Abstract
Background and Objectives
Though exercise for care recipients receives considerable emphasis, few dyadic studies focus on caregivers. This systematic review identified dyadic exercise interventions, which measured outcomes for older adult caregivers. Studies that met inclusion criteria were examined to better understand whether caregivers derived greater benefit from exercising with care recipients, or not exercising at all.
Research Design and Methods
PRISMA guidelines were followed to identify quantitative studies of dyadic exercise interventions in which caregivers enrolled with care recipients, and either coparticipated in exercise; or while their care recipients exercised independently, caregivers received a separate, nonexercise intervention or usual care (UC). To be included, studies had to measure physical or psychosocial outcomes for caregivers. Study quality was assessed via the Downs and Black checklist.
Results
Eleven studies met inclusion criteria. In six, the dyad exercised; in five, care recipients exercised while caregivers received a separate program, or UC. Results suggest that caregivers may improve both psychosocial and physical health when exercising together with care recipients. Caregivers who did not exercise but received a separate, nonexercise intervention, such as support, education, or respite, showed psychosocial benefits. Those who received UC were less likely to derive physical or psychosocial benefits. Included studies were fair to good quality with moderate to high risk of bias.
Discussion and Implications
Often examined secondarily, caregivers are overlooked for participation in interventions with care recipients. This analysis suggests that caregivers may benefit from dyadic interventions in which they either exercise together with their care recipients or receive a separate nonexercise intervention or respite.
Keywords: Family caregivers, physical activity, Psychosocial health, physical health
Background and Objectives
As many as 36 million people in the United States provide unpaid, informal care for older adults (Giovannetti & Wolff, 2010; Riffin, Van Ness, Wolff, & Fried, 2017). Among informal caregivers, 47% are adult children and 11% are spouses or partners of the care recipients (NAC & AARP, 2015). Compared to adult child caregivers, spouses and partners (hereafter referred to collectively as spouses) typically provide more hours per week of care (Pinquart & Sorensen, 2011), and feel a greater sense of obligation to be carers (Riffin et al., 2017; Wolff, Spillman, Freedman, & Kasper, 2016).
Higher-hour spousal caregivers report worse physical health, greater stress, anxiety, and depression, a diminished sense of well-being and self-efficacy (Pinquart & Sorensen, 2003; Riffin et al., 2017), and poorer performance of activities of daily living (Jenkins, Kabeto, & Langa, 2009). Additionally, longer-term spousal caregivers report progressively higher levels of burden (Swinkels, Broese van Groenou, Boer, & Tilburg, 2019). They are also at greater risk of morbidities including frailty (Dassel & Carr, 2016), hypertension, cardiovascular disease (Capistrant, Moon, Berkman, & Glymour, 2012), dementia (Dassel, Carr, & Vitaliano, 2017), and premature mortality (Fredman et al., 2008, 2010; Schulz & Beach, 1999).
Moreover, older spousal caregivers who have provided care for a longer period of time are less likely to engage in activities that improve their health (Queen, Butner, Berg, & Smith, 2019). Taken together, the increased risks associated with being spousal caregivers not only affects their own health, but may ultimately limit their ability to continue providing care to loved ones. As such, it is essential to identify, evaluate, design, and implement effective interventions that address caregiver health and well-being; and physical activity-focused interventions are one area of research that merits further exploration.
Physical activity (PA) interventions, including exercise, have proven efficacious for older adults; reducing their risk of chronic diseases, preserving functional capabilities, enhancing cognition and psychological well-being, and enriching community and social engagement—all of which are essential to healthy aging among an ever-increasing older adult population (Bauman, Merom, Bull, Buchner, & Singh, 2016). Similarly for caregivers of adults with a variety of chronic diseases, recent reviews suggest that PA has a favorable effect on burden (Lambert et al., 2016; Orgeta & Miranda-Castillo, 2014), and some psychosocial outcomes (Lambert et al., 2016; Loi et al., 2014); but results were less robust for physical health (Lambert et al., 2016). Spousal caregivers cite a number of barriers to PA including their own mental and physical health (Cao et al., 2010; Etkin, Prohaska, Connell, Edelman, & Hughes, 2008; Hirano et al., 2011a and b; Marquez, Bustamante, Kozey-Keadle, Kraemer, & Carrion, 2012), perceptions of increased burden due to caregiving (Hirano et al., 2011b), and limited time to engage in their own self-care (Etkin et al., 2008). Interestingly, some spousal caregivers indicate they do not enjoy exercising alone (Cao et al., 2010). Many are interested in physical and leisure time activities they can engage in with their care recipients (Cao et al., 2010; Malthouse & Fox, 2014; Van’t Leven et al., 2013) to enhance their time together, and gain social participation and support (Anton, Partridge, & Morrissy, 2013).
Dyadic exercise interventions, in which both caregivers and care recipients are involved, may enhance social participation and overcome other barriers to engaging in PA, while benefitting both partners. Spousal dyads can mutually influence mental and physical health, including perceptions of well-being and quality of life, development of depression, hypertension, and cardiovascular disease (Meyler, Stimpson, & Peek, 2007); and facilitate adoption of preventative health behaviors (Falba & Sindelar, 2008; Meyler et al., 2007; Pai, Godboldo-Brooks, & Edington, 2010). Chronic disease and functional limitations in one member of a spousal dyad often result in decreases in PA between both members of the couple (Li, Cardinal, & Acock, 2013). However, spouses who remain physically active in the face of a partner’s disease can positively influence physical activity maintenance for the dyad (Li et al., 2013). This is more commonly seen in wives who were physically active prior to their partner’s disease (Li et al., 2013). Dyadic interventions also have the potential to ameliorate spousal caregivers’ restricted social participation (Baanders & Heijmans, 2007; Riffin et al., 2017; Wolff et al., 2016), and weakened relationships with friends, relatives, and especially their spousal care recipients (Anton et al., 2013; Baanders & Heijmans, 2007; Davis, Gilliss, Deshefy-Longhi, Chestnutt, & Molloy, 2011).
Lending support to the positive impact of dyadic interventions targeting couples living with chronic illnesses, a 2010 review and meta-analysis examined a range of behavioral and psychosocial, couple-oriented interventions compared to patient-only interventions (Martire, Schulz, Helgeson, Small, & Saghafi, 2010). Programs such as education, partner support, relationship counseling, coping, problem-solving skills, and health behaviors were among the included dyadic interventions, in which care recipients and caregivers either participated together, or each member of the couple received treatments separately. For both types of dyadic interventions, 80% of studies yielded promising results over and above patient-only interventions for care recipients who experienced greater improvements in pain and depression, as well as marital relationships. In contrast, only 25% of the reviewed studies indicated similar improvements to caregivers’ well-being and relationships; the remaining studies either found no significant differences (30%) or did not report on caregiver outcomes (45%).
A 2016 review of PA interventions primarily targeting only caregivers (for care recipients with Alzheimer’s, cancer, stroke, and mental illness) reported on two dyadic exercise studies that also found improvements in caregiver psychological health, as well as enhanced functional fitness in both members of the dyad (Lambert et al., 2016). However, in one study, it was unclear whether dementia caregivers were coparticipating or receiving separate treatment (Canonici et al., 2012); in the other study, stroke caregivers were coparticipating, but authors reported only descriptive statistics (Marsden et al., 2012).
Given the limited evidence for dyadic exercise interventions, especially for the effects on caregivers, this systematic review contributes to and expands the body of literature by identifying additional dyadic exercise interventions for caregivers and their older adult care recipients. Specifically, the purpose of this systematic review was to examine whether caregivers realize greater physical and psychosocial health and well-being benefits when: (a) the caregiver–care recipient dyad enrolls and exercises together, or (b) the dyad enrolls together, but then separates with the care recipient exercising and the caregiver completing a nonexercise intervention or usual care (UC). Based upon the studies that met the inclusion criteria, we examined the literature to better understand whether caregivers derived greater benefit from exercising with care recipients, or not exercising at all.
Methods
Methodological Structure
Based on the 2009 Checklist of the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) (Moher, Liberati, Tetzlaff, Altman, & Grp, 2009), we conducted a descriptive systematic review of the literature.
Eligibility Criteria
Studies
This review included randomized controlled trials (RCT), quasi-experimental, case–control, and cohort studies of dyadic exercise or physical activity interventions, in which adult caregivers of older adult care recipients were evaluated for physical and psychosocial indicators of well-being. To be included, studies had to be published in peer-reviewed journals or in press. Excluded were systematic reviews and meta-analyses, descriptive or qualitative studies, meeting abstracts, conference abstracts, editorial introductions, letters to the editor, opinions, and position statements.
Participants
Targeted participants were informal, unpaid adult caregivers, which could include spouses, adult children, and family members. Studies had to define caregivers and their older adult care recipients with physical conditions, chronic diseases, and/or memory problems. Studies examining informal caregivers of infants, children, and adolescents, as well as paid and institutional caregivers were excluded. Caregivers had to be enrolled or participating with care recipients as a dyad; or they were required as part of eligibility criteria for care recipients to participate. Studies also had to provide demographic data for caregivers, which, at a minimum, needed to include gender distribution and average age. Outcome measures for caregivers had to be reported in the results of included studies.
Interventions
Included studies had to involve interventions using some form of physical activity or exercise, where according to Caspersen, Powell, & Christenson (1985), “PA is defined as any bodily movement produced by skeletal muscles resulting in energy expenditure; and exercise is a subset of PA that is planned, structured and repetitive to improve physical fitness” (Caspersen et al., 1985). (For the purpose of this review, the two terms are used interchangeably hereafter.) Mindfulness-based activities (e.g., meditation and breathing), pharmaceutical and surgical trials were excluded, unless part of a multicomponent physical activity intervention.
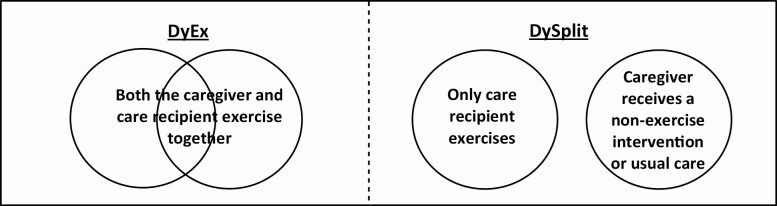
The intervention was required to target dyads in which: (a) caregivers and care recipients enrolled and coparticipated in exercise together (hereafter referred to as DyEx), or (b) the dyad enrolled together, but then separated or split into different groups, such that care recipients exercised, while caregivers received a nonexercise intervention or UC (hereafter referred to as DySplit). See Figure 1 for descriptive diagrams of DyEx versus DySplit. Interventions in which caregivers were involved primarily to assist care recipients with exercise were excluded.
Figure 1.
Descriptive diagrams of DyEx versus DySplit studies.
