Abstract
Modafinil, a widely used psychoactive drug, has been shown to exert a positive impact on cognition and is used to treat sleep disorders and hyperactivity. Using time‐of‐flight secondary ion mass spectrometric imaging, we studied the changes of brain lipids of Drosophila melanogaster induced by modafinil to gain insight into the functional mechanism of modafinil in the brain. We found that upon modafinil treatment, the abundance of phosphatidylcholine and sphingomyelin species in the central brain of Drosophila is significantly decreased, whereas the levels of phosphatidylethanolamine and phosphatidylinositol in the brains show significant enhancement compared to the control flies. The alteration of brain lipids caused by modafinil is consistent with previous studies about cognition‐related drugs and offers a plausible mechanism regarding the action of modafinil in the brain as well as a potential target for the treatment of certain disorders.
Keywords: cognitive enhancer, drosophila melanogaster, lipids, modafinil, secondary ion mass spectrometry
Modafinil, used for sleep disorder treatment, appears to enhance attention as well as improve learning and memory. Imaging mass spectrometry is used to investigate the effects of modafinil on the alteration of lipid composition on fly brain. Modafinil induces a decrease in PC and SM contents and increases the levels of PEs and PIs. The lipid changes caused by modafinil are similar to the effects of methylphenidate, which also improves cognition.
Modafinil (diphenylmethyl‐sulfinyl‐2 acetamide) is a nonamphetaminergic psychoactive drug with multiple positive effects on wakefulness, locomotor activity, and cognitive ability.[1] Modafinil is primarily prescribed for the treatment of several kinds of sleep disorders.[2] Recently, the therapeutic efficacies of modafinil in the improvement of cognition in Parkinson's disease, attention deficit/hyperactivity disorder, depression, and drug addiction have been demonstrated.[3] Despite its stimulant effect, modafinil depicts a limited potential for abuse.[4] Currently, there is a rise in the use of modafinil by healthy individuals, such as students or on‐call physicians, who need to boost their concentration in hard work situations.[5]
The mechanism of chemical action of modafinil is complex and still uncertain after more than three decades of research.[6] Studies on neurochemical levels have shown that modafinil increases extracellular levels of dopamine, norepinephrine, glutamate, and serotonin.[1] On the other hand, it attenuates GABAergic neurotransmission in the brain.[1] Some researchers have suggested that the presynaptic bindings of modafinil to the dopamine transporter (DAT) and norepinephrine transporter are key pharmacological events in mediating the wake‐promoting effects of modafinil.[7] In addition to that, several other studies have shown that modafinil also modulates the activity of histamine, hypocretin, adrenergic, and glutamate receptors. However, modafinil interacts with DAT differently than classical cocaine‐like inhibitors.[8] Based on in vitro experiments, modafinil exhibits weak selective affinity to DAT, and not at all to any other binding sites and receptors.[9]
Beyond acting on the catecholaminergic system, modafinil induces significant alterations in the lipid composition of the brain.[10] The brain is a lipid‐rich organ and various lipids distribute in different regions of the brain. In the fly brain, there are a variety of lipids distributed in the central brain, optical lobes, and proboscis.[11] The lipid composition of the brain is of great importance to different aspects of cellular metabolism and function.[12] Alteration and perturbation of fatty acid composition in the brain have been linked to several neurological disorders and neurodegenerative diseases.[13] Hence, understanding the knowledge about chemical lipid composition in the brain is essential to pursue possible mechanisms that occur in diseases. Although some papers have discussed the effect of modafinil on the lipid alterations in the brain and blood,[10a, 14] this is the first report of modafinil's effect on the regional composition of the phospholipids, triacylglycerols (TAG), and sphingolipid in the fly brain.
The major development having spurred advances in lipid analysis is mass spectrometry.[15] Time‐of‐flight secondary ion mass spectrometry (ToF‐SIMS) imaging[11] is well suited for the study of lipids in biological samples from single cells to large biological tissue sections,[16] allowing parallel and label‐free detection of a wide range of molecular species with m/z up to 1500 Da, including small molecules, intact lipids, and small peptides.[11] Liquid chromatography mass spectrometry (LC‐MS) can also be used to determine the alteration of the brain's lipid composition as it is very sensitive and can be quantitative; however, it provides no localization information since the lipids need to be extracted from the homogenate matrix of the brain. Regardless of the analytical approach, the LC‐MS's success rate, in this case, depends on the completeness of lipids extraction from the brain tissue. Moreover, coextracted components from the complex matrix of the brain will affect both the sensitivity and specificity of the lipid extraction and analysis.
