Abstract
Background:
The development of colon cancer has been described as a multistep process of carcinogenesis. Understanding molecular and cellular changes underlying this process is required to determine potential biomarkers and therapeutic targets in colon cancers. Several molecular entities, including glypicans, are implicated in cancer development. Among these is glypican-6, which is overexpressed in a limited number of cancers. This study aims to characterise the glypican-6 expression in different types of colon cancer.
Methods:
Immunohistochemistry was used to characterise glypican-6 expression in a panel of archived formalin-fixed, paraffin-embedded colon tissue types. These types included 39 normal colon tissues, 10 colon tubular adenomas, 60 colon adenocarcinomas without metastasis and 60 colon adenocarcinomas with metastasis. Glypican-6 expression relation to demographic and clinicopathologic features was also examined.
Results:
Glypican-6 was strongly expressed in benign, primary and metastatic colon tumours. Normal tissue samples exhibited low to undetectable levels of glypican-6. A significantly high glypican-6 expression was displayed in colon cancers with lymph node metastasis, high depth of invasion, distant metastasis, high histological grades and late stages of the disease (P < 0.05). Importantly, a significant differential in glypican-6 expression was found between normal tissues and different types of colon cancer tissues. Moreover, the highest glypican-6 expression was more frequently found in metastatic tumours, followed by primary tumours and the least in benign tumours (P < 0.05).
Conclusions:
Selective expression of glypican-6 may establish a basis for potential use as a tissue biomarker or as a novel therapeutic target in treatment of colon cancer.
Keywords: Cancer, colon cancer, glypicans, glypican-6, immunohistochemistry, proteoglycans
Introduction
Colon cancer arises from progressive development of genetic and epigenetic mutations leading to a malignant stepwise transformation of normal colon epithelial cells to invasive and metastatic colon adenocarcinomas. Such mutations trigger colon cancer by targeting genes involved in DNA repair mechanisms, tumour suppressor genes, and oncogenes.1 Based on the type of mutation, colon cancers are categorised as inherited (5%), familial (25%) and mostly sporadic (70%).2 Within these forms of colon cancer, prevalent mutations, chromosomal modifications and translocations have been identified to influence significant pathways like TP53 and TGF. Moreover, some gene mutations, such as BRAF for example could be used as predictors of patient outcome.1,2 Consequently, these alterations at the molecular and cellular level may help in identifying alternative mechanisms for the development of potential colon cancer therapy.
Nowadays, 1 member of the glypican family that has attracted recent interest is glypican-6. Its role in cancer progression and metastasis has been the focus of many studies. In gastric cancer, levels of glypican-6 mRNA are significantly elevated in cancer tissues compared with normal tissues. Nevertheless, its levels do not strongly correlate to clinicopathological features.3 Concomitantly, glypican-6 mRNA is overexpressed in early-stage ovarian cancer and its levels are strongly associated with the overall survival of patients with early-stage ovarian cancer.4 Moreover, the expression levels of the glypican-6 gene distinguish primary melanoma from regional cutaneous/subcutaneous metastases and are implicated as a biomarker for metastatic melanoma.5 This gene has also been found to be more frequently mutated in nasopharyngeal carcinoma.6 Importantly, functional assays using cancer cell line models have demonstrated that overexpression of glypican-6 enhances cell proliferation, invasion and migration.6,7 All of these effects are inhibited upon glypican-6 silencing,7 suggesting its use as a potential therapeutic target for treating nasopharyngeal carcinoma.6 However, the exact biological role of glypican-6 remains unclear.
The present study aims to characterise the glypican-6 expression in different colon tissue types, including normal, benign and malignant tumoural, primary and metastatic, and to examine its relation to demographic and clinicopathologic features.
