Abstract
Background:
Circulating tumor cells (CTCs) with an epithelial-mesenchymal transition phenotype in peripheral blood may be a useful marker of carcinomas with poor prognosis. The aim of this study was to determine the prognostic significance of CTCs expressing Krüppel-like factor 8 (KLF8) and vimentin in pancreatic cancer (PC).
Methods:
CTCs were isolated by immunomagnetic separation from the peripheral blood of 40 PC patients before undergoing surgical resection. Immunocytochemistry was performed to identify KLF8+ and vimentin+ CTCs. The associations between CTCs and time to recurrence (TTR), clinicopathologic factors, and survival were assessed. Univariate and multivariate analyzes were performed to identify risk factors.
Results:
Patients with CTCs (n = 30) had a higher relapse rate compared to those without (n = 10) (70.0% vs 20.0%; P < 0.01). The proportion of KLF8+/vimentin+ CTCs to total CTCs was inversely related to TTR (r = −0.646; P < 0.01); TTR was reduced in patients with > 50% of CTCs identified as KLF8+/vimentin+ (P < 0.01). Independent risk factors for recurrence were perineural invasion and > 50% KLF8+/vimentin+ CTCs (both P < 0.05).
Conclusion:
Poor prognosis can be predicted in PC patients when > 50% of CTCs are positive for KLF8 and vimentin.
Keywords: circulating tumor cells, Krüppel-like factor 8, pancreatic cancer, prognosis, recurrence, vimentin
Introduction
Pancreatic adenocarcinoma is the most lethal of the solid tumors and the fourth leading cause of cancer-related death in North America,1 with a 5-year relative survival rate of ∼6%.2 Pancreatic cancer (PC) has a poor prognosis due to delayed diagnosis. Early diagnosis is the most important factor for improving prognosis.3 Fewer than 20% of patients are eligible for surgery because the disease is often diagnosed at an advanced stage,4,5 and neoadjuvant/adjuvant chemotherapy is often inadequate. However, the treatment outcome can be dramatically improved if PC is detected at an early stage, prior to metastasis.6-8 Nevertheless, early detection of PC is limited by the absence of specific clinical biomarkers and noninvasive screening tests.7
Circulating tumor cells (CTCs) in peripheral blood are linked to the development of metastases,9 and have been used for monitoring patients with solid tumors.10,11 CTCs are increasingly being used as a prognostic tool for PC12 because of their specificity and stability.13 However, CTCs are a heterogeneous population comprised of various molecular subtypes.14-16 Therefore, markers of epithelial–mesenchymal transition (EMT), which regulates the generation, survival, and recolonization of CTCs, represent a potential target for clinical evaluation.17,18 One such marker of EMT is the intermediate filament protein vimentin, which is involved with microtentacle formation during EMT.19 The abundance of clinical data linking vimentin to poor patient outcome.20 Additionally, Krüppel-like factor 8 (KLF8) has been implicated in EMT and invasion.21 KLF8 is a GT-box-binding dual transcription factor that also regulates cell cycle progression22-26 and transformation.27 Indeed, KLF8 is highly expressed in breast,28 stomach,29 ovary,24 brain,30 kidney,31 and liver32,33 cancers, but barely detectable in normal epithelial cells.21,27 KLF8 overexpression promotes the growth of human lung cancer cells by promoting the expression of JMJD2A34 and regulates glycolysis by targeting GLUT4 in gastric cancer.35 The level of KLF8 expression predicts prognosis and metastasis in various carcinomas.36
Few studies have investigated the expression of KLF8 and vimentin in PC. The aim of this study was to assess their expression in CTCs from peripheral blood and their association with patient prognosis. We found that poor prognosis can be predicted in PC patients when > 50% of CTCs staining positive for KLF8 and vimentin.
Materials and Methods
Patient Population and Clinical Data
This study included 40 randomly selected patients (53 ± 10 years of age) preoperatively diagnosed with PC between July 2011 and June 2013 at the Department of General Surgery, Affiliated Changzheng Hospital of the Second Military Medical University, Shanghai, China. The inclusion criteria were: 1) patients undergoing pancreaticoduodenectomy, extended pancreaticoduodenectomy, pylorus-preserving pancreaticoduodenectomy, or total pancreatectomy; 2) patients without metastatic or residual disease in other organs before surgery as shown by whole-body computed tomography, magnetic resonance cholangiopancreatography, or 18F-fluorodeoxyglucose positron emission tomography; 3) patients who had not received preoperative radiotherapy or chemotherapy. Patients were treated postoperatively (within 2 mo) with gemcitabine or gemcitabine-based chemotherapy, which continued for at least 6 mo. For comparison, 10 healthy donors and 5 patients with benign pancreatic disease were enrolled as controls.
Detailed clinicopathologic and outcome data were collected from medical records and radiographic images obtained at our hospital (Table 1). PC was classified according to the American Joint Committee on Cancer/International Union against Cancer. All subjects provided written informed consent prior to study enrollment. The study was approved by local institutional review boards.
Table 1.
Clinical Characteristics of Pancreatic Cancer Patients (n = 40).
| Characteristic | n | % |
|---|---|---|
| Sex | ||
| Male | 30 | 75 |
| Female | 10 | 25 |
| Age (yr) | ||
| < 50 | 13 | 33 |
| > 50 | 27 | 67 |
| Tumor size (cm) | ||
| ≤ 4 | 16 | 40 |
| > 4 | 24 | 60 |
| TNM stage | ||
| I-II | 21 | 53 |
| III-IV | 29 | 47 |
| CA19-9 (kU/L) | ||
| ≤ 37 | 18 | 45 |
| > 37 | 22 | 55 |
| Tumor location | ||
| Head | 16 | 40 |
| Body/tail | 24 | 60 |
| Vascular invasion | ||
| Absent | 18 | 45 |
| Present | 22 | 55 |
| Perineural invasion | ||
| Absent | 18 | 45 |
| Present | 22 | 55 |
Abbreviations: CA19-9, Cancer antigen 19-9; TNM, Tumor-Node-Metastasis.
