Abstract
Metabolomics in the Alzheimer’s Disease Neuroimaging Initiative cohort provides a powerful tool for mapping biochemical changes in Alzheimer’s disease, and a unique opportunity to learn about the association between circulating blood metabolites and brain amyloid-β deposition in Alzheimer’s disease. We examined 140 serum metabolites and their associations with brain amyloid-β deposition, cognition and conversion from mild cognitive impairment to Alzheimer’s disease in the Alzheimer’s Disease Neuroimaging Initiative. Processed [18F] Florbetapir PET images were used to perform a voxel-wise statistical analysis of the effect of metabolite levels on amyloid-β accumulation across the whole brain. We performed a multivariable regression analysis using age, sex, body mass index, apolipoprotein E ε4 status and study phase as covariates. We identified nine metabolites as significantly associated with amyloid-β deposition after multiple comparison correction. Higher levels of one acylcarnitine (C3; propionylcarnitine) and one biogenic amine (kynurenine) were associated with decreased amyloid-β accumulation and higher memory scores. However, higher levels of seven phosphatidylcholines (lysoPC a C18:2, PC aa C42:0, PC ae C42:3, PC ae C44:3, PC ae C44:4, PC ae C44:5 and PC ae C44:6) were associated with increased brain amyloid-β deposition. In addition, higher levels of PC ae C44:4 were significantly associated with lower memory and executive function scores and conversion from mild cognitive impairment to Alzheimer’s disease dementia. Our findings suggest that dysregulation of peripheral phosphatidylcholine metabolism is associated with earlier pathological changes noted in Alzheimer’s disease as measured by brain amyloid-β deposition as well as later clinical features including changes in memory and executive functioning. Perturbations in phosphatidylcholine metabolism may point to issues with membrane restructuring leading to the accumulation of amyloid-β in the brain. Additional studies are needed to explore whether these metabolites play a causal role in the pathogenesis of Alzheimer’s disease or if they are biomarkers for systemic changes during preclinical phases of the disease.
Keywords: Alzheimer’s disease, amyloid-β, metabolomics, cognition, phosphatidylcholine metabolism
Nho et al. report that serum-based metabolites are associated with brain amyloid-β deposition, cognition and disease progression from mild cognitive impairment to Alzheimer’s disease in a large cohort of older adults. Results suggest that dysregulation of very long chain peripheral phospholipid metabolism is associated with earlier pathological changes in Alzheimer’s disease.
Graphical Abstract
Graphical Abstract.
Introduction
Late-onset Alzheimer’s disease is an age-related neurodegenerative disease with a long preclinical period extending at least two decades.1 Understanding the aetiology of Alzheimer’s disease has proved to be challenging due to the complexity of the disease including its long pre-symptomatic period and variability in clinical symptoms. Targeted and non-targeted metabolomic and lipidomic platforms are being used to investigate the molecular underpinnings of pathological hallmarks of Alzheimer’s disease relevant to the pathophysiology of the disease in greater detail. Compared to cognitively normal controls, Alzheimer’s disease patients have impairments in phospholipid homeostasis,2–5 up-regulated degradation of membrane phospholipids and sphingolipids,2,6–8 and impairments in neurotransmission.6,7 Furthermore, several metabolomics studies report overlap between associations of multiple metabolite classes (e.g. phospholipids, sphingomyelins, acylcarnitines, branched chain and aromatic amino acids) with known risk factors of Alzheimer’s disease and cognitive impairment including insulin resistance.9–12
Although there is extensive literature related to metabolic perturbations in Alzheimer’s disease and its risk factors using metabolomic platforms, it is still unclear how circulating serum metabolites are associated with brain amyloid-β deposition. Evidence suggests there are metabolic interactions between peripheral and central compartments that go beyond substrate transport,5,13–17 however, our understanding of these interactions is limited. Work by the Alzheimer’s Disease Metabolomics Consortium (ADMC) identified the role of the gut microbiome and liver in cognitive decline and changes in the brain that are hallmarks of the disease, highlighting the cross-talk between peripheral and central compartments.13–15 Amyloid deposition is one of the central neuropathological features of Alzheimer’s disease, yet little is known about its association with the metabolome. Understanding the associations between circulating metabolites and amyloid-β deposition could shed light on the mechanisms underlying the association between metabolic perturbations and Alzheimer’s disease risk as well as lead to the identification of novel biomarkers. Here, we performed association analyses of circulating serum metabolites with measures of amyloid-β deposition, cognition and conversion to Alzheimer’s disease dementia from mild cognitive impairment (MCI) in a large national multi-centre cohort, the Alzheimer’s Disease Neuroimaging Initiative (ADNI).
Materials and methods
Study cohort
Serum samples and data analyzed in the present report were obtained from the ADNI cohort. The initial phase (ADNI-1) was launched in 2003 to test whether serial MRI, PET, other biological markers, and clinical and neuropsychological assessment could be combined to measure the progression of MCI and early Alzheimer’s disease. ADNI-1 was extended to subsequent phases (ADNI-GO, ADNI-2 and ADNI-3) for follow-up for existing participants and additional new enrolments. Inclusion and exclusion criteria, clinical and neuroimaging protocols and other information about ADNI can be found at www.adni-info.org (last accessed 6 july 2021).18,19 Demographic information, raw neuroimaging scan data, apolipoprotein E (APOE) and clinical information are available and were downloaded from the ADNI data repository (www.loni.usc.edu/ADNI/; last accessed 6 july 2021). Written informed consent was obtained according to the Declaration of Helsinki at the time of enrolment that included permission for analysis and data sharing and consent forms were approved by each participating sites’ Institutional Review Board (IRB).
AbsoluteIDQ-p180 kit metabolites
Metabolites were measured using a targeted metabolomics approach using the AbsoluteIDQ-p180 kit (BIOCRATES Life Science AG, Innsbruck, Austria), with an ultra-performance liquid chromatography (UPLC)/MS/MS system [Acquity UPLC (Waters), TQ-S triple quadrupole MS/MS (Waters)], which provides measurements of up to 186 endogenous metabolites quantitatively (amino acids and biogenic amines) and semi-quantitatively [acylcarnitines, sphingomyelins, phosphatidylcholines (PCs) and lyso-glycero-phosphatidylcholines (a = acyl) [lysoPCs] across multiple classes]. The AbsoluteIDQ-p180 kit has been fully validated according to European Medicine Agency Guidelines on bioanalytical method validation. In addition, plates include an automated technical validation to approve the validity of the run and provide verification of the actual performance of the applied quantitative procedure including instrumental analysis. The technical validation of each analyzed kit plate was performed using MetIDQ software based on results obtained and defined acceptance criteria for blank, zero samples, calibration standards and curves, low/medium/high-level QC samples, and measured signal intensity of internal standards over the plate.20
Metabolomics data and pre-processed data are accessible through the AMP-AD Knowledge Portal (https://ampadportal.org; last accessed 6 july 2021). The AMP-AD Knowledge Portal is the distribution site for data, analysis results, analytical methodology and research tools generated by the AMP-AD Target Discovery and Preclinical Validation Consortium and multiple Consortia and research programs supported by the National Institute on Aging. Information on data availability and accessibility is available in the Data availability section.
