical sleep spindles (i.e. short bursts of waxing and waning oscillations occurring during stage 2 and slow-wave sleep) might orchestrate consolidation of spatio-temporal sequences. There is accumulating evidence that sleep spindles are involved in memory processing [37–39], and several studies have reported modality-specific correlations between spindle activity and behavioral performance: Nishida and Walker [40], Tamaki et al. [41], and Johnson et al. [42] found that sleep spindles over premotor regions contribute to motor learning. In the visual domain, similar results have been reported for high-level declarative learning [43, 44] and low-level perceptual learning [45], with increased spindle activity over posterior cortices correlating with better retention. Importantly, spindle activity has been shown to be coupled not only to learning success but also to subsequent reactivation of task-relevant cortices during sleep [46–48]. Here, our goal was to extend these findings by testing whether sleep spindles over visual cortices are specifically related to active predictions of spatio-temporal regularities.
As to the question of how sleep affects responses to predicted vs. unpredicted stimuli, our previous behavioral results showed that sleep, while enhancing responses to predicted stimuli, impairs performance (i.e. increases error rates and reaction times) for unpredicted stimuli [35]. An explanation for this impairment arising in the context of predictive coding theory is that unpredicted stimuli elicit heightened mismatch responses once knowledge of the sequence is more consolidated after sleep. In the present study, our aim was to examine this hypothesis, that is, to investigate the electrophysiological correlates of attention regulation towards predicted vs. unpredicted stimuli. We were especially interested in early (N1 and N2), as well as later (P3) event-related potential (ERP) components based on a large body of evidence showing that these components are most closely linked to attention [49–53]. Importantly, these components have also been suggested to play a role in the prediction of upcoming stimuli, especially within the literature on sequence learning [54–58]. A few studies have suggested an association between prediction and allocation of attentional resources, such that there is a reduced need to allocate attention to predicted vs. unpredicted stimuli [58–60]. More generally, predictions have been suggested to interact with attention, thereby enhancing the precision of perceptual inference [61–63]. However, this complex relationship is not well understood, especially with respect to sleep-dependent consolidation. Our study aimed to shed light on this matter. We expected to observe increases in early (N1 and N2) as well as later components (P3) of the visual evoked potential in response to unpredicted stimuli specifically after sleep.
To investigate these hypotheses, we developed a novel sequence learning protocol and recorded behavioral performance and high-density EEG during initial task training and during a test session two days later. Two groups of healthy participants either slept or were deprived of sleep during the night after initial training. In the sleep group, we also recorded polysomnography including high-density EEG. Importantly, the test session did not take place until two days later, allowing for two nights of recovery sleep. We refer to sleep-deprived participants as the Wake group, because our goal was to study effects of sleep versus wakefulness while controlling for both circadian factors and acute effects of sleep deprivation.
Most previous studies investigating sequence learning, also in the context of sleep, employ some version of the serial reaction-time task (SRTT [13, 14, 20, 25, 30, 64]), in which perceptual and motor learning can be difficult to disentangle [65, 66]. However, the few studies investigating sleep-dependent consolidation of perceptual sequences suggest that perceptual representations profit especially from sleep [17, 67–69] (but see [70]). Our protocol aimed to focus on perceptual sequence learning by having participants perform an explicit motor task based on a visual feature (stimulus orientation) that was essentially random and, importantly, independent of the sequence of stimulus locations that was to be learned implicitly. This was done to isolate the contribution of the visual system to sequence learning. While our protocol and control measures do not allow us to separate perceptual from oculomotor learning, the present study is, to our knowledge, the first to investigate the role of sleep spindles and event-related potentials in visual sequence learning. Our results indicate that sleep supports visual sequence learning via a sleep-spindle-based mechanism and a subsequent reduction of attentional resources allocated to predicted stimuli.
Methods
Participants
Thirty-three young healthy male adults participated in the study (mean age: 25 years; range: 19–36). The sample size in our experimental design was determined by a statistical power calculation based on previous perceptual sequence learning studies [17, 67, 69] (mean effect size for a significant sleep/wake × pre/post × stimulus type interaction: = 0.21 [range: 0.07–0.42]), a power of 1 – β = 0.8, and α = 0.05. The choice of an all-male sample was made to reduce variance due to the female hormonal cycle known to affect sleep-dependent memory processing (e.g. [71, 72]). However, this choice limits the generalizability of our results and future studies should specifically investigate whether our findings apply to women as well. Half of them were assigned to a Sleep group (nsleep = 16), the other half to a Wake group (nwake = 17). Note that due to bad wake EEG quality in one participant we recorded data from one additional participant in the Wake group and included them both in the behavioral analyses. The Sleep group was trained before night-time sleep, and the Wake group before a nocturnal period of wakefulness (i.e. sleep deprivation). Importantly, participants did not know if they were going to sleep or stay awake during the night until the end of the Training session and right before bedtime. They were told that there was an equal chance they would sleep or stay awake. This was done to ensure there would be no effects of experimental group assignment on training performance. Both groups were tested again two days later, that is, after the Wake group had two nights of recovery sleep to counteract acute effects of sleep deprivation (Figure 1). Participants did not take any medication, did not report any neurological or psychological disorders, were right-handed, and had normal or corrected-to-normal visual acuity. They were asked not to ingest caffeine or alcohol and not to take naps during the days of the experimental sessions. Musicians and professional typists were excluded from the study. All participants were included in the final analyses of behavioral data (reaction times) and sleep spindles (Sleep group only). Three participants in each group were excluded from analyses of event-related potentials due to bad wake-EEG quality (non-stereotypic artifacts, potentially related to head movements and not present during sleep). Importantly, we found that average spindle events in the three Sleep participants excluded from event-related potential analyses were not different from other participants (all values within the range of ± 2 SD of the mean across all other participants for fast and slow spindle count, density, amplitude and power density). All participants gave written informed consent and were paid for participation. The experiment was approved by the ethics committee of the Medical Faculty at the University of Tübingen and conducted in accordance with the approved guidelines.
Figure 1.
Task and experimental design. (A) Participants’ explicit motor task was to indicate (by button press) as fast and accurately as possible at which of two possible orientations (45° or 135°) each Gabor grating is tilted, while fixating the cross in the middle of the screen at all times. (B) Upper panel displays the perceptual sequence to be learned implicitly. Middle panel shows an example random sequence. Note that location and color are correlated in sequence but uncorrelated in random blocks. Bottom panel illustrates predictable orientation trials of the explicit motor task (i.e. stimulus orientation changed predictably to the other direction following a triplet of equal orientations). (C) Three to ten days after an adaptation session including an adaptation night in the sleep laboratory (Sleep group only), sequence knowledge was acquired during the Training session in the evening. Following a normal night of sleep (Sleep group) or night-time wakefulness (Wake group) in the sleep laboratory, participants had two (recovery) nights of regular sleep at home. In the evening of the next day, participants were tested on their sequence knowledge (Test session).
Stimuli and task
Visual stimuli were shown on a 19-inch, 5:4 TFT display, with the layout consisting of a central fixation cross and four possible peripheral stimulus locations (Figure 1, A). Participants saw sequences of colored (red, blue, green, or yellow) Gabor gratings occurring at one of the four locations. The Gabor gratings were tilted either at an angle of 45° or 135° (stimulus size: 5° of visual angle, eccentricity: 7.5° [span: 5°–10°], spatial frequency: 1.5 cycles per degree, FWHM contrast: 1.7°; mean luminance equivalent to the gray background; see http://www.cogsci.nl/software/online-gabor-patch-generator). Participants’ explicit motor task was to respond to the (essentially random) orientation of each stimulus with a corresponding key press (left-hand index finger for 135°, right index for 45°) on a standard computer keyboard. The next stimulus appeared after a response-to-stimulus interval (RSI) of 200 ± 50 ms, with the jitter period selected randomly from a uniform distribution. To establish the visual sequence to be learned implicitly, in “sequence” blocks, the locations at which the gratings appeared followed a predictable sequence. In control blocks, this sequence was unpredictable (“random”). More specifically, sequence blocks consisted of a repeating sequence of eight locations, a regularity the participants were not informed about (depicted in Figure 1, B). Given that button presses (corresponding to stimulus orientations) were essentially random, differences between reaction times for random vs. sequence blocks may be interpreted as visual rather than (peripheral) motor learning. To facilitate learning [12, 73, 74], stimulus color was fully correlated with the location sequence in sequence blocks (Figure 1, B); in random blocks, there was no correlation between location and color (r = 0.0, p = 1.00).
All participants were trained on the same spatial sequence, which adhered to the following constraints: (1) stimuli occurred equally often at each of the four locations; (2) stimuli at a given location were always followed by at least two stimuli at other locations before the first location could be stimulated again (no triplets; e.g. 1-4-2-1, not 1-4-1-2); (3) stimuli never occurred at more than two neighboring locations in a row (no runs; e.g. not 1-2-3 or 4-3-2); and (4) stimuli at a given location did not predict stimuli at any of the other locations more than once within the sequence (no double predictions). We selected one grammar fulfilling these conditions (a-b-c-a-d-c-b-d) which can be translated into eight possible permutations. Of these, we selected one to be presented to all participants (i.e. 2-1-3-2-4-3-1-4). Thus, our location sequence followed a second-order conditional (SOC) rule [35, 75], i.e. a stimulus at a given location can fully and only be predicted by a combination of the two preceding stimulus locations.
In order to minimize behavioral and electrophysiological adaptation effects, for the explicit motor task, we implemented the additional constraint that the same stimulus orientation must not occur more than three times in a row. Given that stimulus orientation was the task-relevant feature, this constraint implies a deterministic regularity that is arguably more powerful than the location sequence, particularly because it applied to both sequence and random blocks. We therefore report additional analyses in which we separate these “predictable orientation trials” (17.4% of all trials on average; Figure 1, B) from “unpredictable orientation trials.”
Each experimental block consisted of 96 trials made up of 11 repetitions of the 8-item location sequence plus an additional, randomly split sequence whose two parts were added to the beginning and the end of each block to minimize the risk of the participants becoming aware of the regularity [35]. Between blocks, there were programmed breaks of 30 seconds, after which participants were free to initiate the next block whenever they chose. Participants were explicitly instructed to continue only when they felt ready to do so, in order to reduce the effects of fatigue. We encouraged participants to respond as fast and as accurately as possible, and both mean reaction times and the number of errors were displayed after each block to boost participants’ motivation. Stimuli disappeared immediately after each button press, independently of whether the response was correct or incorrect. Stimulus presentation was performed using Presentation (Neurobehavioral Systems; RRID:SCR_002521).