Comparisons
Comparison groups were not required but could include other types of physical activity, psychoeducation, support groups, counseling, dyadic training (unrelated to exercise), nutrition, day care, or other single or multicomponent interventions. “Usual care” (UC) and “treatment as usual” were also accepted as comparison groups.
Outcomes of Interest
Outcomes of interest included caregiver physical health (e.g., heart rate, body mass, biomarkers), psychosocial health (e.g., depression, burden, strain), and well-being (e.g., quality of life, sleep quality), all of which could be primary or secondary as identified by researchers in the respective studies. At a minimum, studies had to use at least one standardized and validated outcome measure. For studies coreporting on care recipients, we also examined their physical, psychosocial, and well-being outcomes. Excluded were studies only reporting descriptive statistics.
Information Sources and Search Strategy
Sources of Information
A search strategy was initially developed and executed in PubMed, then modified and conducted in Web of Science, CINAHL Plus (to include ERIC, SocINDEX Full, and SPORTDiscus), Cochrane Library, OT Seeker, Psych Info, and Scopus. The last search was run on April 17, 2017. Additional articles were identified during a limited literature update performed up to and including January 4, 2019, based on the published protocol and method papers found in the original search.
Search Strategy
To optimize search results, a combination of Medical Subjects Headings (MeSH) and field tags were used to exclude studies with infants or children, and to describe variations of key topics, namely caregivers, exercise, exercise movement techniques, and adults. Additionally, specific key words were used to describe typical exercise interventions for caregivers or older adult care recipients (e.g., walking, hiking, stretching, swimming, cycling, treadmill, strength and resistance training, yoga, tai chi, dance, and Pilates). See Table 1 for a sample search strategy. Only human subject studies using the English language were searched. To capture as many relevant articles as possible, no timeframe limit was imposed. The search yielded journal articles between January 1978 and April 17, 2017. Following completion of the searches, all references were uploaded to EndNote for further processing.
Table 1.
Sample Search Used in PubMed
| 1 | (caregivers[mh] OR caregiv*[tw] OR carer[tw] OR care giver*[tw] OR informal care*[tw]) |
| 2 | AND (exercise[mh] OR exercise[tw] OR “physical activity” OR “physical fitness” OR “leisure activity” OR walking OR hiking OR stretching OR swimming OR cycling OR treadmill OR “exercise movement techniques”[mh] OR yoga OR “tai chi” OR “tai ji” OR dance OR Pilates) |
| 3 | NOT (infant[mh] OR child[mh]) NOT (adult[mh]) |
| 4 | Filters: English |
Note: Adding “resistance training” as a MeSH [mh] or text word [tw] did not change the number of PubMed studies, because the term falls under the MeSH of “exercise.” However, “resistance training” did increase the records sufficiently in other databases to merit adding it as a key term. MeSH = Medical Subjects Headings.
Procedures for Identification and Data Collection
Study Selection
Search results were compiled and uploaded to EndNote. Duplicates were eliminated using EndNote, and by culling through each title to search for juxtaposing of full names and initials. The review team of seven people included four graduate students and three faculty members. Articles were screened for inclusion first by title, then abstract, and then full text. At each stage of screening, the article (title, abstract, or full text) was independently reviewed by at least two reviewers. Three teams of two reviewers conducted the title search with each team member independently reviewing one third of all titles. Retained titles from each reviewer were recombined and redistributed to different teams for independent abstract review. Abstracts retained from each team member were again recombined and redistributed for full article review and data extraction. To avoid rejection of relevant articles, three independent reviewers each reviewed one third of the articles; and the first author reviewed all full-text articles. All articles stemming from a single study were assessed independently for inclusion. Interrater agreement was 78% on full-text articles. Regular arbitration meetings were held to establish consensus on acceptance and rejection of all articles. For those articles needing further arbitration, the full team was consulted and the principal investigator made final decisions.
Data Collection Process
Using the 2011 Cochrane Handbook for Systematic Reviews of Interventions as guidance, a data extraction form was developed (Higgins & Green, 2011). Reviewers were trained and practiced using the developed form on three studies. Three reviewers each extracted data from one third of the full-text articles. To verify extracted data on included studies, four authors independently checked data, and then met to compare and develop consensus. All authors reviewed the final version of the evidence table before submission for publication. See Tables 2 and 3 for a full description of included data.
Table 2.
DyEx—Characteristics of Included Studies in Which Both Members of the Dyad Exercised Together (N = 6)
| Study | Participants | Caregiver interventions | Caregiver measures and outcomes | Care recipient interventions | Care recipient measures and outcomes |
|---|---|---|---|---|---|
| Badger et al. (2007) RCTPre, post (6 weeks), F/U (1 month) N dyads = 96 | CG: Partners (P), family, friends; N = 96 (71P-M/25F); age = 51.68 yearsCR with breast cancer:N = 96 (F); age = 54.11 years | CG interventions: Home via telephone delivery TIP-C: (N = 38) Telephone counseling and cancer education 6 weeks/1×/ biwk/34 min/call EX: (N = 19) Self-managed walking 4×/week; and counseling calls 6 weeks/1×/ biwk/11 min/callAC: (N = 30) Attention control printed info and calls; 6 weeks/1×/biwk/7 min/call | Depression: CES-DMEtime: All improved (p < .001)MEgrp: NSMEgxt: NS CG anxiety: Composite of 8 anxiety-related questionsMEtime: All improved (p = .02)MEgrp: NSMEgxt: NS; trend (p = .09); TIP-C and EX improved at 6 weeks, all NS at F/U | CR interventions: Same as CG, different doses TIP-C: (N = 38) 6 weeks/1×/ week/34 min/call EX: (N = 21) 6 weeks/4×/week and counseling calls 1×/week/11 min/call AC: (N = 33) 6 weeks/1×/ week/7 min/call | Depression: CES-D MEtime: NS; trend (p = .08) for all to improveMEgrp: TIP-C highest depression, but improved (p = .03) from B/L to F/U MEgxt: NSAnxiety: Eight-question compositeMEtime: All improved (p < .001)MEgrp: NSMEgxt: TIP-C (p < .001) and EX (p = .01) improved at 6 weeks only |
| Burgener et al. (2011)RCT and quasi-experimentalBaseline, post-test (20 weeks) N dyads = 38 | CG: Family members Multimodal-Dyad (MMD): N = 10 (6F/4M); age = 75.8 years Multimodal-CR (MMCR): N = 8 (5F/3M); age = 79.3 yearsControl: N = 14 (9F/5M); age = 74.6 years CR with dementia: early to middle stage (no other info) | CG interventions: CommunityMMD: (N = 10) CG chooses to exercise w/CR; Taiji, 20 weeks/3×/ week/1 hr; and support group for CGs only; 20 weeks/biwk/1.5 hr MMCR: (N = 8) Only CR exercises, CG only attends support; same dosesUC (CG only): (N = 14) UC plus educ; 20 weeks/bimon | Stress:RSSMMD vs MMCR: NS; trend (p = .12) for lower personal distress in exercising CGs Multimodal vs UC: NS CG family relationship: ECFRMMD vs MMCR: NS Multimodal vs UC: CG in multimodal fewer negative feelings toward CR (p = 0.03)All comparisons BtwGrp | CR interventions: Similar to CG Multimodal: Addition of CBT, w/cognitive training for CR only UC: Delayed intervention | CR outcomes described elsewhere |
| Lowery et al. (2014)RCT Pre, post (6 weeks), follow-up (6 weeks) N dyads = 131 | CG: Partner/spouse (p = 62.7%)Walking: N = 67 (50F/17M); age = 65.4 yearsUC: N = 64 (39F/25M); age = 60.9 years CR dementia (1 min BPSD): Walking: N = 67 (35F/32M); age = 79 yearsUC: N = 64 (39F/25M); age = 78 years | CG interventions: Home-basedWalking: (N=59); 12 weeks/5×/week/20–30 min at RPE of 12–14; 6 weeks guided by therapist; 6 weeks independentUC: (N = 57) | Burden: ZBI, at 12 weeks walking decreased burden, UC doubled (p = .01) Distress: NPI-Q, NSMental health: GHQ, NS CG on CR QOL: Dem-QOL, NSAll comparisons BtwGrp | CR interventions: Same as CG Walking: (N = 59) UC: (N = 57) | Behavior: NPI-Q, NS Health: GHQ, NS QOL: Dem-QOL, NSAll comparisons BtwGrp |
| Milbury et al. (2015) Single-arm Feasibility Trial Pre, post (5–6 weeks) N dyads = 15 | CG: Spouse (N = 6), family member (N = 3), other (N = 6); N = 15 (6F/9M); age = 58.95 years CR with lung cancer:N = 15 (4F/11M); age = 62.16 years | CG intervention: Clinic- basedVivekananda Yoga: (N = 9); yoga exercises, breathing, relaxation, and meditation; 5–6 weeks/2–3×/ week/60 min; home practice encouraged; printed materials provided | Psychological distress: BSI-18, NS depression (ES = 0.25); NS anxiety (ES = 0.03); NS somatization (ES = 0.06) Fatigue: BFI, NS (ES = 0.08) Sleep: PSQI, reduction in sleep disturbances (p = .02, ES = 1.01) Health-related QOL: SF-36, NS physical (ES = 0.50); NS mental (ES = 0.08) Spiritual well-being: FACT, NS (ES = 0.24) Benefit finding: BFCS, NS (ES = 0.12) Emotional intimacy: CLOSE, NS (ES = 0.07)All comparisons t-tests | CR intervention: Same as CG Vivekananda Yoga: (N = 9) | Psychological distress: BSI-18Anxiety decreased (p = .04, ES = 0.81); NS depression (ES = 0.28); NS somatization (ES = 0.65) Fatigue: BFI, NS, (ES = 0.12) Sleep: PSQI, NS (ES = 0.36) Health-related QOL: SF-36, mental improved (p = .04, ES = 0.84); NS physical (ES = 0.15) Spiritual well- being: FACT, NS (ES = 0.31) Benefit finding: BFCS, NS (ES = 0.64)Emotional intimacy: CLOSE, NS (ES = 0.19)All comparisons t-tests |
| Milbury et al. (2018) Single-arm Feasibility Trial Pre, post (5–6 weeks)N dyads = 5 | CG: Spouse (N = 3), sibling, adult child (N = 2); N = 5 (3F, 2M); age = 58.16 years CR with high-grade glioma:N = 5 (4F/1M); age = 51.94 years | CG intervention: Clinic-basedVivekananda Yoga: (N = 5); yoga exercises, breathing, relaxation, and meditation; 5–6 weeks/2–3×/ week/60 min; home practice encouraged; DVD provided at week 5 | Fatigue: BFI, NS (ES = 0.21)Depression: CES-D, improved (p = .08, ES = 1.04)Report CR Sx severity: MDASI, Improved perception of CRs’ Sx (p = .05, ES = 1.25); NS total score (ES = .67); NS Sx interference (ES = 0.32) Health-related QOL: SF-36, NS physical (ES = 0.48); NS mental (ES = 0.64) Sleep: PSQI, NS (ES = 0.49)All comparisons t-test; authors set alpha p = .10 | CR intervention: Same as CGVivekananda Yoga: (N = 5) | Fatigue: BFI, NS Depression: CES-D, NS (ES = 0.59)Sx severity: MDASI, improved total score (p = .08, ES = 1.03); NS Sx inventory (ES = 0.81); NS Sx interference (ES = 0.79)Health-related QOL: SF-36, NS physical (ES = 0.07); NS mental (ES = 0.60) Sleep: PSQI; better sleep quality (p = .10, ES = 1.17)All comparisons t-test; authors set alpha p = .10 |
| Winters-Stone et al. (2016) RCTPre, post (3 months), F/U (6 months) N dyads = 64 |
CG: Female spouses; N = 64FExercising Together (ET)Strength: age = 66.5 yearsUC: age = 69.7 years CR-prostate cancer survivor:N = 64MET-Strength: age = 70.6 yearsUC: age = 72.9 years |
CG interventions: University facilityET: (N = 32); progressive strength training with partnered aspects; 6 months/2×/week/60 min with 8–10 upper and lower body exercises/8–15 reps/ea/4%–15% body weightUC: (N = 32); at conclusion of study, received instructional video for home use and a workshop | Body composition: DXAET gained lean mass (p = 0.05) Maximal muscle strength: 1RMET improved upper body (p < .01), lower body (p < .01), and chair stand (p = .02), but NS for gait speed Physical function: PPBET improved (p = .01)Self-reported health: SF-36NS in physical health, mental health, or vitality/fatigue Self-reported PA: CHAMPS, NSAll comparisons BtwGrp | CR interventions: Same as CGET: (N = 32)UC: (N = 32) | Body composition: DXA, NSMaximal muscle strength: 1RMET improved upper body (p < .01); but NS in lower body, chair stand, and gait speedPhysical function: PPB, NSSelf-reported health: SF-36, NS in physical health, or vitality/fatigue, but trend (p = .06) for improved mentalSelf-reported PA: CHAMPS ET more PA, UC decreased (p < .01)All comparisons BtwGrp |
Key - General Abbreviations: B/L = baseline; BPSD = behavioral and psychological symptoms of dementia; CG=caregiver; CR=care recipient; F/U=follow up; PA=physical activity; RPE=rating of perceived exertion;Sx=symptoms; UC=usual care (also treatment as usual).
