Abstract
Background
Data from the Improving Outcomes and Antibiotic Stewardship for Patients with Bloodstream Infections: Accelerate PhenoTest™ BC Kit (AXDX) Registry Study were analysed to determine the impact of rapid organism identification and antimicrobial susceptibility testing (AST) for Gram-positive bacteraemia.
Patients and methods
This multicentre, quasi-experimental study evaluated clinical and antimicrobial stewardship metrics following the implementation of AXDX. Data from hospitalized patients with bacteraemia were compared between groups, one that underwent testing on AXDX (post-AXDX) and one that underwent traditional identification and AST (pre-AXDX). An analysis of patients with Gram-positive bacteraemia was performed. The primary outcome was time to optimal therapy (TTOT). Secondary outcomes included time to first antibiotic modification (overall and Gram-positive), duration of unnecessary MRSA coverage, incidence of adverse events, length of stay and mortality.
Results
A total of 219 (109 pre-AXDX, 110 post-AXDX) patients with Gram-positive bacteraemia were included. Median TTOT was 36.3 h (IQR, 16.9–56.7) in the pre-AXDX group and 20.4 h (IQR, 7.5–36.7) in the post-AXDX group (P = 0.01). Compared with pre-AXDX, median time to first antibiotic modification (29.1 versus 15.9 h; P = 0.002), time to first Gram-positive antibiotic modification (33.2 versus 17.2 h; P = 0.003) and median duration of unnecessary MRSA coverage (58.4 versus 29.7 h; P = 0.04) were reduced post-AXDX. A trend towards decreased acute kidney injury (24% versus 13%; P = 0.06) was observed in the post-AXDX group. Groups did not differ in other secondary outcomes.
Conclusions
Implementation of AXDX testing for patients with Gram-positive bacteraemia shortened the TTOT and reduced unnecessary antibiotic exposure due to faster antibiotic modifications.
Introduction
Gram-positive bacteria are the predominant cause of bloodstream infections (BSIs) and a significant cause of morbidity and mortality among hospitalized patients in the USA.1,2 Delayed administration of effective antibiotic treatment, particularly among patients with Staphylococcus aureus bacteraemia, can have deleterious effects on clinical outcomes.3,4 Conventional methods for organism identification (ID) typically provide results 16 to 24 h following recovery of organisms in blood culture bottles, and an additional 16 to 24 h are required to perform antimicrobial susceptibility testing (AST) of bacterial isolates.5,6 Molecular methods can be used for more rapid identification of organisms recovered from blood culture bottles, and in some technologies also assess for the presence of antimicrobial resistance genes.7 Several studies have demonstrated a positive benefit of these technologies in the treatment of patients with bacteremia.8,9
More recently, rapid AST methods have emerged that can provide phenotypic MIC results within hours, directly from positive blood cultures.10 Faster AST results have been shown to allow for expedited therapy adjustments that theoretically improve patient outcomes and subsequently reduce costs.11–16 However, evidence supporting real-world clinical impact of rapid ID/AST tests for Gram-positive bacteraemia is limited.
The Improving Outcomes and Antibiotic Stewardship for Patients with Bloodstream Infection: Accelerate PhenoTest™ BC Kit Registry Study (IOAS), is a multicentre, quasi-experimental study designed to compare clinical and antimicrobial stewardship metrics, prior to and after the implementation of the Accelerate Diagnostics PhenoTest™ BC Kit (AXDX), a platform that provides both rapid identification (∼2 h) and MIC results (∼7 h) from positive blood cultures up to 40 h faster than conventional methods. The present study utilized data from this database to assess the impact of rapid identification and susceptibility testing on patient management and outcomes for those with Gram-positive bacteraemia.
Patients and methods
Study design
For the present analysis, IOAS data were collected from participating centres (n = 2) who implemented AXDX for testing of blood cultures positive for Gram-positive bacteria on Gram stain. This analysis is a subgroup of IOAS, which includes additional centres that implemented AXDX for testing of blood cultures with other Gram stain findings (e.g. Gram-negative), for which enrolment is ongoing. Both centres in this analysis implemented AXDX for testing of blood cultures positive for Gram-positive and Gram-negative bacteria on Gram stain. This study was submitted to and approved by the institutional review board at each participating site. Patients with positive blood cultures prior to the implementation of AXDX (pre-AXDX) were compared with patients who had blood culture testing using AXDX (post-AXDX).
Study population and inclusion criteria
Hospitalized patients with positive blood cultures that contained pathogens deemed clinically significant by the participating sites (i.e. not a contaminant) were eligible for inclusion in the IOAS study. Patients with blood cultures deemed to be contaminants were excluded to minimize the level of heterogeneity in the study population and because AST information, the primary differentiator between study groups, is not routinely reported for contaminants. Blood cultures were considered contaminated if one of the following organisms was present in ≤50% of all blood culture sets obtained from one patient on the same day: CoNS, alpha-haemolytic streptococci, Micrococcus species, Cutibacterium species, Corynebacterium species, and Bacillus species (not anthracis).17 Blood cultures that contained Gram-negative bacteria or yeast on final culture were eligible for the IOAS study, but were excluded from the present analysis of Gram-positive bacteria only. The following exclusion criteria were used for this analysis: patient discharged from the hospital at the time of positive blood culture, history of positive blood culture in the prior 14 days with the same organism, patient expired within 48 h of positive blood culture, and patient treated with palliative care and not expected to survive. Patients were enrolled into the study in an intention to treat manner based on whether the positive blood culture met criteria to be run on AXDX in the post-AXDX group, or theoretically would have been tested in the pre-AXDX group, including isolates not included in the AXDX panel of organisms (i.e. ‘off-panel’).
Sites and conventional microbiological diagnostics
The study was conducted at two US sites (University of Arkansas for Medical Sciences, Little Rock, AR, USA; University of Iowa Hospitals & Clinics, Iowa City, IA, USA) over a period time to enrol 100 eligible patients (∼2–3 months) into each study period. Pre-AXDX blood culture testing methods at Hospital A were MALDI-TOF MS (VITEK MS®, bioMérieux, Durham, NC, USA) for identification and VITEK® 2 (bioMérieux) for AST whereas Hospital B used MALDI-TOF MS (Bruker Daltonics, Billerica, MA, USA) for identification, VITEK® 2 and Sensititre™ (Thermo Scientific™, Waltham, MA, USA) for AST. PBP2a latex agglutination testing (Abbott Laboratories, Abbott Park, IL, USA) was performed for S. aureus isolates. Both institutions had active antimicrobial stewardship programmes (ASP) throughout the study period. Details on microbiology workflow, communication of results, and ASP intervention by each hospital can be found in the supplementary material (Table S1A and S1B, available as Supplementary data at JAC Online).
