Summary
Background
Tegoprazan is a novel, fast‐ and long‐acting potassium‐competitive acid blocker that suppresses gastric acid secretion, which could benefit patients with non‐erosive reflux disease (NERD), a type of gastroesophageal reflux disease.
Aim
To evaluate the efficacy and safety profiles of tegoprazan compared with those of a placebo in Korean patients with NERD.
Methods
In this phase 3, double‐blind, placebo‐controlled, multicentre study, 324 Korean patients with NERD were randomised into three treatment groups: tegoprazan 50 mg, tegoprazan 100 mg and placebo. These drugs were provided once daily for 4 weeks. The primary endpoint was the proportion of patients with complete resolution of major symptoms (both heartburn and regurgitation) for the last 7 days of the 4‐week treatment period. Other outcomes related to efficacy, safety and tolerability were also evaluated.
Results
Among all, 42.5% (45/106), 48.5% (48/99) and 24.2% (24/99) of patients showed complete resolution of major symptoms at week 4 after receiving tegoprazan 50 mg, tegoprazan 100 mg, and placebo, respectively. Both doses of tegoprazan showed superior efficacy than the placebo (P = 0.0058 and P = 0.0004, respectively). The complete resolution rates of heartburn and proportions of heartburn‐free days (as other efficacy outcomes) were significantly higher in both tegoprazan groups than in the placebo group (P < 0.05 for all). No significant difference in the incidence of treatment‐emergent adverse events were noted.
Conclusions
Tegoprazan 50 and 100 mg showed superior therapeutic efficacy compared with the placebo, as well as a favourable safety profile in patients with NERD.
Registration number: ClinicalTrials.gov identifier NCT02556021.
Tegoprazan 50 and 100 mg showed superior therapeutic efficacy compared with the placebo, as well as a favourable safety profile in patients with non‐erosive reflux disease.

1. INTRODUCTION
About 4.4%‐14.0% of the adult population experience symptoms of gastroesophageal reflux disease (GERD), such as acid regurgitation and heartburn.1, 2, 3, 4 Among them, more than 50% exhibit normal oesophageal mucosa on upper endoscopy, diagnosed as non‐erosive reflux disorder (NERD).5, 6, 7, 8 NERD, which significantly compromises the patient quality of life (QoL), is commonly and daily seen in the clinical setting.5, 6, 8, 9
Proton pump inhibitors (PPIs) are currently used as a first‐line therapy for NERD,10, 11 with the clinical goal of reducing reflux symptoms. However, previous studies showed that reflux symptoms were not completely resolved in approximately 30%‐55% of patients with NERD.12, 13 Therefore, a new drug is needed to improve the clinical outcomes for patients with NERD.
Tegoprazan is a novel potassium‐competitive acid blocker (P‐CAB) that exhibits rapid and effective anti‐secretory activity by reversibly binding to the H+/K+‐ATPase on the parietal cell.13, 14 P‐CAB inhibits the proton pump through a competitive interaction with the potassium site of the enzyme without acid activation.14, 15, 16, 17 P‐CAB blocks the active and the inactive forms of the proton pump. Thus, P‐CAB inhibits the gastric acid secretion rapidly and for longer time, which could also improve the clinical outcomes, such as heartburn and regurgitation, in patients with NERD. Animal studies and clinical pharmacology studies in healthy volunteers demonstrated that tegoprazan exhibited its maximum acid inhibitory effect rapidly and for a longer period than esomeprazole or revaprazan.18, 19, 20 We assumed that these properties of tegoprazan would improve the clinical outcomes, such as heartburn and regurgitation, in patients with NERD.
Therefore, in this study, we evaluated the efficacy—in terms of the proportion of patients with complete resolution of symptoms including heartburn and regurgitation (primary objective)—and the safety (secondary objective) of tegoprazan in patients with NERD compared with those of a placebo. The recommended clinical dose of tegoprazan was also determined.
2. MATERIALS AND METHODS
2.1. Study subjects
Male or female patients were out‐patients aged ≥20 years old who had recurrent typical GERD symptoms (heartburn and regurgitation) for ≥3 months before screening, both heartburn and regurgitation for 7 days prior to randomisation, and with mild severity for ≥2 days/week or with moderate and severe ones for ≥1 day/week.
The major exclusion criteria included complications associated with erosive reflux disease (ERD), acute upper gastrointestinal bleeding, acute gastritis, gastric or duodenal ulcer within 2 months before screening esophagogastroduodenoscopy (EGD), history of gastric or oesophageal surgery, Barrett's oesophagus or oesophageal stricture, eosinophilic oesophagitis, Zollinger‐Ellison syndrome, diagnosis of depression, abnormal laboratory test values at the screening (blood urea nitrogen and serum creatinine level, >1.5 upper limit of normal [ULN]; total bilirubin levels and serum levels of alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase and gamma glutamyltransferase, >2 ULN) or any other conditions or diseases that an investigator considered not appropriate for this study. Pregnant and lactating female subjects were excluded from this study.
Patients were not allowed to concomitantly use any medications that could affect the efficacy evaluation, such as PPIs (within 2 weeks before endoscopy), histamine receptor 2 blockers, prokinetics or antacids (within 7 days before endoscopy), anti‐depressants, anti‐psychotics and anti‐anxiety drugs.
2.2. Study design and treatments
This was a phase 3, randomised, double‐blind, multicentre (17 centres, 17 investigators in South Korea), placebo‐controlled, parallel‐group, three‐arm study, conducted from September 2015 to November 2016. In this study, the therapeutic effect of tegoprazan 50 and 100 mg, compared with that of the placebo, was analysed in patients with NERD who displayed normal mucosa at the screening EGD (endoscopic appearance of NERD defined as normal (N), according to the Los Angeles classification). The clinical protocol was approved by the Institutional Review Boards of each institute and followed the Declaration of Helsinki and the International Congress on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use—Good Clinical Practice (ICH‐GCP) guidelines. The study was registered in ClinicalTrials.gov under the number NCT02556021. All patients signed the informed consent form before inclusion in the study and initiation of study procedures.
