Abstract
Background:
Low-cycle fatigue damage accumulating to the point of structural failure has been recently reported at the origin of the human anterior cruciate ligament under strenuous repetitive loading. If this can occur in a ligament, low-cycle fatigue damage may also occur in the connective tissue of muscle-tendon units. To this end, we reviewed what is known about how, when, and where injuries of muscle-tendon units occur throughout the body.
Purpose:
To systematically review injuries in the muscle-tendon-bone complex; assess the site of injury (muscle belly, musculotendinous junction [MTJ], tendon/aponeurosis, tendon/aponeurosis–bone junction, and tendon/aponeurosis avulsion), incidence, muscles and tendons involved, mechanism of injury, and main symptoms; and consider the hypothesis that injury may often be consistent with the accumulation of multiscale material fatigue damage during repetitive submaximal loading regimens.
Methods:
PubMed, Web of Science, Scopus, and ProQuest were searched on July 24, 2019. Quality assessment was undertaken using ARRIVE, STROBE, and CARE (Animal Research: Reporting In Vivo Experiments, Strengthening the Reporting of Observational Studies in Epidemiology, and the Case Report Statement and Checklist, respectively).
Results:
Overall, 131 studies met the inclusion criteria, including 799 specimens and 2,823 patients who sustained 3,246 injuries. Laboratory studies showed a preponderance of failures at the MTJ, a viscoelastic behavior of muscle-tendon units, and damage accumulation at the MTJ with repetitive loading. Observational studies showed that 35% of injuries occurred in the tendon midsubstance; 28%, at the MTJ; 18%, at the tendon-bone junction; 13%, within the muscle belly and that 6% were tendon avulsions including a bone fragment. The biceps femoris was the most injured muscle (25%), followed by the supraspinatus (12%) and the Achilles tendon (9%). The most common symptoms were hematoma and/or swelling, tenderness, edema and muscle/tendon retraction. The onset of injury was consistent with tissue fatigue at all injury sites except for tendon avulsions, where 63% of the injuries were caused by an evident trauma.
Conclusion:
Excluding traumatic tendon avulsions, most injuries were consistent with the hypothesis that material fatigue damage accumulated during repetitive submaximal loading regimens. If supported by data from better imaging modalities, this has implications for improving injury detection, prevention, and training regimens.
Keywords: muscle injuries, fatigue damage, muscle-tendon-bone injuries, systematic review
The archetypical construction of muscle-tendon-bone units includes a muscle that has an origin and an insertion; a tendon or an aponeurosis that interdigitates with muscle via a musculotendinous junction (MTJ); and an enthesis, where the tendon/aponeurosis inserts into the bone.140,153 Injuries to muscle-tendon/aponeurosis-bone units are common115,138 and have 2 anatomic sites where failure is most likely to occur. These include junctions between tissues with substantially different mechanical properties, such as the tendon/aponeurosis-bone junction and the MTJ.140 Engineers recognize junctions between dissimilar materials as being locations where mechanical stress concentrates, meaning that these junctions have to sustain higher stresses even when the muscle-tendon/aponeurosis-bone unit is uniformly loaded.153 When mechanical stress concentrates, it can reach values that lead to failure on a nanoscale. When the loading cycle is repeated, the damage can spread to the microscale and, under certain conditions, result in partial tears on the ultrastructural scale or even in a complete rupture on the macroscopic scale. Such a failure has been reported in muscle near the MTJ153 as well as in the tendon/aponeurosis midsubstance.8
Chen et al recently found molecular, cellular, and ultrastructural evidence of low-cycle fatigue damage accumulation in type I collagen at the origin of the anterior cruciate ligament at its femoral enthesis after repeated submaximal loading.23 This multiscale finding is significant since this entheseal location is also where the preponderance of anterior cruciate ligament ruptures occurs clinically166 as well as experimentally under certain forms of large repeated knee loading.83
Any structure can fail in tension under a single supramaximal loading cycle that exceeds its so-called ultimate tensile strength. However, a characteristic of low-cycle fatigue failure is that the structure can fail under 2 or more tensile loading cycles, each less than the ultimate tensile strength that by itself would not cause failure. In other words, multiscale damage can accumulate in substructures to weaken the structure to the point that it fails upon the next submaximal loading cycle, often without warning. This is the engineering concept of fatigue, also termed material fatigue, which provides an underlying mechanism for describing how damage can accumulate and be driven to propagate across length scales, from the molecular to the ultrastructural, under repetitive submaximal loading exceeding a certain magnitude. This is the mechanistic hypothesis that we consider in this article as underlying many overuse injuries.
The purpose of this work was to systematically review injuries in the muscle-tendon-bone complex to assess the site of injury (muscle belly, MTJ, tendon/aponeurosis, tendon/aponeurosis–bone junction, and tendon/aponeurosis avulsion), incidence, muscles and tendons involved, mechanism of injury, and main symptoms. Hopefully, this work will provide the necessary framework to evaluate our hypothesis that injury may often be consistent with the accumulation of multiscale material fatigue damage during repetitive submaximal loading regimens.
For simplicity, we generalize the term “tendon/aponeurosis” to “tendon” throughout the Methods and Results sections.
Methods
Search Strategy
A systematic literature search was performed following the PRISMA guidelines98 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) on July 24, 2019. The PubMed, Web of Science, Scopus, and ProQuest databases were searched. Only peer-reviewed studies published in full English text between January 1960 and May 2019 were included. The search strings that we employed are listed in Appendix A.
Data Extraction
The results from the initial search (citations including title and abstract) were exported to Mendeley Desktop (Version 1.19.4; Elsevier). After removing duplicates, M.V.P. screened all titles and abstracts and excluded records if the title and/or abstract was irrelevant. A full article review was performed for the final inclusion. The references of eligible articles were then screened for relevant studies. Review articles were excluded.
In our definition of injury, we included partial or complete tears, whether of the tendon, the MTJ, the tendon-bone junction, or the muscle belly; tendinopathy, whether in the tendon-bone junction or tendon midsubstance; and tendon avulsions (defined as a complete detachment of the tendon from the bone while containing a small fragment of bone) and MTJ strains if verified using magnetic resonance imaging, computed tomography scans, or surgery. To clarify the term, if a patient sustained bilateral tendon avulsion (or a muscle belly and a tendon tear), the patient was considered to have sustained 2 injuries.
The injury site locations within the muscle-tendon-bone unit were labeled as follows: muscle (M) to refer to midsubstance muscle belly injury, musculotendinous (MTJ) to refer to injuries within the musculotendinous (or myotendinous junction), tendon (T) to refer to intratendinous injuries (within the tendon midsubstance), tendon-bone (TB) to refer to injuries within the tendon-bone interface, and avulsion (AV) to refer to a complete detachment of the tendon that includes a bony fragment. Although tendon-bone injuries and avulsions can occur at the same interface, the failure mechanism is distinct because avulsion failures involve failure within the bone.
The classification of the muscle/tendons was mostly performed using groups of muscle-tendon units—for example, injuries within the proximal or distal tendon of the biceps femoris are classified as a biceps femoris tendon injury. Some exceptions were made. For instance, injuries in the Achilles tendon are so prominent that we decided to separate them from the proximal injuries of the gastrocnemius unit, so they have their own category. We based our classification according to Netter.100
To evaluate the possibility of injuries being caused by submaximal repetitive loading (material fatigue), the onset of injury was divided into 2 categories: “evident trauma” and “possible contribution of material fatigue.” Evident trauma was chosen in cases of falls, work accidents (eg, crushed shoulders, hands caught in machines), car accidents, recreational sports activities (first-time or casual players; eg, skiing and bowling), and professional sports (if the mechanism of injury was a twisting motion of the knee, a tackle in American football, or a forceful hit). Possible contribution of material fatigue was chosen for high school, collegiate, or professional well-trained athletes; avid weightlifters; and people who were actively or previously engaged in sports activities or the military. As such, this category included injuries sustained by those active in sports, whatever their age, where no evident trauma was reported. Particularly in older patients, other factors might be at play, such as degenerative changes attributed to aging. However, in older adults active in sports, age-related changes and material fatigue can be present together. Thus, although we cannot state that the injuries in this category are exclusively caused by material fatigue, we argue that it can play a role.
Baseline data extraction for laboratory studies included number of specimens, type of model, tested muscle, site of injury, and major relevant findings. For observational studies, data included number of participants, country where the study was performed, age of the patients, relevant medical conditions, methods of diagnosis, muscle or tendon injured, site of injury, onset of injury, and main symptoms.
Quality Assessment
To access quality and risk of bias, 3 checklists were followed. ARRIVE (Animal Research: Reporting In Vivo Experiments)77 was used for the laboratory studies. It consists of a 20-item checklist that describes the minimum information that all scientific publications reporting research with animals should include. The observational studies were case reports and cohort studies. The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology)152 checklist was chosen to evaluate the cohort studies. It consists of 22 items that highlight the essential information to include in observational studies. Eighteen items are common to cohort studies, case-control studies, and cross-sectional studies, and 4 are specific to each of the 3 study designs. CARE (Case Report Statement and Checklist)46 was followed for case reports. It consists of a 13-item checklist that provides a framework to satisfy the need for completeness and transparency for published case reports.
With the ARRIVE, STROBE, and CARE checklists, a number of points was attributed to each item, depending on how many topics the item covered. For instance, if the item regarding the abstract recommended that the study provide a summary of the background, research objectives, methods, results, and conclusions, 5 points were attributed to this item. Each study was reviewed, and the items were scored from 0 to the maximum number of points attributed. The points were added for each study and recorded as a percentage. Nonapplicable items were deducted from the maximum possible score in that study. Study quality was assessed via percentage: ≥80%, very good; 50%-80%, good; 30%-50%, fair; and ≤30%, poor. A more extensive description and a table with the score of each study are presented in Appendix B.
Statistical Analysis
Statistical analysis was performed using MATLAB (Version R2018a; MathWorks) for injury site and age comparison. One-way analysis of variance was used, and P = .001 was the threshold for significance.
Quantitative values are presented as the mean and standard error of the mean unless stated otherwise.