Implementation of AXDX
AXDX was performed according to manufacturer instructions at both sites in the post-AXDX group. Identification and AST were performed using AXDX for on-panel organisms (see Table S2). Off-panel organisms or antimicrobials were evaluated by conventional methods described above. AXDX testing was performed 24/7 on the first positive blood culture bottle per patient with a unique Gram stain. MICs were interpreted using CLSI M100 breakpoints.18–20
Data collection
Patients were evaluated for study inclusion by site investigators at each participating institution. Sites evaluated patients from corresponding time periods of the year (i.e. pre-AXDX October–December 2017 and post-AXDX group October–December 2018) to minimize any differences in seasonality. Once patients were identified, retrospective review of patient charts was performed, and data were entered into a study-specific case report form (CRF) using Clindex® Electronic Data Capture software (Fortress Medical Systems LLC, Hopkins, MN, USA). Data collected from each enrolled patient included demographics and baseline characteristics, location of care, relevant past medical history, disease characteristics (including source of bacteraemia, PITT bacteraemia score), microbiology data, antibiotic use information and clinical outcomes. All included patients’ CRFs were reviewed for accuracy and completeness by S.H.M or A.A.B.
Outcomes
Primary outcome was median time to optimal therapy (TTOT) in the first 96 h after blood culture positivity. Optimal therapy was calculated as hours from blood culture positivity until first administered dose of an optimal antibiotic and was determined by the investigators at each site using institution-specific preferred treatment for the patient based on AST, patient condition and comorbidities, and hospital policy. The assessment of optimal therapy was determined retrospectively by either an infectious diseases physician or a pharmacist. Patients who received optimal therapy prior to blood culture positivity and patients who did not receive optimal therapy during the first 96 h from blood culture positivity were excluded from the TTOT analysis.
Secondary outcome measures included time to first antibiotic modification (either escalation or de-escalation) within 96 h of blood culture positivity; time to first Gram-positive antibiotic modification within 96 h of blood culture positivity; duration of unnecessary MRSA coverage within 96 h of blood culture positivity; incidence of laboratory-documented Clostridioides difficile infection (CDI) within 30 days after blood culture positivity; incidence of acute kidney injury (AKI) within 14 days after blood culture positivity; acquisition of new MDR organisms (MDROs) within 30 days; length of stay in the hospital after blood culture positivity; and in-hospital mortality. Antipseudomonal β-lactam therapy was included in the study as a nonequivalent dependent variable to help identify the true impact of AXDX testing on anti-MRSA therapy.21,22 Necessity for anti-MRSA therapy was determined based on patient blood culture pathogen(s) and an assessment of a concurrent infection that required antibiotic therapy. AKI was defined using Risk, Injury, Failure, Loss, End-stage renal disease (RIFLE) criteria for patients ≥18 years of age and paediatric RIFLE (pRIFLE) for patients <18 years of age.23,24 Patients who were receiving renal replacement therapy (i.e. haemodialysis) at the time of blood culture positivity were excluded from the AKI assessment.
Statistical analysis
Primary and secondary outcomes were assessed in the intention-to-treat population, which included all patients who met inclusion and exclusion criteria. Baseline comparison of categorical variables between the two groups was performed using Pearson's χ2 test or Fisher’s exact test. Statistical comparisons were performed between study groups with Student’s t-test or Mann–Whitney U test for continuous variables, where appropriate. Time-to-event data were also evaluated by the Kaplan–Meier method and compared using the log-rank test. Statistical analyses were performed using JMP Version 13.0 (SAS Institute, Inc., Cary, NC, USA).
We determined the sample size for the IOAS study based on the number of patients needed to have 80% power to conclude that 30 day mortality was different between the two groups based on the log-rank test. Based on existing literature, it was estimated a pre-AXDX 30 day mortality rate of 16% would require 1000 patients (500 per group) to detect a relative risk (post-AXDX to pre-AXDX) of 0.6, with a 2-sided α = 0.05 test.25–27 Because this analysis was for Gram-positive bacteria, a fully enrolled subset of patients in the IOAS study, the sample size was anticipated to be too small to evaluate 30 day mortality.
Results
Patients
Data from 389 patients at the two participating centres that implemented AXDX for testing of Gram-positive bacteria were enrolled in IOAS; 219 (109 pre-AXDX, 110 post-AXDX) of these patients met criteria for this Gram-positive bacteria sub-analysis. Patient demographics, co-existing conditions, and baseline clinical characteristics at the time of blood culture positivity were similar between groups except for moderate-to-severe chronic kidney disease (CKD) being more prevalent in the pre-AXDX group (Table 1). However, baseline serum creatinine, Modification of Diet in Renal Disease (MDRD) glomerular filtration rate, and proportion of patients on haemodialysis were comparable between groups (Table 1). Markers of clinical severity were also similar between groups with more than a third of patients residing in the intensive care unit (37.9%) at time of blood culture positivity, 18% on mechanical ventilation and 11% requiring an IV vasopressor agent.
Table 1.