Following a screening period (2 weeks), patients were randomised 1:1:1 (block randomisation, block size = 6) to receive orally and once daily either tegoprazan (50 or 100 mg) or placebo for 4 weeks. This study was assigned by central enrolment, and Interactive Web Response System (IWRS) was used for randomisation. All study drugs were manufactured by HK inno.N Corp. Patients with intolerable pain caused by NERD symptoms were allowed to take up to one dose per day of the rescue medication (Gelfos‐M Suspension, Boryung Pharmaceutical Co., Ltd.).
Patients received tegoprazan 50 mg, 100 mg, or placebo, and rescue medication on day 1 and returned for visits at weeks 2, 4, and 6 (follow‐up) for the assessment of NERD symptoms (Figure 1). Unused study drug and rescue medication were collected at weeks 2 and 4, and new ones were dispensed at week 2. All study patients underwent a complete physical examination and were evaluated for treatment‐emergent adverse events (TEAEs). Laboratory analyses and pregnancy tests for female patients were performed at screening, week 4, and follow‐up visits.
FIGURE 1.
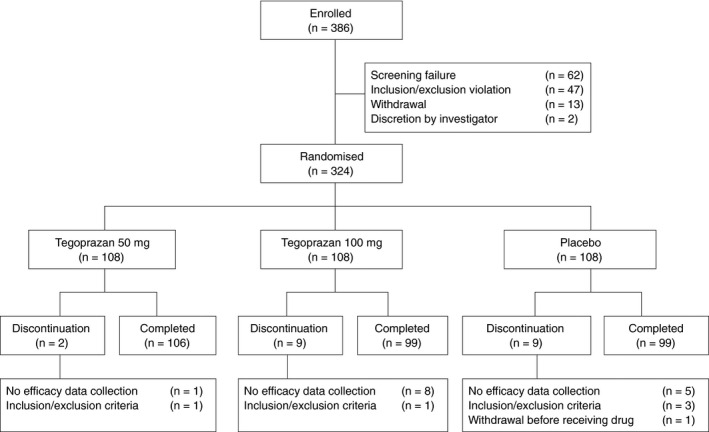
Randomisation protocol and patient disposition
Patient symptoms were assessed with the Reflux Disease Questionnaire (RDQ), in which patients were asked to report six symptoms covering three items (heartburn, regurgitation and dyspepsia) using a 12‐item self‐administered questionnaire, designed to assess the frequency and severity of the symptoms. The mean RDQ score was calculated at baseline and at weeks 2 and 4 and follow‐up visit. Heartburn and regurgitation were defined according to the Montreal definition. Heartburn was defined as a "burning sensation in the posterior bony thorax area (back of the bone, near the breast and in the epigastric region)"; meanwhile, regurgitation was defined as a "feeling of movement from the stomach to the mouth of the hypopharynx (the part adjacent to the oesophagus, below the airway), that resulted in a bitter or sour taste in the mouth."21
Patients recorded once a day the occurrence of heartburn and regurgitation without distinction of onset time (day or night time) in the patient diary (paper) before going to bed. The following 5‐point scale, defined by the patient, assessed the severity of heartburn and regurgitation symptoms: no symptoms, mild: symptom did not interfere with routine activities including sleep; moderate: slight discomfort and interference with routine activities including sleep; severe: recurring symptoms that frequently interfered with routine activities including sleep; and very severe: consistent symptoms that substantially interfered with routine activities including sleep.
2.3. Outcome measures
The primary efficacy endpoint was the proportion of patients with complete resolution of major symptoms (both heartburn and regurgitation) for the last 7 days of week 4 (treatment period), according to the RDQ score.22, 23 Complete resolution was defined as no symptoms. The secondary efficacy endpoints included RDQ‐based complete resolution of the major symptoms at week 2, and of heartburn at weeks 2 and 4, and the proportions of days without heartburn during the 4‐week treatment period, as reported in the patient's diary.
As patients with severe heartburn may be more likely to have acid‐related NERD (true NERD), subgroup analyses of the proportions of heartburn‐free days during the 4‐week treatment period and daily proportions of patients without heartburn were performed in patients with NERD, who experienced moderate and severe heartburn during the screening period (n = 41, n = 42 and n = 39 in tegoprazan 50 mg, 100 mg and placebo groups, respectively).
Safety was evaluated by TEAEs at weeks 2 and 4, and follow‐up visits as well as physical examination, electrocardiogram, vital signs (blood pressure, heart rate and temperature) and laboratory test results (haematology, blood chemistry, blood coagulation test and urinalysis). Laboratory data were monitored for clinically significant changes from baseline. Adverse drug reactions were defined as adverse events for which a causal relationship could not be ruled out. All adverse events reported during the study, regardless of their relationship with the study drug, were recorded in detail in terms of the date of onset, duration (if applicable), seriousness, severity, the required treatment modification, the causal relationship with the study medication and the outcome. Adverse events, adverse drug reactions and serious adverse events were recorded using MedDRA 19.1 and classified by System Organ Class and Preferred Term.
2.4. Statistical analysis
Based on previous study results,24 assuming 19.9% difference in the outcome proportion from placebo group, number of patients with this assumption was 86 in each treatment group to observe intergroup differences in the proportion of patients with complete resolution of major symptoms (heartburn and regurgitation) at week 4 of the treatment period with a power of 80% at a significance level of 0.05. Additionally, the total sample size was 324 subjects with 108 patients per group considering 20% dropout.