Materials and Methods
Tissue specimens
Before conducting the study, an exemption of written informed consent for the use of colon tissue samples was obtained from the Institutional Review and Ethics Committee, Faculty of Medicine, University of Mutah (Reference No.7012021, Date: January 20, 2021). The study complies with regulations set by the Declaration of Helsinki (2013). We performed an immunohistochemical analysis of 169 colon clinical specimens. All samples were collected from the Pathology Department, King Hussein Medical Hospital and King Abdullah University Hospital, Jordan. Fresh tissue samples were fixed in 10% formalin for 24 hours, embedded in paraffin, cut into 5-µm thick tissues and stained with haematoxylin and eosin for routine diagnosis. Tissue specimens consisted of 60 primary colon adenocarcinomas without metastasis, 10 colon tubular adenomas and 60 colon adenocarcinomas with metastasis (55 samples with lymph node metastasis and 5 samples with the liver metastasis). The control group consisted of 39 normal colon tissues in which 29 normal colon tissues were postoperatively taken from patients who underwent colonic resection for colon adenocarcinoma as regular follow-up. These samples were taken 5 cm a part from the site of previous anastomosis that was identified during colonoscopy. The remaining 10 normal colon tissues were taken from patients who underwent colonoscopy having single polyp less than 3 mm and found histopathologically to be normal colon tissue. Normal colon tissue samples who were taken from patients with inflammatory bowel disease, colorectal polyposis syndromes and hereditary non-polyposis colorectal cancer were excluded from the study. Moreover, patients who had received pre-surgical radiotherapy or chemotherapy were excluded from this study. Clinical parameters regarding age, tumour histopathology, histological grade and stage of the disease were retrospectively retrieved from the patients’ pathology reports. The patients’ clinical and pathological information was anonymised and kept confidential.
Immunohistochemistry
A peroxidase polymer-based detection system was used for the immunohistochemical localisation of glypican-6. Tissue sections were de-waxed, rehydrated and immersed in 3% hydrogen peroxide solution for 5 minutes. Antigen retrieval was performed by heating samples in a microwave in a citrate buffer for 20 minutes. Following incubation with 2.5% normal goat serum, sections were covered with a rabbit polyclonal antibody specific to glypican-6 (ab251716) (Abcam, Cambridge, UK) at a concentration of 10 µg/ml (dilution 1:100) at 4°C overnight. After that, ImmPRESS (Peroxidase) Polymer Goat Anti-Rabbit IgG Reagent was applied for 30 minutes (MP-7451; Vector Laboratories, Burlingame, CA, USA). Sections were then incubated with diaminobenzidine substrate to visualise the immunoreactivity. The tissues were subsequently counterstained with Harris’s haematoxylin solution, dehydrated and mounted with coverslips. As positive control, ovarian cancer tissue was used to demonstrate specific antibody binding to glypican-6 protein. Moreover, specific binding of primary antibody was validated by pre-incubating glypican-6 antibody with human glypican-6 blocking peptide (kindly gifted from Dr Fatemah Alshammari) for 45 minutes at room temperature. The resulting mixture was then used instead of glypican-6 antibody to inhibit subsequent primary antibody binding to glypican-6 epitope in sample. The staining intensity of samples stained with glypican-6 antibody and samples stained with blocked antibody were compared. The negative control samples were performed by incubating tissue sections with normal goat serum instead of a primary antibody. Mounted slides were assessed and analysed using a Leica DMRB microscope (Leica DMRB, Wetzlar, Germany). Digital images were captured and processed using the AcQuis imaging capture system (Synoptics, Cambridge, UK).
Scoring
Glypican-6 expression was semi-quantitatively examined by 2 independent, experienced pathologists.
Cells showing membranous or cytoplasmic immunostaining were considered positive. Tissue specimens were scored based on the percentage of tumour cells showing positive expression and staining intensity as follows: negative (no expression), weak (<33%), moderate (33%–66%) and strong (>67%).
Statistical analysis
Statistical analysis was performed using the Statistical Packages for Social Sciences (SPSS) software version 16.0 (SPSS Inc, Chicago, IL, USA). Data were expressed in simple measures of frequency and percentage. The Pearson’s chi-square test, with the application of an analysis of variance (ANOVA) test, was used to examine the difference between the discrete variables. The results were considered statistically significant only if P < 0.05.
