Abstract
Background
WHO revised their HIV drug resistance (HIVDR) monitoring strategy in 2014, enabling countries to generate nationally representative HIVDR prevalence estimates from surveys conducted using this methodology. In 2016, we adopted this strategy in Uganda and conducted an HIVDR survey among adults initiating or reinitiating ART.
Methods
A cross-sectional survey of adults aged ≥18 years initiating or reinitiating ART was conducted at 23 sites using a two-stage cluster design sampling method. Participants provided written informed consent prior to enrolment. Whole blood collected in EDTA vacutainer tubes was used for preparation of dried blood spot (DBS) specimens or plasma. Samples were shipped from the sites to the Central Public Health Laboratory (CPHL) for temporary storage before transfer to the Uganda Virus Research Institute (UVRI) for genotyping. Prevalence of HIVDR among adults initiating or reinitiating ART was determined.
Results
Specimens from 491 participants (median age 32 years and 61.5% female) were collected between August and December 2016. Specimens from 351 participants were successfully genotyped. Forty-nine had drug resistance mutations, yielding an overall weighted HIVDR prevalence of 18.2% with the highest noted for NNRTIs at 14.1%.
Conclusions
We observed a high HIVDR prevalence for NNRTIs among adults prior to initiating or reinitiating ART in Uganda. This is above WHO’s recommended threshold of 10% when countries should consider changing from NNRTI- to dolutegravir-based first-line regimens. This recommendation was adopted in the revised Ugandan ART guidelines. Dolutegravir-containing ART regimens are preferred for first- and second-line ART regimens.
Introduction
Pretreatment HIV drug resistance (PDR) may be observed with widespread use of antiretroviral (ARV) drugs like NNRTIs for treating and preventing HIV infection.1 As the global scale-up of ART increases, there are growing concerns about an increase in the prevalence of HIV drug resistance (HIVDR)2 among people on ART with unsuppressed viral load or people not currently receiving treatment but with prior exposure to ARV drugs, as part of prevention of mother-to-child transmission (PMTCT), previous treatment or, less likely, post-exposure prophylaxis and pre-exposure prophylaxis.3,4 The WHO global reports have demonstrated an increase in HIVDR among ART initiators in low- and middle-income countries (LMICs) from 6.8% in 20105 to 10% and above in 2017.1 High prevalence of PDR to NNRTIs negatively affects individual healthcare and the success of the public health response to treatment of HIV and potentially endangers the attainment of the global targets to end the AIDS epidemic as a global threat.1 Obtaining population-level data on HIVDR in different populations can inform programme-level decision-making, for choosing optimal first-line and second-line ARV drugs, for both children and adults.6
Regional HIVDR reports have shown varying levels of PDR. For example, a survey conducted between 2008 and 2010 in Zimbabwe reported a prevalence of 6.3%.7 A study conducted in Western Kenya in 2013 reported an overall PDR prevalence of 8.8% (8.3% for NNRTIs and 2.1% for NRTIs) among participants initiating ART.8 PDR prevalence was reported to be 10.4% in Cameroon9 and 12.7% in Namibia10 between 2015 and 2016. National prevalence estimates of PDR rates were reported as 11.6% in Eswatini (in 2016) and 10.9% in Zimbabwe (in 2015).11 Uganda rolled out the PMTCT programme in 2001, initially with single-dose nevirapine12 then Option A in 201013 (zidovudine starting at 14 weeks of gestation then single-dose nevirapine at onset of labour and zidovudine/lamivudine for 7 days postpartum plus daily nevirapine at birth until 1 week after cessation of breastfeeding) and then eventually Option B+ with ART for life in 2013.14 Provision of free ART for people living with HIV (PLHIV) began for individuals with CD4 T cell counts ≤200 cells/mm3 in 2004, then ≤350 cells/mm3 in 2008, ≤500 cells/mm3 in 2014 and eventually treatment for all in 2016.
The few studies conducted in Uganda among adults initiating ART at specific sites demonstrated varying baseline HIVDR results to any ARV: 11.6% in 2010;15 4.5% in 2012;16 and more recently over 15% in a 2016 meta-analysis.17 A longitudinal cohort study conducted in Western Uganda reported an overall PDR prevalence of 3.5% with an increased rate of PDR among only women—1.8% among those enrolling in the clinic from 2001 to 2006 to 7% among those enrolling from 2007 to 2013.18
The most common mutations observed in most of the Ugandan studies included M184V/I and thymidine analogue mutations (TAMS), mainly M41L, T215Y/F and D67N for NRTIs and K103N/S and Y181C/I for NNRTIs. Mutations to PIs were not common.
