Summary
The use of steatotic livers in liver transplantation (LT) is controversial. Ischaemia‐free liver transplantation (IFLT) has obvious advantages for the recovery of allograft function. The aim of this study was to examine the effect of liver grafts with steatosis on outcome and the effect of IFLT with steatotic livers. 360 patients with LT were enrolled in this study. Perioperative characteristics and differences in outcome among different grades of steatotic groups, and between the IFLT and conventional LT (CLT) groups were analysed. Occurrence of early allograft dysfunction (EAD; 50%) and primary nonfunction (PNF; 20%) was significantly higher in the severe steatosis group (P < 0.001 and <0.001, respectively). Survival rate is significantly low in severe steatosis group (3‐year: 60%, P = 0.0039). The IFLT group had a significantly lower occurrence of EAD than the CLT group (0% vs. 60%, P = 0.01). The level of postoperative peak AST, GGT and creatine were significantly lower in IFLT group (P = 0.009, 0.032 and 0.024, respectively). In multivariable analysis, IFLT and EAD were independent factors affecting postoperative survival. Severe steatotic livers lead to severe complications and poor outcomes in LT. IFLT has obvious advantages for reducing the rate of EAD in LT with steatotic livers.
Keywords: early allograft dysfunction, ischaemia‐free liver transplantation, primary nonfunction, steatosis
Introduction
Liver transplantation (LT) is one of the most effective treatment options for both tumour and cirrhosis patients [1]. Patients with hepatocellular cancer who meet the Milan criteria and the University of California San Francisco (UCSF) criteria can achieve encouraging survival outcomes after LT [2]. However, organ shortage limits the selection of LT treatment and is associated with a higher waiting‐list mortality rate [3, 4]. Therefore, expansion of the pool of available liver grafts is of great significance to save more lives. Attempts are being made to use extended criteria donor (ECD) organs in LT. They are defined as grafts with steatosis greater than 30%, donor age over 60 years, long cold ischaemia times, donors with hypernatremia, positive serologies for hepatitis B virus (HBV) or hepatitis C virus (HCV), deceased donor split livers and living donors [5].
In recent studies, steatotic livers were seen in up to 9–26% of donors [6]. However, the usage of fatty liver in LT remains controversial. Previous reports suggested that it was associated with a higher risk of PNF, EAD and poor graft survival [7, 8]. Otherwise, several studies also demonstrated similar perioperative and long‐term outcomes for liver allografts with steatosis >30%. A retrospective study conducted by Soejima et al. [9] reported that grafts with moderate steatosis showed comparable 1‐year graft survival and patient survival.
Whether moderate or even severe steatotic donor livers can be used for transplantation remains unclear and needs to be further investigated. Fortunately, it has been identified that donor livers with more than 60% steatosis can be transplanted to recipients using a new technique: IFLT [10]. Compared with conventional procedures, IFLT has obvious advantages for the recovery of allograft function and complication incidence. Randomized clinical trials (RCTs) are underway to confirm its feasibility [11]. In this study, we aimed to examine the effect of liver grafts of different steatotic grades on outcome after LT and the effect of IFLT with steatotic liver grafts.
Materials and methods
All the procedures were performed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. The study was approved by the Institutional Ethics Committee for Clinical Research and Animal Trials of the First Affiliated Hospital of Sun Yat‐sen University, and an informed consent waiver was granted by the IEC given the retrospective, minimal‐risk nature of the study (Approval ID: [2020]370). No organs from executed prisoners were transplanted into any of the patients reported in this study.
Study population, data collection and outcome parameters
Between January 2015 and June 2020, 410 liver grafts from donors maintained in our centre were procured for transplantation and 360 deceased donor LT were enrolled in this retrospective study. The inclusion and exclusion criteria were presented in Fig. 1. The preoperational data, including donors' age, gender, body mass index (BMI), cause of death, laboratory tests result, warm ischaemia time (WIT), cold ischaemia time (CIT), indocyanine green (ICG) test and recipients’ age, gender, model for end‐stage liver disease (MELD) score, BMI, diagnosis were collected. The perioperative outcomes, including anhepatic time, type of operation, blood loss, transfusion of red blood cells (RBCs) and length of stay in the intensive care unit (ICU), were recorded and compared between different grades of steatotic groups. Additionally, graft functions, postoperative complications and patient survival at 3 years after transplantation were assessed and analysed. Differences in outcome between the IFLT and conventional LT (CLT) groups were also analysed.
Figure 1.
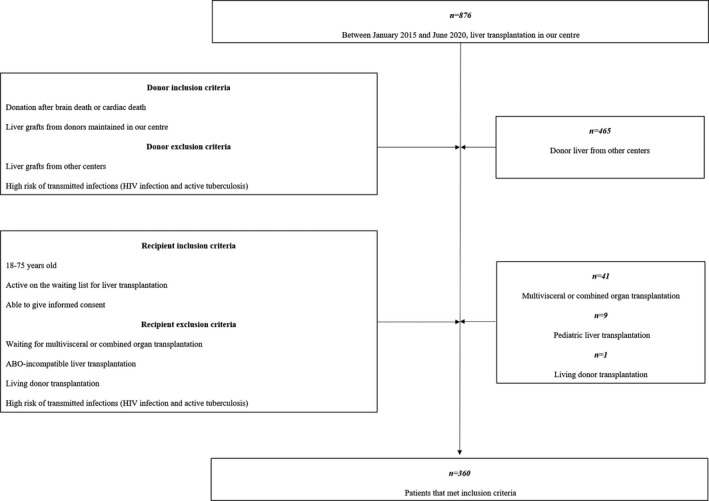
A brief flow chart of patients’ selection.