Demographics and baseline characteristics of patients
| Parameter | Pre-AXDX (n = 109) | Post-AXDX (n = 110) | P value |
|---|---|---|---|
| Demographics | |||
| male sex | 60 (55.1) | 62 (56.4) | 0.84 |
| age, years, median (IQR) | 55 (33-67) | 56 (30-65) | 0.63 |
| age <18 years old | 11 (10.1) | 18 (16.4) | 0.17 |
| Co-existing conditions | |||
| Charlson comorbidity score, mean ± SD | 4.8 ± 4.1 | 4.3 ± 4.1 | 0.40 |
| myocardial infarction | 9 (8.4) | 7 (6.4) | 0.56 |
| chronic heart failure | 27 (24.8) | 23 (20.9) | 0.50 |
| cerebrovascular accident | 18 (16.5) | 11 (10.0) | 0.16 |
| COPD | 35 (32.1) | 26 (23.6) | 0.16 |
| malignancy | 0.92 | ||
| leukaemia, lymphoma, local tumour | 28 (25.7) | 31 (28.2) | |
| metastatic tumour | 7 (6.4) | 7 (6.4) | |
| diabetes mellitus | 0.43 | ||
| uncomplicated | 17 (15.6) | 16 (14.6) | |
| end-organ damage | 26 (23.9) | 19 (17.3) | |
| chronic kidney disease | 29 (26.6) | 17 (15.5) | 0.04 |
| baseline serum creatinine > 3 mg/dL | 6 (5.9) | 3 (2.9) | |
| on dialysis | 7 (6.4) | 8 (7.3) | |
| chronic liver disease | 0.36 | ||
| mild | 8 (7.3) | 12 (11.0) | |
| moderate to severe | 3 (2.8) | 6 (5.5) | |
| transplant | 11 (10.1) | 8 (7.3) | 0.46 |
| bone marrow transplant | 5 (4.6) | 5 (4.6) | |
| Clinical characteristics at blood culture positivity | |||
| source of bacteraemiaa | 0.16 | ||
| bone/joint | 12 (11.0) | 14 (12.7) | |
| cardiovascular | 10 (9.2) | 10 (9.1) | |
| central venous catheter | 20 (18.3) | 12 (10.0) | |
| intra-abdominal | 5 (2.8) | 9 (8.2) | |
| respiratory | 9 (8.3) | 3 (2.7) | |
| skin/soft tissue | 5 (4.6) | 8 (7.3) | |
| urinary | 2 (1.8) | 3 (2.7) | |
| other | 3 (2.8) | 1 (0.9) | |
| unidentified | 39 (35.8) | 49 (44.6) | |
| immunosuppressant useb | 17 (15.6) | 14 (12.7) | 0.54 |
| concurrent infection requiring antibiotic therapyc | 30 (27.5) | 26 (23.6) | 0.51 |
| culture-confirmed infection | 19 (17.4) | 15 (13.6) | |
| suspected infection | 11 (10.1) | 11 (10.0) | |
| acquisition type | |||
| community acquiredd | 76 (69.7) | 81 (73.6) | 0.52 |
| ICU residence | 44 (40.3) | 39 (35.5) | 0.45 |
| Pitt bacteraemia scoree | 2.6 ± 2.8 | 2.3 ± 2.3 | 0.47 |
| quick SOFA (qSOFA) scoree | 1.0 ± 0.7 | 0.8 ± 0.7 | 0.17 |
| serum creatinine, mg/dLe ± SD | 1.4 ± 1.3 | 1.2 ± 1.0 | 0.30 |
| estimated glomerular filtration rate, mL/min/1.73 m² (MDRD)e, mean ± SD | 72.6 ± 53.3 | 77.2 ± 47.8 | 0.53 |
| requiring mechanical ventilation | 19 (17.4) | 21 (19.1) | 0.75 |
| hypotension (systolic blood pressure <90 mm Hg) | 27 (24.8) | 32 (29.1) | 0.47 |
| required IV vasopressors | 14 (12.8) | 11 (10.0) | 0.51 |
Data are presented as n (%) of patients, unless specified otherwise.
Significant differences are highlighted in bold.
Source of bacteraemia: (i) for a bloodstream infection to be determined secondary to another site of infection, at least one organism from the blood specimen must match an organism identified from the site-specific infection; (ii) if there is not another site of infection with organism growth, a clinician may determine the likely source of the bacteraemia based on their clinical judgement; and (iii) unidentified: unknown or no clear source of bacteria.
Immunosuppression included any of the following: (i) active systemic chemotherapy, tacrolimus, mycophenolate mofetil, azathioprine, cyclosporine (or equivalent therapy), for more than 7 days OR a systemic steroid for more than 10 days in the previous month; or (ii) absolute neutrophil count <1500.
A patient was classified as with a concurrent infection when a culture from the concomitant infection site grew at least one organism that was not isolated from blood or had a suspected infection that required additional antibiotic therapy.
Occurred prior to hospitalization or within ≤2 days of hospital admission.
Evaluated for patients ≥18 years of age.
Microbiological characteristics
There were 134 isolates identified in the pre-AXDX group and 126 in the post-AXDX group. Of these, 85% from pre-AXDX and 84% from post-AXDX were organisms present on the AXDX panel (on-panel). AST was performed ≥1 blood culture isolate for 92.5% of patients (Table 2). AST was performed for 89% of patients with CoNS isolated. The most prevalent organisms were S. aureus (29.2%) and CoNS (29.2%), followed by Streptococcus spp. (22.7%) (Table 2). MRSA was more prevalent in the post-AXDX group (P = 0.03); whereas CoNS were numerically more common in the pre-AXDX group (P = 0.10). The proportion of patients with polymicrobial blood cultures was slightly greater in the pre-AXDX group than the post-AXDX group, although this difference was not statistically significant (P = 0.08; Table 2).
Table 2.
Blood culture organisms
| Organism isolated | Pre-AXDX (n = 109) | Post-AXDX (n = 110) |
|---|---|---|
| CoNS | 43 (32.1) | 33 (26.2) |
| S. aureus | 33 (24.6) | 43 (34.1) |
| MRSA | 9 (6.7) | 20 (15.9) |
| Enterococcus spp. | 19 (14.2) | 20 (15.9) |
| Enterococcus faecalis | 10 (7.5) | 13 (10.3) |
| Enterococcus faecium | 8 (6.0) | 6 (4.8) |
| Enterococcus spp., vancomycin-resistant | 7 (5.2) | 3 (2.4) |
| Streptococcus spp. | 29 (21.6) | 30 (23.8) |
| Streptococcus agalactiae | 4 (3.0) | 4 (3.2) |
| Streptococcus anginosus group | 3 (2.2) | 5 (3.7) |
| Streptococcus mitis | 5 (3.7) | 9 (7.1) |
| Streptococcus pneumoniae | 12 (9.0) | 4 (3.2) |
| Streptococcus pyogenes | 2 (1.5) | 0 |
| Other | 10 (7.5) | 0 |
| Total organisms isolated | 134 | 126 |
| Polymicrobial | 21 (19.3) | 12 (10.9) |
| Proportion on rapid ID/AST panel | 114 (85.0) | 106 (84.1) |
| AST performed on ≥1 isolate | 92 (84.4) | 106 (96.4) |
Other organisms in the pre-AXDX group: Abiotrophia defectiva (1), Clostridium septicum (1), Clostridium tertium (2), Corynebacterium spp. [not otherwise specified] (2), Finegoldia magna (1), Nocardia farcinica (1), Peptoniphilus harei (1), Bacillus spp. [not otherwise specified] (1).