Procedure
Before the experiment proper, participants came to the laboratory for initial testing and adaptation (Sleep group at 10:00 pm, Wake group at 08:00 pm; see Control analyses for details). Participants in the Sleep group spent an adaptation night in the sleep laboratory, including wearing a high-density EEG net. Participants were further asked to keep a sleep journal (asking for bedtimes and sleep quality) for the seven nights preceding the experiment.
Three to 10 days after the adaptation session, participants in both Sleep and Wake groups came to the laboratory at 08:00 pm for the Training session. After placement of the high-density EEG net, participants were tested on their vigilance and subjective sleepiness (Stanford Sleepiness Scale [76, 77]). The sequence learning task started at 10:00 pm: after a short warm-up of 48 random trials, participants performed 11 training blocks that consisted mostly of sequence blocks, with three interleaved random blocks (i.e. the fourth, sixth, and eighth training block), to probe for sequence learning during Training.
Participants in the Sleep group were then given the opportunity to sleep in the sleep laboratory for eight hours from 11:00 pm to 07:00 am, including the EEG net. The next morning, they completed a sleep quality questionnaire (SF-A/R). Conversely, participants in the Wake group stayed awake for the whole night (i.e. they were sleep deprived). Wake participants did not wear high-density EEG nets during the deprivation period. However, night-time wakefulness was accompanied and closely monitored by the experimenter and included watching documentaries and playing easy board and computer games with lights dimmed. Importantly, the experimenters did not report any sleep bouts in any of the Wake participants during this time. After the experimental night, all participants left the laboratory and were asked to stay awake during the day and to adhere to their normal sleep/wake rhythms over the course of the two following days and recovery nights. This served to counteract the effects of sleep deprivation in the Wake group. They were further asked to document their bedtimes and sleep quality in a sleep journal, and not to take any daytime naps during the retention interval (i.e. between Training and Test). Activity patterns were controlled by actimetry (Actiwatch 2, Philips Respironics). Additionally, day activities were recorded via a questionnaire (for more information, see Control analyses).
Following the two recovery nights, all participants came to the laboratory at 04:30 pm for the Test session. After placement of the EEG electrodes, they performed a warm-up of 48 random trials, followed by 10 task blocks (alternating random and sequence blocks, with the starting condition counterbalanced across subjects). Finally, we probed participants’ explicit sequence knowledge with a series of additional tests (see Control analyses).
Data reduction and analysis
Behavioral performance
Sequence learning was assessed in terms of individual median reaction times. General task learning during Training was analyzed using a mixed analysis of variance (ANOVA) with between-subjects factor Sleep/wake and within-subjects factor Block (Block 1 to Block 11). To test for sequence-specific learning during Training, we further used a mixed ANOVA with the additional within-subjects factor Block type (sequence vs. random blocks), for which we used the mean of the medians of each block type. Sleep-dependent consolidation of visual sequence learning was assessed via mixed ANOVAs on data from the Test session with between-subjects factor Sleep/wake, and within-subjects factor Block type.
In additional analyses, we tested whether participants also learned the rule that the same orientation of a grating must not occur more than three times in a row. In these analyses, we focused on the predictable fourth stimulus following a triplet of equal orientations (predictable orientation trials). In total, 350 of 2,016 trials were predictable orientation trials (i.e. 17.4% of all trials; 189 during Training and 161 during Test). Variance caused by the different alternations of sequence and random blocks during Test (sequence first vs. random first) was controlled for by inserting “test order” as a covariate, which led to a significant improvement of the statistical model (p = .036). Conversely, to test for unpredictable orientation trials, we performed an analysis excluding predictable orientation trials.
To assess whether subjects acquired explicit sequence knowledge as well as how participants slept during the recovery nights, we ran additional tests and collected actigraphy data reported in the Control analyses.
Event-related potentials
During task performance, EEG data were recorded unfiltered using 128-channel HydroCel Geodesic Sensor Nets (Electrical Geodescs, Inc., Amsterdam, The Netherlands), at a sampling rate of 500 Hz. All EEG data processing was performed offline using EEGLAB v14.1.2 and ERPLAB v7.0.0. Data were bandpass (between 0.1 and 70 Hz) and notch filtered (between 45 and 55 Hz) using the EEGLAB function “pop_eegfiltnew” (i.e. Hamming windowed zero-phase since FIR filters with roll-offs at 6 dB). The bandpass filtering between 0.1 and 70 Hz was used to reduce distortion of the ERP waveforms [78]; the width of the notch filtering of 50 ± 5 Hz was used to cover the width of the line noise and its interaction with the analog-to-digital converter. Afterwards, data were downsampled to 250 Hz. Non-stereotypical artifacts were removed manually, and bad channels were interpolated (using the “spherical” algorithm as implemented in EEGLAB). Bad channel detection was guided by the “automatic channel rejection” tool from EEGLAB, by visually screening channel power spectra, and by visually screening the raw signal. On average, 1.3 (range: 0–5) and 2.1 (range: 0–7) channels were interpolated per participant in the Training and Test sessions, respectively. This was followed by re-referencing from Cz to the average of all electrodes. Independent component analysis (ICA) was performed using the extended “runica” infomax algorithm as implemented in EEGLAB. To yield better results, this was done on separate data that were only notch- (between 45 and 55 Hz) and high-pass filtered (at 1 Hz), but were otherwise identical (i.e. the same non-stereotypical artifacts were removed, and the same channels were interpolated before performing ICA). ICA weights were then transferred to the 0.1-Hz high-pass filtered data [79], which were used in all further analyses. Independent-component-based correction was performed for muscle and heartbeat artifacts. Epochs containing stereotypical eye blink and eye movement artifacts were detected and rejected at a later stage using algorithms implemented in ERPLAB (see below) [78, 80]. This was done to account for changes in sensory input caused by eye blinks and eye movements (e.g. an eye blink during stimulus presentation may lead to not properly seeing the respective stimulus, and eye movements themselves create sensory ERP responses caused by the changing visual input), which are not addressed by ICA correction [78].
In ERPLAB, epochs were extracted from −200 to 500 ms around stimulus onset. Baseline correction was performed for the period preceding stimulus onset (−200 to 0 ms). Then, bad epochs were flagged using different algorithms for simple voltage threshold (if the amplitude at any channel exceeded ± 120 µV); moving window peak-to-peak threshold and step-like artifacts (different algorithms for the detection of eye blinks and eye movements, respectively; both applied at a threshold of ±100 µV in moving windows of 200 ms width and 50 ms step size). Averaged event-related potentials (ERPs) were then computed per condition, excluding flagged epochs and epochs containing boundary events (i.e. events that resulted from prior data rejection), and ERPs were then used to calculate grand averages. After rejection of artifactual epochs, an average of 182 (range: 45−270; Training, random), 451 (range: 81−691; Training, sequence), 338 (range: 56−447; Test, random), and 344 (range: 143−473; Test, sequence) artifact-free epochs were included for ERP analyses. Electrodes and latencies for each component were determined visually using scalp maps of the Training session (baseline). Specifically, we created sequences of grand average scalp maps at a high temporal resolution and visually searched for activity within expected time windows for the components (N1 was detected within a time window of 150−210 ms; N2: 242−302 ms; P3: 310−380 ms). Within these time windows, we selected electrodes of interest that contributed strongest to the components with the highest (P3) or lowest (N1 and N2) peak voltages (i.e. electrodes 61, 62, 67, 72, 77, and 78 of the EGI system for N1; electrodes 54, 55, 61, 62, 78, and 79 for N2; electrodes 61, 62, 67, 77, and 78 for the P3). Electrodes of interest with the highest (lowest) peak voltage and the respective approximate time windows were used to determine peak latencies for each component using ERPLAB’s “peak latency” function of the “ERP Measurement Tool” (i.e. 180, 272, and 320 ms for N1, N2, and P3, respectively). Then, mean amplitudes (peak latency ±30 ms for N1 and N2 components; peak latency ±50 ms for the P3 component) were calculated for each component, electrode of interest, block type (random and sequence blocks), and participant in the Test session. For statistical analyses, we used mixed designs for each component with between-subject factor Sleep/wake and within-subject factors Block type (sequence vs. random blocks) and Electrode (depending on the number of electrodes of interest).
Sleep recordings
During sleep, participants continued to wear the 128-channel electrode net. The nets cover parts of the face, so that sleep scoring can be performed without additional electrodes. Thus, we selected electrodes corresponding to channels C3 and C4, mastoids A1 and A2, diagonal EOG electrodes (electrodes E126 and E25 of the EGI system) and two electrodes positioned on the jaw (E48 and E119). We used FieldTrip (RRID:SCR_004849) [81] to filter EEG, EOG and mastoid electrodes between 0.5 and 40 Hz, and EMG electrodes between 5 and 90 Hz (fourth-order two-pass Butterworth bandpass filters). Additionally, all channels were notch filtered between 45 and 55 Hz (fourth-order two-pass Butterworth bandstop filter). Then, data of all channels were downsampled to 250 Hz, EEG electrodes were re-referenced to the average of the two mastoids, and bipolar derivations were calculated for the diagonal EOG arrangement as well as for the two EMG electrodes. Sleep scoring was then performed manually based on 30-second epochs, according to standard criteria [82] (see Control analyses).
Sleep spindle detection was performed using an algorithm implemented in SpiSOP (RRID:SCR_015673). Based on the individual power spectra for sleep stages S2, S3, and S4, the frequencies for slow (mean center frequency, 11.45 Hz) and fast spindles (mean center frequency, 13.57 Hz) were determined for each subject individually. These frequencies were then used for individualized spindle detection in each subject and electrode, based on data for sleep stages S2, S3, and S4. Minimum and maximum spindle detection length were set to 0.5 and 3 s, respectively. Our main focus in this visual task was on occipital spindles. To test the specificity of spindles detected at occipital sites, we calculated the overlap of spindle occurrence between electrode Oz and all other electrodes. A spindle detected at another electrode site was defined as co-occurring with a spindle at Oz when it was detected within ±0.25 seconds from the maximum spindle peak at Oz. Correlation analyses were performed on a pre-defined occipital cluster including electrode Oz and directly neighboring electrodes (i.e. electrodes E70, E71, E74, E75, E76, E82, and E83 of the EGI system). Spindle measures (amplitude, power density, count, and density for fast and slow spindles, respectively) were averaged across these electrodes and then correlated with behavioral performance (i.e. the reaction time difference between random and sequence blocks during Test). We further tested the topographical specificity of occipital correlations by transforming Pearson correlation coefficients (r) to z values (Fisher’s r-to-z transformation) and comparing the distribution of the occipital cluster with the distribution of all electrodes. To correct for multiple correlations with the different spindle measures, we used false discovery rate (FDR)-correction following the Benjamini–Hochberg procedure and report FDR-corrected p values for all correlation measures, both for the correlations and the topographical specificity tests [83, 84]. Additional robustness tests were performed by comparing the occipital electrode cluster with all other 84 clusters outside of the occipital region of interest that have a similar size (7 ± 1 electrodes) and spatial arrangement (one central electrode and surrounding electrodes that lie within the same radius as in the occipital cluster). Specifically, we calculated the averaged z values per cluster and subtracted each of them from the averaged z values of the occipital cluster. Finally, based on the resulting distribution of difference values we calculated 95% confidence intervals (CI) for each spindle measure. To test for differences between correlations of Training and Test, we used Steiger’s Z test [85, 86]. ERPLAB’s plotting function was used to display topographic distributions of spindle count, power density and correlations between behavioral sequence learning success and spindle power density for each electrode.