Results
Baseline demographic and clinicopathologic features
There were 110 males and 59 females with a mean age of 54.1 ± 14.3 years (Table 1). Less than half of the patients were below the age of 50 years (n = 77, 45.6%), and the remainder were over the age of 50 years (n = 92, 54.4%). Clinically normal colon specimens consisted of 29 (17.1%) cases adjacent to tumours, whereas others were normal healthy tissues (10 cases, 5.9%). Additionally, tubular adenomas (5.9%, 10 cases) were the benign colorectal tumours in this study. Of 60 (35.5%) primary colon cancers, 55 (27.2%) cases were colon adenocarcinomas, whereas other cancers were specifically mucinous adenocarcinomas (5 cases, 8.3%). All the metastatic tumours (60 cases, 35.5%) were lymph node metastases. Of 60 patients with primary colon cancer, most were grade II (30.8%, 52 cases) and grade III (23.7%, 40 cases), whereas grade I constituted only 10.7% (18 cases). The majority of colorectal cancer patients were stage II (26.7%, 16 cases) and stage IIB (21.7%, 13 cases); the other stages were IIIB (15%, 9 cases), III (10%, 6 cases), I (10%, 6 cases), IIA (6.7%, 4 cases), IIIC (5%, 3 cases) and IV (5%, 3 cases). Regarding the primary tumour depth of invasion, most were at T4 (36 cases, 60%), whereas others were at T1 (1 case, 1.7%), T2 (5 cases, 8.3%) and T3 (18 cases, 30%). Of the primary tumours, 60% (36) of the cases had no lymph node metastasis (N0), whereas 25% (15) of cases showed metastasis to 3 regional lymph nodes and 15% (nine cases) showed metastasis to 6 regional lymph nodes. Moreover, distant metastasis only presented in 6.7% (4 cases) of patients, whereas other patients were free from distant metastasis (56 cases, 93.3%).
Table 1.
Association between glypican-6 expression and clinicopathologic features of colon cancer patients.
| Characteristic | Negative | Weak | Moderate | High | P-value |
|---|---|---|---|---|---|
| Gender | |||||
| Male (n = 110, 65.1%) | 10 (9.1%) | 34 (30.9%) | 13 (11.8%) | 53 (48.2%) | .513 |
| Female (n = 59, 34.9%) | 3 (5.1%) | 14 (23.7%) | 9 (15.3%) | 33 (55.9%) | |