Blood Samples
Peripheral blood from vein puncture was collected after discarding the first 8 mL to eliminate epithelial cell contamination from the skin. Blood samples (7 mL in EDTA) were stored at room temperature and processed within 8 h after collection. Peripheral blood was collected from PC patients prior to undergoing surgery.
Cell Culture and Spiking Experiments
The AsPC-1 cell line was obtained from the American Culture Type Collection (Rockville, MD, United States) and maintained according to their guidelines. Cells were cultured in 1:1 (v/v) DMEM: Ham’s F12 medium supplemented with 5% horse serum, 30 mM NaHCO3, 10 mg/mL insulin, 500 ng/mL hydrocortisone, 100 ng/mL cholera toxin, 20 ng/mL epidermal growth factor, and 50 mg/mL penicillin/streptomycin in a humidified atmosphere of 5% CO2. Subcultivation was performed with 0.25% trypsin and 5 mM EDTA. The cell suspensions were only used at a viability > 90% as assessed by trypan blue exclusion.
Immunomagnetic Separation of CTCs
Peripheral blood mononuclear cells (PBMCs) and tumor cells were enriched from the whole blood samples by density gradient Ficoll–Paque PLUS (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) and then further enriched using the RosetteSep Human CD45 Depletion Cocktail (dilution at 1:50; StemCell Technologies, Vancouver, BC, Canada) as previously described.37,38 All procedures were performed according to the manufacturer’s instructions. A total volume of 100 µL Dynal CELLection beads (Invitrogen of Thermo Fisher Scientific, Waltham, MA, United States) coated with anti-CD45 monoclonal antibody were added to 1 × 107/mL PBMCs in PBS containing 0.1% bovine serum albumin and 2 mM EDTA. After incubation for 30 minutes at 4°C, the supernatant was transferred into fetal bovine serum-coated tubes and cells were cytocentrifuged at 2000 rpm for 5 min onto polylysine-coated slides. Slides were stored at 4°C until further use. For method validation, 5 mL of peripheral blood from healthy donors was spiked with 100 AsPC-1 cells. The specificity and sensitivity of this method have been validated previously.39
Immunocytochemical Identification of CTC Subtypes
CTCs slides were fixed with ethyl alcohol for 10 min at room temperature and incubated overnight at 4°C with mouse anti-human CK (to detect normal and neoplastic pancreatic cells) monoclonal antibodies (dilution at 1:100; Santa Cruz Biotechnology, Inc., Dallas, TX, United States) along with rat anti-human CD45 (to detect hematologic cells; dilution at 1:50; Santa Cruz Biotechnology, Inc.) or rabbit anti-human KLF8 (dilution at 1:100; Abcam, Cambridge, United Kingdom) and rat anti-human vimentin (dilution at 1:100; Santa Cruz Biotechnology, Inc.). The following fluorescence-conjugated secondary antibodies were used: Cy5 rabbit anti-mouse IgG (dilution at 1:400; Pierce of Thermo Fisher Scientific), and Alexa Fluor 488 rabbit anti-rat IgG, Alexa Fluor 647 goat anti-mouse IgG, Cy3 goat anti-rat IgG, or FITC goat anti-rabbit IgG (dilution at 1:400; Invitrogen). All slides were subsequently stained with DAPI (dilution at 1:1000; Pierce) and coverslipped in antifade solution (Vector Laboratories, Inc., Burlingame, CA, United States). Slides containing a mixture of human PBMCs and AsPC-1 cells were used for positive controls. The slides were imaged on a confocal laser-scanning microscope (Leica TCS SP5 MP; Leica Microsystems, Wetzlar, Germany). Cell counts are expressed as the number of cells per 7 mL of blood.
Follow-Up and Tumor Recurrence
Postoperative patient surveillance was performed as described previously.40 The follow-up began the day after the operation and ended on 30 December 2014 or at the time of recurrence or death. Patients underwent computed tomography and assessment of cancer antigen 19-9 levels every 3 month the first year after surgery, and then every 6 months over the next 2 years. Recurrence was diagnosed based on these results, with or without histologic confirmation. The interval between the surgery date and the diagnosis of recurrence (within or outside the pancreas) was defined as the time to recurrence (TTR).
Statistical Analysis
All data were analyzed using SPSS 19.0 for Windows (IBM Corp., Armonk, NY, United States). Continuous variables are presented as mean ± SD. Spearman rank correlation analysis was used to examine the associations between CTC subtypes and clinical characteristics of the patients. Associations between the cell type ratios and TTR were tested using the Kaplan-Meier method. Survival rates were compared using the log-rank test. Univariate and multivariate analyzes were based on the Cox proportional hazard regression model. Statistical significance was determined at P < 0.05.