Histological assessment of steatosis
Liver biopsies were taken from the donor livers before and after reperfusion during transplantation (Fig. 2). Biopsies were obtained as the size of 0.5 × 0.5 cm and wedge‐shaped from the left lobe of donor liver for clear and representative pathology diagnosis. Biopsy specimens were fixed in formalin, embedded in paraffin and subsequently stained with haematoxylin–eosin. All histological slides were evaluated by an experienced pathologist who was unaware of the clinical assessment of steatosis. Macrovesicular steatosis was defined as fat vesicles larger than the cell nucleus, often displacing the nucleus. Microvesicular steatosis was defined as fat vesicles with similar size or smaller than the liver cell nucleus [12]. Depending on the degree of macrovesicular steatosis, liver biopsies were graded in mild (10–30%), moderate (between 30% and 60%) or severe (>60%) steatotic infiltration according to the histological scoring system designed by the Nonalcoholic Steatohepatitis Clinical Research Network (NASH) [13].
Figure 2.
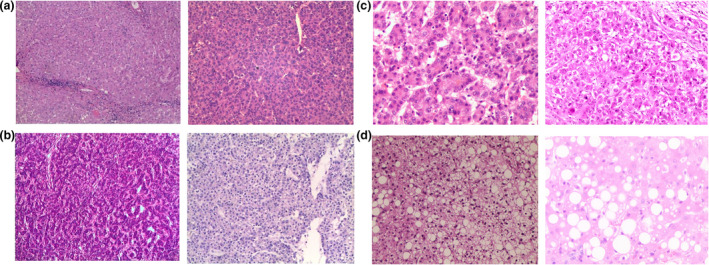
Example of biopsies taken from the donor livers before and after reperfusion during transplantation. (a) Nonsteatosis. (b) Mild steatosis. (c) Moderate steatosis. (d) Severe steatosis.
Description of IFLT
We previously described the produce of IFLT in several published articles [10, 11, 14]. All livers used in IFLT are from donor after brain death (DBD). In the procession, the donor liver is procured, preserved and implanted under continuous normothermic machine perfusion (NMP) without a cold perfusion process. During procurement, a tube was placed in the common bile duct for bile drainage and the cystic duct was ligated. A caval cannula was placed in the infrahepatic inferior vena cava (IHIVC) for outflow to the organ reservoir of Liver Assist (Organ Assist, Groningen, the Netherlands). Another cannula was inserted into the portal vein (PV) via a bridge vein. An arterial cannula was inserted into the gastroduodenal artery without interruption of arterial supply. The venous drainage of suprahepatic inferior vena cava (SHIVC) was blocked before procurement. All cannulas were then connected to Liver Assist. After the circuit of NMP was established, the liver was dissected and moved to the organ reservoir under NMP. Parameter including pH value, lactate, ion concentration (Sodium, calcium and potassium), and enzymes are monitored, adjusted and kept stable during the NMP procession. For implantation, the donor liver was transferred from the machine to the abdominal cavity. Under continuous perfusion in situ, vascular anastomosis (superior hepatic vena cava, portal vein and artery) was performed. After revascularization, the NMP was stopped and the catheterization was removed. Afterwards, the inferior hepatic vena cava and bile duct were anastomosed. With this technique, the CIT can be reduced to 0.
Postoperative management and follow‐up
Basiliximab was used for induction during the operation and postoperative day (POD) 4. The immunosuppressive regimen was tacrolimus (Tac) and mycophenolate mofetil (MMF). Corticosteroid therapy was not included in the routine regimen. The blood concentration of Tac was controlled at 8–12 ng/ml in the early stage after the operation. Doppler ultrasound of the liver graft blood flow and biliary tract was performed once every 2 days for 7 days. Routine outpatient follow‐up was performed every month in the first year and every three to six months in the second and third year.
Statistical analysis
All statistical analyses of the data were performed using spss version 26.0. All data are expressed as the mean ± standard deviation or the number and percentage of patients. For comparisons between groups, the chi‐square and Fisher’s exact tests were performed for frequencies and continuous data, respectively. A Cox proportional hazards model was used for multivariate analysis. Overall survival was compared using the Kaplan–Meier method with a log‐rank test. A P‐value <0.05 was considered statistically significant.