Efficacy assessments were analysed primarily in the full‐analysis set (FAS). Safety assessments were analysed in the safety set. For the primary endpoint, the proportion of patients with complete resolution of the major symptoms for the last 7 days of week 4 (treatment period), according to the RDQ score, was calculated in the FAS. Chi‐square and Fisher's exact tests were used to compare the primary endpoints, the complete resolution rate of heartburn or regurgitation, the proportion of heartburn‐free days and the daily proportion of patients without heartburn between tegoprazan and placebo administrations. Hochberg method was used to adjust the significance level for multiple comparisons.
Statistical analyses were performed using SAS version 9.3 (SAS Institute), and two‐sided P‐values of < 0.05 were considered statistically significant.
3. RESULTS
3.1. Study subjects
Out of the 386 patients enrolled in the study, 47 subjects were ineligible based on the inclusion/exclusion criteria, 13 subjects withdrew the consent and two subjects were determined ineligible for other reasons by the investigator. A total of 324 eligible patients were randomised into the three treatment arms, including tegoprazan 50 mg (n = 108), 100 mg (n = 108) and placebo (n = 108). Twenty patients (6.2%) did not complete the study. One subject, assigned to the placebo arm, withdrew consent before taking the study drug and was excluded from the safety set analysis. Additionally, 19 (5.9%) were discontinued from the study due to inclusion/exclusion criteria violation (n = 4, 1.2%) and no collection of efficacy data (n = 14, 4.3%) (Figure 2).
FIGURE 2.
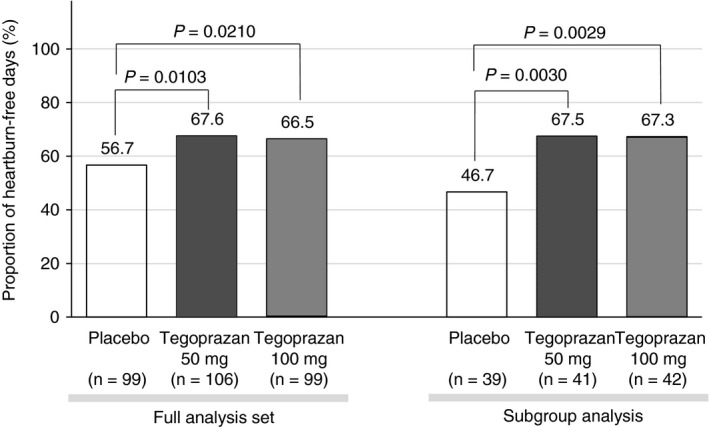
Proportion of heartburn‐free days, according to the full‐analysis set or subgroup analysis of patients, who experienced moderate or severe heartburn symptom during the screening period
The patients' and baseline characteristics are summarised in Table 1. No significant differences in baseline characteristics were observed between the treatment groups.
TABLE 1.
Demographic characteristics (full‐analysis set)
| Demographics | Tegoprazan | Placebo | |
|---|---|---|---|
| 50 mg (n = 106) | 100 mg (n = 99) | (n = 99) | |
| Age (years) | 45.4 (12.2) | 44.1 (13.3) | 45.6 (13.3) |
| Male (%) | 31 (29.3) | 37 (37.4) | 31 (31.3) |
| Height (cm) | 163.5 (8.8) | 164.4 (8.4) | 163.9 (8.2) |
| Weight (kg) | 61.3 (10.8) | 63.3 (11.8) | 62.7 (12.1) |
| Body mass index (kg/m2) | 22.9 (3.2) | 23.3 (3.4) | 23.2 (3.0) |
| ≥25 and ≤30, n (%) | 22 (20.8) | 20 (20.2) | 27 (27.3) |
| >30, n (%) | 2 (1.9) | 4 (4.0) | 1 (1.0) |
| Smoking, n (%) | 12 (11.3) | 19 (19.2) | 11 (11.1) |
| Alcohol consumption, n (%) | 33 (31.1) | 39 (39.4) | 33 (33.3) |
Values are presented as mean (standard deviation) except for gender, smoking, and alcohol consumption, which were presented as mean (%).
Abbreviation: n, number of patients.
3.2. Efficacy analysis
3.2.1. Complete resolution rates of major symptoms (both heartburn and regurgitation)
The proportion of patients with complete resolution of major symptoms (heartburn and regurgitation) for the last 7 days of the treatment (week 4) were 42.5% (45/106) with tegoprazan 50 mg, 48.9% (48/99) with the tegoprazan 100 mg and 24.2% (24/99) with placebo, respectively (Table 2). All doses of tegoprazan were superior in terms of completely resolving major symptoms compared with the placebo (P = 0.0058 and P = 0.0004 for tegoprazan 50 and 100 mg, respectively).
TABLE 2.
Summary of efficacy results (full‐analysis set)
| Parameters | Tegoprazan | Placebo | P‐value (vs placebo) | |
|---|---|---|---|---|
| 50 mg (n = 106) | 100 mg (n = 99) | (n = 99) | [tegoprazan 50 mg; 100 mg] | |
| Complete resolution of major symptoms (RDQ score) | ||||
| Week 4, n (%) | 45 (42.5) | 48 (48.5) | 24 (24.2) | [P = 0.0058; P = 0.0004]a |
| [Difference from placebo, n (%)] | [23 (18.3)] | [24 (24.3)] | ||
| Week 2, n (%) | 17 (16.0) | 23 (23.2) | 10 (10.1) | [P = 0.2091; P = 0.0264]a |
| [Difference from placebo, n (%)] | [7 (5.9)] | [13 (13.1)] | ||
| Complete resolution of heartburn (RDQ score) | ||||
| Week 4, n (%) | 66 (62.3) | 65 (65.7) | 43 (43.4) | [P = 0.0069; P = 0.0017]a |
| [Difference from placebo, n (%)] | [20 (18.9)] | [22 (22.3)] | ||
| Week 2, n (%) | 43 (40.6) | 42 (42.4) | 26 (26.3) | [P = 0.0303; P = 0.0166]a |
| [Difference from placebo, n (%)] | [17 (14.3)] | [16 (16.1)] | ||
| Complete resolution of regurgitation (RDQ score) | ||||
| Week 4, n (%) | 58 (54.7) | 60 (60.6) | 48 (48.5) | [P = 0.3722; P = 0.1736]a |
| [Difference from placebo, n (%)] | [10 (6.2)] | [12 (12.1)] | ||
| Complete resolution of dyspepsia (RDQ score) | ||||
| Week 4, n (%) | 67 (63.2) | 66 (66.7) | 64 (64.7) | [P = 0.8303; P = 0.7647]a |
| [Difference from placebo, n (%)] | [3 (−1.5)] | [2 (2.0)] | ||
| Proportion of heartburn‐free days, % (SD) | 67.6 (29.8) | 66.5 (29.1) | 56.7 (30.3) | [P = 0.0103; P = 0.0210]b |
| [Difference from placebo, %] | [10.9] | [9.8] | ||
Abbreviations: n, number of patients; SD, standard deviation.