However, these studies in Uganda were conducted mainly in urban research settings and hence the results are difficult to generalize to populations beyond such settings. Initial guidance provided by WHO in 2004 for HIVDR surveillance among LMICs consisted of performing targeted surveillance for transmitted HIVDR (TDR) among newly infected individuals and acquired HIVDR among individuals receiving ART. These surveys were conducted at conveniently located sites where cold chain facilities required for sample storage were available. Such surveys were difficult and expensive to implement. Furthermore, identification of recently infected individuals for the TDR studies was difficult due to limitations of available techniques that allowed for detection of recent HIV infections. In 2014, WHO revised the HIVDR surveillance guidance to address some of these challenges. Countries could use WHO-created concept notes that recommended a ‘proportional to clinic size’ sampling approach and also allowed the use of existing national HIVDR testing systems to determine a national estimate of PDR instead of TDR prevalence.6 Based on this WHO recommendation and because routine PDR testing wasn’t available in Uganda, we conducted a cross-sectional survey among adults initiating and reinitiating ART at sites across the country to estimate the national prevalence of PDR and to describe the pattern of drug-resistant mutations (DRMs) in adults initiating and reinitiating ART. In this report, we present the findings of this survey, which have now been used to inform the national ART guidelines on the appropriate first-line ART regimens.19
Methods
Study design and participants
This was a cross-sectional survey conducted using a two-stage cluster design among adult (≥18 years old) populations initiating or reinitiating first-line ART for their own health (after an interruption longer than 3 months) or for PMTCT. The participants provided written informed consent. Participants who had interrupted their ART for <3 months as well as those who initiated ART in a different clinic were excluded.
Site selection
This survey was conducted from 1 August to 30 November 2016. Prior to sampling, study sites were selected from a list of all clinics in the country that had been dispensing ART for at least 12 months. The smallest sites, which made up <10% of the total population of patients on ART, were excluded. The clinics were then stratified into five geographical regions and sites randomly selected proportionally to the size of the region. We used the following estimates when determining our sample size: 10% prevalence of HIVDR among all ART initiators, 25% of ART initiators with prior exposure to ARVs, 80% rate of PCR amplification, intra-cluster correlation for HIVDR of 0.8%, design effect of 1.5 and a 20% site drop-out rate; 24 sites with 21 participants from each site were chosen for the survey. One site was subsequently removed due to a protocol violation, leaving 23 sites to participate in the survey.
Participant enrolment
Eligible participants were consecutively enrolled until the sample size of at least 21 consenting adults was reached at each of the survey sites. Consenting participants were assigned a survey identification (SID) number, survey data were collected and blood specimens obtained. Blood collection was done prior to ART initiation or reinitiation (i.e. before ARVs were dispensed to the participants).
We trained the site laboratory staff in specimen collection, processing and other handling procedures prior to survey commencement, with emphasis on sample quality and the consequences of poor specimen processing to ensure successful genotyping of at least 80% of the specimens.
Venous whole blood was collected in a 4 mL EDTA vacutainer tube for preparation of two dried blood spot (DBS) cards,20 each containing five blood spots (at 22 sites) and two plasma aliquots (at one site) from each participant. The DBS specimens were packaged in gas-permeable sealable plastic bags, which were placed in specially labelled envelopes and shipped at room temperature from the sites to the Central Public Health Laboratories (CPHL) in Kampala within 7 days of blood collection, for temporary storage (at 2°C–8°C), using the national sample and results transport network,21 whose delivery period to the genotyping laboratory was expected within another 7 days from specimen pick-up from site or 2 weeks from specimen collection. The DBS specimens were later transferred from CPHL to the MRC/Uganda Virus Research Institute (UVRI) and London School of Hygiene and Tropical Medicine (LSHTM) genotyping laboratory on dry ice. Plasma specimens were stored at −80°C at the site and transferred in liquid nitrogen within 1 week to the genotyping laboratory.
Demographic and clinical information was abstracted from the medical records of each participant. This included date of birth or age, gender, any previous and type of ART exposure, ART regimen prescribed, CD4 T cell count, WHO clinical stage and date of ART initiation or reinitiation.
HIVDR genotyping
Genotyping of the full protease gene and partial reverse transcriptase regions of the HIV-1 pol gene was done using a modified and validated in-house method22 at the MRC/UVRI and LSHTM laboratory, which is a WHO HIVResNet-designated reference laboratory for HIVDR genotyping. The 1300 bp segment of the 5′ region of the pol gene was generated by RT–PCR, followed by nested PCR using total nucleic acid extracted from DBS or plasma. The fragment was purified, sequenced using a BigDye Terminator v3.1 cycle sequencing kit (Life Technologies, Foster City, CA, USA) and analysed on an ABI Prism 3500 Genetic Analyzer (Life Technologies). A combination of the Sequencher software (Sequencher® v5.4.1 sequence analysis software, Gene Codes Corporation, Ann Arbor, MI, USA) and a customized RECall software program23 was used to edit the raw sequence data and generate consensus sequences. DRMs were analysed using the Stanford HIVdb program according to the 2009 WHO mutation list.24 For quality control purposes, our laboratory is enrolled in the Virology Quality Assurance programme funded by the National Institutes of Health located in Baltimore, MD, USA. All sequences generated in the laboratory are assessed for cross-contamination by phylogenetic analysis. Sequences from this study were deposited in GenBank under accession numbers MN625980–6330.
