Abstract
Background:
We investigated the association between glucose excursions and the dawn phenomenon, and the effects of oral-glucose lowering drugs on the dawn phenomenon in patients with type 2 diabetes (T2D).
Methods:
We conducted a post hoc analysis using data from a previous randomized trial. Patients with T2D on metformin monotherapy were randomized to receive add-on acarbose or glibenclamide for 16 weeks. Ambulatory continuous glucose monitoring (CGM) was conducted before randomization and at the end of the study. Using the CGM data, we assessed glucose excursions as indicated by mean amplitude of glycemic excursions (MAGE). The magnitude of the dawn phenomenon was calculated as the difference between the nocturnal nadir (0:00 to 6:00 a.m.) and prebreakfast glucose level.
Results:
A total of 50 patients with T2D [mean age 53.5 ± 8.2 years, mean glycated hemoglobin (HbA1c) 8.4 ± 1.2%] were analyzed. There was an independent association between MAGE and the dawn phenomenon [β coefficient 0.199, 95% confidence interval (CI) 0.074–0.325, p = 0.003]. HbA1c improved significantly after treatment with acarbose or glibenclamide. However, only treatment with acarbose significantly improved glucose excursions. The dawn phenomenon decreased significantly only in patients treated with acarbose (from 35.9 ± 15.7–28.3 ± 16.5 mg/dl, p = 0.037), but not in those treated with glibenclamide (from 35.9 ± 20.6–34.6 ± 17.0 mg/dl, p = 0.776).
Conclusion:
Glucose excursions were independently associated with the dawn phenomenon in patients with T2D on metformin monotherapy. Both glucose excursions and the dawn phenomenon improved after treatment with acarbose, but not after treatment with glibenclamide.
Keywords: continuous glucose monitoring, dawn phenomenon, glucose excursions, type 2 diabetes
Introduction
More than 35 years ago, the term “dawn phenomenon” was introduced to describe the increase of blood glucose level during the period from nocturnal nadir to early morning in patients with type 1 diabetes.1,2 Similar observations were reported in patients with type 2 diabetes (T2D),3 and even in those with prediabetes or normal glucose tolerance.4 The dawn phenomenon may contribute to postprandial hyperglycemia in the morning,1,5 and its impact on diurnal glycemic control in patients with T2D could be assessed using data collected through ambulatory continuous glucose monitoring (CGM).6
The pathogenesis of the dawn phenomenon involves nocturnal increases of counter-regulatory hormones, including growth hormone, cortisol, and catecholamines.2,3,7–9 In people with insulin resistance or β-cell dysfunction, the secretion of insulin during the nocturnal period is not enough to suppress hepatic glucose overproduction in response to the increases of counter regulatory hormones.10 Therefore, the dawn phenomenon is frequently present not only in patients with T2D (~50%), but also in individuals with prediabetes (~30%).4 Moreover, nocturnal hypoglycemia may also lead to hyperglycemia in the early morning period.11
Although the dawn phenomenon is frequently present in patients with T2D,4 there are limited data regarding the effects of oral glucose-lowering drugs on the dawn phenomenon.6,12 To quantify the magnitude of the dawn phenomenon, researchers subtracted nocturnal nadir glucose (between 0:00 and 6:00 a.m.) from prebreakfast glucose using data collected through frequent glucose monitoring or ambulatory CGM.1,4,12,13 As the dawn phenomenon is characterized by glucose excursions, we hypothesized that glucose excursions would be associated with the dawn phenomenon in patients with T2D. In this study, we investigated the association between glucose excursions and the dawn phenomenon, and assessed the effects of oral glucose lowering drugs on the dawn phenomenon in patients with T2D.