| Age | |||||
| <50 (n = 77, 45.6%) | 8 (10.4%) | 28 (36.4%) | 8 (10.4%) | 33 (42.9%) | .006 |
| ⩾50 (n = 92, 54.4%) | 5 (5.4%) | 20 (21.7%) | 14 (15.2%) | 53 (57.6%) | |
| Histological type | |||||
| Normal (n = 39, 23.1%) | 8 (20.5%) | 25 (64.1%) | 4 (10.3%) | 2 (5.1%) | .008 |
| Benign (n = 10, 5.9%) | 0 (0%) | 6 (60%) | 2 (20%) | 2 (20%) | |
| Primary (n = 60, 35.5%) | 3 (5%) | 15 (25%) | 12 (20%) | 30 (50%) | |
| Metastasis (n = 60, 35.5%) | 2 (3.3%) | 2 (3.3%) | 4 (6.7%) | 52 (86.7%) | |
| Histological grade | |||||
| I (n = 18, 10.7%) | 2 (11.1%) | 5 (27.8%) | 4 (22.2%) | 7 (38.9%) | .005 |
| II (n = 52, 30.8%) | 2 (3.8%) | 5 (9.6%) | 7 (13.5%) | 38 (73.1%) | |
| III (n = 50, 29.6%) | 1 (2%) | 7 (14%) | 5 (10%) | 37 (74%) | |
| TNM histological stage | |||||
| I (n = 6, 10%) | 0 (0%) | 1 (16.7%) | 1 (16.7%) | 4 (66.7%) | .003 |
| II (n = 33, 55%) | 2 (6.1%) | 8 (24.2%) | 5 (15.2%) | 18 (54.5%) | |
| III (n = 18, 30%) | 1 (5.6%) | 6 (33.3%) | 4 (22.2%) | 7 (38.9%) | |
| IV (n = 3, 5%) | 0 (0.0%) | 0 (0.0%) | 2 (66.7%) | 1 (33.3%) | |
| p T stage | |||||
| T1+T2 (n = 6, 10%) | 0 (0%) | 1 (16.7%) | 1 (16.7%) | 4 (66.7%) | .011 |
| T3+T4 (n = 54, 90%) | 3 (5.6%) | 14 (25.9%) | 11 (20.4%) | 26 (48.1%) | |
| p N stage | |||||
| N0 (n = 36, 60%) | 2 (5.6%) | 8 (22.2%) | 6 (16.7%) | 20 (55.6%) | .009 |
| N1 (n = 15, 25%) | 1 (6.7%) | 4 (26.7%) | 5 (33.3%) | 5 (33.3%) | |
| N2 (n = 9, 15%) | 0 (0%) | 3 (33.3%) | 1 (11.1%) | 5 (55.6%) | |
| p M stage | |||||
| M0 (n = 56, 93.3%) | 3 (5.4%) | 14 (25%) | 10 (17.9%) | 56 (51.8%) | .002 |
| M1 (n = 4, 6.7%) | 0 (0.0%) | 1 (25%) | 2 (50%) | 1 (25%) | |
Glypican-6 expression in normal colon samples
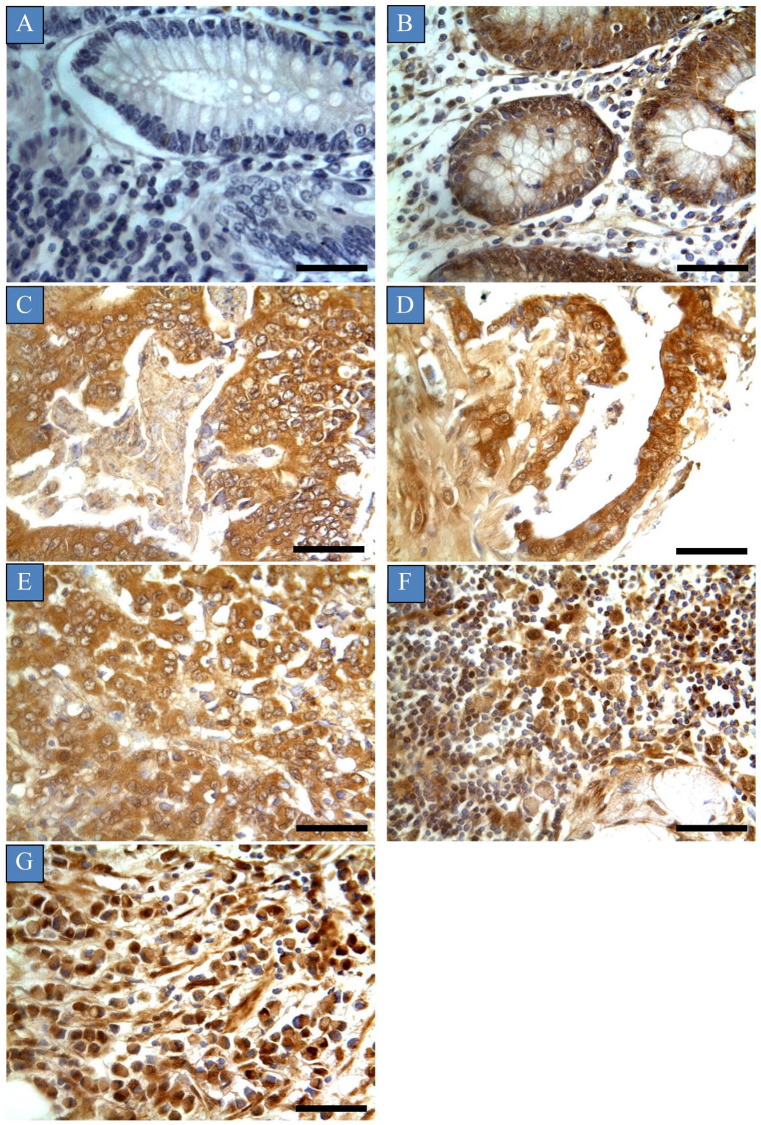
The phenotypic expression pattern of glypican-6 is shown in Figure 1. Glypican-6 positivity was observed in 79.8% (31 cases) of the normal samples in the form of dark-brown membranous or cytoplasmic immunostaining without any considerable staining in cell nuclei. Of 31 positive cases, the expression was low in 25 cases (64.1%), and only 2 cases (5.1%) exhibited high expression. Importantly, almost all the positive normal cases were normal colon tissues adjacent to tumours (79.4%), whereas the remainder were normal healthy tissues (20.6%). This expression was characterised as patchy and heterogeneous, and many areas showed complete negative expression (Table 1). Importantly, validation of glypican-6 expression was established by inhibition of immunoreactivity using glypican-6 protein as a blocking step and by using appropriate positive and negative controls. Colon cancer tissues incubated with mixture of primary antibody and blocking peptide showed weak to no glypican-6 expression. Moreover, the positive control ovarian cancer tissue showed high glypican-6 expression, whereas negative control exhibited no expression at all ( Supplemental Figure S1).
Figure 1.
Glypican-6 expression in different types of colon cancers. Immunohistochemical staining was recognised as dark-brown membranous or cytoplasmic immunostaining without any considerable staining in cell nuclei. Tumours were classified based on the histological type: (A) normal colon tissue, (B) colon tubular adenoma, (C) colon adenocarcinoma, (D) mucinous adenocarcinoma, (E) metastatic adenocarcinoma from the colon, (F) metastatic mucinous adenocarcinoma and (G) metastatic signet-ring cell carcinoma.
Scale bar = 50 µm. Magnification (×400).
Glypican-6 expression in benign colon tumours
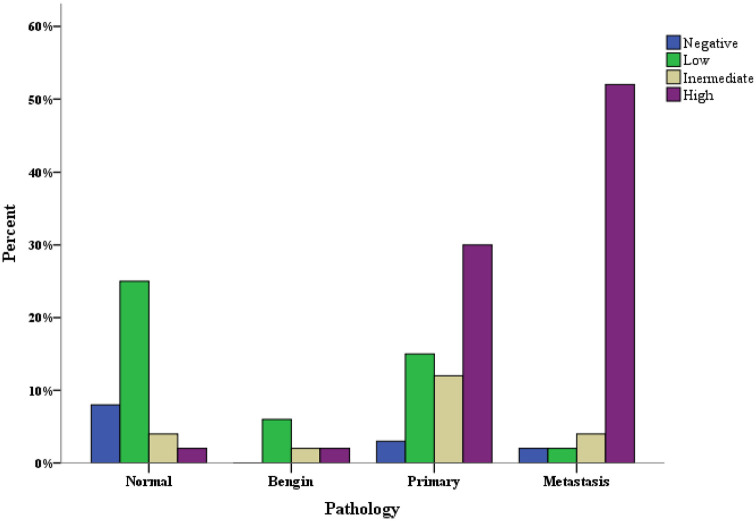
All the benign colon tumours exhibited glypican-6 expression (Figure 1). However, this expression was found to be low in 60% (6 cases) of patients, and only 20% (2 cases) of patients exhibited high expression. Moreover, the expression was focal and heterogeneous, and it varied in staining intensity between different sections and sometimes from one area to another in the same section. Importantly, there was a significant difference in glypican-6 expression between normal colon tissues and benign tumours (P = 0.023) (Figure 2).
Figure 2.
Glypican-6 expression in normal, benign, primary and metastatic colon samples.
