Abstract
Non‐alcoholic fatty liver disease (NAFLD) is the fastest growing cause of chronic liver disease worldwide. Although only a small proportion of NAFLD patients will progress to end‐stage liver disease and death, the clinical burden of NAFLD is substantial due the sheer number of individuals affected worldwide. In fact, recent estimates suggest that 25% of the world have NAFLD, which is now one of the leading causes of cirrhosis and indications for liver transplantation. Although liver‐related mortality is common, the most common cause of death in patients with NAFLD is related to cardiovascular diseases, followed by extra‐hepatic cancers. There is a significant interindividual variability in the susceptibility to liver disease. The severity of metabolic alterations is the main risk factor for progressive NAFLD, but the qualitative components of diet, physical activity and genetic factors also play an important role. In particular, common variants in patatin‐like phospholipase domain‐containing 3 (PNPLA3), transmembrane 6 superfamily member 2 (TM6SF2), membrane bound O‐acyl transferase 7 (MBOAT7) and glucokinase regulator (GCKR) have been shown to contribute to the full spectrum of NAFLD. In those at risk of a potentially progressive form of NAFLD or non‐alcoholic steatohepatitis or in those with hepatic fibrosis, additional assessment must be made.
Keywords: cirrhosis, fibrosis of the liver, genetics, hepatocellular carcinoma, natural history, non‐alcoholic fatty liver disease, non‐alcoholic steatohepatitis
Abbreviations
- ALT
alanine aminotransferase
- BMI
body mass index
- CI
confidence interval
- CVD
Cardiovascular disease
- GCKR
Glucokinase regulator
- GGT
gammaglutamiltransferase
- HCC
hepatocellular carcinoma
- MBOAT7
Membrane bound O‐acyl transferase 7
- MS
metabolic syndrome
- NAFL
non‐alcoholic fatty liver
- NAFLD
Non‐alcoholic fatty liver disease
- NASH
non‐alcoholic steatohepatitis
- PNPLA3
Patatin‐like phospholipase domain‐containing 3
- RCTs
randomized controlled trials
- SEERD
Surveillance, Epidemiology and End Results Database
- SRTR
Scientific Registry of Transplant Recipients
- T2DM
type 2 diabetes mellitus
- TM6SF2
Transmembrane 6 superfamily member 2
- VLDL
very low‐density lipoproteins
Key points.
NAFLD can progress to advanced liver disease, hepatocellular carcinoma, liver transplantation and death.
NAFLD is associated with an increased mortality rate, with the three top causes of death being cardiovascular disease, extra‐hepatic cancer and liver disease.
Prediction models for NAFLD forecast a 30% increase in total NAFLD cases in the next decade.
Worsening of liver damage depends on both environmental and genetic factors, with fluctuant phases of fibrosis progression and regression.
Advanced fibrosis bears a seven times higher risk for developing hepatocellular carcinoma, which has become the fastest growing indication for liver transplantation in the USA.