In this work, we applied ToF‐SIMS imaging to identify the lipid changes in the fly brain following modafinil treatment. We have discovered that modafinil induces alterations in the concentration and metabolism of brain lipids, which might be associated with the cognitive‐enhancing effects brought by modafinil. These changes are similar to those for the cognition enhancing methylphenidate and opposite to cognition‐diminishing cocaine.[11]
For over a century, the fruit fly, Drosophila melanogaster, has been a key model for studying biological processes involved in cognitive functions, memory, and learning.[17] Furthermore, the fruit fly has a relatively simple nervous system but possesses high physiological similarities to humans.[18] Hence, we used Drosophila to investigate the effects of modafinil on the chemical lipid structure of the brain. We hypothesize that this psychostimulant acts in part by altering the lipidic structure of the brain.
We fed the flies with a yeast‐based food containing 10 mM of modafinil for three days followed by TOF‐SIMS imaging (Figure 1). The ToF‐SIMS data offer semi‐quantification of the relative abundance of several lipid species in fly brains as well as their spatial distribution across the sample surface. Full spectra and a peak assignment list of ToF‐SIMS analysis for fly head sections are provided in the Supporting Information, Figure S1, and Table S1.
Figure 1.
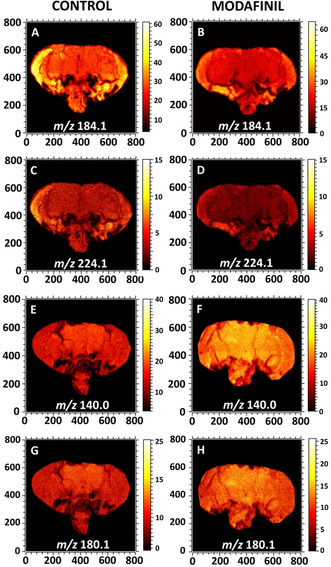
ToF‐SIMS images display the distribution of lipid headgroup fragments in fly brain sections of control (left column) and modafinil‐treated (right column) flies analyzed with ToF‐SIMS. A),B) Ion images of the PC and SM head group at m/z 184.1 in the positive ion mode; C),D) PC fragment at m/z 224.1 in the positive ion mode; and E)–H) PE fragments at m/z 140.0 and 180.1 in the negative ion mode. All the images were recorded with the ToF.SIMS V instrument equipped with 25 keV Bi3 ++ as a primary ion beam. The primary ion beam current was 0.3 pA and the total ion dose was 2×1012 ions cm−2.
Figure 1 reveals the localization of different lipid fragments across the fly brain of control and modafinil treatment groups. Phosphatidylcholine (PC) and sphingomyelin (SM) have the same structure for their phosphate head group and thereby, produce the same head group ion at m/z 184.1 corresponding to phosphocholine. The peak at m/z 224.1, however, is characteristic of the PC fragment and is distinct from SM.[19] ToF‐SIMS images of freeze‐dried fly brain sections show that the fragments at m/z 184.1 and 224.1 are distributed evenly across the entire surface of the brain but appear with stronger intensities in the optical lobe regions. After modafinil administration, there is no significant difference in the distribution of these peaks, but the intensities decrease. In contrast to the PC fragments, modafinil induces an elevation of the levels of phosphatidylethanolamine (PE) fragments at m/z 140.0 and 180.1. The ion images of some intact lipids are also provided in Figure S2.