Glypican-6 expression in primary colon cancers
Glypican-6 was intensely expressed in 95% (57 cases) of primary colon tumours. High expression was recognised in 50% (30 cases) of the patients, whereas other patients showed low (25%, 15 cases) to moderate (20%, 12 cases) expression. The expression was consistent through the tissue sections and no intratumour variation was apparent (Table 1) (Figure 1).
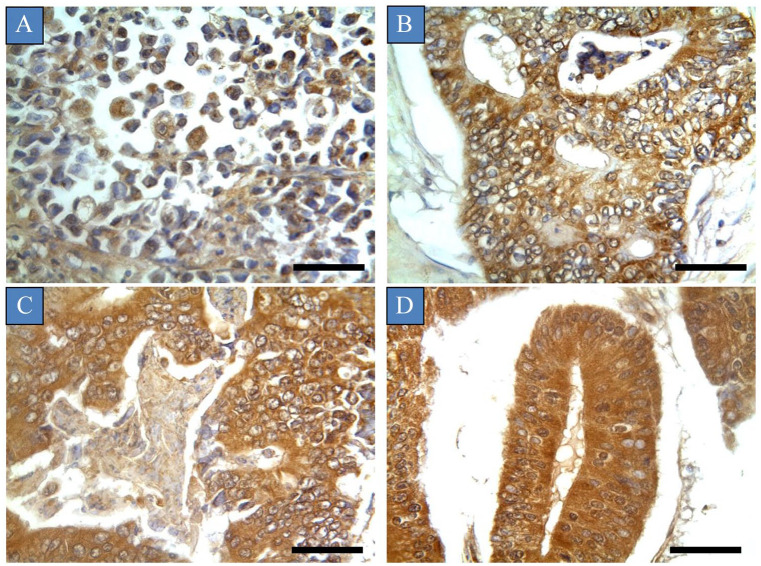
The relationships between immunohistochemical expression of glypican-6 and different demographic and clinicopathologic features are shown in Table 1. There were significant associations between glypican-6 expression and patient age, histological grade, histological stage, depth of invasion, lymph node metastasis, distant metastasis and the pathology type of colon tissue (P-value < 0.05). Glypican-6 expression was more frequently found in colon cancers with lymph node metastasis, high depth of invasion, distant metastasis, high histological grades and late stages of the disease (P < 0.05). For instance, Figure 3 shows pattern of glypican-6 expression in different stages of colon cancers. In contrast, no significant association was found between glypican-6 expression and the patient’s gender. Crucially, there was a significant difference in glypican-6 expression between normal colon samples and primary tumour samples (P-value = 0.001). A higher frequency of glypican-6 expression was observed in primary tumour samples than in normal samples. Additionally, a similar differential of glypican-6 expression was observed between benign tumour samples and primary tumour samples, although this finding did not reach statistical significance (P = 0.155) (Figure 2).
Figure 3.
Glypican-6 expression in different histological stages of colon cancers. Tumours were classified based on the histological stage: (A) Stage I, (B) Stage II, (C) Stage III and (D) Stage IV.
Scale bar = 50 µm. Magnification (×400).
Glypican-6 expression in metastatic colon cancers
Glypican-6 expression was determined in almost all metastatic samples (96.7%, 58 cases) (Table 1) (Figure 1). All tissue sections of metastatic deposit samples showed consistent staining, and no intratumour heterogeneity or variation in staining intensity was observed. Of 58 patient cases, glypican-6 was highly expressed in 52 cases (86.7%), whereas other patient cases displayed low (3.3%, 2 cases) to moderate (6.7%, 4 cases) expression. A significant difference in glypican-6 expression was observed between metastatic samples and normal, benign and primary tumour samples (P < 0.05). A higher frequency of glypican-6 expression was observed in metastatic samples compared with benign and primary tumour samples and normal tissues (Figure 2).