Results
Search Results
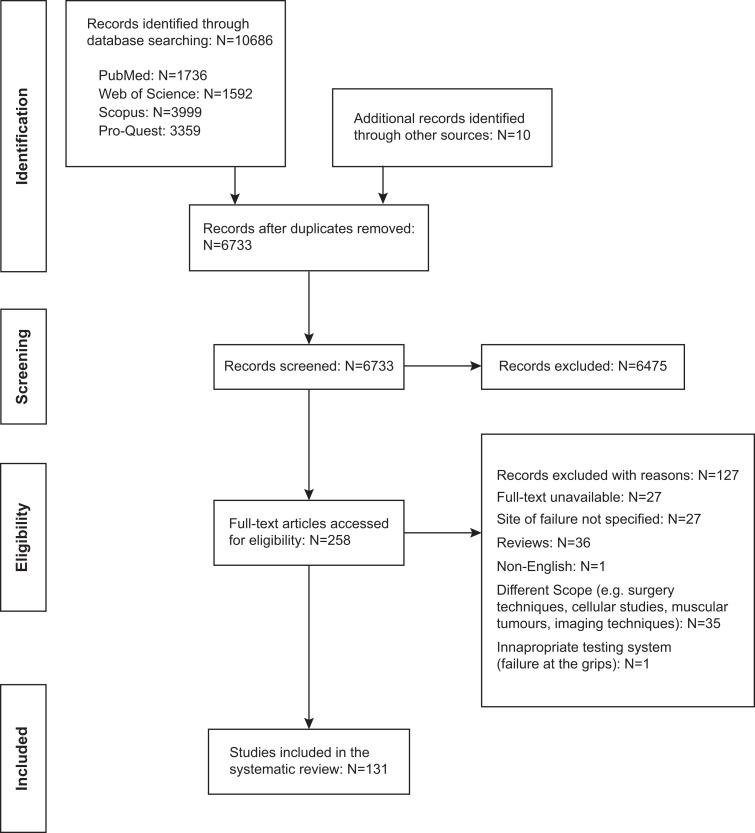
The combined search terms yielded 10,686 results: 1,736 from PubMed, 1,592 from Web of Science, 3,999 from Scopus, and 3,359 from ProQuest. Ten additional records were added from other sources. A primary review of titles and abstracts resulted in 258 documents. Of these, 131 studies met the inclusion criteria (Figure 1).
Figure 1.
PRISMA flowchart detailing study identification and record-screening process.98 PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
In total, 131 studies were included in the final analysis: 11 laboratory studies∥ and 120 observational studies,¶ of which there were 50 cohort studies and 70 case reports or case series. Of the laboratory studies, 10 were rated as good quality (50%-80%), and 1 was rated as fair (30%-50%). Of the observational cohort studies, 9 were rated as very good quality (80%-100%); 39, good; and 2, fair. Of the case reports or case series, 4 were rated as very good quality; 60, good; and 6, fair. None of the studies was rated as poor quality (≤30%). Details can be found in Appendix B.
Of the laboratory studies (n = 11), 8 used New Zealand White rabbits as a model,# whereas each of the remaining 3 studies used mice,79 mongrel dogs,54 and cadaveric human specimens.121 A total of 799 specimens were tested to evaluate muscle-tendon-bone units of different muscle complexes. The musculotendinous units included the extensor digitorum longus, the tibialis anterior, the rectus femoris, the gastrocnemius, the soleus, and the supraspinatus tendon and muscle. Histologic features were noted in 5 articles.54,59,104,121,143 The laboratory studies are summarized in Appendix C.
In the observational studies (n = 120), the population was injury specific in 107 studies (analyzed patients with specific injuries or pain in specific sites) and injury/population specific in 13 studies (analyzed athletes with specific injuries). A total of 2,823 patients with injuries in muscle-tendon-bone units were observed: 2,047 from the injury-specific studies and 776 from injury/population–specific studies.
The injured muscle and tendon units are listed in Table 1, and the number of studies per country is shown in Table 2. From the studies with age data,** the patients were 41.4 ± 13.5 years old (mean ± standard deviation). Observational studies are summarized in Appendix D.
TABLE 1.
Injured Muscles and Tendons Reported by the Included Observational Studies and the Abbreviations Used
| ACT: Achilles tendon | PL: peroneus longus |
| ADL: adductor longus | PM and PMT: pectoralis major muscle and tendon |
| BB: biceps brachii | POP: popliteus tendon |
| BCH: brachialis | PT: patellar tendon |
| BF: bicep femoris | QF: quadratus femoris |
| DLT: deltoid | QT: quadriceps tendon |
| ED: extensor digitorum | RF: rectus femoris |
| EDM: extensor digiti minimi | SC and SCT: subscapularis muscle and tendon |
| EHL: extensor hallucis longus | SM: semimembranosus |
| EIP: extensor indicis proprius | SP and SPT: supraspinatus muscle and tendon |
| EPL: extensor pollicis longus | ST: semitendinosus |
| FPL: flexor pollicis longus | TFL: tensor fascia lata |
| FPT: flexor profundus tendon | TMIN: teres minor |
| GMEDT: gluteus medius tendon | TMJ: teres major |
| GMINT: gluteus minimus tendon | TR and TT: triceps muscle and tendon |
| GRC: gracilis | TS: triceps surae |
| GST: gastrocnemius | TT: tibial tendon |
| IP and IPT: infraspinatus muscle and tendon | VI: vastus intermedius tendon |
| LDT: latissimus dorsi tendon | VL: vastus lateralis |
| PB and PBT: peroneus brevis muscle and tendon | VM: vastus medialis |
TABLE 2.
Distribution of the Observational Studies by Country
| Country | No. of Studies |
|---|---|
| United States | 55 |
| Japan | 10 |
| United Kingdom | 10 |
| Australia | 7 |
| France | 7 |
| Finland | 5 |
| Italy | 5 |
| Switzerland | 4 |
| India | 3 |
| Netherlands | 3 |
| Turkey | 3 |
| Brazil | 1 |
| Canada | 1 |
| Croatia | 1 |
| Denmark | 1 |
| Serbia | 1 |
| Taiwan | 1 |
Laboratory Studies Employing Mechanical Failure Tests
Ten studies used animal models, 7 in vivo47,48,88,104,139,143,149 and 3 in vitro,54,59,79 and 1 used cadaveric human specimens.121 Eight studies used an Instron Testing Instrument (Instron Corp),†† and 3 used an MTS systems instrument (MTS Bionix 858 Test System; MTS Corp).54,139,149
Different muscle groups were tested at different strain rates, but when pulled to failure, the site of injury was at the MTJ in 8 studies (73%),‡‡ independent of the strain rate. This behavior was verified for muscles with different architectures (extensor digitorum longus, tibialis anterior, rectus femoris, gastrocnemius, the 4 peroneus muscles, and the supraspinatus), whether the muscles were tested passively or actively using electrical stimulation. Failure was seen at the distal MTJ in all specimens in 6 studies48,59,104,139,143,149 and in 95% of the cases in 1 study.47 One article indicated that most failures occurred at the proximal MTJ,79 although the number of specimens was not specified. Failure was confirmed when histologic assessment was performed54,59,104,143 by areas of fiber rupture, hemorrhage, edema, and acute inflammatory cells being found at the MTJ. One study121 (9%) in cadaveric human specimens (mean age, 62 years; range, 39-83 years) focused on the supraspinatus tendon, without including the muscle and the MTJ. In that study, failure was seen at the tendon-bone interface in 65% of the cases and at the tendon midsubstance in 35%. Histology showed thinning of tendon fibers and granulation of the tissue more frequently in the insertion group than in the midsubstance group. One article (9%) did not state the specific injury site.88 Viscoelastic effects were also demonstrated,139 with a larger ultimate tensile force (peak force at failure) being found at higher strain rates. Material fatigue (damage accumulation that can lead to failure attributed to a submaximal cyclic load) was analyzed in 4 studies.88,104,143,149 Based on the ultimate tensile properties of control specimens (whether tested via controlled force or displacement), nondisruptive injuries were mechanically caused at a percentage of the failure force or displacement. Nondisruptive injuries at 20% ultimate tensile force did not influence the failure parameters, and the histology showed no differences as compared with the controls. At 30% ultimate tensile force, histology showed areas of fiber rupture and hemorrhage near the MTJ, although the failure parameters were not affected. After a nondisruptive injury at a stretch just before failure, the ultimate tensile force was decreased by 37%; the ultimate tensile displacement, by 21%. Histology then showed incomplete disruption at the MTJ.
Observational Studies
From the observational studies, 1 did not specify the injured muscles, referring only to the lower extremities43; 2 did not cite the age of the patients41,43; and 3 reported just the age range.32,111,117 Fifteen did not specify the activities at the time of injury, §§ although 2 of those studies85,156 did differentiate between progressive and traumatic onset. In the 2,823 patients, a total of 3,246 injuries were observed. Age was specified in 2,106 injuries; onset of injury, in 1,772. The activity at the time of injury was sports in 66% of the patients when the onset of injury was indicated. Among other events were falls, working accidents, car accidents, and moving heavy objects. Hematoma and/or swelling were described in 49 (41%) of the studies∥∥; retraction of either the muscle or the tendon in 34 (28%)¶¶; tenderness in 33 (28%)##; edema in 32 (27%)a; ecchymosis in 16 (13%)b; a mass of soft tissue in 14 (12%)c; and hemorrhage in 10 (8%).d
Injury Site
Of the 3,246 injuries, 1,130 (35%) were within the tendon, 918 (28%) were in the MTJ, 584 (18%) were at the tendon-bone junction, 434 (13%) were within the muscle belly, and 180 (6%) were tendon avulsions (Figure 2).
Figure 2.
Injury site distribution among the patient cohort (n = 3,246). AV, tendon avulsion; M, muscle belly; MTJ, musculotendinous junction; T, tendon; TB, tendon-bone junction.
Patient age was reported for 2,106 injuries. The mean age was 28.5 ± 2.1 years for muscle belly injuries, 32.5 ± 0.8 years for tendon avulsions, 33.7 ± 0.5 years for MTJ injuries, 47.4 ± 0.4 years for tendon injuries, and 51.4 ± 0.5 years for tendon-bone junction injuries. The age of the patients by injury site is displayed in a box-and-whisker plot in Figure 3. With the exception of age comparisons for MTJ versus avulsions, MTJ versus muscle belly injuries, and avulsions versus muscle belly injuries, all the mean ages were significantly different between injury sites (P < .001, 1-way analysis of variance).
Figure 3.
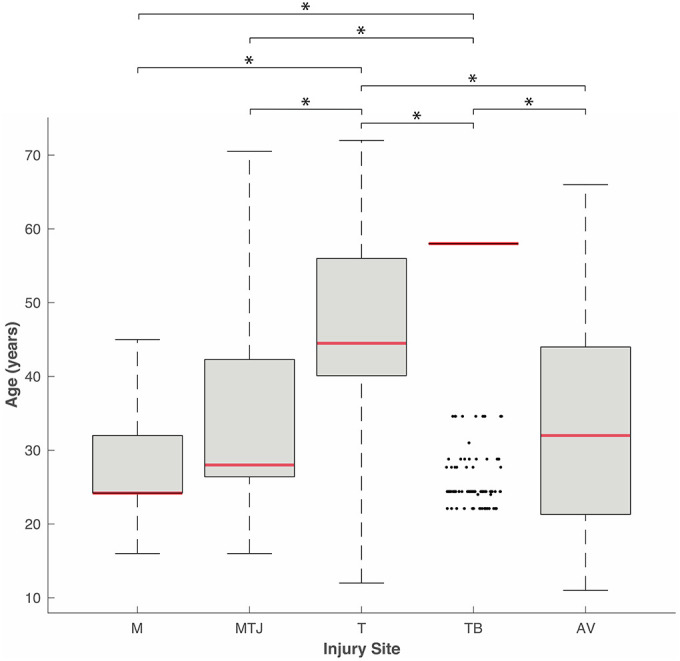
Box-and-whisker plot (median, 25th percentile; range, 75th percentile) of patient age by injury site (n = 2,106). *P < .001. One-way analysis of variance. Dots appearing outside the whisker are outliers (observations numerically distant from the rest of data). AV, tendon avulsion; M, muscle belly; MTJ, musculotendinous junction; T, tendon; TB, tendon-bone junction.