Key - Outcomes & Measures Abbreviations:1RM = 1-repetition maximum; BFCS = Benefit Finding in Cancer Scale; BFI = Brief Fatigue Inventory; BSI-18 = Brief Symptoms Inventory-18; CES-D = Center for Epidemiological Studies-Depression Scale; CHAMPS = Community Healthy Activities Model Program for Seniors; CLOSE = Perceived Closeness and Responsiveness Measure; Dem-QOL = Dementia Health-Related Quality of Life; DXA = Dual-energy X-ray Absorptiometry; ECFR = Elder-Caregiver-Family Relationship scale; FACT = Functional Assessment of Cancer Therapy; GHQ = General Health Questionnaire; MDASI = MD Anderson Symptom Inventory; SF-36 = Medical Outcomes Study 36-item Short-Form Survey; NPI-Q = Neuropsychiatric Inventory; PPB = Physical Performance Battery; PSQI = Pittsburgh Sleep Quality Index; RSS = Relatives Stress Scale.
Key - Statistical Tests Abbreviations: BtwGrp = between groups; ES = effect size; MEtime = main effect of time; MEgrp = main effect of group; MEgxt = effect of grp x time; t-test = paired t-test.
Table 3.
DySplit—Characteristics of Included Studies in Which Care Recipients Exercised, and Caregivers Received a Nonexercise Intervention or UC (N = 5)
| Study | Participants | Caregiver interventions | Caregiver measures and outcomes | Care recipient interventions | Care recipient measures and outcomes |
|---|---|---|---|---|---|
| Barnes et al. (2015)Pilot, non RCTB/L, cross-over (18 weeks), post (36 weeks) N dyads = 12 | CG: Partners (N = 2M), Daughters (N = 9D); N = 11 (2M/9F)FM: N = 6 (5D/1M-P); age = 57.5 yearsUC: N = 5 (4D/1M-P); age = 54.6 yearsCR with mild to moderate dementia:N = 11FM: N = 6 (5F/1M); age = 85.67 yearsUC: N = 5 (4F/1M); age = 81.6 years | CG interventions: HomeGrp 1 FM: (N = 6 at 18 weeks, and N = 3 at 36 weeks); UC for CG, but instructors met with the dyad to provide exer instruction for CR and assess CR goals and interests; four in-home visits over 18 weeks, plus biwkly calls to CG for reporting of CR adverse events Grp 2 UC: (N = 4 at 18 weeks, and N = 6 at 36 weeks); biwkly calls for adverse events Cross-over design: From 1 to 18 weeks, Grp 1 in FM and Grp 2 in UC; from 19–36 weeks, Grp 1 in UC and Grp 2 in FM | Distress: NPI-QBtwGrp: NS (ES + 0.21) w/iGrp1: NS (ES + 0.26) w/iGrp2: NS (ES + 0.49) Burden: CBIBtwGrp: NS (ES + 0.49)w/iGrp1: NS (ES + 1.92)w/iGrp2: NS (ES − 0.05) CR Func’l Ability: ADCS-ADLBtwGrp: NS (ES = 0.07)w/iGrp1: NS (ES 0.12) w/iGrp2: NS (ES − 0.31) CR Behavior: NPI-QBtwGrp: NS (ES + 0.02)w/iGrp1: NS (ES + 0.59)w/iGrp2: NS (ES − 1.22)CR QOL: QOL-AD BtwGrp: NS (ES + 0.33) w/iGrp1: NS (ES + 0.50) w/iGrp2: NS (ES + 0.47) All BtwGrp comparisons at 18 weeks. For effect sizes, +Favors FM, –Favors UC, =Both groups same; authors defined ES ≥ 0.25 as clinically meaningful. | CR interventions: Adult day care Grp 1 Functional Movement (FM): (N = 6 at 18 weeks; N = 4 at 36 weeks); combination of physical therapy occupational therapy, yoga, Tai Chi, dance; 18weeks/2 days/ week/45 min Grp 2 UC: (N = 5 at 18 weeks, N = 6 at 36 weeks); usual care of chair-based exercises, art, music; 18 weeks/2 days/ week/20 min Cross-over design: same as CGs | Physical function: PPB and SFT Lower extremity function BtwGrp: NS (ES + 0.34) w/iGrp1: NS (ES + 0.25) w/iGrp2: NS (ES + 0.34) Sit and reach BtwGrp: NS (ES – 0.32) w/iGrp1: NS (ES – 0.49) w/iGrp2: NS (ES + 0.71) Back scratch BtwGrp: NS (ES + 0.35) w/iGrp1: NS (ES + 0.99) w/iGrp2: NS (ES – 0.40) Mobility-8ft up and go BtwGrp: NS (ES + 0.24) w/iGrp1: NS (ES + 0.29) w/iGrp2: NS (ES + 0.32) Cognitive function: ADAS BtwGrp: NS (ES + 0.76) w/iGrp1: NS (ES + 0.55) w/iGrp2: NS (ES + 0.38) Quality of life: QOL-AD BtwGrp: NS (ES + 0.83) w/iGrp1: NS (ES + 1.61) w/iGrp2: NS (ES – 1.06) |
| Lamb et al. (2018) RCT Pre, post (6 months), F/U (12 months) N dyads = 459 | CG: Spouse (S), Adult Child (AC), or Other (O) Ex ± UC: N = 305 (218F/87M); 239(S), 55(AC), 11(O); age = 69.1 years UC: N = 154 (125F/29M), 117(S), 32(AC), 4(O); age = 70.2 years CR with mild to moderate dementia:Ex ± UC: N = 329 (195M/134F); age = 76.9 years UC: N = 165 (106M/59F); age = 78.4 years | CG interventions: Community and home Ex ± UC: (N = 258; 184F/74M) UC: (N = 129; 104F/25M) All CGs received UC | Burden: ZBI, NS Health-related QOL: EQ-5D, NS All comparisons are main effects at 12 months. | CR interventions: Same as CGs Ex ± UC: (N = 278; 112F/166M); cycling (25 min) and strength training (3 sets/20 reps), moderate to hard intensity, plus usual care; doses: (1) gym: 4 months/2×/ week/60–90 min; (2) home: 4 months/1 hr/week; (3) postintervention home: 150 min/week with behavioral strategies UC: (N = 137; 51F/86M); usual care included clinical assessment, carer counseling, Rx treatments, brief PA advice | Cognition: ADAS, both groups declined, exer significantly worse (p = .03) Praxis/memory/ language: ADAS, NS ADLs: BADL-proxy by CG, NS Behavior: NPI-proxy by CG, NS Health-related QOL: EQ-5D, NS Quality of life: QOL-AD, NS Falls and fractures: NS 6-min walk test: 6MWD, Ex group improved by 18.1 m (p = .001); only tested in Ex group |
| Maci et al. (2012) Pilot RCT B/L, post (3 months) N dyads = 14 | CG: Family members; wives (W), husbands (H), daughters (D), sons (S) PA ± Cog ± Soc: N = 7 (3W/3D/1S); age = 54.6 years UC: N = 7 (3W/2H/2D); age = 60.4 years CR with Alzheimer’s Disease:PA ± Cog ± Soc: N = 7 (4F/3M); age = 75 yearsUC: N = 7 (4F/3M); age = 70.3 years | CG interventions: Respite PA ± Cog ± Soc: CGs received 12 weeks/3–4 hrs/day (15–20 hr/week); researchers transported CRs to and from gym setting UC: Usual care | Burden: CBI, PA+ improved (p < .05); UC worsened (p < .05) Depression: BDI, PA + improved (p < .05); UC worsened (p < .05) QOL of CR: QOL-AD, PA + improved (p < .05); UC worsened (p < .05) All comparisons w/iGrp. | CR interventions: Community PA ± Cog ± Soc: Physical activity (aerobic, balance, gait, coordination) at mild intensity, plus cognitive stimulation, and socialization; 12 weeks/5×/ week/60 min (PA) + 60 min(cog) +60 min(soc) UC: Treatment as usual | Cognitive: MMSE, NS either group ADLs: NS either group Executive function: FAB, UC worse (p < .05); NS PA+ Anxiety: HAM, PA+ improved (p < .05); NS UC Depression: CSDD, PA + improved (p < .05); UC worsened (p < .05) Apathy: AES, PA+ improved (p < .05); NS UC QOL: QOL-AD (total and patient), PA + improved (p < .05); UC worsened (p < .05) QOL: CBS, PA + improved (p < .05); NS UC |
| Marques et al. (2015) Mixed Methods w/a Single Arm Feasibility Trial B/L, post (12 weeks) N dyads = 9 | CG: Family members; N = 9 (8F/ 1M); age = 63.8 years CR with COPD: COPD, N = 35 (2F/7M); age = 69.6 years Demographics provided only on the portion of participants involved in the feasibility trial. | CG intervention: Primary care clinic Psychosoc ± Educ: psychosocial support and education for CG and CR together; 12 weeks/1×/week/90 min | Family coping: F-COPES, improved in passive appraisal (p = .043), and total score (p = .011); NS in social support, reframing, spiritual support, and accepting helpAdjustment to illness: PAIS-SR, improved in sexual relationship (p = .013), and psychological distress (p = .012); NS in health care orientation, domestic environment, extended family relationships, social environment, and total score All comparisons t-tests. | CR intervention: Primary care clinic Rehab ± Psychosoc ± Educ: Pulmonary rehabilitation (endurance, resistance, balance), 12 weeks/3×/ week/60 min; plus psychosocial support and education for CR and CG together, 12 weeks/1×/ week/90 min | Dyspnea: mMRC, NS Quadriceps strength: 10RM, increased strength (p = .002) Walking distance: 6MWD, increased by 26.8 m (p = .023) Mobility and balance: TUG, improved (p = .002) Health-related QOL: SGRQ, NS Family coping: F-COPES, improved in social support (p = .018), accepting help (p = .027), passive appraisal (p = .043), and total score (p = .026); NS in reframing and spiritual support. Adjustment to illness: PAIS-SR, NS in all subscales and total All comparisons t-tests. |
| Yu et al. (2015) Pilot, Feasibility Study w/a Single. Arm B/L, post (3 months), F/U (6 months) N dyads = 26 | CG: Family members; N = 26 (20F/6M); age = 64 years CR with Alzheimer’s Disease:N = 26 (16F/10M) community- dwelling, age = 78 years | CG intervention: Respite CGs received 8–10 hr/ week; researchers transported CRs to and from gym setting | Distress: NPI-Q, B/L to 3 months: NS, though an 8% decrease 3 to 6 months: Decrease of 32% (p < .05) CR behavior: NPI-Q, 3 and 6 months: NS, but maintained B/L score All comparisons MEtime. | CR interventions: Community Cycling: Moderate intensity cycling, supervised, individualized, 6 months/3×/week/15–45 min, plus 10 min each of warm-up and cool down | Cognition: ADAS, 3 and 6 months: NS, but maintained B/L score ADLs: DAD 3 and 6 months: NS, but maintained B/L score All comparisons MEtime. |
Key - General Abbreviations: ADLs = activities of daily living; B/L = baseline; CG = caregiver; CR = care recipient; F/U = follow up; PA = physical activity; Rx = prescription; UC = usual care (also treatment as usual).