1. INTRODUCTION
The acronym non‐alcoholic fatty liver disease (NAFLD) defines a spectrum of clinico‐pathological liver diseases that ranges from non‐alcoholic fatty liver (NAFL or simple steatosis) to non‐alcoholic steatohepatitis (NASH) and cirrhosis with its complications.1 NAFLD clusters with obesity and T2DM and is commonly considered to be the hepatic manifestation of the metabolic syndrome (MS), reflecting shared pathogenic factors. The pathogenesis of the transition from simple steatosis to progressive disease is still not completely understood, and is probably multifactorial.1 NAFLD is rapidly becoming the leading cause of liver disease worldwide and one of the top causes of cirrhosis and hepatocellular carcinoma (HCC). Currently, 25% of the general population is thought to have NAFLD worldwide, with the highest prevalence found in the Middle East and South America (31.79% and 30.45% respectively) and the lowest in Africa (13.48%).2 The prevalence of NAFLD is higher (57.80%) in patients with T2DM.3 Furthermore, the prevalence of NAFLD in the morbidly obese can be as high as 95%.4
The true prevalence of NASH is uncertain because the diagnosis of NASH is based on histology. Estimated prevalence rates of NASH in the general population range from 1.5% to 6.45%.2 The highest rates of NASH are found in diabetic patients (65.26%, 15.05% with advanced fibrosis ≥F3),3 and in morbidly obese subjects (20%‐50%, 10% with advanced fibrosis).4 Recent global modelling analyses based on changes in adult obesity and T2DM, suggest that the prevalence of NAFLD is set to grow exponentially over the next decade.5
2. NATURAL HISTORY OF NAFLD
A diagnosis of NAFLD is associated with an increased rate of mortality, with the three top causes of death being cardiovascular disease, extra‐hepatic cancer and liver disease.6 On the other hand, liver‐related mortality predominates in patients with NASH and advanced fibrosis. Data on the natural history of NAFLD are mainly from tertiary centres including histological cohorts with mortality data or repeated liver biopsies performed during clinical follow‐up.7 Other evidence shows that most patients with cryptogenic cirrhosis have the metabolic profile of patients with NASH and a high recurrence of NASH post‐liver transplantation.8 More recent data from placebo arms of clinical trials of NASH with sequential protocol biopsies provide a much more dynamic picture. These results show that 20%‐30% of patients with NAFLD will have NASH and that 10%‐15% of these can progress to cirrhosis. Overall, the histological worsening of liver damage is not a smooth transition from simple fatty liver to NASH and fibrosis, but rather the composite result of episodes of inflammation leading to the progression of fibrosis alternating with periods of regression. This pattern is especially evident in the placebo arms of randomized controlled trials (RCTs), where the primary endpoint of the regression of NASH with no worsening of fibrosis or the regression of fibrosis regression with no worsening of NASH, is achieved in more than 20% of subjects.9 The exact reasons for these fluctuating patterns in the progression and regression of fibrosis are not completely understood, but lifestyle changes probably play a major role.
3. HEPATIC COMPLICATIONS OF NAFLD
The severity of liver fibrosis is the main prognostic factor in patients with NAFLD.10 Compared to NAFLD patients without fibrosis, those with fibrosis are at an increased risk of all‐cause mortality, while the risk of liver‐related mortality increases exponentially with each increase in the stage of fibrosis. The estimated mortality rate ration for stage 1 is 1.41 (95% confidence interval (CI) 0.17‐11.95); stage 2, 9.57 (95% CI 1.67‐54.93); stage 3, 16.69 (95% CI 2.92‐95.36); and for stage 4 (cirrhosis), 42.30 (95% CI 3.51‐510.34).10 A meta‐analysis of early studies has shown that the progression of one stage of fibrosis takes an average of 14 years in patients with steatosis and 7 years in those with NASH.11 However, the progression of fibrosis progression varies widely; a significant proportion of patients without histological NASH can progress rapidly, especially those with visceral obesity, T2DM, older age and Hispanic ethnicity. NASH without liver fibrosis does not seem to result in an increased risk of mortality, but it is probably associated with a faster progression of liver fibrosis12 because of the role of necroinflammatory changes in the development of fibrosis. Liver‐specific mortality in those with NAFLD has also been reported to be 0.77 per 1000 person‐years, but this rate is almost 10 times higher in patients who develop NASH, with a reported rate of 11.77 per 1000 person‐years.13, 14 Liver disease becomes the leading cause of death in patients with cirrhosis. Furthermore, the risk of HCC related to NAFLD has increased substantially. In fact, the estimated incidence of HCC in patients with NAFLD is 0.44 per 1000 person‐years.15 Patients with NAFLD stage 3 and 4 fibrosis have an almost seven times higher risk of developing HCC than those without significant liver disease.16 The presence of the metabolic syndrome, especially obesity and T2DM, may hasten the development of HCC. The Surveillance, Epidemiology and End Results Database (SEERD) study suggests that although NAFLD is among the top 3 causes of HCC, the mortality in patients with NAFLD HCC is higher 1‐year after diagnosis because of a higher rate of diagnosis outside of surveillance programs.15 NAFLD/NASH is also rapidly becoming a major indication for liver transplantation in the USA. A recent analysis of the US Scientific Registry of Transplant Recipients (SRTR) from 2012 to 2016 found that NASH was the fastest growing indication for liver transplantation in listed patients, positioning NASH to become the most common cause of liver transplantation in the near future.17 Another analysis of SRTR suggests that NASH‐related HCC is the fasting growing indication for HCC listing for liver transplantation in the USA.18 Because of the lack of systematic screening or failure to screen for HCC in these individuals, it is possible that most patients with NASH‐related HCC do not get listed for liver transplantation or die while waiting for an organ.16
Prediction models for NAFLD in Asia and Europe show that there could be an increase of up to 30% in total NAFLD cases between 2016 and 20305 in relation to the increase in obesity and T2DM. Modelling shows a slow growth in total cases and greater increase in advanced cases.
