Abstract
Objective
To investigate whether tumor necrosis factor inhibitors (TNFi) impact spinal radiographic progression in patients with axial spondyloarthritis (SpA) and whether this is coupled to their effect on inflammation.
Methods
Patients with axial SpA fulfilling the modified New York criteria were included in a prospective cohort (the ALBERTA Follow Up Research Cohort in Ankylosing Spondylitis Treatment). Spine radiographs, performed every 2 years for up to 10 years, were scored by 2 central readers, using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS). The indirect effect of TNFi on mSASSS was evaluated with generalized estimating equations by testing the interaction between TNFi and Ankylosing Spondylitis Disease Activity Score (ASDAS) at the start of each 2‐year interval (t). If significant, the association between ASDAS at t and mSASSS at the end of the interval (t+1) was assessed in 1) patients treated with TNFi at all visits, 2) patients treated with TNFi at some visits, and 3) patients who were never treated with TNFi. In addition, the association between TNFi at t and mSASSS at t+1 (adjusting for ASDAS at t) was also tested (direct effect).
Results
In total, 314 patients were included. A gradient was seen for the effect of ASDAS at t on mSASSS at t+1 (interaction P = 0.10), with a higher progression in patients never treated with TNFi (β = 0.41 [95% confidence interval (95% CI) 0.13, 0.68]) compared to those continuously treated (β = 0.16 [95% CI 0.00, 0.31]) (indirect effect). However, TNFi also directly slowed progression, as treated patients had on average an mSASSS 0.85 units lower at t+1 compared to untreated patients (β = −0.85 [95% CI −1.35, −0.35]).
Conclusion
Our findings indicate that TNFi reduce spinal radiographic progression in patients with radiographic axial SpA, which might be partially uncoupled from their effects on inflammation as measured by the ASDAS.
INTRODUCTION
Axial spondyloarthritis (SpA) is a chronic inflammatory rheumatic disease that preferentially involves the axial skeleton. In axial SpA, systemic inflammation is usually measured with clinical measures of disease activity, such as the Ankylosing Spondylitis Disease Activity Score (ASDAS) (1). Local inflammation (e.g., bone marrow edema in a vertebral corner) is seen with imaging modalities such as magnetic resonance imaging (MRI) (2). The association between inflammation, measured either by ASDAS or MRI, and pain, impaired mobility, disability, and poor health‐related quality of life (HRQoL) is well known (3). In addition, evidence supporting the link between inflammation and axial damage, usually measured on spine radiographs according to the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) (4), has also been consistently reported (5, 6, 7, 8, 9, 10).
Abrogation of inflammation has been shown to improve signs and symptoms of the disease and to have a positive effect on mobility, function, and HRQoL (11). Thus, drugs, such as nonsteroidal antiinflammatory drugs (NSAIDs) and tumor necrosis factor inhibitors (TNFi), play a central role in the management of axial SpA (12). However, and despite significant efforts, it remains to be clarified whether there is also an effect of these drugs on axial damage accrual. Conflicting evidence emerged from trials testing NSAIDs, with some studies supporting the hypothesis of a positive effect, especially among patients with elevated C‐reactive protein (CRP) level (13, 14), while others rejected this hypothesis (15). Randomized placebo‐controlled trials testing the structural effect of TNFi are, currently, unfeasible (16). Data stemming mostly from historical comparisons and from nonrandomized experiments have attempted to fill the gap but have proved inconclusive. Some studies showed a protective effect, especially if treatment was taken for at least 4 years, while others failed to demonstrate any impact (17, 18, 19).
Inconsistencies in the literature might be explained by differences in how the methodologic challenges posed by the above‐mentioned studies have been dealt with (16). The strategies to address confounding, to limit loss to follow‐up, and the low sensitivity to change of the mSASSS are, among others, factors that are likely to interfere with the detection of treatment effects. In addition, the complex mechanisms of structural damage in axial SpA pose further challenges (20). For instance, recent observational studies suggest that TNFi interfere with radiographic progression solely by reducing inflammation (21, 22). However, it has been shown that inflammation that is captured either by repeated measurements of the ASDAS or by sequential MRIs only partially explains new bone formation in axial SpA (5, 6). Thus, the question remains of whether TNFi have a “true” effect on damage accrual in axial SpA and, if so, whether this effect is dependent on or independent of their inhibitory effect on inflammation.
We therefore aimed to investigate whether TNFi reduce spinal radiographic progression in patients with axial SpA and, if so, whether this occurs indirectly through their effect on inflammation as assessed by the ASDAS, or whether a direct effect uncoupled from ASDAS inflammation can also be demonstrated.