Other organisms in the post-AXDX group: none.
Among patients who had AXDX on-panel organisms, time from blood culture positivity to organism identification was 24.3 h faster in the post-AXDX than pre-AXDX group (mean [SD] 3.1 [2.3] versus 27.4 [12.5] h, P < 0.0001, Table S3). AST (18.7 [24.1] versus 48.7 [16.2] h, P < 0.0001, Table S3) was 30 h faster in the post-AXDX than pre-AXDX group.
Antimicrobial measures
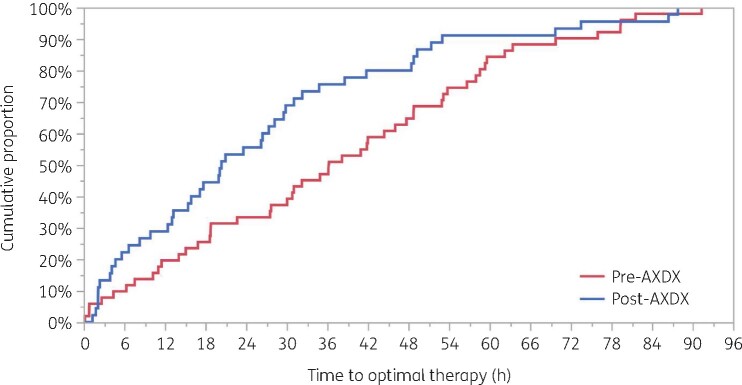
TTOT (Figure 1) was significantly shorter in the post-AXDX group than in the pre-AXDX group. Median [IQR] TTOT in the post-AXDX cohort was 20.4 [7.5–36.7] h, compared with 36.3 [16.9–56.7] h in the pre-AXDX group, a difference of 15.9 h (P = 0.01). The proportion of patients who achieved optimal therapy within 24 h after blood culture positivity was slightly higher in the post-AXDX group (61.8%) than in the pre-AXDX group (53.2%) but did not reach statistical significance (Table 3). The proportion of patients receiving optimal antibiotic therapy prior to blood culture positivity (37.6% pre-AXDX and 39.1% post-AXDX), the proportion of patients who received optimal therapy more than 96 h after blood culture positivity (3.7% pre-AXDX and 6.4% post-AXDX), and the proportion of patients who never received optimal antibiotic therapy (11.9% pre-AXDX and 13.6% post-AXDX) did not differ between groups.
Figure 1.
Kaplan–Meier analysis of the time from blood culture positivity to optimal antibiotic therapy. Log-rank P = 0.01. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Table 3.
Antibiotic-related outcomes
| Antibiotic modifications | Pre-AXDX (n = 109) | Post-AXDX (n = 110) | P value |
|---|---|---|---|
| Time to optimal therapy (n = 96) | 36.3 (16.9–56.7) | 20.4 (7.5–36.7) | 0.01 |
| Achievement of optimal therapy within 24 h after blood culture positivity, n (%) | 58 (53.2) | 68 (61.8) | 0.20 |
| Time to first antibiotic modification (n = 179) | 29.1 (11.4–47.8) | 15.9 (3.8–34.4) | 0.002 |
| Time to first Gram-positive antibiotic modification (n = 141) | 33.2 (14.1–55.1) | 17.2 (4.7–35.9) | 0.003 |
| Duration of therapy, h | |||
| vancomycin | |||
| vancomycin, all patients (n = 219) | 51.4 (17.7–91.2) | 55 (16.2–96.0) | 0.53 |
| vancomycin, all patients who received therapy (n = 190) | 62.1 (32.5–94.3) | 75.3 (26.4–96) | 0.37 |
| vancomycin, organisms not requiring vancomycina (n = 100) | 47.1 (24.9–79.3) | 25.5 (12.3–64.2) | 0.03 |
| vancomycin, organisms not requiring vancomycin, no concurrent infection (n = 72) | 48.2 (25.5–68.0) | 26.0 (14.9–56.9) | 0.06 |
| anti-MRSAb | |||
| anti-MRSA, all patients (n = 219) | 65.8 (34.0–96.0) | 71.9 (23.3–96) | 0.96 |
| anti-MRSA, all patients who received therapy (n = 199) | 70.8 (43.7–96) | 87.4 (33.5–96) | 0.75 |
| anti-MRSA, organisms not requiring vancomycin (n = 100) | 56.2 (32.2–92.2) | 33.5 (16.0–71.9) | 0.03 |
| anti-MRSA, organisms not requiring vancomycin, no concurrent infection (n = 72) | 58.4 (33.9–80.4) | 29.7 (17.0–64.4) | 0.04 |
| antipseudomonal β-lactamsc | |||
| antipseudomonal β-lactams, all patients | 30.3 (0–76.0) | 21.9 (0–78.0) | 0.27 |
| antipseudomonal β-lactams, all patients who received therapy (n = 154) | 55.0 (24.0–96.0) | 55.1 (21.9–94.7) | 0.89 |
| antipseudomonal β-lactams, no concomitant infection (n = 163) | 27.8 (0–61.3) | 17.5 (0–71.8) | 0.27 |
Data points were evaluated at 96 h after blood culture positivity and are reported as median (IQR), unless specified otherwise. Number of observations for each variable are included as (n=).
Significant differences are highlighted in bold.
Organisms not requiring vancomycin (MSSA; group A, B, C, or G streptococci; S. anginosus group; and ampicillin-susceptible E. faecalis).
Anti-MRSA agents: vancomycin, daptomycin, linezolid, ceftaroline, telavancin.
Antipseudomonal β-lactams: aztreonam, cefepime, ceftazidime, ceftazidime/avibactam, ceftolozane/tazobactam, imipenem/cilastatin, meropenem, piperacillin/tazobactam, meropenem/vaborbactam.
