Abstract
The mammalian female is born with a limited ovarian reserve of primordial follicles. These primordial follicles are slowly activated throughout the reproductive lifecycle, thereby determining lifecycle length. Once primordial follicles are exhausted, women undergo menopause, which is associated with several metabolic perturbations and a higher mortality risk. Long before exhaustion of the reserve, females experience severe declines in fertility and health. As such, significant efforts have been made to unravel the mechanisms that promote ovarian aging and insufficiency. In this review, we explain how long-living murine models can provide insights in the regulation of ovarian aging. There is now overwhelming evidence that most life-span–extending strategies, and long-living mutant models simultaneously delay ovarian aging. Therefore, it appears that the same mechanisms that regulate somatic aging may also be modulating ovarian aging and germ cell exhaustion. We explore several potential contributing mechanisms including insulin resistance, inflammation, and DNA damage—all of which are hallmarks of cellular aging throughout the body including the ovary. These findings are in alignment with the disposable soma theory of aging, which dictates a trade-off between growth, reproduction, and DNA repair. Therefore, delaying ovarian aging will not only increase the fertility window of middle age females, but may also actively prevent menopausal-related decline in systemic health parameters, compressing the period of morbidity in mid-to-late life in females.
Keywords: Calorie restriction, Fertility, Follicle, IGF, Menopause
Aging is the progressive decline of cellular and molecular functions throughout the body thereby impeding the maintenance of homeostasis. These declines promote the onset of several chronic diseases in parallel leading to multimorbidity and frailty (1). In the female, aging is also associated with the decline of ovarian function, which will be the main focus of the current review. This decline in ovarian function is linked to reduced fertility and increased incidence of chronic diseases in older women. The field of Geroscience aims to understand the molecular pathways that promote aging so that therapeutic approaches that target these pathways can be developed. Several interventional strategies have successfully extended life span in rodents. Among these, calorie restriction (CR) is the most commonly studied. Several studies have demonstrated that subjecting mice to 20%–40% CR slows the aging process, resulting in increased life span and healthspan compared as to littermate controls (2). Treatment with rapamycin (3), metformin (4), and nicotinamide adenine dinucleotide (NAD+) (5) has also been found to increase life span. Additionally, a reduction in growth hormone (GH) production and/or signaling have also been shown to significantly attenuate biological aging and extend life span (6,7).
Interestingly, several trials have revealed that the rate of aging and responsiveness to a myriad of interventional strategies differs between sexes, although the mechanisms responsible remain unclear (8). Therefore, there is a clear need to unravel the mechanisms that promote these sexually divergent outcomes so that its implication on gonadal aging can be elucidated. In the female, aging is associated with the decrease of the ovarian follicular reserve. Females are born with a lifelong supply of oocytes, therefore, the term “ovarian reserve” reflects the number of oocytes enclosed in the ovarian tissue and, therefore, the functional reproductive potential of the ovary (9). The ovarian reserve is represented mainly by the primordial follicles, an oocyte surrounded by a single layer of granulosa cells, which are in a quiescent state (10). Once activated, the primordial follicles start developing in an irreversible process and may undergo ovulation or become atretic. The exhaustion of the ovarian reserve will result in diminished ovarian activity, characterized by reduction of estradiol output and cessation of ovarian cycles, also known as menopause in humans (9). Menopause is associated with metabolic declines that increase susceptibility to chronic diseases (11). Furthermore, increased age at menopause is linked to reduced mortality and increased life span in women (12), thereby confirming the importance of the ovary in regulating aging. Therefore, this review will focus on pathways that are affected by life-span–extending therapies and what role they play in the ovarian life span.
Ovarian Reserve Exhaustion and Activation of Primordial Follicles
The number of primordial follicles gradually decreases as females age. In C57BL/6 mice, the ovarian reserve is reduced to half at 10 months of age and at 18 months of age the reserve is reduced approximately 10 times (13). Additionally, female C57BL/6 mice will stop producing pups at around 18 months of age, while significant fertility reduction is already observed at 10 months of age (14). These data indicate that, although a clear menopausal transition is not observed in mice, there is a severe reduction in the ovarian reserve followed by reduced fertility and reduced fidelity of reproductive cycles, similar to what is observed in humans (9). Therefore, mice serve as a relevant model for testing therapies that may prolong the reproductive life span and impact overall longevity and healthy aging.
Activation of the primordial follicles from the ovarian reserve represents a tightly regulated process. Activation of the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway and the phosphorylation of forkhead box protein O 3a (FoxO3a) are essential steps in the activation of the primordial follicles in ovaries (15). Hyperphosphorylation of FOXO3a in oocytes results in its nuclear exclusion, culminating in the global activation of primordial follicles and premature ovarian failure (15). Therefore, the presence of FOXO3a in its non-phosphorylated form is crucial to maintaining the primordial follicles in their quiescent state. Interestingly, this process depends on the regulation of pathways in granulosa cells and oocytes. Activation of dormant oocytes through the PI3K/Akt/FOXO3a signaling in oocytes depends on granulosa KIT ligand production (16). One of the regulators of granulosa cell KIT ligand (KITL) production is the mechanistic target of rapamycin complex 1 (mTORC1) (16). Therefore, granulosa cells mTORC1 activation promotes KITL production, which in the oocyte activates KIT and PI3K/Akt signaling and FOXO3a phosphorylation, activating the primordial follicle irreversibly (16). Knockout of tuberous sclerosis 1 (TSC1), an inhibitor of mTORC1 signaling, in primordial follicles granulosa cells resulted in global follicular activation and premature ovarian failure (16). Similarly, FOXO3a knockout results in primordial follicle hyperactivation and premature exhaustion of the ovarian reserve (15). Therefore, it becomes clear that mTOR and FOXO3a pathways act in concert as main regulators of primordial follicle activation and ovarian aging.