Key - Measures Abbreviations: 6MWD = 6-meter walking distance; 10RM = 10-repetition maximum; ADAS = Alzheimer’s Disease Assessment Scale; ADCS-ADL = Alzheimer’s Disease Cooperative Study—Activities of Daily Living; AES = Apathy Evaluation Scale; BADL = Bristol Activity of Daily Living Index; BDI = Beck Depression Inventory; CBI = Caregiver Burden Inventory; CBS = Cornell-Brown Scale for Quality of Life in Dementia; CSDD = Cornell Scale for Depression in Dementia; DAD = Disability Assessment for Dementia; EQ-5D = EuroQOL-5 dimension; FAB = Frontal Assessment Battery; F-COPES = Family-Crisis Oriented Personal Scale; HAM = Hamilton Anxiety Rating Scale; mMRC = Modified Medical Research Council Dyspnea Scale; MMSE = Mini-Mental State Exam; NPI-Q = Neuropsychiatric Inventory Questionnaire; PAIS-SR = Psychosocial Adjustment to Illness Scale-Self-Report; PPB = Physical Performance Battery; QOLAD = Quality of Life Scale in Alzheimer’s Disease; SFT = Senior Fitness Test; SGRQ = St. George’s Respiratory Questionnaire; TUG = Timed Up and Go test; ZBI = Zarit Burden Interview.
Key - Statistical Tests Abbreviations: BtwGrp = between groups; ES = effect size; MEtime = main effect of time; SD = standard deviation; w/iGrp = within group.
Assessment of Quality and Risk of Bias in Individual Studies
Given the diversity of the included research, we opted to use the Downs and Black checklist to provide a common scoring system for assessing quality and risk of bias in both nonrandomized and randomized control trials (Downs & Black, 1998). Question 27, which addresses statistical power, was modified from a possible score of five points to one point for analyzing and achieving adequate power or to zero points for no power calculations. Thus, studies are rated excellent (26–28), good (20–25), fair (15–19), and poor (≤14) (Chudyk, Jutai, Petrella, & Speechley, 2009). Regular meetings were held to establish consensus on scoring. No studies were excluded on the basis of score.
Data Synthesis
Given the heterogeneity of caregivers and care recipients, as well as included levels of evidence and methodologies, narrative synthesis was used to report results and discuss intervention effectiveness.
Results
Study Selection
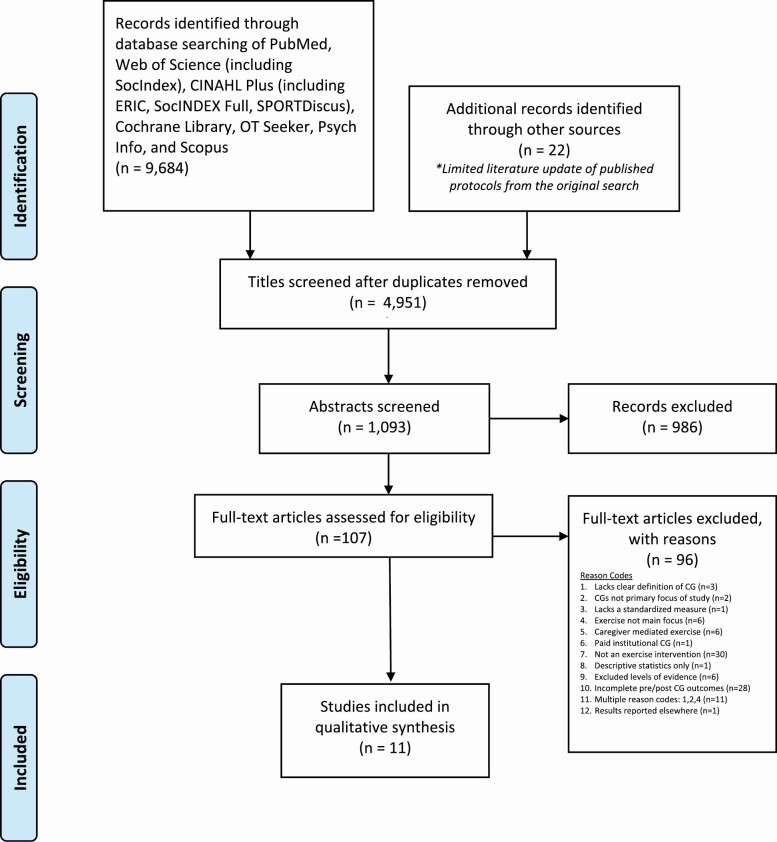
The database search yielded 9,684 articles, of which 4,733 articles were duplicates, which occurred due to the replication of studies catalogued across all searched databases. An additional 22 articles were identified based upon published protocol and method papers found in the original database search. After the removal of duplicates, reviewers screened the remaining 4,951 titles. Using the research question and inclusion criteria to determine selection, title screening yielded 1,093 abstracts meeting the established criteria (Figure 2). Following abstract screening, 107 articles remained and went through full-text review. Of the full-text articles, 96 did not meet the inclusion criteria, and thus were excluded for the reasons noted in Figure 2 (e.g., studies lacked a clear definition of the caregiver; exercise was not the primary focus of the study, etc.). Eleven articles met inclusion criteria. All final articles were cross-referenced within Retraction Watch, and researched in PubMed and Google Scholar on January 17, 2019 with no retractions issued for any included articles.
Figure 2.
PRISMA flow chart of study selection.
Study Characteristics
Final studies included five RCTs (Badger, Segrin, Dorros, Meek, & Lopez, 2007; Lamb et al., 2018; Lowery et al., 2014; Maci et al., 2012; Winters-Stone et al., 2016), and six nonRCTs (Barnes et al., 2015; Burgener, Marsh-Yant, & Nega, 2011; Canonici et al., 2012; Marques et al., 2015; Milbury et al., 2015, 2018; Yu et al., 2015). Study designs and characteristics can be found in Tables 2 and 3. Sample sizes ranged from 5 to 459 participants. To best interpret the results, studies were grouped into two categories: (a) caregiver–care recipient dyads exercised together (DyEx; N = 6; Table 2) and (b) caregiver–care recipient dyads were enrolled, but only care recipients exercised while caregivers received a separate, nonexercise intervention or UC (DySplit; N = 5; Table 3).
Participants
Overall, studies enrolled 862 family caregivers (DyEx = 343, DySplit = 518) with a mean age of 67.1 years (DyEx = 66.1 years, DySplit = 70.5 years), and 69.4% were female caregivers (DyEx = 63.2%, DySplit = 75.3%). DyEx had a higher percentage of male caregivers due to one study of 96 dyads conducted for females with breast cancer and male spouses (Badger et al., 2007). Across studies, spouses comprised 68.1% of caregivers (DyEx = 58.9%, DySplit = 70.5%); 29.4% were adult children (DyEx = 39.4%, DySplit = 26.4%).
Care recipients were older adults with Alzheimer’s disease or dementia (DyEx = 2, DySplit = 4), cancer (DyEx = 4), and chronic obstructive pulmonary disease (DySplit = 1). The 865 care recipients (DyEx = 311, DySplit = 554) were a mean age of 72.3 years (DyEx = 66.9 years, DySplit = 76.9 years); 46.9% were female (DyEx = 57.2%, DySplit = 40.7%), and 53.1% male (DyEx = 42.8%, DySplit = 58.8%). One study reported demographics for care recipients elsewhere (Burgener et al., 2011).
Interventions and Comparison Groups
Interventions and exercise prescriptions in DyEx and DySplit varied in length from 5 weeks to 6 months, 2–5 days per week for 45 min to 1.5 hr per session. Across the included studies, exercise protocols varied in intensity. Five studies prescribed the exercises as low intensity or low impact (Badger et al., 2007; Barnes et al., 2015; Burgener et al., 2011; Milbury et al., 2015, 2018), but none provided a specific definition based on exercise physiology measures. Of the studies employing moderate- to high-intensity protocols for aerobic exercise, measures varied and included the use of a perceived exertion rating of 12–14 (Lowery et al., 2014; Yu et al., 2015), 55% VO2max (Maci et al., 2012), 65%–75% heart rate reserve (Yu et al., 2015), and 60%–80% of a 6-min walk test (6MWT) at baseline assessment (Marques et al., 2015). One study used a more general definition and noted that the moderate to high-intensity aerobic and strength training components were based on participants’ tolerance, in combination with baseline performance of the 6MWT (Lamb et al., 2018). Studies using more specific measures of resistance training protocols noted an 8–15 repetition maximum (RM) for upper body, an 8–15 RM at 4%–15% of body weight for lower body (Winters-Stone et al., 2016), and 50%–85% of the 1RM for upper and lower body (Marques et al., 2015).
