Abstract
Background:
There is limited understanding of tracheal carcinoma (TC) because of its rarity. We examined the efficacy of radiotherapy (RT) for patients with primary TC.
Methods:
We analyzed the records of 32 patients with primary TC who received RT at our center between November 1996 and December 2016.
Results:
Thirteen patients received adjuvant RT and 18 received definitive RT. Eight patients achieved complete remission (CR) after definitive RT. Among all patients, the 5-year overall survival (OS) rate was 46.9% and the locoregional progression free survival (LRPFS) rate was 68.1%. Univariate analysis indicated the 5-year OS was better in those with adenoid cystic adenocarcinoma than squamous cell carcinoma (P = 0.001); the 5-year LRPFS was better in patients who received surgical resection than those who did not (92.9% vs 46.4%, P = 0.013) and in patients who received postoperative RT than in those who received definitive RT (91.7% vs 50.1%, P = 0.038). A sub-group univariate analysis indicated the 5-year PFS was better for those who received at least 68 Gy of radiation (44.4% vs 13.0%, P = 0.044). Patients who achieved CR had a better 5-year PFS than those who did not (57.1% vs 10%, P = 0.006). No patients had a toxicity of grade 3 or more.
Conclusions:
Adjuvant and definitive RT are safe and effective treatments for TC. Patients who received dosages of 68 Gy or more and who had complete tumor regression following definitive RT seemed to have better long-term survival.
Keywords: tracheal carcinoma, radiotherapy, dose, IMRT, survival
Introduction
The incidence of primary tracheal carcinoma (TC) is extremely low, and this cancer accounts for only about 0.1% of all malignancies.1 The most common histological type is squamous cell carcinoma (SCC) followed by adenoid cystic adenocarcinoma (ACC).2,3 Because there are no specific symptoms, diagnosis typically occurs in patients with advanced tumors. There is no definitive staging system and no comprehensive treatment guidelines due to the rarity of this cancer. Bhattacharyya et al proposed a TNM staging system,3 but this system has not been widely accepted. Because the study is based on the result of the SEER database. As it is a survey database, problems with missing data may be encountered. In addition, the staging system lacks the subdivision of N staging and does not include M staging, which leads to difficulties in comparison between different studies.
Surgery is considered the main treatment. Radiotherapy (RT) plays an important role as a postoperative adjuvant treatment because the surgery margin may be insufficient.4-11 For this reason, most clinicians administer postoperative RT after resection for locally advanced tumors, even in the absence of any prospective evidence. There is no evidence from clinical trials regarding the optimal approach for patients who are inoperable or unsuitable for surgery, and clinicians typically propose definitive RT for these patients.4-6,12 Data on long-term complete remission suggest that outcome is related to radiation dose.4,6 Although there are some reports on the use of different RT regimens, there is little information on this topic, and it is difficult to develop personalized RT planning and dosages.
The purpose of this study is to evaluate our institutional experience with RT, either as adjuvant or definitive treatment, on the outcome of patients with primary TC, and to compare our results with previous studies of this cancer.
Materials and Methods
Subject Selection and Data Collection
Following approval by our Institutional Review Board, we identified patients by searching the institutional tumor registry of our Cancer Center for all patients with neoplasms of the trachea from 1996 to 2016. Eligible patients had primary TC confirmed by pathology and received RT at our institution. Patients were excluded if they had benign tumors, tumors that might have arisen from other sites, did not receive anti-tumor therapy or RT at our institution, or had distant metastases at diagnosis.
The medical charts were reviewed to collect clinical and demographic data, including age, sex, carcinogen exposures, presenting symptoms, histologic information, tumor location and size, treatment modality, and outcome.
Treatment
The surgical approach was endoscopic resection or open surgical resection. Open surgical resection included tracheal resection, laryngotracheal resection, stent implantation, and tracheotomy. Tumor resection was not performed for the latter 2 methods.
The goal of the surgical resection was complete resection. The main objectives were to protect the blood supply of the trachea and to perform safe reconstruction by avoiding excessive anastomotic tension. The eligibility for surgical resection was determined via a multidisciplinary clinical conference after review of the patient’s condition and analysis of tumor characteristics, including size and location. Contraindications to resection of the major airways were respiratory failure and presence of a major co-morbidity. The surgical approach that was used depended on the surgeon’s experience.
RT was administered with a linear accelerator using a 6 MV X-ray beam. Adjuvant RT was usually initiated 4-6 weeks after surgery, and the total dose was 50-54 Gy. A boost dose was administered if there was microscopic residual tumor or an insufficient surgical margin. The definitive RT dose was 60-70 Gy. The dose per fraction was 180-200 c Gy, 5 times per week.
Gross tumor volume (GTV) was defined as all gross visible disease based on imaging and endoscopy. The clinical target volume (CTV) was usually expanded 3-5 cm longitudinally and 1-2 cm axially from the tumor bed or primary tumor, and adjacent lymph node stations were also included. The planning target volume (PTV) was generally expanded 1 cm beyond the CTV. Dose constraints were implemented for organs at risk (OAR), such as the spinal cord, esophagus, and lung.