The Aging/Reproduction Trade-off
The Reproductive Cell Cycle Theory of Aging states that the hormones that regulate reproduction act in an antagonistic pleiotropic manner to control aging via cell cycle signaling (17). Therefore, this theory proposes that promoting growth and development early in life to achieve reproduction results in increased senescence and age-related diseases later in life. Reproduction is critical for the survival of the species, therefore reproductive cell cycle signaling factors will also regulate growth, development, fertility, and senescence, ultimately regulating the rate of aging and life span. Many pathways can be implicated in this trade-off. However, existing evidence indicates that FOXO3a and mTOR are central to this trade-off. The role of FOXO3a and mTOR in the regulation of the ovarian reserve is critical as explained in the previous section. In the same way, these pathways are critical regulators of overall body growth and aging (18).
The FOXO3a protein is regulated by the insulin/PI3K/Akt signaling pathway (19). FOXO members are linked to multiple physiological and pathological processes by inducing transcription of target genes involved in apoptosis, proliferation, cell cycle progression, survival, and DNA damage (18). FOXO3a signaling is associated with longevity in several species (18). Similarly, the mTOR pathways is a central regulator of complex biological functions, including cell growth, proliferation, and survival in response to nutrients and hormones (20). Downstream effectors of mTOR include mTORC1 and mTORC2 (21). mTORC1 disruption increased longevity in mice (22), as well as pharmacological inhibition of mTORC1 by rapamycin decreases body weight gain and increases longevity in mice (23). Therefore, both mTOR and FOXO3a are regulated by nutrient-sensing pathways and can result in reduced cell growth and increased longevity. At the same time, overactivation of these pathways will result in premature exhaustion of the ovarian reserve and inability to reproduce in adulthood (15,16).
To exemplify this trade-off, lower GH/IGF levels correlate with increased life span and delayed sexual maturation in different strains of mice, which indicates the existence of a trade-off between growth rate, sexual maturation, and longevity (24). GH-deficient Ames dwarf mice (df/df) live longer than normal littermates, are smaller, have delayed puberty, and have a prolonged reproductive life span (25,26). This model clearly displays the trade-off between longevity and reproduction. It is important to emphasize that while reproducing for a longer period, mice receiving life-extending interventions have reduced number of offspring, as observed for CR mice (14). This further reflects the evolutionary trade-off that diverges energy from reproductive efficiency to prioritize self-preservation.
The Effect of Life-Extending Interventions on Reproductive Life Span
Caloric Restriction and Mimetics
Caloric restriction is one of the first and most studied life-extending therapies. It can be defined as a reduction in caloric intake (27), usually between 20% and 40% (without malnutrition), compared to ad libitum controls (28). CR can increase longevity by up to 50% in different species (2). Mice subjected to CR have a significant increase in insulin sensitivity, decrease in body fat, and reduced systemic inflammation (29). CR reduces liver IGF-I secretion (30), therefore several beneficial actions of CR are also observed in GH (31) and GH receptor (GHR) (32) deficient mice. At the cellular level, the life-extending effects of CR are dependent on FOXO3 and decrease mTOR activation (33,34). Nevertheless, it is believed that the collective effects of CR in several pathways, including inflammation and oxidative stress-related pathways, exerts positive effects on longevity (35).
Due to limitations in the use of CR for an extended period to increase longevity, the search for CR mimetics that benefit longevity through similar pathways has been pursued (29). In this sense, rapamycin is an immunosuppressive drug (21), used in clinical practice for organ transplant recipients. Rapamycin is recognized as a promising mimetic of CR (3). When provided continuously, it promotes an increase in longevity similar to that observed for CR mice (36). Rapamycin has also been shown to have wide-spread anti-inflammatory effects (37), even in the ovary (38). Long-term rapamycin treatment resulted in reduced body weight and body fat despite an increase in insulin resistance (23). Although CR and rapamycin produce similar effects in longevity, the specific mechanisms of action may diverge between the 2, especially regarding insulin sensitivity (36).
In a previous study by our group, we found that young female mice exposed to CR (30%) and rapamycin (4 mg/kg of weight) for a 3-month period maintained twice as many primordial follicles in the ovarian reserve compared to control mice (39). In contrast to primordial follicles, the number of primary follicles was reduced, although there was no difference in the number of total follicles, suggesting that the transition from primordial to primary follicles was reduced (39). In this study, both CR and rapamycin had similar effects on ovarian function, but dissimilar systemic effects. CR promoted reduced body weight and fat, and increased insulin sensitivity, while rapamycin treatment did not influence body weight or fat content and promoted insulin resistance. This suggests a mechanism of action that is independent of insulin signaling and most likely dependent on mTORC1. Our results indicate that mice subjected to CR and rapamycin exhibit higher ovarian expression of FOXO3a in primordial follicles compared to control females (39).
The beneficial effect of CR on ovarian reserve preservation is well established in the literature. In young mice and rats submitted to CR (30%–40%), the number of quiescent primordial follicles increased 2-fold, compared to ad libitum controls (14,40). Interestingly, CR mice remained fertile for longer and had a higher pregnancy rate and litter size compared to age-matched control mice receiving an ad libitum diet (40). Similarly, young and old female mice and rats treated with rapamycin at 2–5 mg/kg maintained twice the number of primordial follicles compared to controls, and produced twice as many pups (38). Additionally, chemotherapy can result in primordial follicle activation and premature ovarian failure (41). In this sense, both CR and rapamycin protected ovaries of mice submitted to chemotherapy from premature follicle exhaustion (42,43). Furthermore, the simultaneous use of mTOR and Akt activators promote follicle growth and exhaustion of the follicular reserve (44).
As mentioned before, activation of the mTORC1 in granulosa cells promotes FOXO3a phosphorylation in oocytes to stimulate the awakening of primordial follicles (16). However, there is still much to be studied about this interaction. The complex mechanisms by which rapamycin and CR act beneficially in the ovary are not yet fully understood. The evidence presented here suggest that the mTORC1 pathway may be an important mediator of the beneficial effects of CR and rapamycin at the ovarian level.