Results
KLF8 and Vimentin Expression in AsPC-1 Cells and PBMCs of Normal Blood Donors
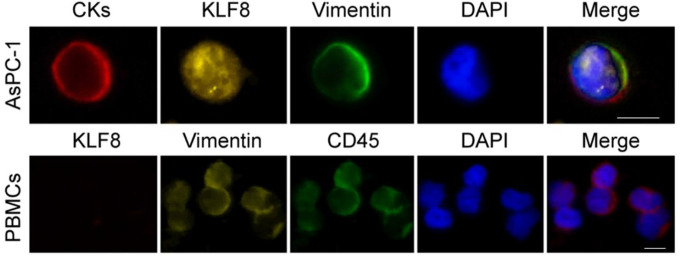
Due to technical limitations, the isolation of CTCs may also have some PBMCs.39 Although definitions vary between studies, it is widely accepted that CTCs lack CD45 and express cytokeratins.41 In order to determine if the expression of KLF8 and vimentin in PBMCs, PBMC samples from healthy volunteers were spiked with AsPC-1 cells and immunostained for KLF8 and vimentin (Figure 1). Cells positive for CK with a morphologically intact nucleus were AsPC-1 cells, whereas CD45+ cells were considered normal hematologic cells. AsPC-1 cells, but not PBMCs, were immunopositive for KLF8, whereas both cells types expressed vimentin. There were no CK+/KLF8+, CK+/vimentin+, vimentin+/KLF8+ cells present in samples from healthy volunteers and no triple-positive (CK+/CD45+/ KLF8+ or CK+/CD45+/ vimentin+) cells identified in patient samples (data not shown). These results demonstrated that we can distinguish the expression of KLF8 and vimentin in pancreatic cancer cells and PBMCs, imply the reliability of expression of KLF8 and vimentin as a marker for further analysis.
Figure 1.
KLF8 and vimentin expression in AsPC-1 cells spiked in blood of normal volunteers. Representative images showing immunocytochemistry for CK (to detect epithelial cells), CD45 (to discriminate normal hematologic cells), KLF8, and vimentin. KLF8 indicates Krüppel-like factor 8; PBMCs, peripheral blood mononuclear cells, scale bar, 20 μm.
Isolation and Molecular Characterization of CTCs
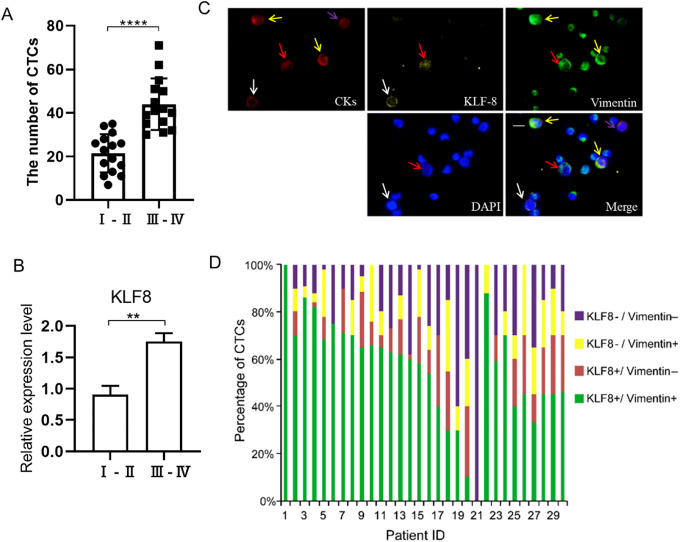
CTCs were identified in 30/40 (75.0%) PC patients, with an average of 33 ± 15 CTCs/7 mL (range: 7-71 CTCs/7 mL). We classified the TNM stage of 30 PC patients into Ⅰ-Ⅱ stage and Ⅲ-Ⅳ stage (Table 1). The number of CTCs had a positive correlation with the TNM stage (Figure 2A). Furthermore, the expression of KLF8 had a positive correlation with the TNM stage (Figure 2B). No CTCs were detected in samples from the 10 healthy donors or 5 patients with benign liver disease. Among the 30 patients with CTCs, 4 phenotypes were identified by immunocytochemistry: KLF8+/vimentin+ (Figure 2C, the red arrow indicated), KLF8+/vimentin- (Figure 2C, the white arrow indicated), KLF8-/vimentin+ (Figure 2C, the yellow arrow indicated), and KLF8-/vimentin- (Figure 2C, the purple arrow indicated), the remaining smaller cells are PBMCs. KLF8 was detected both in the cytoplasm and in the nuclei of CTCs, whereas vimentin was only located in the cytoplasm. The numbers and proportion of phenotypes varied among the patients (Figure 2D, Table 2). We found the ratio of KLF8+/vimentin+ CTCs is higher than KLF8+/vimentin-, KLF8-/vimentin+ and KLF8-/vimentin- CTCs in 30 patients (Figure 2D). By analyzing original data. The average ratio of KLF8+/vimentin+ CTCs is 53.6%, the average ratio of KLF8+/vimentin- is 12%, the average ratio of KLF8-/vimentin+ is 10.9%, the average ratio of KLF8-/vimentin- is 20.6%. Besides, the total average ratio of KLF8+ CTCs is 65.6%, the total average ratio of vimentin+ is 64.5% (Figure 2D). Previous study reported KLF8 overexpression upregulate Vimentin to promote the proliferation and invasion of cancer cells.32,42 So, we speculate KLF8 have a close relationship with Vimentin in CTCs. In other words, KLF8 may upregulate Vimentin in CTCs. Combining with the higher ratio of KLF8+/vimentin+ CTCs. So, we think KLF8+/vimentin+ CTCs may have more important clinical value. Then we next focus on KLF8+/vimentin+ CTCs.
Figure 2.
The expression of KLF8 and vimentin in CTCs. A, The number of CTCs in I-II stage and III-IV stage from 30 PC patients. B, The expression of KLF8 in CTCs from I-II stage and III-IV stage 30 PC patients. C, Representative images showing immunocytochemistry for CK, KLF, and vimentin; KLF8+/vimentin+ (red arrows), KLF8+/vimentin- (white arrows), KLF8-/vimentin+ (yellow arrows), and KLF8-/vimentin- (purple arrows) CTCs. D, The ratio of various positive cells in CTCs from 30 pancreatic cancer patients. CTC indicates circulating tumor cell; KLF8, Krüppel-like factor 8, Scale bar, 20 μm.