RT techniques have changed during the 20-year period of this analysis. Thus, patients received 2-dimensional radiotherapy (2DRT), 3-dimensional conformal radiotherapy (3DCRT), or intensity-modulated radiotherapy (IMRT).
Chemotherapy was combined with RT as concurrent or adjuvant chemotherapy in some cases. The most common therapeutic regimen was 1-7 cycles of cisplatin.
Evaluation Standards
Response evaluation and routine follow-up was performed by use of CT imaging and bronchoscopy with or without biopsy after treatment. Tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria.13 The Radiation Therapy Oncology Group (RTOG) toxicity grading system was used to score acute and chronic complications.14
Statistical Analysis
The primary endpoints were overall survival (OS), locoregional progression free survival (LRPFS), progression free survival (PFS), and distant metastasis free survival (DMFS). OS was measured from the date of treatment to the date of death or last follow-up. LRPFS, PFS, and DMFS were measured from the date of treatment to the date of each event, death, or last follow-up.
The survival rate was calculated using the Kaplan-Meier survival analysis method. Univariate and multivariate analyses of prognostic factors were performed using the log rank and Cox’s regression methods, respectively. Variables associated with a P value < 0.2 were included in the multivariate analysis. All tests were two-sided and P-values of less than 0.05 were considered to indicate statistical significance. SPSS Statistics 17.0 software was used for the survival analysis.
Results
Patient and Treatment Characteristics
We initially reviewed the records of 96 patients who had diagnoses of tracheal neoplasms, and excluded 11 patients with benign tumors and 29 patients who had tumors that might have arisen from other sites. Among the 56 patients with primary malignant neoplasms of the trachea, 8 patients did not receive anti-tumor therapy at our institution and 16 patients did not receive RT or had distant metastases before treatment. Thus, we ultimately examined the records of 32 patients.
Analysis of the characteristics of the 32 patients (Table 1) indicated that most lesions were in the thoracic trachea (78.1%), and 22 patients had extra-tracheal invasion. The most common presenting symptoms were hemoptysis (37.5%) and dyspnea (28.1%). Twenty-three patients (71.9%) were male, and SCC (56.3%) and ACC (31.2%) were the predominant histological types. Sixteen patients with SCCs had histories of smoking, but none of the patients with ACCs had histories of smoking.
Table 1.
Characteristics of Patients and Treatments.
| Patients | No. (%) or median (Range) | Treatments | No. (%) or median (Range) |
|---|---|---|---|
| Age, years | 48 (28-68) | Surgery | 19 (59.4) |
| Sex | Type | ||
| Male | 23 (71.9) | Endoscopic rsct | 3 (3/19,15.8) |
| Female | 9 (28.1) | Tracheal rsct | 9 (9/19,47.3) |
| Histologic type | Laryngotracheal rsct | 4 (4/19,21.1) | |
| SCC | 18 (56.3) | Stent implantation | 2 (2/19,10.5) |
| ACC | 10 (31.2) | Tracheotomy | 1 (1/19,5.3) |
| Undifferentiated | 3 (9.4) | Rsct margin status | |
| Adenocarcinoma | 1 (3.1) | R1 | 7 (7/16,43.8) |
| Smoking status | R0 | 9 (9/16,56.3) | |
| Never | 19 (59.4) | Radiotherapy | 32 (100) |
| Ever | 13 (40.6) | Type | |
| Location | PORT | 13 (40.6) | |
| Cervical | 7 (21.9) | Definitive RT | 18 (56.3) |
| Thoracic | 25 (78.1) | Palliative RT | 1 (3.1) |
| Extension | Technique | ||
| Carina | 10 (31.3) | 2DRT | 9 (28.1) |
| Nodal involvement | 5 (15.6) | 3DCRT | 12 (37.5) |
| Esophagus | 3 (9.4) | IMRT | 11 (34.4) |
| Main stem bronchus | 3 (9.4) | Median dose, Gy | |
| Thyroid | 1 (3.1) | PORT | 54.0 (50-60) |
| Presenting symptoms | Definitive RT | 67.5 (60-70) | |
| Hemoptysis | 12 (37.5) | Chemotherapy | 12 (37.5) |
| Dyspnea | 9 (28.1) | Induction | 3 (9.4) |
| Stridor | 6 (18.8) | Concurrent | 7 (21.9) |
| Cough | 5 (15.6) | Adjuvant | 2 (6.3) |
Abbreviations: Rsct, resection; SCC, squamous cell carcinoma; ACC, adenoid cystic carcinoma; PORT, post-operative radiotherapy; RT, radiotherapy; 2DRT, 2-dimensional radiotherapy; 3DCRT, 3-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy.
Sixteen patients (50.0%) received surgical resection, 13 of whom received open surgical resection and 3 of whom received endotracheal resection. Evidence of microscopic residual tumor (R1 at the resection margin) was present in 7 patients after resection. Two patients received stent implantation and 1 patient received a tracheotomy for palliation, but none of these 3 patients received any form of tracheal resection.