P180 quality control
Metabolites with >40% of measurements below the lower limit of detection (LOD) were excluded from the analysis. Metabolite values were scaled across the different plates using the quality control (QC) pool duplicates. Metabolite measurements below LOD were imputed using each metabolite’s LOD/2 value. Using the blinded duplicates, we selected metabolites with a coefficient of variation <20% and an intraclass correlation coefficient >0.65.20 We checked for the presence of multivariate outlier participants by evaluating the first and second principal components in each platform. For the participants with duplicated measurements, we used the average values of the two measured values in further analyses. The QC process resulted in 140 metabolites for further analysis. The QC-passed preprocessed metabolite measurements were adjusted for the effect of medication use at baseline on metabolite levels (see Toledo et al.20 for adjustment description details). All metabolites were log2 transformed and scaled to mean zero and variance 1.
Cognitive performance
Composite scores were used to measure memory and executive functioning in ADNI. A composite score for memory (ADNI-MEM) was created from memory-related tasks and included the following: memory tasks from the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-Cog), the Rey Auditory Verbal Learning Test, memory components of the Mini-Mental State Examination and the Logical Memory task.21 A composite score for executive functioning (ADNI-EF) included the following tests: the WAIS-R Digit Symbol Substitution task and Digit Span backwards task, Trail Making Test Parts A and B, category fluency (animals and vegetables), and five clock drawing items. ADNI composite scores have a mean of 0 and a standard deviation of 1.22
PET processing
Pre-processed [18F] Florbetapir PET scans (co-registered, averaged, standardized image and voxel size, uniform resolution) were downloaded from the ADNI LONI site (http://adni.loni.usc.edu; last accessed 6 july 2021) as described in previously reported methods for acquisition and processing of PET scans from the ADNI sample.23,24 For [18F] Florbetapir PET, scans were intensity-normalized using a whole cerebellum reference region to create SUVR images.
Statistical analyses
For [18F] Florbetapir PET, a mean SUVR value was extracted using MarsBaR from a global cortical region generated from an independent comparison of ADNI-1 [11C] Pittsburgh Compound B SUVR scans [regions where Alzheimer’s disease > cognitively normal older adults (CN)]. In this study, we used R version 3.5.1 (R-project.org) for analyses unless otherwise specified.
We performed a linear regression association analysis of P180 metabolites with brain amyloid-β deposition and with composite scores for memory and executive functioning using a linear regression model (glm in R) with age, sex, years of education, body mass index, study phase (ADNI-1 or ADNI GO/2) and APOE ɛ4 as covariates. In addition, we explored the group differences between MCI-Converter and MCI-Stable groups stratified by baseline diagnosis and 2-year conversion from MCI to Alzheimer’s disease. Of the 615 MCI patients with both P180 metabolites and a follow-up clinical diagnosis, 182 MCI patients progressed to Alzheimer’s disease dementia over the 2-year period from the baseline visit (MCI-Converter) and 433 MCI patients remained stable over 2-year period from the baseline visit (MCI-Stable). We performed association analyses of levels of nine identified metabolites at baseline with MCI to Alzheimer’s disease dementia conversion over 2 years (MCI-stable and MCI-Converter) using a logistic regression model (glm in R). Age, sex, years of education, body mass index, study phase (ADNI-1 or ADNI-GO/2) and APOE ε4 status were used as covariates. False discovery rate (FDR)-based multiple comparison adjustment with the Benjamini–Hochberg procedure was used because the metabolites and Alzheimer’s disease biomarker phenotypes were strongly correlated with each other.25 Not accounting for this high collinearity of dependent variables would lead to an overly stringent correction for multiple testing.
Whole brain imaging analysis
The processed [18F] Florbetapir PET images were used to perform a voxel-wise statistical analysis of the effect of P180 metabolite levels on amyloid-β accumulation across the whole brain using SPM8 (www.fil.ion.ucl.ac.uk/spm/; last accessed 6 july 2021). We performed a multivariable regression analysis using age, sex, body mass index, APOE ε4 status and study phase (ADNI-1 or ADNI-GO/2) as covariates. In the voxel-wise whole brain analysis, the significant statistical parameters were selected to correspond to a threshold of P < 0.05 (FDR-corrected).
Data availability
Metabolomics datasets from the AbsoluteIDQ-p180 metabolomics kit used in the current analyses for the ADNI-1 and ADNI-GO/-2 cohorts are available via the Accelerating Medicines Partnership-Alzheimer’s Disease (AMP-AD) Knowledge Portal and can be accessed at http://dx.doi.org/10.7303/syn5592519 (ADNI-1; last accessed 6 july 2021) and http://dx.doi.org/10.7303/syn9705278 (ADNI-GO/-2; last accessed 6 July 2021). The full complement of clinical and demographic data for the ADNI cohorts are hosted on the LONI data sharing platform and can be requested at http://adni.loni.usc.edu/data-samples/access-data/ (last accessed 6 july 2021).
Results
Study sample
Demographic information for all diagnosis groups is presented in Table 1. This included 1531 ADNI participants [370 cognitively normal older adult controls (CN), 95 with significant memory concern (SMC), 271 with early mild cognitive impairment (EMCI), 491 with late MCI (LMCI) and 304 with Alzheimer’s disease].
Table 1.