Results
Demographics
One hundred and thirty‐seven (38%) donor livers were diagnosed with steatosis. These 137 donor livers were categorized according to the severity of steatosis and included in one of the following groups: mild (<30%; n = 111), moderate (30–60%; n = 16) and severe (>60%; n = 10) steatosis. The other 223 livers were categorized as the nonsteatotic group. During the study period, the mean age and BMI of these 360 recipients was 50.39 ± 0.60 years old and 22.97 ± 0.19, respectively. The male‐to‐female ratio was 3.9–1. The most frequent causes of end‐stage liver diseases (ESLDs) requiring transplantation were hepatocellular carcinoma (HCC; n = 197, 54.7%; HCC with cirrhosis: n = 49, 13.6%; HCC without cirrhosis: n = 148, 41.1%), followed by cirrhosis without tumours (n = 112, 31%). The mean age and BMI of donors were 38.28 ± 0.66 years and 22.20 ± 0.17, respectively. A total of 332 (92.2%) donor livers were from DBD. The most frequent causes of death were trauma (n = 197, 54.7%), followed by vascular accidents (n = 132, 36.6%; Table 1).
Table 1.
Donor and recipient characteristics in different grades of steatotic groups.
| Variables | Mild steatosis (N = 111) | Moderate steatosis (N = 16) | Severe steatosis (N = 10) | Nonsteatosis (N = 223) | P |
|---|---|---|---|---|---|
| Preoperative donor parameters | |||||
| Donor age, years | 40.03 ± 1.03 | 38.38 ± 3.28 | 41.30 ± 5.06 | 37.29 ± 0.88 | 0.254 |
| Donor sex male n (%) | 89 | 15 | 10 | 173 | 0.162 |
| Donor type | |||||
| DBD | 102 | 15 | 10 | 205 | 0.972 |
| DCD | 8 | 1 | 0 | 17 | |
| DBCD | 1 | 0 | 0 | 1 | |
| BMI (kg/m2) | 22.17 ± 0.30 | 21.03 ± 0.95 | 20.95 ± 0.76 | 22.37 ± 0.22 | 0.250 |
| Cause of death | |||||
| Anoxia | 7 | 0 | 1 | 13 | 0.534 |
| Trauma | 53 | 11 | 7 | 126 | |
| Vascular | 49 | 5 | 2 | 76 | |
| Other | 2 | 0 | 0 | 8 | |
| AST (U/l) | 150.76 ± 24.50 | 84.56 ± 27.27 | 142.00 ± 21.29 | 107.30 ± 10.65 | 0.215 |
| ALT (U/l) | 77.88 ± 8.36 | 47.50 ± 9.22 | 90.50 ± 21.18 | 79.12 ± 6.68 | 0.589 |
| GGT (U/l) | 85.76 ± 10.02 | 74.25 ± 20.14 | 76.60 ± 29.26 | 86.88 ± 7.48 | 0.963 |
| Bilirubin (mmol/l) | 24.41 ± 1.63 | 22.08 ± 3.03 | 31.28 ± 7.79 | 27.27 ± 1.74 | 0.544 |
| CIT, h | 5.79 ± 0.3 | 5.50 ± 0.96 | 5.10 ± 1.22 | 5.99 ± 0.31 | 0.877 |
| Donor WIT, min | 1.17 ± 0.49 | 1.25 ± 0.69 | 0 | 0.62 ± 0.12 | 0.387 |
| ICG test result % | 4.77% ± 0.64% | 4.20% ± 2.0% | 3.52% ± 0.97% | 4.21% ± 0.41% | 0.882 |
| Preoperative recipient parameters | |||||
| Recipient age, years | 49.22 ± 1.12 | 53.56 ± 2.70 | 51.00 ± 2.50 | 60.67 ± 0.75 | 0.450 |
| Recipient sex male n (%) | 103 | 15 | 10 | 200 | 0.560 |
| BMI (kg/m2) | 23.37 ± 0.34 | 21.18 ± 0.90 | 21.73 ± 1.14 | 22.94 ± 0.25 | 0.101 |
| MELD | 18.58 ± 0.87 | 16.56 ± 2.33 | 19.30 ± 3.06 | 19.34 ± 0.71 | 0.708 |
| Diagnosis | |||||
| HCC | 64 | 9 | 3 | 121 | 0.330 |
| Cirrhotic | 31 | 6 | 7 | 68 | |
| Acute liver failure | 5 | 1 | 0 | 14 | |
| Others | 11 | 0 | 0 | 20 | |
| Transplantation parameters | |||||
| Anhepatic time, min | 55.06 ± 1.98 | 56.25 ± 5.58 | 60.50 ± 6.75 | 53.56 ± 1.29 | 0.661 |
| Intraoperative transfusions (U) | 6.31 ± 0.53 | 7.40 ± 1.84 | 8.23 ± 2.56 | 7.31 ± 0.53 | 0.637 |
| Blood loss (ml) | 2030.54 ± 165.96 | 3150.00 ± 842.12 | 2280.00 ± 254.65 | 2346.32 ± 184.23 | 0.351 |
| Type of vena cava anastomosis | |||||
| Classic | 52 | 5 | 3 | 90 | 0.821 |
| Classic piggyback | 8 | 2 | 1 | 21 | |
| Modified piggyback | 51 | 9 | 6 | 112 | |
| ICU length‐of‐stay (h) | 83.44 ± 12.66 | 43.28 ± 8.70 | 111.90 ± 73.70 | 73.31 ± 7.74 | 0.477 |
| Postoperative outcome parameters | |||||
| EAD | 33 | 7 | 5 | 22 | <0.001 |
| PNF | 1 | 0 | 2 | 1 | <0.001 |
| Biliary anastomotic strictures | 2 | 0 | 0 | 2 | 0.833 |
| Biliary leakage | 1 | 0 | 0 | 2 | 0.972 |
| Hepatic artery complications | 2 | 0 | 0 | 5 | 0.889 |
| Retransplantation | 1 | 0 | 0 | 2 | 0.972 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CIT, cold ischaemia time; DBCD, Donor after brain and cardiac death; DBD, donor after brain death; DCD, donor after cardiac death; EAD, early allograft dysfunction; GGT, γ‐glutamyl transpeptidase; HCC, hepatocellular carcinoma; ICG, indocyanine green; ICU, intensive care unit; IFLT, ischaemia‐free liver transplantation; MELD, model for end‐stage liver diseases; PNF, Primary nonfunction; WIT, warm ischaemia time.