All patients who received open surgical resection received postoperative RT (PORT), and all patients who received endoscopic resection received definitive RT. Resection and definitive RT was not possible in 1 patient because the tumor was too large; thus, palliative RT (dose: 56 Gy) was applied after tracheotomy in this patient. The mean duration of adjuvant RT was 5.5 weeks and the mean dose was 55.2 Gy (median: 54.0 Gy; range: 50 to 60 Gy). The mean duration of definitive RT was 6.6 weeks and the mean dose was 66.5 Gy (median: 67.5; range: 60 to 70 Gy).
Twelve patients received 3DCRT, 11 patients received IMRT, and 9 patients received 2DRT (Table 1).
Twelve patients received chemotherapy, 7 of them were treated with cisplatin as a single-drug regimen for concurrent chemotherapy combined with radiotherapy. Three patients who received induction chemotherapy before and 2 patients who received adjuvant chemotherapy after surgical resection used the PF regimen (Cisplatin plus 5-fluorouracil).
Tumor Response and Pattern of Failure
Patients were evaluated for short-term efficacy at 3 months after the end of RT. Among the 18 patients who received definitive RT, 8 (44.4%) achieved complete remission (CR) and 10 (55.6%) achieved partial remission (PR) (Table 2). None of the 32 patients experienced progressive disease (PD) during the initial response evaluation, and the overall response rate was 100%.
Table 2.
Patient and Treatment Characteristics of Definitive RT Group.
| Sex | Age (yrs) | Histologic type | Size (cm) | Dose (Gy) | RT technique | Chemotherapy type | RESP | LP | T-LP (mo.) | DM | T-DM (mo.) | Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | 41 | ACC | 1.8 | 67 | 2D-RT | / | PR | – | + | 92 | DM | |
| F | 38 | ACC | 2.0 | 68 | 3DCRT | / | CR | – | + | 23 | DM | |
| M | 39 | ACC | 4.5 | 70 | IMRT | / | CR | – | – | Alive | ||
| F | 32 | ACC | 1.0 | 66 | IMRT | / | CR | – | – | Other | ||
| M | 50 | SCC | 4.2 | 68 | 3DCRT | Induction | CR | – | – | Alive | ||
| M | 60 | SCC | 3.5 | 70 | 3DCRT | Concurrent | CR | – | – | Alive | ||
| M | 36 | SCC | 4.0 | 64 | IMRT | Concurrent | PR | + | 13 | – | LP | |
| M | 56 | SCC | 3.8 | 63 | IMRT | Concurrent + adjuvant | PR | – | + | 5 | DM | |
| F | 38 | SCC | 6.0 | 68 | 3DCRT | / | CR | + | 30 | – | LP | |
| M | 52 | SCC | 2.9 | 66 | 2D-RT | / | CR | + | 14 | – | LP | |
| M | 68 | SCC | 3.5 | 60 | 2D-RT | Concurrent + adjuvant | PR | + | 6 | – | LP | |
| M | 57 | ADC | 6.5 | 68 | 2D-RT | Concurrent | PR | + | 7 | – | LP | |
| M | 39 | SCC | 2.5 | 68 | 2D-RT | Concurrent | PR | – | + | 12 | DM | |
| M | 57 | SCC | 3.2 | 66 | 2D-RT | Induction | PR | + | 10 | – | LP | |
| M | 65 | ACC | 4.1 | 68 | 3DCRT | / | CR | – | – | Alive | ||
| M | 53 | SCC | 3.3 | 66 | 2D-RT | / | PR | – | + | 56 | DM | |
| M | 59 | SCC | 1.6 | 70 | IMRT | Concurrent + adjuvant | PR | + | 48 | – | LP | |
| M | 44 | SCC | 2.0 | 63 | IMRT | / | PR | + | 22 | – | LP |
Abbreviations: ADC, adenocarcinoma; F, female; M, male; ACC, adenoid cystic carcinoma; SCC, squamous cell carcinoma; RT, radiotherapy; 2DRT, 2-dimensional radiotherapy; 3DCRT, 3-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; RESP, initial response after RT; CR, complete remission; PR, partial remission; LP, local progression; DM, distant metastasis; T, time since start of treatment; mo., months.
Locoregional progression (LP) occurred in 9 patients (28.1%) during the follow-up period, and 8 of these patients received definitive RT. Seven patients (21.9%) developed distant metastases (4 with lung metastases and 3 with bone metastasis).
Survival and Prognostic Factors
We followed up patients by telephone and clinical visits. The median follow-up duration was 60 months (range: 7 to 202 months) and 20 patients (62.5%) died during the follow-up period. No patients were lost to follow up. The cause of death was LP in 9 patients, lung metastasis in 4 patients, bone metastasis in 2 patients, and was unrelated to cancer in 5 patients. Analysis of survival data indicated the 5-year OS was 46.9%, the LRPFS was 68.1%, the DMFS was 77.6%, and the PFS was 47.9%.
Univariate analysis (Table 3) indicated that histologic type (ACC vs SCC) was significantly associated with OS and LRPFS, and that surgical resection (yes vs no) and RT type (PORT vs Definitive RT) were significantly associated with LRPFS. The 5-year OS rate was better for patients with ACC than SCC (80.0% vs 29.4%, P = 0.001; Figure 1) and the 5-year LRPFS rate was 100% in patients with ACC and 44.2% in patients with SCC (P < 0.001). The 5-year LRPFS rate was better for patients who received surgical resection than those did not (92.9% vs 46.4%, P = 0.013; Figure 2), and the 5-year LRPFS rate was better for patients who received PORT than those received definitive RT (91.7% vs 50.1%, P = 0.038; Figure 3).