Demographics of ADNI participants stratified by baseline diagnosis
| CN (N = 370) | SMC (N = 95) | EMCI (N = 271) | LMCI (N = 491) | AD (N = 304) | P-value | |
|---|---|---|---|---|---|---|
| Age | 74.6 (5.8) | 72.3 (5.7) | 71.3 (7.6) | 74.0 (7.6) | 74.8 (7.8) | 2.6 × 10−9 |
| Sex: (Male/Female) | 181/189 | 40/55 | 149/122 | 299/192 | 166/138 | 9.2 × 10−4 |
| Education, years | 16.3 (2.8) | 16.7 (2.6) | 16.0 (2.7) | 15.8 (2.9) | 15.2 (3.0) | 1.3 × 10−6 |
| BMI (Kg/M2) | 27.0 (4.5) | 28.4 (6.2) | 28.0 (5.4) | 26.4 (4.3) | 25.9 (4.7) | 2.9 × 10−8 |
| APOE ε4 (0/1/2) | 266/95/9 | 66/28/1 | 156/96/19 | 224/201/66 | 106/139/59 | <1 × 10−15 |
| Amyloid positivity (−/+) | 114/53 | 49/26 | 137/130 | 49/97 | 15/113 | <1 × 10−15 |
| Amyloid PET SUVR | 1.11 (0.17) | 1.12 (0.18) | 1.18 (0.21) | 1.28 (0.24) | 1.39 (0.22) | <1 × 10−15 |
| Memory composite | 1.03 (0.57) | 1.08 (0.54) | 0.58 (0.60) | −0.08 (0.62) | −0.85 (0.54) | <1 × 10−15 |
| Executive function composite | 0.78 (0.79) | 0.79 (0.85) | 0.50 (0.85) | 0.01 (0.86) | −0.90 (0.93) | <1 × 10−15 |
| MMSE | 29.1 (1.1) | 29.0 (1.2) | 28.3 (1.60) | 27.1 (1.83) | 23.3 (2.0) | <1 × 10−15 |
| C3 | −0.02 (1.01) | −0.06 (0.73) | −0.15 (0.91) | −0.04 (0.90) | −0.23 (0.93) | 1.9 × 10−3 |
| Kynurenine | −0.08 (0.98) | −0.31 (0.87) | −0.32 (0.88) | −0.12 (0.96) | −0.24 (1.04) | 5.1 × 10−3 |
| LysoPC a C18:2 | 0.17 (0.96) | −0.11 (0.89) | 0.04 (0.92) | 0.37 (0.97) | 0.27 (0.92) | 3.4 × 10−7 |
| PC aa C42:0 | 0.11 (0.98) | 0.01 (1.01) | 0.01 (0.90) | 0.26 (0.95) | 0.24 (0.91) | 1.4 × 10−3 |
| PC ae C42:3 | 0.44 (0.91) | 0.17 (1.00) | 0.27 (0.95) | 0.49 (0.91) | 0.40 (0.92) | 2.5 × 10−3 |
| PC ae C44:3 | 0.34 (0.92) | 0.28 (0.97) | 0.07 (0.91) | 0.45 (0.93) | 0.41 (0.94) | 3.1 × 10−6 |
| PC ae C44:4 | 0.28 (0.95) | 0.12 (0.96) | 0.17 (0.99) | 0.46 (0.93) | 0.45 (0.91) | 1.3 × 10−5 |
| PC ae C44:5 | 0.15 (1.01) | 0.02 (0.94) | 0.09 (1.01) | 0.30 (0.94) | 0.32 (0.90) | 1.2 × 10−3 |
| PC ae C44:6 | 0.13 (1.00) | −0.03 (0.96) | 0.06 (0.94) | 0.34 (0.94) | 0.31 (0.92) | 1.7 × 10−5 |
Data are reported as mean (SD) unless otherwise indicated.
AD, Alzheimer’s disease; ADAS-Cog 13, modified 13-item Alzheimer’s Disease Assessment Scale, cognitive subscale; BMI, Body mass index; CDR-SB, Clinical Dementia Rating-Sum of Boxes; CN, cognitively normal older adults; EMCI, early mild cognitive impairment; LMCI, late mild cognitive impairment; SMC, subjective memory complaint; MMSE, Mini-Mental State Examination.
Region of interest-based analysis of amyloid-β PET
Using 783 ADNI participants (167 CN, 75 SMC, 267 EMCI, 146 LMCI and 128 Alzheimer’s disease) with both P180 metabolites and [18F] Florbetapir PET scans, we evaluated whether metabolites were associated with region of interest-based brain amyloid-β load by performing an association analysis with each metabolite. We used global cortical amyloid deposition measured by amyloid PET scans. We identified nine metabolites significantly associated with global cortical amyloid deposition after applying FDR-based multiple comparison correction (Table 2). Increased levels of one acylcarnitine (C3; propionylcarnitine) and one biogenic amine (kynurenine) were associated with decreased brain amyloid-β accumulation; whereas increased levels of seven PCs (lysoPC a C18:2, PC aa C42:0, PC ae C42:3, PC ae C44:3, PC ae C44:4, PC ae C44:5 and PC ae C44:6) were associated with increased brain amyloid-β accumulation.
Table 2.
Results of association of P180 metabolites with a global cortical amyloid deposition measured from amyloid PET scans (FDR-corrected P-value < 0.05)
| Metabolite | β (SE) | Corrected P-value |
|---|---|---|
| C3 | −0.009 (0.003) | 0.042 |
| Kynurenine | −0.010 (0.003) | 0.036 |
| LysoPC a C18:2 | 0.009 (0.003) | 0.042 |
| PC aa C42:0 | 0.009 (0.003) | 0.042 |
| PC ae C42:3 | 0.009 (0.003) | 0.042 |
| PC ae C44:3 | 0.010 (0.003) | 0.036 |
| PC ae C44:4 | 0.008 (0.003) | 0.042 |
| PC ae C44:5 | 0.008 (0.003) | 0.042 |
| PC ae C44:6 | 0.009 (0.003) | 0.036 |
Detailed whole brain analysis of amyloid-β PET
In addition to the priori region-based amyloid-β PET analysis, we performed a detailed whole-brain analysis of brain amyloid-β accumulation on a voxel-wise level measured from amyloid-β PET scans for nine metabolites (C3, kynurenine, lysoPC a C18:2, PC aa C42:0, PC ae C42:3, PC ae C44:3, PC ae C44:4, PC ae C44:5 and PC ae C44:6) that were significantly associated with a global cortical amyloid deposition. We used a general linear model approach to identify brain regions where amyloid-β accumulation was associated with levels of each metabolite. The results of the voxel-wise association between brain amyloid-β load and metabolites are shown in Figs. 1 and 2. Higher levels of C3 were associated with decreased amyloid-β accumulation in the bilateral frontal and temporal lobes, with a global maximum association in the left temporal cortex [Brodmann area (BA) 38] (Fig. 1A). Higher levels of kynurenine were associated with decreased amyloid-β accumulation in a widespread pattern, especially the bilateral temporal, parietal and frontal lobes, with a global maximum association in the left dorsolateral prefrontal cortex (BA 9) (Fig. 1B). In contrast, higher levels of lysoPC a C18:2. PC aa C 42:0, PC ae C42:3, PC ae C44:3, PC ae C44:4, PC ae C44:5 and PC ae C44:6 were associated with increased amyloid-β accumulation in a widespread pattern of significant voxels, especially in the bilateral frontal, temporal and parietal lobes. Global maximum associations were noted in the right parietal cortex (BA 7) (Fig. 1C) for lysoPC a C18:2, the right dorsolateral prefrontal cortex (BA 9) (Fig. 1D) for PC aa C42:0, the right parietal cortex (BA 39) (Fig. 1E) for PC ae C42:3, the right primary auditory cortex (BA 41) (Fig. 2A, C and D) for PC ae C44:3, PC ae C44:5, and PC ae C44:6, and the left parietal cortex (BA 40) (Fig. 2B) for PC ae C44:4.
Figure 1.