PATIENTS AND METHODS
Patients and study design
Consecutive patients from community‐based and academic rheumatology practices in Northern Alberta, Canada with a clinical diagnosis of axial SpA according to their treating rheumatologists were referred to the University of Alberta for inclusion in the Follow Up Research Cohort in Ankylosing Spondylitis Treatment (ALBERTA FORCAST) observational cohort study. Patients had to fulfill the modified New York classification criteria (i.e., with radiographic axial SpA) (23), and recruitment started in 2008. Clinical and imaging data were collected at baseline and every 2 years for up to 10 years of follow‐up. A window of up to 12 months between imaging and clinical visits was allowed. In addition, to be included patients had to have a baseline mSASSS of <71, ≥1 postbaseline spinal radiograph available, and complete data on ASDAS and exposure to TNFi at the start of and during the 2‐year interval. The database used for the current analysis was locked on September 6, 2018. The study was conducted according to Good Clinical Practice Guidelines and has been approved by the University of Alberta Health Research Ethics Committee. All patients provided written informed consent before inclusion.
Scoring procedures and definition of radiographic progression
All available lateral radiographs of the cervical and lumbar spine for each patient were independently scored by 2 trained central readers using the mSASSS. The readers were aware of the chronology of the radiographs but were blinded with regard to clinical data. Only scores for radiographs with ≤3 missing vertebral corners per segment (either cervical or lumbar) were used. Individual missing vertebral corners were imputed according to a method previously described in detail (24). One adjudicator scored all films of each patient where there was a discrepancy between the 2 primary readers of ≥5 units for the change in mSASSS in at least one 2‐year interval. The main outcome was the total mSASSS score (range 0–72) at each visit. In addition, the following binary definitions were used, considering the time between 2 consecutive vists: any change in mSASSS (Δ>0 yes/no); change in mSASSS ≥2 (yes/no); and ≥1 new syndesmophyte (yes/no).
Treatment with TNFi
Treatment with a TNFi (adalimumab, certolizumab, etanercept, golimumab, or infliximab) at each visit (yes/no; time‐varying) was the main explanatory variable of interest. In addition, we analyzed treatment with TNFi according to the following definitions: treatment with TNFi at any time during the follow‐up interval (yes/no; time‐varying), duration of treatment with any TNFi during the follow‐up interval (continuous variable in years; time‐varying), proportion of time receiving TNFi treatment during the follow‐up interval (continuous variable as a proportion of follow‐up; time‐varying), duration of TNFi treatment <50% versus ≥50% of the follow‐up interval (yes/no; time‐varying), and duration of continuous TNFi treatment (allowing for interruptions of a maximum of 6 months) ≤4 years versus >4 years (yes/no; time‐fixed).
Statistical analysis
Reliability
Reliability between readers was determined, at the patient level, by calculating the smallest detectable change (SDC). A two‐way analysis of variance with the change in mSASSS over time as the outcome and with time and reader as independent variables was used to estimate the SEM of the change in mSASSS score, which was then used to calculate the SDC according to the following formula: . In addition, the interreader intraclass correlation coefficient (ICC) for the mSASSS at baseline and for the change in mSASSS per each 2‐year interval were calculated. The latter derived from a mixed model with time as independent variable and with a random‐effect for patient and for reader.
Main analysis
First, we evaluated whether there was an indirect effect of treatment with TNFi at each visit on mSASSS over time, by testing the interaction between exposure to TNFi and ASDAS at the start of each 2‐year interval on the mSASSS 2 years later. In the case of a significant interaction (P < 0.15), meaning that the association between ASDAS and mSASSS was modified by exposure to TNFi, the relationship between ASDAS and mSASSS 2 years later was assessed in the following 3 groups of patients exposed to TNFi: 1) patients who were receiving treatment at all visits (100% of visits); 2) patients who were receiving treatment at some visits (>0% and <100% of visits), and 3) patients who were never treated with TNFi (0% of visits). In addition, interactions between treatment with TNFi and 1) achieving inactive disease according to the ASDAS (ASDAS <1.3) after 1 year (yes/no); 2) NSAIDs (yes/no), 3) symptom duration, 4) smoking (yes/no), and 5) time between diagnosis and start of TNFi were also tested. Second, we tested whether there was a direct effect of receiving TNFi at the start of the interval on mSASSS 2 years later, with and without adjustment for ASDAS at the start of the interval. The indirect and direct effects were also tested with the binary definitions of progression as outcome in separate models.