Table 3.
Univariate Analysis of the Relationship of Clinical and Treatment-Related Factors With OS and LRPFS.
| Factors | Comparison | P-value | |
|---|---|---|---|
| OS | LRPFS | ||
| Clinical factors | |||
| Sex | Male vs female | 0.689 | 0.594 |
| Age | ≤ 40 years vs > 40 years | 0.361 | 0.681 |
| Smoking history | Never vs ever | 0.184 | 0.575 |
| Histologic type | SCC vs ACC | 0.001 | <0.001 |
| Location | Cervical vs thoracic | 0.170 | 0.056 |
| Tumor size | ≤ 4 cm vs > 4 cm | 0.102 | 0.389 |
| Extension | Yes vs no | 0.545 | 0.608 |
| Treatment-related factors | |||
| Surgical resection | Yes vs No | 0.823 | 0.013 |
| RT type | PORT vs definitive RT | 0.324 | 0.038 |
| RT technique | 3DCRT/IMRT vs 2D-RT | 0.193 | 0.062 |
| Chemotherapy | Yes vs No | 0.543 | 0.189 |
| Tumor response | CR vs PR | 0.160 | 0.205 |
Abbreviations: SCC, squamous cell carcinoma; ACC, adenoid cystic carcinoma; RT, radiotherapy, PORT, post-operative radiotherapy; 2DRT, 2-dimensional radiotherapy; 3DCRT, 3-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; OS, overall survival; LRPFS, locoregional progression free survival.
Figure 1.
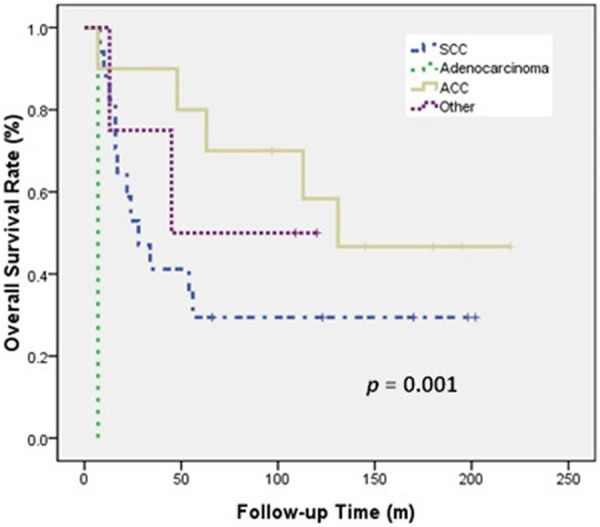
OS of patients with SCC, ACC, and other histology.
Figure 2.
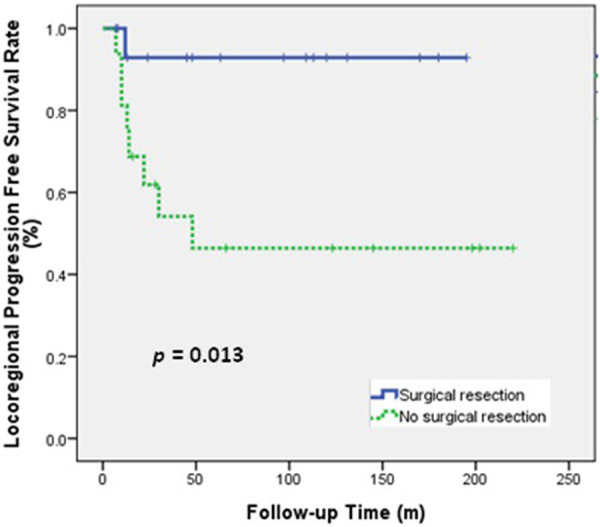
LRPFS of patients who received or did not receive surgical resection.
Figure 3.
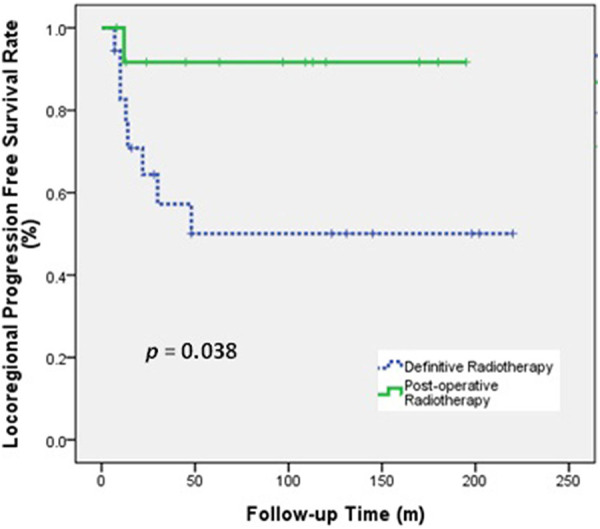
LRPFS of patients who received post-operative radiotherapy or definitive radiotherapy.