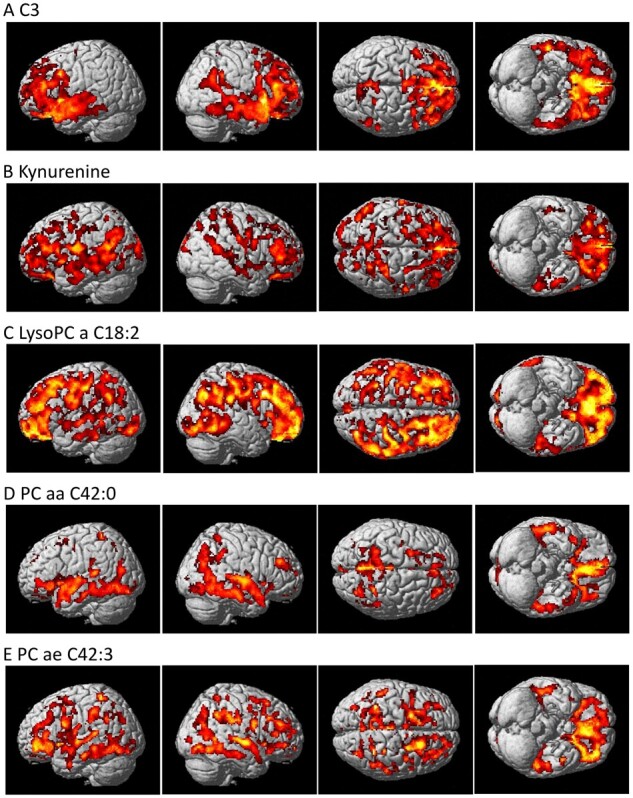
Detailed whole-brain voxel-based imaging analysis for amyloid-β accumulation using [18F]Florbetapir PET scans. Higher levels of (A) C3 and (B) kynurenine were associated with decreased amyloid-β accumulation. Higher levels of (C) lysoPC a C18:2, (D) PC aa C42:0 and (E) PC ae C42:3 were associated with increased amyloid-β accumulation (corrected P-value < 0.05).
Figure 2.
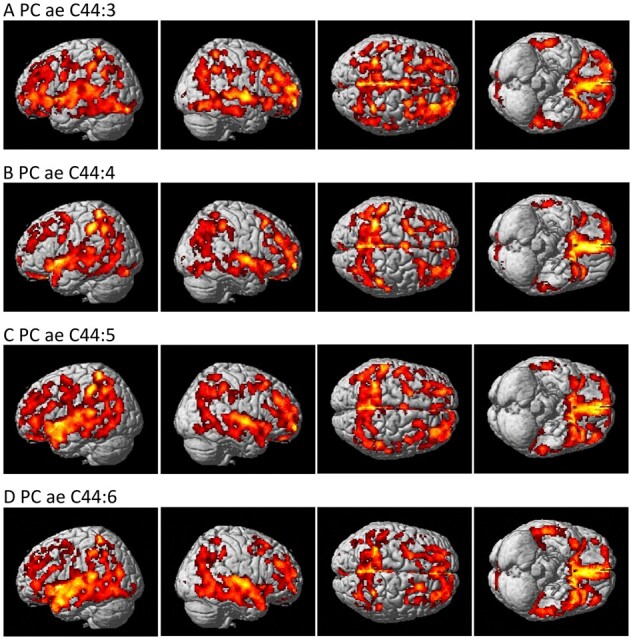
Detailed whole-brain voxel-based imaging analysis for amyloid-β accumulation using [18F]Florbetapir PET scans. Higher levels of (A) PC ae C44:3, (B) PC ae C44:4, (C) PC ae C44:5 and (D) PC ae C44:6 were associated with increased amyloid-β accumulation (corrected P-value < 0.05).
Association of significant metabolites with cognition
Using composite scores for memory (ADNI-MEM) and executive functioning (ADNI-EF), we performed a further analysis of nine metabolites (C3, kynurenine, lysoPC a C18:2, PC aa C42:0, PC ae C42:3, PC ae C44:3, PC ae C44:4, PC ae C44:5 and PC ae C44:6) that were significantly associated with a global cortical amyloid deposition. We identified significant associations of the selected metabolites with cognition after adjusting for multiple comparison correction using FDR (Table 3). Higher levels of C3 and kynurenine were associated with higher memory scores. In contrast, higher levels of PC ae C44:4 and PC ae C44:6 were associated with lower memory scores. Higher kynurenine levels were associated with higher executive functioning scores, whereas higher levels of PC aa C42:0, PC ae C44:4, PC ae C44:5 and PC ae C44:6 were associated with lower executive functioning scores.
Table 3.
Results of association of the nine metabolites significantly associated with brain amyloid-β deposition with composite cognitive performance measures
| Metabolite | Memory composite score |
Executive function composite score |
||
|---|---|---|---|---|
| β (SE) | Corrected P-value | β (SE) | Corrected P-value | |
| C3 | 0.07 (0.02) | 0.016 | 0.05(0.03) | 0.148 |
| Kynurenine | 0.05 (0.02) | 0.042 | 0.07 (0.03) | 0.021 |
| LysoPC a C18:2 | −0.01 (0.02) | 0.893 | 0.04 (0.03) | 0.213 |
| PC aa C42:0 | −0.05 (0.02) | 0.074 | −0.08 (0.03) | 0.016 |
| PC ae C42:3 | −0.0004 (0.02) | 0.987 | −0.004 (0.03) | 0.940 |
| PC ae C44:3 | −0.03 (0.02) | 0.263 | −0.05 (0.03) | 0.132 |
| PC ae C44:4 | −0.06 (0.02) | 0.038 | −0.08 (0.03) | 0.015 |
| PC ae C44:5 | −0.03 (0.02) | 0.226 | −0.08 (0.03) | 0.015 |
| PC ae C44:6 | −0.05 (0.02) | 0.040 | −0.10 (0.03) | 0.004 |
Association of significant metabolites with conversion to Alzheimer’s disease dementia in MCI
Of the 615 MCI patients with both P180 metabolites and a follow-up clinical diagnosis, 182 MCI patients progressed to Alzheimer’s disease dementia over the 2-year period from the baseline visit (MCI-Converter) and 433 MCI patients remained stable over 2-year period from the baseline visit (MCI-Stable). We performed an association of levels of the nine identified metabolites at baseline with MCI-to-Alzheimer’s disease dementia conversion over 2 years (MCI-Stable and MCI-Converter). Only PC ae C44:4 showed a significant group difference between MCI-Stable and MCI-Converter groups after FDR-correction (Fig. 3). The MCI-Converter group has significantly higher levels of PC ae C44:4 at baseline compared to the MCI-Stable group.
Figure 3.
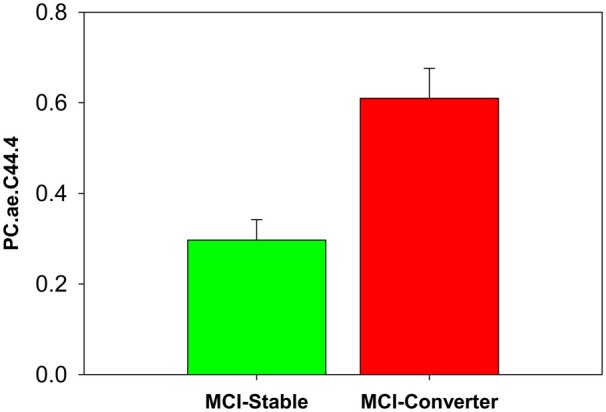
Association of PC ae C44:4 with conversion to Alzheimer’s disease dementia in MCI. Group comparison of mild cognitive impairment groups (MCI-Stable and MCI-Converter) based on baseline diagnosis and 2-year conversion status for PC ae C44:4.