Discussion
In the last decade, several attempts have been made to find new molecules that play an important role in colon cancer tumorigenesis. Since an expression prototype of glypican-6 is presently lacking and has yet to be defined in colon cancer, we aimed to examine, for the first time, the expression of glypican-6 in colon cancers, including benign and malignant and also healthy colon tissues. Our study indicated that glypican-6 expression was found in normal healthy tissues, as well as in cancer tissues taken from colon cancer patients, with the levels in cancer tissues being significantly elevated. Importantly, glypican-6 expression was determined to be weak or absent in normal healthy tissues. These observations are similar to earlier studies demonstrating high glypican-6 levels in gastric, ovarian, nasopharyngeal and melanoma cancers compared with normal healthy tissues.3-6
There are limited studies linked to the expression of glypican-6 with different demographic and clinicopathologic features of cancer patients.3,4 Our study is the first to find significant associations with different demographic and clinicopathologic features of cancer patients. Conversely, no significant associations have been found between glypican-6 expression and demographic and pathological parameters of gastric cancer patients.3 In our study, a higher frequency of glypican-6 expression was found in patients aged over 50 years than in patients aged below 50 years. Moreover, patients with high-grade and advanced stages of the disease displayed stronger glypican-6 expression than those with low-grade and early stages of the disease. Additionally, tumours with a high glypican-6 expression exhibited a greater depth of invasion (T3 and T4) than those with low levels of glypican-6 expression (T1 and T2). Another interesting observation was that lymph node metastases were identified more frequently in colon cancer patients with elevated glypican-6 expression. The same tendency was found for distant metastases. These interesting results may reflect the implication of glypican-6 in the molecular mechanisms of different cancers.
The idea of targeted therapy development lies on a specific molecular target that has differential expression between tumour tissues and normal tissues. Here, a strong differential in glypican-6 expression was observed between tumour tissues and normal tissues, which is a novel finding. The highest glypican-6 expression was particularly found in tumour tissues compared with almost negative expression in normal tissues. Additionally, a higher rate of glypican-6 expression was found in metastatic tumours than in primary tumours. Benign tumours showed the least glypican-6 expression compared with primary and metastatic tumours. These observations are consistent with other studies reporting this differential in glypican-6 expression between tumour tissues and normal tissues. For instance, there was a higher incidence of glypican-6 in the melanoma cancer cell line than in normal melanocytes. Moreover, metastatic melanoma cancer cell lines demonstrated more elevated glypican-6 expression than primary melanoma cell lines.5,8 Importantly, glypican-6 expression was able to differentiate regional subcutaneous metastases from primary melanoma tumours and normal skin tissues, suggesting its use as a clinical biomarker for metastatic melanoma.5 Research on this differential in glypican-6 expression between different types of tumours and normal tissues remains elusive.
Several studies investigated the role of glypican-6 expression in the carcinogenesis process. For instance, glypican-6 was highly expressed in breast cancer, where its expression was transcriptionally regulated by the nuclear factor of activated T cells (NFAT). This, in turn, enhanced breast cancer cell invasion and migration by altering the non-canonical Wnt5a signaling.9 Additionally, glypican-6 activated Hedgehog (Hh) signalling through the interaction of Hh and protein phosphatase 2C homolog 1 (Ptc1) via its core protein and chains of glycosaminoglycan.7,10 This activation promoted the invasion, proliferation and spread of gastric cancer cells.7 Similar effects were observed with nasopharyngeal cancer cells.6 Importantly, all these glypican-6-mediated effects decreased upon glypican-6 silencing.7 More recently, glypican-6 was required for R-spondin-promoted Wnt signalling due to low levels of Wnt.11 On this basis, it seems that glypican-6 is involved in molecular mechanisms of cancer progression and metastasis, and further investigations of this, particularly in colon cancer, are needed.