Table 2.
KLF8/Vimentin Expression in CTCs From 30 Pancreatic Cancer Patients.
| Patient no. | CTCs, n | KLF8/vimentin expression, % | |||
|---|---|---|---|---|---|
| +/+ | +/− | −/+ | −/− | ||
| 1 | 62 | 100 | 0 | 0 | 0 |
| 2 | 71 | 70 | 10 | 10 | 10 |
| 3 | 55 | 86 | 0 | 5 | 9 |
| 4 | 51 | 82 | 2 | 4 | 12 |
| 5 | 41 | 68 | 10 | 20 | 2 |
| 6 | 40 | 75 | 0 | 0 | 25 |
| 7 | 36 | 71 | 19 | 0 | 10 |
| 8 | 32 | 70 | 0 | 15 | 15 |
| 9 | 31 | 68 | 25 | 7 | 5 |
| 10 | 35 | 66 | 10 | 24 | 0 |
| 11 | 50 | 65 | 5 | 10 | 20 |
| 12 | 45 | 63 | 10 | 0 | 27 |
| 13 | 30 | 62 | 15 | 10 | 13 |
| 14 | 26 | 60 | 2 | 0 | 38 |
| 15 | 28 | 58 | 20 | 20 | 2 |
| 16 | 31 | 54 | 10 | 10 | 26 |
| 17 | 42 | 4 | 30 | 0 | 30 |
| 18 | 39 | 3 | 25 | 30 | 15 |
| 19 | 26 | 3 | 0 | 10 | 60 |
| 20 | 34 | 10 | 30 | 20 | 40 |
| 21 | 25 | 0 | 0 | 0 | 100 |
| 22 | 7 | 88 | 0 | 12 | 0 |
| 23 | 35 | 59 | 11 | 0 | 30 |
| 24 | 23 | 70 | 0 | 10 | 20 |
| 25 | 19 | 40 | 20 | 10 | 30 |
| 26 | 16 | 45 | 25 | 30 | 0 |
| 27 | 11 | 33 | 12 | 20 | 35 |
| 28 | 17 | 45 | 20 | 20 | 15 |
| 29 | 13 | 45 | 25 | 20 | 10 |
| 30 | 11 | 46 | 24 | 10 | 20 |
Abbreviations: CTC, Circulating tumor cell; KLF8, Krüppel-like factor 8.
CTCs as an Indicator of Prognosis in PC Patients
The median duration of follow-up was 13 months (range: 1-18 month). Recurrence was observed in 23/40 (57.5%) patients; the rate of recurrence was higher in patients with CTCs (21/30; 70.0%) than in those without (2/10; 20.0%) (r = 0.438; P < 0.01). Furthermore, patients with CTCs had a shorter recurrence-free survival than those without (log-rank = 10.384; P < 0.01) (Figure 3).
Figure 3.
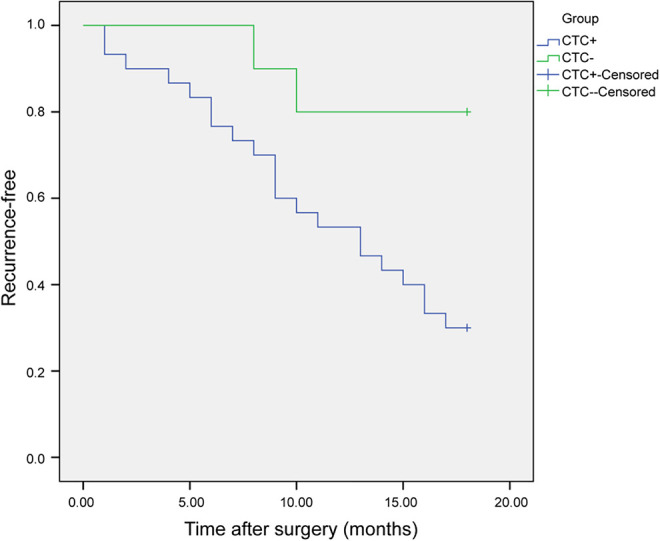
Recurrence-free survival of pancreatic cancer patients as assessed by the Kaplan-Meier method. Recurrence-free survival in pancreatic cancer patients with circulating tumor cells (CTC+; n = 30) and without (CTC-; n = 10).
CTC Subtypes and Clinical Outcome
TTR was correlated with proportion of KLF8+/vimentin+ CTCs (r = −0.646; P < 0.01), but not with the other 3 phenotypes or with the total numbers of CKs+, KLF8+, or vimentin+ cells. Further analysis revealed that TTR was significantly reduced in patients in whom > 50% of CTCs were KLF8+/vimentin+ compared with those with ≤ 50% (log-rank = 10.017; P < 0.01) (Figure 4).
Figure 4.