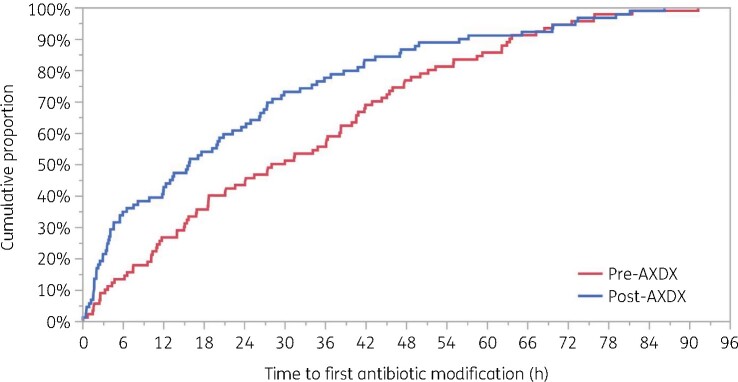
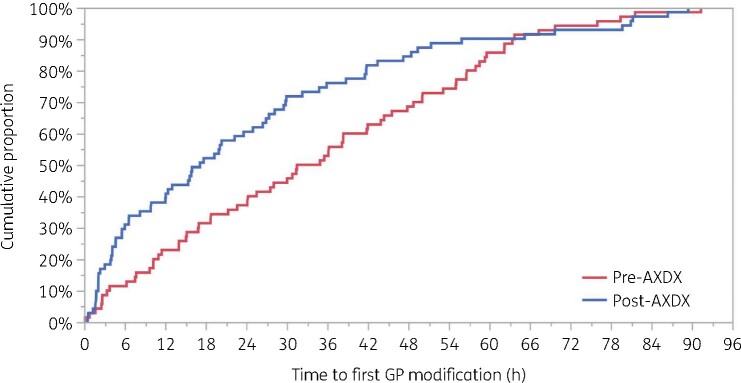
Time to first antibiotic modification (Figure 2) was 13.2 h faster in the post-AXDX group. The median [IQR] post-AXDX time to first antibiotic modification was 15.9 [3.8–34.4] h compared with the pre-AXDX group, where the median was 29.1 [11.4–47.8] h (P = 0.002). Among antibiotics with Gram-positive activity, the median initial antibiotic modification was nearly twice as fast for post-AXDX as pre-AXDX, with median times of 17.2 [4.7–35.9] versus 33.2 h [14.1–55.1] (Table 3 and Figure 3). There was no difference between groups in terms of the proportion of patients with any antibiotic modification within 96 h (82.6% pre-AXDX and 80.9% post-AXDX) and the proportion of patients with a Gram-positive antibiotic modification within 96 h (64.2% pre-AXDX and 64.5% post-AXDX).
Figure 2.
Kaplan–Meier analysis of the time from blood culture positivity to first antibiotic modification. Log-rank P = 0.03. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 3.
Kaplan–Meier analysis of the time from blood culture positivity to first Gram-positive (GP) antibiotic modification. Log-rank P = 0.04. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
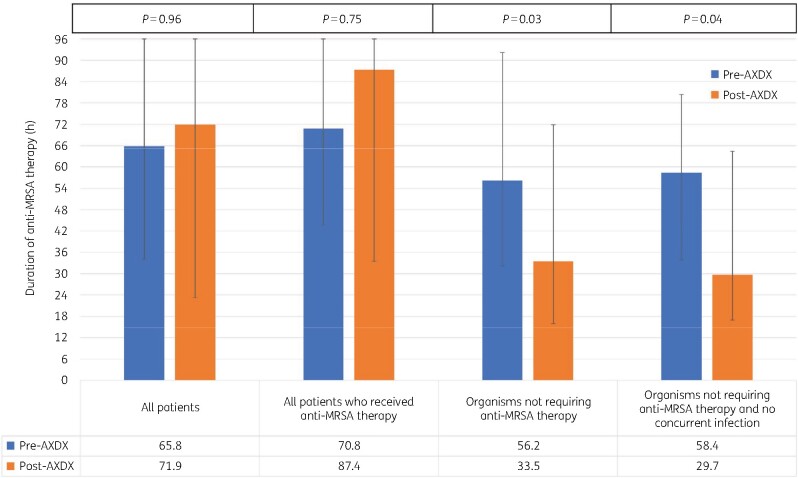
Duration of anti-MRSA and antipseudomonal β-lactam therapy received 96 h after blood culture positivity were similar between groups (Table 3). When restricted to patients with isolates that did not require MRSA therapy (n = 100), the duration of unnecessary anti-MRSA therapy was lower in the post-AXDX group by 22.7 h (P = 0.03; Table 3 and Figure 4). There was >24 h reduction of unnecessary anti-MRSA therapy in the post-AXDX group when restricting the analysis to patients (n = 72) who did not have a concurrent infection at another body site (P = 0.04; Table 3). These differences were largely driven by a reduction in vancomycin use (Table 3).
Figure 4.
Duration of anti-MRSA therapy during the initial 96 h following blood culture positivity. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Clinical endpoints
For the secondary endpoints, groups did not differ in mortality (in-hospital and 30 day), post-culture length of stay, proportion of patients who developed CDI, acquisition of new MDROs and 30 day readmission rates (Table 4).
Table 4.
Clinical outcomes
| Clinical outcome | Pre-AXDX (n = 109) | Post-AXDX (n = 110) | P value |
|---|---|---|---|
| In-hospital mortality | 6 (5.5) | 5 (4.6) | 0.75 |
| 30 day mortality | 8 (7.3) | 6 (5.5) | 0.57 |
| Patient disposition | 0.24 | ||
| deceased/hospice/comfort care | 8 (7.3) | 6 (5.5) | |
| home (no additional antibiotics) | 20 (18.3) | 25 (22.7) | |
| home with outpatient antimicrobial therapy | 54 (49.5) | 48 (44.0) | |
| nursing home/SNF/LTACH | 25 (22.9) | 18 (16.4) | |
| remains in hospital | 1 (0.9) | 4 (3.7) | |
| remains in ICU | 1 (0.9) | 4 (3.7) | |
| transfer to outside hospital | 0 | 4 (3.7) | |
| Total hospital length of stay, days, median (IQR) | 10.7 (5.8–18.1) | 11.9 (7.0–23.0) | 0.37 |
| Post-blood culture length of stay, days, median (IQR) | 9.1 (4.6–14.2) | 9.6 (5.8–18.0) | 0.21 |
| Acute kidney injury (all) | 24/100 (24.0) | 13/97 (13.4) | 0.06 |
| without CKD | 19/78 (24.4) | 12/88 (13.6) | 0.08 |
| with CKD and not on dialysis | 5/22 (22.7) | 1/9 (11.1) | 0.46 |
| acute kidney injury (<18 years old) | 3/10 (30.0) | 1/13 (7.7) | 0.16 |
| Acute kidney injury (≥18 years old) | 21/90 (23.3) | 12/84 (14.3) | 0.13 |
| without CKD | 16/68 (23.5) | 11/75 (14.7) | 0.17 |
| with CKD and not on dialysis | 5/22 (22.7) | 1/9 (11.1) | 0.46 |
| 14 day RRT | 3 (2.8) | 3 (2.7) | 0.99 |
| 30 day CDI | 2 (1.8) | 3 (2.7) | 0.66 |
| Acquisition of new MDROs within 30 days | 3 (2.8) | 2 (1.8) | 0.68 |
| Readmission within 30 days | 22 (21.4) | 27 (25.7) | 0.46 |
| Readmission within 30 days from bacteraemia | 7 (6.4) | 4 (3.6) | 0.37 |
All data are reported as n (%), unless specified otherwise.