Eye tracking
We additionally collected eye tracker data throughout the measurements (The Eye Tribe Tracker, The Eye Tribe ApS, Copenhagen, Denmark). Fixation coordinates were extracted for single events (corresponding to the interval stimulus onset and offset, with the latter dependent on participants’ response time). ROIs were defined as the area around the fixation cross (central fixation) as well as the respective stimulus location (stimulus gazing). Percentage of gaze time was then calculated as the number of fixations within each ROI divided by the number of eye tracking data points per event.
Statistics
Two-tailed tests were chosen for all statistical analyses. The level of significance was set to p = .05. Mixed ANOVAs as implemented in the GLM module of SPSS 24 (RRID:SCR_002865) were used, in combination with follow-up t-tests. Greenhouse–Geisser correction of degrees of freedom was applied when the assumption of sphericity was violated. We report original degrees of freedom and corrected p values in these cases.
Results
Sleep enhances behavioral signs of sequence learning for predictable but not unpredictable motor responses
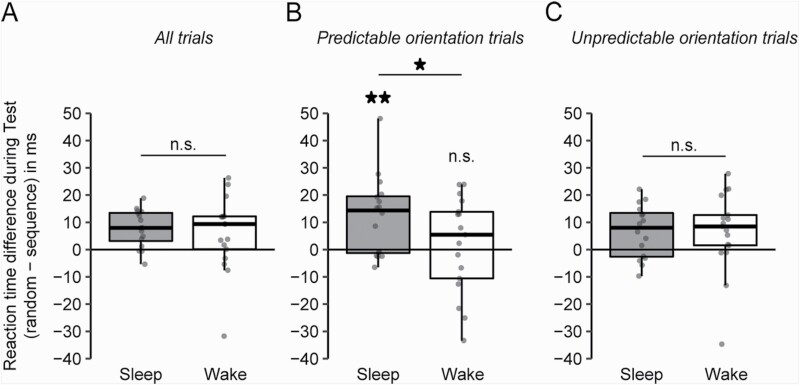
The main aim of our study was to assess the effect of post-training sleep vs. wakefulness on implicit visual sequence learning. To account for the change across the retention periods of sleep and wakefulness, averaged reaction times of the last two training blocks (see Control analyses) were subtracted from reaction times at the Test session for each individual. As a behavioral measure of sequence learning during the Test session, we used the individual gain (i.e. the difference) in reaction time for sequence blocks as compared to random blocks. At the Test session, participants demonstrated knowledge of the implicit location sequence (indicated by a significant difference between reaction times for random and sequence blocks) independently of whether they belonged to the Sleep or the Wake group (F(1,31) = 12.814, = 0.29, p = .001; estimated mean difference ± SEM: 6.78 ± 1.90 ms), with no difference in magnitude of this effect between the groups (Sleep/wake × Block type interaction, F(1,31) = 0.29, p = .597; Figure 2, A) and no Sleep/wake difference on general task learning (irrespective of Block type; F(1,31) = 0.25, p = .624).
Figure 2.
Reaction time differences between random and sequence blocks during Test. When considering all trials (A), participants showed a significant difference between random and sequence blocks after the retention period, when considering both Sleep and Wake groups together. However, we failed to find a significant difference between groups. A more fine-grained analysis on predictable orientation trials (B) (i.e. the fourth stimulus after a triplet of equal orientations) showed that participants had a greater difference for random vs. sequence blocks after sleep compared with wakefulness. In addition, only after sleep but not after wakefulness was there a significant difference between random and sequence blocks. However, when focusing on unpredictable orientation trials (C), there was no longer a significant difference between groups. Boxplots and individual data points are shown. **p < .01; *p < .05; n.s., not significant. N = 16 and 17 for Sleep and Wake groups, respectively.
We suspected that the absence of a clear-cut sleep effect on sequence learning was a consequence of the task paradigm designed to make visual learning implicit and independent of peripheral motor learning. Indeed, participants’ primary motor task was to respond as fast as possible to the orientation of each grating, and here the only sequential constraint was that the same orientation did not occur more than three times in a row. This was implemented to minimize behavioral and neural adaptation effects, but added a predictable, component to the explicit motor task. Exploratory post-hoc analyses confirmed that participants in both groups learned this rule, responding faster to the predictable fourth stimulus following a triplet of equal orientations (“predictable orientation trials”) compared to all other trials (i.e. “unpredictable orientation trials”; F(1,31) = 15.87, = 0.34, p < .001; estimated means ± SEM: 502.66 ± 10.35 and 513.38 ± 10.83 ms for predictable and unpredictable orientation trials, respectively). Indeed, some subjects noticed this regularity explicitly (see Control analyses).
We did not find a significant Sleep/wake × Predictability (predictable vs. unpredictable orientation trials) × Block type interaction (F(1,31) = 2.62, p = .115). Nevertheless, we performed additional exploratory analyses to examine signs of sequence learning (i.e. the gain in response speed for sequence vs. random blocks) separately for trials with predictable and unpredictable orientation of the grating. Analyses of trials with predictable grating orientation indeed showed a significantly greater gain in response speed for sequence blocks in the Sleep group than in the Wake group (Figure 2, B; Sleep/wake × Block type, F(1,30) = 4.62, p = .040; t(15) = 3.34, Cohen’s d = 0.22, p = .004 and t(16) = 0.41, p = .687, for post-hoc pairwise comparisons of sequence vs. random blocks for the Sleep and Wake groups, respectively). By contrast, when restricting the analysis to unpredictable orientation trials, Sleep and Wake groups performed virtually identical (Sleep/wake × Block type, F(1,31) = 0.00, p = 0.992; Figure 2, C). Again, we did not observe a main effect of Sleep/wake irrespective of Block type (p ≥ .570 for both predictable and unpredictable orientation trials). These results indicate that sleep enhances behavioral signs of implicit visuo-motor sequence knowledge. However, this effect only emerges when explicit motor responses are predictable, a condition that presumably absorbs fewer attentional resources than unpredictable trials. Given the exploratory nature of our analyses on predictable and unpredictable orientation trials and our primary focus on sequence learning irrespective of predictability on the explicit motor component, we performed all further analysis including all trials.
Occipital sleep spindles predict improvement of sequence learning performance
The main hypothesis of our study was that sleep spindles contribute to the consolidation of visual sequence knowledge during sleep. We were especially interested in fast spindles (~12–15 Hz; Figure 3, A), which are most pronounced over posterior regions [87], are thought to be involved in memory systems consolidation during sleep [11, 33, 38], and have been shown to be implicated in visuo-spatial processing [43, 44]. We expected that especially occipital spindles would contribute to the consolidation of visuo-motor sequence knowledge (as indicated by the reaction times difference between random and sequence blocks).
Figure 3.
Fast sleep spindles during the experimental night correlate with performance improvement 2 days later. (A) Upper panel displays an average fast spindle (black line) at electrode Oz across all participants ± SD (gray shading). Lower panel shows the percentage of overlap between occipital fast spindles (at Oz) and other electrodes. (B) Fast spindle count (left), amplitude (middle) and power density (right) are all highest at posterior electrodes. (C) Positive correlation between fast spindle amplitude (upper panel) or power density in the fast spindle band (lower panel) and behavioral performance after sleep (reaction time for random minus sequence blocks during Test) was highest at occipital electrodes, as shown in correlation topographies (left), histograms of z-transformed correlation coefficients (comparison of the distribution of occipital electrodes in gray vs. all electrodes in white; middle), and scatterplots for occipital electrodes (right). Black dots in scalp maps of (B, C) correspond to C3, Cz, and C4 (horizontally) and Fz, Cz, Pz, and Oz (vertically), respectively; gray squares in scalp maps of (C) correspond to the pool of occipital electrodes used for analyses (i.e. Oz and surrounding electrodes). The figure shows FDR-corrected p-values. N = 16.
The number of fast spindles during stage 2 sleep and slow-wave sleep (SWS) over the course of the night was maximal at electrode Pz (mean: 1,710 fast spindles, Figure 3, B), and both fast spindle amplitude (i.e. the mean amplitude of discrete fast spindle events, measured from trough to peak) and power density in the fast spindle band was highest between electrodes Cz and Pz (Figure 3, B). Still, large numbers of fast spindles were evident more occipitally (mean: 1,468 fast spindles at Oz, see Figure 3, A for an averaged fast spindle at this location). The overlap between occipital fast spindles (at Oz) and other electrodes was low and declined uniformly with distance from Oz. This suggests that occipital spindle events are primarily local phenomena (Figure 3, A). Importantly, we found that fast spindle amplitude correlated with behavioral performance in a locally specific manner (reaction time difference between random and sequence blocks during Test; r = 0.632, FDR-corrected p = .034 at a pre-defined cluster of occipital electrodes highlighted in Figure 3, C). This correlation was topographically specific to the occipital region (Z-test comparing z-transformed correlation coefficients of the cluster of occipital electrodes with the sample distribution of all electrodes, z = 3.83, Cohen’s d = 1.45, FDR-corrected p < .001). We did not find such correlations for fast spindle count (r = 0.337 FDR-corrected, p = .269); or fast spindle density (fast spindles per 30-s epochs; r = 0.403, FDR-corrected p = .195).
Similar results were observed for slow spindles (~9–12 Hz; r = .634, FDR-corrected p = .034 for slow spindle amplitude at the cluster of occipital electrodes; see Figure 4), even though their number was much lower (mean count: 769 at Oz), as well as for power density in the fast and slow spindle band (r = 0.588, FDR-corrected p = .034 and r = 0.596, FDR-corrected p = .034 for fast and slow spindle power density, respectively, at the cluster of occipital electrodes; see Figure 3, C and Figure 4, C), suggesting that spindles do not necessarily act only within their dominant loci [45].