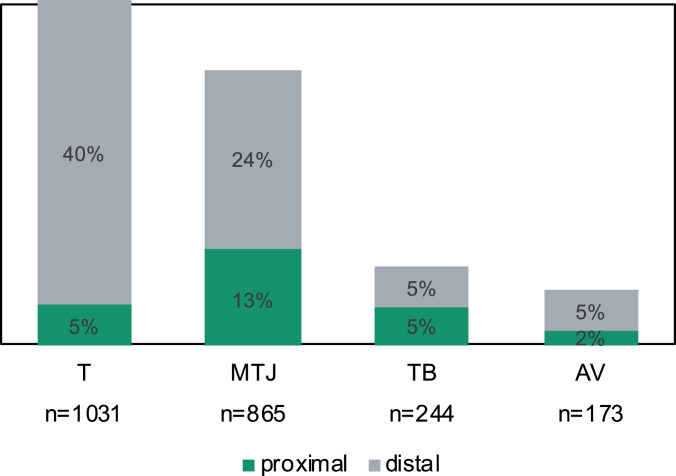
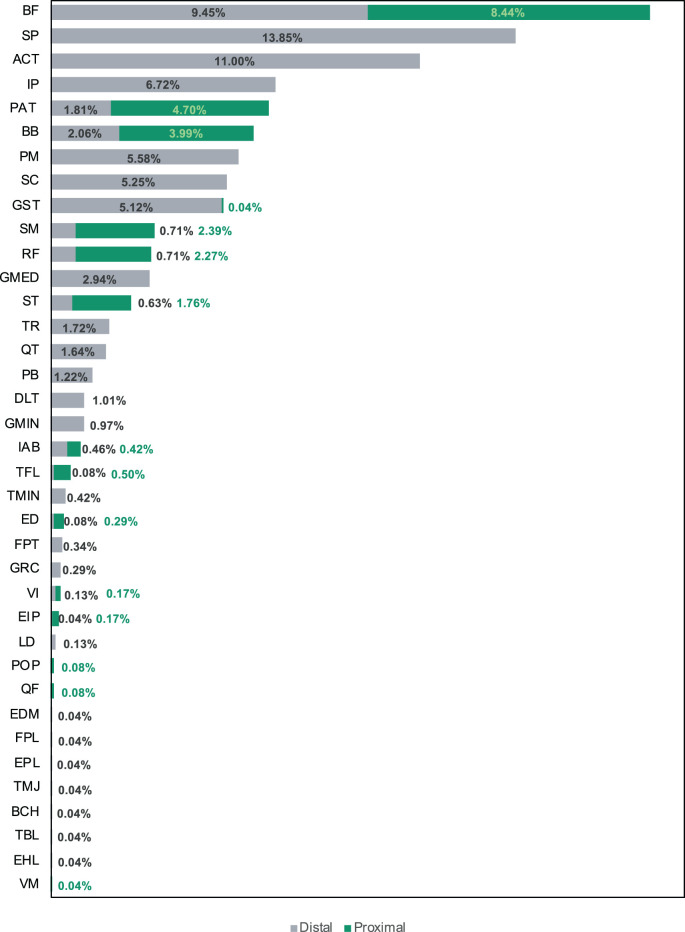
Musculoskeletal injuries (with the exception of muscle belly injuries) occur where the muscle originates from the bone (proximal location) and where the muscle inserts into the bone (distal location). In Figure 4, we differentiate the injury site (tendon avulsion, tendon, tendon-bone, and MTJ) into proximal and distal locations within each “muscle.” Muscle belly injuries were assumed to be in midsubstance, and so they were not included in this analysis, which comprised 2,328 injuries. The primary site of injury was at the distal attachment for all injury sites. Overall, 74% of the injuries occurred at the distal location, and 26% occurred at the proximal location. This was especially marked for tendon injuries, 40% of which occurred at the distal attachment as opposed to 5% at the proximal attachment.
Figure 4.
Distribution of proximal and distal locations within each muscle by injury site (n = 2,328). AV, tendon avulsion; MTJ, musculotendinous junction; T, tendon; TB, tendon-bone junction.
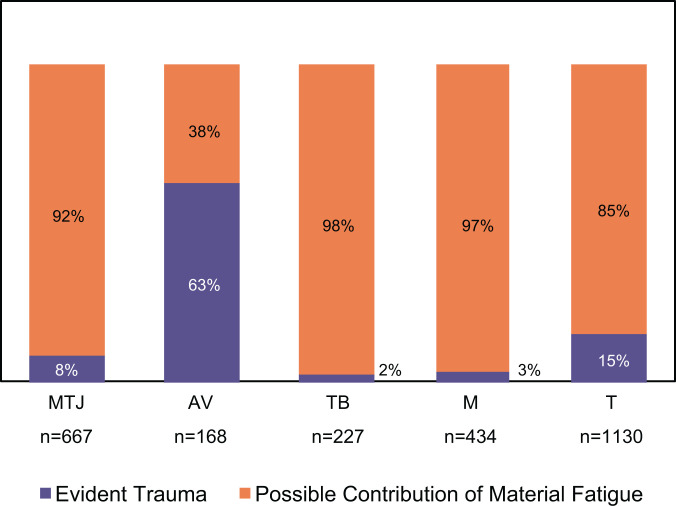
The analysis of onset of injury, divided in the 2 categories (evident trauma and possible contribution of material fatigue), comprised 1,772 injuries. Every one of the injuries categorized into the “possible contribution of material fatigue” group was associated with exercise/sports activity practiced on a regular basis. These sports/exercise injuries (after those with known trauma were excluded) were the ones that we could comfortably include in this category since these activities typically involve repetitive loading. The results by injury site are shown in Figure 5. Material fatigue could be involved in the cause of injury at all sites, with the exception of tendon avulsions, where evident trauma was the primary cause of injury.
Figure 5.
Comparison of onset of injury category by injury sites (n = 1,772). AV, tendon avulsion; M, muscle belly; MTJ, musculotendinous junction; T, tendon; TB, tendon-bone junction.
Injured Muscle/Tendon Groups
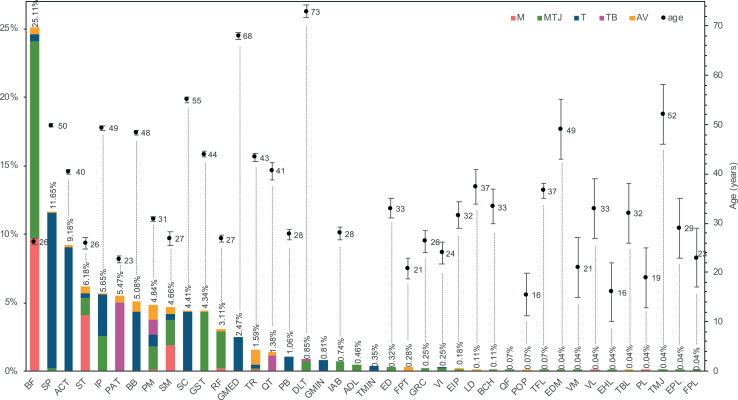
The distribution of injuries by muscle/tendon and injury site is illustrated in Figure 6 and included 2,832 injuries. As described in the Methods section, the injuries in this category were primarily grouped by muscle-tendon units, with tendon injuries grouped as proximal and distal injuries. A few exceptions were made for certain tendons, which were assigned their own category (eg, Achilles tendon).
Figure 6.
Injury distribution by muscle/tendon and injury site (n = 2,832) and mean ± SEM age by muscle/tendon (n = 2,106). Patient age was not reported for ADL, TMIN, and QF injuries. ACT, Achilles tendon; ADL, adductor longus; AV, tendon avulsion; BB, biceps brachii; BCH, brachialis; BF, biceps femoris; DLT, deltoid; ED, extensor digitorum; EDM, extensor digiti minimi; EHL, extensor hallucis longus; EIP, extensor indicis proprius; EPL, extensor pollicis longus; FPL, flexor pollicis longus; FPT, flexor profundus tendon; GMED, gluteus medius; GMIN, gluteus minimus; GRC, gracilis; GST, gastrocnemius; IAB, internal abdominal; IP, infraspinatus; LD, latissimus dorsi; M, muscle belly; MTJ, musculotendinous junction; PAT, patellar tendon; PB, peroneus brevis; PL, peroneus longus; PM, pectoralis major; POP, popliteus; QF, quadratus femoris; QT, quadriceps tendon; RF, rectus femoris; SC, subscapularis; SM, semimembranosus; SP, supraspinatus; ST, semitendinosus; T, tendon; TB, tendon-bone junction; TBL, tibialis posterior; TFL, tensor fasciae latae; TMIN, teres minor; TMJ, teres major; TR, triceps brachii; VI, vastus intermedius; VL, vastus lateralis; VM, vastus medialis.
The biceps femoris was the most commonly injured musculotendinous unit, representing 25.1% of the injuries. Of those, 14.4% were in the MTJ, and 9.7% were in the muscle belly. The highest incidence of MTJ injuries was in the biceps femoris (14.4%); that of tendon midsubstance injuries was in the supraspinatus (11.4%); that of tendon-bone junction injuries was in the patellar tendon (5.0%); that of muscle belly was once again in the biceps femoris (9.7%); and complete tendon avulsions occurred more frequently in the pectoralis major and triceps brachii (1.1%). There were 3 muscle groups more predominantly involved: the hamstring, the quadriceps, and the rotator cuff. From the hamstring group, the biceps femoris was the most frequently injured (25.1%); from the quadriceps group, the rectus femoris was the most injured unit (3.1%); and from the rotator cuff, the supraspinatus was the most prone to injury (11.7%).
The age of the patients at the time of injury was verified for the different muscle/tendon units. This information was not always reported, especially in studies involving injuries in different muscle/tendon groups, since the norm is to provide the mean age of the entire cohort. Nevertheless, of the 3,246 injuries, it was possible to associate age with the muscle-tendon unit in 1,681. Mean and standard error of the mean are presented as well in Figure 6. Injuries in the biceps femoris, which was the most commonly injured unit, occurred frequently in young adults (26.3 ± 0.5 years). The muscle units associated with older patients were the deltoid (73.0 ± 1.2 years) and the gluteus medius (68.0 ± 0.7 years), while the popliteus (15.5 ± 4.3 years) and the extensor hallucis longus (16.0 ± 6.1 years) were seen in the younger patients of the cohort. Between muscle groups, hamstring injuries occurred more frequently in younger adults, as did quadriceps injuries, while rotator cuff injuries were more typical in middle-aged patients.