Comparison between the different steatosis grade groups
Preoperative characteristics of donors and recipients in the different steatotic grade groups are presented in Table 1. The laboratory results were similar in all groups (P = 0.215, 0.589 and 0.963, respectively). There were also no statistically significant differences in the ICG results among these four groups (P = 0.882). The CITs were 5.79 ± 0.3, 5.50 ± 0.96, 5.10 ± 1.22 and 5.99 ± 0.31 h (P = 0.877), and the WIT (The period from the cessation of donor blood supply to the beginning of cold preservation) was 1.17 ± 0.49, 1.25 ± 0.69, 0 and 0.62 ± 0.12 min (P = 0.387). In addition, no differences were found in the diagnosis and MELD score. The median postoperative follow‐up was 24.3 months (range from 1 to 67 months). Perioperative outcomes and postoperative complications were also compared and are presented in Table 1. No significant differences were found in intraoperative transfusions, blood loss, ICU length of stay, IFLT or not and type of vena cava anastomosis (P > 0.05). The occurrence rate of EAD and PNF was significantly higher in the severe steatosis group as compared to mild and moderate steatosis groups (P < 0.001 and <0.001, respectively), and the rest of complications were similar in all groups (P > 0.05).
Comparison between the IFLT and CLT groups with moderate or severe steatosis
Six patients and 20 patients of 26 patients with moderate or severe steatotic donor livers underwent IFLT and CLT, respectively. The preoperative characteristics of the donors and recipients in the two groups are presented in Table 2. The IFLT group had a significantly shorter CIT and donor WIT than those in the CLT group (0 vs. 6.52 ± 0.24 h, P < 0.001 and 0 vs. 1.00 ± 0.56 min, P = 0.030, respectively). In contrast, the outcomes of the laboratory results, WIT, ICG results, MELD scores and diagnoses were not significantly different (P > 0.05). The perioperative outcomes and postoperative complications in both the IFLT and CLT groups are also compared and presented in Table 2. The level of postoperative peak aspartate aminotransferase (AST), γ‐glutamyl transpeptidase (GGT) and creatine within 7 days were significantly lower in IFLT group (P = 0.009, 0.032 and 0.024, respectively). Compared with the CLT group, the IFLT group had a significantly lower occurrence rate of EAD (0% vs. 60%, P = 0.01). Occurrences rate of PNF in these two groups were 0 and 10%, respectively, and there was no significantly difference (P = 0.420).
Table 2.
Donor and recipient characteristics in patients with moderate and severe steatotic livers who received IFLT versus CLT.