Growth Hormone
The GH/IGF-I axis is crucial to determine longevity in mice. GH-deficient df/df mice are known for living 35%–75% longer than normal littermates due to their recessive homeobox protein prophet of Pit-1(Prop1) loss-of-function mutation that results in deficient GH, thyroid-stimulating hormone, and prolactin secretion (6). In addition, GHRKO mice are long-lived and share several metabolic phenotypes with df/df mice which are likely responsible for their extended longevity (7). In contrast, transgenic mice overexpressing bovine GH (bGH), have high plasma GH levels, increased serum IGF-I levels, and a life span about 50% shorter than normal mice (45). Interestingly, submitting GHRKO mice to 30% CR does not benefit insulin sensitivity and longevity, suggesting similar mechanisms of action (32). Importantly, treatment with GH in early life shortens the life span of long-living df/df mice (46). This suggests that GH exposure affects developmental programming of aging producing persistent changes through adult life. Conversely, GHR disruption in adult mice did not improve median life span, with only a modest increase in maximal life span of females (47).
It is well established in reproductive biology studies that the GH/IGF-I axis is important for normal ovary function, especially the later stages of follicle development before ovulation (48). df/df mice are unable to maintain pregnancy without prolactin treatment, while GHRKO mice have decreased pregnancy rate and number of pups/litter (49). However, several studies from our group showed that GH can also modulate the initial stages of follicle development and, therefore, ovarian aging. Long-lived df/df mice have almost 4 times more primordial follicles at 6 months (50), 3 times more at 12 months (51), and twice as many at 18 months of age compared to normal littermates (26). This suggests that the difference in the ovarian primordial reserve between df/df and normal mice counterparts is larger at the beginning of life and decreases with age, as the reserve gradually becomes smaller in both genotypes. In addition, the treatment with exogenous GH for a short period increased primordial follicle activation and reduced the ovarian reserve in df/df and normal mice (26). Similar to df/df mice, GHRKO mice have a larger ovarian reserve than normal mice (52,53) and the treatment of GHRKO mice with IGF-I decreased the primordial follicle reserve (53). bGH mice, otherwise, had accelerated ovarian aging and a decreased ovarian primordial follicle reserve (26). Overall, this indicates that the size of the ovarian primordial follicle reserve can be a direct reflection of the circulating levels of GH/IGF-I, indicating a central role of the GH/IGF-I axis to regulate primordial follicle recruitment and ovarian aging. More importantly, these findings indicate strong association between GH/IGF-I, ovarian reserve, and life span. In the clinical setting, the use of GH adjunct therapy in women is controversial. Although it increases the number of oocytes retrieved in poor responders, it did not improve the number of live births (54).
The expression of FOXO3a mRNA was 10-fold higher in the ovaries of 20-month-old df/df mice when comparing to normal littermates (55). At the protein level, FOXO3a is also increased in oocytes from primordial follicles of df/df mice compared to normal littermates (26). GH treatment reduced the FOXO3a protein level, while it increased its phosphorylated form in primordial follicles oocytes (26). Oocytes from primordial/primary follicles of df/df mice also had a lower level of p-FOXO3 protein compared to normal littermates (55). Therefore, suggesting that higher FOXO3a levels can help maintain follicles in the primordial stage. In the ovaries of young GHRKO mice, we also observed a tendency for increased expression of FOXO3a (56). In contrast with df/df and GHRKO models, bGH mice had higher level of pFOXO3a protein in primary follicle oocytes compared to normal littermates, which could be related to the higher rate of primordial follicle activation observed in bGH mice (26). Therefore, this suggests that GH/IGF-I dependent FOXO3a activation could be a key pathway for primordial follicle activation. This suggests that this pathway can have a central role in the regulation of the trade-off between somatic and reproductive aging.
We previously discussed that GH-deficient long-lived mice maintain a larger ovarian reserve, even at older ages. Besides quantity, several factors can influence oocyte quality during aging. Mammalian oocytes enclosed in primordial follicles remain arrested in prophase I of meiosis up to several decades in women (57,58). This long dormancy period increases the chances of accumulating DNA damage, as shown in mice and humans, where DNA double-strand breaks accumulate in primordial follicle oocytes with aging (59). At 6 months of age, df/df mice had less DNA damage in oocytes and granulosa cells of primordial and primary follicles compared to normal littermates (50). The GH deficiency not only preserves the quantity, but also the quality of the remaining follicles and oocytes in the reserve. Therefore, along with FOXO3a activation, reduced DNA double-strand breaks in primordial follicle oocytes can be a mechanism of increased preservation of the ovarian reserve in long-living GH-deficient mice. Overall, these important observations confirm that the delayed aging phenotype in these unique long-living mice is strongly associated with the beneficial characteristics of longevity in the female reproductive organs.
Other Life-Extending Therapies
Although CR and GH deficiency are the most studied life-extending therapies, several other therapies have been successfully tested. Among these, another drug that effectively increases life span is metformin (4,60). In humans, clinical trials regarding antiaging effects of metformin in healthy individuals are being developed. A decrease in the mortality rate of diabetic patients treated with metformin was showed, regardless of the effects on diabetes control (61). Metformin’s mechanisms of action are similar to those of CR, such as decreased insulin and IGF-I levels (60), and therefore it is mainly used for the treatment of type 2 diabetes mellitus (62). Metformin promotes reduction of blood glucose concentrations by decreasing intestinal glucose absorption, improving peripheral glucose uptake, lowering fasting plasma insulin levels, and increasing insulin sensitivity (63). It also inhibits the mTOR pathway (64), and reduces the production of reactive oxygen species (65), thereby reducing DNA damage (60). As the mechanism of action is similar to CR, similar effects on the ovarian reserve should be expected. In fact, this has recently been proved true. Metformin administration decreased primordial follicle activation in mice (66). Also, metformin has been reported to improve estrous cycle and hormonal profile in rodents, specially by decreasing testosterone and FSH/LH ratio (67). Even though it did not influence estradiol serum levels, metformin suppressed irregular estrous cycles, usually observed with aging (68). Metformin is also used in the treatment of polycystic ovary syndrome (PCOS), restoring ovulation and decreasing androgenesis (67,69). Overall, decades worth of studies in animals and humans showed that metformin has a significant impact on female reproductive organs. In addition to treating PCOS and improving insulin resistance in type 2 diabetes patients, metformin also delayed reproductive senescence, improved fertility rate, and increased longevity in animal studies.