Information regarding injury of the proximal or distal attachments was reported in 2,328 injuries and is shown in Figure 7. Regarding specific musculotendinous units, the biceps femoris showed a balanced distribution, with 9.7% at the distal location and 8.6% at the proximal location. However, injury of the other hamstring muscles occurred more frequently at proximal locations. The rotator cuff injuries all occurred at distal locations, as did injuries in the pectoralis major muscle, while in the quadriceps group, they occurred more frequently at proximal locations.
Figure 7.
Distribution of injuries by musculotendinous group and proximal and distal locations (n = 2,328). ACT, Achilles tendon; BB, biceps brachii; BCH, brachialis; BF, biceps femoris; DLT, deltoid; ED, extensor digitorum; EDM, extensor digiti minimi; EHL, extensor hallucis longus; EIP, extensor indicis proprius; EPL, extensor pollicis longus; FPL, flexor pollicis longus; FPT, flexor profundus tendon; GMED, gluteus medius; GMIN, gluteus minimus; GRC, gracilis; GST, gastrocnemius; IAB, internal abdominal; IP, infraspinatus; LD, latissimus dorsi; PAT, patellar tendon; PB, peroneus brevis; PM, pectoralis major; POP, popliteus; QF, quadratus femoris; QT, quadriceps tendon; RF, rectus femoris; SC, subscapularis; SM, semimembranosus; SP, supraspinatus; ST, semitendinosus; TBL, tibialis posterior; TFL, tensor fasciae latae; TMIN, teres minor; TMJ, teres major; TR, triceps brachii; VI, vastus intermedius; VM, vastus medialis.
The onset of injury in musculotendinous units was reported in 1,415 injuries analyzed in the categories of evident trauma and possible contribution of material fatigue. The results are shown in Figure 8.
Figure 8.
Comparison of onset of injury category (in percentages) by injured muscle/tendon (n = 1,415). ACT, Achilles tendon; BB, biceps brachii; BCH, brachialis; BF, biceps femoris; ED, extensor digitorum; EDM, extensor digiti minimi; EHL, extensor hallucis longus; EIP, extensor indicis proprius; EPL, extensor pollicis longus; FPL, flexor pollicis longus; FPT, flexor profundus tendon; GRC, gracilis; GST, gastrocnemius; IAB, internal abdominal; IP, infraspinatus; LD, latissimus dorsi; PAT, patellar tendon; PB, peroneus brevis; PL, peroneus longus; PM, pectoralis major; POP, popliteus; QF, quadratus femoris; QT, quadriceps tendon; RF, rectus femoris; SC, subscapularis; SM, semimembranosus; SP, supraspinatus; ST, semitendinosus; TBL, tibialis posterior; TFL, tensor fasciae latae; TMJ, teres major; TR, triceps brachii; VI, vastus intermedius; VL, vastus lateralis; VM, vastus medialis.
Discussion
This review included data from 131 studies, with 799 specimens in laboratory studies and 2,823 patients in observational studies. To the best of our knowledge, this is the largest series of muscle and tendon injuries analyzed since the report of 1,014 cases by Anzel et al4 in 1959. In the ensuing 60 years, participation in sports has burgeoned, with increased female participation, a broader age range, and many more types of sports. Since sports are now the main cause of musculoskeletal injuries, it is natural that the patterns of these type of injuries may have changed with time. As such, we believe that the present review provides an update on current trends in musculoskeletal injuries.
In the laboratory studies, failure and disruption occurred mainly at the MTJ, independent of strain rate and muscle architecture. Moreover, the site of failure was more commonly the distal attachment, independent of whether the soft tissues of the specimen were gripped via a clamp at the proximal or distal end of the muscle. This supports the muscle-tendon junction being the weakest link of the muscle-tendon unit because tensile-testing machine grips can often induce local failures of their own, owing to artifactual stress concentrations.101
The behavior of the muscle-tendon unit has been described as viscoelastic.140 Viscoelasticity has an important functional significance for biological soft tissues because it allows them to stretch more at a constant load (known as creep behavior) as well as to decrease mechanical stresses over time when held at a constant length (known as relaxation behavior). These behaviors help soft tissues withstand larger deformations without injury.2
Four studies analyzed the influence of repetitive loading.88,104,143,149 Small nondisruptive injuries caused by a single tensile loading cycle to 30% of failure force were evidenced by microdamage in the MTJ detectable histologically. For larger nondisruptive injuries, the mechanical properties were more severely affected. This implies that repeated activities are a risk factor for musculotendinous tears. Moreover, it suggests that if the movement requires large enough muscle forces, a small number of loading cycles can suffice to disrupt the tissue in a way that partial or even complete failure occurs without warning in an otherwise normal movement.
The observational studies showed that the most common injury site was the tendon substance (35%), followed by the MTJ. These results are not in accordance with the animal studies, which showed a preponderance for failure at the MTJ. This is interesting since midsubstance tendon injuries are mostly described as rare in normal tendons, especially considering that they can withstand higher loads than those the muscle can generate or the bone can sustain.140,148 However, since tendinopathy was also considered an injury in the tendon midsubstance, this could explain the discrepancy with the laboratory tests, which used tissue from mostly younger/healthier individuals where tissue degeneration and aging effects were not a variable.
Of the reported injuries, 28% were at MTJs, and patients were significantly younger (P < .001) than were those with tendon and tendon-bone junction injuries. Only 8% of these injuries were associated with evident trauma, which means that 92% were instigated by a noncontact event and occurred in well-trained athletes. This information, with the fact that in laboratory studies the MTJ showed signs of disruption at submaximal loads,88,104,143,149 strongly suggests that material fatigue damage can play a role in MTJ injuries.
The patients with tendon-bone junction injuries were significantly older (P < .001) than were patients with injuries at all the other analyzed sites. This could be indicative that aging and the associated degenerative changes involve the tendon-bone junction besides the tendon itself, weakening the tissue. However, tendon-bone junction injuries, particularly insertional tendinopathies or enthesopathies, have been associated with overuse injury,12,13,22,37,125 which, as we hypothesized, may be related to material fatigue. Since just 2% of the cases were caused by evident trauma, the degenerative changes can be also be a result of damage accumulation attributed to overuse.
Tendon avulsion was the least common injury, and although it is usually considered the most typical in children and adolescents,94,140 the mean age of these patients was actually 32.5 ± 0.8 years. As such, it should not be exclusively associated with children and adolescents. When the onset of injury was analyzed, 63% of tendon avulsions occurred because of an evident trauma. Moreover, it was the only injury site at which evident trauma was the primary cause of damage, which could explain the older patients with this type of injury. It seems that in cases of traumatic fall, vehicle accidents, and work-related accidents, avulsion fractures can occur independent of age. It is interesting to observe that although tendon-bone junction injuries and tendon avulsions occur in the same interface—with the distinction that in tendon avulsions there is a complete detachment of the tendon with a bony fragment—they seem to have different mechanisms. Not only was the age of the patients significantly different (P < .001), but tendon-bone injuries had a low rate of “evident trauma” injuries, although they were the primary cause of tendon avulsions.
When patient results were analyzed by muscle/tendon groups, the hamstring and rotator cuff groups stood out, as did the gastrocnemius, the rectus femoris, and the Achilles and patellar tendon groups. It was confirmed, as described in the literature,28,29,117,134,135,150 that the biceps femoris is the most prone to injury in the hamstring group; the supraspinatus, in the rotator cuff; the Achilles tendon, in the triceps surae; and the rectus femoris, in the quadriceps. It was surprising that the triceps brachii musculotendinous unit represented 1.59% of the injuries. Although not a large proportion, it was not the least injured unit.
It is not clear why certain musculotendinous units are more susceptible to injury at a specific site, the underlying causes being likely multifactorial. Many factors are known to affect the stresses at myofascial junctions, including the relative lengths and widths of the muscle’s proximal and distal MTJ114; however, it seems that MTJ and muscle belly injuries might be more common in the lower extremities. A wonderfully illustrative example of a hamstring material fatigue injury is the one recorded in real time at the proximal MTJ of the long head of the biceps femoris in a professional athlete running at 5.4 m/s (or about 80% of an average sprinter speed but less than half Usain Bolt’s maximum sprinting speed) up a 15% incline on a treadmill.60 After appropriately warming up, the athlete had just completed several trials on the treadmill at the same and steeper inclines. The motion capture data showed that the injury occurred during an eccentric contraction of the muscle (when stresses are known to be higher than are isometric values) at the end of a stride, as the muscle decelerated the limb for the next heel contact. Clearly, in this example of constant submaximal speed running, even though the muscle forces were likely substantial, (1) they were still submaximal, (2) they had already been repeated many times within the past hour, and (3) evidently sufficient microdamage had accumulated at the biceps femoris MTJ to weaken it to the point of a partial failure under the submaximal loading. Although the exact anatomic and physiological reason for the failure remains unknown, material fatigue was certainly involved because of the many preceding loading cycles that did not cause injury.
An anatomic feature that makes muscle-tendon units more prone to injury at the tendon is the ratio between the tendon and the muscle cross-sectional area (CSA). For instance, a study reported that in the anterior portion of the supraspinatus, the physiological CSA was measured as 140 ± 44 mm2 and the tendon CSA was measured as 26.4 ± 11.3 mm2.118 This is equivalent to a tendon/muscle CSA ratio of 0.19. In the posterior portion of the supraspinatus, which is much less prone to injury,118 the tendon/muscle ratio was 0.5, meaning that the difference in CSAs was not so accentuated. Since the mechanical stress is defined in terms of force per unit area, a smaller tendon CSA, combined with a large muscle CSA, will increase the stresses in the tendon above those at the muscle or the MTJ for the same force. The higher the mechanical stress, generally the higher the risk for incurring fatigue damage under repetitive submaximal loading.