The prevalence of NASH will increase by 15%‐56%, while liver‐related mortality and advanced liver disease will more than double. In the European countries, the greatest increase in NASH and HCC cases is expected in Germany while France is projected to have the most cases of compensated and decompensated cirrhosis by 2030. China is expected to have the greatest increase in NAFLD cases, with an estimated 29% increase from 243.7 million in 2016 to 314.6 million in 2030. The USA will experience the highest rate of decompensated cirrhosis with an estimated 56% increase from 17.3 million cases in 2016 to 27.0 million cases in 2030.5
4. EXTRAHEPATIC COMPLICATIONS IN NAFLD
Cardiovascular disease and extra‐hepatic cancer predominate in subjects with lower stages of fibrosis and NAFLD can also increase the risk of morbidity and mortality related to T2DM and to cardiovascular disease (CVD).6 A systematic review and a meta‐analysis of 21 prospective, population‐based studies in different ethnic groups found that ultrasound‐diagnosed NAFLD and increased liver function tests (alanine aminotransferase [ALT] and gammaglutamiltransferase [GGT]) were associated with an increased risk of incident T2DM.19 In subjects with T2DM, the presence of NAFLD further increases the risk of incident CVD and the presence of complications of T2DM.20 NAFLD is associated with an increased prevalence of CVD, as well as incident non‐fatal CVD events and CVD mortality. Among liver enzymes, GGT rather than ALT levels are most closely associated with incidental CVD events, even when they are within the normal range. In a systematic review and a meta‐analysis of 10 studies in different ethnic groups, 1 U/L higher GGT (on a log scale) was associated with a 20% increase in the risk of CVD, a 54% increase in the risk of stroke, and a 34% increase in the risk of CVD and stroke combined.21 In most cases, the association between NAFLD and mortality from CVD was independent of classical CVD risk factors and, in a few cases, of the diagnosis of MS. In biopsy‐proven NAFLD, the presence of hepatic fat accumulation was associated with increased carotid artery intima‐media thickness and the presence of carotid plaques,22 with significant carotid atherosclerosis occurring approximately 5‐10 years earlier in subjects with NAFLD, independently of T2DM and endothelial dysfunction. Cardiac involvement in NAFLD is not limited to coronary artery disease. Fatty liver is also associated with increased intrapericardial and extrapericardial fat and a reduced phosphocreatine/adenosine triphosphate ratio, a recognized in vivo marker of myocardial energy metabolism, even in subjects without risk factors for cardiovascular diasease.23 Subjects with high liver fat have lower insulin‐stimulated myocardial glucose uptake and lower coronary flow reserve compared to the low liver fat group,24 suggesting that liver fat content is an independent indicator of myocardial insulin resistance and reduced coronary functional capacity.