Another pharmacological intervention able to increase life span that has been intensively studied is the enhancement of the NAD+, through supplementation with its metabolic precursor nicotinamide mononucleotide (NMN). NAD+ is an important redox cofactor and enzyme substrate, involved on the maintenance of epigenetic homeostasis, since it serves as a required substrate for the deacetylase activity of sirtuin (70). Increased NAD+ levels lead to increased activation of sirtuins, thereby decreasing the aging process (5). NAD+ levels naturally decrease with age in somatic tissues, including the ovary (71). Recently, it was demonstrated that NAD+ levels in ovulated oocytes also decrease with age, and supplementation with NMN restored these levels in oocytes of aged mice. Oocytes from NAD+ supplemented females also, had better rates of in vitro maturation and embryo production, and greater pregnancy and birth rates in aged animals (72). However, although these data suggest a positive impact of NMN on the ovarian reserve, no direct measurement of the effects of NMN supplementation on primordial follicles has been performed.
17α-Estradiol (17αE2) is another drug that has been shown to increase life span of male mice (73). 17α-E2 is considered a non-feminizing estrogen due to its minimal activation of classical estrogen receptors (74). Treatment with 17α-E2 reduces energy intake due to activation of hypothalamic anorexigenic pathways (75), resulting in reduced body mass, visceral adiposity, and ectopic lipid deposition (76), similarly to CR. However, these effects are stronger in males, while in females, 17α-E2 has little or no effect (75). As discussed throughout this review, most drugs that contribute to a longer life span also result in a slower exhaustion of ovarian reserve. Interestingly, 17α-E2 appears to have no effect on the ovarian reserve of normal mice, while in the long-lived GHRKO mice, it decreased the number of primordial follicles (52). These results are interesting and show that drugs proven to be more effective in males, have no effect in the ovarian aging of females. This also demonstrates that in females, the positive effects of many life-span–extending therapies are closely linked with the preservation of the ovarian reserve.
Other Determinants of the Ovarian Reserve
Obesity/High-Fat Diets and Aging
As mentioned previously, CR results in extended longevity. In contrast, obesity reduces life span among human populations (77). Leptin deficiency, due to a mutation in the ob gene, results in severe obese phenotype (78). The ob/ob mice have severe obesity and insulin resistance, among other comorbidities (79). The life span of ob mice is also compromised, averaging 18 months for obese mice and 27 months for normal lean littermates (80). While life-prolonging therapies such as CR (29), rapamycin (37), and GH deficiency (6,7) lead to a diminished inflammatory state, the obese phenotype is associated with a greater secretion of proinflammatory cytokines (81). These proinflammatory cytokines are elevated in adipose tissue and in systemic circulation during obesity (82).
The ob mice are infertile and have a lower gonadal weight in both males and females (83). This condition is mainly associated with the lack of leptin action at the hypothalamic level, decreasing the release of gonadotropins (84) and impairing final follicle growth (84) and ovulation (85). As a consequence, ob mice have a reduced number of corpora luteum and increased follicular atresia (86). However, fertility can be restored when exogenous leptin is supplemented (84) or when females are transplanted with adipose tissue from normal littermates (87). Although gonadotropin independent, the early stages of follicle development are also impaired in the ob mice. Studies have shown a decreased number of primordial and total follicles in ob mice (83) and that exogenous leptin treatment rescued the number of primordial follicles (84). This suggests that leptin is involved in the survival of primordial follicles, as ob mice have an increased rate of granulosa cell apoptosis in preantral follicles (83). Interestingly, prolonged exposure to a high-fat diet in young mice also caused a significant reduction in the total number of primordial follicles, even though these mice did not develop obesity (88). These effects seem to be mediated trough increased activation of the mTOR pathway and suppression of sirtuin signaling (89). Additionally, the high-fat diet was associated with increased systemic proinflammatory cytokines and macrophage infiltration in the ovary, resulting in impaired fertility (88).
In addition to impaired ovulation and reduced ovarian reserve, deformed oocytes with irregular granulosa cell layers are observed in preantral and antral follicles from ob mice (90). Increased oxidative stress is observed in ob mice and impairs mitochondrial function and distribution in oocytes, resulting in apoptosis (91). Obesity, induced by a high-fat diets or ob gene mutation, is also associated with oocyte epigenetic modification, disruption of meiotic spindle formation, and reduced oocyte polarity, resulting in reduced oocyte maturation and germinal vesicle breakdown (91). Exposure of mice to a high-fat diet for 4 weeks results in increased lipid accumulation in oocytes and in cumulus cells (92), and a 6-week exposure had irreversible effects on oocyte quality (93). Overall, this evidence indicates that obesity in mice accelerates several negative aspects of ovarian aging, presenting effects opposite to those observed in CR and GH-deficient mice.
Intrauterine Origins of the Ovarian Reserve
The primordial ovarian reserve is formed in utero in rodents. Primordial germ cells colonize the gonads at around 10 days of gestation in mice, forming cysts that cease mitosis and enter meiosis I around day 14 of gestation (94). Just after birth, the cysts break down and form the primordial follicle pool (94). Transcription factors play crucial roles in this process, such as factor in the germline alpha (FIGLA), which is responsible for coordinating the expression of oocyte-specific zona pellucida genes (94) and the newborn ovary homeobox (NOBOX), essential for the survival of the oocyte and assembly of the primordial follicle (94). Increased expression of FIGLA was correlated with loss of primordial follicles and premature ovarian failure in women (95).