Both the direct and indirect effects were tested in 2 types of multivariable longitudinal generalized estimating equation (GEE) models. In model 1, individual mSASSS scores (continuous and binary) per reader were used as the outcome in a multilevel model adjusted for the correlation of mSASSS within each reader, an assumption‐free approach we have previously proved to be robust in the analysis of long‐term imaging data (25). In model 2, we used the average score, either between the 2 primary readers or between the adjudicator’s score and the closest score of the 2 primary readers, for the main outcome (mSASSS continuous); the agreement between the 2 readers, at the vertebral unit level, was used for the binary scores. For syndesmophytes, the following 2 definitions were used: 1) the new syndesmophyte was seen by both readers; 2) the new syndesmophyte was seen by at least 1 reader.
Both types of model were adjusted for the outcome (mSASSS) at the start of the interval (autoregression), which isolates the “within‐patient” effects and thus allows for a truly longitudinal interpretation of the data. Models were also adjusted for potential confounders defined a priori on clinical grounds: symptom duration (years), sex, HLA–B27, and number of TNFi used before inclusion. In addition, treatment with conventional synthetic disease‐modifying antirheumatic drugs (csDMARDs) (yes/no; time‐varying), treatment with NSAIDs (yes/no; time‐varying), smoking (yes/no; time‐varying), number of csDMARDs and number of NSAIDs used before inclusion (both time‐fixed) were tested in univariable models, and if significant (P < 0.20), were added to the multivariable model and finally selected if proved significant (P < 0.05) or to confound the association of interest.
Sensitivity analyses
We also tested 1) the direct and indirect effect of TNFi on mSASSS (continuous) using a database with a 6‐month window between imaging and clinical visits; 2) the direct effect of TNFi after adjusting for a propensity score (PS), to take confounding by indication into account (details on the estimation and balancing diagnostics of the PS are provided in the Supplementary Methods, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41667/abstract); and 3) the direct and indirect effect using alternative definitions of exposure to TNFi.
RESULTS
Baseline characteristics
Of the 427 patients with radiographic axial SpA included in FORCAST, 314 fulfilled the inclusion criteria for the present study. Baseline characteristics were typical of patients with radiographic axial SpA: 74% were men, the mean ± SD symptom duration was 18 ± 12 years, and 83% were HLA–B27 positive. The majority (63%) had high or very high disease activity according to the ASDAS and had a high level of damage according to the mSASSS (mean ± SD 14 ± 19). Compared to excluded patients, those included were more likely to smoke (56% versus 48%), had a lower mean Bath Ankylosing Spondylitis Metrology Index (BASMI) score (2 versus 4), had a lower mean mSASSS (14 versus 22), and were more likely to be treated with NSAIDs (53% versus 36%) at baseline (Table 1).
Table 1.
Baseline characteristics of the patients with axial SpA in FORCAST who met the inclusion criteria for the present study and those who were excluded from the present study*
|
Included (n = 314) |
Excluded (n = 113) |
|
|---|---|---|
| Age at baseline, years | 41.1 ± 13 | 43.5 ± 13 |
| Symptom duration, years† | 18 ± 12 | 20 ± 12 |
| Sex, no. (%) male | 233 (74) | 83 (73) |
| HLA–B27 positive, no. (%)† | 262 (83) | 90 (83) |
| Ever smoked, no. (%)‡ | 149 (56) | 15 (48) |
| CRP, mg/liter§ | 10 ± 17 | 13 ± 16 |
| ASDAS‐CRP§ | 3 ± 1 | 3 ± 1 |
| ASDAS‐CRP category, no. (%) | ||
| Inactive disease (<1.3) | 48 (16) | 7 (14) |
| Low disease activity (≥1.3 and <2.1) | 66 (21) | 10 (20) |
| High disease activity (≥2.1 and ≤3.5) | 101 (33) | 19 (38) |
| Very high disease activity (>3.5) | 94 (30) | 14 (28) |
| BASFI (range 0–10)§ | 4 ± 3 | 4 ± 3 |
| BASMI (range 0–10)¶ | 2 ± 2 | 4 ± 3 |
| mSASSS (range 0–72)§# | 14 ± 19 | 22 ± 24 |
| ≥1 syndesmophyte, no. (%)§** | 165 (53) | 32 (67) |
| Use of TNFi, no. (%)§ | 151 (49) | 24 (48) |
| Use of csDMARDs, no. (%)§ | 9 (3) | 4 (8) |
| Use of NSAIDs, no. (%)§ | 165 (53) | 18 (36) |
| ≥1 TNFi before inclusion, no. (%) | 21 (7) | 3 (3) |
| ≥1 NSAID before inclusion, no. (%) | 201 (64) | 71 (63) |
| ≥1 csDMARD before inclusion, no. (%) | 11 (4) | 10 (9) |
Except where indicated otherwise, values are the mean ± SD. SpA = spondyloarthritis; FORCAST = Follow Up Research Cohort in Ankylosing Spondylitis Treatment; CRP = C‐reactive protein; ASDAS‐CRP = Ankylosing Spondylitis Disease Activity Score using the CRP level; BASFI = Bath Ankylosing Spondylitis Functional Index; BASMI = Bath Ankylosing Spondylitis Metrology Index; mSASSS = modified Stoke Ankylosing Spondylitis Spine Score; TNFi = tumor necrosis factor inhibitor; csDMARDs = conventional synthetic disease‐modifying antirheumatic drugs; NSAIDs = nonsteroidal antiinflammatory drugs.