Figure 4.
Slow sleep spindles during the experimental night correlate with performance improvement two days later. (A) Upper panel displays an average slow spindle (black line) at electrode Oz across all participants ± SD (gray shading). Lower panel shows the percentage of overlap between occipital slow spindles (at Oz) and other electrodes. (B) Slow spindle count (left), amplitude (middle) and power density (right) are all highest at frontal electrodes. (C) Positive correlation between slow spindle amplitude (upper panel) or power density in the slow spindle band (lower panel) and behavioral performance after sleep (reaction time for random minus sequence blocks during Test) was highest at occipital electrodes, as shown in correlation topographies (left), histograms of z-transformed correlation coefficients (comparison of the distribution of occipital electrodes in gray vs. all electrodes in white; middle), and scatterplots for occipital electrodes (right). Black dots in scalp maps of (B, C) correspond to C3, Cz, and C4 (horizontally) and Fz, Cz, Pz, and Oz (vertically), respectively; gray squares in scalp maps of (C) correspond to the pool of occipital electrodes used for analyses (i.e. Oz and surrounding electrodes). The figure shows FDR-corrected p-values. N = 16.
These correlations were specific for the Test session and were not observed during Training (r ≤ 0.14, p ≥ .615 at occipital electrodes), although the difference between correlations during Training vs. Test only reached significance for power density in the slow spindle band (Steiger’s Z test, z = 2.16, Cohen’s d = 0.54, p = .031) and remained a trend for slow spindle amplitude (z = 1.83, Cohen’s d = 0.46, p = .068). Taken together, these data suggest highly specific occipital sleep spindle-dependent processing of spatial sequence information, possibly due to experience-dependent neural plasticity involved in memory consolidation during non-REM sleep.
Sleep modulates attention-related N1 and N2 components of the ERP
Event-related potential components were identified based on data from the Training session. Analysis of data from the Test session then focused on N1, N2, and P3, all of which have previously been linked to sequence learning [54–58, 60, 88–91]. To account for known asymmetries in visual evoked responses [92–95], ERP components were extracted separately for the lower and upper parts of the visual field (i.e. ERPs for stimuli presented in the lower and upper parts of the screen were extracted and analyzed separately). Their comparison revealed effects of sleep to concentrate on the lower visual field, as indicated by significant interactions between Visual field, Block type, and Sleep/wake for N1 (F(1,25) = 8.98, p = .006) and N2 (F(1,25) = 5.52, p = .027), and a corresponding trend for the P3 (F(1,25) = 3.64, p = .068).
The ERPs from the lower visual field showed more negative N1 and N2 amplitudes for random compared to sequence blocks after sleep vs. wakefulness (Sleep/wake × Block type, F(1,25) = 6.16, p = .020 and F(1,25) = 4.95, p = .035, for N1 and N2, respectively; Figure 5). Follow-up comparisons within groups indicated significant differences between random and sequence blocks for both components after sleep (main effect of Block type, N1: F(1,12) = 5.38, p = .039; N2: F(1,12) = 5.80, p = .33), but not after wakefulness (N1: F(1,13) = 0.72, p = .411; N2: F(1,13) = 0.45, p = .516). Strikingly, the P3 component was more pronounced for sequence than random blocks after sleep but, conversely, more pronounced in random than sequence blocks in the Wake group (Sleep/wake × Block type, F(1,25) = 8.23, p = .008). Within the groups, these opposing dynamics approached significance (sleep: F(1,12) = 4.67, p = .052; Wake: F(1,13) = 3.46, p = .086). To account for possible confounding effects of locking stimulus colors to spatial locations in sequence blocks, supplementary analyses were restricted to those trials of the random blocks where color and location were matched in the same way as in the sequence blocks. These analyses revealed very similar results (see Control analyses).
Figure 5.
Event-related potentials. Grand averages for random and sequence blocks pooled over peak electrodes of the N1 component (A), the N2 component (B) and the P3 component (C), for Sleep and Wake group during Test, respectively. Red dots on scalp map show pooled electrodes per component; black dots correspond to C3, Cz, and C4 (horizontally) and Fz, Cz, Pz and Oz (vertically), respectively. Inserts next to the scalp maps show mean differences (± SEM; in µV) over shaded areas in (A-C) for random minus sequence blocks, for all three components and Sleep (black bars) and Wake groups (white bars), respectively. *p < .05, **p < .01. N = 13 and 14 for Sleep and Wake groups, respectively.
N1 and N2 components further showed an increased negativity for random compared to sequence blocks after sleep compared to wakefulness in additional baseline-corrected analyses (Sleep/wake x Block type interaction, N1: F(1,25) = 4.87, p = .037; N2: F(1,25) = 5.85, p = .023). For the P3, on the other hand, the Sleep group showed a trend towards increased amplitudes in sequence vs. random blocks in comparison with the Wake group (F(1,25) = 3.31, p = .081). In post-hoc tests, the significant interactions for the N1 and N2 appeared to be mostly driven by the increased negativity for random blocks after sleep compared to wakefulness (N1: t(25) = 1.80, Cohen’s d = 0.69, p = .084; N2: t(25) = 2.33, Cohen’s d = 0.90, p = .029; all others, p ≥ .345). We did not find any correlation between ERP components and sleep spindles (|r| ≤ 0.457, p ≥ .117 for amplitude, power density, count, and density, of fast and slow spindles, respectively, at the cluster of occipital electrodes).
Control analyses
Training performance
Results during Test were not affected by training performance: participants in both groups were successfully trained on the sequence, as indicated by significantly reduced median reaction times over the course of the Training session (main effect of Block, F(7,217) = 28.47, p < .001; linear contrast, F(1,31) = 94.62, p < .001; quadratic contrast, F(1,31) = 17.35, p < .001; analysis across sequence blocks; Figure 6). There were no significant differences between Sleep and Wake groups in terms of general task learning (analysis across sequence blocks: Sleep/wake × Block, F(7,217) = 0.58, p = .650; Sleep/wake, F(1,31) = 0.77, p = .386) or in terms of sequence-specific learning (analysis including the mean of the medians of all sequence and random blocks: Sleep/wake × Block type, F(1,31) = 2.71, p = .110; Sleep/wake, F(1,31) = 0.41, p = .525). In order to equate the number of trials being compared between conditions, we additionally calculated analyses based on three directly neighboring sequence blocks yielding equivalent results (for sequence blocks # 3, 5, and 7: Sleep/wake, p = .638; Sleep/wake × Block type, p = .557; for sequence blocks # 5, 7, and 9: Sleep/wake, p = .509; Sleep/wake × Block type, p = .119).
Figure 6.
Reaction times during Training. Participants were successfully trained on the sequence as seen by a steady decrease of reaction times over the course of the training blocks. See main text for details. Mean ± SEM are shown. ***p < .001. N = 16 and 17 for Sleep and Wake groups, respectively.
Assessment of explicit sequence knowledge
After testing performance on the sequence learning task, participants were asked increasingly specific questions regarding their knowledge of any regularity, including a paper-and-pencil free recall test. Then, they were informed about the presence of an eight-location sequence in the task, and were further tested on their sequence knowledge in three computerized explicitness tests: (1) a free recall task, (2) a triplet-recognition task, and (3) a triplet-completion task (for details, see Table 1).
Table 1.
Explicitness tasks
| Sleep group | Wake group | |
|---|---|---|
| Paper-and-pencil free recall task | 28.91 ± 3.17 | 27.94 ± 4.36 |
| Computerized free recall task (conservative) | 28.91 ± 2.73 | 24.26 ± 2.83 |
| Computerized free recall task (liberal) | 43.75 ± 1.80 | 40.81 ± 2.69 |
| Triplet-recognition task (d’) | 0.21 ± 0.23 | 0.35 ± 0.21 |
| Triplet-completion task (hits) | 25.00 ± 3.02 | 25.00 ± 2.46 |
Mean ± SEM are shown. In the paper-and-pencil free recall task participants were asked to explicitly reconstruct the 8-item sequence on a piece of paper; the value reflects the percentage of correct continuous items. In the computer-based free recall task, participants were asked to reconstruct the location sequence twice with eight inputs each; here, “conservative” and “liberal” denote analysis of the percentage of correct continuous items from the first button press or from any given button press, respectively. In the triplet-recognition task, participants watched sequences of three stimuli that were either part of the trained longer sequence (correct) or not (foils; eight triplets each) and were asked for each sequence if they found it familiar or not; due to its recognition test characteristic, d’ was used as the dependent variable. In the triplet-completion task, participants were asked to watch sequences of two stimuli (all of which were correct) and then to generate the following stimulus location. All possible triplets were completed twice (16 triplets in total); here, the percentage of correct choices is shown. In both triplet-recognition and triplet-completion tasks, participants were additionally asked to indicate for each response their confidence on a 4-point scale. N = 16 and 17 for Sleep and Wake groups, respectively.
None of the participants indicated any knowledge about the spatio-temporal structure of the task, and there were no significant differences between Sleep and Wake groups on any of the explicitness tests (all p ≥ .248). Interestingly, however, when summing individual rank scores across all tests, rank sums correlated positively with sequence performance (random minus sequence blocks) at the Test session after sleep (Spearman’s ρ = .612, p = .012), but not after wakefulness (Spearman’s ρ = .214, p = .409). Although the differences in coefficients between the Sleep and Wake groups remained non-significant (all p > 0.2), these findings suggest that Sleep participants may have gained a rudimentary explicit knowledge that supported task performance with regard to the discrimination of sequence vs. random blocks. Nevertheless, these data offer only tentative evidence that sleep may transform implicit to explicit knowledge [30, 96] in the present task.
As to the rule in the motor task (namely, that the same orientation of a grating never occurred more than three times in a row, seven participants in the Sleep group and five participants in the Wake group gained full explicit knowledge. These ratios did not differ between groups (Fisher’s exact test, p = .481). Importantly, it seems unlikely that explicit knowledge of the orientation rule can explain implicit learning of the location sequence, as our main analyses were conducted on all trials, irrespective of whether stimulus orientation was predictable or not. Moreover, both spindle-behavior correlations and ERP analyses yielded similar results when they were restricted to unpredictable orientation trials (p ≤ .009 for spindle-behavior correlations; p ≤ .048 for N1 and P3 ERP analyses; the N2 effect failed to reach significance: p = .105).