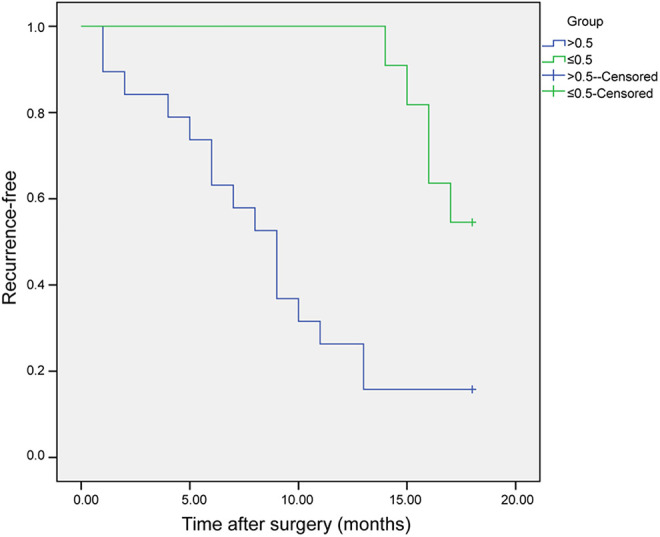
Recurrence-free survival of pancreatic cancer patients according to CTC phenotype. Recurrence-free survival in 30 pancreatic cancer patients with > 50% (> 0.5) and ≤ 50% (≤ 0.5) of CTCs double positive for Krüppel-like factor 8 and vimentin.
Five clinicopathologic factors were identified as prognostic indicators using univariate analysis: tumor size, Tumor-node-metastasis stage, vascular invasion, perineural invasion and > 50% KLF8+/vimentin+ CTCs (Table 3). A multivariate analysis of these factors confirmed that perineural invasion (relative risk = 2.984, 95% confidence interval: 1.179-7.553; P < 0.05) and > 50% KLF8+/vimentin+ CTCs (relative risk = 4.414, 95% confidence interval: 1.545-12.611; P < 0.01) were significant independent predictors of poor survival in PC.
Table 3.
Univariate Analysis for Factors Predicting Recurrence.
| Variable | n | Recurrence-free interval, mo | ||
|---|---|---|---|---|
| Mean | 95% CI | P value | ||
| Sex | ||||
| Male | 23 | 12.2 | 9.9-14.5 | 0.646 |
| Female | 7 | 10.4 | 6.1-14.7 | |
| Age (yr) | ||||
| < 50 | 9 | 11.3 | 6.8-15.8 | 0.562 |
| ≥ 50 | 21 | 12.0 | 9.8-14.2 | |
| Tumor size (cm) | ||||
| ≤ 4 | 12 | 14.3 | 11.0-17.7 | 0.028 |
| > 4 | 18 | 10.1 | 7.8-12.4 | |
| TNM stage | ||||
| I-II | 15 | 14.2 | 11.4-17.0 | 0.014 |
| III-IV | 15 | 9.4 | 6.9-12.0 | |
| CA19-9 (kU/L) | ||||
| ≤ 37 | 18 | 13.0 | 10.7-15.2 | 0.190 |
| > 37 | 12 | 9.1 | 5.3-12.9 | |
| Tumor location | ||||
| Head | 16 | 12.2 | 9.0-15.4 | 0.999 |
| Body/tail | 14 | 11.6 | 8.9-14.2 | |
| Vascular invasion | ||||
| Absent | 18 | 14.1 | 11.4-16.9 | 0.005 |
| Present | 12 | 9.5 | 6.9-12.0 | |
| Perineural invasion | ||||
| Absent | 18 | 13.9 | 11.2-16.7 | 0.008 |
| Present | 12 | 9.7 | 7.0-13.9 | |
| KLF8+/vimentin+ CTCs (%) | ||||
| ≤ 50 | 11 | 16.9 | 16.1-17.7 | 0.002 |
| >50 | 19 | 8.8 | 6.5-11.2 | |
Abbreviations: CA19-9, Cancer antigen 19-9; CTCs, Circulating tumor cells; KLF8, Krüppel-like factor 8; TNM, Tumor-Node-Metastasis.
Discussion
The association of poor prognosis in PC patients with the presence of CTCs has been debated in the literature, with earlier studies showing a weak association,43,44 and recently more studies indicating a strong correlation.13,45-48 We collected the samples of 40 PC patients. These patients had many characteristics, including sex, age, tumor size, TNM stage, CA19-9, Tumor location, Vascular invasion, Perineural invasion (Table 1). Among them, there are 30 patients harboring CTCs. As showed in Table 3, there are 21 age ≤ 50 and 9 age < 50, 18 tumor size > 4 (cm) and 12 tumor size ≤ 4, 19 KLF8+/vimentin+ CTCs >50 and 11 KLF8+/vimentin+ CTCs ≤ 50. The results of this study also provide further evidence in support of close correlation, showing a significantly higher recurrence rate and shorter recurrence-free survival in patients with CTCs. Thus, the detection of CTCs in PC patients may be useful for identifying those at high risk for relapse. CTCs were examined in 75.0% of PC patients enrolled in the current study, which was consistent with values reported by Chausovsky49 and Marrinucci,50 but higher than others.51,52 The discrepancies may be due to the small sample sizes or differences in detection and treatment methods.
To provide further information concerning the phenotype of this heterogeneous population of cells, CTCs were classified into 4 subtypes based on their expression of EMT markers. Initial experiments confirmed that CTCs can be distinguished from PBMCs by their expression of both CKs and vimentin. To our knowledge, this is the first study to evaluate KLF8 and vimentin phenotypes in CTCs of peripheral blood from PC patients. Overall, the patient population exhibited various combinations of the 4 possible CTC phenotypes. A larger percentage of patients (19/30; 63.3%) had > 50% of their CTCs double-positive for KLF8 and vimentin, whereas 36.7% (11/30) had ≤ 50% KLF8+/vimentin+ CTCs. Nel et al53 showed that vimentin+/CK+ ratio higher than 0.5, meaning that there were half as many vimentin positive cells as CK+ cells, was associated with a longer median time to progression (hazard ratio = 0.18; 95% confidence interval: 0.01-2.75; P = 0.03). Similarly, our results show that the double-positive phenotype is inversely associated with TTR, such that patients with a > 50% proportion of double-positive CTCs have a shorter TTR. Moreover, this was identified as an independent risk factor for recurrence by univariate and multivariate analyzes. These data indicate that the proportion of KLF+/vimentin+ CTCs may serve as a potential prognostic marker for PC patients treated by resection.