Methods
In this study, a post hoc analysis was conducted using data from a previous randomized trial [ClinicalTrials.gov identifier: NCT00417729].14 The study protocol was approved by the Institutional Review Board of Taichung Veterans General Hospital, Taichung, Taiwan (approval number C06211). The study was conducted in accordance with the Declaration of Helsinki, and all participants provided written informed consent. Details of the study design and the primary results were reported previously.14 Briefly, patients with T2D who had an HbA1c 7.0–11.0% on one or two oral glucose-lowering drugs were enrolled. They were treated with metformin 1500 mg daily for 8 weeks, followed by randomization to add-on acarbose or glibenclamide for 16 weeks. The initial dosage of glibenclamide was 2.5 mg thrice daily for 4 weeks, followed by up-titration to 5 mg thrice daily for 12 weeks.14 Six patients could not tolerate dose titration due to concern or symptoms of hypoglycemia. The final mean dosage of glibenclamide was 13.0 ± 3.4 mg/day. Before randomization and after treatment with acarbose or glibenclamide for 16 weeks, an ambulatory CGM was conducted for the assessment of glucose excursions. In this study, we investigated the association between glucose excursions and the dawn phenomenon determined using data from the CGM.
Ambulatory continuous glucose measurements were conducted using a Medtronic MiniMed CGM system (Northridge, CA, USA) before randomization and at the end of the study.14,15 Patients were instructed to calibrate the system using capillary blood glucose testing, and to mark the time when they ate meals. Glucose excursions were measured and expressed as mean amplitude of glycemic excursions (MAGE), as previously reported.16 Insulin resistance and β-cell function were assessed using the homeostasis model assessment (HOMA-IR and HOMA-β, respectively).17 HOMA-IR = fasting insulin (μU/l) × fasting glucose (mmol/l)/22.5. HOMA-β = 20 × fasting insulin (μU/l)/[fasting glucose (mmol/l)–3.5].
To determine the magnitude of the dawn phenomenon, a nadir glucose level during the nocturnal period (0:00–6:00 a.m.) was identified using the CGM data. The magnitude of the dawn phenomenon was then calculated as the difference between the nocturnal nadir and prebreakfast glucose level (Figure 1).12,13 To avoid the possible confounding effect of nocturnal hypoglycemia,11 we did not assess the dawn phenomenon on days with nocturnal hypoglycemia (CGM reading <70 mg/dl during 0:00–6:00 a.m.). The mean value of eligible dawn phenomenon for each patient was used for analyses.
Figure 1.
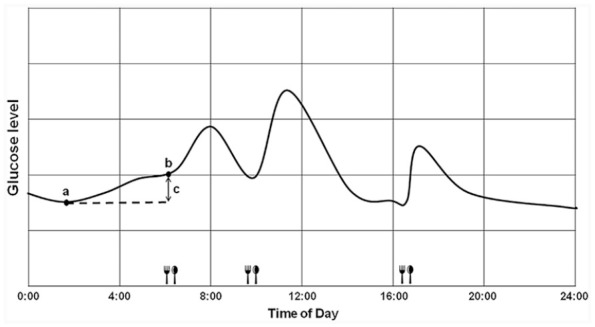
Schematic representation of calculation of the dawn phenomenon (difference between nocturnal nadir and pre-breakfast glucose levels) using data from CGM. (a) Nocturnal nadir. (b) Pre-breakfast. (c) Dawn phenomenon.
CGM, continuous glucose monitoring.
Statistical analyses
All statistical analyses were conducted using the Statistical Package for the Social Sciences (IBM SPSS version 22.0; International Business Machines Corporation, Armonk, NY, USA). Categorical and continuous data are expressed as numbers (percentages) and mean ± standard deviation (SD), respectively. To examine the association between MAGE and the dawn phenomenon, a linear regression analysis was used with adjustment for age, sex, duration of diabetes, body mass index, HOMA-β, and HOMA-IR. To determine the statistical differences in variables between baseline and after treatment, a paired Student’s t test was used. A two-sided p value of less than 0.05 was considered statistically significant.
Results
Table 1 shows the baseline characteristics of the study population. A total of 50 patients with T2D [mean age 53.5 ± 8.2 years, female 52.0%, mean body mass index (BMI) 25.6 ± 3.4 kg/m2] were analyzed. The mean duration of CGM was 3.1 ± 0.6 days. A total of 13 episodes of nocturnal glucose <70 mg/dl with no symptoms were identified (5 at baseline, 8 at the end of study, and 5 were treated with glibenclamide), and dawn phenomenon was not assessed on the day with nocturnal glucose <70 mg/dl. All the patients had uncontrolled glycemia (mean fasting plasma glucose 153.8 ± 40.9 mg/dl, mean HbA1c 8.4 ± 1.2%) on metformin monotherapy. Data from CGM also revealed poor glycemic control (mean glucose 174.3 ± 43.8 mg/dl, percentage of time in glucose range 70–180 mg/dl 61.5 ± 28.2 %), significant glucose excursions (MAGE 105.3 ± 39.3 mg/dl), and the dawn phenomenon (35.9 ± 17.9 mg/dl) in the study population.