Robustness check of occipital spindle correlations
To test for robustness of the occipital spindle correlation results, we compared the z-transformed distribution of correlations in the occipital cluster with the distribution of all other 84 clusters outside of the occipital region of interest that have a similar size (7 ± 1 electrode) and spatial arrangement (one central electrode and surrounding electrodes that lie within the same radius as in the occipital cluster). We found highly similar results to those reported for occipital vs. all electrodes in the main section (see above) for fast spindle amplitude (95% CI of the mean difference between occipital and all other clusters, Δz95%-CI = [0.20, 0.25]), fast spindle power density (Δz95%-CI = [0.33, 0.37]), slow spindle amplitude (Δz95%-CI = [0.24, 0.33]), and slow spindle power density (Δz95%-CI = [0.33, 0.39]).
Event-related potentials: location/color-matching trials
Whereas in random blocks, stimulus color was balanced for all four stimulus locations, in sequence blocks, stimulus color was invariantly bound to specific locations (e.g. stimuli at the left upper location were always yellow). To control for possible confounding effects resulting from color-location locking, we re-analyzed ERP data based on location/color-matching trials for both sequence and random blocks where only the ¼ of the trials from random blocks were used that matched in location and color with that in the sequence blocks. Results are qualitatively equivalent to those reported in the main text: ERPs from the lower visual field showed a greater separation between sequence and random blocks for N1 (mean difference of random minus sequence blocks, Sleep: −0.46 µV; Wake: 0.25 µV), N2 (Sleep: −0.42 µV; Wake: −0.01 µV) and P3 (Sleep: −0.35 µV; Wake: 0.13 µV) ERP components for Sleep compared with Wake participants. However, these differences were only a trend for the N1 component (Sleep/wake × Block type, N1: F(1,25) = 3.86, = 0.13, p = .061) and not significant for both the N2 and P3 components (N2: F(1,25) = 1.48, p = .236; P3: F(1,25) = 2.02, p = .168), probably due to the lack of statistical power given the small number of trials in this analysis.
Actigraphy and sleep journals
To assess if participants had normal sleep/wake cycles, they were asked to keep a sleep journal (asking for bedtimes and sleep quality) for the seven nights preceding the experiment. Moreover, to assess if and how participants slept during the two recovery nights, we collected actigraphy data and had the participants keep an additional sleep journal (see Table 2 for details).
Table 2.
Actigraphy and sleep journals
| Sleep group | Wake group | |
|---|---|---|
| Irregular nights (out of 7) prior to first experimental session | 1.43 ± 0.40 | 1.63 ± 0.39 |
| TiB of night before first experimental session (min) | 497.86 ± 12.67 | 517.81 ± 13.42 |
| First recovery night | ||
| TiB (min) | 595.88 ± 31.37 | 601.49 ± 28.74 |
| Sleep period (min) | 512.62 ± 32.75 | 572.87 ± 29.71 |
| WASO (min) | 30.88 ± 5.66 | 26.68 ± 4.24 |
| Sleep efficiency (%) | 81.16 ± 3.52 | 90.52 ± 1.64* |
| Second recovery night | ||
| TiB (min) | 557.17 ± 47.89 | 540.18 ± 27.97 |
| Sleep period (min) | 470.55 ± 25.62 | 509.43 ± 29.18 |
| WASO (min) | 37.63 ± 10.30 | 41.54 ± 11.27 |
| Sleep efficiency (%) | 81.32 ± 3.79 | 87.00 ± 1.96 |
Mean ± SEM are shown. Participants were asked to press a marker on an Actiwatch when getting to bed in the evening and getting up in the morning. In the sleep journals, participants should further indicate the time they switched off the lights, the time when they fell asleep, how often and how long they were awake during the night, as well as the time when they got up. Time in bed (TiB) was calculated as the time between lights off and getting up; sleep period was calculated as the time between falling asleep and waking up; wake after sleep onset (WASO) was calculated as the time being awake after falling asleep; and sleep efficiency was calculated as the percentage of sleep during bed time (i.e. sleep period/TiB × 100). For raw data analysis of the actigraphy data, we used a standard algorithm as implemented in Respironics Actiware 5 (RRID:SCR_016440), with a medium threshold for the estimation of wake phases (40 activity counts/epoch for epoch lengths of 15 s), and a detection algorithm of sleep intervals based on min of inactivity (10 min of inactivity were set as threshold for the estimation of start and end phases of a sleep interval). N = 16 and 17 for Sleep and Wake groups, respectively. *p < .05; significant differences between the Sleep group and the Wake group.
There was no significant difference between Sleep and Wake groups for sleep irregularity the seven nights before participating in the study (i.e. nights with more than 1 h deviation from normal bed time (t(28) = 0.35, p = .728 [97]). Moreover, Sleep and Wake participants did not differ the night before the experiment in terms of their time in bed (TiB) (t(28) = 1.07, p = .293). This is crucial since the night before the experiment can be considered critical for cognitive performance the day after.
All participants showed normal sleep patterns during the first and second recovery nights, respectively. However, sleep efficiency was higher for the Wake group compared to the Sleep group in the first recovery night, reflecting the sleep rebound after deprivation in the Wake group (t(30) = 2.41, Cohen’s d = 0.87, p = .026). In contrast, there were no significant differences between Sleep and Wake groups on any of these measures in the second recovery night (i.e. the night before the Test session, p ≥ .179). Subjective sleep questionnaire data largely confirmed these results.
Eye tracking
To control for eye movements, we collected eye tracker data throughout the measurements. However, we were unable to achieve a level of data quality sufficient for proper eye tracking analysis in most participants, probably due to lighting conditions in combination with suboptimal hardware. For the subset of participants with good calibration results (Training, n = 9; Test, n = 8), we found that the proportion of fixations falling within a 5° diameter around the fixation cross during stimulus presentation was (mean ± SEM) 49 ± 12% during Training and 71 ± 9% during Test, with only 0.7 ± 0.1% and 0.2 ± 0.1% direct stimulus fixation (i.e. fixations falling within the surface of the respective stimulus location). Nevertheless, these incomplete data do not allow us to assess whether and to what extent oculomotor learning [19, 59] may explain the results observed.
No association between the interval from adaptation to experimental night and sleep parameters
Participants had their experimental night planned between three to 10 days following the adaptation session. However, due to organizational constraints, this interval was shorter for two participants (two days) and longer for one participant (18 days), with a mean ± SD of 5.75 ± 3.96 days. Importantly, there were no significant associations between the interval from the adaptation to the experimental nights and percentage or the time spent in SWS or rapid-eye-movement (REM) sleep (r ≥ −0.20, p ≥ .460) or any of the spindle parameters (i.e. amplitude, power density, count, and density for fast and slow spindles, respectively; |r| ≤ 0.248, p ≥ .355). Total sleep time correlated positively with this interval (r = 0.50, p = .049), suggesting that longer intervals between the adaptation and the experimental session leads to even longer sleep durations. However, when excluding the outlier participant with an 18-day interval, this correlation was no longer significant (r = 0.24, p = .386).
Control tests
During the adaptation session before the main experiment, participants were screened using several tests: a visual acuity test (Freiburg Vision Test, [FrACT] [98], RRID:SCR_016439), an ocular dominance test (based on the Dolman method [99, 100]), an online color vision test (http://www.color-blindness.com/color-arrangement-test/), a standard handedness test (Edinburgh Handedness Inventory [101]), a chronotype test (Morningness-Eveningness Questionnaire Self-Assessment Version [MEQ-SA] [102]), and a standard vigilance test [103].
In addition, before Training and Test, all participants performed a vigilance task and a subjective sleepiness questionnaire (Stanford Sleepiness Scale, SSS [77]). They further performed tests of their visuo-spatial memory span and supra span (adopted from Corsi [104] and implemented in The Psychology Experiment Building Language (RRID:SCR_014794)), as well as their chronotype [102]. There were no significant differences between Sleep and Wake groups on any measure (all p ≥ .419; Table 3).
Table 3.
Control tests
| Sleep group | Wake group | ||
|---|---|---|---|
| Adaptation | Vigilance | 443.88 ± 11.86 | 437.76 ± 8.12 |
| Training | SSS | 3.08 ± 0.17 | 3.15 ± 0.21 |
| Vigilance | 448.56 ± 9.45 | 449.44 ± 8.16 | |
| Test | SSS | 2.63 ± 0.21 | 2.56 ± 0.24 |
| Vigilance | 441.41 ± 9.67 | 443.53 ± 9.83 | |
| Chronotype | 2.88 ± 0.09 | 2.94 ± 0.10 | |
| Corsi (block span) | 6.50 ± 0.33 | 6.12 ± 0.33 | |
| Corsi (supra span) | 3.25 ± 0.58 | 2.71 ± 0.60 |
Mean ± SEM are shown. Vigilance is measured as reaction time (ms). Chronotype is measured based on the MEQ and ranges from 1 (definitely evening type) to 5 (definitely morning type). The Corsi block span is a measure for the number of visual items that can be remembered in sequence; the supra span test examines the number of repetitions needed until a sequence of visual items is learned implicitly. SSS, Stanford Sleepiness Scale (7-point-scale on sleepiness). N = 16 and 17 for Sleep and Wake groups, respectively.
Discussion
The present study suggests that sleep supports the extraction of regularities from implicitly learned visual stimulus sequences. Behaviorally, this sleep benefit was evident only for predictable stimuli of the explicit motor task (i.e. when three stimuli with the same grating orientation were followed by a stimulus with the other grating orientation). Crucially, behavioral improvements after sleep were predicted by occipital sleep spindles during non-REM sleep. In addition, participants who slept during the night after training showed a modulation of early visual evoked N1 and N2 components for random vs. sequence stimuli. The P3 after sleep tended to be increased to stimuli presented in sequence as compared to randomly presented stimuli, whereas in the Wake group, the post-training P3 was higher to random than sequence blocks. Taken together, these results are in accordance with the notion that sleep extracts spatio-temporal regularities from previously encoded information via spindle-mediated localized reactivation of sensory cortices. The enhanced representation of such regularities would then reduce the amount of attention required to process similar sequences in the future. Our results extend a growing body of evidence suggesting that sleep is critically involved in generating holistic representations, that is, internal models of critical environmental features, thereby allowing for predictions of future events and adaptive behaviors [3–6, 8, 9, 34, 105, 106].