Only 1 other study has examined the association between KLF8 and the prognosis of PC,54 which found that patients with positive expression had significantly shorter overall survival. However, overexpression of KLF8 has been correlated with prognoses of other malignant tumors.24,29-31,55 Li32 reported a significantly higher tumor recurrence rate in hepatocellular carcinoma patients with positive KLF8 expression compared to those with negative expression (59.8% vs 41.9%), and KLF8 showed prognostic significance in the early recurrence group. Yang33 also showed that KLF8 overexpression was positively correlated with progression of HCC. Another study showed an association between KLF8 expression and breast cancer metastasis and patient survival.28 KLF8 was also associated with poor prognosis in gastric cancer.35 Vimentin is overexpressed in various epithelial cancers, including prostate cancer, gastrointestinal tumors, tumors of the central nervous system, breast cancer, malignant melanoma, and lung cancer.56-58 Vimentin’s overexpression in cancer correlates well with accelerated tumor growth, invasion, and poor prognosis.59-63 Vimentin could be as potential target for different cancer treatment.64,65 Previous study reported KLF8 overexpression upregulate Vimentin to promote the proliferation and invasion of cancer cells.32,42 So, we believe KLF8 have a close relationship with Vimentin in CTCs. Our study combined the 2 independent prognosis Markers to detect its correlation with prognosis, ensuring its accuracy and clinical value.
Taken together, these findings indicate that classification of KLF8+/vimentin+ CTCs may have an important clinical application, it can provide potential indication for treatment of PC patients before distant metastasis. However, further investigations with a larger population and a longer follow-up period are needed to verify these results.
Acknowledgments
The authors thank Professor. Zheng-Feng Yin in the department of Molecular Oncology, Affiliated Eastern Hepatobiliary Surgery Hospital of the Second Military Medical University for his technical support. We also are very grateful for the sincere help and excellent technical support by the Translational Medicine Research Center of the Second Military Medical University.
Abbreviations
- PC
Pancreatic cancer
- CTCs
circulating tumor cells
- EMT
epithelial–mesenchymal transition
- KLF8
Krüppel-Like Factor 8
- PBMNCs
peripheral blood mononuclear cells
- TTR
time to recurrence.
Authors’ Note: Peng Zhu, Hui-ying Liu and Fu-chen Liu contributed equally to this work. All patients provided written informed consent prior to enrollment in the study. These experiments were approved by the Ethical Committee of Naval Medical University (approval no. EHBHKY2020-K-057) following the declaration of Helsinki ethical guidelines. The research has been carried out in accordance with the World Medical Association Declaration of Helsinki. All patients provided written informed consent prior to enrollment in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Open Project of Key Laboratory of Pancreatic Diseases of Shanghai, Contract grant numbers: P2012007.
ORCID iD: Peng Zhu, MSc  https://orcid.org/0000-0002-5883-672X
https://orcid.org/0000-0002-5883-672X
References
- 1.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7(3):163–172. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. [DOI] [PubMed] [Google Scholar]
- 3.Kikuyama M, Kamisawa T, Kuruma S, et al. Early diagnosis to improve the poor prognosis of pancreatic cancer. Cancers (Basel). 2018;10(2):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao H, LE D, Yang LX. Current status in chemotherapy for advanced pancreatic adenocarcinoma. Anticancer Res. 2013;33(5):1785–1791. [PubMed] [Google Scholar]
- 5.Lakkakula BVKS, Farran B, Lakkakula S, et al. Small molecule tyrosine kinase inhibitors and pancreatic cancer-trials and troubles. Semin Cancer Biol. 2019;56:149–167. [DOI] [PubMed] [Google Scholar]
- 6.Riall TS, Nealon WH, Goodwin JS, et al. Pancreatic cancer in the general population: improvements in survival over the last decade. J Gastrointest Surg. 2006;10(9):1212–1223. Discussion 1223-1214. [DOI] [PubMed] [Google Scholar]
- 7.Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10(1):88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunner M, Wu Z, Krautz C, Pilarsky C, Grützmann R, Weber GF. Current clinical strategies of pancreatic cancer treatment and open molecular questions. Int J Mol Sci. 2019;20(18):4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coghlin C, Murray GI. Current and emerging concepts in tumour metastasis. J Pathol. 2010;222(1):1–15. [DOI] [PubMed] [Google Scholar]
- 10.Lianidou ES, Strati A, Markou A. Circulating tumor cells as promising novel biomarkers in solid cancers. Crit Rev Clin Lab Sci. 2014;51(3):160–171. [DOI] [PubMed] [Google Scholar]
- 11.Cortés-Hernández LE, Eslami SZ, Pantel K, Alix-Panabières C. Molecular and functional characterization of circulating tumor cells: from discovery to clinical application. Clin Chem. 2020;66(1):97–104. [DOI] [PubMed] [Google Scholar]
- 12.Pantel K, Alix-Panabières C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med. 2010;16(9):398–406. [DOI] [PubMed] [Google Scholar]
- 13.Khoja L, Backen A, Sloane R, et al. Pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer. 2012;106(3):508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner NC, Reis-Filho JS. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012;13(4):e178–e185. [DOI] [PubMed] [Google Scholar]