Table 1.
Baseline characteristics of the study participants. Data are presented as mean ± SD or numbers (percentages).
| Number of patients | 50 |
| Age, years | 53.5 ± 8.2 |
| Female, n (%) | 26 (52.0) |
| BMI, kg/m2 | 25.6 ± 3.4 |
| Duration of diabetes, years | 6.8 ± 4.6 |
| Fasting plasma glucose, mg/dl | 153.8 ± 40.9 |
| HbA1c, % | 8.4 ± 1.2 |
| HOMA-IR | 3.7 ± 2.9 |
| HOMA-β | 45.1 ± 40.8 |
| Parameters from continuous glucose monitoring | |
| Mean glucose level, mg/dl | 174.3 ± 43.8 |
| Percentage of time in glucose range >180 mg/dl, % | 37.9 ± 28.6 |
| Percentage of time in glucose range 70–180 mg/dl, % | 61.5 ± 28.2 |
| Percentage of time in glucose range <70 mg/dl, % | 0.6 ± 1.6 |
| Mean amplitude of glycemic excursions, mg/dl | 105.3 ± 39.3 |
| Prebreakfast glucose level, mg/dl | 159.2 ± 40.6 |
| Nocturnal nadir glucose level, mg/dl | 123.9 ± 40.7 |
| Dawn phenomenon, mg/dl | 35.9 ± 17.9 |
BMI, body mass index; HbA1c, glycosylated hemoglobin; HOMA, homeostasis model assessment; IR, insulin resistance; SD, standard deviation.
Figure 2 displays the association between glucose excursions and the dawn phenomenon. We observed a significant association between MAGE and the dawn phenomenon (r = 0.424, p = 0.002) in the study population. We then examined whether the association was independent of age, sex, BMI, duration of diabetes, and HOMA-β/HOMA-IR. As shown in Table 2, the association between MAGE and the dawn phenomenon (β coefficient 0.193, 95% CI 0.073–0.313, p = 0.002) remained significant after adjustment for the aforementioned parameters (β coefficient 0.199, 95% CI 0.074–0.325, p = 0.003). The findings were similar when we replaced MAGE using glucose standard deviation (β coefficient 0.526, 95% CI 0.177–0.875, p = 0.004) or coefficient of variation (β coefficient 1.081, 95% CI 0.466–1.696, p = 0.001) of the CGM readings.
Figure 2.
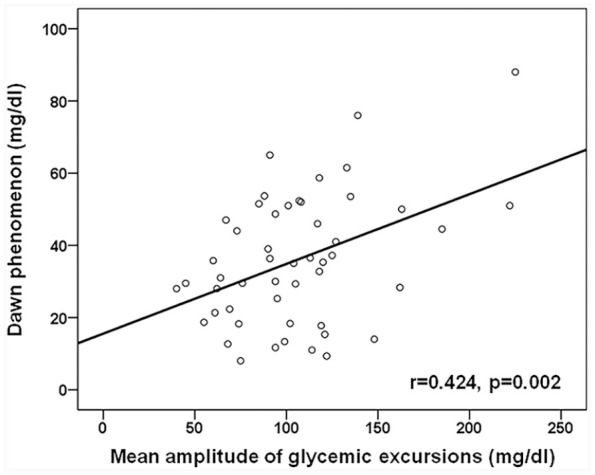
The association of MAGE with the dawn phenomenon in the study population.
MAGE, mean amplitude of glycemic excursions.
Table 2.
