Abstract
Until recently, there had been little attempt in the literature to identify and quantify the underlying mechanics of tooth durability in terms of materials engineering concepts. In humans and most mammals, teeth must endure a lifetime of sustained occlusal mastication—they have to resist fracture and wear. It is well documented that teeth are resilient, but what are the unique features that make this possible? The present article surveys recent materials engineering research aimed at addressing this fundamental question. Elements that determine the mechanics and micromechanics of tooth fracture and wear are analysed: at the macrostructural level, the geometry of the enamel shell and cuspal configuration; and at the microstructural level, interfacial weakness and property gradients. Inferences concerning dietary history in relation to evolutionary pressures are discussed.
Keywords: tooth enamel, cusps, decussation, interfacial weakness, fracture, wear
1. Introduction
The teeth of humans and other primates have to break down a wide range of foods over millions of chewing cycles, often at high bite forces. They are subject to degradation from fracture and wear, among other attacks from disease and chemical erosion. But for the most part, they manage to survive. While it is well appreciated that teeth owe that resilience to their unique structure [1–5], the underlying materials features responsible for this longevity are only recently coming to light. The issue is critical to inferring dietary history and associated implications concerning hominin evolution. Fracture and wear markings can be regarded as diagnostic ‘foodprints’ [6–10].
In the current article, we explore the mechanical properties of tooth enamel from a materials engineering perspective. We begin with consideration of fracture at both the microscopic and macroscopic levels. The fundamental manner in which the basic enamel microstructure acts to constrain cracks in humans and like primates is first detailed. This is followed by analysis of the way fracture patterns develop around the outer tooth walls, treating the enamel structure as a dome-like shell, with or without occlusal cusps. Complementary in vitro laboratory testing on extracted teeth and computer modelling are used to place the essential fracture mechanics on a sound analytical footing. Wear is then considered, with distinction between macrowear and microwear. How natural wear patterns may be simulated by simple indentation tests on tooth specimens is analysed in terms of micromechanical contact models. Attention is given to the role of hard particulates in the food source, exogenous silica grit and endogenous phytoliths. Finally, how a materials approach may elucidate aspects of hominin evolution is discussed.
2. Fracture
2.1. Microstructure
Tooth enamel in humans and other primates has a complex, hierarchical structure [2]. At the microstructural scale, it consists of tightly packed mineral prisms of diameter approximately 5 µm. Analysis of crack path deflections in figure 1a around a contact site made using a conical indenter with rounded tip on the surface of a human tooth specimen (inset) indicates that the prisms are weakly bound, with interfacial bonding energy about one-half that of bulk hydroxyapatite—i.e. sufficiently strong to bond the structure but sufficiently weak to provide easy crack and slip paths [11,12]. In the teeth of many primates, the prisms are aligned with their long axis perpendicular to the occlusal surface in the outer enamel, but are intertwined in a ‘decussation’ pattern in the inner regions. Wavy crack-like ‘tufts’ of the kind illustrated in figure 1b emanate from the dento-enamel junction (DEJ) into the enamel along inter-prism boundaries [13,14]. These tufts are partially healed with intrusive proteinaceous matter [15], but can nevertheless act as sources of macrocrack initiation [16]. Their waviness reflects crack path disruption within the decussation zone. Enamel in primates with low-crowned (bunodont) dentition also contains outer-surface longitudinal fissures running between occlusal surface and margin [9]. Remarkably, despite all these defects, teeth generally remain functional—they are damage-tolerant. Inter-prism weakness [11] and through-thickness property gradients [17,18], the former directing any inward intruding cracks along disruptive bifurcating paths within the decussation zone and the latter confining the cracks to the outer surface, act in concert to confer durability on the tooth assembly.
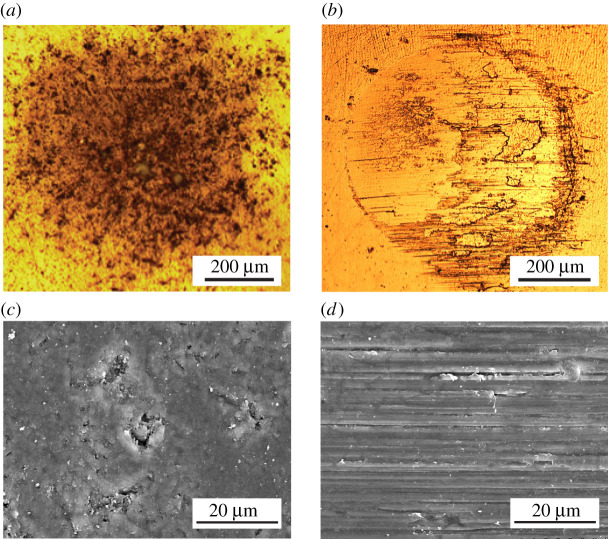
Figure 1.