LTACH, long-term acute care hospital; RRT, renal replacement therapy; SNF, skilled nursing facility.
The isolation of an MDR organism includes vancomycin-resistant enterococci, MRSA, extended-spectrum cephalosporin-resistant Enterobacteriaceae and Pseudomonas aeruginosa and Acinetobacter species non-susceptible to at least 1 agent in ≥3 antimicrobial categories as described by Magiorakos et al.39 (i) Extended-spectrum cephalosporin-resistant Enterobacteriaceae will be defined as the as intermediate or resistant to a third-generation cephalosporin. (ii) Carbapenem-resistant Enterobacteriaceae will be defined as intermediate or resistant to imipenem, doripenem, ertapenem (R only) or meropenem. If the susceptibility test indicated the specimen was resistant to any of those medications the specimen was categorized as ‘carbapenem non-susceptible’.
The proportion of patients who developed an AKI within 14 days after blood culture positivity were lower in the post-AXDX group (13%) than in the pre-AXDX group (24%), but this did not reach statistical significance (P = 0.06). A sub-analysis of adults (≥18 years old) was performed based on pre-existing CKD status and found the rates of AKI were higher for pre-AXDX in both patients with pre-existing CKD (22.7% pre-AXDX versus 11.1% post-AXDX) and patients without pre-existing CKD (23.5% pre-AXDX versus 14.7% post-AXDX).
Discussion
This pragmatic study showed real-world evidence that the implementation of AXDX significantly improved the treatment of patients with Gram-positive bacteraemia. Rapid organism identification and phenotypic AST led to significant reductions in TTOT, first antibiotic modification and duration of unnecessary MRSA coverage compared with a historical pre-implementation group. Several recent studies have shown that rapid organism identification and phenotypic AST with AXDX enables clinicians to make faster antibiotic modifications which in turn may improve the outcomes of patients with bacteremia.11,12,14,16,28 While some of these studies have included patients with Gram-positive bacteraemia, no studies have focused exclusively on this patient population.
We found that the implementation of AXDX decreased the time from blood culture positivity to identification and AST by >24 h compared with conventional diagnostics used at the two participating centres, which is within the range of previous studies including Gram-positive bacteria.16,29–31 These observed time savings in time to results are consistent with other rapid, Gram-positive, direct from positive blood culture diagnostics.32 As a result of the expedited information provided by the diagnostic test, clinicians modified Gram-positive antibiotics a median of 16 h earlier and delivered optimal therapy to patients with bacteraemia nearly 50% faster than before use of AXDX. This time-savings in antibiotic optimization is comparable to other evaluations of rapid detection of Gram-positive bacteria by AXDX and other assays. Ehren et al.12 saw a 12 to 16 h reduction in TTOT with use of AXDX but did not analyse this endpoint for Gram-positive bacteria separately from other pathogens. Dare et al.16 reported a > 1 day reduction in TTOT, which was relatively consistent across all pathogen sub-analyses. In a landmark randomized evaluation of rapid multiplex PCR for detection of bloodstream pathogens, Banerjee et al. (2015)9 saw similar reductions in appropriate antibiotic de-escalations and use of broad-spectrum antibiotics to what was observed in this study.9
We did not observe any differences in total duration of anti-MRSA or antipseudomonal β-lactam therapy between groups during the early (96 h) period post-positive blood cultures. Conversely, when focusing specifically on unnecessary anti-MRSA therapy, i.e. those patients without documented MRSA in blood, patients in the post-AXDX group received approximately 24 h less unnecessary anti-MRSA therapy than patients in the pre-AXDX group. Similarly, Banerjee et al. (2015)9 observed a shorter duration of vancomycin use in patients not requiring vancomycin only, but no difference in total vancomycin use in their prospective evaluation of a rapid multiplex PCR platform.9 A subgroup analysis of CoNS bacteraemia reported by Dare et al.16 (NB none of those patients was included in the current paper) observed a reduction in broad Gram-positive antibiotic therapy of >1 day with AXDX. It is important to note that approximately 40% of the patients with positive blood cultures in Dare et al.16 were identified as potential contaminants, which would have been excluded from this study. Ehren et al.12 observed an increased duration of anti-MRSA therapies following implementation of AXDX, which may have been explained by a greater number of patients with Enterococcus faecium bacteraemia (72% vancomycin-susceptible) in the post-AXDX group. Similar to these studies, neither of the participating sites in this interim analysis employed molecular methods for organism identification in the pre-AXDX group, however Hospital B performed ‘scum plate’ MALDI-TOF and 6 h PBP2a testing (for S. aureus), which may have minimized the impact of AXDX on anti-MRSA therapy as pre-AXDX and post-AXDX groups were able to differentiate MRSA/MSSA within approximately the same timeframe. It is important to note there were significant differences in study inclusion criteria and antimicrobial stewardship intervention between our study and that of Banerjee et al.,9 Dare et al.16 and Ehren et al.12 that make any comparison of antimicrobial use data challenging. For instance, there are several important confounding variables other than organism identification and AST results that might affect antibiotic prescribing practices. To assess for these confounding variables, antipseudomonal β-lactam therapy duration was included as a dependent control variable. Specifically, as the duration of anti-MRSA therapy decreased following implementation of AXDX, but the duration of antipseudomonal β-lactam therapy did not, this increases the likelihood the observed decrease in anti-MRSA therapy was attributed to AXDX testing, and less likely due to other factors. Similarly, there is a large body of evidence demonstrating that rapid results from microbiological technologies are of little value if not actively communicated and acted upon in a timely manner through antimicrobial stewardship intervention.32 Antimicrobial stewardship intervention was unchanged at Site A throughout the study period, but Site B implemented real-time notification of organism identification and AST results in the post-AXDX time period. While it is plausible the addition of real-time notification could have impacted some of the study endpoints, a recent quasi-experimental study that observed improved clinical outcomes with implementation of AXDX found that the addition of real-time notification did not further improve several metrics such as time to optimal therapy as compared with an already established active ASP (i.e. routine monitoring of positive blood culture and intervention).16 A similar study design on the implementation of the Verigene™ Gram-Positive Blood Culture nucleic acid microarray assay observed sustainability of shorter time to optimal therapy even after real-time antimicrobial stewardship interventions were discontinued.33 Important to note, the authors attribute the sustainability of impact to the initial implementation involving clear guidance on antimicrobial selection, presence of an established antimicrobial stewardship programme and the relative ease of interpretability of the assay results. Taken together, these findings support that a considerable portion of the impact observed in the post-AXDX group is attributable to AXDX testing.