Aside from the injuries attributed to trauma via a single overload in muscle, tendon, or bone, the results of the present review are consistent with the idea that fatigue damage accumulation may play an important role in musculoskeletal injuries. Although we cannot prove that the observed injuries were caused by material fatigue, we can prove that most of them were not caused by evident trauma. This information, with the fact that sports and exercise activity were the main cause of injury in all of the cases, indicates that material fatigue can be one of the mechanisms of injury, which is supported by the experimental studies. The widely used expression “overuse injury” implicitly acknowledges this damage mechanism but is often associated with specific type of injuries, such as tendinopathies.20,82,95
In the case of the tendon injuries, where “overuse” is linked clinically to the development of tendinopathy, microdamage has been noted in the form of collagen fibril disruption at 6% strain.136 Moreover, fatigue resistance decreases with age in the interfascicular matrix and in the fascicules.145 If the loading is repeated at a high-enough intensity, this damage accumulation can become severe since tendon and tendon-bone injuries naturally have limited healing potential70 and there is almost no tissue renewal after complete formation of the tendon.61 Moreover, the tendon-bone interface is a distinctive and specialized structure that almost never recovers completely to its undamaged state, even after surgical treatment.120
In the case of muscle injuries, several experimental88,104,143,149 and even some observational studies85,156 have presented the idea of repetitive loading causing severe damage in muscle near the MTJ. Since muscles are well known to have an intrinsic capacity to adapt and minor injuries heal spontaneously in healthy adults,112 we believe that the concept of “overuse” is not clinically recognized when associated with muscle or MTJ injuries. However, beyond a certain injury threshold, natural muscle repair can prove insufficient, leading to loss of contractile tissue, fatty degeneration, and fibrotic scar tissue.120 If the injury occurs near the MTJ, the tips of the regenerating muscle cells begin to penetrate into the scar tissue. This penetration is soon halted by the formation of new mini-myotendinous junctions with the scar. Although the scar diminishes with time, it is possible that some connective tissue remains.70
The ability to heal is what distinguishes these tissues from engineering materials, and it can make the difference when dealing with this injury mechanism. Each tissue in the muscle-tendon-bone unit needs its own time to heal, so after the injured tissue type is identified, allowing adequate time to heal is a reasonable prevention strategy. Also, given the cumulative feature of this damage mechanism, it is a possibility that earlier symptoms occur, which happens, for example, with tendinopathies. In the future, this could be used to aid preventive measures by allowing more time to heal. Therefore, it is important to recognize this damage mechanism helps with prevention. We do not exclude other damage mechanisms and factors at play (nutrition, aging, type of training, intensity of training, recovery, etc). Rather, we point out that fatigue damage accumulation is yet another damage mechanism of which to be aware, particularly regarding sports or exercise activity.
Therefore, more research is needed, aided by improved noninvasive imaging methods, to answer the question of how many musculoskeletal structures can accumulate damage owing to too many submaximal loads of a certain intensity being imposed within a given time frame. Although material fatigue failure plays a role in many fields of research,23,153 recognition of this type of mechanism of failure in musculoskeletal injuries should prove beneficial by improving the diagnosis and prevention of such injuries.
Strengths
To our knowledge, this is the only systematic review to focus on all structures composing the muscle-tendon-bone unit and then to assess injury incidence by site and muscular group, age, attachment location, and onset of injury. Four databases were searched using broad search criteria to allow the capture of a wide range of articles. We included laboratory and observational studies, which helped to corroborate trends found in each and together strengthen the conclusions. The large numbers of studies (n = 131), specimens in laboratory studies (n = 799), and injuries reported in the observational studies (n = 3,246) increase the reliability of the results.
Limitations
Limitations include the screening and evaluation phase being performed by a single person (M.V.P.). However, to provide complete transparency of how the quality of each study was evaluated, an extensive description is presented in Appendix B, which includes the score assigned to each article. Last, while there was an inherent risk of selection bias, we tried to counteract this by including broad search terms and searching in 4 databases.
Conclusion
While midsubstance tendon injuries were the most common injury, mainly affecting middle-aged patients, MTJ injuries, the second-most frequent injury, affected younger adults. Tendon-bone junction injuries occurred in significantly older patients when compared with all other sites of injury. Tendon avulsion, the least common injury, occurred in young adults. The biceps femoris was the most frequently injured structure, usually at its proximal MTJ, while the second-most injured structure was the midsubstance of the supraspinatus tendon.
With the exception of tendon avulsions, which were primarily linked with evident trauma, most injuries did not seem to be caused by a single traumatic event. This is consistent with our hypothesis that fatigue damage accumulation may play a role, particularly in well-trained athletes, although we recognize that many other factors can be at play. If material fatigue is indeed involved, this has implications for improving injury detection, prevention, and training regimens.
Supplemental material for this article is available at http://journals.sagepub.com/doi/suppl/10.1177/23259671211020731.
Supplemental Material
Supplemental Material, sj-pdf-1-ojs-10.1177_23259671211020731 for Injuries in Muscle-Tendon-Bone Units: A Systematic Review Considering the Role of Passive Tissue Fatigue by Maria C.P. Vila Pouca, Marco P.L. Parente, Renato M. Natal Jorge and James A. Ashton-Miller in Orthopaedic Journal of Sports Medicine
¶References 1, 3, 5-7, 9-11, 14-19, 21, 24-36, 38-45, 49-53, 55-58, 62-69, 71-76, 78, 80, 81, 84-87, 89-93, 96, 97, 99, 102, 103, 105-111, 113, 116, 117, 119, 122-124, 126-135, 137, 141, 142, 144, 146, 147, 150, 151, 154-165
**References 1, 3, 5 -7, 9 -11, 14 -19, 21, 24 -31, 33 -36, 38 -40, 42, 44, 45, 49 -53, 55 -58, 62 -69, 71 -76, 78, 80, 81, 84 -87, 89 -93, 96, 97, 99, 102, 103, 105 -110, 113, 116, 119, 122 -124, 126 -135, 137, 141, 142, 144, 146, 147, 150, 151, 154 -165
∥∥References 6, 11, 14-16, 18, 19, 21, 25, 31, 32, 34, 39, 44, 51, 52, 56-58, 62, 65, 68, 71, 74, 76, 80, 81, 87, 99, 103, 105, 108, 109, 113, 116, 122-124, 126, 127, 132, 147, 151, 154, 157-159, 163, 164
¶¶References 5, 14, 18, 19, 35, 38, 44, 56-58, 62-64,66, 67, 69, 75, 80, 85, 89, 97, 105, 108, 124, 126, 135, 137, 144, 146, 151, 156, 157, 159, 164
##References 3, 6, 10, 11, 14, 21, 24, 27, 29, 40, 41, 62, 65, 68, 72, 74, 81, 86, 87, 97, 109, 113, 116, 119, 122, 124, 130, 141, 142, 146, 147, 158, 161
Footnotes
Final revision submitted March 11, 2021; accepted March 25, 2021.
The content is solely the responsibility of the authors and does not represent the official views of the US National Institutes of Health.
One or more of the authors declared the following potential conflict of interest or source of funding: Research reported in this publication was supported by US Public Health Service awards P30 AG024824, P30AR069620, and R01 AR054821. The authors also acknowledge support from the Portuguese Foundation for Science and Technology under grant SFRH/BD/136213/2018 and funding provided by the Associated Laboratory for Energy, Transports and Aeronautics (Portugal) under project UIDB/50022/2020. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1.Äärimaa V, Rantanen J, Heikkilä J, Helttula I, Orava S. Rupture of the pectoralis major muscle. Am J Sports Med. 2004;32(5):1256–1262. [DOI] [PubMed] [Google Scholar]
- 2.Abramowitch S, Easley D. Introduction to classical mechanics. In: Hoyte L, Damaser M, eds. Biomechanics of the Female Pelvic Floor. Academic Press; 2016:89–107. [Google Scholar]
- 3.Andersen E. Triceps tendon avulsion. Injury. 1986;17(4):279–280. [DOI] [PubMed] [Google Scholar]
- 4.Anzel SH, Covey KW, Weiner AD, Lipscomb PR. Disruption of muscles and tendons: an analysis of 1,014 cases. Surgery. 1959;45(3):406–414. [PubMed] [Google Scholar]
- 5.Asinger DA, el-Khoury GY. Tensor fascia lata muscle tear: evaluation by MRI. Iowa Orthop J. 1998;18:146–149. [PMC free article] [PubMed] [Google Scholar]
- 6.Aso K, Torisu T. Muscle belly tear of the triceps. Am J Sports Med. 1984;12(6):485–487. [DOI] [PubMed] [Google Scholar]
- 7.Bach BR, Warren RF, Wickiewicz TL. Triceps rupture: a case report and literature review. Am J Sports Med. 1987;15(3):285–289. [DOI] [PubMed] [Google Scholar]
- 8.Balius R, Alomar X, Pedret C, et al. Role of the extracellular matrix in muscle injuries: histoarchitectural considerations for muscle injuries. Orthop J Sports Med. 2018;6(9):2325967118795863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basic-Jukic N, Juric I, Racki S, Kes P. Spontaneous tendon ruptures in patients with end-stage renal disease. Kidney Blood Press Res. 2009;32(1):32–36. [DOI] [PubMed] [Google Scholar]
- 10.Bass CJ, Connell DA. Sonographic findings of tensor fascia lata tendinopathy: another cause of anterior groin pain. Skeletal Radiol. 2002;31(3):143–148. [DOI] [PubMed] [Google Scholar]