For OS the smoking history, histological type, tumor size, location, RT technique and tumor response enter to multivariate analysis. For LPRFS the histological type, location, surgical resection, RT type, RT technique and chemotherapy enter to multivariate analysis. However, no factor could reach statistical significance for both (Table 4).
Table 4.
Prognostic Factors for OS and LRPFS in Multivariate Analysis.
| HR | 95% CI | P-value | |
|---|---|---|---|
| OS | |||
| Smoking history | 0.478 | 0.166-1.379 | 0.172 |
| Histologic type | 0.800 | 0.513-1.248 | 0.325 |
| Location | 0.821 | 0.264-2.547 | 0.733 |
| Tumor size | 0.532 | 0.167-1.701 | 0.287 |
| RT technique | 0.941 | 0.296-2.821 | 0.876 |
| Tumor response | 1.429 | 0.493-4.138 | 0.511 |
| LRPFS | |||
| Histologic type | 0.254 | 0.053-1.224 | 0.088 |
| Location | 0.297 | 0.055-1.620 | 0.161 |
| Surgical resection | 1.033 | 0.020-52.370 | 0.987 |
| RT type | 0.335 | 0.011-9.799 | 0.526 |
| RT technique | 0.876 | 0.196-3.906 | 0.862 |
| Chemotherapy | 1.857 | 0.292-11.831 | 0.512 |
Abbreviations: HR, hazard ratio; CI, confidence interval; OS overall survival; LRPFS local progression free survival; RT radiotherapy.
Sub-group analysis of patients who received definitive RT indicated the 5-year OS was 33.3%, the LRPFS was 50.1%, the DMFS was 67.7%, and the PFS was 29.8%.
Univariate analysis indicated that RT dose and tumor response were associated with PFS (Table 5). The 5-year PFS was 44.4% for patients who received at least 68 Gy during RT, but was 13.0% for those who received less than 68 Gy (P = 0.044; Figure 4). In addition, patients who achieved CR had a better 5-year PFS than those who did not (57.1% vs 10%, P = 0.006; Figure 5). In multivariate analysis, no factor could reach statistical significance for OS and PFS (Table 5).
Table 5.
Univariate and Multivariate Analysis of the Relationship of Treatment-Related Factors With 5-Year OS and PFS in Definitive RT Group.
| Factors | Description | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| %, P-value | HR (95% CI) | P-value | |||||
| OS | PFS | OS | PFS | OS | PFS | ||
| RT technique | 3DCRT/IMRTvs 2D-RT | 36.4% vs28.6% 0.128 |
40.4% vs 14.3% 0.053 |
0.828 (0.205-3.351) | 0.896 (0.211-3.807) | 0.792 | 0.881 |
| RT dose | ≥ 68 Gy vs <68Gy | 44.4% vs 22.2% 0.067 |
44.4% vs 13.0% 0.044 |
0.638 (0.118-3.450) | 0.951 (0.202-4.471) | 0.602 | 0.949 |
| Tumor response | CR vs PR | 50.0% vs 20.0% 0.065 |
57.1% vs 10.0% 0.006 |
1.959 (0.388-9.903) | 4.741 (0.843-26.654) | 0.416 | 0.077 |
Abbreviations: RT, radiotherapy; HR, hazard ratio; CI, confidence interval; OS, overall survival; PFS, progression free survival; 2DRT, 2-dimensional radiotherapy; 3DCRT, 3-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; CR, complete remission; PR, partial remission.
Figure 4.
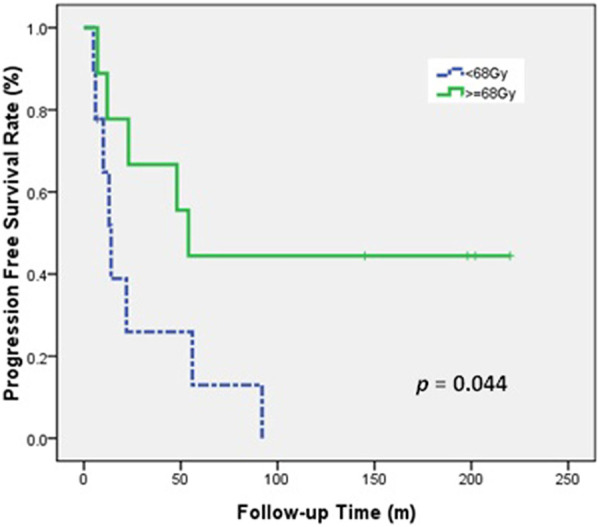
PFS of patients who received high-dose and low-dose definitive RT.
Figure 5.
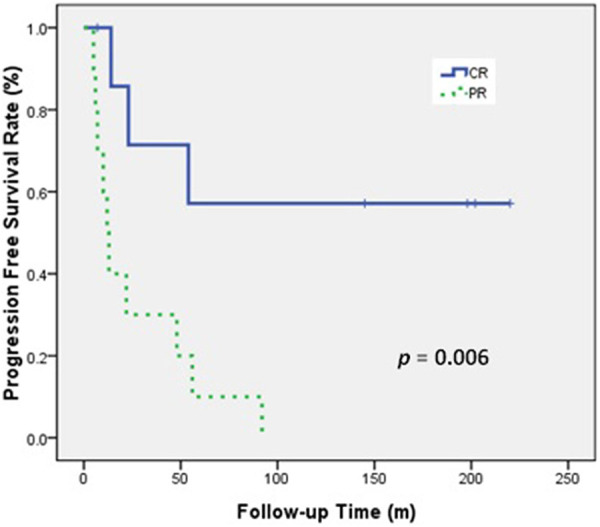
PFS of patients who received definitive RT and achieved or did not achieve CR.