| Variables | IFLT (N = 6) | CLT (N = 20) | P |
|---|---|---|---|
| Preoperative donor parameters | |||
| Donor age, years | 45.00 ± 8.03 | 37.85 ± 2.70 | 0.057 |
| Donor sex male n (%) | 6 | 19 | 0.576 |
| BMI (kg/m2) | 19.66 ± 0.76 | 21.40 ± 0.80 | 0.166 |
| Donor type, DBD | 6 | 19 | 0.576 |
| AST (U/l) | 183.17 ± 63.40 | 80.70 ± 20.17 | 0.058 |
| ALT (U/l) | 82.00 ± 17.76 | 58.65 ± 12.58 | 0.671 |
| GGT (U/l) | 56.67 ± 24.02 | 80.70 ± 20.17 | 0.380 |
| Bilirubin (mmol/l) | 31.70 ± 6.34 | 23.79 ± 4.20 | 0.812 |
| CIT, h | 0 | 7.30 ± 0.47 | 0.009 |
| Donor WIT, min | 0 | 1.00 ± 0.56 | 0.030 |
| ICG % | 5.0 ± 0.9 | 3.0 ± 1.5 | 0.321 |
| Preoperative recipient parameters | |||
| Recipient age, years | 50.83 ± 5.02 | 53.10 ± 2.03 | 0.751 |
| Recipient sex male n (%) | 6 | 19 | 0.576 |
| BMI (kg/m2) | 20.01 ± 1.53 | 21.80 ± 0.77 | 0.877 |
| MELD | 18.83 ± 4.54 | 17.25 ± 2.02 | 0.848 |
| Transplantation parameters | |||
| Intraoperative transfusions (U) | 7.63 ± 1.41 | 7.74 ± 1.88 | 0.094 |
| Blood loss (ml) | 2800.00 ± 331.66 | 2820.00 ± 683.24 | 0.149 |
| Anhepatic time, min | 56.50 ± 11.45 | 58.30 ± 4.51 | 0.197 |
| ICU length‐of‐stay (h) | 28.50 ± 7.92 | 82.02 ± 37.03 | 0.343 |
| Postoperative outcome parameters | |||
| ALT (U/l) | 196.83 ± 62.16 | 869.55 ± 159.45 | 0.133 |
| AST (U/l) | 280.67 ± 80.63 | 2947.90 ± 532.69 | 0.009 |
| GGT (U/l) | 174.83 ± 34.69 | 338.60 ± 56.42 | 0.032 |
| Bilirubin (mmol/l) | 85.47 ± 22.18 | 119.19 ± 31.73 | 0.275 |
| Creatine (mmol/l) | 83.83 ± 10.82 | 172.12 ± 22.14 | 0.024 |
| EAD | 0 | 12 | 0.01 |
| PNF | 0 | 2 | 0.420 |
| Acute kidney injury | 0 | 8 | 0.063 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CIT, cold ischaemia time; CLT, conventional liver transplantation; CLT, conventional liver transplantation; DBCD, donor after brain and cardiac death; DBD, Donor after brain death; DCD, donor after cardiac death; EAD, early allograft dysfunction; GGT, γ‐glutamyl transpeptidase; HCC, hepatocellular carcinoma; ICG, Indocyanine green; ICU, intensive care unit; IFLT, ischaemia‐free liver transplantation; IFLT, ischaemia‐free liver transplantation; MELD, model for end‐stage liver diseases; PNF, primary nonfunction; WIT, warm ischaemia time.
Analysis of overall survival after LT in different situations
During the study period, the median patient survival times in the steatosis and nonsteatosis groups was 23.6 and 25.6 months, respectively, and the survival rate was not significantly different (3‐year: 84.8% vs. 89.9%, P = 04096, Fig. 3a). Comparisons were also made among different grades of steatosis, and recipients in the severe steatosis group showed a significantly low survival rate (3‐year: 60%, P = 0.0039), while recipients in the mild and moderate steatosis groups showed similar survival rates to those in the nonsteatosis group (3‐year: 86.7%, 89.9% and 93.7%, respectively, Fig. 3b).
Figure 3.
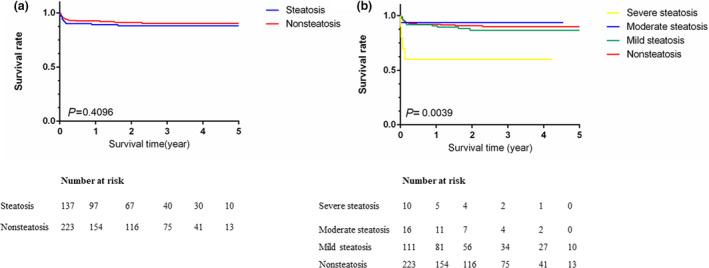
Comparison of patient survival (a) between the steatosis group and nonsteatosis group and (b) among different grades of steatosis.
The median survival times of the IFLT and CLT patients with moderate and severe steatotic donor liver were 22.0 and 9.17 months, respectively, and the survival rates were similar in the two groups (P = 0.786, Fig. 4). In the multivariable analysis, we adopted PNF [15], EAD [16], HAT [17], AKI [18] and with steatosis or not as predictors for they are proved to affect the survival after LT. We also added IFLT into analysis to investigate its effect. The result showed that IFLT (RR: 2.085–4.507, P < 0.001) and EAD (RR: 0.237–0.472, P < 0.001) were independent factors affecting patient postoperative survival (Table 3).
Figure 4.
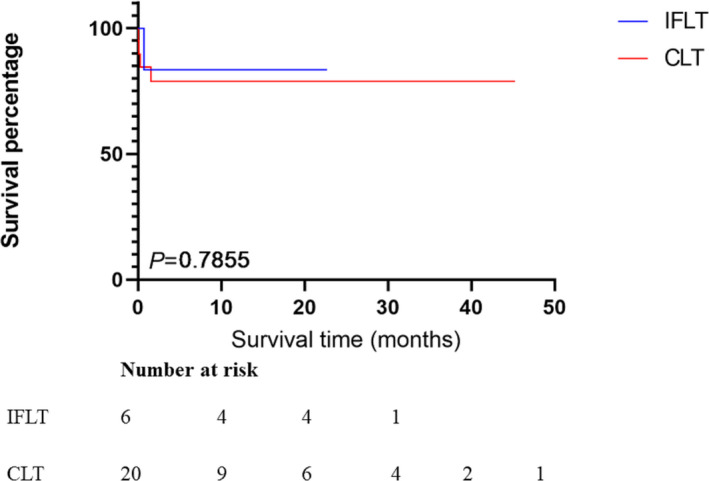
Comparison of patient survivals between IFLT group and CLT group in recipients with moderate or severe steatotic livers.