Data management and statistical methods
The extracted participant data were entered into a Microsoft Access 2016 (Microsoft Corporation, Redmond, WA, USA) database. Laboratory data of HIVDR results were linked to clinical data using the participant SID.
All statistical analyses were performed following the WHO guidelines for surveillance of HIVDR in adults initiating ART6 and were done using STATA v14 (StataCorp LP, College Station, TX, USA). Weighted prevalence rates for HIVDR and ART exposure, with 95% CIs, were calculated. Frequency distributions for survey characteristics were not weighted. The prevalence of HIVDR was determined using only specimens that were successfully genotyped, whereas the prevalence for ART exposure was determined using all enrolled participants. All analyses accounted for stratification and clustering, and survey weights were accounted for explicitly by using the svyset function. Weights accounted for unequal selection probabilities, for both selection of sites and selection of patients. A base weight was constructed by taking the reciprocal of a site’s or participant’s probability of selection into the sample, a two-stage design (sites at the regional level and individuals at the site level) and the base weights reflected probabilities of selection at each stage. Stratification was done to reduce sampling variation to account for stratification by geographical region during selection of sites. Clustering accounted for the two clustering units: the sites and the participants.
Analyses were done for the following study outcomes: prevalence of HIVDR among adults initiating first-line ART regardless of prior exposure to ARVs; prevalence of HIVDR among adults initiating first-line ART without prior exposure to ARVs; proportion of all ART initiators without prior exposure to ARVs; proportion of all ART initiators with prior exposure to ARVs; and proportion of all ART initiators with unknown exposure to ARVs.
Ethics
This study was approved by the UVRI Research and Ethics Committee [UVRI-REC, Federal Wide Assurance (FWA) No. 00001354] and the Uganda National Council for Science and Technology (FWA No. 00001293). The study was also reviewed in accordance with the CDC human research protection procedures and determined to be research, but CDC investigators did not interact with human subjects or have access to identifiable data or specimens for research purposes. All participants gave written informed consent prior to enrolment into the study.
Results
Participant characteristics
Between August and December 2016, a total of 491 participants who met the study eligibility criteria and consented to participate in the study were enrolled at 23 sites distributed across the country. Female participants totalled 302 (61.5%) and the median age was 32 years (IQR: 30–33). Most participants (93.9%) were initiated on tenofovir, lamivudine and efavirenz as their first-line ART regimen. The median CD4 T cell count at ART initiation was 228 cells/mm3 (IQR: 166–271). Most (95.1%) of the participants did not report prior exposure to ARVs. (Table 1).
Table 1.
Characteristics of HIV-positive adults initiating ART in 23 sites in Uganda in 2016
| Characteristics | Total n (%) |
|---|---|
| Sex | |
| Male | 189 (38.5) |
| Female | 302 (61.5) |
| Age (years), median (IQR) | 32 (30.0–33.0) |
| Region | |
| Central | 148 (30.1) |
| Eastern | 84 (17.1) |
| Kampala | 31 (6.3) |
| Northern | 84 (17.1) |
| Western | 144 (29.3) |
| CD4 count at ART initiation or reinitiation (cells/mm3), median (IQR) | 228 (166–271) |
| Regimen initiateda | |
| ZDV + 3TC + EFV | 11 (2.3) |
| ZDV + 3TC + NVP | 11 (2.3) |
| TDF + 3TC + EFV | 458 (93.9) |
| TDF + 3TC + NVP | 8 (1.6) |
| Past ARV Exposure | |
| Yes | 18 (3.7) |
| No | 467 (95.1) |
| Unknown | 6 (1.2) |
| Type of past ARV exposure | |
| ART | 15 (83.3) |
| PMTCT | 2 (11.1) |
| Unknown | 1 (5.6) |
ZDV, zidovudine; 3TC, lamivudine; EFV, efavirenz; NVP: nevirapine; TDF, tenofovir disoproxil fumarate.
Three patients transferred out before initiating ART.