DyEx interventions included self-managed walking programs (Badger et al., 2007; Lowery et al., 2014), yoga (Milbury et al., 2015, 2018), strength training (Winters-Stone et al., 2016), and taiji (Burgener et al., 2011). In three studies, dyads engaged in some exercises as a co-occupation (Milbury et al., 2015, 2018; Winters-Stone et al., 2016), such that they entailed interactive and interdependent participation as a couple. The other three studies entailed the dyads performing the same exercises, but did not require coordinated interaction (Badger et al., 2007; Burgener et al., 2011; Lowery et al., 2014). Of the DyEx interventions, four utilized comparison cohorts (Badger et al., 2007; Burgener et al., 2011; Lowery et al., 2014; Winters-Stone et al., 2016). One study, Badger et al. (2007), compared exercise to telephone counseling and an attention control. The other three studies employed UC cohorts (Burgener et al., 2011; Lowery et al., 2014; Winters-Stone et al., 2016); however, none defined what UC entailed. Burgener and colleagues (2011) offered bimonthly educational programs in conjunction with UC to control for the attention given to the treatment cohort and Winters-Stone and colleagues (2016) noted that UC participants were also directed to maintain their typical physical activities. The remaining two DyEx studies (Milbury et al., 2015, 2018) did not utilize a comparison group.
Four DySplit studies employed mixed modalities of exercise for care recipients (Barnes et al., 2015; Lamb et al., 2018; Maci et al., 2012; Marques et al., 2015); the fifth study involved cycling (Yu et al., 2015). In all five studies, care recipients exercised, while caregivers received separate, nonexercise interventions or UC. Three DySplit studies provided a separate intervention to caregivers (Marques et al., 2015; Maci et al., 2012; Yu et al., 2015). Of the studies providing a separate intervention, one study targeted both members of the dyad to receive psychosocial support and education together once weekly for 90 min over 12 weeks (Marques et al., 2015). The two other DySplit studies that offered a separate intervention for caregivers arranged for them to receive 8–20 hr per week of respite by transporting care recipients to and from intervention settings (Maci et al., 2012; Yu et al., 2015). Caregivers received UC in two studies (Barnes et al., 2015; Lamb et al., 2018). The study by Barnes and colleagues (2015) also utilized a UC cohort, which was undefined for the caregiver, but was supplemented with four in-home visits and biweekly calls to the dyad; however, the emphasis was on the care recipient’s exercise, goals, and adverse events (Barnes et al., 2015). For care recipients in Barnes and colleagues (2015), the UC cohort continued with seated exercises and activities typical of an adult day care. In Lamb and colleagues (2018), the UC-only cohort received the typical clinical guidance offered to caregiver–care recipient dyads.
Outcomes
Caregiver Psychosocial Well-Being
All studies (N = 11) examined psychosocial well-being of caregivers with emphasis on mental health (depression, anxiety, distress, stress), quality of life (burden, fatigue, sleep, and general QOL), relationships (couple, family, social, spiritual), and perceptions of care recipients (symptoms, behavior, QOL). Results were mixed across and within DyEx and DySplit studies.
Of the DyEx studies, six examined caregiver psychosocial well-being. Beneficial outcomes in four studies indicated significant improvements in mental health (Badger et al., 2007; Canonici et al., 2012), QOL (Burgener et al., 2011; Canonici et al., 2012; Lowery et al., 2014), relationship quality (Burgener et al., 2011), and perceptions of the care recipients’ symptoms (Milbury et al., 2018). Though nonsignificant, two studies reported trends or moderate effect sizes suggesting enhancements to some aspects of mental health (Burgener et al., 2011; Milbury et al., 2018). However, no significant findings were reported for other mental health indicators (Burgener et al., 2011; Lowery et al., 2014; Milbury et al., 2015; Winters-Stone et al., 2016), QOL (Milbury et al., 2015, 2018), relationships (Milbury et al., 2015), or perceptions of care recipients (Lowery et al., 2014). One small pilot study of five dyads doing yoga reported significant worsening of depression with a large effect size (Milbury et al., 2018); researchers surmised the results may have been due to the intervention’s secondary focus on mindfulness, which could have resulted in caregivers accepting the poor prognosis for their loved ones.
In three DySplit interventions, caregivers experienced significant improvements to mental health when they were offered nonexercise interventions of either respite (Maci et al., 2012; Yu et al., 2015) or a dyadic support group (Marques et al., 2015), while their care recipients exercised. The support group intervention also saw significant relationship benefits (Marques et al., 2015). Two studies that provided UC did not realize any significant changes to caregiver mental health or QOL (Barnes et al., 2015; Lamb et al., 2018). Three studies examined caregivers’ perceptions of care recipients’ health, but results were mixed such that one study reported improvements (Maci et al., 2012), while two others saw no significant differences (Barnes et al., 2015; Yu et al., 2015).
Caregiver Physical Well-Being
Three DyEx studies measured caregiver physical health. A resistance training intervention noted significant increases in muscle mass, strength, and physical function, but no significant difference in gait speed (Winters-Stone et al., 2016). Self-reported physical outcomes were equivocal. One study demonstrated significant increases in physical health and activity (Winters-Stone et al., 2016), whereas two yoga studies conducted by the same researchers reported nonsignificant, oppositional findings in physical well-being—one indicating improvements (Milbury et al., 2015), the other showing decrements (Milbury et al., 2018). In their later study, Milbury and colleagues (2018) did not specifically address why caregivers’ physical well-being may have decreased; however, researchers did note the lack of a control group and small sample size were limitations to ascertaining the strength of results. No DySplit interventions examined physical well-being in caregivers.
Care Recipient Outcomes
Given the review’s emphasis on caregivers, outcomes for care recipients are reported in Tables 2 and 3. To synthesize, authors reported mixed findings with some beneficial effects for care recipients in varying indicators of psychosocial, physical, and functional well-being across both DyEx and DySplit studies.
Quality Assessment and Risk of Bias
The modified Downs and Black quality assessment scores ranged from 13 to 22 points (mean = 17) out of 27 possible (Table 4). Three met criteria for good methodological quality, seven fair, and one poor. Of the three rated as good quality, two were DyEx studies (Lowery et al., 2014; Winters-Stone et al., 2016), and one DySplit study (Lamb et al., 2018); all three were medium to large-scale RCTs scoring higher for reporting and internal validity, with adequate power and analyses. Due to the nature of the interventions, no included RCTs blinded study subjects; no study met the criteria for external validity.
Table 4.
Quality and Risk of Bias Assessment Using the Modified Downs and Black Checklist
| DyEx studies (n = 6) | DySplit studies (n = 5) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Badger et al. (2007) | Burgener et al. (2011) | Lowery et al. (2014) | Milbury et al. (2015) | Milbury et al. (2018) | Winters-Stone et al. (2016) | Barnes et al. (2015) | Lamb et al. (2018) | Maci et al. (2012) | Marques et al. (2015) | Yu et al. (2015) | |
| Reporting | 10 | 8 | 10 | 0 | 11 | 11 | 9 | 11 | 7 | 9 | 8 |
| External Validity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Internal Validity—Bias | 5 | 6 | 5 | 3 | 4 | 6 | 5 | 6 | 6 | 4 | 6 |
| Internal Validity— Confounding | 1 | 1 | 5 | 2 | 2 | 4 | 3 | 4 | 3 | 0 | 2 |
| Power | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Total Score and | 16 | 15 | 21 | 15 | 17 | 22 | 17 | 22 | 16 | 13 | 16 |
| Quality Rating | Fair | Fair | Good | Fair | Fair | Good | Fair | Good | Fair | Poor | Fair |
Note: Quality rating: Excellent (26–28), Good (20–25), Fair (15–19), Poor (≤14).
Discussion
This systematic review examined whether caregivers, who enroll with their care recipients in dyadic exercise interventions, realize greater health benefits when they coparticipate in exercise, or when their care recipients exercise independently while the caregivers receive another nonexercise treatment or UC. Results of this review are limited by the number and quality of studies that have specifically addressed and included caregivers as the primary focus of the study, and by the varied assessment techniques used for each study.
Although some results were mixed and outcome measures varied across studies, our findings indicate that when exercising together with care recipients, caregivers were more likely to experience improvements in both psychosocial and physical health. In comparison, caregivers who did not exercise, but did receive a separate, nonexercise intervention—specifically planned respite or a dyadic support group—were only measured for psychosocial outcomes, and thus more likely to show psychosocial benefits. Those caregivers who received UC were less likely to derive either physical or psychosocial health benefits. In both DyEx and DySplit studies, care recipients also improved in physical and psychosocial outcomes following exercise, although outcome measures were heterogeneous and results were mixed. Most studies were of low to moderate quality and moderate to high risk of bias. Results suggest that spousal and family caregivers may gain more from engaging in dyadic exercise compared to when their care recipients exercise independently.
The bulk of research focuses on interventions for individuals with a given pathology, but addresses caregivers only secondarily, if at all. The critical problem this introduces is a cadre of studies that have not been well designed to address outcomes for caregivers. Randomization has occurred based on the care recipient, selected outcome measures are inconsistent, and often caregiver demographics are not considered as part of the study. Although it is certainly understandable why researchers have elected to focus the effects of an intervention on individuals with pathologies, the current approaches often count, as ancillary, a key member of the team that determines the effectiveness of an intervention.
Moreover, the studies included in this review encompass only a small segment of pathologies experienced by caregiver–care recipient dyads, namely those with Alzheimer’s disease, cancer, dementia, and chronic obstructive pulmonary disease. Undoubtedly, the caregiving requirements and burdens vary—not only for the ones covered in the present review, but also for many other pathologies affecting older adults. Unfortunately, studies excluded from the present review covered additional pathologies, such as cardiovascular disease, diabetes, osteoarthritis, Parkinson disease, and stroke (see Supplementary Table 1 for a list of excluded full-text articles). Though the titles and abstracts of these studies mentioned caregivers, the study designs did not fully address caregiver outcomes. Lastly, the heterogeneous nature of the interventions, which included cycling, strength training, taiji, walking, yoga, and mixed modes of exercise—all with varying outcomes for caregivers and care recipients—makes it difficult to advocate for one form of PA over another. Given these considerations, results of the present review should be interpreted with caution.