- 15.Powell AA, Talasaz AH, Zhang H, et al. Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS One. 2012;7(5):e33788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller L, Pantel K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat Rev Cancer. 2019;19(10):553–567. [DOI] [PubMed] [Google Scholar]
- 17.Chin VL, Lim CL. Epithelial-mesenchymal plasticity-engaging stemness in an interplay of phenotypes. Stem Cell Investig. 2019;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou J, Guo C, Lyu G. Clinical significance of epithelial-mesenchymal transition typing of circulating tumour cells in colorectal cancer. Colorectal Dis. 2020;22(5):581–587. [DOI] [PubMed] [Google Scholar]
- 19.Whipple RA, Balzer EM, Cho EH, Matrone MA, Yoon JR, Martin SS. Vimentin filaments support extension of tubulin-based microtentacles in detached breast tumor cells. Cancer Res. 2008;68(14):5678–5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson AM, Havel LS, Koyen AE, et al. Vimentin is required for lung adenocarcinoma metastasis via heterotypic tumor cell-cancer-associated fibroblast interactions during collective invasion. Clin Cancer Res. 2018;24(2):420–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Zheng M, Liu G, et al. Krüppel-like factor 8 induces epithelial to mesenchymal transition and epithelial cell invasion. Cancer Res. 2007;67(15):7184–7193. [DOI] [PubMed] [Google Scholar]
- 22.Mehta TS, Lu H, Wang X, et al. A unique sequence in the N-terminal regulatory region controls the nuclear localization of KLF8 by cooperating with the C-terminal zinc-fingers. Cell Res. 2009;19(9):1098–1099. [DOI] [PubMed] [Google Scholar]
- 23.Urvalek AM, Wang X, Lu H, Zhao J. KLF8 recruits the p300 and PCAF co-activators to its amino terminal activation domain to activate transcription. Cell Cycle. 2010;9(3):601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Urvalek AM, Liu J, Zhao J. Activation of KLF8 transcription by focal adhesion kinase in human ovarian epithelial and cancer cells. J Biol Chem. 2008;283(20):13934–13942. [DOI] [PubMed] [Google Scholar]
- 25.Wei H, Wang X, Gan B, et al. Sumoylation delimits KLF8 transcriptional activity associated with the cell cycle regulation. J Biol Chem. 2006;281(24):16664–16671. [DOI] [PubMed] [Google Scholar]
- 26.Zhao J, Bian ZC, Yee K, Chen BP, Chien S, Guan JL. Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin D1 and cell cycle progression. Mol Cell. 2003;11(6):1503–1515. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Zhao J. KLF8 transcription factor participates in oncogenic transformation. Oncogene. 2007;26(3):456–461. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Lu H, Urvalek AM, et al. KLF8 promotes human breast cancer cell invasion and metastasis by transcriptional activation of MMP9. Oncogene. 2011;30(16):1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, Liu N, Xu M, et al. Lentivirus-delivered Krüppel-like factor 8 small interfering RNA inhibits gastric cancer cell growth in vitro and in vivo. Tumour Biol. 2012;33(1):53–61. [DOI] [PubMed] [Google Scholar]
- 30.Wan W, Zhu J, Sun X, Tang W. Small interfering RNA targeting Krüppel-like factor 8 inhibits U251 glioblastoma cell growth by inducing apoptosis. Mol Med Rep. 2012;5(2):347–350. [DOI] [PubMed] [Google Scholar]
- 31.Fu WJ, Li JC, Wu XY, et al. Small interference RNA targeting Krüppel-like factor 8 inhibits the renal carcinoma 786-0 cells growth in vitro and in vivo. J Cancer Res Clin Oncol. 2010;136(8):1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li JC, Yang XR, Sun HX, et al. Up-regulation of Krüppel-like factor 8 promotes tumor invasion and indicates poor prognosis for hepatocellular carcinoma. Gastroenterology. 2010;139(6):2146–2157.e12. [DOI] [PubMed] [Google Scholar]
- 33.Yang T, Cai SY, Zhang J, et al. Krüppel-like factor 8 is a new Wnt/beta-catenin signaling target gene and regulator in hepatocellular carcinoma. PLoS One. 2012;7(6):e39668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma D, Liu H, Qin Y, Tian Z, Li S, Liang N. KLF8 overexpression promotes the growth of human lung cancer cells by promoting the expression of JMJD2A. Cancer Cell Int. 2019;19:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao A, Zhou X, Liu Y, Ding J, Miao A, Pan G. KLF8 is associated with poor prognosis and regulates glycolysis by targeting GLUT4 in gastric cancer. J Cell Mol Med. 2019;23(8):5087–5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Zheng H, Ding Y, et al. The level of Krüppel-like factor 8 expression predicts prognosis and metastasis in various carcinomas. Medicine. 2019;98(18):e15519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reinholz MM, Kitzmann KA, Tenner K, et al. Cytokeratin-19 and mammaglobin gene expression in circulating tumor cells from metastatic breast cancer patients enrolled in North Central Cancer Treatment Group Trials, N0234/336/436/437. Clin Cancer Res. 2011;17(22):7183–7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu W, Cao L, Chen L, et al. Isolation of circulating tumor cells in patients with hepatocellular carcinoma using a novel cell separation strategy. Clin Cancer Res. 2011;17(11):3783–3793. [DOI] [PubMed] [Google Scholar]
- 39.Kallergi G, Markomanolaki H, Giannoukaraki V, et al. Hypoxia-inducible factor-1alpha and vascular endothelial growth factor expression in circulating tumor cells of breast cancer patients. Breast Cancer Res. 2009;11(6):R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oshima M, Okano K, Muraki S, et al. Immunohistochemically detected expression of 3 major genes (CDKN2A/p16, TP53, and SMAD4/DPC4) strongly predicts survival in patients with resectable pancreatic cancer. Ann Surg. 2013;258(2):336–346. [DOI] [PubMed] [Google Scholar]