To investigate the alteration of the lipid composition in fly brains after modafinil administration, we applied region of interest (ROI) selection to different regions of the fly section including the central fly brain and optic lobes using lipid membrane signals (Figure S3). The data obtained from ToF‐SIMS imaging are usually complex and large in size containing perhaps thousands of peaks. Principal component analysis (PCA), therefore, is used to simplify data with a minimal loss of information. Modafinil induces an alteration of the lipids of the central area of the fly brain. There is no significant difference in the optic regions after modafinil treatment. Interestingly, psychostimulants, such as cocaine and methylphenidate, also showed no effect on the lipid contents in the fly optic lobes.[11, 16b] PCA in the positive ion mode displays a clear separation across the principal component 2 (Figure 2 A). The contributions to the loading plot generated from principal component 2 identifies the main chemical species that change after modafinil exposure (Figure 2 B). Modafinil induces depletion of PC and SM species in the central fly brain regions. For example, species SM (d32:1)+Na at m/z 697.5, SM (d34:1)+Na at m/z 725.5, PC (34:1) at m/z 760.6, PC (34:1)+K at m/z 798.5, PC (36:4)+Na at m/z 804.5, and PC (36:3)+K at m/z 822.5 are less abundant in modafinil‐treatment samples compared to control. The decrease in the levels of PCs and SMs are associated with their fragments at m/z 184.1 and 224.1 observed in Figure 1. The ratios of Na/K adducts over [M+H]+ for different PC species are also compared after modafinil administration, as seen in Figure S4. The treatment of modafinil showed no effect of the salt levels in fly brains. In contrast to PC species, Na/K salts of TAGs, such as TAG (46:2)+K at m/z 815.7, TAG (48:1)+Na at m/z 827.7, TAG (48:2)+K at m/z 841.7, and TAG (50:2)+K at m/z 869.7, are more abundant in modafinil‐treated fly brains compared to control.
Figure 2.
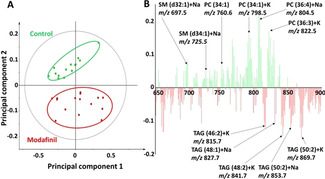
PCA of the positive ion mode data of the central brain regions of control versus modafinil‐treated flies from the ToF‐SIMS experiments. A) Score plot of principal component 1 versus principal component 2 from the spectra. B) Corresponding loading plot of the principal component 2 shows the peaks contributing to the separation of the two fly groups. The data from 12 control and 15 treated flies were used for PCA.
In the negative ion mode, PCA separates control and modafinil‐treated flies completely across principal component 2, as seen in Figure 3 A. The loading plot associated with principal component 2 suggests that modafinil‐treated flies have higher levels of PE and PI species, such as PE (34:1) at m/z 716.5, PE (36:1) at m/z 744.6, PE (38:2) at m/z 770.6, PI (32:1) at m/z 807.5, PI (34:1) at m/z 835.5, and PI (36:3) at m/z 859.5 (Figure 3 B). Similar to the positive ion mode, unsaturated SM species, which are detected as [M−CH3]−,[20] appear to be reduced following modafinil administration, including SM (d32:1) at m/z 659.5, SM (d34:1) at m/z 687.5, SM (d36:1) at m/z 715.6, and SM (d42:2) at m/z 797.6.
Figure 3.
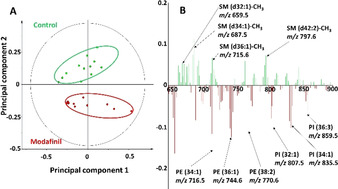
PCA of negative ion mode data of the central brain regions of control versus modafinil‐treated flies from the ToF‐SIMS experiments. A) Score plot of principal component 1 versus principal component 2 from the spectra. B) Corresponding loading plot of the principal component 2 showing the peaks contributing to the separation of the two fly groups. Twelve samples of control and treated flies were used for PCA.