Exposure to a stimulus or stress during the prenatal period can result in long-term adaptations of the physiological functions of the offspring, a process called fetal programming (96). In addition, protein-caloric malnutrition during pregnancy can result in delayed fetal development and obesity in adult rats and mouse offspring (97). There is also evidence that reproductive maturation is influenced by early life events. Fewer primordial follicles are observed in the ovaries of offspring from mothers subjected to CR during gestation (98). Additionally, CR offspring display increased follicular apoptosis at later stages of follicle development (98). In humans, data from the Dutch hunger cohort showed that women exposed to prenatal hunger were more likely to start menopause at an earlier age (99). Despite this, exposure to hunger had no effect on the age of menarche, the proportion of women without children, the age of first birth, or the size of the family (100).
Concluding Remarks
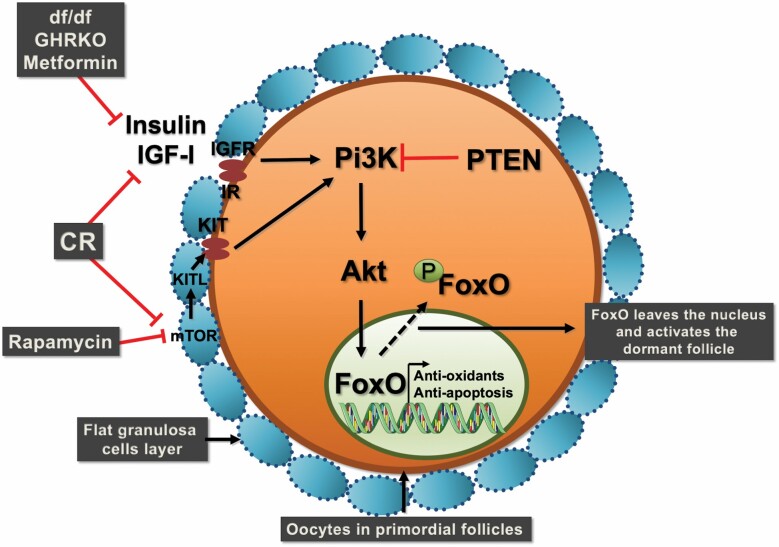
Based on the evidence presented in this review, we can conclude that there is overwhelming data suggesting that in females increased longevity is associated with delayed ovarian aging. Several animal models of extended female longevity also display increased numbers of primordial follicles in the ovaries. Activation of mTOR and FOXO3a pathways also appear as important links between somatic and gonadal aging (Figure 1). Overwhelming evidence supports the observation that the most successful life-extending therapies for females also reduce ovarian aging, suggesting an important active role for the ovary in the life span and healthspan of females.
Figure 1.
Schematic representation of pathways that regulate primordial follicle activation, which are simultaneously regulated by life-extending therapies. Calorie restriction (CR) and rapamycin inhibit the mTOR pathway in granulosa cells, which in turns regulates oocyte activation of the Pi3K/Akt/FOXO3a pathway. FOXO3a phosphorylation results in its nuclear extrusion and activation of the primordial follicle. Reduced IGF-I and insulin signaling in metformin-treated mice, GH-deficient Ames dwarf, and GH receptor knockout (GHRKO) mice also contribute to reduced Pi3K/Akt activation and reduced FOXO3a phosphorylation, maintaining primordial follicle in the quiescent stage.
Funding
This work was supported by Científico e Tecnológico (A.S.), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (A.S.), Fundação de Amparo à pesquisa do Estado do RS (A.S.), and the National Institutes of Health (R01 AG069742 to M.B.S., R56 AG061414, R03 AG059846, and R15 AG059190 to M.M.M.).
Conflict of Interest
None declared.
References
- 1.De la Fuente M. Role of the immune system in aging. Inmunología. 2008;27(4):176–191. doi: 10.1016/s0213-9626(08)70066-0 [DOI] [Google Scholar]
- 2.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012 [DOI] [PubMed] [Google Scholar]
- 3.Powers RW 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soukas AA, Hao H, Wu L. Metformin as anti-aging therapy: is it for everyone? Trends Endocrinol Metab. 2019;30:745–755. doi: 10.1016/j.tem.2019.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD(+) and sirtuin-activating compounds. Nat Rev Mol Cell Biol. 2016;17(11):679–690. doi: 10.1038/nrm.2016.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0 [DOI] [PubMed] [Google Scholar]
- 7.Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374 [DOI] [PubMed] [Google Scholar]
- 8.Austad SN, Bartke A. Sex differences in longevity and in responses to anti-aging interventions: a mini-review. Gerontology. 2015;62:40–46. doi: 10.1159/000381472 [DOI] [PubMed] [Google Scholar]
- 9.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465–493. doi: 10.1210/er.2009-0006 [DOI] [PubMed] [Google Scholar]
- 10.te Velde ER, Scheffer GJ, Dorland M, Broekmans FJ, Fauser BC. Developmental and endocrine aspects of normal ovarian aging. Mol Cell Endocrinol. 1998;145(1–2):67–73. doi: 10.1016/s0303-7207(98)00171-3 [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Jones M, Mishra GD. Age at natural menopause and development of chronic conditions and multimorbidity: results from an Australian prospective cohort. Hum Reprod. 2020;35:203–211. doi: 10.1093/humrep/dez259 [DOI] [PubMed] [Google Scholar]
- 12.Ossewaarde ME, Bots ML, Verbeek AL, et al. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology. 2005;16:556–562. doi: 10.1097/01.ede.0000165392.35273.d4 [DOI] [PubMed] [Google Scholar]
- 13.Kevenaar ME, Meerasahib MF, Kramer P, et al. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology. 2006;147:3228–3234. doi: 10.1210/en.2005-1588 [DOI] [PubMed] [Google Scholar]
- 14.Selesniemi K, Lee HJ, Tilly JL. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging Cell. 2008;7:622–629. doi: 10.1111/j.1474-9726.2008.00409.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336 [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Risal S, Gorre N, et al. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr Biol. 2014;24:2501–2508. doi: 10.1016/j.cub.2014.09.023 [DOI] [PubMed] [Google Scholar]
- 17.Atwood CS, Bowen RL. The reproductive-cell cycle theory of aging: an update. Exp Gerontol. 2011;46:100–107. doi: 10.1016/j.exger.2010.09.007 [DOI] [PubMed] [Google Scholar]
- 18.Martins R, Lithgow GJ, Link W. Long live FOXO: unraveling the role of FOXO proteins in aging and longevity. Aging Cell. 2016;15:196–207. doi: 10.1111/acel.12427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science. 2002;298:830–834. doi: 10.1126/science.1074240 [DOI] [PubMed] [Google Scholar]