Prevalence of PDR
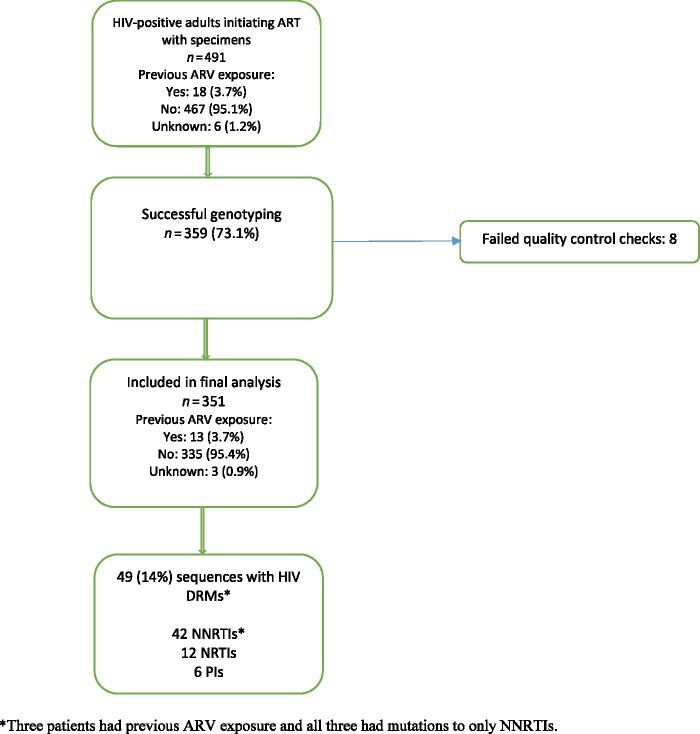
Three hundred and fifty-nine (73.1%) samples from the 491 participants were successfully genotyped and 351 (71.4%) passed the quality control checks. Data from the 351 participants were included in the analysis. HIV-1 subtypes identified included A (61%), C (2.8%), D (21.9%), G (2.6%) and unique recombinant forms (11.7%). Forty-nine (14.0%) of 351 participants had DRMs. (Figure 1).
Figure 1.
Enrolment profile of HIV-positive adults initiating ART from 23 sites in Uganda. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
The overall weighted prevalence of HIVDR among the 351 participants genotyped was 18.2% (95% CI: 11.3–25.2). The weighted prevalence of NNRTI, NRTI and PI HIV DRMs was 14.1%, 5.8% and 1.5%, respectively. The weighted prevalence of resistance to NNRTIs was comparable for both female (14.1%) and male (14.0%) participants. (Table 2).
Table 2.
Prevalence estimates of HIV DRMs among HIV-positive adults initiating ART in 23 sites in Uganda
| Survey outcome | n/N | Prevalence % (95% CI) |
|---|---|---|
| All participants | ||
| Any mutations | 49/351 | 18.2 (11.3–25.2) |
| NNRTI mutations | 42/351 | 14.1 (10.2–18.0) |
| NRTI mutations | 12/351 | 5.8 (0.0–12.6) |
| PI mutations | 6/351 | 1.5 (0.0–3.9) |
| NRTI + NNRTI mutations | 8/351 | 3.1 (0.5–5.7) |
| Female participants | ||
| Any mutations | 32/215 | 20.3 (9.7–30.9) |
| NNRTI mutations | 26/215 | 14.1 (8.5–19.7) |
| NRTI mutations | 11/215 | 7.9 (0.0–16.9) |
| PI mutations | 5/215 | 2.2 (0.0–3.0) |
| NRTI + NNRTI mutations | 7/215 | 3.7 (0.3–7.2) |
| Male participants | ||
| Any mutations | 17/136 | 14.1 (7.3–20.0) |
| NNRTI mutations | 16/136 | 14.0 (7.2–20.9) |
| NRTI mutations | 1/136 | 1.8 (0.0–5.1) |
| PI mutations | 1/136 | 0.1 (0.0–0.4) |
| NRTI + NNRTI mutations | 1/136 | 1.8 (0.0–5.1) |
| No ARV drug exposure | ||
| Any mutations | 46/335 | 18.0 (10.9–25.1) |
| NNRTI mutations | 39/335 | 13.7 (1.0–17.5) |
| NRTI mutations | 12/335 | 6.0 (0.0–12.9) |
| PI mutations | 6/335 | 1.5 (0.0–4.0) |
| NRTI + NNRTI mutations | 8/335 | 3.2 (0.5–5.8) |
| ARV drug exposure | ||
| Any mutations | 3/13 | 54.2 (29.9–78.6) |
| NNRTI mutations | 3/13 | 54.2 (29.9–78.6) |
| NRTI mutations | 0/13 | — |
| PI mutations | 0/13 | — |
| NRTI + NNRTI mutations | 0/13 | — |
All prevalences are weighted and accounted for two-stage clustered survey design.
The overall weighted HIVDR prevalence was not statistically different from 18.0% (95%CI: 10.9–25.1) among those without prior exposure but was statistically different from 54.2% (95% CI: 29.9–78.6) among those with prior exposure, as shown in Table 3.