PCA analysis is one of the best approaches to indicate the peaks associated with the separation. However, it is hard to interpret the precise differences based on the scores and loadings. Hence, the changes in the level of each molecular species are measured and evaluated with statistical analysis. The lipid composition has been primarily identified using the relative intensities of different lipid molecules. We observe a statistically significant decrease (p<0.05, probability value) in the abundances of PCs and their Na/K adducts in modafinil‐treated fly brains (Figure 4). Likewise, modafinil induces a depletion in the abundance of the Na adducts of unsaturated SM species, as seen in Figure S5A. A decrease in the signals of head group ions at m/z 184.1 and 224.1 correlating to PC and SM species is obtained following modafinil exposure, as (Figure 4). In contrast, we find that the relative amounts of Na/K adducts of TAG species increase in flies fed with modafinil (Figure S5A). We speculate modafinil enhances TAG levels by enhancement of cyclic adenosine monophosphate (cAMP) levels. Modafinil blocks the KCa3.1 channel, resulting in the elevation of the intracellular cAMP levels.[21] Also, it has been reported that modafinil promotes cAMP levels as a G protein‐coupled adenosine receptor agonist.[22] As the cAMP signal pathway is involved in the regulation of TAG biosynthesis,[23] we speculate that the upregulation of the TAG induced by modafinil might result from G protein‐coupled adenosine receptor activation.
Figure 4.
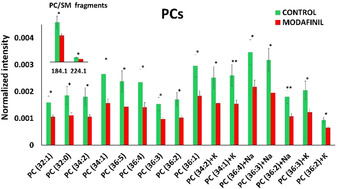
Relative quantification of PC species in the central brains of control and modafinil‐treated samples. Data were collected from 23 flies for control and 24 flies for modafinil‐treated group. Green and red bars correspond to control and modafinil‐treated groups, respectively. Peak intensities of lipid species were normalized as discussed in the SI. The error bars represent standard error of the mean (SEM). PC species were detected as [M+H]+ unless specified as Na/K adduct species. *: p<0.05, **: p<0.01.
The data obtained from PCA analysis of ToF‐SIMS spectra in the negative ion mode show opposite alterations for the PE and PI levels compared to those for PC and SM. There is a significant elevation in the relative abundances of unsaturated PEs and PIs, as shown in Figure 5. Specifically, the PE and PI fragments at m/z 140.0, 180.1, and 241.1 are elevated after modafinil exposure. Consistent with the results obtained from the positive ion mode, modafinil causes a decrease in the levels of unsaturated SM species with 32, 34, 36, and 42 carbon fatty acid chains (Figure S5B). PC is a precursor for SM synthase that transfers phosphorylcholine from PC to ceramide during the synthesis of SM.[24] Hence, a decrease of SM in the modafinil‐treated flies could be the result of PC attenuation. A role for SM in neuroinflammatory regulation has been suggested.[25] Enhanced levels of SMs have been associated with neuroinflammation and heart disease.[26] In addition to wake‐promoting properties, the chemical mechanisms of modafinil could protect against dopamine toxicity, cell death, and especially neuroinflammation.[27] Our data reveal that modafinil induces a reduction in SM levels in fly brains, suggesting an action pathway using the anti‐inflammation properties of modafinil in the brain.
Figure 5.
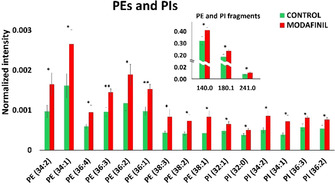
Relative quantification of PE and PI species in the central brains of control and modafinil‐treated Drosophila. Data were collected from 24 flies for both groups. Green and red bars correspond to control and modafinil‐treated groups, respectively. The error bars represent SEM. PE and PI species were detected as [M−H]−. *: p<0.05, **: p<0.01.