Treatment-Related Complications
Analysis of complications during RT (data not shown) indicated that they were mainly non-severe (grade 1 or 2), and included odynophagia (n = 19), esophagitis (n = 6), radiation-induced lung fibrosis (n = 3), and tracheal stenosis (n = 2). The patients with odynophagia were able to tolerate eating after taking analgesics. None of the patients had esophageal stenosis or perforation after treatment.
Discussion
During the 20-year history of this major cancer center in South China, only 56 patients were diagnosed with primary TC. We analyzed 32 of these patients, after exclusion of 8 patients who did not receive anti-tumor therapy at our institution and 16 patients who did not receive RT or had distant metastases before treatment. The sex, age, histologic type, and carcinogen exposures were similar among our 32 patients and among patients in most other series.4-6,8,9 In particular, most of our patients were male and the main histological types were SCC and ACC. In addition, SCC was associated with a history of smoking, but ACC was not. Most patients tolerated their treatments, and there was a low rate of complications.
The thoracic trachea was the most common tumor location among our patients. Patients with tumors in this location are often misdiagnosed. Thus, we suggest that primary TC be included in the differential diagnosis when a patient presents with symptoms of hemoptysis, dyspnea, and stridor, particularly when the patient has a history of smoking.
The length of a tumor, the presence of extracapsular extension, and the difficulty in performing reconstruction are often responsible for insufficient surgical margins. Thus, most clinicians agree on the necessity for PORT following resection for locally advanced tumors.4,12 Definitive RT remains important for patients with unresectable tumors. In our study, the 5-year OS rate was 46.9% in the entire group. Other large series reported 5-year OS rates of 15% to 73%.3,8,9,15,16 However, it is difficult to directly compare these results because there are no agreements regarding the staging system and treatment strategies.
Previous studies of primary TC have reported longer survival times for patients with ACC than SCC. In particular, the 5-year OS rate ranged from 40% to 88% for ACC and 13% to 45% for SCC.7-10,16,17 Our results are consistent with these previous results. In fact, none of our patients with ACC experienced LP during the follow-up, regardless of the receipt of PORT or definitive RT. This might be because ACC tends to be well-differentiated and slow-growing, although previous research reported the appearance of metastases after 10 years.11,12 One of our patients with ACC developed distant metastases during the first decade after treatment.
We observed only one patient who received PORT after surgical resection who had LP, although 7 (43.8%) patients who received surgical resection had R1 resections and 4 (25.0%) others had surgical margins less than 1 cm. This result suggests that adjuvant RT provides an important benefit. In the definitive RT group, 44.4% (8/18) of the patients had LP. This indicates that surgery may be important for local control, even if the tracheal tumor cannot be completely removed. Our study also showed that the 5-year LRPFS was significantly longer in patients who received surgical resection than those who did not, and that the 5-year LRPFS was better in the PORT group than in the definitive RT group (Figure 3).
Our sub-group analysis of the definitive RT group indicated that patients treated with high RT doses (≥ 68 Gy) had better long-term progression-free control than those receiving low doses (<68 Gy), and this difference was marginally significant in univariate analysis (P = 0.044). Selection of the most appropriate RT dose for unresectable TC can be difficult. Several previous retrospective studies examined the effect of RT on the outcome of patients with primary TC (Table 6). The available literature indicates that an RT dose of at least 60 Gy was associated with improved outcome.3-6,8-11,16 Makarewicz et al 5 reported that RT doses of 60 to 70 Gy are needed to achieve CR.
Table 6.
Previous Studies That Examined the Effect of RT on Primary TC.
| Author | Year | Patients, n (PORT/DRT) |
Histology SCC/ACC |
Dose (Gy) PORT/DRT |
CR, % | Survival |
|---|---|---|---|---|---|---|
| Fields4 | 1989 | 24 (6/18) | 13/4 | <40-70 | 86(6/7) | 5-year OS: 25% |
| Makarewicz5 | 1998 | 23 (NP/8) | 13/7 | >60 | 75 | Mean survival: 26 months |
| Chao6 | 1998 | 42 (9/32) | 28/3 | 10-64 | 50 (4/8) | Median survival: 5.7 months |
| Bhattacharyya3 | 2004 | 92 (39/21) | 41/19 | NP | NP | Mean survival: 63.3 months |
| Webb8 | 2006 | 45 (10/18) | 26/7 | 29.8-70/18-130 | NP | 5-year DSM: 72.9% |
| Honing16 | 2007 | 293 (23/156) | 156/21 | NP | NP | 5-year OS: 15% |
| Xie9 | 2012 | 78 (46/30) | 33/12 | NP | NP | 5-year OS: 42.3% |
| Je11 | 2017 | 22 (13/9) | All ACC | 50.4/60-66 | 11.1 (1/9) | 5-year OS: 81.8% |
| Levy10 | 2018 | 31 (22/9) | All ACC | 45-65/66-70 | NP | 5-year OS: 88% |
Abbreviations: PORT, post-operative radiotherapy; DRT, definitive radiotherapy; SCC, squamous cell carcinoma; ACC, adenoid cystic carcinoma; CR, complete remission; DSM, disease-specific mortality; NP, not provided.