5. ENVIRONMENTAL AND GENETIC RISK FACTORS FOR DISEASE PROGRESSION
The severity of metabolic abnormalities, insulin resistance, and in particular the presence of T2DM, represent the major risk factors for the development of advanced liver disease, and the progression of fibrosis in prospective studies in patients with NAFLD1 (Figure 1). Variations in body weight and associated metabolic abnormalities are the main clinical predictors for the progression of liver disease during follow‐up. A key mediator of the progression of liver disease induced by metabolic risk factors can be represented by the severity of hepatic fat accumulation, which has been linked to short‐ and long‐term progression of fibrosis independently of several confounders.25 Industrial fructose intake has been associated with a higher risk of both the development and progression of NAFLD, probably by stimulating de novo lipogenesis,26 as well as an increase in the ratio of dietary saturated/unsaturated fat intake. On the other hand, the role of red meat consumption has not been clearly established.
FIGURE 1.
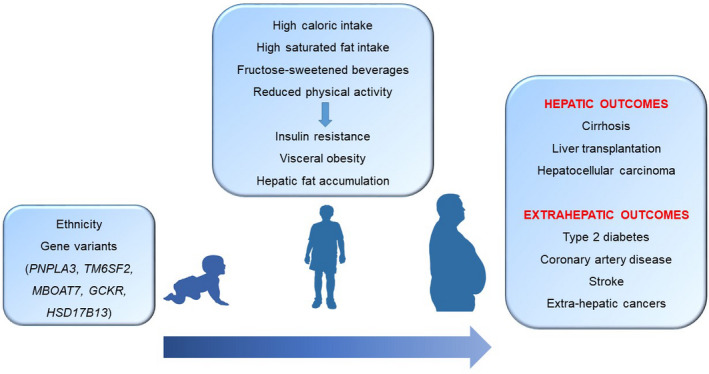
Environmental and genetic factors implied in the development of NAFLD and its complications, according to different ages of life
Accumulating evidence shows that hepatic fat and NAFLD are strongly inheritable conditions.27 Multi‐ethnic cohort studies show that there is a strong interethnic variability in the susceptibility to the development of NAFLD, which is higher in Hispanics, intermediate in Europeans and lower in African‐Americans, independent of weight, T2DM and socioeconomic factors. The risk of progressive NAFLD is higher in first‐degree relatives of patients with NAFLD cirrhosis compared to the general population, independent of several confounders. In the past few years, the most important common genetic determinants of hepatic fat variability and the susceptibility to develop NAFLD have been identified with the advent of genome‐wide association studies. The major determinant is the rs738409 C > G encoding for the I148 M protein variant of patatin‐like phospholipase domain‐containing 3 (PNPLA3), which accounts for a large fraction of the increased risk of this condition in Hispanics. The I148 M variant increases susceptibility to the whole spectrum of NAFLD‐related liver damage, from simple steatosis, to NASH, fibrosis and cirrhosis, thus representing a general modifier in the progression of liver disease. Furthermore, the I148 M variant increases the risk of progression to HCC independently from the effect on fibrosis. In Europeans, homozygosity for the mutation is enriched almost nine‐fold in patients who develop NAFLD‐HCC compared to the general population, while an absence of this variant can exclude the risk of HCC with a high specificity in the general population; polygenic risk scores can help to gain insight into the causal relationship between NAFLD and HCC and to improve HCC risk stratification.27, 28 Carriage of this variant influences the risk of liver disease especially during developmental ages, interacting with dietary factors such as the intake of fructose‐enriched drinks, and a lack of physical activity.29 Other common genetic mutations regulating hepatocellular lipid contribute to the risk of NAFLD. The rs58542926 C > T encoding for the E167K variant in transmembrane 6 superfamily member 2 (TM6SF2) favours hepatic fat accumulation by decreasing lipid secretion in very low‐density lipoproteins (VLDL), also leading to increased susceptibility to liver damage. At the same time, this genetic factor protects from CVD by reducing circulating lipids.27 Variants in glucokinase regulator (GCKR) and in membrane bound O‐acyl transferase 7 (MBOAT7) also contribute to the risk, by increasing