Chi‐square test, the P‐values were adjusted for multiple comparisons (overall familywise type I error of 5%) by the Hochberg procedure.
Unpaired t test, the P‐values were adjusted for multiple comparisons (overall familywise type I error of 5%) by the Hochberg procedure.
The complete resolution rates of the major symptoms at week 2 were 16.0% (17/106), 23.2% (23/99) and 10.0% (10/99) in the tegoprazan 50 mg, tegoprazan 100 mg and placebo groups, respectively. However, a statistically significant difference was only observed in the tegoprazan 100 mg group (P = 0.0264).
3.2.2. Complete resolution rates of heartburn
The proportion of patients with complete resolution of heartburn for the last 7 days was significantly higher in all tegoprazan groups than that in the placebo group at both weeks 2 and 4 (Table 2). The complete resolution rates of heartburn were 40.6% (43/106) with tegoprazan 50 mg, 42.4% (42/99) with tegoprazan 100 mg, and 26.3% (26/99) with placebo at week 2, respectively. At week 4, the overall rates were further increased than those of week 2; 62.3% (66/106) with tegoprazan 50 mg, 65.7% (65/99) with tegoprazan 100 mg and 43.4% (43/99) with placebo, respectively.
3.2.3. Complete resolution rates of regurgitation or dyspepsia
The proportion of patients with complete resolution of regurgitation for the last 7 days showed higher trend in all tegoprazan groups than that in the placebo group at week 4; 54.7% (58/106) with tegoprazan 50 mg, 60.6% (60/99) with tegoprazan 100 mg and 48.5% (48/99) with placebo, respectively, but the differences were not statistically significant (Table 2).
The proportion of patients with complete resolution of dyspepsia for the last 7 days were similar in all groups at week 4; 63.2% (67/106) with tegoprazan 50 mg, 66.7% (66/99) with tegoprazan 100 mg and 64.7% (64/99) with placebo, respectively (Table 2).
3.2.4. Proportion of heartburn‐free days
The proportion of heartburn‐free days during the 4‐week treatment period was 67.6% with tegoprazan 50 mg, 66.5% with tegoprazan 100 mg and 56.7% with placebo, respectively (Table 2, Figure 2). The proportions of heartburn‐free days with both tegoprazan 50 and 100 mg were higher to those of the placebo (P = 0.0103 and P = 0.0210, respectively).
3.3. Subgroup analyses
Subgroup analysis for proportions of heartburn‐free days during a 4‐week treatment period and daily proportions of patients without heartburn were evaluated in patients with NERD, who experienced heartburn with moderate and severe severity during screening period.
The proportions of heartburn‐free days during a 4‐week treatment period in a subgroup analysis were 67.5% with tegoprazan 50 mg, 67.3% with tegoprazan 100 mg and 46.7% with placebo, respectively (Figure 2). The proportions of heartburn‐free days after administering both tegoprazan 50 and 100 mg were higher than those of the placebo (P = 0.0030 and P = 0.0029, respectively).
The daily proportions of patients without heartburn in a subgroup analysis were overall higher in all tegoprazan groups than in the placebo group, from day 1 and throughout the 4‐week treatment period (Figure 3).
FIGURE 3.
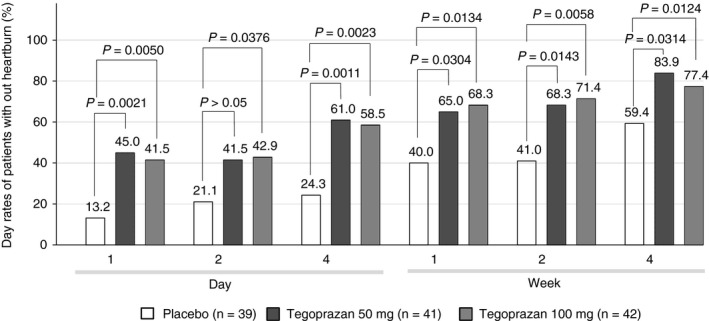
Daily proportions of patients without heartburn during treatment period, who experienced moderate or severe heartburn symptom during the screening period (subgroup analysis)
3.4. Safety analysis
A total of 323 patients received at least one dose of the study drug and were included in the safety analysis. Neither deaths nor unexpected serious TEAEs were reported. Most TEAEs (79.6%, 78/98) were mild in severity; meanwhile, severe ones were not detected in any of the groups (Table 3). The percentages of patients with more than one TEAE were 19.4%, 22.2% and 20.6% for the tegoprazan 50 mg, 100 mg and placebo groups, respectively (Table 4). Five patients with TEAEs discontinued treatments (one, two and two patients in tegoprazan 50 mg, 100 mg and placebo groups, respectively). Nausea (2.8%, 3/108) in the tegoprazan 50 mg group, headache (4.6%, 5/108) in the tegoprazan 100 mg group and nasopharyngitis (2.8%, 3/107) in the placebo group were the most frequently reported drug‐related TEAEs. A serious TEAE was reported in the tegoprazan 100 mg (pyrexia) group and another one in the placebo group (haemoptysis), which were considered drug‐related. No significant changes in vital signs or ECG findings were observed during the study period.