However, some reports showed that higher RT doses led to increased complications and did not improve survival.17,18 We believe that this discrepancy may be related to the use of different RT technologies. When a tumor is near a natural air cavity, the RT dose may be insufficient for the gross tumor volume (GTV), due to electronic disequilibrium around the air-tissue interfaces. Joshi et al 19 demonstrated that the magnitude of dose reduction in water near the air-water interface increases with photon energy and the number and size of the radiation beam. They also demonstrated no interface dose reductions when using 3DCRT. Use of Monte Carlo simulation may provide better estimates of the doses delivered at these interfaces.20 In our study, 77.8% (7/9) of patients received 68 Gy or more by 3DCRT or IMRT, and 2 patients (22.2%, 2/9) received 68 Gy by 2DRT. The 3DCRT/IMRT group had a better 5-year PFS than 2DRT group, but this was not statistically significant (P = 0.053). The effect of conformal technology on increasing the dose for tracheal tumors requires further research.
All of our patients who received definitive RT also received at least 60 Gy, and this group had a high remission rate (100%; CR: 44.4%). Those with complete tumor regression had a significantly better 5-year PFS. Fields et al 4 reported that CR was significantly related to dose. In particular, 6 of their 7 patients (86%) who received more than 60 Gy achieved CR, but 1 of 11 (9.0%) who received less than 60 Gy achieved CR (P < 0.001). Although the association between CR and RT dose needs verification, CR was reported as a favorable prognostic factor for survival in several case series.4,5,21
We found that the major pattern of distant failure was lung metastasis, regardless of whether surgery or high-dose definitive RT was administered. The development of systemic therapeutics in this setting seems to be important.22 Concurrent or adjuvant chemotherapy tends to prolong survival, but provided no significant effect in our group. Thus, further studies are needed to determine the benefit of chemotherapy. In addition, it is meaningful to analyze different chemotherapy regimens, cycles and combination modes if we expand the sample size in follow-up research. The search for genetic mutation targets is also a priority for future research.
We examined patients in our cancer center with primary TC who received RT over a period of 20 years. This is a retrospective analysis of a relatively small group of patients, which affects the reliability of the results. Need to expand the sample size to further confirm. The RT techniques have changed substantially over this period, and this could have led to bias. The lack of a standardized staging system and the rarity of this cancer make comparisons among studies difficult.
Conclusions
In conclusion, our retrospective analysis of patients with primary TC indicated that RT was a safe and effective adjuvant or definitive method of treatment. Patients who received dosages of 68 Gy or more and who had complete tumor regression following definitive RT seemed to have better long-term survival. Further studies are needed to evaluate the use of modern RT techniques, including conformity, dose distribution, and protection of organs at risk.
Abbreviations
- ACC
adenoid cystic adenocarcinoma
- ADC
adenocarcinoma
- CI
confidence interval
- CR
complete remission
- CTV
clinical target volume
- DMFS
distant metastasis free survival
- F
female
- GTV
Gross tumor volume
- HR
hazard ratio
- IMRT
intensity-modulated radiotherapy
- LP
Locoregional progression
- LRPFS
locoregional progression free survival
- M
male
- NP
not provided
- OAR
organs at risk
- OS
overall survival
- PD
progressive disease
- PFS
progression free survival
- PORT
postoperative RT
- PR
partial remission
- PTV
planning target volume
- RECIST
Response Evaluation Criteria in Solid Tumors
- Rsct
resection
- RT
radiotherapy
- RTOG
Radiation Therapy Oncology Group
- SCC
squamous cell carcinoma
- TC
tracheal carcinoma
- 2DRT
2-dimensional radiotherapy
- 3DCRT
3-dimensional conformal radiotherapy
Footnotes
Authors’ Note: Ruifang Zeng and Hanyu Wang contributed equally to this work. This study was approval by Ethics Committee of Cancer Center Integrated Hospital of Traditional Chinese Medicine (No. NFZXY20170618). The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Science and Technology Project Fund of Guangdong China [Grant No. 2017ZC0059].