Data were missing for <1% of patients.
n = 297
Data were missing for <10% of patients.
n = 306.
Average of the scores of 2 readers.
Agreement between the 2 readers on the presence of a syndesmophyte at the vertebral corner level.
Main analysis
In total, 442 intervals were included in the analysis, with 223 patients contributing 1 interval, 58 patients contributing 2 intervals, 30 patients contributing 3 intervals, 2 patients contributing 4 intervals, and 1 patient contributing 5 intervals. The mean ± SD progression was 1.33 ± 2.68 mSASSS units per 2‐year interval. The SDC was 3.6, the ICC at baseline was 0.96, and the change score ICC was 0.47.
The interaction between ASDAS and TNFi at the start of the interval was significant (model 1; P = 0.100) with mSASSS continuous as the outcome (Table 2) but not with the binary outcomes (data not shown). A gradient was seen for the effect of ASDAS at the start of the interval on mSASSS 2 years later, which was >2 times higher in patients never treated with TNFi (β = 0.41 [95% confidence interval (95% CI) 0.13, 0.68]) compared to those treated with TNFi at all visits (β = 0.16 [95% CI 0.00, 0.31]) (Table 2 and Figure 1). Results were similar for model 2. No other interactions were significant.
Table 2.
Indirect effect of TNFi on mSASSS, analyzed by the longitudinal association between ASDAS at the start of the 2‐year interval and mSASSS 2 years later, according to the type of exposure to TNFi (multivariable models)
| Exposure to TNFi | Adjusted β (95% CI)* | Interaction P † |
|---|---|---|
| Model 1 (n = 313)‡ | ||
| All visits (n = 119) | 0.16 (0.00, 0.31) | |
| Some visits (n = 93) | 0.28 (0.12, 0.45)§ | 0.100 |
| Never (n = 101) | 0.41 (0.13, 0.68)§ | |
| Model 2 (n = 306)¶ | ||
| All visits (n = 119) | 0.10 (−0.07, 0.27) | |
| Some visits (n = 89) | 0.29 (0.09, 0.48)§ | 0.057 |
| Never (n = 99) | 0.47 (0.13, 0.82)§ |
95% CI = 95% confidence interval.
Interaction between treatment with tumor necrosis factor inhibitors (TNFi) (yes/no) and Ankylosing Spondylitis Disease Activity Score (ASDAS) at the start of the interval. Significant interaction was prespecified as P < 0.15.
Multivariable multilevel longitudinal generalized estimating equation (GEE) model with individual modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) per reader as the outcome, adjusted for the correlation of mSASSS within each reader, mSASSS at the start of the interval, symptom duration (years), sex, HLA–B27, and the number of TNFi used before inclusion.
Significant effect.
Multivariable longitudinal GEE model with the average of the 2 readers’ mSASSS scores as the outcome, adjusted for mSASSS at the start of the interval, symptom duration (years), sex, HLA–B27, and the number of TNFi used before inclusion.
Figure 1.