- 20.Caron E, Ghosh S, Matsuoka Y, et al. A comprehensive map of the mTOR signaling network. Mol Syst Biol. 2010;6:453. doi: 10.1038/msb.2010.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016 [DOI] [PubMed] [Google Scholar]
- 22.Lamming DW, Ye L, Katajisto P, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang Y, Westbrook R, Hill C, et al. Duration of rapamycin treatment has differential effects on metabolism in mice. Cell Metab. 2013;17:456–462. doi: 10.1016/j.cmet.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan R, Meng Q, Nautiyal J, et al. Genetic coregulation of age of female sexual maturation and lifespan through circulating IGF1 among inbred mouse strains. Proc Natl Acad Sci USA. 2012;109:8224–8229. doi: 10.1073/pnas.1121113109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartke A, Brown-Borg H, Mattison J, Kinney B, Hauck S, Wright C. Prolonged longevity of hypopituitary dwarf mice. Exp Gerontol. 2001;36:21–28. doi: 10.1016/s0531-5565(00)00205-9 [DOI] [PubMed] [Google Scholar]
- 26.Saccon TD, Moreira F, Cruz LA, et al. Ovarian aging and the activation of the primordial follicle reserve in the long-lived Ames dwarf and the short-lived bGH transgenic mice. Mol Cell Endocrinol. 2017;455:23–32. doi: 10.1016/j.mce.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genaro P, Sarkis K, Martini L. O efeito da restrição calórica na longevidade. Arq Bras Endocrinol Metab. 2009;53(5):667–672. doi: 10.1590/S0004-27302009000500019 [DOI] [PubMed] [Google Scholar]
- 28.Cantó C, Auwerx J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol Metab. 2009;20:325–331. doi: 10.1016/j.tem.2009.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikolai S, Pallauf K, Huebbe P, Rimbach G. Energy restriction and potential energy restriction mimetics. Nutr Res Rev. 2015;28:100–120. doi: 10.1017/S0954422415000062 [DOI] [PubMed] [Google Scholar]
- 30.Dunn SE, Kari FW, French J, et al. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 1997;57:4667–4672. [PubMed] [Google Scholar]
- 31.Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the lifespan of long-lived mice. Nature. 2001;414:412. doi: 10.1038/35106646 [DOI] [PubMed] [Google Scholar]
- 32.Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci USA. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimokawa I, Komatsu T, Hayashi N, et al. The life-extending effect of dietary restriction requires Foxo3 in mice. Aging Cell. 2015;14:707–709. doi: 10.1111/acel.12340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unnikrishnan A, Kurup K, Salmon AB, Richardson A. Is rapamycin a dietary restriction mimetic? J Gerontol A Biol Sci Med Sci. 2020;75:4–13. doi: 10.1093/gerona/glz060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Araki K, Ellebedy AH, Ahmed R. TOR in the immune system. Curr Opin Cell Biol. 2011;23:707–715. doi: 10.1016/j.ceb.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dou X, Sun Y, Li J, et al. Short-term rapamycin treatment increases ovarian lifespan in young and middle-aged female mice. Aging Cell. 2017;16:825–836. doi: 10.1111/acel.12617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia DN, Saccon TD, Pradiee J, et al. Effect of caloric restriction and rapamycin on ovarian aging in mice. Geroscience. 2019;41:395–408. doi: 10.1007/s11357-019-00087-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L, Fu YC, Xu JJ, et al. Caloric restriction promotes the reserve of follicle pool in adult female rats by inhibiting the activation of mammalian target of rapamycin signaling. Reprod Sci. 2015;22:60–67. doi: 10.1177/1933719114542016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalich-Philosoph L, Roness H, Carmely A, et al. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5:185ra62. doi: 10.1126/scitranslmed.3005402 [DOI] [PubMed] [Google Scholar]
- 42.Zhou L, Xie Y, Li S, et al. Rapamycin prevents cyclophosphamide-induced over-activation of primordial follicle pool through PI3K/Akt/mTOR signaling pathway in vivo. J Ovarian Res. 2017;10:56. doi: 10.1186/s13048-017-0350-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiang Y, Xu J, Li L, et al. Calorie restriction increases primordial follicle reserve in mature female chemotherapy-treated rats. Gene. 2012;493:77–82. doi: 10.1016/j.gene.2011.11.019 [DOI] [PubMed] [Google Scholar]
- 44.Cheng Y, Kim J, Li XX, Hsueh AJ. Promotion of ovarian follicle growth following mTOR activation: synergistic effects of AKT stimulators. PLoS ONE. 2015;10(2):e0117769. doi: 10.1371/journal.pone.0117769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartke A. Can growth hormone (GH) accelerate aging? Evidence from GH-transgenic mice. Neuroendocrinology. 2003;78:210–216. doi: 10.1159/000073704 [DOI] [PubMed] [Google Scholar]
- 46.Sun LY, Fang Y, Patki A, et al. Longevity is impacted by growth hormone action during early postnatal period. Elife. 2017;6:e24059. doi: 10.7554/eLife.24059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Junnila RK, Duran-Ortiz S, Suer O, et al. Disruption of the GH receptor gene in adult mice increases maximal lifespan in females. Endocrinology. 2016;157:4502–4513. doi: 10.1210/en.2016-1649 [DOI] [PubMed] [Google Scholar]
- 48.Chandrashekar V, Zaczek D, Bartke A. The consequences of altered somatotropic system on reproduction. Biol Reprod. 2004;71:17–27. doi: 10.1095/biolreprod.103.027060 [DOI] [PubMed] [Google Scholar]