- 11.Benazzo F, Marullo M, Pietrobono L. Supraspinatus rupture at the musculotendinous junction in a young woman. J Orthop Traumatol. 2014;15(3):231–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR. The skeletal attachment of tendons—tendon “entheses.” Comp Biochem Physiol A Mol Integr Physiol. 2002;133(4):931–945. [DOI] [PubMed] [Google Scholar]
- 13.Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: attachment sites (“entheses”) in relation to exercise and/or mechanical load. J Anat. 2006;208(4):471–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianchi S, Martinoli C, Waser NP, Bianchi-Zamorani MP, Federici E, Fasel J. Central aponeurosis tears of the rectus femoris: sonographic findings. Skeletal Radiol. 2002;31(10):581–586. [DOI] [PubMed] [Google Scholar]
- 15.Bowers KDJ. Patellar tendon avulsion as a complication of Osgood-Schlatter’s disease. Am J Sports Med. 1981;9(6):356–359. [DOI] [PubMed] [Google Scholar]
- 16.Bunshah JJ, Raghuwanshi S, Sharma D, Pandita A. Triceps tendon rupture: an uncommon orthopaedic condition. BMJ Case Rep. 2015;2015:bcr2014206446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butcher JD, Siekanowicz A, Pettrone F. Pectoralis major rupture: ensuring accurate diagnosis and effective rehabilitation. Phys Sportsmed. 1996;24(3):37. [DOI] [PubMed] [Google Scholar]
- 18.Canovas F, Nicolau F, Bonnel F. Avulsion of the flexor digitorum profundus tendon associated with a chondroma of the distal phalanx. J Hand Surg Br. 1998;23(1):130–131. [DOI] [PubMed] [Google Scholar]
- 19.Carrino JA, Chandnanni VP, Mitchell DB, Choi-Chinn K, DeBerardino TM, Miller MD. Pectoralis major muscle and tendon tears: diagnosis and grading using magnetic resonance imaging. Skeletal Radiol. 2000;29(6):305–313. [DOI] [PubMed] [Google Scholar]
- 20.Casanova R, Chuang A, Goepfert AR, et al. Beckmann and Ling’s Obstetrics and Gynecology. 8th ed. Wolters Kluwer; 2019. [Google Scholar]
- 21.Cetinkaya E, Aydin CG, Akman YE, et al. A rare knee extensor mechanism injury: vastus intermedius tendon rupture. Int J Surg Case Rep. 2015;14:186–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chauvin NA, Ho-Fung V, Jaramillo D, Edgar JC, Weiss PF. Ultrasound of the joints and entheses in healthy children. Pediatr Radiol. 2015;45(9):1344–1354. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Kim J, Shao W, et al. An anterior cruciate ligament failure mechanism. Am J Sports Med. 2019;47(9):2067–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clay NR. A pulled muscle: partial avulsion of the extensor indicis proprius tendon following a hyperflexion injury. Injury. 1988;19(2):127–128. [DOI] [PubMed] [Google Scholar]
- 25.Connell DA, Jhamb A, James T. Side strain: a tear of internal oblique musculature. AJR Am J Roentgenol. 2003;181(6):1511–1517. [DOI] [PubMed] [Google Scholar]
- 26.Connell DA, Potter HG, Sherman MF, Wickiewicz TL. Injuries of the pectoralis major muscle: evaluation with MR imaging. Radiology. 1999;210(3):785–791. [DOI] [PubMed] [Google Scholar]
- 27.Cooper ME, Selesnick FH. Partial rupture of the distal insertion of the patellar tendon: a report of two cases in professional athletes. Am J Sports Med. 2000;28(3):402–406. [DOI] [PubMed] [Google Scholar]
- 28.Crema MD, Guermazi A, Tol JL, Niu J, Hamilton B, Roemer FW. Acute hamstring injury in football players: association between anatomical location and extent of injury—a large single-center MRI report. J Sci Med Sport. 2016;19(4):317–322. [DOI] [PubMed] [Google Scholar]
- 29.Cross TM, Gibbs N, Houang MT, Cameron M. Acute quadriceps muscle strains: magnetic resonance imaging features and prognosis. Am J Sports Med. 2004;32(3):710–719. [DOI] [PubMed] [Google Scholar]
- 30.Depalma MJ, Perkins RH. Patellar tendinosis: acute patellar tendon rupture and jumper’s knee. Phys Sportsmed. 2004;32(5):41–45. [DOI] [PubMed] [Google Scholar]
- 31.De Roos WK, Zeeman RJ. A flexor tendon rupture in the palm of the hand. J Hand Surg Am. 1991;16(4):663–665. [DOI] [PubMed] [Google Scholar]
- 32.De Smet AA, Best TM. MR imaging of the distribution and location of acute hamstring injuries in athletes. AJR Am J Roentgenol. 2000;174(2):393–399. [DOI] [PubMed] [Google Scholar]
- 33.de Waal Malefijt MC, Beeker TW. Avulsion of the triceps tendon in secondary hyper parathyroidism: a case report. Acta Orthop. 1987;58(4):434–435. [DOI] [PubMed] [Google Scholar]
- 34.Dürig M, Schuppisser JP, Gauer EF, Müller W. Spontaneous rupture of the gastrocnemius muscle. Injury. 1977;9(2):143–145. [DOI] [PubMed] [Google Scholar]
- 35.Entwisle T, Ling Y, Splatt A, Brukner P, Connell D. Distal musculotendinous T junction injuries of the biceps femoris: an MRI case review. Orthop J Sports Med. 2017;5(7):2325967117714998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans NA, Bowrey DJ, Newman GR. Ultrastructural analysis of ruptured tendon from anabolic steroid users. Injury. 1998;29(10):769–773. [DOI] [PubMed] [Google Scholar]
- 37.Fan L, Xu B, Liu N, Wang L. Histopathological changes in patellar tendon enthesis of rabbit induced by electrical stimulation intensity. J Orthop Sci. 2020;25(2):344–348. [DOI] [PubMed] [Google Scholar]
- 38.Farley TE, Neumann CH, Steinbach LS, Jahnke AJ, Petersen SS. Full-thickness tears of the rotator cuff of the shoulder: diagnosis with MR imaging. AJR Am J Roentgenol. 1992;158(2):347–351. [DOI] [PubMed] [Google Scholar]
- 39.Farrar EL, III, Lippert FG, III. Avulsion of the triceps tendon. Clin Orthop Relat Res. 1981;161:242–246. [PubMed] [Google Scholar]
- 40.Ferretti A, Ippolito E, Mariani P, Puddu G. Jumper’s knee. Am J Sports Med. 1983;11(2):58–62. [DOI] [PubMed] [Google Scholar]
- 41.Ferretti A, Puddu G, Mariani PP, Neri M. The natural history of jumper’s knee: patellar or quadriceps tendonitis. Int Orthop. 1985;8(4):239–242. [DOI] [PubMed] [Google Scholar]
- 42.Fitzpatrick D, Cagle P, Flatow E. Isolated teres major rupture: a case report with a suggested dedicated imaging protocol and review of the literature. J Radiol Case Rep. 2016;10(4):31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleckenstein JL, Weatherall PT, Parkey RW, Payne JA, Peshock RM. Sports-related muscle injuries: evaluation with MR imaging. Radiology. 1989;172(3):793–798. [DOI] [PubMed] [Google Scholar]
- 44.Floor S, Van Der Veen AH, Devilee RJ. Two patients with a complete proximal rupture of the hamstring. Arch Orthop Trauma Surg. 2010;130(4):523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freccero DM, Berkowitz MJ. The relationship between tears of the peroneus brevis tendon and the distal extent of its muscle belly: an MRI study. Foot Ankle Int. 2006;27(4):236–239. [DOI] [PubMed] [Google Scholar]
- 46.Gagnier JJ, Riley D, Altman DG, Moher D, Sox H, Kienle G. The CARE guidelines: consensus-based clinical case reporting guideline development. Dtsch Arztebl Int. 2013;110(37):603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garrett WEJ, Nikolaou PK, Ribbeck BM, Glisson RR, Seaber AV. The effect of muscle architecture on the biomechanical failure properties of skeletal muscle under passive extension. Am J Sports Med. 1988;16(1):7–12. [DOI] [PubMed] [Google Scholar]
- 48.Garrett WEJ, Safran MR, Seaber AV, Glisson RR, Ribbeck BM. Biomechanical comparison of stimulated and nonstimulated skeletal muscle pulled to failure. Am J Sports Med. 1987;15(5):448–454. [DOI] [PubMed] [Google Scholar]
- 49.Gaskin CM, Anderson MW, Choudhri A, Diduch DR. Focal partial tears of the long head of the biceps brachii tendon at the entrance to the bicipital groove: MR imaging findings, surgical correlation, and clinical significance. Skeletal Radiol. 2009;38(10):959–965. [DOI] [PubMed] [Google Scholar]
- 50.Gidwani S, Bircher MD. Avulsion injuries of the hamstring origin—a series of 12 patients and management algorithm. Ann R Coll Surg Engl. 2007;89(4):394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giuffre BM, Lisle DA. Case report: tear of the distal biceps branchii tendon—a new method of ultrasound evaluation. Australas Radiol. 2005;49(5):404–406. [DOI] [PubMed] [Google Scholar]
- 52.Godoy IRB, Martinez-Salazar EL, Simeone FJ, Bredella MA, Palmer WE, Torriani M. MRI of pectoralis major tears: association between ancillary findings and tear severity. Skeletal Radiol. 2018;47(8):1127–1135. [DOI] [PubMed] [Google Scholar]
- 53.Goodier D, Maffulli N, Good CJ, Good J. Tibial tuberosity avulsion associated with patellar tendon avulsion. Acta Orthop Belg. 1994;60(2):336–338. [PubMed] [Google Scholar]
- 54.Gottsauner-Wolf F, Grabowski JJ, Chao EY, An KN. Effects of freeze/thaw conditioning on the tensile properties and failure mode of bone-muscle-bone units: a biomechanical and histological study in dogs. J Orthop Res. 1995;13(1):90–95. [DOI] [PubMed] [Google Scholar]
- 55.Gruel JB. Isolated avulsion of the popliteus tendon. Arthroscopy. 1990;6(2):94–95. [DOI] [PubMed] [Google Scholar]
- 56.Gyftopoulos S, Rosenberg ZS, Schweitzer ME, Bordalo-Rodrigues M.Normal anatomy and strains of the deep musculotendinous junction of the proximal rectus femoris: MRI features. AJR Am J Roentgenol. 2008;190(3):W182–W186. [DOI] [PubMed] [Google Scholar]
- 57.Hart ES. Acute quadriceps tendon rupture. Orthop Nurs. 2013;32(1):53–54. [DOI] [PubMed] [Google Scholar]
- 58.Hasegawa K, Schofer JM. Rupture of the pectoralis major: a case report and review. J Emerg Med. 2010;38(2):196–200. [DOI] [PubMed] [Google Scholar]
- 59.Hasselman CT, Best TM, Seaber AV, Garrett WEJ. A threshold and continuum of injury during active stretch of rabbit skeletal muscle. Am J Sports Med. 1995;23(1):65–73. [DOI] [PubMed] [Google Scholar]
- 60.Heiderscheit BC, Hoerth DM, Chumanov ES, Swanson SC, Thelen BJ, Thelen DG. Identifying the time of occurrence of a hamstring strain injury during treadmill running: a case study. Clin Biomech (Bristol, Avon). 2005;20(10):1072–1078. [DOI] [PubMed] [Google Scholar]