Table 3.
Prevalence of past ARV exposure among HIV-positive adults initiating ART in 23 sites in Uganda in 2016
| Survey outcome | n/N | Prevalence % (95% CI) |
|---|---|---|
| ARV drug exposure | ||
| Yes | 18/491 | 2.3 (0.6–8.1) |
| No | 467/491 | 96.4 (90.8–98.6) |
| Unknown | 6/491 | 1.4 (0.3–6.0) |
| ARV drug exposure (female participants) | ||
| Yes | 11/302 | 2.8 (0.6–12.4) |
| No | 289/302 | 96.8 (88.0–99.2) |
| Unknown | 2/302 | 0.4 (0.0–2.8) |
| ARV drug exposure (male participants) | ||
| Yes | 7/189 | 1.2 (0.3–4.8) |
| No | 178/189 | 95.6 (84.7–98.8) |
| Unknown | 4/189 | 3.3 (0.5–17.6) |
All prevalences are weighted and account for two-stage clustered survey design.
Prevalence of prior ARV exposure
Eighteen of the 491 participants had prior ARV exposure (15 started ART and stopped; 2 had exposure via PMTCT; 1 had no reason provided).
The weighted prevalence of past ARV exposure was 2.3%. Although point prevalence of prior ARV exposure was higher for female participants compared with male participants, this was not statistically significant (Table 3).
Pattern of DRMs among adults initiating or reinitiating ART
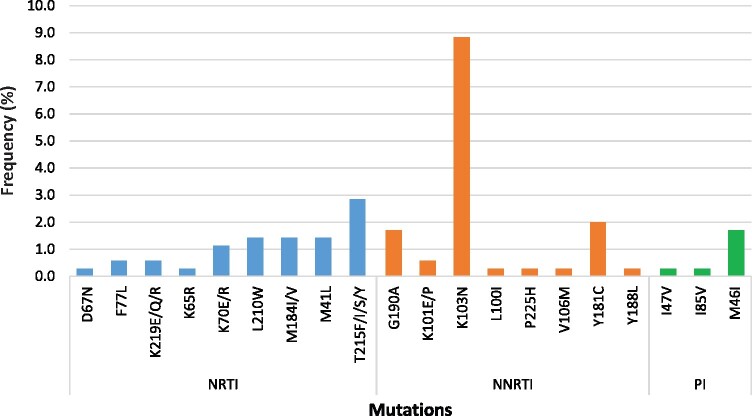
Of the 49 participants with mutations, 42 (11.9%) had mutations to NNRTIs, 12 (3.4%) had NRTI mutations, 6 (1.7%) had PI mutations and 8 (2.8%) had both NRTI and NNRTI mutations (Figure 1). None of the participants had mutations to all three (NRTI, NNRTI and PI) classes of ARVs. The most common NNRTI mutation was K103N, which was observed in 31 participants (8.8%), followed by Y181C in 7 participants (2.0%); NRTI mutations included M184V/I in 5 participants and TAMs in 10 participants, with M41L occurring in 5 of these participants (1.4%). The most common PI mutation was M46I/L, which was present in all six participants who had PI mutations. The K65R mutation was present in the specimen of one participant (Table 4 and Figure 2).
Table 4.
Distribution of HIV DRMs among 49 patients initiating ART who had HIVDR mutations detected
| Mutation | n (%) |
|---|---|
| NRTI | |
| D67N | 1 (0.3) |
| F77L | 2 (0.6) |
| K219E/Q/R | 2 (0.6) |
| K65R | 1 (0.3) |
| K70E/R | 4 (1.1) |
| L210W | 5 (1.4) |
| M184I/V | 5 (1.4) |
| M41L | 5 (1.4) |
| T215F/I/S/Y | 10 (2.8) |
| NNRTI | |
| G190A | 6 (1.7) |
| K101E/P | 2 (0.6) |
| K103N | 31 (8.8) |
| L100I | 1 (0.3) |
| P225H | 1 (0.3) |
| V106M | 1 (0.3) |
| Y181C | 7 (2.0) |
| Y188L | 1 (0.3) |
| PI | |
| I47V | 1 (0.3) |
| I85V | 1 (0.3) |
| M46I | 6 (1.7) |
Figure 2.