Discussion
In this report, we analyzed 140 targeted serum-based metabolites in the ADNI cohort to investigate the relationship between metabolite levels and amyloid-β deposition, cognition, and disease progression. We identified nine metabolites (C3, kynurenine, lysoPC a C18:2, PC aa C42:0, PC ae C42:3, PC ae C44:3, PC ae C44:4, PC ae C44:5 and PC ae C44:6) significantly associated with global cortical amyloid deposition, including glycerophospholipids, an acylcarnitine and a biogenic amine. Of the nine amyloid-β-associated metabolites, six were significantly associated with cognitive performance (C3, kynurenine, PC aa C42:0, PC ae C44:4, PC ae C44:5 and PC ae C44:6), and one metabolite (PC ae C44:4) was associated with conversion to Alzheimer’s disease dementia from MCI.
Phosphatidylcholines
Our findings suggest that dysregulation of peripheral PC metabolism is associated with earlier pathological changes noted in Alzheimer’s disease as measured by amyloid-β deposition as well as later clinical changes including changes in memory and executive functioning. Higher levels of seven PCs were associated with increased amyloid deposition (lysoPC a C18:2, PC aa C42:0, PC ae C42:3, PC ae C44:3, PC ae C44:4, PC ae C44:5 and PC ae C44:6). Of these seven PCs, four were significantly associated with cognitive performance. Higher levels of PC ae C44:4 and PC ae C44:6 were associated with lower memory scores, whereas higher levels of PC aa C42:0, PC ae C44:4, PC ae C44:5 and PC ae C44:6 were associated with lower executive functioning scores. The MCI-Converter group had significantly higher levels of PC ae C44:4 at baseline compared to the MCI-Stable group.
In our previous publication using p180 data from the ADNI cohort, we reported PC ae C44:4, PC ae C44:5 and PC ae C44:6 were all associated with CSF Aβ pathology, and PC ae C44:4 and PC ae C44:5 were further associated with brain glucose metabolism as measured by FDG PET.26 Effect directions were consistent with our current findings. Other studies reported associations of PCs with changes in cognition that become apparent later in the disease.20,27,28 In addition, there is research to suggest PC metabolism is perturbed in Alzheimer’s disease. Gonzalez-Dominguez et al.2 reported an increase of PCs in serum samples of patients with Alzheimer’s disease. Results from previous studies suggest dysregulation in the biosynthesis, turnover and acyl chain remodelling of phospholipids that is in line with increased phospholipid breakdown due to phospholipase A2 (PLA2) over activation.2,29,30 Perturbations in PC metabolism appear to play a role in several key molecular pathways intrinsic to cognitive decline and Alzheimer’s disease including neuroinflammation through arachidonic acid signalling, amyloid precursor protein processing through phospholipase A2, and cholesterol transport through high-density lipoproteins.31–33 Furthermore, PCs play a critical role in the balance between cell proliferation and death which has clear implications for the pathogenesis of Alzheimer’s disease.34 Ether-linked PCs may be located in membrane rafts and could support the hypothesis that lipid rafts play a critical role in Alzheimer’s disease through the promotion of amyloid-β peptide and its aggregation.35 Amyloid-β peptides are derived from the proteolytic processing of amyloid precursor protein within lipid rafts.36 In addition, many proteins associated with Alzheimer’s disease, including β- and γ-secretase as well as amyloid-β protein precursor have been found in lipid rafts.37 Abnormal lipid rafts have been noted in post-mortem brain samples from Alzheimer’s disease patients.38 While these associations with lipid metabolites and amyloid-β accumulation may shed led on mechanisms related to Aβ pathology in Alzheimer’s disease, given the limitations of the current study including its cross-sectional design and measurement of blood in the periphery, we cannot make assumptions about directionality.
LysoPC C18:2 was significantly associated with increased amyloid accumulation in a widespread pattern especially in the bilateral frontal, temporal, and parietal lobes. Lyso PC C18:2 was proposed as part of a biomarker panel that predicted conversion to Alzheimer’s disease.39 Levels of lysoPC C18:2 were reduced at baseline in future converters, but tended to increase after conversion to Alzheimer’s disease,39 similar findings were reported in the Vienna Transdanube Aging (VITA) study.40 However, it should be noted that the VITA study reported elevated levels after diagnosis of probable Alzheimer’s disease compared to non-demented controls, whereas the values reported by Mapstone et al. were below those of age-matched cognitively normal controls even after diagnosis of Alzheimer’s disease. The levels of these lipids may be heavily dependent on the study population, including the age distribution and the length of time between the measurements of lipids and conversion. LysoPCs are known to play a role in inflammation41 and have both pro- and anti-atherogenic properties.42 Low levels of plasma lysoPC 18:2 predict memory impairment39 and declines in gait speed in older adults,43 both of which are known to be associated with Alzheimer’s disease. Amyloid-β may directly disrupt integrity of the lipid bilayer by interactions with phospholipids.44
Propionylcarnitine
We found that higher levels of propionylcarnitine (C3) were associated with less amyloid-β deposition in the bilateral temporal and frontal lobes. Previous studies reported perturbations in acylcarnitines in Alzheimer’s disease and MCI patients compared to cognitively normal controls as well as diagnostic converters.20,26,39,45 In our previous publication examining effects of sex and APOE ɛ4 on the metabolome using ADNI data,26 we reported significant sex differences in MCI patients in multiple acylcarnitines (i.e. C0, C3, C9 and C18:2). Mapstone et al.39 reported significantly lower plasma levels of C3 in the converter group, before and after phenoconversion to MCI/Alzheimer’s disease, and in the MCI/Alzheimer’s disease group. In a separate study, levels of C3 were significantly lower in MCI compared to cognitively normal controls.45
Acylcarnitines are involved in energy metabolism, mitochondrial function, neurotransmission and neuroprotection.46 Acylcarnitines directly reflect the oxidation rate of amino acids and fatty acids.47–50 González-Dominguez et al.51 demonstrated the deficit of several acylcarnitines, including C3, in the brain (hippocampus, cortex, and cerebellum) of APP/PS1 transgenic mouse model of Alzheimer’s disease. Short- and medium-chain acyl groups are catalyzed by acyltransferases that are located in microsomes and peroxisomes, whereas long-chain acyl groups are catalyzed by carnitine palmitoyltransferase I and II located on the mitochondria membranes. Our results could suggest selective perturbed metabolism of short-chain acylcarnitines in the peroxisomes.50,52 Propionylcarnitine reflects the propionyl CoA pool which is a byproduct of isoleucine and valine catabolism. A direct link between branch chain amino acids (BCAA) and C3 has been demonstrated by a rise in circulating levels of C3 in response to BCAA supplementation.53 We and others have implicated BCAAs in cognitive decline, Alzheimer’s disease diagnosis, and Alzheimer’s disease risk.20,26,54–56
Kynurenine
We report that higher levels of kynurenine were associated with widespread decreased amyloid-β accumulation, especially in the bilateral temporal, parietal, and frontal lobes as well as higher memory and executive function scores. An independent study of older adults with normal global cognition reported a positive correlation between plasma Aβ1-42 and metabolites in the kynurenine pathway.57 These same metabolites were also associated with plasma neurofilament light chain, an emerging marker of neurodegeneration and inflammation.57 Plasma neurofilament light chain, a marker of axonal cytoskeletal damage, and plasma Aβ have been shown to reflect brain Aβ deposition.58,59 However, it is possible that axonal damage may be due to other aetiologies related to axonal injury including vascular pathologies or traumatic brain injuries. The kynurenine pathway is dysregulated in neuroinflammation, and chronic activation of the kynurenine pathway has been implicated in Alzheimer’s disease and other neuropsychiatric disorders, including depression, suicidality and schizophrenia.60–65 Neuropsychiatric symptoms are common among Alzheimer’s disease patients with up to 80% reporting at least one neuropsychiatric symptom.66 This could suggest that in Alzheimer’s disease, the neuroinflammatory consequences of chronic activation of the kynurenine pathway could be a factor in cognitive changes and/or neuropsychiatric symptoms noted in the disease. Metabolites in the kynurenine pathway, including kynurenine, readily cross the blood brain barrier suggesting that circulating levels may contribute significantly to cerebral pools.67 However, it should be noted that while mechanistic data (e.g. quinolinic acid neurotoxicity and 3-hydroxyanthranilic acid mediated reactive oxidative species production68,69) support the role of kynurenine pathway metabolites in Alzheimer’s disease pathogenesis, it is still unclear if these are causative of disease or merely the result of chronic neuroinflammation.