TABLE 3.
Summary of safety outcomes (safety analysis set)
| TEAE | Tegoprazan | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 50 mg | 100 mg | ||||||||
| (n = 108) | (n = 108) | (n = 107) | |||||||
| n | (%) | [F] | n | (%) | [F] | n | (%) | [F] | |
| TEAE | 21 | (19.4) | [29] | 24 | (22.2) | [32] | 22 | (20.6) | [37] |
| Related | 13 | (12.0) | [19] | 12 | (11.1) | [14] | 13 | (12.2) | [23] |
| Not related | 8 | (7.4) | [10] | 12 | (11.1) | [14] | 10 | (9.4) | [14] |
| Mild | 18 | (16.7) | [21] | 23 | (21.3) | [28] | 20 | (18.7) | [29] |
| Moderate | 4 | (3.7) | [8] | 3 | (2.8) | [4] | 6 | (5.6) | [8] |
| Severe | 0 | (0.0) | [0] | 0 | (0.0) | [0] | 0 | (0.0) | [0] |
| Leading to discontinuation | 0 | (0.0) | [0] | 2 | (1.9) | [2] | 1 | (1.985) | [2] |
| SAE | 0 | (0.0) | [0] | 1 | (0.9) | [1] | 1 | (0.9) | [1] |
| Related | 0 | (0.0) | [0] | 1 | (0.9) | [1] | 1 | (0.9) | [1] |
| Not related | 0 | (0.0) | [0] | 0 | (0.0) | [0] | 0 | (0.0) | [0] |
| Leading to discontinuation | 0 | (0.0) | [0] | 1 | (0.9) | [1] | 0 | (0.0) | [0] |
| Deaths | 0 | (0.0) | [0] | 0 | (0.0) | [0] | 0 | (0.0) | [0] |
Abbreviations: [F], Frequency of TEAEs; SAEs, serious adverse events; TEAEs, treatment‐emergent adverse events.
TABLE 4.
Summary of treatment‐emergent adverse events (TEAEs) and drug‐related TEAEs (safety analysis set)
| TEAE | Tegoprazan | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 50 mg | 100 mg | ||||||||
| (n = 108) | (n = 108) | (n = 107) | |||||||
| n | (%) | [F] | n | (%) | [F] | n | (%) | [F] | |
| Most frequently reported TEAEs by system organ class and preferred terma | |||||||||
| Nervous system disorders | 2 | (1.9) | [2] | 6 | (5.6) | [6] | 3 | (2.8) | [5] |
| Headache | 2 | (1.9) | [2] | 4 | (3.7) | [4] | 5 | (4.7) | [5] |
| Infections and infestations | 4 | (3.7) | [4] | 5 | (4.6) | [5] | 6 | (5.6) | [6] |
| Nasopharyngitis | 3 | (2.8) | [3] | 4 | (3.7) | [4] | 5 | (4.7) | [5] |
| Most frequently reported drug‐related TEAEs by system organ class and preferred termb | |||||||||
| Gastrointestinal disorders | 8 | (7.4) | [13] | 3 | (2.8) | [3] | 7 | (6.5) | [8] |
| Nausea | 3 | (2.8) | [5] | 1 | (0.9) | [1] | 2 | (1.9) | [2] |
| Infections and infestations | 1 | (0.9) | [1] | 2 | (1.9) | [2] | 3 | (2.8) | [3] |
| Nasopharyngitis | 1 | (0.9) | [1] | 2 | (1.9) | [2] | 3 | (2.8) | [3] |
| Nervous system disorders | 1 | (0.9) | [1] | 5 | (4.6) | [5] | 2 | (1.9) | [3] |
| Headache | 1 | (0.9) | [1] | 5 | (4.6) | [5] | 2 | (1.9) | [3] |
Abbreviations: [F], Frequency of TEAEs; SAEs, serious adverse events.
≥4%.
≥2%.
4. DISCUSSION
This was the first randomised, double‐blind, controlled phase 3 study that evaluated the effectiveness of tegoprazan, a novel P‐CAB, in 324 Korean patients with NERD. Four weeks of treatment with tegoprazan 50 and 100 mg once daily significantly increased the resolution rate of major symptoms (both heartburn and regurgitation) compared with placebo administration.
Tegoprazan 50 and 100 mg also achieved significantly higher complete heartburn resolution rates at weeks 2 and 4 and proportion of heartburn‐free days during 4‐week of treatment period compared with the placebo. The responses for complete resolution rates of heartburn observed in this study were comparable with those previously obtained with PPIs in patients with NERD.25, 26
NERD, characterised by the presence of typical GERD symptoms without oesophageal erosion at upper endoscopy, negatively influences the QoL of patients.6, 8 PPIs are currently used as the most effective treatment for GERD, but reflux symptoms are not completely controlled in a significant number of patients with NERD.7 Additionally, the responses to PPI therapy were higher in patients with ERD than those with NERD,12, 27, 28 which suggested that these two disorders might have different underlying pathogeneses.7, 13 The characteristics and pathophysiology of NERD led to administration of higher dose of tegoprazan than the approved ones for treatment of ERD in this study. Our data showed that an increased dose of tegoprazan was more helpful to patients with NERD if it provided a significant relief of the major symptoms, in cases where typical GERD symptoms were not achieved with the standard dose.