- 41.Ning Y, Hanna DL, Zhang W, et al. Cytokeratin-20 and survivin-expressing circulating tumor cells predict survival in metastatic colorectal cancer patients by a combined immunomagnetic qRT-PCR approach. Mol Cancer Ther. 2015;14(10):2401–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Liu L, Wang Y, et al. KLF8 involves in TGF-beta-induced EMT and promotes invasion and migration in gastric cancer cells. J Cancer Res Clin Oncol. 2013;139(6):1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uchikura K, Takao S, Nakajo A, et al. Intraoperative molecular detection of circulating tumor cells by reverse transcription-polymerase chain reaction in patients with biliary-pancreatic cancer is associated with hematogenous metastasis. Ann Surg Oncol. 2002;9(4):364–370. [DOI] [PubMed] [Google Scholar]
- 44.Soeth E, Grigoleit U, Moellmann B, et al. Detection of tumor cell dissemination in pancreatic ductal carcinoma patients by CK 20 RT-PCR indicates poor survival. J Cancer Res Clin Oncol. 2005;131(10):669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Albuquerque A, Kubisch I, Breier G, et al. Multimarker gene analysis of circulating tumor cells in pancreatic cancer patients: a feasibility study. Oncology. 2012;82(1):3–10. [DOI] [PubMed] [Google Scholar]
- 46.Bidard FC, Huguet F, Louvet C, et al. Circulating tumor cells in locally advanced pancreatic adenocarcinoma: the ancillary CirCe 07 study to the LAP 07 trial. Ann Oncol. 2013;24(8):2057–2061. [DOI] [PubMed] [Google Scholar]
- 47.Sparano J, O’Neill A, Alpaugh K, et al. Association of circulating tumor cells with late recurrence of estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(12):1700–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyamoto DT, Lee RJ, Kalinich M, et al. An RNA-based digital circulating tumor cell signature is predictive of drug response and early dissemination in prostate cancer. Cancer Discov. 2018;8(3):288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chausovsky G, Luchansky M, Figer A, et al. Expression of cytokeratin 20 in the blood of patients with disseminated carcinoma of the pancreas, colon, stomach, and lung. Cancer. 1999;86(11):2398–2405. [PubMed] [Google Scholar]
- 50.Marrinucci D, Bethel K, Kolatkar A, et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys Biol. 2012;9(1):016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurihara T, Itoi T, Sofuni A, et al. Detection of circulating tumor cells in patients with pancreatic cancer: a preliminary result. J Hepatobiliary Pancreat Surg. 2008;15(2):189–195. [DOI] [PubMed] [Google Scholar]
- 52.Sergeant G, Roskams T, van Pelt J, Houtmeyers F, Aerts R, Topal B. Perioperative cancer cell dissemination detected with a real-time RT-PCR assay for EpCAM is not associated with worse prognosis in pancreatic ductal adenocarcinoma. BMC Cancer. 2011;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nel I, Baba HA, Ertle J, et al. Individual profiling of circulating tumor cell composition and therapeutic outcome in patients with hepatocellular carcinoma. Transl Oncol. 2013;6(4):420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei Y, Chen G, You L, Zhao Y. Krüppel-like factor 8 is a potential prognostic factor for pancreatic cancer. Chin Med J (Engl). 2014;127(5):856–859. [PubMed] [Google Scholar]
- 55.Zheng Y, Zheng B, Meng X, Yan Y, He J, Liu Y. LncRNA DANCR promotes the proliferation, migration, and invasion of tongue squamous cell carcinoma cells through miR-135a-5p/KLF8 axis. Cancer Cell Int. 2019;19:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68(18):3033–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyer SN, Galván JA, Zahnd S, et al. Co-expression of cytokeratin and vimentin in colorectal cancer highlights a subset of tumor buds and an atypical cancer-associated stroma. Hum Pathol. 2019;87:18–27. [DOI] [PubMed] [Google Scholar]
- 58.Zhang H, Wu X, Xiao Y, et al. Coexpression of FOXK1 and vimentin promotes EMT, migration, and invasion in gastric cancer cells. J Mol Med (Berl). 2019;97(2):163–176. [DOI] [PubMed] [Google Scholar]
- 59.Kaschula CH, Tuveri R, Ngarande E, et al. The garlic compound ajoene covalently binds vimentin, disrupts the vimentin network and exerts anti-metastatic activity in cancer cells. BMC Cancer. 2019;19(1):248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma P, Alsharif S, Fallatah A, Chung BM. Intermediate filaments as effectors of cancer development and metastasis: a focus on keratins, vimentin, and nestin. Cells. 2019;8(5):497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li W, Cho MY, Lee S, Jang M, Park J, Park R. CRISPR-Cas9 mediated CD133 knockout inhibits colon cancer invasion through reduced epithelial-mesenchymal transition. PLoS One. 2019;14(8):e0220860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, Ji S, Ma Y, et al. Vimentin plays an important role in the promotion of breast cancer cell migration and invasion by leucine aminopeptidase 3. Cytotechnology. 2020;72(5):639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lavenus SB, Tudor SM, Ullo MF, Vosatka KW, Logue JS. A flexible network of vimentin intermediate filaments promotes migration of amoeboid cancer cells through confined environments. J Biol Chem. 2020;295(19):6700–6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strouhalova K, Přechová M, Gandalovičová A, Brábek J, Gregor M, Rosel D. Vimentin intermediate filaments as potential target for cancer treatment. Cancers. 2020;12(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Makol A, Kaur H, Sharma S, Kanthaje S, Kaur R, Chakraborti A. Vimentin as a potential therapeutic target in sorafenib resistant HepG2, a HCC model cell line. Clin Mol Hepatol. 2020;26(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]