de novo lipogenesis and altering the remodelling of phospholipids respectively. All these factors result in fat accumulation and a higher risk of liver disease.27, 28 Conversely, the most recent HSD17B13 variant T > TA confers protection from liver damage in NAFLD.30 The impact of the genetic variants on hepatic fat content, the risk of NAFLD and that of cirrhosis increases exponentially with an increasing body mass index (BMI), indicating the presence of a synergy among these components of the disease. It is important note that in individuals at a high genetic risk, a healthy dietary pattern modelled on the Mediterranean diet as well as regular physical activity can reduce the risk of NAFLD.31
6. CONCLUSIONS
Non‐alcoholic fatty liver disease is not a benign disease because it can progress to advanced liver disease, hepatocellular carcinoma, liver transplantation and death. The prevalence and incidence of NAFLD is increasing globally. Although the number of patients with disease progression is small, the global disease burden is substantial. Further studies are needed to develop interventions to reverse the course of NAFLD, especially as we increase our understanding of NAFLD.
CONFLICT OF INTEREST
The authors declare no conflict of interest with the present article.
Armandi A, Bugianesi E. Natural history of NASH. Liver Int. 2021;41:78–82. 10.1111/liv.14910
REFERENCES
- 1.Marengo A, Jouness RI, Bugianesi E. Progression and natural history of nonalcoholic fatty liver disease in adults. Clin Liver Dis. 2016;20(2):313‐324. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73‐84. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Tampi R, Priyadarshini M, Nader F, Younossi IM, Racila A. Burden of illness and economic model for patients with nonalcoholic steatohepatitis in the United States. Hepatology. 2019;69(2):564‐572. [DOI] [PubMed] [Google Scholar]
- 4.Ong JP, Elariny H, Collantes R, et al. Predictors of nonalcoholic steatohepatitis and advanced fibrosis in morbidly obese patients. Obes Surg. 2005;15(3):310‐315. [DOI] [PubMed] [Google Scholar]
- 5.Estes C, Anstee QM, Arias‐Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896‐904. [DOI] [PubMed] [Google Scholar]
- 6.Vanni E, Marengo A, Mezzabotta L, Bugianesi E. Systemic complications of nonalcoholic fatty liver disease: when the liver is not an innocent bystander. Semin Liver Dis. 2015;35(3):236‐249. [DOI] [PubMed] [Google Scholar]
- 7.McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing‐steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148‐1155. [DOI] [PubMed] [Google Scholar]
- 8.Thuluvath PJ, Hanish S, Savva Y. Waiting list mortality and transplant rates for NASH cirrhosis when compared with cryptogenic, alcoholic, or AIH cirrhosis. Transplantation. 2019;103(1):113‐121. [DOI] [PubMed] [Google Scholar]
- 9.Han MAT, Altayar O, Hamdeh S, et al. Rates of and factors associated with placebo response in trials of pharmacotherapies for nonalcoholic steatohepatitis: systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2019;17(4):616‐629.e26. [DOI] [PubMed] [Google Scholar]
- 10.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta‐analysis. Hepatology. 2017;65(5):1557‐1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pais R, Charlotte F, Fedchuk L, et al. A systematic review of follow‐up biopsies reveals disease progression in patients with non‐alcoholic fatty liver. J Hepatol. 2013;59(3):550‐556. [DOI] [PubMed] [Google Scholar]