Table 3.
Multivariate analysis of relevant factors for survival in 360 patients.
| Variable | Multivariate analysis | |
|---|---|---|
| RR (95% CI) | P‐value | |
| PNF | 0.530 (0.074–3.808) | 0.528 |
| EAD | 0.335 (0.237–0.472) | <0.001 |
| HAT | 0.951 (0.635–1.422) | 0.806 |
| AKI | 1.336 (0.847–2.108) | 0.213 |
| Steatosis | 1.051 (0.832–1.328) | 0.677 |
| IFLT | 3.065 (2.085–4.507) | <0.001 |
AKI, acute kidney injury; EAD, early allograft dysfunction; HAT, hepatic artery thrombosis; IFLT, ischaemia‐free liver transplantation; PNF, primary nonfunction.
Discussion
Attempts are being made to use ECD organs in LT due to organ shortages and higher waiting‐list mortality rates [19]. Grafts with steatosis greater than 30% are associated with an increased risk of PNF, EAD, and poor graft survival. It was the aim of our study to examine the effect of liver grafts of different steatotic grades on outcomes after OLT.
Evaluation of whether there was steatosis by the surgeons, according to appearance and hardness, during procurement is subjective and susceptible to errors [20]. Additionally, liver grafts from other centres have insufficient information, such as the history and laboratory results of the donors. Therefore, only liver grafts from donors maintained in our centre were enrolled in this study so that we could obtain sufficient preoperative information. The use of ultrasound or CT scanning may also be useful for diagnosis of steatosis [21]. Furthermore, biopsies were taken from the donor livers before and after reperfusion during transplantation to evaluate the percentage of steatosis.
Our data showed that 38% donor livers were diagnosed with steatosis in this study period, and this is consistent with epidemiological findings [22]. We divided them into three groups: mild, moderate and severe steatosis groups according to the severity of steatosis, and we made comparisons among groups. We adopted EAD and PNF as criteria to evaluate postoperative liver function in the groups. EAD [16] was defined as the presence of one or more of the following criteria: TBil >10 mg/dl on day 7, international normalized ratio (INR)>1.6 on day 7 and alanine aminotransferase (ALT) or AST >2000 IU/l within the first week. PNF [23] was defined as recipient death or retransplantation within 7 days after operation. The results in our study showed that in comparison with that of nonsteatotic livers, the postoperative function of mild and moderate steatotic livers was not impaired. We reported similar postoperative incidences of severe complications and patient survival between recipients with mild, moderate and nonsteatotic livers. Nevertheless, our data show that recipients with severe steatotic livers had a higher rate of PNF and EAD. Additionally, survival in the severe steatotic groups was significantly lower.
A series of previous studies revealed that moderate and severe steatosis are independent prognostic factors for poor outcomes after LT [24, 25]. Zhang et al. [26] reported in a meta‐analysis that recipients with moderate and severe steatotic donor livers have higher rates of EAD and PNF. Deroose et al. [27] published a retrospective study and showed that livers with severe steatosis combined with a long CIT had a high risk of developing EAD and shorter graft survival. The differences in postoperative outcome in these studies could depend on other donor‐related risk factors. Steatotic livers are more fragile with respect to the effects of cold ischaemia during organ preservation and reperfusion [28]. Westerkamp et al. [29] reported another retrospective study and suggested that moderately steatotic and nonsteatotic livers could achieve similar outcomes only if the CIT was <8 h. In our study, the mean CITs in the nonsteatotic, mild, moderate and severe steatotic groups were all less than 6 h, and the results of both EAD and survival rate support Westerkamp’s results. Vodkin et al. [30] suggested in their review that promising outcomes can be achieved by having a short CIT, selecting recipients with MELD scores <25, when steatotic livers are used in LT. Nevertheless, severely steatotic livers still correlated with poor outcome in our study. Stricter selection criteria and more intervention strategies such as defatting are needed to achieve better results in CLT.
In July 2017, a new technique, called IFLT, was reported to ensure complete avoidance of ischaemia injury during transplantation [10]. The donor liver is procured, preserved and implanted under continuous NMP without a cold perfusion process. The first case of IFLT showed obvious advantages in the recovery of allograft function and the reduction of complications compared with the conventional procedure. Zhao et al. [14] reported minimal hepatocyte and biliary epithelium injury during the preservation stage of IFLT. An RCT is underway to confirm its feasibility [11]. Ischaemia‐reperfusion injury (IRI) is the cause of EAD as it leads to cellular damage [31]. Our data show that recipients undergoing IFLT experienced no CIT and that IR was avoided. Comparison in recipients with moderate or severe steatosis between the IFLT and CLT groups showed that the rate of EAD was remarkably low in the ILFT groups. The level of postoperative peak enzyme and creatine were also significantly lower in IFLT group. The results reveal obvious advantages of IFLT in allograft function recovery and reducing complication incidence. In multivariable analysis, IFLT was an independent factor impacting postoperative survival. For the short application time and short follow‐up period of IFLT, no significantly difference was found in survival rate in these two group and this may be the reason that the results of survival curve contradict those of multivariate analysis. Further follow‐up is needed for long‐term survival comparison. Results from RCTs will provide reliable information on the feasibility and safety of the use of IFLT.