Differences from placebo for complete resolution rates of heartburn in tegoprazan groups (14.3% and 16.2% for tegoprazan 50 and 100 mg, respectively) were higher than those for the major symptoms, both heartburn and regurgitation (5.9% and 13.1%, respectively) at week 2, whereas the differences were comparable at week 4. Moreover, complete resolution rates of regurgitation were higher in tegoprazan groups compared with the response of placebo but those differences were not statistically significant, which was consistent with previous reports that regurgitation was less responsive to acid suppression than heartburn in patients with GERD.29 Interestingly, responses to tegoprazan 50 and 100 mg were comparable in terms of heartburn but not for the complete resolution of major symptoms. Because regurgitation was less responsive to acid suppressant, the complete resolution rate of the major symptoms at week 2 was not significantly different between administration of tegoprazan 50 mg and placebo.
Even though several attempts to improve the development of P‐CAB and thus overcome the major disadvantages of PPIs have been made, the evidence of the P‐CAB therapeutic potential in the treatment of NERD is limited. Recently, two randomised, placebo‐controlled phase 3 studies reported that vonoprazan was not significantly superior to the placebo in achieving the primary efficacy outcome (proportion of days without heartburn during the 4‐week treatment period) in the FAS of patients with NERD.30, 31 It is unexpected that vonoprazan did not achieve the superiority in the primary efficacy outcome for treatment of NERD compared with placebo, although it was reported the superior inhibition of gastric acid secretion by P‐CAB did not translate into an improved clinical benefit over PPI (active comparator) in patients with NERD.32 Yoshikazu et al explained that the partial response of vonoprazan monotherapy may be due to the heterogeneous pathophysiology of NERD.30, 31 Additionally, Ryota et al and Satoshi et al reported that vonoprazan was effective in relieving the gastroesophageal reflux symptoms in patients with PPI‐resistant NERD.33, 34 These studies do not discard the possibility that P‐CAB could be effectively used in NERD; however, these results should be confirmed in larger randomised controlled trials.
Tegoprazan was approved in Korea for the treatment of ERD, NERD, gastric ulcer and eradication of Helicobacter pylori 35, 36 and became the first P‐CAB clinically available for patients with NERD. Because tegoprazan exhibited a rapid acid inhibition and long‐lasting effect in previous studies,18, 19 it is plausible that tegoprazan may have a better therapeutic effect in acid‐related diseases. This study confirmed that tegoprazan was superior to the placebo in the achieving the primary efficacy outcome and heartburn‐related secondary efficacy outcomes (complete resolution rates of heartburn for the last 7 days of weeks 2 and 4, and proportion of days without heartburn during the 4‐week treatment period) in patients with NERD. Although patients who participated in this study had milder heartburn symptoms than those in the vonoprazan studies, subgroup analyses of the proportions of heartburn‐free days and the daily proportions of patients without heartburn revealed that tegoprazan provided rapid and sustained heartburn relief to patients with NERD, who experienced heartburn symptom with moderate and higher severity in the screening period, from day 1 and throughout the treatment period (Figures 2 and 3). This rapid and sustained symptom relief supports that tegoprazan may provide on‐demand therapeutic option to patients with NERD.
According to the safety analysis, no significant differences in the incidence of TEAEs between tegoprazan and placebo groups were noted (19.4%, 22.2% and 20.6% for tegoprazan 50 mg, 100 mg and placebo, respectively). Moreover, no significant drug‐related TEAEs were reported throughout the study, confirming a favourable safety profile for the oral administration of tegoprazan.
This study has several limitations. First, it is difficult to generalise the study results for non‐Korean populations. The design of this study was not active‐controlled. The beneficial features of tegoprazan, such as fast onset, no food effect, nocturnal acid breakthrough control and less inter‐individual variation due to different PPIs metabolism pathways, were not reflected in the design of this study. There was no information on disposition of patients infected with H. pylori infection in this randomised study although it is still controversy if status of H. pylori infection affects symptom outcomes of PPIs in patients with NERD. In addition, due to the multifactorial pathophysiology of NERD and no intra‐oesophageal pH monitoring before enrolment, the patient population recruited in this study may have included patients with true NERD, oesophageal hypersensitivity, functional heartburn and functional dyspepsia, which could affect the outcome responses.
In conclusion, oral administration of 50 and 100 mg of the novel P‐CAB tegoprazan, once daily for 4 weeks, resulted in a statistically superior efficacy over the placebo in terms of complete resolution of RDQ‐based major symptoms in patients with NERD. Tegoprazan also significantly increased the complete heartburn relief rates at both weeks 2 and 4 and the percentage of heartburn‐free days, compared with those observed in the placebo group. Tegoprazan provided effective and sustained symptom relief to NERD patients, constituting an effective therapeutic option for the treatment of NERD.
AUTHORSHIP
Guarantor of the article: Soo Heon Park.
Author contributions: All authors were involved in the acquisition of data and interpretation of study results. Prof. Soo Heon Park participated in the study design, manuscript draft and critical revision of the manuscript. Dr Seung Han Kim wrote the manuscript. All authors approved the final version of the manuscript, including the authorship list.
ACKNOWLEDGEMENTS
The authors would like to thank all the investigators who contributed to this study.
Declaration of personal interest: The authors thank all the investigators who contributed to this study. Statistical analysis was supported by Jae Hoon Kwon, Hyun Wook Park. Kyeong Min Oh, Ji Won Lee, Ah Rong Kim, Eun Ji Kim, Bong Tae Kim and Geun Seog Song who are employees of HK inno.N Corp., Seoul, Korea.