Linear regression analysis with the dawn phenomenon as the dependent variable.
| Independent variable | β coefficient | 95% CI | p |
|---|---|---|---|
| MAGE (mg/dl) | |||
| Model 1a | 0.193 | 0.073, 0.313 | 0.002 |
| Model 2b | 0.193 | 0.071, 0.316 | 0.003 |
| Model 3c | 0.190 | 0.067, 0.313 | 0.003 |
| Model 4d | 0.190 | 0.063, 0.317 | 0.004 |
| Model 5e | 0.199 | 0.074, 0.325 | 0.003 |
Unadjusted.
Adjusted for age and sex.
Adjusted for variables in model 2 plus body mass index and duration of diabetes.
Adjusted for variables in model 3 plus HOMA-β.
Adjusted for variables in model 3 plus HOMA-IR.
HOMA, homeostasis model assessment; IR, insulin resistance; MAGE, mean amplitude of glycemic excursions.
Table 3 shows the treatment effects on glycemic parameters by treatment allocation. Both fasting plasma glucose and HbA1c significantly improved after randomization to acarbose (n = 27) or glibenclamide (n = 23) for 16 weeks. However, only treatment with acarbose significantly improved glucose excursions (MAGE from 100.4 ± 27.8–71.4 ± 25.2 mg/dl, p < 0.001). Similar findings were noted for changes in the SD and coefficient of variation of the CGM readings. These results were supported by the 24-h glucose profiles in Figure 3. Regarding the treatment effects on the dawn phenomenon, both acarbose and glibenclamide decreased prebreakfast glucose levels, but only glibenclamide significantly decreased nocturnal nadir glucose levels. As a result, the dawn phenomenon significantly decreased only in patients treated with acarbose (from 35.9 ± 15.7–28.3 ± 16.5 mg/dl, p = 0.037), but not in those treated with glibenclamide (from 35.9 ± 20.6–34.6 ± 17.0 mg/dl, p = 0.776, Table 3).
Table 3.
Treatment effects on glycemic parameters by treatment allocation. Data are presented as mean ± SD.
| Variable | Acarbose (n = 27) |
p | Glibenclamide (n = 23) |
p | ||
|---|---|---|---|---|---|---|
| Baseline | After treatment | Baseline | After treatment | |||
| Fasting plasma glucose, mg/dl | 146.9 ± 23.7 | 132.1 ± 22.3 | 0.012 | 161.9 ± 54.1 | 130.3 ± 38.1 | <0.001 |
| Change from baseline | −14.8 ± 28.6 | −31.6 ± 36.2 | 0.202** | |||
| HbA1c, % | 8.2 ± 0.8 | 7.5 ± 0.8 | <0.001 | 8.6 ± 1.6 | 7.4 ± 1.2 | <0.001 |
| Change from baseline | −0.7 ± 0.7 | −1.2 ± 0.8 | 0.025** | |||
| Parameters from CGM | ||||||
| Mean glucose level, mg/dl | 165.0 ± 29.6 | 139.4 ± 24.8 | 0.001 | 185.2 ± 54.7 | 148.9 ± 35.6 | <0.001 |
| Change from baseline | −25.5 ± 34.4 | −36.3 ± 42.1 | 0.514** | |||
| % of time in glucose range >180 mg/dl | 32.0 ± 22.9 | 14.9 ± 19.6 | 0.006 | 44.8 ± 33.4 | 25.7 ± 22.3* | 0.002 |