Conclusion
As the number of studies assessing glypican-6 expression in cancer is limited, this is the first study to characterise glypican-6 expression in different colon tumour types. Glypican-6 was strongly expressed in benign, primary and metastatic colon tumours. Normal healthy tissues exhibited weak to no expression of glypican-6. Importantly, there was a strong differential in glypican-6 expression between tumour and normal tissues. Moreover, glypican-6 expression was found at high frequency in colon tumours with lymph node metastasis, high depth of invasion, distant metastasis, high histological grades and late stages of the disease. These findings provide a strong basis for the potential use of glypican-6 as a tissue biomarker or as a novel therapeutic target.
Supplemental Material
Supplemental material, sj-docx-1-onc-10.1177_11795549211036419 for Screening of Glypican-6 Expression in Benign, Primary and Metastatic Colon Cancers by Yousef M Al-Saraireh, Fatemah OFO Alshammari, Ahmed MM Youssef, Sameeh Al-Sarayreh, Yahya M Al-Sarayra, Emad Aborajooh, Jehad Al-Shuneigat and Hamzeh M Alrawashdeh in Clinical Medicine Insights: Oncology
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Yousef M Al-Saraireh  https://orcid.org/0000-0002-8120-9118
https://orcid.org/0000-0002-8120-9118
Ahmed MM Youssef  https://orcid.org/0000-0002-3299-6047
https://orcid.org/0000-0002-3299-6047
Emad Aborajooh  https://orcid.org/0000-0003-1090-1083
https://orcid.org/0000-0003-1090-1083
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Harada S, Morlote D.Molecular pathology of colorectal cancer. Adv Anat Pathol. 2020;27:20-26. [DOI] [PubMed] [Google Scholar]
- 2.Kheirelseid EA, Miller N, Kerin MJ.Molecular biology of colorectal cancer: review of the literature. Am J Mol Biol. 2013;3:72-80. [Google Scholar]
- 3.Dinccelik-Aslan M, Gumus-Akay G, Elhan AH, Unal E, Tukun A.Diagnostic and prognostic significance of glypican 5 and glypican 6 gene expression levels in gastric adenocarcinoma. Mol Clin Oncol. 2015;3:584-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karapetsas A, Giannakakis A, Dangaj D, et al. Overexpression of GPC6 and TMEM132D in early stage ovarian cancer correlates with CD8+ T-lymphocyte infiltration and increased patient survival. Biomed Res Int. 2015;2015:712438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Li M, Shats I, et al. Glypican 6 is a putative biomarker for metastatic progression of cutaneous melanoma. PLoS One. 2019;14:e0218067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan C, Tu C, Qi P, et al. GPC6 promotes cell proliferation, migration, and invasion in nasopharyngeal carcinoma. J Cancer. 2019;10:3926-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng C, Yan R, Yang G, Xiang S, Zhao F.Hedgehog signaling activation required for glypican-6-mediated regulation of invasion, migration, and epithelial-mesenchymal transition of gastric cancer cells. Biosci Rep. 2020;40:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loftus SK, Baxter LL, Cronin JC, Fufa TD, Pavan WJ.Hypoxia-induced HIF1alpha targets in melanocytes reveal a molecular profile associated with poor melanoma prognosis. Pigment Cell Melanoma Res. 2017;30:339-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yiu GK, Kaunisto A, Chin YR, Toker A.NFAT promotes carcinoma invasive migration through glypican-6. Biochem J. 2011;440:157-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capurro M, Izumikawa T, Suarez P, et al. Glypican-6 promotes the growth of developing long bones by stimulating Hedgehog signaling. J Cell Biol. 2017;216:2911-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lebensohn AM, Dubey R, Neitzel LR, et al. Comparative genetic screens in human cells reveal new regulatory mechanisms in WNT signaling. Elife. 2016;5:1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-onc-10.1177_11795549211036419 for Screening of Glypican-6 Expression in Benign, Primary and Metastatic Colon Cancers by Yousef M Al-Saraireh, Fatemah OFO Alshammari, Ahmed MM Youssef, Sameeh Al-Sarayreh, Yahya M Al-Sarayra, Emad Aborajooh, Jehad Al-Shuneigat and Hamzeh M Alrawashdeh in Clinical Medicine Insights: Oncology