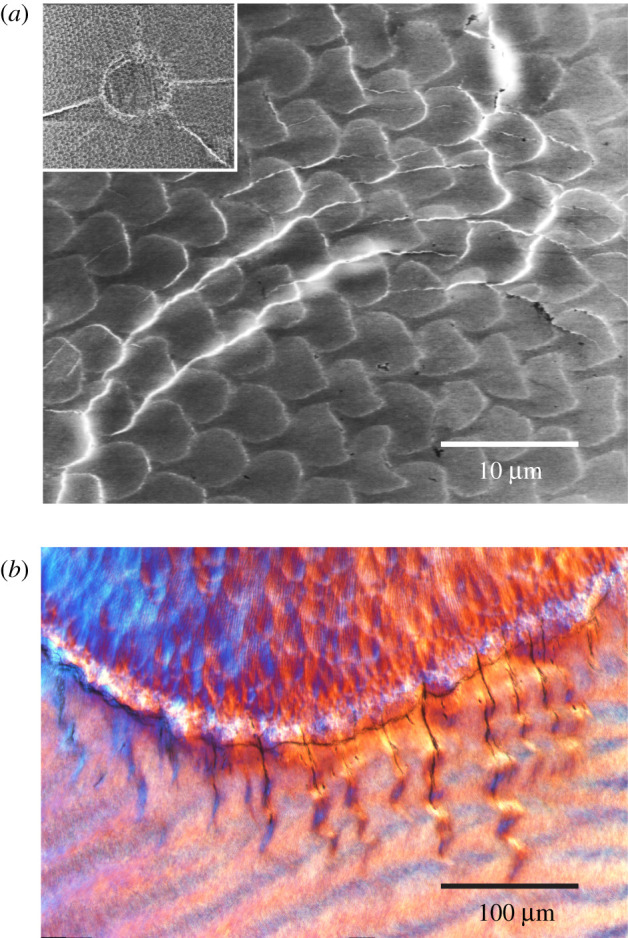
(a) Crack paths at indentation on polished enamel plate from human molar tooth with conical indenter of tip radius r = 200 µm at load P = 20 N. Inset shows the indentation zone. Note deflections around some of the protruding prisms. After [11]. (b) Tufts penetrating downwards from DEJ into enamel. Courtesy Tim Bromage.
2.2. Longitudinal fracture
Longitudinal cracks can start from the occlusal surface and run down to the margin, or vice versa, depending on the nature of the contacting surface. Hard contacts tend to start cracks from the occlusal surface (radial, R, cracks) [19], soft contacts from the margins (margin, M, cracks) [20]. Which of these two configurations dominates can provide clues to dietary history [9]. Examples of longitudinal fractures are shown in figure 2, R cracks from occlusal loading of an extracted human molar (figure 2a) and M cracks in a gorilla museum specimen (figure 2b). The bite forces needed to generate these cracks can approach or even exceed 1 kN in humans, depending on the tooth dimensions.
Figure 2.
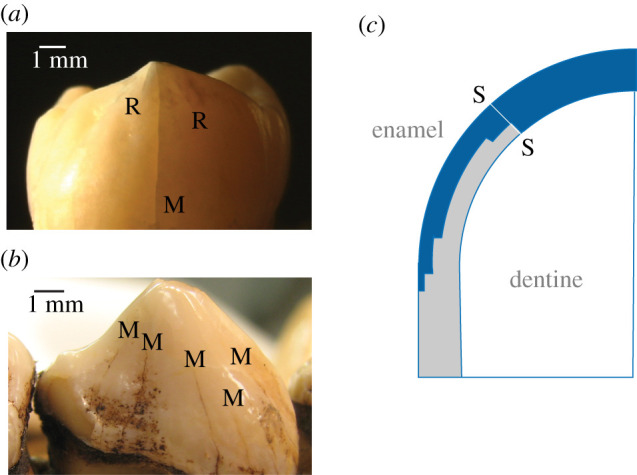
(a) Longitudinal R (radial) and M (margin) cracks in extracted human molar tooth. Tooth loaded at upper cusp with a flat metal plate at load P = 390 N. (b) Multiple M cracks in a museum gorilla tooth. (c) XFEM computed R crack profile in top-surface loaded dome tooth of height 7.5 mm, base radius 5 mm and enamel thickness 1.5 mm, with graded elastic modulus and fracture toughness. S–S is the starter crack. Note confinement of ensuing crack propagation to outer walls of the enamel shell.
Longitudinal fractures can be modelled analytically by conventional fracture mechanics [9,21] or by extended finite-element modelling (XFEM) [22,23]. The simplest models treat the enamel shell as a dome on a cylindrical base. An analytical solution for the fracture force PF to propagate a longitudinal crack completely around the tooth wall gives
| 2.1 |
where T is the mean enamel toughness (crack resistance), R is the dome radius, d is the enamel thickness and C is a constant. Assuming that full development of an R or M crack signals prospective failure by crack coalescence at any ensuing overload, this explicit relation affords a convenient estimate of maximum bite force for any species with bunodont dentition, simply from the tooth dimensions, circumventing the need for elaborate jaw mechanics [9].