Behaviorally, we found shorter reaction times for sequence compared with random blocks. Notably, this effect differed between Sleep and Wake groups when grating orientation and the associated motor response were predictable. Compared with unpredictable orientation trials, processing of these predictable orientation trials likely requires less attention [58, 60, 107–109]. This would allow for additional attentional resources to be devoted to implicit aspects of the task, thereby unmasking sleep benefits on location-sequence learning. In this interpretation, the motor task impairs sequence learning, similar to dual-task conditions investigated in many SRTT studies (for a review, see [66]). Alternatively, our protocol may be described in terms of multidimensional learning [110], as the location sequence uses the same modality as the motor task and contains reliable information for performing the latter. In this context, our results are in agreement with previous work [91] indicating reaction time differences only for task-relevant motor, but not for task-irrelevant perceptual deviants in a sequence learning task.
The role of sleep in consolidating sequence learning has long remained unclear (for reviews, see [32, 33]). Sleep effects have most consistently been demonstrated for perceptual sequence learning [17, 67–69] (but see [70]). Our findings support this view and help clarify this discussion by suggesting that the behavioral expression of sleep-dependent perceptual sequence learning is coupled to participants’ explicit motor task. However, we note that implicit learning of the location sequence may reflect a combination of perceptual and oculomotor learning [19, 59], as we cannot exclude a role of eye movements in the current dataset.
High-density EEG data obtained during sleep revealed that overnight behavioral improvements in the Sleep group were predicted by sleep-spindle activity in a topographically specific manner. Current models suggest that sleep spindles contribute to the reprocessing of discrete memory traces in local networks [11, 111] and thus play a key role in active systems consolidation during sleep [38, 39, 112–114]. Correlations between spindle activity and memory consolidation are well established in different learning paradigms [38, 115, 116], with robust evidence in the motor domain (e.g. [29, 40–42]), but effects on perception being less clear. Most studies have focused on explicit learning on various declarative tasks [43, 44, 46], whereas investigations of perceptual learning have been rare [45]. Our results extend this literature by showing that topographically specific spindle activity contributes to consolidation of implicitly learned visual sequences. Activity in early visual areas is known to change in response to visual-perceptual learning [93, 117–119], and especially during sleep after learning [120, 121]. It has recently been shown that such changes may depend on spindle-based interactions between thalamus and cortex, particularly during sleep [122, 123]. We propose that in our protocol, higher tiers of the visual system implicitly encode transition probabilities across distant locations. The networks involved would then be reactivated during sleep, with increased synchrony in cortico-cortical and cortico-thalamic networks [123] supporting consolidation of the regularities encoded during wakefulness. These considerations extend current models of stimulus-induced sequence replay in the primary visual cortex [124]. Future investigations will need to address the relative contribution of perceptual and oculomotor [125, 126] learning to this spindle-based mechanism of visual memory consolidation. In a similar vein, additional studies are needed to clarify whether the spindles detected over occipital areas originate from visual or parietal cortices [127].
Following the retention interval, we observed more negative N1 and N2 amplitudes for random compared to sequence blocks specifically in the sleep group. It is well established that stimuli of high compared to low predictability elicit smaller amplitudes for both the N1 [58] and the N2 [54–57, 60, 88, 90, 91, 128]. This effect has been linked to attentional processing, with reduced amplitudes reflecting reduced spatial attention allocated to predictable stimuli (e.g. [58–60]). Against this backdrop, the observed effects of post-training sleep on N1 and N2 components most likely reflect a shift in the allocation of attentional resources.
For the P3 component, we observed opposing dynamics, with stimuli embedded in predictable sequences eliciting higher P3 amplitudes than random stimuli in the Sleep group, but lower P3 amplitudes to sequential than random stimuli in the Wake group. Effects of sequence learning on P3 have been reported in numerous studies [55–57, 60, 90, 128], in accordance with the component’s role in stimulus-driven memory processing (for reviews, see [53, 129]). The increased P3 to sequential stimuli presented in the Sleep group is in accordance with the notion that sleep enhanced the internal representation of the memorized sequence.
These ERP effects were only present when stimuli were shown in the lower visual field. Retinotopic asymmetries in visual processing are frequently observed in humans [92–94, 130–132] and other species [95, 133]. They are commonly attributed to anisotropic sensitivity profiles starting at the level of the retina and extending to higher visual cortices [134–137]. Such anisotropies are particularly pronounced in the spatial-frequency domain [138–140]. Importantly, results from complementary studies in our lab have revealed significantly reduced upper visual field responses to textures containing spatial frequencies similar to the stimuli used here [141], as well as unmasking of attentional modulations of upper visual field responses by individually adapting spatial frequency content (Herde et al., in preparation). Against this backdrop, the present results suggest a distinctly reduced sensitivity of the upper visual field to attentional modulation.
Our results are in general agreement with studies finding sleep effects on memory consolidation when compared to either daytime or nighttime wakefulness [11]. However, while circadian differences during encoding and retrieval may confound results when comparing nighttime sleep to daytime wakefulness [142–145], sleep deprivation disrupts the normal function of nighttime sleep and is associated with severe cognitive deficits [146, 147]. In the present study, young and healthy participants recovered from the deprivation procedure during two nights of sleep at home, after which normal cognitive functioning is thought to be fully restored [148]. Moreover, previous studies using comparable sleep deprivation procedures in humans have shown that the increase in stress hormone levels which might affect consolidation during the nocturnal vigil (e.g. cortisol and norepinephrine) is numerically small and remains far below the levels typical for responses to stressful stimuli (e.g. [149–152]). Thus, we are convinced that our protocol selectively affected consolidation without impairing encoding or retrieval. This allows us to draw robust conclusions about the functional role of sleep in consolidating visual sequence learning.
In conclusion, our findings support the notion that sleep underpins the construction of internal models of implicitly learned visual sequences. Behaviorally, this effect only emerged for stimuli that were predictable with regard to the explicit motor task, thus absorbing less attention. The improved extraction of spatio-temporal regularities was linked to sleep spindles occurring over the occipital cortex during post-training sleep, in accordance with the notion of sleep-dependent neural reactivation enhancing the representation of the implicitly learned sequence. Sleep also modulated amplitudes of the N1, N2, and P3 components of the visual evoked response in a manner consistent with improved sequence knowledge, and increased allocation of attention to unpredictable stimuli. Future studies will need to determine whether these effects depend on perceptual learning, oculomotor learning, or a combination of both.
Acknowledgments
We thank Jens G. Klinzing for providing code for pre-processing of sleep EEG data. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 1233 “Robust Vision: Inference Principles and Neural Mechanisms,” Project no. 276693517, TP8) and the European Research Council (ERC, AdG 883098, “SleepBalance”). The authors declare no competing financial interests.
The work was performed at the Institute of Medical Psychology and Behavioral Neurobiology, University of Tübingen, 72076 Tübingen, Germany.
Disclosure Statement
Financial Disclosure: none.
Non-financial Disclosure: none.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Turk-Browne NB. Statistical learning and its consequences. In: Dodd M, Flowers J, eds. The Influence of Attention, Learning, and Motivation on Visual Search. New York, NY: Springer; 2012: 117–146. [DOI] [PubMed] [Google Scholar]
- 2. Barlow H. Redundancy reduction revisited. Network. 2001;12(3):241–253. [PubMed] [Google Scholar]
- 3. Stefanics G, et al. Visual mismatch negativity: a predictive coding view. Front Hum Neurosci. 2014;8(September):666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clark A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav Brain Sci. 2013;36(3):181–204. [DOI] [PubMed] [Google Scholar]
- 5. Southwell R, et al. Is predictability salient? a study of attentional capture by auditory patterns. Philos Trans R Soc B Biol Sci. 2017;372(1714):20160105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bar M. The proactive brain: using analogies and associations to generate predictions. Trends Cogn Sci. 2007;11(7):280–289. [DOI] [PubMed] [Google Scholar]
- 7. Rauss K, et al. Top-down effects on early visual processing in humans: a predictive coding framework. Neurosci Biobehav Rev. 2011;35(5):1237–1253. [DOI] [PubMed] [Google Scholar]
- 8. Rao RP, et al. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat Neurosci. 1999;2(1):79–87. [DOI] [PubMed] [Google Scholar]
- 9. Friston K. Predictive coding, precision and synchrony. Cogn Neurosci. 2012;3(3–4):238–239. [DOI] [PubMed] [Google Scholar]
- 10. Diekelmann S, et al. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. [DOI] [PubMed] [Google Scholar]
- 11. Rasch B, et al. About sleep’s role in memory. Physiol Rev. 2013;93(2):681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spencer RM, et al. Sleep-dependent consolidation of contextual learning. Curr Biol. 2006;16(10):1001–1005. [DOI] [PubMed] [Google Scholar]
- 13. Maquet P, et al. Experience-dependent changes in cerebral activation during human REM sleep. Nat Neurosci. 2000;3(8):831–836. [DOI] [PubMed] [Google Scholar]
- 14. Peigneux P, et al. Learned material content and acquisition level modulate cerebral reactivation during posttraining rapid-eye-movements sleep. Neuroimage. 2003;20(1):125–134. [DOI] [PubMed] [Google Scholar]
- 15. Robertson EM, et al. Off-line learning and the primary motor cortex. J Neurosci. 2005;25(27):6372–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ertelt D, et al. Skill memory escaping from distraction by sleep–evidence from dual-task performance. PLoS One. 2012;7(12):e50983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pace-Schott EF, et al. Age-related changes in consolidation of perceptual and muscle-based learning of motor skills. Front Aging Neurosci. 2013;5:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cousins JN, et al. Cued memory reactivation during slow-wave sleep promotes explicit knowledge of a motor sequence. J Neurosci. 2014;34(48):15870–15876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Albouy G, et al. Implicit oculomotor sequence learning in humans: time course of offline processing. Brain Res. 2006;1090(1):163–171. [DOI] [PubMed] [Google Scholar]