Support for Exercising Together as a Dyad
A growing body of literature supports the use of dyadic psychosocial and behavioral interventions as a means for addressing the negative outcomes experienced by couples living with various chronic illnesses (Martire et al., 2010). The present review aligns with the meta-analysis of 33 educational and behavioral interventions for couples conducted by Martire and colleagues (2010), which reported small, but significant effects on psychosocial outcomes for care recipients, and when measured, caregivers. However, many of the studies placed an emphasis on care recipients and offered limited insights on the effects of such interventions for caregivers. Moreover, Martire and colleagues (2010) included just three exercise studies, of which only one assessed outcomes in both caregivers and care recipients, and is included in the present review (Badger et al., 2007). The other two exercise studies paired couple-oriented behavioral therapy with patient-only exercise for older adults with osteoarthritis (Keefe et al., 2004) and low back pain (Turner, Clancy, McQuade, & Cardenas, 1990).
The present review expands the evidence available to support dyadic exercise interventions, and in particular, to lend support to the efficacy of such interventions for caregivers when they coparticipate in the exercise and are assessed for outcomes. In our review, five DyEx studies reported significance or trends for improvement in caregiver psychosocial health, and two DyEx studies reported significant enhancements to physical and functional outcomes for caregivers. Similar to our findings, a recent review of four dyadic exercise interventions supported some favorable health outcomes for dementia caregivers with two studies showing decreased burden (Lamotte, Shah, Lazarov, & Corcos, 2016). However, Lamotte and colleagues (2016) noted mood states were inconsistent such that one small controlled trial described improvements (Canonici et al., 2012); whereas two larger RCTs reported no significant differences (Lowery et al., 2014; Prick, de Lange, Twisk, & Pot, 2015). Of the four studies comprising Lamotte and colleagues (2016), three were excluded from the present systematic review due to a lack of pre- and post-test outcomes for caregivers (Pitkala et al., 2013), an emphasis on caregivers assisting with the exercises (Prick et al., 2015), and in the third study, it was ambiguous as to whether caregivers were co participating or receiving separate treatment (Canonici et al., 2012).
Interestingly, results of DyEx studies are also similar to outcomes reported in a review of PA interventions tailored to caregivers, such that caregivers are the focus, and the only ones to exercise (not to be confused with DySplit studies, in which the care recipient exercised independently and the caregiver received a separate, nonexercise intervention or UC). Similar to DyEx interventions, caregiver-only exercise studies, targeted to family caregivers of adults living with a wide range of chronic illnesses, primarily emphasized psychosocial health yielding mixed results with significant improvements and varying efficacy in selected outcomes (Lambert et al., 2016; Loi et al., 2014; Orgeta & Miranda-Castillo, 2014). Findings for physical health were also equivocal. Lambert and colleagues (2016) concluded caregiver-only interventions increased physical activity levels, and improved blood pressure, but only half of the reviewed studies found a positive impact on other physical health indicators (Lambert et al., 2016).
Given the semblance of results between dyadic and caregiver-only exercise, it then becomes relevant to consider whether caregivers benefit more from dyadic exercise or from caregiver-only exercise. It is not surprising that caregivers attained benefits from participating in exercise, whether the intervention involved the dyad or caregivers only, because the literature supports the role of physical activity in the healthy aging of older adults (Bauman et al., 2016). However, among barriers to caregiver-only exercise interventions are perceptions of increased burden (Hirano et al., 2011b), inability to leave care recipients, and few opportunites to partake in physical activities with them (Janevic & Connell, 2004; Malthouse & Fox, 2014). These barriers pose limitations to caregiver-only exercise studies, making it difficult to translate and maintain them (Cuthbert, King-Shier, Ruether, Tapp, & Culos-Reed, 2017), and as such may suggest a possible advantage of dyadic exercise interventions.
Further supporting dyadic interventions, Burgener and colleagues (2011) postulated that dementia caregivers gained feelings of empowerment and improved the quality of their relationships because they coparticipated in interventions to enhance care recipients’ well-being. Also relevant, Badger and colleagues (2007) cited mutual spousal influences on health as a reason for reciprocal decreases in depression and anxiety for women with breast cancer and their spouses. Dyadic exercise may also prove helpful in overcoming caregivers’ diminished social interaction as a barrier to physical activity. For example, Lowery and colleagues (2014) noted dyadic walking afforded dementia caregivers an opportunity to receive and provide psychosocial support, which may have been enough to precipitate decreases in their burden, despite no change in care recipients’ behavioral symptoms (Lowery et al., 2014). Further, Winters-Stone and colleagues (2016) attributed improvements to physical and mental health seen in cancer dyads to the emphasis on co-occupational exercises that encouraged interdependent and interactive participation as a couple (Winters-Stone et al., 2016).
Dyadic interventions may offer many advantages to spousal caregivers and care recipients when both exercise. However, previous work is limited in scope and methodology, which is further discussed subsequently. Recently, though, a study published outside the timeframe of this review, implemented a large community-based intervention combining multicomponent exercise with behavioral treatment for dementia dyads; both members of the dyad increased the days they engaged in physical activity together, and caregivers improved in depression, but not physical measures of health (Teri, Logsdon, McCurry, Pike, & McGough, 2018). In general, more work is needed to understand the efficacy and applicability to a broader spectrum of caregiver–care recipient dyads.
If Not Exercising, Respite or a Separate Intervention
For researchers interested in focusing the exercise on the care recipient, the present review suggests it may be advantageous to provide a separate intervention for caregivers. Three pilot DySplit studies in the present review were identified as specifically enrolling a dyad, and providing respite or a separate, nonexercise activity for the caregivers, while their care recipients exercised. These three pilot studies reported psychosocial benefits for caregivers; however, none measured physical health. In contrast, caregivers only receiving UC did not observe significant psychosocial benefits, and neither measured physical outcomes.
Corroborating findings of the present review, two systematic reviews examining various forms of respite and caregiver support indicated positive, but small to moderate effects on psychosocial outcomes for family caregivers of frail elderly individuals (Lopez-Hartmann, Wens, Verhoeven, & Remmen, 2012; Shaw et al., 2009). A qualitative study noted that an often-overlooked need for older adult caregivers is respite, which provides temporary relief from their responsibilities (Johnson, Hofacker, Boyken, & Eisenstein, 2016). Caregivers indicated a strong interest in interventions that specifically designate respite and access to support groups (Johnson et al., 2016).
Planned respite or a caregiver-specific portion of the intervention may be more advantageous to caregivers than simply not engaging in an exercise program. Certainly, one could argue that caregivers receive respite when care recipients are solely engaged in exercise interventions, whether that respite is planned or not. However, caregivers are often the ones who must help prepare and transport care recipients to and from the interventions. As such, this may add to their typical caregiving workload, and put additional onus on them to ensure care recipients’ participation and adherence, which detracts from the value of respite (Shaw et al., 2009).
In the review by Shaw and colleagues (2009) of respite effects on caregivers of older adults, the authors recommended respite be made available in a range of services, and that it be flexible and responsive to caregivers’ and care recipients’ needs. Two studies in the present dyadic exercise review provided planned respite for caregivers by transporting dementia care recipients to and from the intervention sites. Combined duration of the transportation and the intervention gave caregivers between 8 and 10 hr (Yu et al., 2015) and up to 20 hr (Maci et al., 2012) per week of respite. Maci and colleagues (2012) reported that caregivers significantly improved in mood and perception of care recipients’ quality of life. Similarly, caregivers in Yu and colleagues (2015) experienced a 40% decrease in burden, which they attributed to both the respite and improvements made by their care recipients, and facilitated easier caregiving. In the study by Marques and colleagues (2015), caregivers of individuals with chronic obstructive pulmonary disease improved in family coping strategies and psychosocial adjustment, which they ascribed to the dyadic support group helping them cope with the illness as a team, thus enhancing their relationships with care recipients.
As with exercise interventions targeting the dyad, there may be many advantages to spousal and family caregivers when they are offered planned respite or a simultaneously occurring intervention while their care recipients exercise. Again, though, the small number of studies reviewed herein limit generalizations, but merit further investigation that places greater emphasis on designing studies specifically with caregivers in mind.
Limitations of the Included and Excluded Studies
Most of the included studies were scored as fair to good quality and moderate to high risk of bias. These findings can be largely attributed to the fact that caregivers were not the focus of the study but rather a tangential sample to the individuals being studied. Nearly half of the excluded studies were removed based upon how caregiver data were handled. For example, multiple studies were eliminated during the full-text review for failing to provide pre- and post-test outcomes for caregivers; lacking a precise and congruent description of caregivers and their role in the exercise program; and placing an emphasis on caregivers assisting with the exercises, and thus lacking a control for or report on how much all caregivers were actually able to engage in the exercise themselves. See Supplementary Table 1 for a list of excluded full-text articles.
Given the number of studies that mentioned or included the caregiver in the abstract, it is apparent researchers are interested in investigating how exercise impacts caregivers. As the field moves forward, it is important to correct the shortcomings and specifically design interventions around both members of the dyad. This is especially valid given the body of literature that demonstrates the reciprocal influence spouses can have on each other’s mental and physical health, as well as exercise behaviors.
Limitations of the Review
This review is limited in that it cannot be generalized to all caregiver–care recipient dyads. Inclusion criteria required studies to clearly define caregivers and provide baseline demographics along with pre- and post-test outcomes. Additionally, care recipients in the included studies were predominantly diagnosed with cancer and dementia; thus, caregiving demands associated with other chronic conditions may differentially influence results. Moreover, the methodological quality of the studies, combined with heterogeneity of the exercise interventions, outcome measures, and statistical analyses, prevent a meta-analysis of the data, as well as a comparison of intervention efficacy, thus allowing only general conclusions about dyadic exercise and its impact on caregivers. Also, the broad definition used to identify dyadic exercise interventions was constructed to suit the scope of this review, and therefore may not necessarily reflect the intentions of the researchers. Finally, the search included only quantitative studies published in English, thereby overlooking qualitative results, as well as reports in other languages.
Implications
The present systematic review extends the knowledge of dyadic exercise interventions involving caregivers of older adults, and encompassed a cross-disease examination of primarily caregiver outcomes, but also effects on care recipients. Employing a broad definition of the dyad allowed us to compare caregiver outcomes in two different variations of dyadic interventions, namely those in which both members of the dyad exercised, and those in which only care recipients exercised, while caregivers received a separate nonexercise intervention or UC. This enabled a comparison with the goal of identifying what yields the best results for caregivers. PRISMA reporting guidelines were followed to enhance the quality and replication of the results. To address publication bias and provide the most current information available, we conducted a limited literature update of previously published protocols found during our title and abstract review.