- 61.Heinemeier KM, Schjerling P, Heinemeier J, Magnusson SP, Kjaer M. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb (14)C. FASEB J. 2013;27(5):2074–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henry JC, Scerpella TA. Acute traumatic tear of the latissimus dorsi tendon from its insertion: a case report. Am J Sports Med. 2000;28(4):577–579. [DOI] [PubMed] [Google Scholar]
- 63.Hertel R, Lambert SM. Supraspinatus rupture at the musculotendinous junction. J Shoulder Elbow Surg. 1998;7(4):432–435. [DOI] [PubMed] [Google Scholar]
- 64.Hughes C, IV, Hasselman CT, Best TM, Martinez S, Garrett WEJ. Incomplete, intrasubstance strain injuries of the rectus femoris muscle. Am J Sports Med. 1995;23(4):500–506. [DOI] [PubMed] [Google Scholar]
- 65.Huri G, Dubin JM, Ozgonen K, Kaya D, Doral MN. A unique rectus femoris injury in an adolescent professional soccer player: a case report. JBJS Case Connect. 2014;4(4):e115. [DOI] [PubMed] [Google Scholar]
- 66.Ilaslan H, Iannotti JP, Recht MP. Deltoid muscle and tendon tears in patients with chronic rotator cuff tears. Skeletal Radiol. 2007;36(6):503–507. [DOI] [PubMed] [Google Scholar]
- 67.Irmola T, Heikkilä JT, Orava S, Sarimo J. Total proximal tendon avulsion of the rectus femoris muscle. Scand J Med Sci Sports. 2007;17(4):378–382. [DOI] [PubMed] [Google Scholar]
- 68.Jaiswal A, Kacchap ND, Tanwar YS, Kumar D, Kumar B. Rupture of the triceps tendon—a case series. Chin J Traumatol. 2016;19(4):235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jansen JA, Cormier S, Patel JV. Acute rectus femoris rupture at the distal musculotendinous junction in a football player: a case report and surgical technique. Curr Orthop Pract. 2012;23(4):390–392. [Google Scholar]
- 70.Järvinen TAH, Järvinen TLN, Kääriäinen M, Kalimo H, Järvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745–764. [DOI] [PubMed] [Google Scholar]
- 71.Kameyama M, Shiraishi T. Traumatic rupture of the extensor digitorum communis and extensor digiti minimi at the musculotendinous junction associated with volar dislocation of the distal radioulnar joint—a case report. Hand Surg. 2000;5(2):165–168. [DOI] [PubMed] [Google Scholar]
- 72.Kankaya Y, Oruç M, Uysal A, Koçer U. Multiple closed flexor tendon avulsions from their insertions by a high-energy explosion. J Hand Surg Am. 2006;31(6):663–664. [DOI] [PubMed] [Google Scholar]
- 73.Karjalainen PT, Soila K, Aronen HJ, et al. MR imaging of overuse injuries of the Achilles tendon. AJR Am J Roentgenol. 2000;175(1):251–260. [DOI] [PubMed] [Google Scholar]
- 74.Kawashima M, Sato M, Torisu T, Himeno R, Iwabuchi A. Rupture of the pectoralis major: report of 2 cases. Clin Orthop Relat Res. 1975;109:115–119. [DOI] [PubMed] [Google Scholar]
- 75.Khiami F, Tavassoli S, De Ridder Baeur L, Catonné Y, Sariali E. Distal partial ruptures of triceps brachii tendon in an athlete. Orthop Traumatol Surg Res. 2012;98(2):242–246. [DOI] [PubMed] [Google Scholar]
- 76.Kibuule LK, Fehringer EV. Distal triceps tendon rupture and repair in an otherwise healthy pediatric patient: a case report and review of the literature. J Shoulder Elbow Surg. 2007;16(3):e1–e3. [DOI] [PubMed] [Google Scholar]
- 77.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim TK, Rauh PB, McFarland EG. Partial tears of the subscapularis tendon found during arthroscopic procedures on the shoulder: a statistical analysis of sixty cases. Am J Sports Med. 2003;31(5):744–750. [DOI] [PubMed] [Google Scholar]
- 79.Law DJ, Caputo A, Tidball JG. Site and mechanics of failure in normal and dystrophin-deficient skeletal muscle. Muscle Nerve. 1995;18(2):216–223. [DOI] [PubMed] [Google Scholar]
- 80.Lempainen L, Sarimo J, Mattila K, Heikkilä J, Orava S. Distal tears of the hamstring muscles: review of the literature and our results of surgical treatment. Br J Sports Med. 2007;41(2):80–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levine JW, Savoie FH, III. Traumatic rupture of the latissimus dorsi. Orthopedics. 2008;31(8):799. [DOI] [PubMed] [Google Scholar]
- 82.Lian Ø, Scott A, Engebretsen L, Bahr R, Duronio V, Khan K. Excessive apoptosis in patellar tendinopathy in athletes. Am J Sports Med. 2007;35(4):605–611. [DOI] [PubMed] [Google Scholar]
- 83.Lipps DB, Wojtys EM, Ashton-Miller JA. Anterior cruciate ligament fatigue failures in knees subjected to repeated simulated pivot landings. Am J Sports Med. 2013;41(5):1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lukac D, Jakovljevic DK, Klasnja A, Drapsin M, Slavic D, Karan V. Ultrasonographic evaluation of the ruptured medial head of gastrocnemius muscle. Revista Brasileira de Medicina do Esporte. 2016;22(5):381–385. [Google Scholar]
- 85.Lunn JV, Castellanos-Rosas J, Tavernier T, Barthelemy R, Walch G. A novel lesion of the infraspinatus characterized by musculotendinous disruption, edema, and late fatty infiltration. J Shoulder Elbow Surg. 2008;17(4):546–553. [DOI] [PubMed] [Google Scholar]
- 86.Maehara H, Sakaguchi Y. Avulsion fracture of the medial head of the gastrocnemius muscle: a case report. J Bone Joint Surg Am. 2004;86(2):373–375. [DOI] [PubMed] [Google Scholar]
- 87.Maffulli N. The clinical diagnosis of subcutaneous tear of the Achilles tendon: a prospective study in 174 patients. Am J Sports Med. 1998;26(2):266–270. [DOI] [PubMed] [Google Scholar]
- 88.Mair SD, Seaber AV, Glisson RR, Garrett WE, Garrett WE, Jr. The role of fatigue in susceptibility to acute muscle strain injury. Am J Sports Med. 1996;24(2):137–143. [DOI] [PubMed] [Google Scholar]
- 89.Makridis KG, Lequesne M, Bard H, Djian P. Clinical and MRI results in 67 patients operated for gluteus medius and minimus tendon tears with a median follow-up of 4.6 years. Orthop Traumatol Surg Res. 2014;100(8):849–853. [DOI] [PubMed] [Google Scholar]
- 90.Mansat P, Frankle MA, Cofield RH. Tears in the subscapularis tendon: descriptive analysis and results of surgical repair. Joint Bone Spine. 2003;70(5):342–347. [DOI] [PubMed] [Google Scholar]
- 91.Mechrefe AP, Walsh EF, DiGiovanni CW. Anterior tibial tendon avulsion with distal tibial fracture entrapment: case report. Foot Ankle Int. 2006;27(8):645–647. [DOI] [PubMed] [Google Scholar]
- 92.Mellado JM, Calmet J, Giné J, Saurí A. Pectoralis major muscle and tendon tears: report of two cases with surgical correlation and postoperative follow-up. Eur J Radiol Extra. 2004;50(3):101–104. [Google Scholar]
- 93.Menz P, Nettle WJ. Closed rupture of the musculotendinous junction of extensor hallucis longus. Injury. 1989;20(6):378–381. [DOI] [PubMed] [Google Scholar]
- 94.Micheli LJ, Fehlandt AFJ. Overuse injuries to tendons and apophyses in children and adolescents. Clin Sports Med. 1992;11(4):713–726. [PubMed] [Google Scholar]
- 95.Millar NL, Hueber AJ, Reilly JH, et al. Inflammation is present in early human tendinopathy. Am J Sports Med. 2010;38(10):2085–2091. [DOI] [PubMed] [Google Scholar]
- 96.Miller WA. Rupture of the musculotendinous juncture of the medial head of the gastrocnemius muscle. Am J Sports Med. 1977;5(5):191–193. [DOI] [PubMed] [Google Scholar]
- 97.Miranda MO, Bureau NJ. Supraspinatus myotendinous junction injuries: MRI findings and prevalence. AJR Am J Roentgenol. 2019;212(1):W1–W9. [DOI] [PubMed] [Google Scholar]
- 98.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Naito K, Homma Y, Morita M, Mogami A, Obayashi O. Triceps tendon avulsion: a case report and discussion about the olecranon ossification nucleus. Eur J Orthop Surg Traumatol. 2013;23(suppl 2):S193–S196. [DOI] [PubMed] [Google Scholar]
- 100.Netter FH. Atlas of Human Anatomy. 6th ed. Saunders/Elsevier; 2014. [Google Scholar]
- 101.Ng BH, Chou SM, Krishna V. The influence of gripping techniques on the tensile properties of tendons. Proc Inst Mech Eng H. 2005;219(5):349–354. [DOI] [PubMed] [Google Scholar]
- 102.Nimityongskul P, Montague WL, Anderson LD. Avulsion fracture of the tibial tuberosity in late adolescence. J Trauma. 1988;28(4):505–509. [DOI] [PubMed] [Google Scholar]
- 103.Nishida Y, Tsukushi S, Yamada Y, Hosono K, Ishiguro N. Brachialis muscle tear mimicking an intramuscular tumor: a report of two cases. J Hand Surg Am. 2007;32(8):1237–1241. [DOI] [PubMed] [Google Scholar]
- 104.Noonan TJ, Best TM, Seaber AV, Garrett WEJ. Identification of a threshold for skeletal muscle injury. Am J Sports Med. 1994;22(2):257–261. [DOI] [PubMed] [Google Scholar]
- 105.Ohashi K, El-Khoury GY, Albright JP, Tearse DS. MRI of complete rupture of the pectoralis major muscle. Skeletal Radiol. 1996;25(7):625–628. [DOI] [PubMed] [Google Scholar]
- 106.Panni AS, Tartarone M, Maffulli N. Patellar tendinopathy in athletes: outcome of nonoperative and operative management. Am J Sports Med. 2000;28(3):392–397. [DOI] [PubMed] [Google Scholar]
- 107.Pedret C, Balius R, Barcelo P, et al. Isolated tears of the gracilis muscle. Am J Sports Med. 2011;39(5):1077–1080. [DOI] [PubMed] [Google Scholar]
- 108.Pina A, Garcia I, Sabater M. Traumatic avulsion of the triceps brachii. J Orthop Trauma. 2002;16(4):273–276. [DOI] [PubMed] [Google Scholar]
- 109.Pitts RT, Garner HW, Ortiguera CJ. Pectoralis major avulsion in a skeletally immature wrestler: a case report. Am J Sports Med. 2010;38(5):1034–1037. [DOI] [PubMed] [Google Scholar]
- 110.Pochini ADC, Ejnisman B, Andreoli CV, et al. Pectoralis major muscle rupture in athletes: a prospective study. Am J Sports Med. 2010;38(1):92–98. [DOI] [PubMed] [Google Scholar]
- 111.Pomeranz SJ, Heidt RSJ. MR imaging in the prognostication of hamstring injury: work in progress. Radiology. 1993;189(3):897–900. [DOI] [PubMed] [Google Scholar]
- 112.Qazi TH, Duda GN, Ort MJ, Perka C, Geissler S, Winkler T. Cell therapy to improve regeneration of skeletal muscle injuries. J Cachexia Sarcopenia Muscle. 2019;10(3):501–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rajasekhar C, Kakarlapudi TK, Bhamra MS. Avulsion of the triceps tendon. Emerg Med J. 2002;19(3):271–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rehorn MR, Blemker SS. The effects of aponeurosis geometry on strain injury susceptibility explored with a 3D muscle model. J Biomech. 2010;43(13):2574–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Renström P, Hach T. Insertional tendinopathy in sports. In: Maffulli N, Renstrom P, Leadbetter WB, eds. Tendon Injuries. Springer-Verlag London; 2005:70–85. [Google Scholar]