XFEM, with inbuilt provision to follow extending cracks step by step, provides essential confirmation of equation (2.1) [22,23]. It also serves to demonstrate that longitudinal crack growth around the tooth wall is highly stable, meaning that the evolution of fracture is incrementally progressive with increasing load and not abrupt. XFEM can also handle gradients in material properties, notably elastic modulus and toughness, through an enamel thickness. An example of such a computation is illustrated in figure 2c, half-section of a top-loaded dome-like tooth with through-thickness property variations obtained from independent in vitro material evaluation testing on human tooth specimens [24]. (In this figure S–S is a ‘starter crack’, emplaced so that the computation can focus on propagation rather than initiation.) The ensuing crack (blue shading) highlights how the fracture runs around the outside walls without penetrating to the DEJ. Again, it is the combined interfacial weakness and property gradient that is responsible for confinement of the crack to the outer enamel surface.
2.3. Cuspal geometry
Another compelling advantage of XFEM is that it can handle geometrical complexity routinely and objectively [22–26]. For instance, it can deal with the variety of occlusal multicusp configurations shown for different primates in figure 3 [25,27]. Tooth morphology can have a strong influence on how food items are processed [28,29]. Figure 4 illustrates how computed R crack depth for a schematic molar of fixed height 7.5 mm, base radius 5 mm and enamel thickness 1.5 mm varies with increasing bite force for these cusp configurations: (a) one cusp loaded, and (b) all cusps loaded. In the case of single cusp loading, the critical load to grow the crack to completion is well below 1 kN, and is insensitive to the number of cusps. If all cusps are loaded equally, the critical load is greatly enhanced, well in excess of 1 kN, the more so the greater the number of cusps. Of course, the latter condition will prevail only with proper occlusion, where the applied bite force is distributed evenly across the tooth surface.
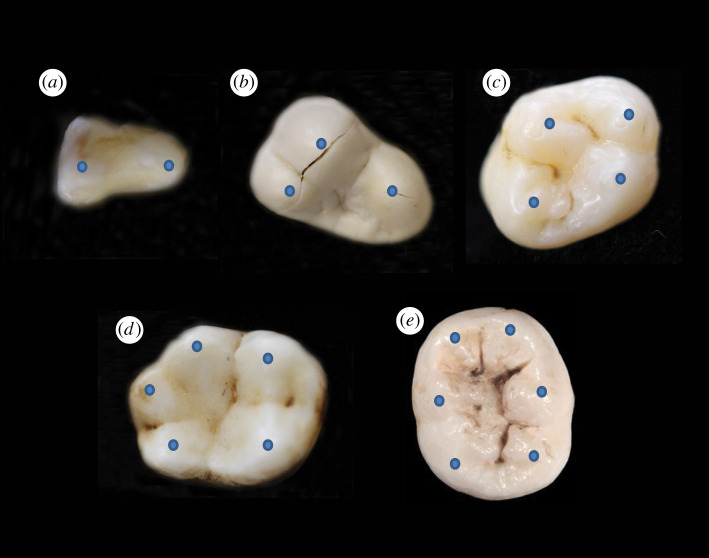
Figure 3.
Cusps on bunodont primate and sea otter teeth [25]. Blue dots highlight the location of cusp apices. (a) Two-cusped slow loris (Nycticebus coucang) premolar; (b) three-cusped sea otter (Enhydra lutris) molar; (c) four-cusped human (Homo sapiens) molar; (d) five-cusped gibbon (Hylobates lar) molar; and (e) six-cusped human (H. sapiens) molar.
Figure 4.
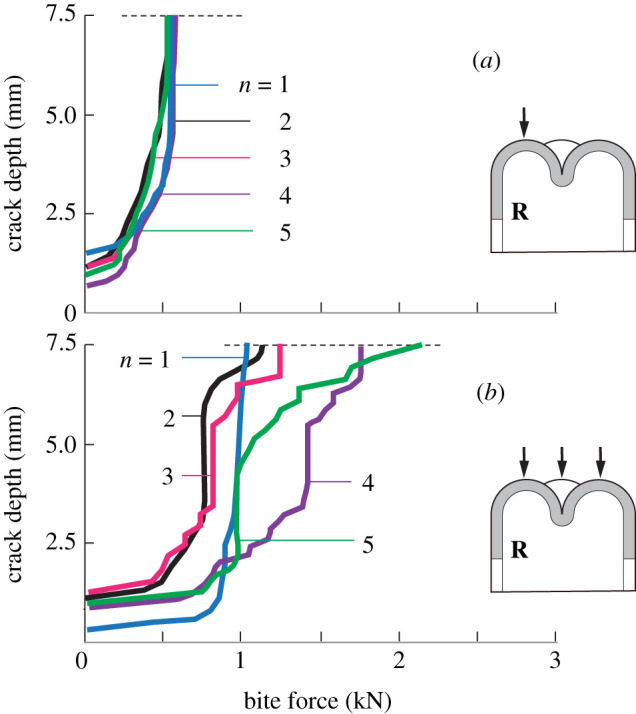
XFEM computed R crack front coordinate measured from the top surface as a function of bite force at occlusal surface, for a tooth with n = 1, 2, 3, 4 and 5 cusps [25]. (a) Single cusp loaded and (b) all cusps loaded.