Phospholipids are unequally localized in the two membrane leaflets of neurons and thereby, the ratio of PC/PE may affect the biophysical properties of the membrane, including bilayer curvature, elasticity, and viscosity. The intrinsic curvature of the phospholipids, depending on the size of their headgroups and their acyl chain compositions (length and saturation degree), confers various membrane shaping needed in several neuronal functions. Previous studies revealed that exocytosis, as an axial intercellular communication process, is impacted by the intrinsic curvature of lipids.[28] The reduction in the low curvature lipids, PC and SM, and the enhancement of high curvature lipids, PE and PI, after modafinil treatment, appear to be similar to the lipid changes induced by methylphenidate, a cognition‐enhancing drug.[11, 16b] Interestingly, our previous studies on fruit flies showed that cocaine and zinc deficiency increase PC levels while reducing the abundance of PE and PI species, which are opposite to the effect of modafinil on lipid composition shown here.[16b, 29]
In summary, our ToF‐SIMS data reveal that chemical student drug modafinil decreases PC and SM levels, while it enhances the abundance of PE and PI species in a fly brain model. Modafinil induces alterations in the concentration and metabolism of brain lipids, which might be associated with the cognitive‐enhancing effects brought by modafinil. While most studies have focused on the effects of modafinil on different proteins, the fundamental results of this work from a lipid perspective might be important for understanding the correlation of modafinil's cognitive impact and the compositional changes of phospholipids in the brain. These data also suggest lipid‐modifying therapies as new possible chemical targets to improve cognitive decline in diseases.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Acknowledgements
All the experiments were performed at the Chalmers Univ./Gothenburg Univ. Chemical Imaging Infrastructure. The authors acknowledge funding from the European Research Council (Advanced Grant Acronym NanoBioNext and project number 787534), the Knut and Alice Wallenberg Foundation, and the Swedish Research Council (VR grant number 2017‐04366). E.R. acknowledges funding from the Human Frontier Science Program Organization (HFSPO; Reference number: LT 000407/2017C).
M. H. Philipsen, E. Ranjbari, C. Gu, A. G. Ewing, Angew. Chem. Int. Ed. 2021, 60, 17378.
References
- 1.Minzenberg M. J., Carter C. S., Neuropsychopharmacology 2008, 33, 1477–1502. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a.Golicki D., Bała M., Niewada M., Wierzbicka A., Med. Sci. Monit. 2010, 16, RA177–RA186; [PubMed] [Google Scholar]
- 2b.Pack A. I., Black J. E., Schwartz J. R., Matheson J. K., Am. J. Respir. Crit. Care Med. 2001, 164, 1675–1681; [DOI] [PubMed] [Google Scholar]
- 2c.Czeisler C. A., Walsh J. K., Roth T., Hughes R. J., Wright K. P., Kingsbury L., Arora S., Schwartz J. R., Niebler G. E., Dinges D. F., N. Engl. J. Med. 2005, 353, 476–486. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a.Turner D. C., Clark L., Dowson J., Robbins T. W., Sahakian B. J., Biol. Psychiatry 2004, 55, 1031–1040; [DOI] [PubMed] [Google Scholar]
- 3b.Kaser M., Deakin J. B., Michael A., Zapata C., Bansal R., Ryan D., Cormack F., Rowe J. B., Sahakian B. J., Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martínez-Raga J., Knecht C., Cepeda S., Curr. Drug Abuse Rev. 2008, 1, 213–221. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a.Franke A. G., Bagusat C., Dietz P., Hoffmann I., Simon P., Ulrich R., Lieb K., BMC Med. 2013, 11, 102; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5b.Greely H., Sahakian B., Harris J., Kessler R. C., Gazzaniga M., Campbell P., Farah M. J., Nature 2008, 456, 702–705. [DOI] [PubMed] [Google Scholar]
- 6.Ramachandra B., Crit. Rev. Anal. Chem. 2016, 46, 482–489. [DOI] [PubMed] [Google Scholar]
- 7.Wisor J. P., Nishino S., Sora I., Uhl G. H., Mignot E., Edgar D. M., J. Neurosci. 2001, 21, 1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitt K. C., Reith M. E., PLoS One 2011, 6, e25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mignot E., Nishino S., Guilleminault C., Dement W., Sleep 1994, 17, 436–437. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a.Ornell F., Valvassori S. S., Steckert A. V., Deroza P. F., Resende W. R., Varela R. B., Quevedo J., Behav. Neurol. 2014, 2014, 1; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10b.Xiao Y.-L., Fu J.-M., Dong Z., Yang J.-Q., Zeng F.-X., Zhu L.-X., He B.-C., Acta Pharmacol. Sin. 2004, 25, 301–305. [PubMed] [Google Scholar]
- 11.Phan N. T., Fletcher J. S., Ewing A. G., Anal. Chem. 2015, 87, 4063–4071. [DOI] [PubMed] [Google Scholar]
- 12.Chavko M., Nemoto E. M., Melick J. A., Mol. Chem. Neuropathol. 1993, 18, 123–131. [DOI] [PubMed] [Google Scholar]
- 13.