Like other investigators, we did not observe any differences in clinical outcomes such as mortality, CDI and readmission to hospital between pre-AXDX and post-AXDX groups.11,12,16 This is not unexpected as this sub-analysis of the IOAS study was not powered to detect subtle differences in secondary outcomes including mortality, length of stay and CDI. Moreover, there was a greater number of patients with bacteraemia caused by MRSA in the post-AXDX group; infection with this organism is known to be associated with a higher rate of complications and mortality, and may have influenced these outcomes.34
In contrast to other studies of AXDX implementation, IOAS data allowed for an assessment of the effect of AXDX on development of AKI, an unintended consequence of antibiotic therapy that some patients experience.35,36 We saw a numerical reduction (∼10% absolute difference) in the proportion of patients who developed an AKI following implementation of AXDX, but this finding was not statistically different between groups. Interestingly, when assessed by age and presence of underlying CKD, ≥10% difference in rates of AKI remained between groups. It is not possible to directly attribute AXDX testing to rates of AKI in this study due to imbalances between groups, the many potential causes of AKI in hospitalized patients that were not assessed and the lack of overall statistical significance. However, the trend towards reduced AKI deserves further evaluation in a larger study, as it is well known that some antibiotics, such as vancomycin, are common inciting causes of AKI and that each day of antibiotic therapy increases the risk of an antibiotic-associated adverse event occurrence.37,38 Therefore, it is plausible that the faster antibiotic modifications and the reduction in unnecessary anti-MRSA therapy enabled by AXDX may have lowered the risk of AKI for some patients and contributed to this intriguing observation.
The retrospective nature and lack of randomization in the study may explain some of the differences in patient characteristics and organism distribution observed between the groups. Most notably, the proportion of patients with CKD was higher in the pre-AXDX group and MRSA was more commonly isolated in the post-AXDX group. Efforts were made to account for these differences during the evaluation of primary and secondary outcomes using subgroup analyses. Additionally, there are many factors outside the microbiology laboratory that affect the management of patients with bacteraemia. We robustly assessed clinical characteristics, severity of illness and concurrent infections as well as having sites evaluate patients from corresponding time periods of the year to minimize the amount of confounding that may exist. Lastly, we were not able to assess the frequency with which antimicrobial stewardship teams intervened and the acceptance rate of their recommendations, as this information was not available at all participating centres for the pre-AXDX group. Despite these limitations, this study contributes novel and informative data on the clinical utility of AXDX in a real-world setting. The multicentre design provides a greater basis for the generalizability of the findings across a broader range of settings and strengthens the argument that AXDX demonstrates clinical benefit seen in other studies. While others have evaluated patients with Gram-positive bacteraemia in their studies, this is the first study to focus on the clinical benefit of rapid, phenotypic AST for patients with only true bloodstream infections (excluding potential contaminants) caused by Gram-positive bacteria in an intention-to-treat manner. We were also able to assess additional clinical endpoints (i.e. AKI) that have not been previously evaluated for AXDX.
In summary, implementation of AXDX offered a comprehensive solution to replace various identification and phenotypic testing methods and had a meaningful impact on the management of patients with Gram-positive bacteraemia in the IOAS study. TTOT and initial antibiotic modifications were significantly faster, and patients received less unnecessary antibiotic therapy compared with conventional microbiology diagnostics. Additional studies with larger sample sizes are needed to evaluate the impact more suitably on clinical outcome parameters. Overall, these findings suggest that rapid ID/AST enhances care of patients with bacteraemia caused by Gram-positive bacteria.
Funding
This work was supported by Accelerate Diagnostics, Inc.
Transparency declarations
S.H.M., A.A.B. and R.M.H. are current/former employees of Accelerate Diagnostics, Inc. and were involved in the design, execution, analysis and reporting of the research. All other authors: none to declare.
Supplementary data
Tables S1 to S3 are available as Supplementary data at JAC Online.