- 20. Fischer S, et al. Developmental differences in sleep’s role for implicit off-line learning: comparing children with adults. J Cogn Neurosci. 2007;19(2):214–227. [DOI] [PubMed] [Google Scholar]
- 21. Cousins JN, et al. Cued reactivation of motor learning during sleep leads to overnight changes in functional brain activity and connectivity. PLoS Biol. 2016;14(5):e1002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Durrant SJ, et al. Sleep-dependent consolidation of statistical learning. Neuropsychologia. 2011;49(5):1322–1331. [DOI] [PubMed] [Google Scholar]
- 23. Durrant SJ, et al. Cross-modal transfer of statistical information benefits from sleep. Cortex. 2016;78:85–99. [DOI] [PubMed] [Google Scholar]
- 24. Durrant SJ, et al. Overnight consolidation aids the transfer of statistical knowledge from the medial temporal lobe to the striatum. Cereb Cortex. 2013;23(10):2467–2478. [DOI] [PubMed] [Google Scholar]
- 25. Robertson EM, et al. Awareness modifies the skill-learning benefits of sleep. Curr Biol. 2004;14(3):208–212. [DOI] [PubMed] [Google Scholar]
- 26. Spencer RM, et al. Age-related decline of sleep-dependent consolidation. Learn Mem. 2007;14(7):480–484. [DOI] [PubMed] [Google Scholar]
- 27. Song S, et al. Practice and sleep form different aspects of skill. Nat Commun. 2014;5:3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diekelmann S, et al. Increasing explicit sequence knowledge by odor cueing during sleep in men but not women. Front Behav Neurosci. 2016;10:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yordanova J, et al. Sleep spindles in the right hemisphere support awareness of regularities and reflect pre-sleep activations. Sleep. 2017;40(11). doi: 10.1093/sleep/zsx151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fischer S, et al. Implicit learning – explicit knowing: a role for sleep in memory system interaction. J Cogn Neurosci. 2006;18(3):311–319. [PubMed] [Google Scholar]
- 31. Zinke K, et al. Children’s initial sleep-associated changes in motor skill are unrelated to long-term skill levels. Dev Sci. 2017;20(6):e12463. [DOI] [PubMed] [Google Scholar]
- 32. Lerner I, et al. Sleep and the extraction of hidden regularities: a systematic review and the importance of temporal rules. Sleep Med Rev. 2019;47:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. King BR, et al. Sleeping on the motor engram: the multifaceted nature of sleep-related motor memory consolidation. Neurosci Biobehav Rev. 2017;80:1–22. [DOI] [PubMed] [Google Scholar]
- 34. Rauss K, et al. A role of sleep in forming predictive codes. In: Axmacher N, Rasch B, eds. Cognitive Neuroscience of Memory Consolidation. Cham, Switzerland: Springer International Publishing; 2017: 117–132. [Google Scholar]
- 35. Lutz ND, et al. Sleep strengthens predictive sequence coding. J Neurosci. 2018;38(42):8989–9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ballesio A, et al. Updating internal cognitive models during sleep. J Neurosci. 2019;39(11):1966–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mednick SC, et al. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci. 2013;33(10):4494–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klinzing JG, et al. Mechanisms of systems memory consolidation during sleep. Nat Neurosci. 2019;22(10):1598–1610. [DOI] [PubMed] [Google Scholar]
- 39. Marshall L, et al. Brain rhythms during sleep and memory consolidation: neurobiological insights. Physiology (Bethesda). 2020;35(1):4–15. [DOI] [PubMed] [Google Scholar]
- 40. Nishida M, et al. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One. 2007;2(4):e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tamaki M, et al. Activation of fast sleep spindles at the premotor cortex and parietal areas contributes to motor learning: a study using sLORETA. Clin Neurophysiol. 2009;120(5):878–886. [DOI] [PubMed] [Google Scholar]
- 42. Johnson LA, et al. Sleep spindles are locally modulated by training on a brain-computer interface. Proc Natl Acad Sci USA. 2012;109(45):18583–18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clemens Z, et al. Twenty-four hours retention of visuospatial memory correlates with the number of parietal sleep spindles. Neurosci Lett. 2006;403(1-2):52–56. [DOI] [PubMed] [Google Scholar]
- 44. Cox R, et al. Local sleep spindle modulations in relation to specific memory cues. Neuroimage. 2014;99:103–110. [DOI] [PubMed] [Google Scholar]
- 45. Bang JW, et al. Location specific sleep spindle activity in the early visual areas and perceptual learning. Vision Res. 2014;99:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bergmann TO, et al. Sleep spindle-related reactivation of category-specific cortical regions after learning face-scene associations. Neuroimage. 2012;59(3):2733–2742. [DOI] [PubMed] [Google Scholar]
- 47. Ramanathan DS, et al. Sleep-dependent reactivation of ensembles in motor cortex promotes skill consolidation. PLoS Biol. 2015;13(9):e1002263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Novitskaya Y, et al. Ripple-triggered stimulation of the locus coeruleus during post-learning sleep disrupts ripple/spindle coupling and impairs memory consolidation. Learn Mem. 2016;23(5):238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Herrmann CS, et al. Mechanisms of human attention: event-related potentials and oscillations. Neurosci Biobehav Rev. 2001;25(6):465–476. [DOI] [PubMed] [Google Scholar]
- 50. Luck SJ, et al. Event-related potential studies of attention. Trends Cogn Sci. 2000;4(11):432–440. [DOI] [PubMed] [Google Scholar]
- 51. Hillyard SA, et al. Event-related brain potentials in the study of visual selective attention. Proc Natl Acad Sci USA. 1998;95(3):781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Folstein JR, et al. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45(1):152–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118(10):2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eimer M, et al. Explicit and implicit learning of event sequences: evidence from event-related brain potentials. J Exp Psychol Learn Mem Cogn. 1996;22(4):970–987. [DOI] [PubMed] [Google Scholar]
- 55. Rüsseler J, et al. Implicit and explicit learning of event sequences: evidence for distinct coding of perceptual and motor representations. Acta Psychol (Amst). 2000;104(1):45–67. [DOI] [PubMed] [Google Scholar]
- 56. Rüsseler J, et al. Differences in incidental and intentional learning of sensorimotor sequences as revealed by event-related brain potentials. Brain Res Cogn Brain Res. 2003;15(2):116–126. [DOI] [PubMed] [Google Scholar]
- 57. Fu Q, et al. Learning without consciously knowing: evidence from event-related potentials in sequence learning. Conscious Cogn. 2013;22(1):22–34. [DOI] [PubMed] [Google Scholar]
- 58. Rose M, et al. ERP correlates of associative learning. Psychophysiology. 2001;38(3):440–450. [PubMed] [Google Scholar]
- 59. Marcus DJ, et al. Oculomotor evidence of sequence learning on the serial reaction time task. Mem Cognit. 2006;34(2):420–432. [DOI] [PubMed] [Google Scholar]
- 60. Kóbor A, et al. ERPs differentiate the sensitivity to statistical probabilities and the learning of sequential structures during procedural learning. Biol Psychol. 2018;135(April):180–193. [DOI] [PubMed] [Google Scholar]
- 61. Rao RPN, et al. Probabilistic models of attention based on iconic representations and predictive coding. In: Itti L, Rees G, Tsotsos JK, eds. Neurobiology of Attention. Amsterdam, the Netherlands: Elsevier; 2005: 553–561. [Google Scholar]
- 62. Summerfield C, et al. Expectation (and attention) in visual cognition. Trends Cogn Sci. 2009;13(9):403–409. [DOI] [PubMed] [Google Scholar]
- 63. Kok P, et al. Attention reverses the effect of prediction in silencing sensory signals. Cereb Cortex. 2012;22(9):2197–2206. [DOI] [PubMed] [Google Scholar]
- 64. Nissen MJ, et al. Attentional requirements of learning: evidence from performance measures. Cogn Psychol. 1987;19(1):1–32. [Google Scholar]
- 65. Robertson EM. The serial reaction time task: implicit motor skill learning? J Neurosci. 2007;27(38):10073–10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schwarb H, et al. Generalized lessons about sequence learning from the study of the serial reaction time task. Adv Cogn Psychol. 2012;8(2):165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Albouy G, et al. Daytime sleep enhances consolidation of the spatial but not motoric representation of motor sequence memory. PLoS One. 2013;8(1):e52805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lewis PA, et al. Keeping time in your sleep: overnight consolidation of temporal rhythm. Neuropsychologia. 2011;49(1):115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cohen DA, et al. Off-line learning of motor skill memory: a double dissociation of goal and movement. Proc Natl Acad Sci USA. 2005;102(50):18237–18241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hallgató E, et al. The differential consolidation of perceptual and motor learning in skill acquisition. Cortex. 2013;49(4):1073–1081. [DOI] [PubMed] [Google Scholar]
- 71. Genzel L, et al. Sex and modulatory menstrual cycle effects on sleep related memory consolidation. Psychoneuroendocrinology. 2012;37(7):987–998. [DOI] [PubMed] [Google Scholar]
- 72. Genzel L, et al. Diminished nap effects on memory consolidation are seen under oral contraceptive use. Neuropsychobiology. 2014;70(4):253–261. [DOI] [PubMed] [Google Scholar]
- 73. Robertson EM, et al. The role of the dorsolateral prefrontal cortex during sequence learning is specific for spatial information. Cereb Cortex. 2001;11(7):628–635. [DOI] [PubMed] [Google Scholar]
- 74. Robertson EM, et al. Aspects of sensory guidance in sequence learning. Exp Brain Res. 2001;137(3-4):336–345. [DOI] [PubMed] [Google Scholar]
- 75. Rosenthal CR, et al. Learning and recognition of a non-conscious sequence of events in human primary visual cortex. Curr Biol. 2016;26(6):834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hoddes E, et al. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10(4):431–436. [DOI] [PubMed] [Google Scholar]
- 77. Hoddes E, et al. The development and use of the Stanford sleepiness scale. Psychophysiology. 1972;9:150. [Google Scholar]
- 78. Luck SJ. An Introduction to the Event-Related Potential Technique. Cambridge, MA; London, UK: The MIT Press; 2005. [Google Scholar]
- 79. Baldwin CL, et al. Detecting and quantifying mind wandering during simulated driving. Front Hum Neurosci. 2017;11:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Johns M, et al. The effect of blinks and saccadic eye movements on visual reaction times. Atten Percept Psychophys. 2009;71(4):783–788. [DOI] [PubMed] [Google Scholar]
- 81. Oostenveld R, et al. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rechtschaffen A, et al. A Manual of Standardised Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Los Angeles: Brain Information Service, University of California; 1968. [Google Scholar]
- 83. Benjamini Y, et al. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57(1):289–300. [Google Scholar]
- 84. Glickman ME, et al. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014;67(8):850–857. [DOI] [PubMed] [Google Scholar]
- 85. Lee IA, et al. Calculation for the test of the difference between two dependent correlations with one variable in common [Computer software]. http://quantpsy.org. Accessed August 1, 2019.