3. Wear
3.1. Microwear
Microwear refers to the microscopic markings on tooth surfaces left from chewing on food items. Dental microwear has become something of a cottage industry among palaeontologists. This is because widespread microwear patterns on fossil teeth can be employed to infer a considerable amount about dietary habits. Two kinds of microwear patterns are identified: ‘pits’, from axial contact loading; and ‘scratches’, from lateral contact motion. It is widely postulated that pits occur from biting on hard foods such as nuts and seeds, scratches from grinding of plant matter and other soft foods. Distinctive microwear patterns on representative museum specimens of two hominins, Paranthropus robustus [30] and Paranthropus boisei [31], are shown in figure 5a,b, revealing what appear to be distinct dietary habits. However, interpretation of such patterns in various animal species has become a lively topic of debate and conjecture.
Figure 5.
Markings on tooth enamel. (a) Pits (P. robustus) [30] and (b) scratches (P. boisei) [31]. Laboratory simulation of damage made on extracted human molar specimen with a conical diamond indenter of tip radius 25 µm at load 1 N: (c) axial and (d) sliding loading.
Individual microwear traces can be simulated by controlled indentation tests in the laboratory. Examples are shown in figure 5c,d, for axial and sliding contacts on an extracted human molar with a conical diamond indenter of tip radius 25 µm [32]. The wear debris around the contact sites is commonplace in such tests. Thus, even micrometre-scale microcontacts are capable of contributing to cumulative wear. The advantage of such simple simulations is that they are amenable to rigorous contact micromechanics modelling [32,33]. Such modelling can enable evaluation of microcontact forces from widths of individual pit or scratch markings. For instance, for the micrometre-scale markings in figure 5, the forces on any individual microcontact are assessed to be in the range of 1 N. The same modelling can account for the role of indenter sharpness and modulus, thus providing a theoretical basis for predicting likely responses from blunt or sharp, hard or soft food particulates [32,33].
3.2. Macrowear
The progressive manifestation of microwear over time is macrowear. Macrowear can seriously degrade the tooth, in extreme cases exposing the underlying soft dentine tissue [34]. This is not uncommon in grazing herbivores [35], and even humans who grind their teeth (bruxing). Some animals in the wild grind their teeth down to the gumline [36]. The bite forces needed to cause deleterious wear are generally much lower than those to fracture an undamaged tooth, but macrowear is cumulative over a lifetime of continual mastication. Excessive wear can also degrade tooth strength, introducing damage and making them more vulnerable to initiation of longitudinal cracks and edge chipping from unexpected overloads [37].
Macrowear can be simulated by in vitro tribological tests. Again, the simplest way is by indentation with a hard sphere, so-called Hertzian contact [38]. This test can readily be conducted in axial or sliding loading, in either single-cycle or multicycle mode. An example for human enamel is shown in figure 6, for cyclic loading with a hard sphere of radius 6.35 mm at 30 N in aqueous solution: (a) axial and (b) sliding contact in a rotating ‘tribometer’ [33]. Both contact modes produce wear, but the material removal rate is markedly higher in sliding because of the superposition of frictional tractions [39,40]. Higher-magnification images of the wear scars in figure 6c,d show damage somewhat reminiscent of the natural pit and scratch markings in figure 5. There are other, more elaborate wear test arrangements, for instance, using dedicated mouth-motion fatigue machines [41], but data interpretation in those cases is not so straightforward.
Figure 6.
Wear scars on human molar made with hard sphere indenter of radius 6.35 mm at load 30 N in slurry of silica grit (mean grit radius 20 µm): (a) axial and (b) rotational (sliding) loading [33]. Higher-magnification images of wear scars are shown in (c,d). Note basic resemblance in (c,d) to natural pits and scratches in figure 5a,b [32].
Models expressing macrowear rate can be constructed by mathematical summation over all individual microcontact asperities within the occlusion zone [42]. In their most basic form, such models assume each contacting asperity to have the form of a sharp particle of fixed profile, and the volume removed in any one event to scale with the contact impression cross section. For sliding contacts, such an analysis yields the total volume V removed,
| 3.1 |
where P is the net load, L is the sliding distance, H is the enamel hardness and K is a ‘wear coefficient’. This is an expression of the generic ‘Archard's Law’ [43]. The value of K is highly sensitive to test conditions, and can increase significantly at elevated contact loads when cracks initiate around the wear tracks. Given a mean bite force, chewing frequency and dimensions for a given bunodont tooth, equation (3.1) can be manipulated to predict the lifetime for a tooth to wear down to its base [42].
3.3. Silica grit versus phytoliths
Laboratory wear tests confirm that the ubiquitous presence of silicate particles in the food source can rapidly accelerate enamel wear in grazers and browsers. Such tests on human enamel in media with additive particulates demonstrate increases of two or more orders of magnitude in wear rate relative to media without additives [42]. This is reflected as a dramatic increase in K in equation (3.1). It becomes apparent that the exposure of food sources to hard particulates can exert a profound influence on tooth endurance [33].