Supplementary Material
References
- 1.Bearman GML, Wenzel RP. Bacteremias: a leading cause of death. Arch Med Res 2005; 36: 646–59. [DOI] [PubMed] [Google Scholar]
- 2.Diekema DJ, Hsueh P-R, Mendes RE et al. The microbiology of bloodstream infection: 20-year trends from the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother 2019; 63: e00355-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khatib R, Saeed S, Sharma M et al. Impact of initial antibiotic choice and delayed appropriate treatment on the outcome of Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis 2006; 25: 181–5. [DOI] [PubMed] [Google Scholar]
- 4.Lodise TP, McKinnon PS, Swiderski L et al. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis 2003; 36: 1418–23. [DOI] [PubMed] [Google Scholar]
- 5.Khan ZA, Siddiqui MF, Park S. Current and emerging methods of antibiotic susceptibility testing. Diagnostics (Basel) 2019; 9: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson ML, Weinstein MP, Reller LB et al. Laboratory detection of bacteremia and fungemia. In: Jorgensen JH, Carroll KC, Funke G., eds. Manual of Clinical Microbiology, 11th edn. Wiley, 2015: 15–28. [Google Scholar]
- 7.Riedel S, Carroll KC. Early identification and treatment of pathogens in sepsis: molecular diagnostics and antibiotic choice. Clin Chest Med 2016; 37: 191–207. [DOI] [PubMed] [Google Scholar]
- 8.Timbrook TT, Morton JB, McConeghy KW et al. The effect of molecular rapid diagnostic testing on clinical outcomes in bloodstream infections: a systematic review and meta-analysis. Clin Infect Dis 2017; 64: 15–23. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee R, Teng CB, Cunningham SA et al. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identification and susceptibility testing. Clin Infect Dis 2015; 61: 1071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pancholi P, Carroll KC, Buchan BW et al. Multicenter evaluation of the Accelerate PhenoTest BC Kit for rapid identification and phenotypic antimicrobial susceptibility testing using morphokinetic cellular analysis. J Clin Microbiol 2018; 56: e01329-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerjee R, Komarow L, Virk A et al. Randomized trial evaluating clinical impact of RAPid IDentification and Susceptibility testing for Gram Negative bacteremia (RAPIDS-GN). Clin Infect Dis 2020; doi:10.1093/cid/ciaa528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehren K, Meißner A, Jazmati N et al. Clinical impact of rapid species identification from positive blood cultures with same-day phenotypic antimicrobial susceptibility testing on the management and outcome of bloodstream infections. Clin Infect Dis 2020; 70: 1285–93. [DOI] [PubMed] [Google Scholar]
- 13.Kim J-H, Kim I, Kang CK et al. Enhanced antimicrobial stewardship based on rapid phenotypic antimicrobial susceptibility testing for bacteraemia in patients with haematological malignancies: a randomized controlled trial. Clin Microbiol Infect 2021; 27: 69–75. [DOI] [PubMed] [Google Scholar]
- 14.Sheth S, Miller M, Prouse AB et al. Pharmacist-driven implementation of fast identification and antimicrobial susceptibility testing improves outcomes for patients with Gram-negative bacteremia and candidemia. Antimicrob Agents Chemother 2020; 64: e00578-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogan CA, Ebunji B, Watz N et al. Impact of rapid antimicrobial susceptibility testing in Gram-negative rod bacteremia: a quasi-experimental study. J Clin Microbiol 2020; 58: e00360-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dare RK, Lusardi K, Pearson C et al. Clinical impact of Accelerate PhenoTM rapid blood culture detection system in bacteremic patients. Clin Infect Dis 2020; doi:10.1093/cid/ciaa649. [DOI] [PubMed] [Google Scholar]
- 17.Roth A, Wiklund AE, Pålsson AS et al. Reducing blood culture contamination by a simple informational intervention. J Clin Microbiol 2010; 48: 4552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CLSI. Performance Standards for Antimicrobial Susceptibility Testing—Twenty-Seventh Edition: M100. 2017.
- 19.CLSI. Performance Standards for Antimicrobial Susceptibility Testing—Twenty-Eighth Edition: M100. 2018.
- 20.CLSI. Performance Standards for Antimicrobial Susceptibility Testing—Twenty-Ninth Edition: M100. 2019.
- 21.Eliopoulos GM, Shardell M, Harris AD et al. Statistical analysis and application of quasi experiments to antimicrobial resistance intervention studies. Clin Infect Dis 2007; 45: 901–7. [DOI] [PubMed] [Google Scholar]
- 22.Eliopoulos GM, Harris AD, Bradham DD et al. The use and interpretation of quasi-experimental studies in infectious diseases. Clin Infect Dis 2004; 38: 1586–91. [DOI] [PubMed] [Google Scholar]
- 23.Bellomo R, Ronco C, Kellum JA et al. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8: R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akcan-Arikan A, Zappitelli M, Loftis LL et al. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 2007; 71: 1028–35. [DOI] [PubMed] [Google Scholar]
- 25.Huang AM, Newton D, Kunapuli A et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis 2013; 57: 1237–45. [DOI] [PubMed] [Google Scholar]
- 26.Perez KK, Olsen RJ, Musick WL et al. Integrating rapid diagnostics and antimicrobial stewardship improves outcomes in patients with antibiotic-resistant Gram-negative bacteremia. J Infect 2014; 69: 216–25. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki H, Hitomi S, Yaguchi Y et al. Prospective intervention study with a microarray-based, multiplexed, automated molecular diagnosis instrument (Verigene system) for the rapid diagnosis of bloodstream infections, and its impact on the clinical outcomes. J Infect Chemother 2015; 21: 849–56. [DOI] [PubMed] [Google Scholar]
- 28.Lee M, Scardina T, Zheng X et al. Clinical performance and impact of Accelerate Pheno for Gram-negative bacteremia in hospitalized children. Clin Ther 2020; doi:10.1016/j.clinthera.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Brazelton de Cárdenas JN, Su Y, Rodriguez A et al. Evaluation of rapid phenotypic identification and antimicrobial susceptibility testing in a pediatric oncology center. Diagn Microbiol Infect Dis 2017; 89: 52–7. [DOI] [PubMed] [Google Scholar]
- 30.Charnot-Katsikas A, Tesic V, Love N et al. Use of the Accelerate Pheno system for identification and antimicrobial susceptibility testing of pathogens in positive blood cultures and impact on time to results and workflow. J Clin Microbiol 2017; 56: e01166-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calderaro A, Buttrini M, Martinelli M et al. Rapid microbial identification and phenotypic antimicrobial susceptibility testing directly from positive blood cultures: a new platform compared to routine laboratory methods. Diagn Microbiol Infect Dis 2020; 96: 114955. [DOI] [PubMed] [Google Scholar]
- 32.Bauer KA, Perez KK, Forrest GN et al. Review of rapid diagnostic tests used by antimicrobial stewardship programs. Clin Infect Dis 2014; 59 Suppl 3: S134–145. [DOI] [PubMed] [Google Scholar]
- 33.Avdic E, Wang R, Li DX et al. Sustained impact of a rapid microarray-based assay with antimicrobial stewardship interventions on optimizing therapy in patients with Gram-positive bacteraemia. J Antimicrob Chemother 2017; 72: 3191–8. [DOI] [PubMed] [Google Scholar]
- 34.Wyllie DH, Crook DW, Peto TEA. Mortality after Staphylococcus aureus bacteraemia in two hospitals in Oxfordshire, 1997-2003: cohort study. BMJ 2006; 333: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chertow GM, Burdick E, Honour M et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005; 16: 3365–70. [DOI] [PubMed] [Google Scholar]
- 36.Kellum JA, Murugan R. Effects of non-severe acute kidney injury on clinical outcomes in critically ill patients. Crit Care 2016; 20: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morales-Alvarez MC. Nephrotoxicity of antimicrobials and antibiotics. Adv Chronic Kidney Dis 2020; 27: 31–7. [DOI] [PubMed] [Google Scholar]
- 38.Tamma PD, Avdic E, Li DX et al. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med 2017; 177: 1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magiorakos A-P, Srinivasan A, Carey RB et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18: 268–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.