- 49.Rocha JS, Bonkowski MS, de França LR, Bartke A. Effects of mild calorie restriction on reproduction, plasma parameters and hepatic gene expression in mice with altered GH/IGF-I axis. Mech Ageing Dev. 2007;128:317–331. doi: 10.1016/j.mad.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 50.Saccon TD, Rovani MT, Garcia DN, et al. Primordial follicle reserve, DNA damage and macrophage infiltration in the ovaries of the long-living Ames dwarf mice. Exp Gerontol. 2020;132:110851. doi: 10.1016/j.exger.2020.110851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider A, Matkovich SJ, Saccon T, et al. Ovarian transcriptome associated with reproductive senescence in the long-living Ames dwarf mice. Mol Cell Endocrinol. 2017;439:328–336. doi: 10.1016/j.mce.2016.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Isola JVV, Zanini BM, Sidhom S, et al. 17alpha-Estradiol promotes ovarian aging in growth hormone receptor knockout mice, but not wild-type littermates. Exp Gerontol. 2020;129:110769. doi: 10.1016/j.exger.2019.110769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slot KA, Kastelijn J, Bachelot A, Kelly PA, Binart N, Teerds KJ. Reduced recruitment and survival of primordial and growing follicles in GH receptor-deficient mice. Reproduction. 2006;131:525–532. doi: 10.1530/rep.1.00946 [DOI] [PubMed] [Google Scholar]
- 54.Hart RJ. Use of growth hormone in the IVF treatment of women with poor ovarian reserve. Front Endocrinol (Lausanne). 2019;10:500. doi: 10.3389/fendo.2019.00500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneider A, Zhi X, Moreira F, Lucia T Jr, Mondadori RG, Masternak MM. Primordial follicle activation in the ovary of Ames dwarf mice. J Ovarian Res. 2014;7:120. doi: 10.1186/s13048-014-0120-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schneider A, Zhi X, Bartke A, Kopchick JJ, Masternak MM. Effect of growth hormone receptor gene disruption and PMA treatment on the expression of genes involved in primordial follicle activation in mice ovaries. Age (Dordr). 2014;36:9701. doi: 10.1007/s11357-014-9701-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130:791–799. doi: 10.1530/rep.1.00793 [DOI] [PubMed] [Google Scholar]
- 58.Chiang T, Schultz RM, Lampson MA. Meiotic origins of maternal age-related aneuploidy. Biol Reprod. 2012;86:1–7. doi: 10.1095/biolreprod.111.094367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Titus S, Li F, Stobezki R, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. 2013;5:172ra21. doi: 10.1126/scitranslmed.3004925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin-Montalvo A, Mercken EM, Mitchell SJ, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campbell JM, Bellman SM, Stephenson MD, Lisy K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev. 2017;40:31–44. doi: 10.1016/j.arr.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 62.Scarpello JH, Howlett HC. Metformin therapy and clinical uses. Diab Vasc Dis Res. 2008;5:157–167. doi: 10.3132/dvdr.2008.027 [DOI] [PubMed] [Google Scholar]
- 63.Grzybowska M, Bober J, Olszewska M. Metformin—mechanisms of action and use for the treatment of type 2 diabetes mellitus. Postepy Hig Med Dosw. 2011;65:277–285. doi: 10.5604/17322693.941655 [DOI] [PubMed] [Google Scholar]
- 64.Anisimov VN. Metformin: do we finally have an anti-aging drug? Cell Cycle. 2013;12:3483–3489. doi: 10.4161/cc.26928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Na HJ, Park JS, Pyo JH, et al. Mechanism of metformin: inhibition of DNA damage and proliferative activity in Drosophila midgut stem cell. Mech Ageing Dev. 2013;134:381–390. doi: 10.1016/j.mad.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 66.Qin X, Du D, Chen Q, et al. Metformin prevents murine ovarian aging. Aging (Albany NY). 2019;11:3785–3794. doi: 10.18632/aging.102016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Furat Rencber S, Kurnaz Ozbek S, Eraldemır C, et al. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: an experimental study. J Ovarian Res. 2018;11:55. doi: 10.1186/s13048-018-0427-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anisimov VN, Berstein LM, Popovich IG, et al. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging. 2011;3(2):148–157. doi: 10.18632/aging.100273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang T, Lord JM, Norman RJ, Yasmin E, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2012(5):CD003053. doi: 10.1002/14651858.CD003053.pub5 [DOI] [PubMed] [Google Scholar]
- 70.Houtkooper RH, Cantó C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2010;31:194–223. doi: 10.1210/er.2009-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mouchiroud L, Houtkooper RH, Moullan N, et al. The NAD(+)/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bertoldo MJ, Listijono DR, Ho WJ, et al. NAD(+) repletion fescues female fertility during reproductive aging. Cell Rep. 2020;30(6):1670–1681, e1677. doi: 10.1016/j.celrep.2020.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harrison DE, Strong R, Allison DB, et al. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13:273–282. doi: 10.1111/acel.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anstead GM, Carlson KE, Katzenellenbogen JA. The estradiol pharmacophore: ligand structure-estrogen receptor binding affinity relationships and a model for the receptor binding site. Steroids. 1997;62:268–303. doi: 10.1016/s0039-128x(96)00242-5 [DOI] [PubMed] [Google Scholar]