- 116.Rochman R, SanGiovanni TP, Uribe JW. Muscle belly tears of the peroneal muscles: a case report. Foot Ankle Int. 2006;27(12):1159–1160. [DOI] [PubMed] [Google Scholar]
- 117.Roe M, Murphy JC, Gissane C, Blake C. Hamstring injuries in elite Gaelic football: an 8-year investigation to identify injury rates, time-loss patterns and players at increased risk. Br J Sports Med. 2018;52(15):982–988. [DOI] [PubMed] [Google Scholar]
- 118.Roh MS, Wang VM, April EW, Pollock RG, Bigliani LU, Flatow EL. Anterior and posterior musculotendinous anatomy of the supraspinatus. J Shoulder Elbow Surg. 2000;9(5):436–440. [DOI] [PubMed] [Google Scholar]
- 119.Rose PS, Frassica FJ. Atraumatic bilateral patellar tendon rupture: a case report and review of the literature. J Bone Joint Surg Am. 2001;83(9):1382–1386. [PubMed] [Google Scholar]
- 120.Rothrauff BB, Tuan RS. Cellular therapy in bone-tendon interface regeneration. Organogenesis. 2014;10(1):13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sano H, Ishii H, Yeadon A, Backman DS, Brunet JA, Uhthoff HK. Degeneration at the insertion weakens the tensile strength of the supraspinatus tendon: a comparative mechanical and histologic study of the bone-tendon complex. J Orthop Res. 1997;15(5):719–726. [DOI] [PubMed] [Google Scholar]
- 122.Schache AG, Koulouris G, Kofoed W, Morris HG, Pandy MG. Rupture of the conjoint tendon at the proximal musculotendinous junction of the biceps femoris long head: a case report. Knee Surg Sports Traumatol Arthrosc. 2008;16(8):797–802. [DOI] [PubMed] [Google Scholar]
- 123.Shah S, Montgomery W, Bastidas M. A unique myotendinous avulsion injury of the Achilles tendon. Curr Orthop Pract. 2009;20(4):464–466. [Google Scholar]
- 124.Sharma P, Vijayargiya M, Tandon S, Gaur S. Triceps tendon avulsion: a rare injury. Ethiop J Health Sci. 2014;24(1):97–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shaw HM, Benjamin M. Structure-function relationships of entheses in relation to mechanical load and exercise. Scand J Med Sci Sports. 2007;17(4):303–315. [DOI] [PubMed] [Google Scholar]
- 126.Sherman OH, Snyder SJ, Fox JM. Triceps tendon avulsion in a professional body builder: a case report. Am J Sports Med. 1984;12(4):328–329. [DOI] [PubMed] [Google Scholar]
- 127.Shields CL, Ashby ME. Diagnosis in patellar tendon avulsion. J Natl Med Assoc. 1975;67(3):231–232. [PMC free article] [PubMed] [Google Scholar]
- 128.Sierra RJ, Weiss NG, Shrader MW, Steinmann SP. Acute triceps ruptures: case report and retrospective chart review. J Shoulder Elbow Surg. 2006;15(1):130–134. [DOI] [PubMed] [Google Scholar]
- 129.Silder A, Heiderscheit BC, Thelen DG, Enright T, Tuite MJ. MR observations of long-term musculotendon remodeling following a hamstring strain injury. Skeletal Radiol. 2008;37(12):1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Simonet WT, Saylor HL, III, Sim L. Abdominal wall muscle tears in hockey players. Int J Sports Med. 1995;16(2):126–128. [DOI] [PubMed] [Google Scholar]
- 131.Singh RK, Pooley J. Complete rupture of the triceps brachii muscle. Br J Sports Med. 2002;36(6):467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Smith JH. Avulsion of a profundus tendon with simultaneous intraarticular fracture of the distal phalanx—case report. J Hand Surg Am. 1981;6(6):600–601. [DOI] [PubMed] [Google Scholar]
- 133.Sollender JL, Rayan GM, Barden GA. Triceps tendon rupture in weight lifters. J Shoulder Elbow Surg. 1998;7(2):151–153. [DOI] [PubMed] [Google Scholar]
- 134.Sonnery-Cottet B, Edwards TB, Noel E, Walch G. Rotator cuff tears in middle-aged tennis players: results of surgical treatment. Am J Sports Med. 2002;30(4):558–564. [DOI] [PubMed] [Google Scholar]
- 135.Speer KP, Lohnes J, Garrett WEJ. Radiographic imaging of muscle strain injury. Am J Sports Med. 1993;21(1):89–95. [DOI] [PubMed] [Google Scholar]
- 136.Stauber T, Blache U, Snedeker JG. Tendon tissue microdamage and the limits of intrinsic repair. Matrix Biol. 2020;85-86:68–79. [DOI] [PubMed] [Google Scholar]
- 137.Street CC, Burks RT. Chronic complete hamstring avulsion causing foot drop: a case report. Am J Sports Med. 2000;28(4):574–576. [DOI] [PubMed] [Google Scholar]
- 138.Sullivan JC, Best TM. Injury of the musculotendinous junction. In: Maffulli N, Renstrom P, Leadbetter WB, eds. Tendon Injuries. Springer-Verlag London; 2005:63–69. [Google Scholar]
- 139.Sun JS, Tsuang YH, Liu TK, Hang YS, Cheng CK. Failure sites and peak tensile forces of the composite triceps surae muscle by passive extension in the rabbit. Clin Biomech (Bristol, Avon). 1994;9(5):310–314. [DOI] [PubMed] [Google Scholar]
- 140.Tadros AS, Huang BK, Pathria MN. Muscle-tendon-enthesis unit. Semin Musculoskelet Radiol. 2018;22(3):263–274. [DOI] [PubMed] [Google Scholar]
- 141.Takami H, Takahashi S, Ando M, Kabata K. Rupture of the flexor pollicis longus tendon at the musculotendinous junction in a bowler. Arch Orthop Trauma Surg. 1998;117(4-5):277–278. [DOI] [PubMed] [Google Scholar]
- 142.Takami H, Takahashi S, Ando M, Suzuki K. Traumatic rupture of the extensor tendons at the musculotendinous junction. J Hand Surg Am. 1995;20(3):474–477. [DOI] [PubMed] [Google Scholar]
- 143.Taylor DC, Dalton JDJ, Seaber AV, Garrett WEJ. Experimental muscle strain injury: early functional and structural deficits and the increased risk for reinjury. Am J Sports Med. 1993;21(2):190–194. [DOI] [PubMed] [Google Scholar]
- 144.Temple HT, Kuklo TR, Sweet DE, Gibbons CL, Murphey MD. Rectus femoris muscle tear appearing as a pseudotumor. Am J Sports Med. 1998;26(4):544–548. [DOI] [PubMed] [Google Scholar]
- 145.Thorpe CT, Riley GP, Birch HL, Clegg PD, Screen HRC. Fascicles and the interfascicular matrix show decreased fatigue life with ageing in energy storing tendons. Acta Biomater. 2017;56:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tiger E, Mayer DP, Glazer R.Complete avulsion of the triceps tendon: MRI diagnosis. Comput Med Imaging Graph. 1993;17(1):51–54. [DOI] [PubMed] [Google Scholar]
- 147.Toczylowski HM, Balint CR, Steiner ME, Boardman M, Scheller AD, Jr. Complete rupture of the distal biceps brachii tendon in female patients: a report of 2 cases. J Shoulder Elbow Surg. 2002;11(5):516–518. [DOI] [PubMed] [Google Scholar]
- 148.Towers JD, Russ EV, Golla SK. Biomechanics of tendons and tendon failure. Semin Musculoskelet Radiol. 2003;7(1):59–66. [DOI] [PubMed] [Google Scholar]
- 149.Tsuang Y-H, Sun J-S, Chen I-H, et al. The effects of cyclic stretching on tensile properties of the rabbit’s skeletal muscle. Clin Biomech (Bristol, Avon). 1998;13(1):48–53. [DOI] [PubMed] [Google Scholar]
- 150.Tung GA, Yoo DC, Levine SM, Brody JM, Green A. Subscapularis tendon tear: primary and associated signs on MRI. J Comput Assist Tomogr. 2001;25(3):417–424. [DOI] [PubMed] [Google Scholar]
- 151.Valente M, Mancuso F, Alecci V. Isolated rupture of biceps femoris tendon. Musculoskelet Surg. 2013;97(3):263–266. [DOI] [PubMed] [Google Scholar]
- 152.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.VanDusen KW, Larkin LM. Muscle-tendon interface. In: Nukavarapu SP, Freeman JW, Laurencin CT, eds. Regenerative Engineering of Musculoskeletal Tissues and Interfaces. Elsevier; 2015:409–429. [Google Scholar]
- 154.Vastamäki M, Brummer H, Solonen KA. Avulsion of the distal biceps brachii tendon. Acta Orthop. 1981;52(1):45–48. [DOI] [PubMed] [Google Scholar]
- 155.Wagner JR, Cooney WP. Rupture of the triceps muscle at the musculotendinous junction: a case report. J Hand Surg Am. 1997;22(2):341–343. [DOI] [PubMed] [Google Scholar]
- 156.Walch G, Nove-Josserand L, Liotard J-P, Noel E. Musculotendinous infraspinatus ruptures: an overview. Orthop Traumatol Surg Res. 2009;95(7):463–470. [DOI] [PubMed] [Google Scholar]
- 157.Wang Y-C, Jeng C-M, Shen P-W, et al. Pectoralis major muscle tear diagnosed with magnetic resonance imaging and ultrasound—a case report. Tzu Chi Med J. 2005;17(6):441–444. [Google Scholar]
- 158.Warme WJ, Whitaker DC. Pectoralis major tendon avulsion from rappelling. Mil Med. 2004;169(2):151–154. [DOI] [PubMed] [Google Scholar]
- 159.Weishaupt D, Schweitzer ME, Morrison WB. Injuries to the distal gastrocnemius muscle: MR findings. J Comput Assist Tomogr. 2001;25(5):677–682. [DOI] [PubMed] [Google Scholar]
- 160.Wenger DR. Avulsion of the profundus tendon insertion in football players. Arch Surg. 1973;106(2):145–149. [DOI] [PubMed] [Google Scholar]
- 161.White GM, Riley LH, Jr. Isolated avulsion of the subscapularis insertion in a child: a case report. J Bone Joint Surg Am. 1985;67(4):635–636. [PubMed] [Google Scholar]
- 162.Williams BD, Schweitzer ME, Weishaupt D, et al. Partial tears of the distal biceps tendon: MR appearance and associated clinical findings. Skeletal Radiol. 2001;30(10):560–564. [DOI] [PubMed] [Google Scholar]
- 163.Winblad JB, Escobedo E, Hunter JC. Brachialis muscle rupture and hematoma. Radiol Case Rep. 2008;3(4):251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yoon MY, Koris MJ, Ortiz JA, Papandrea RF. Triceps avulsion, radial head fracture, and medial collateral ligament rupture about the elbow: a report of 4 cases. J Shoulder Elbow Surg. 2012;21(2):e12–e17. [DOI] [PubMed] [Google Scholar]
- 165.Yoshioka H, Anno I, Niitsu M, Takahashi H, Matsumoto K, Itai Y. MRI of muscle strain injuries. J Comput Assist Tomogr. 1994;18(3):454–460. [DOI] [PubMed] [Google Scholar]
- 166.Zantop T, Brucker PU, Vidal A, Zelle BA, Fu FH. Intraarticular rupture pattern of the ACL. Clin Orthop Relat Res. 2007;454:48–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-ojs-10.1177_23259671211020731 for Injuries in Muscle-Tendon-Bone Units: A Systematic Review Considering the Role of Passive Tissue Fatigue by Maria C.P. Vila Pouca, Marco P.L. Parente, Renato M. Natal Jorge and James A. Ashton-Miller in Orthopaedic Journal of Sports Medicine