APPENDIX 1. COMPLETE AFFILIATIONS OF THE AUTHORS
Seung Han Kim, Department of Gastroenterology, Korea University Guro Hospital, Seoul, South Korea; Kwang Bum Cho, Department of Gastroenterology, Kyemyung University Dongsan Medical Centre, Daegu, South Korea; Hoon Jai Chun, Department of Gastroenterology, Korea University Anam Hospital, Seoul, South Korea; Sang Woo Lee, Department of Gastroenterology, Korea University Ansan Hospital, Gyeonggi‐do, South Korea; Joong Goo Kwon, Department of Gastroenterology, Catholic University of Daegu School of Medicine, Daegu, South Korea; Dong Ho Lee, Department of Gastroenterology, Seoul National University Bundang Hospital, Seongnam, South Korea; Sang Gyun Kim, Department of Gastroenterology, Seoul National University College of Medicine, Seoul, South Korea; Hwoon‐Yong Jung, Department of Gastroenterology, Asan Medical Centre, Seoul, South Korea; Ji Won Kim, Department of Gastroenterology, SMG‐SNU Boramae Medical Centre, Seoul, South Korea; Joon Seong Lee, Department of Gastroenterology, Soon Chun Hyang University Seoul Hospital, Seoul, South Korea; Hyojin Park, Department of Gastroenterology, Gangnam Severance Hospital, Seoul, South Korea; Suck Chei Choi, Department of Gastroenterology, Wonkwang University Hospital, Iksan, South Korea; Sam Ryong Jee, Department of Gastroenterology, Inje University Busan Paik Hospital, Busan, South Korea; Hyun‐Soo Kim, Department of Gastroenterology, Chonnam National University Hospital, Kwangju, South Korea; Kwang Hyun Ko, Department of Gastroenterology, CHA University Bundang Medical Center, Seongnam, South Korea; Seun Ja Park, Department of Gastroenterology, Kosin University Gospel Hospital, Busan, South Korea; Yong Chan Lee, Department of Gastroenterology, Yonsei University Severance Hospital, Seoul, South Korea; Soo Heon Park, Department of Gastroenterology, Catholic University Yeouido St. Mary's Hospital, Seoul, South Korea; Ah Rong Kim, Division of Clinical Development, HK inno.N Corp., Seoul, South Korea; Eun Ji Kim, Division of Clinical Development, HK inno.N Corp., Seoul, South Korea; Hyun Wook Park, Division of Clinical Development, HK inno.N Corp., Seoul, South Korea; Bong Tae Kim, Division of Clinical Development, HK inno.N Corp., Seoul, South Korea; Geun Seog Song, Division of Clinical Development, HK inno.N Corp., Seoul, South Korea.
Kim SH, Cho KB, Chun HJ, et al. Randomised clinical trial: comparison of tegoprazan and placebo in non‐erosive reflux disease. Aliment Pharmacol Ther. 2021;54:402–411. 10.1111/apt.16477
The complete list of affiliations is presented in Appendix 1.
The Handling Editor for this article was Dr Colin Howden, and it was accepted for publication after full peer‐review.
Funding information
This study was funded in full by HK inno.N Corp., Seoul, Korea.
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1.Locke GR 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ 3rd. Prevalence and clinical spectrum of gastroesophageal reflux: a population‐based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448‐1456. [DOI] [PubMed] [Google Scholar]
- 2.El‐Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro‐oesophageal reflux disease: a systematic review. Gut. 2014;63:871‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung LJ, Hsu PI, Yang CY, Wang EM, Lai KH. Prevalence of gastroesophageal reflux disease in a general population in Taiwan. J Gastroenterol Hepatol. 2011;26:1164‐1168. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2017 Gastro‐oesophageal Reflux Disease Collaborators . The global, regional, and national burden of gastro‐oesophageal reflux disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:561‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez SD, Malagon IB, Garewal HS, Cui H, Fass R. Non‐erosive reflux disease (NERD)−acid reflux and symptom patterns. Aliment Pharmacol Ther. 2003;17:537‐545. [DOI] [PubMed] [Google Scholar]
- 6.Fass R, Fennerty MB, Vakil N. Nonerosive reflux disease−current concepts and dilemmas. Am J Gastroenterol. 2001;96:303‐314. [DOI] [PubMed] [Google Scholar]
- 7.Lee ES, Kim N, Lee SH, et al. Comparison of risk factors and clinical responses to proton pump inhibitors in patients with erosive oesophagitis and non‐erosive reflux disease. Aliment Pharmacol Ther. 2009;30:154‐164. [DOI] [PubMed] [Google Scholar]
- 8.Quigley EM, Hungin AP. Review article: quality‐of‐life issues in gastro‐oesophageal reflux disease. Aliment Pharmacol Ther. 2005;22:41‐47. [DOI] [PubMed] [Google Scholar]
- 9.El‐Serag HB. Epidemiology of non‐erosive reflux disease. Digestion. 2008;78:6‐10. [DOI] [PubMed] [Google Scholar]
- 10.Fock KM, Talley NJ, Fass R, et al. Asia‐Pacific consensus on the management of gastroesophageal reflux disease: update. J Gastroenterol Hepatol. 2008;23:8‐22. [DOI] [PubMed] [Google Scholar]
- 11.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308‐328; quiz 329. [DOI] [PubMed] [Google Scholar]
- 12.Miwa H, Sasaki M, Furuta T, et al. Efficacy of rabeprazole on heartburn symptom resolution in patients with non‐erosive and erosive gastro‐oesophageal reflux disease: a multicenter study from Japan. Aliment Pharmacol Ther. 2007;26:69‐77. [DOI] [PubMed] [Google Scholar]
- 13.Fass R. Erosive esophagitis and nonerosive reflux disease (NERD): comparison of epidemiologic, physiologic, and therapeutic characteristics. J Clin Gastroenterol. 2007;41:131‐137. [DOI] [PubMed] [Google Scholar]