| Change from baseline | −17.1 ± 29.5 | −19.0 ± 26.1 | 0.763** | |||
| % of time in glucose range 70–180 mg/dl | 67.6 ± 22.7 | 83.0 ± 19.4 | 0.013 | 54.3 ± 32.6 | 69.7 ± 21.1* | 0.009 |
| Change from baseline | 15.4 ± 30.1 | 15.4 ± 25.8 | 0.633** | |||
| % of time in glucose range < 70 mg/dl | 0.4 ± 1.0 | 2.1 ± 4.8 | 0.074 | 0.9 ± 2.0 | 4.6 ± 6.5 | 0.007 |
| Change from baseline | 1.7 ± 4.8 | 3.7 ± 5.9 | 0.266** | |||
| MAGE, mg/dl | 100.4 ± 27.8 | 71.4 ± 25.2 | <0.001 | 111.0 ± 49.7 | 113.0 ± 42.0* | 0.821 |
| Change from baseline | −29.0 ± 36.5 | 2.0 ± 42.7 | 0.007** | |||
| CGM standard deviation, mg/dl | 39.7 ± 9.6 | 29.3 ± 10.4 | <0.001 | 45.3 ± 17.5 | 47.5 ± 17.6* | 0.497 |
| Change from baseline | −10.4 ± 12.6 | 2.2 ± 15.1 | 0.001** | |||
| CGM coefficient of variation, % | 24.2 ± 4.9 | 21.3 ± 7.8 | 0.088 | 25.3 ± 10.1 | 32.0 ± 10.0* | 0.003 |
| Change from baseline | −2.9 ± 8.4 | 6.7 ± 9.4 | <0.001** | |||
| Prebreakfast glucose level, mg/dl | 153.9 ± 35.5 | 136.6 ± 25.4 | 0.017 | 165.3 ± 45.9 | 128.7 ± 36.3 | <0.001 |
| Change from baseline | −17.3 ± 35.3 | −36.7 ± 34.0 | 0.027** | |||
| Nocturnal nadir glucose level, mg/dl | 118.7 ± 33.0 | 108.9 ± 30.4 | 0.265 | 130.0 ± 48.2 | 94.9 ± 32.6 | <0.001 |
| Change from baseline | −9.8 ± 44.5 | −35.2 ± 33.1 | 0.007** | |||
| Dawn phenomenon, mg/dl | 35.9 ± 15.7 | 28.3 ± 16.5 | 0.037 | 35.9 ± 20.6 | 34.6 ± 17.0 | 0.776 |
| Change from baseline | −7.5 ± 17.8 | −1.3 ± 21.8 | 0.104** | |||
p < 0.05 versus the Acarbose group, **p value compared with changes from baseline in the Acarbose group.
CGM, continuous glucose monitoring; HbA1c, glycosylated hemoglobin; MAGE, mean amplitude of glycemic excursions; SD, standard deviation.
Figure 3.
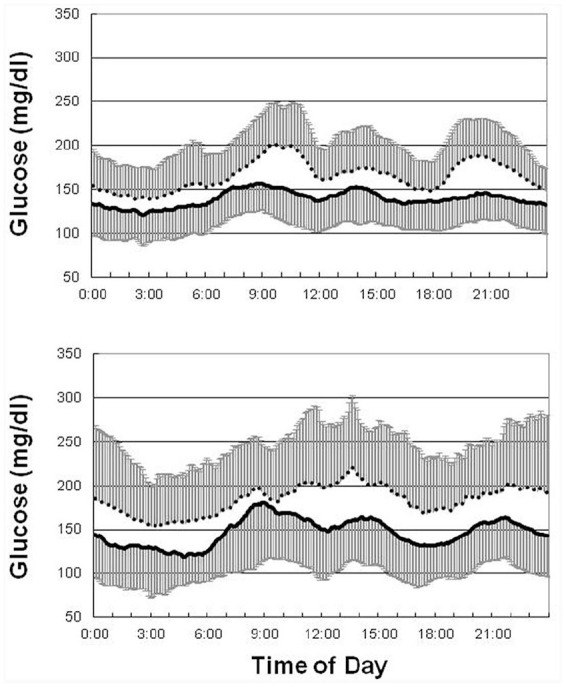
Mean 24-h glucose profiles before (dashed line) and after (solid line) treatment with acarbose (upper panel) and glibenclamide (lower panel). The error bar denotes 1 SD.
SD, standard deviation.
Discussion
Using CGM data, we demonstrated that glucose excursions were associated independently with the dawn phenomenon in patients with poorly controlled T2D on metformin monotherapy (Figure 2 and Table 2). Moreover, the dawn phenomenon decreased significantly in patients randomized to receive acarbose, but not in those randomized to receive glibenclamide (Table 3). The dawn phenomenon is an important part of diurnal glycemic control in patients with T2D.4,6,10 The significant association between glycemic excursions and the dawn phenomenon, and the treatment effects of oral glucose-lowering drugs on the dawn phenomenon in this study are clinically relevant.