Distribution of HIV DRMs among 49 patients initiating ART who had HIVDR mutations detected. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Discussion
To the best of our knowledge, this cross-sectional survey of HIVDR among adults initiating ART in Uganda is the first study to generate population-level estimates of HIVDR prior to initiation of ART for Uganda. The overall HIVDR and NNRTI prevalence rates were 18% and 14%, respectively. This level is higher than the WHO threshold of 10% that is recommended for countries to consider changing from NNRTI- to dolutegravir-based first-line regimens. The level of NNRTI resistance is higher than that previously reported in LMICs, although one study conducted in Uganda at three large clinics between 2007 and 2009 found high (11.6%) levels of baseline HIVDR among ART-naive adult populations initiating ART.15 Furthermore, one TDR survey conducted in 2010 among young adults in Kampala reported a moderate level (8.6%) of HIVDR.25 Although none of the previous findings were representative of adults initiating ART in Uganda at the time they were performed, their findings could be explained by the early introduction of ART in the general population in those settings, as early as the mid-1990s, especially in some urban clinics.
Most of the PMTCT regimens used in the country have included NNRTIs and NRTIs. These classes of drugs have low genetic barriers to resistance, so that mothers and children exposed to these drugs (nevirapine and efavirenz) readily develop HIVDR. The higher prevalence of NNRTI mutations is consistent with the widespread use of this class of drugs as the backbone in first-line regimens, as well as the previous use of single-dose nevirapine for PMTCT. Similar findings of high prevalence of NNRTI PDR have recently been reported in other studies conducted at the national level in Cameroon and Namibia.9,10
The number of participants with prior ARV exposure was very small compared with those without prior exposure, making it difficult to make comparisons of HIVDR between the two groups.
There was low PI resistance in this population. This is reassuring, especially because PIs are currently used as an alternative in first-line ART regimens for children (when dolutegravir is contraindicated or appropriate formulations for specific age categories are unavailable). Moreover, we had observed a high prevalence of HIV DRMs (37.8%), particularly to NNRTIs among children aged less than 18 months recently diagnosed with HIV in a survey conducted in Uganda in 2012.26
Pooled data from 11 nationally representative surveys show that NNRTI PDR in women is nearly twice that in men.27 However, in our study, we did not observe any differences between women and men. This finding has also been reported in a multicentre observational study in sub-Saharan Africa.15 A possible explanation for this observation in our study could be the small number of participants (particularly women) who had prior ARV exposure.
The most frequently observed NNRTI mutations in our study were K103N/S, Y181C and M184V/I and TAMs for the NRTIs. The most frequent PI mutations included M46L/I. M46I/L are non-polymorphic PI-selected mutations that have been reported to reduce susceptibility to a number of drugs such as atazanavir, indinavir and lopinavir when present with other mutations.
This pattern of mutations is not different from what has been described among similar populations in Uganda.15,16 The high frequency of TAMs among participants with NRTI mutations could be a reflection of non-reported prior ARV exposure (especially zidovudine) and could compromise future treatment options for second-line and third-line ART regimens for individuals who have TAMs in Uganda.
Our results suggest that NNRTI (nevirapine/efavirenz)-based first-line regimens may no longer be optimal for treatment of HIV in adults in Uganda. As a result of this study, the consolidated guidelines for prevention and treatment of HIV and AIDS in Uganda have been revised, as recommended by WHO. With levels of PDR to NNRTI exceeding 10%, WHO recommends countries to consider changing the first-line ART regimen from an NNRTI to a dolutegravir backbone.28 In Uganda, dolutegravir has been introduced; this, in addition to the higher genetic barrier to resistance selection compared with NNRTIs,29 has other advantages of superior efficacy30 and tolerability.31 More than 700 000 individuals were receiving dolutegravir-based regimens, representing 57% of the total PLHIV currently on ART by the end of September 2020 in Uganda. As the rollout of dolutegravir continues, plans are underway to perform resistance testing for virally non-suppressed patients on dolutegravir who are identified through the national viral load programme. This will enable surveillance for emerging resistance through early detection of resistance to dolutegravir so that interventions are instituted to mitigate widespread of resistance to this drug.
Our survey had some limitations. There was a higher genotyping failure rate (about 29%) compared with the estimated 20% used in the sample size calculations, probably due to several reasons. Some of these are related to the use of DBS specimens as the primary specimen from all but one site, where we collected plasma. We used Munktell TFN (Munktell Inc., Raleigh, NC, USA) filter paper instead of the preferred Whatman 903 (Whatman, Springfield Mill, UK) filter paper because of availability and this could have reduced the amplification rate, as noted in one study that compared the amplification success for different filter papers.32 Processing of DBS specimens at peripheral health facilities was fraught with challenges, which the investigators had limited control over. Some of these could have included: the use of haemolysed blood and delays in DBS preparation from whole blood; very few specimens (3.2%) being processed immediately after collection into the EDTA vacutainer, as recommended; inadequate blood volume dispensed onto the card; incomplete drying of DBS spots; inappropriate packaging of dried DBS cards; and inadequate desiccant included in the package.