Exercise has the potential to improve health in both members of a caregiver–care recipient dyad. Yet caregivers are often overlooked for participation, and examined only secondarily in exercise interventions for care recipients. This analysis suggests caregivers may benefit both physically and psychosocially from dyadic exercise interventions that intentionally involve their coparticipation. Interventions that offer a separate, nonexercise cohort or planned respite may also benefit caregivers’ mental health, and in the case of planned respite may empower them to self-select how they use their time. However, the mixed benefits of both types of dyadic interventions suggest that the exercise formats and/or the type of respite program offered, as well as the pathologies of care recipients, and the related caregiving demands are all important variables to consider when measuring caregiver outcomes. To foster the uptake and translation of these interventions, future research for caregivers should include larger-scale randomized control trials, more rigorous methodologies that intentionally plan for the caregiver, other populations of older adult caregivers across a broader spectrum of diseases, and comparative investigations of dyadic exercise versus caregiver-only and care recipient-only exercise, as well as respite options. Equally important, if not more so, is taking the time to understand the interests and needs of the caregivers through mixed-method approaches including qualitative assessments to interview and survey caregivers to inform intervention design, dosage, and implementation.
Moreover, from a public health perspective, bolstering the physical and psychosocial well-being of caregivers, through physical activity, will help contain the escalating costs associated with elder care and institutionalization in the United States, which are estimated to range between $470 billion (Reinhard, Feinberg, & Houser, 2015) and $522 billion (Chari, Enberg, Ray, & Mehrotra, 2015). It is important to make advances toward policies, strategies, research funding, and public discourse that supports and promotes community-based and in-home health promotion and wellness programs to encourage physical activity for dyads of older adult caregivers and care recipients. Also necessary are policies that support working family caregivers, such as adult children. More family and medical leave, paid family leave, and financial assistance or incentives to participate in physical activities and other interventions may improve caregiver health and well-being, but also the well-being of their care recipients to help them age in place and avoid institutionalization.
Supplementary Material
Acknowledgments
The authors thank the members of the Sensory Motor Integration Lab at UW-Madison for their assistance with data screening, and manuscript review. The authors also thank Michael Venner and Steve Johnson of the Ebling Library for their guidance and support of our literature search process.
Funding
This work was supported by the National Institutes of Health (UL1TR002373, KL2TR002374); and the University of Wisconsin-Madison through the Virginia Horne Henry Fund; the Blanche Trilling Fellowship; and the Carns, Cronin, Glassow Scholarship.
Conflict of Interest
None reported.
References
- Anton, P. M., Partridge, J. A., & Morrissy, M. J. (2013). Cancer caregivers’ perceptions of an exercise and nutrition program. Supportive Care in Cancer, 21, 803–810. doi: 10.1007/s00520-012-1583-8 [DOI] [PubMed] [Google Scholar]
- Baanders, A. N., & Heijmans, M. J. (2007). The impact of chronic diseases: The partner’s perspective. Family & Community Health, 30, 305–317. doi: 10.1097/01.FCH.0000290543.48576.cf [DOI] [PubMed] [Google Scholar]
- Badger, T., Segrin, C., Dorros, S. M., Meek, P., & Lopez, A. M. (2007). Depression and anxiety in women with breast cancer and their partners. Nursing Research, 56, 44–53. doi: 10.1097/00006199-200701000-00006 [DOI] [PubMed] [Google Scholar]
- Barnes, D. E., Mehling, W., Wu, E., Beristianos, M., Yaffe, K., Skultety, K., & Chesney, M. A. (2015). Preventing loss of independence through exercise (PLIÉ): A pilot clinical trial in older adults with dementia. PLoS One, 10, e0113367. doi: 10.1371/journal.pone.0113367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman, A., Merom, D., Bull, F. C., Buchner, D. M., & Fiatarone Singh, M. A. (2016). Updating the evidence for physical activity: Summative reviews of the epidemiological evidence, prevalence, and interventions to promote “active aging”. The Gerontologist, 56(Suppl 2), S268–S280. doi: 10.1093/geront/gnw031 [DOI] [PubMed] [Google Scholar]
- Burgener, S. C., Marsh-Yant, S., & Nega, K. K. (2011). A combined, multimodal intervention for individuals with dementia. Research in Gerontological Nursing, 4, 64–75. doi: 10.3928/19404921-20100504-01 [DOI] [PubMed] [Google Scholar]
- Canonici, A. P., Andrade, L. P., Gobbi, S., Santos-Galduroz, R. F., Gobbi, L. T., & Stella, F. (2012). Functional dependence and caregiver burden in Alzheimer’s disease: A controlled trial on the benefits of motor intervention. Psychogeriatrics, 12, 186–192. doi: 10.1111/j.1479-8301.2012.00407.x [DOI] [PubMed] [Google Scholar]
- Cao, V., Chung, C., Ferreira, A., Nelken, J., Brooks, D., & Cott, C. (2010). Changes in activities of wives caring for their husbands following stroke. Physiotherapy Canada. Physiotherapie Canada, 62, 35–43. doi: 10.3138/physio.62.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capistrant, B. D., Moon, J. R., Berkman, L. F., & Glymour, M. M. (2012). Current and long-term spousal caregiving and onset of cardiovascular disease. Journal of Epidemiology and Community Health, 66, 951–956. doi: 10.1136/jech-2011-200040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersen, C. J., Powell, K. E., & Christenson, G. M. (1985). Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Reports (Washington, D.C.: 1974), 100, 126–131. [PMC free article] [PubMed] [Google Scholar]
- Chari, A. V., Engberg, J., Ray, K. N., & Mehrotra, A. (2015). The opportunity costs of informal elder-care in the United States: New estimates from the American Time Use Survey. Health Services Research, 50, 871–882. doi: 10.1111/1475-6773.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudyk, A. M., Jutai, J. W., Petrella, R. J., & Speechley, M. (2009). Systematic review of hip fracture rehabilitation practices in the elderly. Archives of Physical Medicine and Rehabilitation, 90, 246–262. doi: 10.1016/j.apmr.2008.06.036 [DOI] [PubMed] [Google Scholar]
- Cuthbert, C. A., King-Shier, K., Ruether, D., Tapp, D. M., & Culos-Reed, S. N. (2017). What is the state of the science on physical activity interventions for family caregivers? A systematic review and RE-AIM evaluation. Journal of Physical Activity & Health, 14, 578–595. doi: 10.1123/jpah.2016-0280 [DOI] [PubMed] [Google Scholar]
- Dassel, K. B., & Carr, D. C. (2016). Does dementia caregiving accelerate frailty? Findings from the health and retirement study. The Gerontologist, 56, 444–450. doi: 10.1093/geront/gnu078 [DOI] [PubMed] [Google Scholar]
- Dassel, K. B., Carr, D. C., & Vitaliano, P. (2017). Does caring for a spouse with dementia accelerate cognitive decline? Findings from the health and retirement study. The Gerontologist, 57, 319–328. doi: 10.1093/geront/gnv148 [DOI] [PubMed] [Google Scholar]
- Davis, L. L., Gilliss, C. L., Deshefy-Longhi, T., Chestnutt, D. H., & Molloy, M. (2011). The nature and scope of stressful spousal caregiving relationships. Journal of Family Nursing, 17, 224–240. doi: 10.1177/1074840711405666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs, S. H., & Black, N. (1998). The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal of Epidemiology and Community Health, 52, 377–384. doi: 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin, C. D., Prohaska, T. R., Connell, C. M., Edelman, P., & Hughes, S. L. (2008). Antecedents of physical activity among family caregivers. Journal of Applied Gerontology, 27, 350–367. doi: 10.1177/0733464808315276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falba, T. A., & Sindelar, J. L. (2008). Spousal concordance in health behavior change. Health Services Research, 43, 96–116. doi: 10.1111/j.1475-6773.2007.00754.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman, L., Cauley, J. A., Hochberg, M., Ensrud, K. E., & Doros, G.; Study of Osteoporotic Fractures . (2010). Mortality associated with caregiving, general stress, and caregiving-related stress in elderly women: Results of caregiver-study of osteoporotic fractures. Journal of the American Geriatrics Society, 58, 937–943. doi: 10.1111/j.1532-5415.2010.02808.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman, L., Cauley, J. A., Satterfield, S., Simonsick, E., Spencer, S. M., Ayonayon, H. N.,...Harris, T. B.; Health ABC Study Group . (2008). Caregiving, mortality, and mobility decline: The Health, Aging, and Body Composition (Health ABC) Study. Archives of Internal Medicine, 168, 2154–2162. doi: 10.1001/archinte.168.19.2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannetti, E. R., & Wolff, J. L. (2010). Cross-survey differences in national estimates of numbers of caregivers of disabled older adults. The Milbank Quarterly, 88, 310–349. doi: 10.1111/j.1468-0009.2010.00602.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. T., Green, S. (Eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Retrieved from www.handbook.cochrane.org. [Google Scholar]
- Hirano, A., Suzuki, Y., Kuzuya, M., Onishi, J., Ban, N., & Umegaki, H. (2011a). Influence of regular exercise on subjective sense of burden and physical symptoms in community-dwelling caregivers of dementia patients: A randomized controlled trial. Archives of Gerontology and Geriatrics, 53, e158–e163. doi: 10.1016/j.archger.2010.08.004 [DOI] [PubMed] [Google Scholar]
- Hirano, A., Suzuki, Y., Kuzuya, M., Onishi, J., Hasegawa, J., Ban, N., & Umegaki, H. (2011b). Association between the caregiver’s burden and physical activity in community-dwelling caregivers of dementia patients. Archives of Gerontology and Geriatrics, 52, 295–298. doi: 10.1016/j.archger.2010.04.011 [DOI] [PubMed] [Google Scholar]
- Janevic, M. R., & Connell, C. M. (2004). Exploring self-care among dementia caregivers: The role of perceived support in accomplishing exercise goals. Journal of Women & Aging, 16, 71–86. doi: 10.1300/J074v16n01_06 [DOI] [PubMed] [Google Scholar]
- Jenkins, K. R., Kabeto, M. U., & Langa, K. M. (2009). Does caring for your spouse harm one’s health? Evidence from a United States nationally-representative sample of older adults. Ageing and Society, 29, 277–293. doi: 10.1017/S0144686X08007824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, R., Hofacker, J., Boyken, L., & Eisenstein, A. (2016). Sustaining Chicago’s informal caregivers: An age-friendly approach. Journal of Urban Health, 93, 639–651. doi: 10.1007/s11524-016-0058-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe, F. J., Blementhal, J., Baucom, D., Affleck, G., Waugh, R., Caldwell, D. S.,...Lefebvre, J. (2004). Effects of spouse-assisted coping skills training and exercise training in patients with osteoarthritic knee pain: A randomized controlled study. Pain, 110, 539–549. doi: 10.1016/j.pain.2004.03.022 [DOI] [PubMed] [Google Scholar]