- 14.Oshima T, Miwa H. Potent potassium‐competitive acid blockers: a new era for the treatment of acid‐related diseases. J Neurogastroenterol Motil. 2018;24:334‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mori H, Suzuki H. Role of acid suppression in acid‐related diseases: proton pump inhibitor and potassium‐competitive acid blocker. J Neurogastroenterol Motil. 2019;25:6‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Echizen H. The first‐in‐class potassium‐competitive acid blocker, vonoprazan fumarate: pharmacokinetic and pharmacodynamic considerations. Clin Pharmacokinet. 2016;55:409‐418. [DOI] [PubMed] [Google Scholar]
- 17.Inatomi N, Matsukawa J, Sakurai Y, Otake K. Potassium‐competitive acid blockers: advanced therapeutic option for acid‐related diseases. Pharmacol Ther. 2016;168:12‐22. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi N, Take Y. Tegoprazan, a novel potassium‐competitive acid blocker to control gastric acid secretion and motility. J Pharmacol Exp Ther. 2018;364:275‐286. [DOI] [PubMed] [Google Scholar]
- 19.Han S, Choi HY, Kim YH, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics, and pharmacodynamics of single and multiple oral doses of tegoprazan (CJ‐12420), a novel potassium‐competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2019;50:751‐759. [DOI] [PubMed] [Google Scholar]
- 20.Sunwoo J, Ji SC, Oh JS, et al. Pharmacodynamics of tegoprazan and revaprazan after single and multiple oral doses in healthy subjects. Aliment Pharmacol Ther. 2020;52:1640‐1647. [DOI] [PubMed] [Google Scholar]
- 21.Vakil N, van Zanten SV , Kahrilas P, Dent J, Jones R, Global Consensus Group . The Montreal definition and classification of gastroesophageal reflux disease: a global evidence‐based consensus. Am J Gastroenterol. 2006;101:1900‐1920; quiz 1943. [DOI] [PubMed] [Google Scholar]
- 22.Shaw M, Dent J, Beebe T, et al. The Reflux Disease Questionnaire: a measure for assessment of treatment response in clinical trials. Health Qual Life Outcomes. 2008;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw MJ, Talley NJ, Beebe TJ, et al. Initial validation of a diagnostic questionnaire for gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:52‐57. [DOI] [PubMed] [Google Scholar]
- 24.Katz PO, Castell DO, Levine D. Esomeprazole resolves chronic heartburn in patients without erosive oesophagitis. Aliment Pharmacol Ther. 2003;18:875‐882. [DOI] [PubMed] [Google Scholar]
- 25.Uemura N, Inokuchi H, Serizawa H, et al. Efficacy and safety of omeprazole in Japanese patients with nonerosive reflux disease. J Gastroenterol. 2008;43:670‐678. [DOI] [PubMed] [Google Scholar]
- 26.Kinoshita Y, Ashida K, Hongo M, Japan Rabeprazole Study Group for NERD . Randomized clinical trial: a multicentre, double‐blind, placebo‐controlled study on the efficacy and safety of rabeprazole 5 mg or 10 mg once daily in patients with non‐erosive reflux disease. Aliment Pharmacol Ther. 2011;33:213‐224. [DOI] [PubMed] [Google Scholar]
- 27.Bate CM, Green JRB, Axon ATR, et al. Omeprazole is more effective than cimetidine for the relief of all grades of gastro‐oesophageal reflux disease‐associated heartburn, irrespective of the presence or absence of endoscopic oesophagitis. Aliment Pharmacol Ther. 1997;11:755‐763. [DOI] [PubMed] [Google Scholar]
- 28.Venables TL, Newland RD, Patel AC, Hole J, Wilcock C, Turbitt ML. Omeprazole 10 milligrams once daily, omeprazole 20 milligrams once daily, or ranitidine 150 milligrams twice daily, evaluated as initial therapy for the relief of symptoms of gastro‐oesophageal reflux disease in general practice. Scand J Gastroenterol. 1997;32:965‐973. [DOI] [PubMed] [Google Scholar]
- 29.Kahrilas PJ, Howden CW, Hughes N. Response of regurgitation to proton pump inhibitor therapy in clinical trials of gastroesophageal reflux disease. Am J Gastroenterol. 2011;106:1149‐1425. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita Y, Sakurai Y, Takabayashi N, et al. Efficacy and safety of vonoprazan in patients with nonerosive gastroesophageal reflux disease: a randomized, placebo‐controlled, phase 3 study. Clin Transl Gastroenterol. 2019;10:e00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinoshita Y, Sakurai Y, Shiino M, et al. Evaluation of the efficacy and safety of vonoprazan in patients with nonerosive gastroesophageal reflux disease: a phase III, randomized, double‐blind, placebo‐controlled, multicenter study. Curr Ther Res Clin Exp. 2016;81–82:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dent J, Kahrilas PJ, Hatlebakk J, et al. A randomised, comparative trial of a potassium‐competitive acid blocker (AZD0865) and esomeprazole for the treatment of patients with nonerosive reflux disease. Am J Gastroenterol. 2008;103:20‐26. [DOI] [PubMed] [Google Scholar]
- 33.Shinozaki S, Osawa H, Hayashi Y, et al. Vonoprazan 10 mg daily is effective for the treatment of patients with proton pump inhibitor‐resistant gastroesophageal reflux disease. Biomed Rep. 2017;7:231‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niikura R, Yamada A, Hirata Y, et al. Efficacy of vonoprazan for gastroesophageal reflux symptoms in patients with proton pump inhibitor‐resistant non‐erosive reflux disease. Intern Med. 2018;57:2443‐2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee KJ, Son BK, Kim GH, et al. Randomised phase 3 trial: tegoprazan, a novel potassium‐competitive acid blocker, vs. esomeprazole in patients with erosive oesophagitis. Aliment Pharmacol Ther. 2019;49:864‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho YK, Choi M‐G, Choi SC, et al. Randomised clinical trial: tegoprazan, a novel potassium‐competitive acid blocker, or lansoprazole in the treatment of gastric ulcer. Aliment Pharmacol Ther. 2020;52:789‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