Limitations
As metabolomics data are only available at one time point, current analyses are cross-sectional and therefore we were unable to explore issues related to causality. The current metabolomics platform does not allow one to determine the acyl chain composition. To address this issue, we have profiled ADNI samples on a highly informative platform that provides semi-quantitative measurement of over 800 lipid species and allows one to determine acyl chain composition. The LC–MS/MS method used in this lipidomics platform allows for improved structural characterization of lipids.70 Owing to the sample recruitment strategy used in the ADNI cohort, which was designed to model a simulated clinical trial for MCI and Alzheimer’s disease, participants were selected for symptoms and early-stage pathology and are likely to differ in significant ways from those recruited into population-based cohorts. As data from both designs become available, the difference between these two sampling strategies is likely to reveal important factors related to early disease and the role of comorbidities.
The present study provides insight into the association of serum-based circulating metabolites with brain amyloid-β accumulation across the whole brain. Perturbations in PC metabolism may point to issues with membrane restructuring leading to the accumulation of amyloid-β in the brain. Additional studies are needed to explore whether these metabolites play a causal role in the pathogenesis of Alzheimer’s disease or if they are biomarkers for systemic changes during preclinical phases of the disease.
Acknowledgements
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. A complete listing of Alzheimer Disease Metabolomics Consortium (ADMC) members is available at https://sites.duke.edu/adnimetab/team/ (last accessed 6 July 2021). Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org; last accessed 6 July 2021). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. The authors are grateful to Lisa Howerton for administrative support and the numerous ADNI study volunteers and their families.
Funding
Funding for ADMC (Alzheimer’s Disease Metabolomics Consortium, led by Dr R.K.-D. at Duke University) was provided by the National Institute on Aging grant 1U01AG061359-01 and R01AG046171, a component of the Accelerating Medicines Partnership for Alzheimer’s disease (AMP-AD) Target Discovery and Preclinical Validation Project (https://www.nia.nih.gov/research/dn/amp-ad-target-discovery-and-preclinical-validation-project; last accessed 6 July 2021) and the National Institute on Aging (NIA) grant RF1 AG0151550, a component of the M2OVE-AD Consortium (Molecular Mechanisms of the Vascular Etiology of AD—Consortium (https://www.nia.nih.gov/news/decoding-molecular-ties-between-vascular-disease-and-alzheimers; last accessed 6 July 2021). The work of various Consortium Investigators is also supported by various NIA grants [U01AG024904-09S4, P50NS053488, R01AG19771, P30AG10133, P30AG10124, K01AG049050], the National Library of Medicine [R01LM011360, R00LM011384], and the National Institute of Biomedical Imaging and Bioengineering [R01EB022574]. Additional support came from Helmholtz Zentrum, the Alzheimer’s Association, the Indiana Clinical and Translational Science Institute and the Indiana University-IU Health Strategic Neuroscience Research Initiative.
Funders listed above had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest
A.J.S. has been supported by multiple grants from the National Institute on Aging (NIA), National Cancer Institute (NCI), and National Collegiatie Athletic Association (NCAA)/Department of Defense (DoD) as well as collaborative research support from Eli Lilly unrelated to the work reported here. He also received nonfinancial support from Avid Radiopharmaceuticals and Neurovision, and has served as a consultant to Arkley BioTek and Bayer. He received support from Springer-Nature as Editor in Chief of Brain Imaging and Behavior, outside the scope of the work submitted here. P.M.D. has received research grants (through Duke University) from Avid/Lilly, Neuronetrix, Avanir, Salix, Alzheimer’s Drug Discovery Foundation, DOD and NIH. P.M.D. has received speaking or advisory fees from Anthrotronix, Neuroptix, Genomind, Clearview, Verily, RBC, Brain Canada, and CEOs Against Alzheimer’s. P.M.D. owns shares in Muses Labs, Anthrotronix, Evidation Health, Turtle Shell Technologies, and Advera Health whose products are not discussed here. P.M.D. served on the board of Baycrest and serves on the board of Apollo Hospitals. P.M.D. is a co-inventor (through Duke) on patents relating to dementia biomarkers, metabolomics, and therapies which are unlicensed. R.K.D. is inventor on key patents in the field of metabolomics including applications for Alzheimer’s disease. M.A., R.B., X.H., A.J.S. and K.N. are co-inventors on patent WO2018049268 in this field. R.K.D. is inventor on key patents in the field of metabolomics including applications for Alzheimer’s disease. All other authors report no disclosures.