It is reasonable to suppose that glucose excursions would be associated significantly with the dawn phenomenon. The definition of the dawn phenomenon and the method we used to define the dawn phenomenon (the difference between the nocturnal glucose nadir and prebreak-fast glucose level) are both involve glucose excursions.1,4,12,13 Our finding was in line with the results recently reported by Li et al.4 In their study, which used data from CGM, patients with the dawn phenomenon had a greater diurnal glucose coefficient of variation and SD than those without the dawn phenomenon.4 The impact of the dawn phenomenon on diurnal glycemic control in patients with T2D has also been reported.6 Nevertheless, data on the effects of oral glucose-lowering drugs on the dawn phenomenon in patients with T2D are limited.6,12 Glucose excursions in patients with T2D could be improved after treatment with acarbose.14 Thus, it is perhaps not surprising that the dawn phenomenon decreased significantly after treatment with acarbose (Table 3) in this study. In support of our findings, dipeptidyl peptidase-4 inhibitors have been reported to improve both glucose excursions and the dawn phenomenon.12,18
Our observation that treatment with sulfonylurea had no effect on the dawn phenomenon (Table 3) is interesting. The dawn phenomenon has been attributed to inadequate insulin action (due to insulin resistance and/or impaired β-cell function) in response to a nocturnal surge of counter-regulatory hormones (growth hormone, cortisol, and catecholamines) in patients with abnormal glucose regulation.1–3,7–10,19–21 As a result, provision of basal insulin has been considered as an optimal treatment to dampen the dawn phenomenon.5,10,22–24 Nevertheless, a stable and peakless insulin action profile is required.10,25 Insulin secretion after treatment with a sulfonylurea26 is not as stable as treatment with basal insulin. Both nocturnal nadir and prebreakfast glucose levels significantly decreased after treatment with glibenclamide (Table 3). However, there was no significant change in the magnitude of the dawn phenomenon. Taken together, our results suggest that treatment with sulfonylurea has no effect on the dawn phenomenon, while treatment with acarbose significantly improved glucose excursions and the dawn phenomenon in T2D patients with poor glycemic control on metformin monotherapy.
There were several limitations to this study. First, this was a post hoc analysis of a randomized trial with a relatively small sample size. Moreover, the mean duration of CGM was only 3.1 ± 0.6 days. Our findings need to be validated in future studies with a larger number of patients and a longer duration of CGM. Second, the extrapolation of our findings to other diabetes populations may be limited by the inclusion and exclusion criteria of the trial. Third, the effect of nocturnal hypoglycemia might not be completely excluded, although we did not assess dawn phenomenon on the day with nocturnal glucose <70 mg/dl. With these limitations in mind, our findings provide novel insight with respect to the effects of oral glucose-lowering drugs on the dawn phenomenon in patients with T2D. Choosing an oral glucose-lowering drug capable of improving glucose excursions may be considered for T2D patients with inadequate glycemic control and the dawn phenomenon. In such patients, treatment with a sulfonylurea may not be appropriate as it might increase the risk of nocturnal hypoglycemia, which may exaggerate the magnitude of glucose increases in the early morning period.
Conclusion
In summary, we demonstrated that glucose excursions were independently associated with the dawn phenomenon in patients with T2D on metformin monotherapy. Both glucose excursions and the dawn phenomenon improved after treatment with acarbose, but not after treatment with glibenclamide.
Footnotes
Author contributions: Conception and design: JSW, WJL, and WHHS. Acquisition of data: ITL, SDL, SLS, STT, and SYL. Analysis and interpretation of data: JSW, WJL, and WHHS. First draft of the manuscript: JSW, WJL, and SDL. Critical revision for intellectual content: ITL, SLS, STT, SYL, and WHHS. Final approval of the version to be published: all authors.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Taichung Veterans General Hospital, Taichung, Taiwan (TCVGH-YM1070102 and TCVGH-1073505C, 2018; TCVGH-1083505C, 2019). The funder was not involved in the study design, data collection, analysis, interpretation of the results, or preparation of the article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Jun-Sing Wang  https://orcid.org/0000-0002-0887-6432
https://orcid.org/0000-0002-0887-6432
Contributor Information
Jun-Sing Wang, Department of Medicine, School of Medicine, National Yang Ming Chiao Tung University, Taipei Rong Hsing Research Center for Translational Medicine, Institute of Biomedical Science, College of Life Science, National Chung Hsing University, Taichung Ph.D. Program in Translational Medicine, National Chung Hsing University, Taichung; Division of Endocrinology and Metabolism, Department of Internal Medicine, Taichung Veterans General Hospital, #1650, Sec. 4, Taiwan Boulevard, Taichung, 407.