- 13a.Farooqui A. A., Horrocks L. A., Farooqui T., Chem. Phys. Lipids 2000, 106, 1–29; [DOI] [PubMed] [Google Scholar]
- 13b.Han X., Holtzman D. M., D. W.McKeel, Jr. , Kelley J., Morris J. C., J. Neurochem. 2002, 82, 809–818. [DOI] [PubMed] [Google Scholar]
- 14.Kumar M., Maqbool S., Neurotoxicology 2020, 78, 106–115. [DOI] [PubMed] [Google Scholar]
- 15.Adibhatla R. M., Hatcher J., Dempsey R., AAPS J. 2006, 8, E314–E321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.
- 16a.Kaya I., Brülls S. M., Dunevall J., Jennische E., Lange S., MÅrtensson J., Ewing A. G., Malmberg P., Fletcher J. S., Anal. Chem. 2018, 90, 13580–13590; [DOI] [PubMed] [Google Scholar]
- 16b.Philipsen M. H., Phan N. T., Fletcher J. S., Malmberg P., Ewing A. G., ACS Chem. Neurosci. 2018, 9, 1462–1468. [DOI] [PubMed] [Google Scholar]
- 17.
- 17a.Greenspan R. J., van Swinderen B., Trends Neurosci. 2004, 27, 707–711; [DOI] [PubMed] [Google Scholar]
- 17b.Zars T., Learn. Mem. 2010, 17, 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jennings B. H., Mater. Today 2011, 14, 190–195. [Google Scholar]
- 19.Tian H., Fletcher J. S., Thuret R., Henderson A., Papalopulu N., Vickerman J. C., Lockyer N. P., J. Lipid Res. 2014, 55, 1970–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angerer T. B., Magnusson Y., Landberg G., Fletcher J. S., Anal. Chem. 2016, 88, 11946–11954. [DOI] [PubMed] [Google Scholar]
- 21.Lee H., Kim K. C., Cho M.-S., Suh S.-h., Hong Y. M., Pediatr. Res. 2016, 80, 119–127. [DOI] [PubMed] [Google Scholar]
- 22.Pal China S., Pal S., Chattopadhyay S., Porwal K., Mittal M., Sanyal S., Chattopadhyay N., Toxicol. Appl. Pharmacol. 2018, 348, 22–31. [DOI] [PubMed] [Google Scholar]
- 23.
- 23a.Holm C., Biochem. Soc. Trans. 2003, 31, 1120–1124; [DOI] [PubMed] [Google Scholar]
- 23b.Miao R., Huang K., J. Oceanol. Limnol. 2020, 38, 517–528. [Google Scholar]
- 24.
- 24a.Marggraf W. D., Zertani R., Anderer F. A., Kanfer J. N., Biochim. Biophys. Acta Lipids Lipid Metab. 1982, 710, 314–323; [DOI] [PubMed] [Google Scholar]
- 24b.Nikolova-Karakashian M., Methods Mol. Biol. 2021, 2187, 113–129. [DOI] [PubMed] [Google Scholar]
- 25.
- 25a.Maceyka M., Spiegel S., Nature 2014, 510, 58–67; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25b.Lee J. Y., Jin H. K., Bae J. S., BMB Rep. 2020, 53, 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.
- 26a.Mielke M. M., Haughey N. J., Bandaru V. V., Weinberg D. D., Darby E., Zaidi N., Pavlik V., Doody R. S., Lyketsos C. G., J. Alzheimer′s Dis. 2011, 27, 259–269; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26b.Kosicek M., Zetterberg H., Andreasen N., Peter-Katalinic J., Hecimovic S., Neurosci. Lett. 2012, 516, 302–305. [DOI] [PubMed] [Google Scholar]
- 27.Raineri M., Gonzalez B., Goitia B., Garcia-Rill E., Krasnova I. N., Cadet J. L., Urbano F. J., Bisagno V., PLoS One 2012, 7, e46599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uchiyama Y., Maxson M. M., Sawada T., Nakano A., Ewing A. G., Brain Res. 2007, 1151, 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philipsen M. H., Gu C., Ewing A. G., ChemBioChem 2020, 21, 2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information