- 75.Garratt M, Leander D, Pifer K, et al. 17-α Estradiol ameliorates age-associated sarcopenia and improves late-life physical function in male mice but not in females or castrated males. Aging Cell. 2019;18:e12920. doi: 10.1111/acel.12920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stout MB, Steyn FJ, Jurczak MJ, et al. 17alpha-Estradiol alleviates age-related metabolic and inflammatory dysfunction in male mice without inducing feminization. J Gerontol A Biol Sci Med Sci. 2017;72(1):3–15. doi: 10.1093/gerona/glv309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vidra N, Trias-Llimos S, Janssen F. Impact of obesity on life expectancy among different European countries: secondary analysis of population-level data over the 1975–2012 period. BMJ Open. 2019;9(7):e028086. doi: 10.1136/bmjopen-2018-028086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Drel VR, Mashtalir N, Ilnytska O, et al. The leptin-deficient (ob/ob) mouse: a new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes. 2006;55:3335–3343. doi: 10.2337/db06-0885 [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0 [DOI] [PubMed] [Google Scholar]
- 80.Ren J, Dong F, Cai GJ, et al. Interaction between age and obesity on cardiomyocyte contractile function: role of leptin and stress signaling. PLoS ONE. 2010;5(4):e10085. doi: 10.1371/journal.pone.0010085 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suganami T, Ogawa Y. Adipose tissue macrophages: their role in adipose tissue remodeling. J Leukoc Biol. 2010;88:33–39. doi: 10.1189/jlb.0210072 [DOI] [PubMed] [Google Scholar]
- 83.Hamm ML, Bhat GK, Thompson WE, Mann DR. Folliculogenesis is impaired and granulosa cell apoptosis is increased in leptin-deficient mice. Biol Reprod. 2004;71:66–72. doi: 10.1095/biolreprod.104.027292 [DOI] [PubMed] [Google Scholar]
- 84.Barash IA, Cheung CC, Weigle DS, et al. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941 [DOI] [PubMed] [Google Scholar]
- 85.Barkan D, Hurgin V, Dekel N, Amsterdam A, Rubinstein M. Leptin induces ovulation in GnRH-deficient mice. FASEB J. 2005;19:133–135. doi: 10.1096/fj.04-2271fje [DOI] [PubMed] [Google Scholar]
- 86.Cabello E, Garrido P, Morán J, et al. Effects of resveratrol on ovarian response to controlled ovarian hyperstimulation in ob/ob mice. Fertil Steril. 2015;103:570–9.e1. doi: 10.1016/j.fertnstert.2014.10.034 [DOI] [PubMed] [Google Scholar]
- 87.Barros CC, Almeida SS, Mori MA, et al. Efficient method for obtaining Lep(ob)/Lep(ob)-derived animal models using adipose tissue transplantations. Int J Obes (Lond). 2009;33:938–944. doi: 10.1038/ijo.2009.95 [DOI] [PubMed] [Google Scholar]
- 88.Skaznik-Wikiel ME, Swindle DC, Allshouse AA, Polotsky AJ, McManaman JL. High-fat diet causes subfertility and compromised ovarian function independent of obesity in mice. Biol Reprod. 2016;94:108. doi: 10.1095/biolreprod.115.137414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang N, Luo LL, Xu JJ, et al. Obesity accelerates ovarian follicle development and follicle loss in rats. Metabolism. 2014;63:94–103. doi: 10.1016/j.metabol.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 90.Serke H, Nowicki M, Kosacka J, et al. Leptin-deficient (ob/ob) mouse ovaries show fatty degeneration, enhanced apoptosis and decreased expression of steroidogenic acute regulatory enzyme. Int J Obes (Lond). 2012;36:1047–1053. doi: 10.1038/ijo.2011.220 [DOI] [PubMed] [Google Scholar]
- 91.Hou YJ, Zhu CC, Duan X, Liu HL, Wang Q, Sun SC. Both diet and gene mutation induced obesity affect oocyte quality in mice. Sci Rep. 2016;6:18858. doi: 10.1038/srep18858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu LL, Dunning KR, Yang X, et al. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151:5438–5445. doi: 10.1210/en.2010-0551 [DOI] [PubMed] [Google Scholar]
- 93.Reynolds KA, Boudoures AL, Chi MM, Wang Q, Moley KH. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod Fertil Dev. 2015;27:716–724. doi: 10.1071/RD14251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grive KJ, Freiman RN. The developmental origins of the mammalian ovarian reserve. Development. 2015;142:2554–2563. doi: 10.1242/dev.125211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao H, Chen ZJ, Qin Y, et al. Transcription factor FIGLA is mutated in patients with premature ovarian failure. Am J Hum Genet. 2008;82:1342–1348. doi: 10.1016/j.ajhg.2008.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1 [DOI] [PubMed] [Google Scholar]
- 97.Picó C, Palou M, Priego T, Sánchez J, Palou A. Metabolic programming of obesity by energy restriction during the perinatal period: different outcomes depending on gender and period, type and severity of restriction. Front Physiol. 2012;3:436. doi: 10.3389/fphys.2012.00436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chan KA, Jazwiec PA, Gohir W, Petrik JJ, Sloboda DM. Maternal nutrient restriction impairs young adult offspring ovarian signaling resulting in reproductive dysfunction and follicle loss. Biol Reprod. 2018;98:664–682. doi: 10.1093/biolre/ioy008 [DOI] [PubMed] [Google Scholar]
- 99.Yarde F, Broekmans FJ, van der Pal-de Bruin KM, et al. Prenatal famine, birthweight, reproductive performance and age at menopause: the Dutch hunger winter families study. Hum Reprod. 2013;28:3328–3336. doi: 10.1093/humrep/det331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lumey LH, Stein AD. In utero exposure to famine and subsequent fertility: the Dutch Famine Birth Cohort Study. Am J Public Health. 1997;87:1962–1966. doi: 10.2105/ajph.87.12.1962 [DOI] [PMC free article] [PubMed] [Google Scholar]