- 12.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta‐analysis of paired‐biopsy studies. Clin Gastroenterol Hepatol. 2015;13(4):643–654.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D, Li AA, Perumpail BJ, et al. Changing trends in etiology‐based and ethnicity‐based annual mortality rates of cirrhosis and hepatocellular carcinoma in the United States. Hepatology. 2019;69(3):1064‐1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vilar‐Gomez E, Calzadilla‐Bertot L, Wai‐Sun Wong V, et al. Fibrosis severity as a determinant of cause‐specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi‐national cohort study. Gastroenterology. 2018;155(2):443‐457.e17. [DOI] [PubMed] [Google Scholar]
- 15.Younes R, Bugianesi E. Should we undertake surveillance for HCC in patients with NAFLD? J Hepatol. 2018;68(2):326‐334. [DOI] [PubMed] [Google Scholar]
- 16.Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62(6):1723‐1730. [DOI] [PubMed] [Google Scholar]
- 17.Cholankeril G, Wong RJ, Hu M, et al. Liver transplantation for nonalcoholic steatohepatitis in the US: temporal trends and outcomes. Dig Dis Sci. 2017;62(10):2915‐2922. [DOI] [PubMed] [Google Scholar]
- 18.Younossi Z, Stepanova M, Ong JP, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17(4):748‐755.e3. [DOI] [PubMed] [Google Scholar]
- 19.Fraser A, Harris R, Sattar N, Ebrahim S, Davey Smith G, Lawlor DA. Alanine aminotransferase, gamma‐glutamyltransferase, and incident diabetes: the British Women's Heart and Health Study and meta‐analysis. Diabetes Care. 2009;32(4):741‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30(8):2119‐2121. [DOI] [PubMed] [Google Scholar]
- 21.Fraser A, Harris R, Sattar N, Ebrahim S, Smith GD, Lawlor DA. Gamma‐glutamyltransferase is associated with incident vascular events independently of alcohol intake: analysis of the British Women's Heart and Health Study and Meta‐Analysis. Arterioscler Thromb Vasc Biol. 2007;27(12):2729‐2735. [DOI] [PubMed] [Google Scholar]
- 22.Fracanzani AL, Burdick L, Raselli S, et al. Carotid artery intima‐media thickness in nonalcoholic fatty liver disease. Am J Med. 2008;121(1):72‐78. [DOI] [PubMed] [Google Scholar]
- 23.Perseghin G, Lattuada G, De Cobelli F, et al. Increased mediastinal fat and impaired left ventricular energy metabolism in young men with newly found fatty liver. Hepatology. 2008;47(1):51‐58. [DOI] [PubMed] [Google Scholar]
- 24.Lautamäki R, Borra R, Iozzo P, et al. Liver steatosis coexists with myocardial insulin resistance and coronary dysfunction in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2006;291(2):E282‐E290. [DOI] [PubMed] [Google Scholar]
- 25.Dongiovanni P, Stender S, Pietrelli A, et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J Intern Med. 2018;283(4):356‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen T, Abdelmalek MF, Sullivan S, et al. Fructose and sugar: a major mediator of non‐alcoholic fatty liver disease. J Hepatol. 2018;68(5):1063‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trépo E, Luca VL. Update on NAFLD genetics: From new variants to the clinic. J Hepatol. 2020;72(6):1196‐1209. [DOI] [PubMed] [Google Scholar]
- 28.Bianco C, Jamialahmadi O, Pelusi S, et al. Non‐invasive stratification of hepatocellular carcinoma risk in non‐alcoholic fatty liver using polygenic risk scores. J Hepatol. 2021;74(4):775‐78228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nobili V, Liccardo D, Bedogni G, et al. Influence of dietary pattern, physical activity, and I148M PNPLA3 on steatosis severity in at‐risk adolescents. Genes Nutr. 2014;9(3):392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abul‐Husn NS, Cheng X, Li AH, et al. A protein‐truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med. 2018;378(12):1096‐1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma J, Hennein R, Liu C, et al. Improved diet quality associates with reduction in liver fat, particularly in individuals with high genetic risk scores for nonalcoholic fatty liver disease. Gastroenterology. 2018;155(1):107‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]