One topic that has aroused considerable debate in the biological literature is the basic source of any such intrusive particulates. Specifically, the argument has raged as to whether the responsible source is exogenous silica grit in the soil or atmosphere, or endogenous phytoliths in plant matter. Some have argued that phytoliths are too soft to cause significant enamel wear [44,45], others present a counter argument [46,47]. Relative volume removal data in figure 7a from pin-on-disc wear tests on human enamel in artificial saliva with and without each kind of particle additive under otherwise identical conditions reveal that phytoliths are indeed able to effect comparable material removal rates to that of silica grit [48]. The question of the relative abundance of each kind of particle in nature remains open, but the images of wear tracks in figure 7b in specimens tested in pure saliva (S), saliva + phytoliths (S + P) and saliva + silica grit (S + S) confirm the efficacy of phytoliths to produce severe abrasion.
Figure 7.
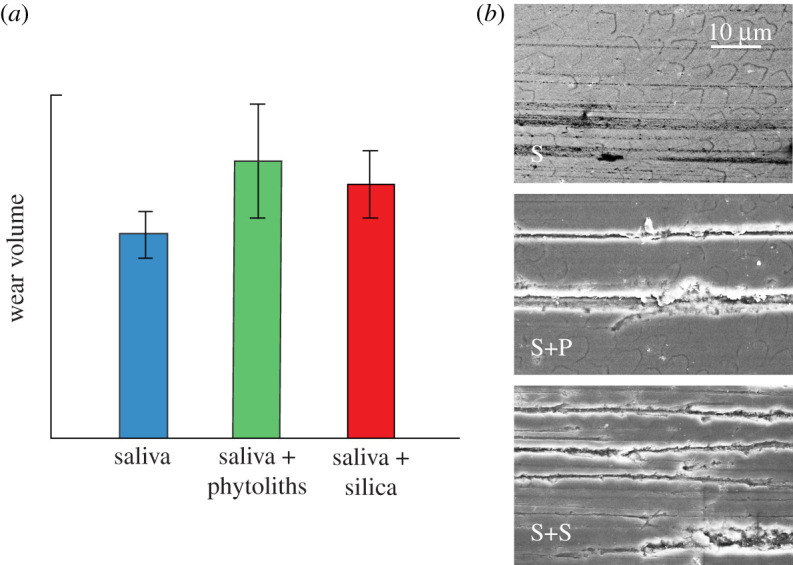
Wear tests on human enamel, using pin-on-disc arrangement with extracted molar as pin and hard ceramic plate as disc [48]. Relative wear volume removed for tests in artificial saliva (S), saliva + phytoliths (S + P) and saliva + silica grit (S + S), for tests at set test duration. Error bars s.d. for five tests per group. (b) Wear scars on specimens tested in S, S + P and S + S media. Note severe wear tracks in the latter two groups.
4. Discussion
Teeth, because of their hardness, comprise the most common component of fossil tissue. Materials engineering analysis of fracture and wear patterns on recovered teeth, using the well-established field of indentation mechanics, can provide useful insights into dietary history of humans, hominins and other primates. It can be useful not only as a diagnostic tool, first in identifying an array of damage modes under various potential mastication conditions, but also in quantifying critical mechanical essentials such as enamel microstructural properties and occlusal bite force, microwear markings and macrowear rate. It can contribute to resolving issues such as the relative roles of exogenous silica grit versus endogenous plant phytoliths in the food source. In short, indentation mechanics affords a fundamental physical (anthroengineering) basis for exploring many aspects of tooth function in the context of evolution.
The question of which form of damage dominates in any given species—fracture or wear—depends largely on the food source. In some primates, especially the males of sexually dimorphic species, the canines used in threat displays and defence are sharp and elongate. Even though they are susceptible to wear at the cusp, canines are more likely to fail by fracture, since they are used intermittently at occasional high axial bite force. Elongation diminishes the prospect of longitudinal cracking, since any such incipient cracks would then have much further to travel to the base [23]. In that case, other forms of cracking, notably transverse fracture from tooth flexure during eating, can take over [10]. In herbivorous primates, chewing is almost continuous, in lateral motion and at comparatively low bite force, so that wear becomes the main threat [35,49]. In omnivorous primates with bunodont dentition, mouth motion is more complex and the food source more varied, so both fracture and wear are potential sources of damage. Apart from longitudinal cracking, chipping at the edges of an occlusal surface can occur; analysis of the chipping force can then be used as another bite force diagnostic [50]. Microwear markings on otherwise intact teeth contain a wealth of information as to diet immediately prior to the demise of an animal.