I-Te Lee, Division of Endocrinology and Metabolism, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung Department of Medicine, School of Medicine, National Yang Ming Chiao Tung University, Taipei Department of Medicine, School of Medicine, Chung Shan Medical University, Taichung.
Wen-Jane Lee, Department of Medical Research, Taichung Veterans General Hospital, Taichung.
Shi-Dou Lin, Division of Endocrinology and Metabolism, Department of Internal Medicine, Chang-Hua Christian Hospital, Chang-Hua.
Shih-Li Su, Division of Endocrinology and Metabolism, Department of Internal Medicine, Chang-Hua Christian Hospital, Chang-Hua.
Shih-Te Tu, Division of Endocrinology and Metabolism, Department of Internal Medicine, Chang-Hua Christian Hospital, Chang-Hua.
Shih-Yi Lin, Division of Endocrinology and Metabolism, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung Department of Medicine, School of Medicine, National Yang Ming Chiao Tung University, Taipei.
Wayne Huey-Herng Sheu, Division of Endocrinology and Metabolism, Taipei Veterans General Hospital, Taipei Department of Medicine, School of Medicine, National Yang Ming Chiao Tung University, Taipei Institute of Medical Technology, College of Life Science, National Chung Hsing University, Taichung.
References
- 1.Schmidt MI, Hadji-Georgopoulos A, Rendell M, et al. The dawn phenomenon, an early morning glucose rise: implications for diabetic intraday blood glucose variation. Diabetes Care 1981; 4: 579–585. [DOI] [PubMed] [Google Scholar]
- 2.Campbell PJ, Bolli GB, Cryer PE, et al. Pathogenesis of the dawn phenomenon in patients with insulin-dependent diabetes mellitus. Accelerated glucose production and impaired glucose utilization due to nocturnal surges in growth hormone secretion. N Engl J Med 1985; 312: 1473–1479. [DOI] [PubMed] [Google Scholar]
- 3.Bolli GB, Gerich JE.The “dawn phenomenon”–a common occurrence in both non-insulin-dependent and insulin-dependent diabetes mellitus. N Engl J Med 1984; 310: 746–750. [DOI] [PubMed] [Google Scholar]
- 4.Li C, Ma X, Yin J, et al. The dawn phenomenon across the glycemic continuum: implications for defining dysglycemia. Diabetes Res Clin Pract 2020; 166: 108308. [DOI] [PubMed] [Google Scholar]
- 5.King AB, Clark D, Wolfe GS.Contribution of the dawn phenomenon to the fasting and postbreakfast hyperglycemia in type 1 diabetes treated with once-nightly insulin glargine. Endocr Pract 2012; 18: 558–562. [DOI] [PubMed] [Google Scholar]
- 6.Monnier L, Colette C, Dejager S, et al. Magnitude of the dawn phenomenon and its impact on the overall glucose exposure in type 2 diabetes: is this of concern? Diabetes Care 2013; 36: 4057–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bright GM, Melton TW, Rogol AD, et al. Failure of cortisol blockade to inhibit early morning increases in basal insulin requirements in fasting insulin-dependent diabetics. Diabetes 1980; 29: 662–664. [DOI] [PubMed] [Google Scholar]
- 8.Skor DA, White NH, Thomas L, et al. Examination of the role of the pituitary-adrenocortical axis, counterregulatory hormones, and insulin clearance in variable nocturnal insulin requirements in insulin-dependent diabetes. Diabetes 1983; 32: 403–407. [DOI] [PubMed] [Google Scholar]
- 9.Geffner ME, Frank HJ, Kaplan SA, et al. Early-morning hyperglycemia in diabetic individuals treated with continuous subcutaneous insulin infusion. Diabetes Care 1983; 6: 135–139. [DOI] [PubMed] [Google Scholar]