Transportation of the samples relied on the national specimen transportation network, which sometimes resulted in delays (of between 3 and 4 weeks instead of 2 weeks) from sample collection date to receipt in the genotyping laboratory. There was no system in place for monitoring temperatures during transportation, therefore high temperatures, especially during the hot seasons, could have caused RNA degradation, hence reducing genotyping success.
These factors affect specimen amplification and subsequently the HIVDR results. This leads to either overestimation of the HIVDR prevalence if the failed samples had no HIVDR, or underestimation if the failed samples had HIVDR.
Moreover, we did not perform viral load testing prior to genotyping to identify and exclude samples with viral loads below 1000 copies/mL. Such specimens were unlikely to be successfully genotyped.
We were unable to perform subanalyses to explain our findings because of the small number of samples with HIV DRMs. For example, one of the main outcomes was prevalence of any HIVDR, among those with and without prior ARV exposure, as well as a description of the mutations observed. However, the number of those with prior exposure was very small, limiting our ability to analyse separately and compare against those without prior exposure.
Conclusions
We observed a high level of HIVDR, particularly to the NNRTI drugs, among adults initiating ART in Uganda in 2016. This level was above the WHO threshold of 10% that is recommended for changing to dolutegravir-based first-line ART regimens. This recommendation has been adopted in Uganda. Resistance to PIs remains low, which is encouraging because this class of drugs is used as an alternative first-line ART regimen for children when dolutegravir is contraindicated, or age-appropriate formulations are unavailable, and also as a second-line ART regimen among adults when dolutegravir has been used in the first-line regimen.
Acknowledgements
We wish to thank the study participants and staff at the 23 HIV treatment centres who contributed the specimens and data for this study.
We thank staff at CPHL who received and temporarily stored samples from sites and later shipped them to the MRC/UVRI and LSHTM laboratory, where genotyping was performed.
An abstract based on this work was published in the proceedings of the Twenty-Sixth International Workshop on HIV Drug Resistance and Treatment Strategies conference, held in 2017.
Funding
This survey was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through CDC under the terms of a Cooperative Agreement, number 5U2GGH000785-02.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Transparency declarations
All authors have no conflicts of interest to declare.
Author contributions
Conceived and designed the study: C.W., P.K., J.A., D.S., W.K., J.M., K.M., G.N., A.N., E.R., C.M. and E.K.M. Collected data: C.W., J.A., D.S., G.S., I.S. and U.K. Conducted laboratory analysis: P.K., D.S., G.S., M.N., J.N. and J.F.S.G. Analysed the data: J.A., C.W., D.S., T.L. and U.K. Wrote the paper: C.W., P.K., J.A., D.S., G.N., A.N., T.L., E.R., W.K., K.M. and E.K.M. All authors reviewed, revised and approved the final paper.
References
- 1.WHO. WHO guidelines on the Public Health Response to Pretreatment HIV Drug Resistance. 2017. https://www.who.int/hiv/pub/guidelines/hivdr-guidelines-2017/en/.
- 2.Coffin JM. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science 1995; 267: 483–9. [DOI] [PubMed] [Google Scholar]
- 3.Nichols BE, Sigaloff KCE, Kityo C et al. Increasing the use of second-line therapy is a cost-effective approach to prevent the spread of drug-resistant HIV: a mathematical modelling study. J Int AIDS Soc 2014; 17: 19164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cambiano V, Bertagnolio S, Jordan MR et al. Predicted levels of HIV drug resistance: potential impact of expanding diagnosis, retention, and eligibility criteria for antiretroviral therapy initiation. AIDS 2014; 28 Suppl 1: S15–23. [DOI] [PubMed] [Google Scholar]
- 5.WHO. WHO HIV Drug Resistance Report. 2012. http://apps.who.int/iris/bitstream/10665/75183/1/9789241503938_eng.pdf.
- 6.WHO. Surveillance of HIV Drug Resistance in Adults Initiating Antiretroviral Therapy (Pre-Treatment HIV Drug Resistance). 2014. https://apps.who.int/iris/bitstream/handle/10665/112802/9789241507196_eng.pdf.
- 7.Mungati M, Mhangara M, Gonese E et al. Pre-treatment drug resistance among patients initiating antiretroviral therapy (ART) in Zimbabwe. BMC Res Notes 2016; 9: 2008–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung MH, Silverman R, Beck IA et al. Increasing HIV-1 pretreatment drug resistance among antiretroviral-naïve adults initiating treatment between 2006 and 2014 in Nairobi, Kenya. AIDS 2016; 30: 1680–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tchouwa GF, Eymard-Duvernay S, Cournil A et al. Prevalence of pretreatment HIV drug resistance in Cameroon following a nationally representative WHO survey. J Antimicrob Chemother 2018; 73: 2468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taffa N, Roscoe C, Sawadogo S et al. Pretreatment HIV drug resistance among adults initiating ART in Namibia. J Antimicrob Chemother 2018; 73: 3137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. HIV drug resistance report. 2019. https://www.who.int/hiv/pub/drugresistance/hivdr-report-2019/en/.