Ultimately, teeth are resilient because of their unique configuration. On a macroscopic level, for their dome-like structure, transferring the bulk of occlusal compression loading through the enamel shell to the tooth base. Tensile stresses responsible for fracture are minimized (but not totally excluded) in such structures. At a microscopic level, the enamel prism structure confers a degree of toughness, ironically from its inter-prism weakness, ‘fooling’ cracks to follow disruptive paths. Tufts at the DEJ can extend to the outer surface under heavy load, but their growth is restricted and shielded by their neighbours [16]. Elastic modulus gradients across the enamel section serve to contain cracks by redirecting applied stresses to the outer tooth regions. Any inward-growing crack fronts slow as they enter the interior decussation zone, where they bifurcate and subsequently arrest. The same weak interfaces responsible for this bifurcation enable ready deformation beneath microcontacts [12], but at the same time inhibit material removal by virtue of the near-perpendicular, parallel orientation at the enamel outer surface [5,51,52]. Large tooth sizes and thicker enamel combine to mitigate against both fracture and wear. These dental features, along with a relatively large brain, are part of a suite of morphological adaptations that have contributed to the evolutionary success of humans and our primate cousins.
In all, teeth are remarkable structures, indeed built for survival. Contact mechanics is a powerful tool for placing these structural characteristics on a solid physical footing.
Ethics
Approval to conduct this research on human tissue was obtained from the Bioethics Committee of the University of Extremadura (permit number 213//2019).
Data accessibility
No new data were created in this study. Data from previously published articles are referenced herein.
Authors' contributions
All persons who meet authorship criteria are listed as authors, and all authors have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, execution, analysis and writing/revision of the article.
Competing interests
We declare we have no competing interests.
Funding
Funding was provided by the Ministry of Science and Innovation, Spain (grant no. PID2019-105377RB-I00), and Junta de Extremadura, Spain, FEDER/ERDF (grant no. IB16139).
References
- 1.Maas MC, Dumont ER. 1999. Built to last: the structure, function, and evolution of primate dental enamel. Evol. Anthrop. 8, 133-152. () [DOI] [Google Scholar]
- 2.Lucas PW. 2004. Dental functional morphology: how teeth work. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Lucas PW, Constantino PJ, Wood BA, Lawn BR. 2008. Dental enamel as a dietary indicator in mammals. Bioessays 30, 374-385. ( 10.1002/bies.20729) [DOI] [PubMed] [Google Scholar]
- 4.Ungar PS. 2010. Mammal teeth. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 5.Wilmers J, Bargmann S. 2020. Nature's design solutions in dental enamel: uniting high strength and extreme damage resistance. Acta Biomater. 107, 1-24. ( 10.1016/j.actbio.2020.02.019) [DOI] [PubMed] [Google Scholar]
- 6.Walker A, Hoeck HN, Perez L. 1978. Microwear of mammalian teeth as an indicator of diet. Science 201, 908-910. ( 10.1126/science.684415) [DOI] [PubMed] [Google Scholar]
- 7.Walker AC. 1981. Diet and teeth: dietary hypotheses and human evolution. Phil. Trans. R. Soc. Lond. B 292, 57-64. ( 10.1098/rstb.1981.0013) [DOI] [PubMed] [Google Scholar]
- 8.Scott RS, Ungar PS, Bergstrom TS, Brown CA, Childs BE, Teaford MF, Walker A. 2006. Dental microwear texture analysis: technical considerations. J. Hum. Evol. 51, 339-349. ( 10.1016/j.jhevol.2006.04.006) [DOI] [PubMed] [Google Scholar]
- 9.Lee JJ-W, Constantino PJ, Lucas PW, Lawn BR. 2011. Fracture in teeth—a diagnostic for inferring bite force and tooth function. Biol. Rev. 86, 959-974. ( 10.1111/j.1469-185X.2011.00181.x) [DOI] [PubMed] [Google Scholar]
- 10.Lawn BR, Bush MB, Barani A, Constantino P, Wroe S. 2013. Inferring biological evolution from fracture patterns in teeth. J. Theor. Biol. 338, 59-65. ( 10.1016/j.jtbi.2013.08.029) [DOI] [PubMed] [Google Scholar]
- 11.Borrero-Lopez O, Constantino PJ, Bush MB, Lawn BR. 2020. On the vital role of enamel prism interfaces and graded properties in human tooth survival. Biol. Lett. 16, 20200498. ( 10.1098/rsbl.2020.0498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He LH, Swain MV. 2007. Contact induced deformation of enamel. Appl. Phys. Lett. 90, 171 911-171 913. ( 10.1063/1.2731659) [DOI] [Google Scholar]