Glossary
- ADMC =
Alzheimer’s Disease Metabolomics Consortium
- ADNI =
Alzheimer’s Disease Neuroimaging Initiative
- APOE =
apolipoprotein E
- EMCI =
early mild cognitive impairment
- FDR =
false discovery rate
- LMCI =
late mild cognitive impairment
- MCI =
mild cognitive impairment
- PC =
phosphatidylcholine
References
- 1. Bateman RJ, Xiong C, Benzinger TL, et al. ; Dominantly Inherited Alzheimer Network. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367(9):795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gonzalez-Dominguez R, Garcia-Barrera T, Gomez-Ariza JL.. Combination of metabolomic and phospholipid-profiling approaches for the study of Alzheimer's disease. J Proteomics. 2014;104:37–47. [DOI] [PubMed] [Google Scholar]
- 3. Li D, Misialek JR, Boerwinkle E, et al. Plasma phospholipids and prevalence of mild cognitive impairment and/or dementia in the ARIC Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amsterdam, Netherlands). 2016;3:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klavins K, Koal T, Dallmann G, Marksteiner J, Kemmler G, Humpel C.. The ratio of phosphatidylcholines to lysophosphatidylcholines in plasma differentiates healthy controls from patients with Alzheimer's disease and mild cognitive impairment. Alzheimers Dement (Amsterdam, Netherlands). 2015;1(3):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Varma VR, Oommen AM, Varma S, et al. Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study. PLoS Med. 2018;15(1):e1002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gonzalez-Dominguez R, Garcia-Barrera T, Gomez-Ariza JL.. Using direct infusion mass spectrometry for serum metabolomics in Alzheimer's disease. Anal Bioanal Chem. 2014;406(28):7137–7148. [DOI] [PubMed] [Google Scholar]
- 7. Gonzalez-Dominguez R, Garcia-Barrera T, Gomez-Ariza JL.. Application of a novel metabolomic approach based on atmospheric pressure photoionization mass spectrometry using flow injection analysis for the study of Alzheimer's disease. Talanta. 2015;131:480–489. [DOI] [PubMed] [Google Scholar]
- 8. Gonzalez-Dominguez R, Garcia-Barrera T, Gomez-Ariza JL.. Metabolomic study of lipids in serum for biomarker discovery in Alzheimer's disease using direct infusion mass spectrometry. J Pharm Biomed Anal. 2014;98:321–326. [DOI] [PubMed] [Google Scholar]
- 9. Floegel A, Stefan N, Yu Z, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;62(2):639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang-Sattler R, Yu Z, Herder C, et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol. 2012;8:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu J, Semiz S, van der Lee SJ, et al. Metabolomics based markers predict type 2 diabetes in a 14-year follow-up study. Metabolomics. 2017;13(9):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fiehn O, Garvey WT, Newman JW, Lok KH, Hoppel CL, Adams SH.. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS One. 2010;5(12):e15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. MahmoudianDehkordi S, Arnold M, Nho K, et al. Altered bile acid profile associates with cognitive impairment in Alzheimer's disease-An emerging role for gut microbiome. Alzheimers Dement. 2019 Jan;15(1):76–92. doi: 10.1016/j.jalz.2018.07.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nho K, Kueider-Paisley A, MahmoudianDehkordi S, et al. Altered bile acid profile in mild cognitive impairment and Alzheimer's disease: Relationship to neuroimaging and CSF biomarkers. Alzheimers Dement. 2019 Feb;15(2):232–244. doi: 10.1016/j.jalz.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nho K, Kueider-Paisley A, Ahmad S, et al. for the Alzheimer’s Disease Neuroimaging Initiative and the Alzheimer Disease Metabolomics Consortium. Association of altered liver enzymes with alzheimer disease diagnosis, cognition, neuroimaging measures, and cerebrospinal fluid biomarkers. JAMA Netw Open. 2019;2(7):e197978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huo Z, Yu L, Yang J, Zhu Y, Bennett DA, Zhao J.. Brain and blood metabolome for Alzheimer's dementia: Findings from a targeted metabolomics analysis. Neurobiol Aging. 2020;86:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang J, Wei R, Xie G, et al. Peripheral serum metabolomic profiles inform antecedent central cognitive impairment in older adults. bioRxiv. 2019;837989. [Google Scholar]
- 18. Saykin AJ, Shen L, Yao X, et al. ; Alzheimer's Disease Neuroimaging Initiative. Genetic studies of quantitative MCI and AD phenotypes in ADNI: Progress, opportunities, and plans. Alzheimers Dement. 2015;11(7):792–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weiner MW, Veitch DP, Aisen PS, et al. ; Alzheimer's Disease Neuroimaging Initiative. Recent publications from the Alzheimer's Disease Neuroimaging Initiative: Reviewing progress toward improved AD clinical trials. Alzheimers Dement. 2017;13(4):e1–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Toledo JB, Arnold M, Kastenmuller G, et al. ; Alzheimer's Disease Neuroimaging Initiative and the Alzheimer Disease Metabolomics Consortium. Metabolic network failures in Alzheimer's disease: A biochemical road map. Alzheimers Dement. 2017;13(9):965–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crane PK, Carle A, Gibbons LE, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav. 2012;6(4):502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gibbons LE, Carle AC, Mackin RS, et al. ; Alzheimer’s Disease Neuroimaging Initiative. A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012;6(4):517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Risacher SL, Kim S, Shen L, et al. The role of apolipoprotein E (APOE) genotype in early mild cognitive impairment (E-MCI). Front Aging Neurosci. 2013;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nho K, Kim S, Risacher SL, et al. Alzheimer's Disease Neuroimaging Initiative. Protective variant for hippocampal atrophy identified by whole exome sequencing. Ann Neurol. 2015;77(3):547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benjamini Y, Hochberg Y.. Controlling the false discovery rate - A practical and powerful approach to multiple testing. J R Stat Soc B Met. 1995;57(1):289–300. [Google Scholar]
- 26. Arnold M, Nho K, Kueider-Paisley A, et al. Sex and APOE epsilon4 genotype modify the Alzheimer's disease serum metabolome. Nat Commun. 2020;11(1):1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simpson BN, Kim M, Chuang YF, et al. Blood metabolite markers of cognitive performance and brain function in aging. J Cereb Blood Flow Metab. 2016;36(7):1212–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whiley L, Sen A, Heaton J, et al. Evidence of altered phosphatidylcholine metabolism in Alzheimer's disease. Neurobiol Aging. 2014;35(2):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Proitsi P, Kim M, Whiley L, et al. Plasma lipidomics analysis finds long chain cholesteryl esters to be associated with Alzheimer's disease. Transl Psychiatry. 2015;5:e494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Proitsi P, Kim M, Whiley L, et al. Association of blood lipids with Alzheimer's disease: A comprehensive lipidomics analysis. Alzheimers Dement. 2017;13(2):140–151. [DOI] [PubMed] [Google Scholar]
- 31. Cramer PE, Cirrito JR, Wesson DW, et al. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science (New York, NY). 2012;335(6075):1503–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sanchez-Mejia RO, Mucke L.. Phospholipase A2 and arachidonic acid in Alzheimer's disease. Biochim Biophys Acta. 2010;1801(8):784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gaudin M, Panchal M, Auzeil N, et al. Choline-containing phospholipids in microdissected human Alzheimer's disease brain senile plaque versus neuropil. Bioanalysis. 2012;4(17):2153–5159. [DOI] [PubMed] [Google Scholar]
- 34. Ridgway ND. The role of phosphatidylcholine and choline metabolites to cell proliferation and survival. Crit Rev Biochem Mol Biol. 2013;48(1):20–38. [DOI] [PubMed] [Google Scholar]