- 10.Porcellati F, Lucidi P, Bolli GB, et al. Thirty years of research on the dawn phenomenon: lessons to optimize blood glucose control in diabetes. Diabetes Care 2013; 36: 3860–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolli GB, Gottesman IS, Campbell PJ, et al. Glucose counterregulation and waning of insulin in the Somogyi phenomenon (posthypoglycemic hyperglycemia). N Engl J Med 1984; 311: 1214–1219. [DOI] [PubMed] [Google Scholar]
- 12.Monnier L, Colette C, Dejager S, et al. “Mild dysglycemia” in type 2 diabetes: to be neglected or not? J Diabetes Complications 2015; 29: 451–458. [DOI] [PubMed] [Google Scholar]
- 13.Monnier L, Colette C, Dejager S, et al. The dawn phenomenon in type 2 diabetes: how to assess it in clinical practice? Diabetes Metab 2015; 41: 132–137. [DOI] [PubMed] [Google Scholar]
- 14.Wang JS, Lin SD, Lee WJ, et al. Effects of acarbose versus glibenclamide on glycemic excursion and oxidative stress in type 2 diabetic patients inadequately controlled by metformin: a 24-week, randomized, open-label, parallel-group comparison. Clin Ther 2011; 33: 1932–1942. [DOI] [PubMed] [Google Scholar]
- 15.Mastrototaro J.The MiniMed Continuous Glucose Monitoring System (CGMS). J Pediatr Endocrinol Metab 1999; 12: 751–758. [PubMed] [Google Scholar]
- 16.Service FJ, Molnar GD, Rosevear JW, et al. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 1970; 19: 644–655. [DOI] [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 18.Rizzo MR, Barbieri M, Marfella R, et al. Reduction of oxidative stress and inflammation by blunting daily acute glucose fluctuations in patients with type 2 diabetes: role of dipeptidyl peptidase-IV inhibition. Diabetes Care 2012; 35: 2076–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perriello G, De Feo P, Torlone E, et al. Nocturnal spikes of growth hormone secretion cause the dawn phenomenon in type 1 (insulin-dependent) diabetes mellitus by decreasing hepatic (and extrahepatic) sensitivity to insulin in the absence of insulin waning. Diabetologia 1990; 33: 52–59. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt MI, Lin QX, Gwynne JT, et al. Fasting early morning rise in peripheral insulin: evidence of the dawn phenomenon in nondiabetes. Diabetes Care 1984; 7: 32–35. [DOI] [PubMed] [Google Scholar]
- 21.Bolli GB, De Feo P, De Cosmo S, et al. Demonstration of a dawn phenomenon in normal human volunteers. Diabetes 1984; 33: 1150–1153. [DOI] [PubMed] [Google Scholar]
- 22.Boden G, Chen X, Urbain JL.Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes 1996; 45: 1044–1050. [DOI] [PubMed] [Google Scholar]
- 23.Perriello G, Pampanelli S, Del Sindaco P, et al. Evidence of increased systemic glucose production and gluconeogenesis in an early stage of NIDDM. Diabetes 1997; 46: 1010–1016. [DOI] [PubMed] [Google Scholar]
- 24.Radziuk J, Pye S.Diurnal rhythm in endogenous glucose production is a major contributor to fasting hyperglycaemia in type 2 diabetes. Suprachiasmatic deficit or limit cycle behaviour? Diabetologia 2006; 49: 1619–1628. [DOI] [PubMed] [Google Scholar]
- 25.Owens DR, Bolli GB.Beyond the era of NPH insulin–long-acting insulin analogs: chemistry, comparative pharmacology, and clinical application. Diabetes Technol Ther 2008; 10: 333–349. [DOI] [PubMed] [Google Scholar]
- 26.Riefflin A, Ayyagari U, Manley SE, et al. The effect of glibenclamide on insulin secretion at normal glucose concentrations. Diabetologia 2015; 58: 43–49. [DOI] [PubMed] [Google Scholar]