- 13.Sognnaes RF. 1950. The organic elements of enamel. IV. The gross morphology and the histological relationship of the lamellae to the organic framework of the enamel. J. Dent. Res. 29, 260-269. ( 10.1177/00220345500290030201) [DOI] [PubMed] [Google Scholar]
- 14.Osborn JW. 1969. The 3-dimensional morphology of the tufts in human enamel. Acta Anat. 73, 481-495. ( 10.1159/000143313) [DOI] [PubMed] [Google Scholar]
- 15.Palamara J, Phakey PP, Rachinger WA, Orams HJ. 1989. The ultrastructure of spindles and tufts in human dental enamel. Adv. Dent. Res. 3, 249-257. ( 10.1177/08959374890030022601) [DOI] [PubMed] [Google Scholar]
- 16.Chai H, Lee JJ-W, Constantino PJ, Lucas PW, Lawn BR. 2009. Remarkable resilience of teeth. Proc. Natl Acad. Sci. USA 106, 7289-7293. ( 10.1073/pnas.0902466106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuy JL, Mann AB, Livi KJ, Teaford MF, Weihs TP. 2002. Nanoindentation mapping of the mechanical properties of human molar tooth enamel. Arch. Oral Biol. 7, 281-291. ( 10.1016/S0003-9969(02)00006-7) [DOI] [PubMed] [Google Scholar]
- 18.Lee JJ-W, Morris D, Constantino PJ, Smith TM, Lawn BR. 2010. Properties of tooth enamel in great apes. Acta Biomater. 6, 4560-4565. ( 10.1016/j.actbio.2010.07.023) [DOI] [PubMed] [Google Scholar]
- 19.Qasim T, Bush MB, Hu X, Lawn BR. 2005. Contact damage in brittle coating layers: influence of surface curvature. J. Biomed. Mater. Res. 73B, 179-185. ( 10.1002/jbm.b.30188) [DOI] [PubMed] [Google Scholar]
- 20.Qasim T, Ford C, Bush MB, Hu X, Malament KA, Lawn BR. 2007. Margin failures in brittle dome structures: relevance to failure of dental crowns. J. Biomed. Mater. Res. 80B, 78-85. ( 10.1002/jbm.b.30571) [DOI] [PubMed] [Google Scholar]
- 21.Lawn BR, Lee JJ-W, Chai H. 2010. Teeth: among nature's most durable biocomposites. Annu. Rev. Mater. Res. 40, 55-75. ( 10.1146/annurev-matsci-070909-104537) [DOI] [Google Scholar]
- 22.Barani A, Keown AJ, Bush MB, Lee JJ-W, Chai H, Lawn BR. 2011. Mechanics of longitudinal cracks in tooth enamel. Acta Biomater. 7, 2285-2292. ( 10.1016/j.actbio.2011.01.038) [DOI] [PubMed] [Google Scholar]
- 23.Barani A, Keown AJ, Bush MB, Lee JJ-W, Lawn BR. 2012. Role of tooth elongation in promoting fracture resistance. J. Mech. Behav. Biomed. Mater. 31, 37-46. ( 10.1016/j.jmbbm.2011.11.014) [DOI] [PubMed] [Google Scholar]
- 24.Barani A, Bush MB, Lawn BR. 2012. Effect of property gradients on enamel fracture in human enamel. J. Mech. Behav. Biomed. Mater. 15, 121-130. ( 10.1016/j.jmbbm.2012.06.014) [DOI] [PubMed] [Google Scholar]
- 25.Barani A, Bush MB, Lawn BR. 2014. Role of multiple cusps in tooth fracture. J. Mech. Behav. Biomed. Mater. 35, 85-92. ( 10.1016/j.jmbbm.2014.03.018) [DOI] [PubMed] [Google Scholar]
- 26.Barani A, Chai H, Lawn BR, Bush MB. 2015. Mechanics analysis of molar tooth splitting. Acta Biomater. 15, 237-243. ( 10.1016/j.actbio.2015.01.004) [DOI] [PubMed] [Google Scholar]
- 27.Constantino P, Bush MB, Barani A, Lawn BR. 2016. On the evolutionary advantage of multi-cusped teeth. J. R. Soc. Interface 13, 20160374. ( 10.1098/rsif.2016.0374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berthaume M, Grosse IR, Patel ND, Strait DS, Wood S, Richmond BG. 2010. The effect of early hominin occlusal morphology on the fracturing of hard food items. Anat. Rec. 293, 594-606. ( 10.1002/ar.21130) [DOI] [PubMed] [Google Scholar]
- 29.Berthaume MA, Dumont ER, Godfrey LR, Grosse IR. 2013. How does tooth cusp radius of curvature affect brittle food item processing. J. R. Soc. Interface 10, 20130240. ( 10.1098/rsif.2013.0240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ungar PS, Grine FE, Teaford MF. 2008. Dental microwear and diet of the Plio-Pleistocene hominin Paranthropus boisei. PLoS ONE 3, e2044. ( 10.1371/journal.pone.0002044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grine FE. 1986. Dental evidence for dietary differences in Australopithecus and Paranthropus: a quantitative analysis of permanent molar microwear. J. Hum. Evol. 15, 783-822. ( 10.1016/S0047-2484(86)80010-0) [DOI] [Google Scholar]
- 32.Borrero-Lopez O, Pajares A, Constantino P, Lawn BR. 2015. Mechanics of microwear traces in tooth enamel. Acta Biomater. 14, 146-153. ( 10.1016/j.actbio.2014.11.047) [DOI] [PubMed] [Google Scholar]