- 86. Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87(2):245–251. [Google Scholar]
- 87. Mölle M, et al. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep. 2011;34(10):1411–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Verleger R, et al. Is insight a godsend? Explicit knowledge in the serial response-time task has precursors in EEG potentials already at task onset. Neurobiol Learn Mem. 2015;125:24–35. [DOI] [PubMed] [Google Scholar]
- 89. Baldwin KB, et al. An ERP analysis of implicit structured sequence learning. Psychophysiology. 1997;34(1):74–86. [DOI] [PubMed] [Google Scholar]
- 90. Ferdinand NK, et al. Error and deviance processing in implicit and explicit sequence learning. J Cogn Neurosci. 2008;20(4):629–642. [DOI] [PubMed] [Google Scholar]
- 91. Rüsseler J, et al. Response anticipation processes in the learning of a sensorimotor sequence. J Psychophysiol. 2001;15(2):95–105. [Google Scholar]
- 92. Di Russo F, et al. Cortical sources of the early components of the visual evoked potential. Hum Brain Mapp. 2002;15(2):95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pourtois G, et al. Effects of perceptual learning on primary visual cortex activity in humans. Vision Res. 2008;48(1):55–62. [DOI] [PubMed] [Google Scholar]
- 94. Rauss KS, et al. Attentional load modifies early activity in human primary visual cortex. Hum Brain Mapp. 2009;30(5):1723–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hafed ZM, et al. Sharper, stronger, faster upper visual field representation in primate superior colliculus. Curr Biol. 2016;26(13):1647–1658. [DOI] [PubMed] [Google Scholar]
- 96. Wagner U, et al. Sleep inspires insight. Nature. 2004;427(6972):352–355. [DOI] [PubMed] [Google Scholar]
- 97. Kang JH, et al. Effects of an irregular bedtime schedule on sleep quality, daytime sleepiness, and fatigue among university students in Taiwan. BMC Public Health. 2009;9:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bach M. The Freiburg Visual Acuity Test-variability unchanged by post-hoc re-analysis. Graefes Arch Clin Exp Ophthalmol. 2007;245(7):965–971. [DOI] [PubMed] [Google Scholar]
- 99. Cheng CY, et al. Association of ocular dominance and anisometropic myopia. Invest Ophthalmol Vis Sci. 2004;45(8):2856–2860. [DOI] [PubMed] [Google Scholar]
- 100. Chaurasia BD, et al. Eyedness. Acta Anat (Basel). 1976;96(2):301–305. [DOI] [PubMed] [Google Scholar]
- 101. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. [DOI] [PubMed] [Google Scholar]
- 102. Horne JA, et al. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 103. Diekelmann S, et al. Sleep to implement an intention. Sleep. 2013;36(1):149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Corsi PM. Human memory and the medial temporal region of the brain. Diss Abstr Int. 1973;34:891. [Google Scholar]
- 105. Gregory RL. Perceptions as hypotheses. Philos Trans R Soc Lond B Biol Sci. 1980;290(1038):181–197. [DOI] [PubMed] [Google Scholar]
- 106. Rauss K, et al. What is bottom-up and what is top-down in predictive coding? Front Psychol. 2013;4:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Fogelson N, et al. Local contextual processing in major depressive disorder. Clin Neurophysiol. 2014;125(3):476–483. [DOI] [PubMed] [Google Scholar]
- 108. Doherty JR, et al. Synergistic effect of combined temporal and spatial expectations on visual attention. J Neurosci. 2005;25(36):8259–8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lum JAG, et al. Visuospatial sequence learning on the serial reaction time task modulates the P1 event-related potential. Psychophysiology. 2019;56(2):e13292. [DOI] [PubMed] [Google Scholar]
- 110. Turk-Browne NB, et al. Multidimensional visual statistical learning. J Exp Psychol Learn Mem Cogn. 2008;34(2):399–407. [DOI] [PubMed] [Google Scholar]
- 111. Antony JW, et al. Sleep spindles and memory reprocessing. Trends Neurosci. 2019;42(1):1–3. [DOI] [PubMed] [Google Scholar]
- 112. Born J, et al. System consolidation of memory during sleep. Psychol Res. 2012;76(2):192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chauvette S, et al. Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity. Neuron. 2012;75(6):1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Niethard N, et al. Cortical circuit activity underlying sleep slow oscillations and spindles. Proc Natl Acad Sci USA. 2018;115(39):E9220–E9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lüthi A. Sleep spindles. Neurosci. 2014;20(3):243–256. [DOI] [PubMed] [Google Scholar]
- 116. Ulrich D. Sleep spindles as facilitators of memory formation and learning. Neural Plast. 2016;2016:1796715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Schwartz S, et al. Neural correlates of perceptual learning: a functional MRI study of visual texture discrimination. Proc Natl Acad Sci USA. 2002;99(26):17137–17142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Walker MP, et al. The functional anatomy of sleep-dependent visual skill learning. Cereb Cortex. 2005;15(11):1666–1675. [DOI] [PubMed] [Google Scholar]
- 119. Yotsumoto Y, et al. Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron. 2008;57(6):827–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yotsumoto Y, et al. Location-specific cortical activation changes during sleep after training for perceptual learning. Curr Biol. 2009;19(15):1278–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Frank MG. Sleep and plasticity in the visual cortex: more than meets the eye. Curr Opin Neurobiol. 2017;44:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chen Z, et al. Thalamic circuit mechanisms link sensory processing in sleep and attention. Front Neural Circuits. 2016;9:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Durkin J, et al. Cortically coordinated NREM thalamocortical oscillations play an essential, instructive role in visual system plasticity. Proc Natl Acad Sci USA. 2017;114(39):10485–10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Klos C, et al. Bridging structure and function: a model of sequence learning and prediction in primary visual cortex. PLoS Comput Biol. 2018;14(6):e1006187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wurtz RH. Neuronal mechanisms of visual stability. Vision Res. 2008;48(20):2070–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Klorfeld-Auslender S, et al. Visual-oculomotor interactions facilitate consolidation of perceptual learning. J Vis. 2019;19(6):11. [DOI] [PubMed] [Google Scholar]
- 127. Breton J, et al. Dual enhancement mechanisms for overnight motor memory consolidation. Nat Hum Behav. 2017;1(6):0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Schlaghecken F, et al. Chunking processes in the learning of event sequences: electrophysiological indicators. Mem Cognit. 2000;28(5):821–831. [DOI] [PubMed] [Google Scholar]
- 129. Duncan CC, et al. Event-related potentials in clinical research: guidelines for eliciting, recording, and quantifying mismatch negativity, P300, and N400. Clin Neurophysiol. 2009;120(11):1883–1908. [DOI] [PubMed] [Google Scholar]
- 130. Zhou Y, et al. Asymmetric representations of upper and lower visual fields in egocentric and allocentric references. J Vis. 2017;17(1):9. [DOI] [PubMed] [Google Scholar]
- 131. Abrams J, et al. Isoeccentric locations are not equivalent: the extent of the vertical meridian asymmetry. Vision Res. 2012;52(1):70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yeshurun Y, et al. Attention improves or impairs visual performance by enhancing spatial resolution. Nature. 1998;396(6706):72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Peichl L. Diversity of mammalian photoreceptor properties: adaptations to habitat and lifestyle? Anat Rec A Discov Mol Cell Evol Biol. 2005;287(1):1001–1012. [DOI] [PubMed] [Google Scholar]
- 134. Silva MF, et al. Radial asymmetries in population receptive field size and cortical magnification factor in early visual cortex. Neuroimage. 2018;167:41–52. [DOI] [PubMed] [Google Scholar]
- 135. Silva MF, et al. Asymmetry of visual sensory mechanisms: electrophysiological, structural, and psychophysical evidences. J Vis. 2010;10(6):26. [DOI] [PubMed] [Google Scholar]
- 136. McAnany JJ, et al. Magnocellular and parvocellular visual pathway contributions to visual field anisotropies. Vision Res. 2007;47(17):2327–2336. [DOI] [PubMed] [Google Scholar]
- 137. Van Essen DC, et al. The visual field representation in striate cortex of the macaque monkey: asymmetries, anisotropies, and individual variability. Vision Res. 1984;24(5):429–448. [DOI] [PubMed] [Google Scholar]
- 138. Previc FH. Functional specialization in the lower and upper visual fields in. Behav Brain Sci. 1990;13:519–575. [Google Scholar]
- 139. Skrandies W. The upper and lower visual field of man: electrophysiological and functional differences. In: Autrum H, Ottoson D, Perl ER, Schmidt RF, Shimazu H, Willis WD, eds. Progress in Sensory Physiology. Berlin, Heidelberg: Springer; 1987: 1–93. [Google Scholar]
- 140. Karim AK, et al. The what and why of perceptual asymmetries in the visual domain. Adv Cogn Psychol. 2010;6:103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Herde L, et al. Anatomic and functional asymmetries interactively shape human early visual cortex responses. J Vis. 2020;20(6):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Hartsock MJ, et al. Memory and the circadian system: identifying candidate mechanisms by which local clocks in the brain may regulate synaptic plasticity. Neurosci Biobehav Rev. 2020;118:134–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Gerstner JR, et al. Circadian rhythms and memory formation. Nat Rev Neurosci. 2010;11(8):577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Keisler A, et al. Time of day accounts for overnight improvement in sequence learning. Learn Mem. 2007;14(10):669–672. [DOI] [PubMed] [Google Scholar]
- 145. Blatter K, et al. Circadian rhythms in cognitive performance: methodological constraints, protocols, theoretical underpinnings. Physiol Behav. 2007;90(2-3):196–208. [DOI] [PubMed] [Google Scholar]
- 146. Killgore WDS. Effects of sleep deprivation on cognition. Progr Brain Res. 2010;185:105–129. [DOI] [PubMed] [Google Scholar]
- 147. Brown LK. Can sleep deprivation studies explain why human adults sleep? Curr Opin Pulm Med. 2012;18(6):541–545. [DOI] [PubMed] [Google Scholar]
- 148. Ikegami K, et al. Recovery of cognitive performance and fatigue after one night of sleep deprivation. J Occup Health. 2009;51(5):412–422. [DOI] [PubMed] [Google Scholar]
- 149. Lange T, et al. Sleep after vaccination boosts immunological memory. J Immunol. 2011;187(1):283–290. [DOI] [PubMed] [Google Scholar]
- 150. Lange T, et al. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010;1193:48–59. [DOI] [PubMed] [Google Scholar]
- 151. Lange T, et al. Sleep-like concentrations of growth hormone and cortisol modulate type1 and type2 in-vitro cytokine production in human T cells. Int Immunopharmacol. 2006;6(2):216–225. [DOI] [PubMed] [Google Scholar]
- 152. Dimitrov S, et al. Number and function of circulating human antigen presenting cells regulated by sleep. Sleep. 2007;30(4):401–411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.