Our study is limited by being from a single‐centre institution, so selection bias may have affected the results. Larger multicentre studies are needed to determine whether similar outcomes can be achieved between steatosis and nonsteatosis groups. Furthermore, postoperative reversal of steatosis was not studied in this study [32]. In some studies, postoperative histologic analysis showed that, in some recipients, steatosis can resolve completely after LT [33]. Follow‐up biopsy is needed to evaluate changes in liver grafts. How to raise the utilization rate of liver grafts with severe steatosis will be interesting and meaningful because it is of great significance to reduce organ shortages and high waiting‐list mortality rates [34, 35]. IFLT has the potential to reduce EAD in LT with steatotic liver grafts. For future studies in IFLT, the 3‐ and 5‐year overall survival values should be calculated to obtain more convincing conclusions.
Conclusion
In conclusion, livers with mild and moderate steatosis can be used successfully for LT while livers with severe steatosis lead to severe complications and poor outcomes. IFLT has obvious advantages in reducing the incidence of EAD in LT with steatotic livers. Larger multicentre studies are needed for the use of steatotic livers in LT, and longer follow‐up outcomes should be used to obtain more convincing results for IFLT.
Authorship
XH and WJ: conception and design. MC and WJ: administrative support. XL, XH and YM: provision of study materials or patients. ZC and MC: collection and assembly of data. XL: data analysis and interpretation. MC, ZC, XL, XH, YM, CH, XH and WJ: manuscript writing. MC, ZC, XL, XH, YM, CH, XH and WJ: final approval of manuscript.
Funding
The authors have declared no funding.
Conflict of interest
The authors declare no competing financial interests. No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication.
Informed consent
Informed consent was obtained from individual participants in the study.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81401324 and 81770410), the Science and Technology Planning Project of Guangdong Province (2016A020215048), the Guangdong Provincial Key Laboratory of Organ Donation and Transplant Immunology (2013A061401007), the Guangdong Basic and Applied Basic Research Foundation (2020A1515011557, 2020A1515010903) and the Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation) (2015B050501002), China.
Maogen Chen, Zhitao Chen and Xiaohong Lin contributed equally to this work.
Contributor Information
Maogen Chen, Email: chenmg3@mail.sysu.edu.cn.
Zhitao Chen, Email: chenzht6@mail2.sysu.edu.cn.
Xiaoshun He, Email: gdtrc@163.com.
Weiqiang Ju, Email: weiqiangju@163.com.
References
- 1.Morris PJ. Transplantation – a medical miracle of the 20th century. N Engl J Med 2004; 351: 2678. [DOI] [PubMed] [Google Scholar]
- 2.Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol 2017; 14: 203. [DOI] [PubMed] [Google Scholar]
- 3.Samuel D, Coilly A. Management of patients with liver diseases on the waiting list for transplantation: a major impact to the success of liver transplantation. BMC Med 2018; 16: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim WR, Lake JR, Smith JM, et al. Liver. Am J Transplant 2016; 16(Suppl 2): 69. [DOI] [PubMed] [Google Scholar]
- 5.Resch T, Cardini B, Oberhuber R, et al. Transplanting marginal organs in the era of modern machine perfusion and advanced organ monitoring. Front Immunol 2020; 11: 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riva G, Villanova M, Cima L, et al. Oil red O is a useful tool to assess donor liver steatosis on frozen sections during transplantation. Transplant Proc 2018; 50: 3539. [DOI] [PubMed] [Google Scholar]
- 7.Chu MJ, Dare AJ, Phillips AR, Bartlett AS. Donor hepatic steatosis and outcome after liver transplantation: a systematic review. J Gastrointest Surg 2015; 19: 1713. [DOI] [PubMed] [Google Scholar]
- 8.McCormack L, Dutkowski P, El‐Badry AM, Clavien PA. Liver transplantation using fatty livers: always feasible? J Hepatol 2011; 54: 1055. [DOI] [PubMed] [Google Scholar]
- 9.Soejima Y, Shimada M, Suehiro T, et al. Use of steatotic graft in living‐donor liver transplantation. Transplantation 2003; 76: 344. [DOI] [PubMed] [Google Scholar]
- 10.He X, Guo Z, Zhao Q, et al. The first case of ischemia‐free organ transplantation in humans: a proof of concept. Am J Transplant 2018; 18: 737. [DOI] [PubMed] [Google Scholar]
- 11.Huang C, Huang S, Tang Y, et al. Prospective, single‐centre, randomised controlled trial to evaluate the efficacy and safety of ischaemia‐free liver transplantation (IFLT) in the treatment of end‐stage liver disease. BMJ Open 2020; 10: e035374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antunes C, Azadfard M, Hoilat GJ, Gupta M. Fatty Liver. Treasure Island, FL: StatPearls Publishing. Copyright © 2020, StatPearls Publishing LLC; 2020. [PubMed] [Google Scholar]