- 12.Ministry of Health. Policy for Reduction of Mother-To-Child HIV Transmission in Uganda. 2003. http://library.health.go.ug/publications/hivaids/policy-reduction-mother-child-hiv-transmission-uganda.
- 13.WHO. Guidelines on antiretroviral drugs for treating pregnant women and preventing HIV and recommendations on infant feeding. 2010. http://www.who.int/hiv/pub/mtct/PMTCTfactsheet/en/. [PubMed]
- 14.WHO. Use of Antiretroviral Drugs for Treating Pregnant women and Preventing HIV Infection in Infants. 2012. http://www.who.int/hiv/pub/mtct/programmatic_update2012/en/.
- 15.Hamers RL, Wallis CL, Kityo C et al. HIV-1 drug resistance in antiretroviral-naive individuals in sub-Saharan Africa after rollout of antiretroviral therapy: a multicentre observational study. Lancet Infect Dis 2011; 11: 750–9. [DOI] [PubMed] [Google Scholar]
- 16.Kaleebu P, Kirungi W, Watera C et al. Virological response and antiretroviral drug resistance emerging during antiretroviral therapy at three treatment centers in Uganda. PLoS One 2015; 10: e0145536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta RK, Gregson J, Parkin N et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis 2018; 18: 346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCluskey SM, Lee GQ, Kamelian K et al. Increasing prevalence of HIV pretreatment drug resistance in women but not men in rural Uganda during 2005-2013. AIDS Patient Care STDS 2018; 32: 257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ministry of Health. Consolidated Guidelines for Prevention and Treatment of HIV in Uganda. 2018. http://library.health.go.ug/publications/hivaids/consolidated-guidelines-prevention-and-treatment-hiv-uganda.
- 20.Grüner N, Stambouli O, Ross RS. Dried blood spots - preparing and processing for use in immunoassays and in molecular techniques. J Vis Exp 2015; 97: 52619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiyaga C, Sendagire H, Joseph E et al. Uganda’s new national laboratory sample transport system: a successful model for improving access to diagnostic services for early infant HIV diagnosis and other programs. PLoS One 2013; 8: e78609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parry CM, Parkin N, Diallo K et al. Field study of dried blood spot specimens for HIV-1 drug resistance genotyping. J Clin Microbiol 2014; 52: 2868–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woods CK, Brumme CJ, Liu TF et al. Automating HIV drug resistance genotyping with RECall, a freely accessible sequence analysis tool. J Clin Microbiol 2012; 50: 1936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis 2006; 42: 1608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ndembi N, Hamers RL, Sigaloff KCE et al. Transmitted antiretroviral drug resistance among newly HIV-1 diagnosed young individuals in Kampala. AIDS 2011; 25: 905–10. [DOI] [PubMed] [Google Scholar]
- 26.Jordan MR, Penazzato M, Cournil A et al. Human immunodeficiency virus (HIV) drug resistance in African infants and young children newly diagnosed with HIV: a multicountry analysis. Clin Infect Dis 2017; 65: 2018–25. [DOI] [PubMed] [Google Scholar]
- 27.Inzaule SC, Jordan MR, Cournil A et al. Increasing levels of pretreatment HIV drug resistance and safety concerns for dolutegravir use in women of reproductive age. AIDS 2019; 33: 1797–9. [DOI] [PubMed] [Google Scholar]
- 28.WHO. Updated Recommendations on First-Line and Second-Line Antiretroviral Regimens and Post-Exposure Prophylaxis and Recommendations on Early Infant Diagnosis of HIV-Interim Guidelines. 2018. https://www.who.int/publications/i/item/WHO-CDS-HIV-18.51.
- 29.Fernandez-Montero JV, Barreiro P, Labarga P et al. Dolutegravir, abacavir and lamivudine as HIV therapy. Expert Opin Pharmacother 2014; 15: 1051–7. [DOI] [PubMed] [Google Scholar]
- 30.Patel DA, Snedecor SJ, Tang WY et al. 48-Week efficacy and safety of dolutegravir relative to commonly used third agents in treatment-naive HIV-1-infected patients: a systematic review and network meta-analysis. PLoS One 2014; 9: e105653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walmsley SL, Antela A, Clumeck N et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369: 1807–18. [DOI] [PubMed] [Google Scholar]
- 32.Rottinghaus E, Bile E, Modukanele M et al. Comparison of Ahlstrom grade 226, Munktell TFN, and Whatman 903 filter papers for dried blood spot specimen collection and subsequent HIV-1 load and drug resistance genotyping analysis. J Clin Microbiol 2013; 51: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]