- Lamb, S. E., Sheehan, B., Atherton, N., Nichols, V., Collins, H., Mistry, D.,...Lall, R.; DAPA Trial Investigators . (2018). Dementia and Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: Randomised controlled trial. BMJ (Clinical Research ed.), 361, k1675. doi: 10.1136/bmj.k1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert, S. D., Duncan, L. R., Kapellas, S., Bruson, A. M., Myrand, M., Santa Mina, D.,...Lambrou, A. (2016). A descriptive systematic review of physical activity interventions for caregivers: Effects on caregivers’ and care recipients’ psychosocial outcomes, physical activity levels, and physical health. Annals of Behavioral Medicine, 50, 907–919. doi: 10.1007/s12160-016-9819-3 [DOI] [PubMed] [Google Scholar]
- Lamotte, G., Shah, R. C., Lazarov, O., & Corcos, D. M. (2017). Exercise training for persons with Alzheimer’s disease and caregivers: A review of dyadic exercise interventions. Journal of Motor Behavior, 49, 365–377. doi: 10.1080/00222895.2016.1241739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, K. K., Cardinal, B. J., & Acock, A. C. (2013). Concordance of Physical Activity Trajectories Among Middle-Aged and Older Married Couples: Impact of Diseases and Functional Difficulties. Journals of Gerontology Series B-Psychological Sciences and Social Sciences, 68, 794–806. doi: 10.1093/geronb/gbt068 [DOI] [PubMed] [Google Scholar]
- Loi, S. M., Dow, B., Ames, D., Moore, K., Hill, K., Russell, M., & Lautenschlager, N. (2014). Physical activity in caregivers: What are the psychological benefits? Archives of Gerontology and Geriatrics, 59, 204–210. doi: 10.1016/j.archger.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Lopez-Hartmann, M., Wens, J., Verhoeven, V., & Remmen, R. (2012). The effect of caregiver support interventions for informal caregivers of community-dwelling frail elderly: A systematic review. International Journal of Integrated Care, 12, e133. doi: 10.5334/ijic.845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery, D., Cerga-Pashoja, A., Iliffe, S., Thuné-Boyle, I., Griffin, M., Lee, J., . . . Warner, J. (2014). The effect of exercise on behavioural and psychological symptoms of dementia: The EVIDEM-E randomised controlled clinical trial. International Journal of Geriatric Psychiatry, 29, 819–827. doi: 10.1002/gps.4062 [DOI] [PubMed] [Google Scholar]
- Maci, T., Pira, F. L., Quattrocchi, G., Nuovo, S. D., Perciavalle, V., & Zappia, M. (2012). Physical and cognitive stimulation in Alzheimer disease. The GAIA Project: A pilot study. American Journal of Alzheimer’s Disease and Other Dementias, 27, 107–113. doi: 10.1177/1533317512440493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malthouse, R., & Fox, F. (2014). Exploring experiences of physical activity among people with Alzheimer’s disease and their spouse carers: A qualitative study. Physiotherapy, 100, 169–175. doi: 10.1016/j.physio.2013.10.002 [DOI] [PubMed] [Google Scholar]
- Marquez, D. X., Bustamante, E. E., Kozey-Keadle, S., Kraemer, J., & Carrion, I. (2012). Physical activity and psychosocial and mental health of older caregivers and non-caregivers. Geriatric Nursing (New York, N.Y.), 33, 358–365. doi: 10.1016/j.gerinurse.2012.03.003 [DOI] [PubMed] [Google Scholar]
- Marques, A., Gabriel, R., Jacome, C., Cruz, J., Brooks, D., & Figueiredo, D. (2015). Development of a family-based pulmonary rehabilitation programme: An exploratory study. Disability and Rehabilitation, 37, 1340–1346. doi: 10.3109/09638288.2014.964376 [DOI] [PubMed] [Google Scholar]
- Marsden, D., Quinn, R., Pond, N., Golledge, R., Neilson, C., White, J., Pollack, M. (2012). A multidisciplinary group programme in rural settings for community-dwelling chronic stroke survivors and their carers: A pilot randomized controlled trial. Clinical Rehabilitation, 24(4), 328–341. doi: 10.1177/0269215509344268 [DOI] [PubMed] [Google Scholar]
- Martire, L. M., Schulz, R., Helgeson, V. S., Small, B. J., & Saghafi, E. M. (2010). Review and meta-analysis of couple-oriented interventions for chronic illness. Annals of Behavioral Medicine, 40, 325–342. doi: 10.1007/s12160-010-9216-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyler, D., Stimpson, J. P., & Peek, M. K. (2007). Health concordance within couples: A systematic review. Social Science & Medicine (1982), 64, 2297–2310. doi: 10.1016/j.socscimed.2007.02.007 [DOI] [PubMed] [Google Scholar]
- Milbury, K., Mallaiah, S., Lopez, G., Liao, Z., Yang, C., Carmack, C.,...Cohen, L. (2015). Vivekananda yoga program for patients with advanced lung cancer and their family caregivers. Integrative Cancer Therapies, 14, 446–451. doi: 10.1177/1534735415583554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbury, K., Mallaiah, S., Mahajan, A., Armstrong, T., Weathers, S. P., Moss, K. E.,...Cohen, L. (2018). Yoga program for high-grade glioma patients undergoing radiotherapy and their family caregivers. Integrative Cancer Therapies, 17, 332–336. doi: 10.1177/1534735417689882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G.; PRISMA Group . (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Annals of Internal Medicine, 151, 264–9, W64. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- NAC, & AARP . (2015). Research report: Caregiving in the U.S., 2015—Focused look at those caring for someone age 50+. Bethesda, MD; Washington, DC: NAC and AARP. [Google Scholar]
- Orgeta, V., & Miranda-Castillo, C. (2014). Does physical activity reduce burden in carers of people with dementia? A literature review. International Journal of Geriatric Psychiatry, 29, 771–783. doi: 10.1002/gps.4060 [DOI] [PubMed] [Google Scholar]
- Pai, C. W., Godboldo-Brooks, A., & Edington, D. W. (2010). Spousal concordance for overall health risk status and preventive service compliance. Annals of Epidemiology, 20, 539–546. doi: 10.1016/j.annepidem.2010.03.020mm [DOI] [PubMed] [Google Scholar]
- Pinquart, M., & Sörensen, S. (2003). Differences between caregivers and noncaregivers in psychological health and physical health: A meta-analysis. Psychology and Aging, 18, 250–267. doi: 10.1037/0882-7974.18.2.250 [DOI] [PubMed] [Google Scholar]
- Pinquart, M., & Sörensen, S. (2011). Spouses, adult children, and children-in-law as caregivers of older adults: A meta-analytic comparison. Psychology and Aging, 26, 1–14. doi: 10.1037/a0021863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkala, K. H., Poysti, M. M., Laakkonen, M. L., Tilvis, R. S., Savikko, N., Kautiainen, H., & Strandberg, T. E. (2013). Effects of the Finnish Alzheimer Disease Exercise Trial (FINALEX) A Randomized Controlled Trial. Jama Internal Medicine, 173, 894–901. doi: 10.1001/jamainternmed.2013.359 [DOI] [PubMed] [Google Scholar]
- Prick, A. E., de Lange, J., Twisk, J., & Pot, A. M. (2015). The effects of a multi-component dyadic intervention on the psychological distress of family caregivers providing care to people with dementia: A randomized controlled trial. International Psychogeriatrics, 27, 2031–2044. doi: 10.1017/S104161021500071X [DOI] [PubMed] [Google Scholar]
- Queen, T. L., Butner, J., Berg, C. A., & Smith, J. (2019). Activity engagement among older adult spousal caregivers. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 74, 1278–1282. doi: 10.1093/geronb/gbx106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard, S. C., Feinberg, L. F., Houser, A. (2015). Valuing the invaluable: 2015 update—Undeniable progress but big gaps remain. AARP Public Policy Institute, July 2015. Retrieved from http://www.aarp.org/content/dam/aarp/ppi/2015/valuing-the-invaluable-2015-update-new.pdf [Google Scholar]
- Riffin, C., Van Ness, P. H., Wolff, J. L., & Fried, T. (2017). Family and other unpaid caregivers and older adults with and without dementia and disability. Journal of the American Geriatrics Society, 65, 1821–1828. doi: 10.1111/jgs.14910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, R., & Beach, S. R. (1999). Caregiving as a risk factor for mortality: The Caregiver Health Effects Study. JAMA, 282, 2215–2219. doi: 10.1001/jama.282.23.2215 [DOI] [PubMed] [Google Scholar]
- Shaw, C., McNamara, R., Abrams, K., Cannings-John, R., Hood, K., Longo, M.,...Williams, K. (2009). Systematic review of respite care in the frail elderly. Health Technology Assessment (Winchester, England), 13, 1–224, iii. doi: 10.3310/hta13200 [DOI] [PubMed] [Google Scholar]
- Swinkels, J. C., Broese van Groenou, M. I., de Boer, A., & Tilburg, T. G. V. (2019). Male and female partner-caregivers’ burden: Does it get worse over time? The Gerontologist, 59, 1103–1111. doi: 10.1093/geront/gny132 [DOI] [PubMed] [Google Scholar]
- Teri, L., Logsdon, R. G., McCurry, S. M., Pike, K. C., & McGough, E. (2018). Translating an evidence-based multicomponent intervention for older adults with dementia and caregivers. The Gerontologist. doi: 10.1093/geront/gny122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, J. A., Clancy, S., McQuade, K. J., & Cardenas, D. D. (1990). Effectiveness of behavioral therapy for chronic low back pain: A component analysis. Journal of Consulting and Clinical Psychology, 58, 573–579. doi: 10.1037//0022-006x.58.5.573 [DOI] [PubMed] [Google Scholar]
- Van’t Leven, N., Prick, A. E., Groenewoud, J. G., Roelofs, P. D., de Lange, J., & Pot, A. M. (2013). Dyadic interventions for community-dwelling people with dementia and their family caregivers: A systematic review. International Psychogeriatrics, 25, 1581–1603. doi: 10.1017/S1041610213000860 [DOI] [PubMed] [Google Scholar]
- Winters-Stone, K. M., Lyons, K. S., Dobek, J., Dieckmann, N. F., Bennett, J. A., Nail, L., & Beer, T. M. (2016). Benefits of partnered strength training for prostate cancer survivors and spouses: Results from a randomized controlled trial of the Exercising Together project. Journal of Cancer Survivorship: Research and Practice, 10, 633–644. doi: 10.1007/s11764-015-0509-0 [DOI] [PubMed] [Google Scholar]
- Wolff, J. L., Spillman, B. C., Freedman, V. A., & Kasper, J. D. (2016). A national profile of family and unpaid caregivers who assist older adults with health care activities. JAMA Internal Medicine, 176, 372–379. doi: 10.1001/jamainternmed.2015.7664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, F., Thomas, W., Nelson, N. W., Bronas, U. G., Dysken, M., & Wyman, J. F. (2015). Impact of 6-month aerobic exercise on Alzheimer’s symptoms. Journal of Applied Gerontology, 34, 484–500. doi: 10.1177/0733464813512895 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.