ORCID iD: Ruifang Zeng, MD  https://orcid.org/0000-0001-6657-1330
https://orcid.org/0000-0001-6657-1330
References
- 1.Macchiarini P. Primary tracheal tumours. Lancet Oncol. 2006;7(1):83–91. doi:10.1016/S1470-2045(05)70541-6 [DOI] [PubMed] [Google Scholar]
- 2.Licht PB, Friis S, Pettersson G. Tracheal cancer in Denmark: a nationwide study. Eur J Cardiothorac Surg. 2001;19(3):339–345. doi:10.1016/s1010-7940(01)00597-8 [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharyya N. Contemporary staging and prognosis for primary tracheal malignancies: a population-based analysis. Otolaryngol Head Neck Surg. 2004;131(5):639–642. doi:10.1016/j.otohns.2004.05.018 [DOI] [PubMed] [Google Scholar]
- 4.Fields JN, Rigaud G, Emami BN. Primary tumors of the trachea. Results of radiation therapy. Cancer. 1989;63(12):2429–2433. [DOI] [PubMed] [Google Scholar]
- 5.Makarewicz R, Mross M. Radiation therapy alone in the treatment of tumours of the trachea. Lung Cancer. 1998;20(3):169–174. [DOI] [PubMed] [Google Scholar]
- 6.Chao MW, Smith JG, Laidlaw C, Joon DL, Ball D. Results of treating primary tumors of the trachea with radiotherapy. Int J Radiat Oncol Biol Phys. 1998;41(4):779–785. [DOI] [PubMed] [Google Scholar]
- 7.Gaissert HA, Grillo HC, Shadmehr MB, et al. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg. 2004;78(6):1889–1896. Discussion 96-7. doi:10.1016/j.athoracsur.2004.05.064 [DOI] [PubMed] [Google Scholar]
- 8.Webb BD, Walsh GL, Roberts DB, Sturgis EM. Primary tracheal malignant neoplasms: the University of Texas MD Anderson cancer center experience. J Am Coll Surg. 2006;202(2):237–246. doi:10.1016/j.jamcollsurg.2005.09.016 [DOI] [PubMed] [Google Scholar]
- 9.Xie L, Fan M, Sheets NC, Chen RC, Jiang GL, Marks LB. The use of radiation therapy appears to improve outcome in patients with malignant primary tracheal tumors: a SEER-based analysis. Int J Radiat Oncol Biol Phys. 2012;84(2):464–470. doi:10.1016/j.ijrobp.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 10.Levy A, Omeiri A, Fadel E, Le Pechoux C. Radiotherapy for tracheal-bronchial cystic adenoid carcinomas. Clin Oncol (R Coll Radiol) . 2018;30(1):39–46. doi:10.1016/j.clon.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 11.Je HU, Song SY, Kim DK, et al. A 10-year clinical outcome of radiotherapy as an adjuvant or definitive treatment for primary tracheal adenoid cystic carcinoma. Radiat Oncol. 2017;12(1):196. doi:10.1186/s13014-017-0933-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madariaga MLL, Gaissert HA. Overview of malignant tracheal tumors. Ann Cardiothorac Surg. 2018;7(2):244–254. doi:10.21037/acs.2018.03.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuchida Y, Therasse P. Response evaluation criteria in solid tumors (RECIST): new guidelines. Med Pediatr Oncol. 2001;37(1):1–3. doi:10.1002/mpo.1154 [DOI] [PubMed] [Google Scholar]
- 14.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. doi:10.1016/0360-3016(95)00060-C [DOI] [PubMed] [Google Scholar]
- 15.Regnard JF, Fourquier P, Levasseur P. Results and prognostic factors in resections of primary tracheal tumors: a multicenter retrospective study. The French Society of Cardiovascular Surgery. J Thorac Cardiovasc Surg. 1996;111(4):808–813. Discussion 13-14. [DOI] [PubMed] [Google Scholar]
- 16.Honings J, van Dijck JA, Verhagen AF, van der Heijden HF, Marres HA. Incidence and treatment of tracheal cancer: a nationwide study in the Netherlands. Ann Surg Oncol. 2007;14(2):968–976. doi:10.1245/s10434-006-9229-z [DOI] [PubMed] [Google Scholar]
- 17.Grillo HC, Mathisen DJ. Primary tracheal tumors: treatment and results. Ann Thorac Surg. 1990;49(1):69–77. [DOI] [PubMed] [Google Scholar]
- 18.Bonner Millar LP, Stripp D, Cooper JD, Both S, James P, Rengan R. Definitive radiotherapy for unresected adenoid cystic carcinoma of the trachea. Chest. 2012;141(5):1323–1326. doi:10.1378/chest.11-0925 [DOI] [PubMed] [Google Scholar]
- 19.Joshi CP, Darko J, Vidyasagar PB, Schreiner LJ. Dosimetry of interface region near closed air cavities for Co60, 6 MV and 15 MV photon beams using Monte Carlo simulations. J Med Phys. 2010;35(2):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagne IM, Zavgorodni S. Evaluation of the analytical anisotropic algorithm in an extreme water-lung interface phantom using Monte Carlo dose calculations. J Appl Clin Med Phys. 2006;8(1):33–46. doi:10.1120/jacmp.v8i1.2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeremic B, Shibamoto Y, Acimovic L, Milisavljevic S. Radiotherapy for primary squamous cell carcinoma of the trachea. Radiother Oncol. 1996;41(2):135–138. [DOI] [PubMed] [Google Scholar]
- 22.Trodella L, Granone P, Valente S, et al. Adjuvant radiotherapy in non-small cell lung cancer with pathological stage I: definitive results of a phase III randomized trial. Radiother Oncol. 2002;62(1):11–19. [DOI] [PubMed] [Google Scholar]