- 13.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41: 1313. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Q, Huang S, Wang D, et al. Does ischemia free liver procurement under normothermic perfusion benefit the outcome of liver transplantation? Ann Transplant 2018; 23: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh N, Washburn K, Schenk A, et al. Rescue hepatectomy and anhepatic phase management after primary nonfunction in a liver transplant. Exp Clin Transplant 2020. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Kok B, Dong V, Karvellas CJ. Graft dysfunction and management in liver transplantation. Crit Care Clin 2019; 35: 117. [DOI] [PubMed] [Google Scholar]
- 17.Kutluturk K, Sahin TT, Karakas S, et al. Early hepatic artery thrombosis after pediatric living donor liver transplantation. Transpl Proc 2019; 51: 1162. [DOI] [PubMed] [Google Scholar]
- 18.Durand F, Francoz C, Asrani SK, et al. Acute kidney injury after liver transplantation. Transplantation 2018; 102: 1636. [DOI] [PubMed] [Google Scholar]
- 19.Zamora‐Valdes D, Leal‐Leyte P, Kim PT, Testa G. Fighting mortality in the waiting list: liver transplantation in North America, Europe, and Asia. Ann Hepatol 2017; 16: 480. [DOI] [PubMed] [Google Scholar]
- 20.Novruzov N, Bayramov N, Mammadov E. Preoperative evaluation of liver parenchyma of potential donors in living donor liver transplantation. Transplant Proc 2019; 51: 2379. [DOI] [PubMed] [Google Scholar]
- 21.Kim JM, Ha SY, Joh JW, et al. Predicting hepatic steatosis in living liver donors via noninvasive methods. Medicine 2016; 95: e2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellentani S. The epidemiology of non‐alcoholic fatty liver disease. Liver Int 2017; 37(Suppl 1): 81. [DOI] [PubMed] [Google Scholar]
- 23.Szilágyi ÁL, Mátrai P, Hegyi P, et al. Compared efficacy of preservation solutions on the outcome of liver transplantation: meta‐analysis. World J Gastroenterol 2018; 24: 1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durand F, Renz JF, Alkofer B, et al. Report of the Paris consensus meeting on expanded criteria donors in liver transplantation. Liver Transpl 2008; 14: 1694. [DOI] [PubMed] [Google Scholar]
- 25.Spitzer AL, Lao OB, Dick AA, et al. The biopsied donor liver: incorporating macrosteatosis into high‐risk donor assessment. Liver Transpl 2010; 16: 874. [DOI] [PubMed] [Google Scholar]
- 26.Zhang QY, Zhang QF, Zhang DZ. The impact of steatosis on the outcome of liver transplantation: a meta‐analysis. Biomed Res Int 2019; 2019: 3962785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deroose JP, Kazemier G, Zondervan P, Ijzermans JN, Metselaar HJ, Alwayn IP. Hepatic steatosis is not always a contraindication for cadaveric liver transplantation. HPB (Oxford) 2011; 13: 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiménez‐Castro MB, Casillas‐Ramírez A, Negrete‐Sánchez E, Avalos‐de León CG, Gracia‐Sancho J, Peralta C. Adipocytokines in steatotic liver surgery/transplantation. Transplantation 2019; 103: 71. [DOI] [PubMed] [Google Scholar]
- 29.Westerkamp AC, de Boer MT, van den Berg AP, Gouw AS, Porte RJ. Similar outcome after transplantation of moderate macrovesicular steatotic and nonsteatotic livers when the cold ischemia time is kept very short. Transpl Int 2015; 28: 319. [DOI] [PubMed] [Google Scholar]
- 30.Vodkin I, Kuo A. Extended criteria donors in liver transplantation. Clin Liver Dis 2017; 21: 289. [DOI] [PubMed] [Google Scholar]
- 31.Berthiaume F, Barbe L, Mokuno Y, MacDonald AD, Jindal R, Yarmush ML. Steatosis reversibly increases hepatocyte sensitivity to hypoxia‐reoxygenation injury. J Surg Res 2009; 152: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Liu B, Yan LN, et al. Reversal of graft steatosis after liver transplantation: prospective study. Transplant Proc 2009; 41: 3560. [DOI] [PubMed] [Google Scholar]
- 33.Machicao VI, Krishna M, Bonatti H, et al. Hepatitis C recurrence is not associated with allograft steatosis within the first year after liver transplantation. Liver Transpl 2004; 10: 599. [DOI] [PubMed] [Google Scholar]
- 34.Wong TC, Fung JY, Chok KS, et al. Excellent outcomes of liver transplantation using severely steatotic grafts from brain‐dead donors. Liver Transpl 2016; 22: 226. [DOI] [PubMed] [Google Scholar]
- 35.Dutkowski P, Schlegel A, Slankamenac K, et al. The use of fatty liver grafts in modern allocation systems: risk assessment by the balance of risk (BAR) score. Ann Surg 2012; 256: 861; discussion 8‐9. [DOI] [PubMed] [Google Scholar]