- 35. Rushworth JV, Hooper NM.. Lipid rafts: Linking Alzheimer's amyloid-beta production, aggregation, and toxicity at neuronal membranes. Int J Alzheimers Dis. 2010;2011:603052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vardy ER, Catto AJ, Hooper NM.. Proteolytic mechanisms in amyloid-beta metabolism: Therapeutic implications for Alzheimer's disease. Trends Mol Med. 2005;11(10):464–472. [DOI] [PubMed] [Google Scholar]
- 37. El Gaamouch F, Jing P, Xia J, Cai D.. Alzheimer's disease risk genes and lipid regulators. J Alzheimers Dis. 2016;53(1):15–29. [DOI] [PubMed] [Google Scholar]
- 38. Martin V, Fabelo N, Santpere G, et al. Lipid alterations in lipid rafts from Alzheimer's disease human brain cortex. J Alzheimers Dis. 2010;19(2):489–502. [DOI] [PubMed] [Google Scholar]
- 39. Mapstone M, Cheema AK, Fiandaca MS, et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med. 2014;20(4):415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dorninger F, Moser AB, Kou J, et al. Alterations in the plasma levels of specific choline phospholipids in Alzheimer's disease mimic accelerated aging. J Alzheimers Dis. 2018;62(2):841–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schmitz G, Ruebsaamen K.. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis. 2010;208(1):10–18. [DOI] [PubMed] [Google Scholar]
- 42. Aikawa S, Hashimoto T, Kano K, Aoki J.. Lysophosphatidic acid as a lipid mediator with multiple biological actions. J Biochem. 2015;157(2):81–89. [DOI] [PubMed] [Google Scholar]
- 43. Gonzalez-Freire M, Moaddel R, Sun K, et al. Targeted metabolomics shows low plasma lysophosphatidylcholine 18:2 predicts greater decline of gait speed in older adults: The Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2019;74(1):62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Haughey NJ, Bandaru VV, Bae M, Mattson MP.. Roles for dysfunctional sphingolipid metabolism in Alzheimer's disease neuropathogenesis. Biochim Biophys Acta. 2010;1801(8):878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cristofano A, Sapere N, La Marca G, et al. Serum levels of acyl-carnitines along the continuum from normal to Alzheimer's dementia. PLoS One. 2016;11(5):e0155694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tarasenko TN, Cusmano-Ozog K, McGuire PJ.. Tissue acylcarnitine status in a mouse model of mitochondrial beta-oxidation deficiency during metabolic decompensation due to influenza virus infection. Mol Genet Metab. 2018;125(1-2):144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mihalik SJ, Goodpaster BH, Kelley DE, et al. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring, Md). 2010;18(9):1695–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kien CL, Everingham KI, Stevens RD, Fukagawa NK, Muoio DM.. Short-term effects of dietary fatty acids on muscle lipid composition and serum acylcarnitine profile in human subjects. Obesity (Silver Spring, Md.). 2011;19(2):305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Soeters MR, Serlie MJ, Sauerwein HP, et al. Characterization of D-3-hydroxybutyrylcarnitine (ketocarnitine): An identified ketosis-induced metabolite. Metabolism. 2012;61(7):966–973. [DOI] [PubMed] [Google Scholar]
- 50. Schooneman MG, Vaz FM, Houten SM, Soeters MR.. Acylcarnitines: Reflecting or inflicting insulin resistance? Diabetes. 2013;62(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gonzalez-Dominguez R, Garcia-Barrera T, Vitorica J, Gomez-Ariza JL.. Metabolomic screening of regional brain alterations in the APP/PS1 transgenic model of Alzheimer's disease by direct infusion mass spectrometry. J Pharm Biomed Anal. 2015;102:425–435. [DOI] [PubMed] [Google Scholar]
- 52. Reuter SE, Evans AM.. Carnitine and acylcarnitines: Pharmacokinetic, pharmacological and clinical aspects. Clin Pharmacokinet. 2012;51(9):553–572. [DOI] [PubMed] [Google Scholar]
- 53. Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tynkkynen J, Chouraki V, van der Lee SJ, et al. Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer's disease: A prospective study in eight cohorts. Alzheimers Dement. 2018;14(6):723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Polis B, Samson AO.. Role of the metabolism of branched-chain amino acids in the development of Alzheimer's disease and other metabolic disorders. Neural Regen Res. 2020;15(8):1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Larsson SC, Markus HS.. Branched-chain amino acids and Alzheimer's disease: A Mendelian randomization analysis. Sci Rep. 2017;7(1):13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chatterjee P, Zetterberg H, Goozee K, et al. Plasma neurofilament light chain and amyloid-beta are associated with the kynurenine pathway metabolites in preclinical Alzheimer's disease. J Neuroinflammation. 2019;16(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-beta biomarkers for Alzheimer's disease. Nature. 2018;554(7691):249–254. [DOI] [PubMed] [Google Scholar]
- 59. Ovod V, Ramsey KN, Mawuenyega KG, et al. Amyloid beta concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13(8):841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yao JK, Dougherty GG Jr., Reddy RD, et al. Altered interactions of tryptophan metabolites in first-episode neuroleptic-naive patients with schizophrenia. Mol Psychiatry. 2010;15(9):938–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reus GZ, Becker IRT, Scaini G, et al. The inhibition of the kynurenine pathway prevents behavioral disturbances and oxidative stress in the brain of adult rats subjected to an animal model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:55–63. [DOI] [PubMed] [Google Scholar]
- 62. Brundin L, Sellgren CM, Lim CK, et al. An enzyme in the kynurenine pathway that governs vulnerability to suicidal behavior by regulating excitotoxicity and neuroinflammation. Transl Psychiatry. 2016;6(8):e865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bryleva EY, Brundin L.. Kynurenine pathway metabolites and suicidality. Neuropharmacology. 2017;112(Pt B):324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ogyu K, Kubo K, Noda Y, et al. Kynurenine pathway in depression: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:16–25. [DOI] [PubMed] [Google Scholar]
- 65. Plitman E, Iwata Y, Caravaggio F, et al. Kynurenic acid in schizophrenia: A systematic review and meta-analysis. Schizophr Bull. 2017;43(4):764–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S.. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the cardiovascular health study. JAMA. 2002;288(12):1475–1483. [DOI] [PubMed] [Google Scholar]
- 67. Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR.. Blood-brain barrier transport of kynurenines: Implications for brain synthesis and metabolism. J Neurochem. 1991;56(6):2007–2017. [DOI] [PubMed] [Google Scholar]
- 68. Castellano-Gonzalez G, Jacobs KR, Don E, et al. Kynurenine 3-monooxygenase activity in human primary neurons and effect on cellular bioenergetics identifies new neurotoxic mechanisms. Neurotox Res. 2019;35(3):530–541. [DOI] [PubMed] [Google Scholar]
- 69. Reyes Ocampo J, Lugo HR, Gonzalez-Esquivel D, et al. Kynurenines with neuroactive and redox properties: Relevance to aging and brain diseases. Oxid Med Cell Longev. 2014;2014:646909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Huynh K, Barlow CK, Jayawardana KS, et al. High-throughput plasma lipidomics: detailed mapping of the associations with cardiometabolic risk factors. Cell Chem Biol. 2019;26(1):71–84.e74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Metabolomics datasets from the AbsoluteIDQ-p180 metabolomics kit used in the current analyses for the ADNI-1 and ADNI-GO/-2 cohorts are available via the Accelerating Medicines Partnership-Alzheimer’s Disease (AMP-AD) Knowledge Portal and can be accessed at http://dx.doi.org/10.7303/syn5592519 (ADNI-1; last accessed 6 july 2021) and http://dx.doi.org/10.7303/syn9705278 (ADNI-GO/-2; last accessed 6 July 2021). The full complement of clinical and demographic data for the ADNI cohorts are hosted on the LONI data sharing platform and can be requested at http://adni.loni.usc.edu/data-samples/access-data/ (last accessed 6 july 2021).