- 33.Constantino P, Borrero-Lopez O, Pajares A, Lawn BR. 2016. Simulation of enamel wear for reconstruction of diet and feeding behavior in fossil animals: a micromechanics approach. Bioessays 38, 89-99. ( 10.1002/bies.201500094) [DOI] [PubMed] [Google Scholar]
- 34.Rose JC, Ungar PS. 1998. Gross dental wear and dental microwear in historical perspective. In Dental anthropology: fundamentals, limits and prospects (eds Alt KW, Rosing FW, Teschler-Nicola M), pp. 349-386. Stuttgart, Germany: Springer. [Google Scholar]
- 35.Damuth J, Janis CM. 2014. A comparison of observed molar wear rates in extant herbivorous mammals. Ann. Zool. Fenn. 51, 188-200. ( 10.5735/086.051.0219) [DOI] [Google Scholar]
- 36.Logan M, Sanson GD. 2002. The effects of tooth wear on the feeding behaviour of free-ranging koalas (Phascolarctos cinereus Goldfuss). Aust. J. Zool. 256, 63-69. ( 10.1017/S0952836902000080) [DOI] [Google Scholar]
- 37.Keown A, Bush MB, Ford C, Lee JJ-W, Constantino PJ, Lawn BR. 2012. Fracture susceptibility of worn teeth. J. Mech. Behav. Biomed. Mater. 5, 247-256. ( 10.1016/j.jmbbm.2011.08.028) [DOI] [PubMed] [Google Scholar]
- 38.Lawn BR. 1998. Indentation of ceramics with spheres: a century after Hertz. J. Am. Ceram. Soc. 81, 1977-1994. ( 10.1111/j.1151-2916.1998.tb02580.x) [DOI] [Google Scholar]
- 39.Hamilton GM, Goodman LE. 1966. The stress field created by a circular sliding contact. J. Appl. Mech. 33, 371-376. ( 10.1115/1.3625051) [DOI] [Google Scholar]
- 40.Hamilton GM. 1983. Explicit equations for the stresses beneath a sliding spherical contact. Proc. Inst. Mech. Eng. 197C, 53-59 ( 10.1243/PIME_PROC_1983_197_076_02) [DOI] [Google Scholar]
- 41.DeLong R, Douglas WH. 1983. Development of an artificial oral environment for the testing of dental restoratives: bi-axial force and movement control. J. Dent. Res. 62, 32-36. ( 10.1177/00220345830620010801) [DOI] [PubMed] [Google Scholar]
- 42.Borrero-Lopez O, Pajares A, Constantino P, Lawn BR. 2014. A model for predicting wear rates in tooth enamel. J. Mech. Behav. Biomed. Mater. 37, 226-234. ( 10.1016/j.jmbbm.2014.05.023) [DOI] [PubMed] [Google Scholar]
- 43.Hutchings IM. 1992. Tribology: friction and wear of engineering materials. Boca Raton, FL: CRC Press. [Google Scholar]
- 44.Lucas PW, et al. 2013. Mechanisms and causes of wear in tooth enamel: implications for hominin diets. J. R. Soc. Interface 10, 20120923. ( 10.1098/rsif.2012.0923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lucas PW, et al. 2014. The role of dust, grit and phytoliths in tooth wear. Ann. Zool. Fenn. 51, 143-152. ( 10.5735/086.051.0215) [DOI] [Google Scholar]
- 46.Rabenold D, Pearson OM. 2011. Abrasive, silica phytoliths and the evolution of thick molar enamel in primates, with implications for the diet of Paranthropus boisei. PLoS ONE 6, e28379. ( 10.1371/journal.pone.0028379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rabenold D, Pearson OM. 2014. Scratching the surface: a critique of Lucas et al. (2013)'s conclusion that phytoliths do not abrade enamel. J. Hum. Evol. 74, 130-133. ( 10.1016/j.jhevol.2014.02.001) [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Rojas F, Borrero-Lopez O, Constantino PJ, Henry AG, Lawn BR. 2020. Phytoliths can cause tooth wear. J. R. Soc. Interface 17, 20200613. ( 10.1098/rsif.2020.0613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fortelius M, Solounias N. 2000. Functional characterization of ungulate molars using the abrasion–attrition wear gradient: a new method for reconstructing paleodiets. Am. Mus. Novit. 3301, 1-36. () [DOI] [Google Scholar]
- 50.Constantino PJ, Lee JJ-W, Chai H, Zipfel B, Ziscovici C, Lawn BR, Lucas PW. 2010. Tooth chipping can reveal bite forces and diets of fossil hominins. Biol. Lett. 6, 826-829. ( 10.1098/rsbl.2010.0304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maas MC. 1991. Enamel structure and microwear: an experimental study of the response of enamel to shearing force. Am. J. Phys. Anthropol. 85, 31-49. ( 10.1002/ajpa.1330850106) [DOI] [PubMed] [Google Scholar]
- 52.Zheng J, Zhou ZR, Zhang J, Li H, Yu HY. 2003. On the friction and wear behavior of human tooth enamel and dentin. Wear 255, 967-974. ( 10.1016/S0043-1648(03)00079-6) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created in this study. Data from previously published articles are referenced herein.