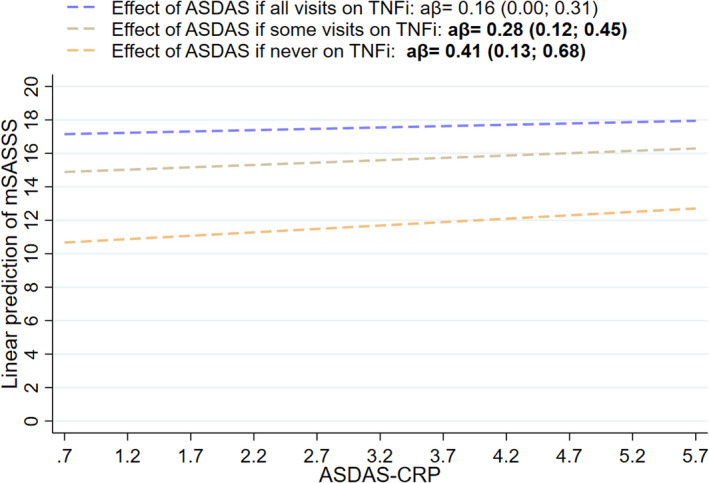
Longitudinal association between Ankylosing Spondylitis Disease Activity Score using the C‐reactive protein level (ASDAS‐CRP) at the start of the 2‐year interval and modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) 2 years later, according to the type of exposure to tumor necrosis factor inhibitors (TNFi). Effects were tested using a multivariable multilevel longitudinal generalized estimating equation model with individual mSASSS scores per reader as the outcome, adjusted for the correlation of mSASSS within each reader (model 1; n = 313), and for mSASSS at the start of the interval, symptom duration (years), sex, HLA–B27, and number of TNFi used before inclusion. Values are the adjusted β (aβ) (95% confidence interval).
Treatment with TNFi was also directly associated with less mSASSS progression. After 2 years, patients who were receiving a TNFi at the start of the interval had on average an mSASSS 0.85 units lower compared to those not treated with a TNFi (model 1; β = −0.85 [95% CI −1.35, −0.35]), independently of ASDAS (Table 3). Results were similar with or without adjustment for ASDAS and with binary definitions of progression, including development of ≥1 new syndesmophyte. The same findings were seen in model 2, except for change in mSASSS ≥2 and for new syndesmophytes, which were not significant.
Table 3.
Direct effect of TNFi on mSASSS, analyzed by association between treatment with TNFi (yes/no) at each visit and radiographic progression 2 years later (multivariable models)*
|
mSASSS, adjusted β (95% CI)† |
Change in mSASSS >0, adjusted OR (95% CI)† |
Change in mSASSS ≥2, adjusted OR (95% CI)† |
≥1 new syndesmophyte, adjusted OR (95% CI)‡ |
≥1 new syndesmophyte (≥1 reader), adjusted OR (95% CI) |
|
|---|---|---|---|---|---|
| Model 1§ | |||||
| Not adjusted for ASDAS (n = 313) | −0.86 (−1.37, −0.35)¶ | 0.62 (0.44, 0.89)¶ | 0.59 (0.40, 0.87)¶ | 0.67 (0.45, 0.99)¶ | – |
| Adjusted for ASDAS (n = 313) | −0.85 (−1.35, −0.35)¶ | 0.62 (0.43, 0.89)¶ | 0.59 (0.40, 0.87)¶ | 0.67 (0.45, 0.99)¶ | – |
| Model 2# | |||||
| Not adjusted for ASDAS (n = 306) | −0.80 (−1.38, −0.22)¶ | 0.60 (0.39, 0.93)¶** | 0.63 (0.39, 1.01) | 0.69 (0.35, 1.36) | 0.78 (0.50, 1.23)** |
| Adjusted for ASDAS (n = 306) | −0.81 (−1.39, −0.24)¶ | 0.60 (0.38, 0.93)¶** | 0.62 (0.39, 1.01) | 0.69 (0.35, 1.35) | 0.76 (0.48, 1.21)** |
95% CI = 95% confidence interval; OR = odds ratio; ASDAS = Ankylosing Spondylitis Disease Activity Score.
Adjudicated in model 2.
For model 2, both readers agreed on “≥1 new syndesmophyte.”
Multivariable multilevel longitudinal generalized estimating equation (GEE) model with individual modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) per reader as the outcome, adjusted for the correlation of mSASSS within each reader, mSASSS at the start of the interval, symptom duration (years), sex, HLA–B27, and the number of tumor necrosis factor inhibitors (TNFi) used before inclusion.
Significant effect.
Multivariable longitudinal GEE model with the average of the 2 readers’ mSASSS scores as the outcome, adjusted for mSASSS at the start of the interval, symptom duration (years), sex, HLA–B27, and the number of TNFi used before inclusion.
Also adjusted for use of nonsteroidal antiinflammatory drugs before baseline (P < 0.05).
Sensitivity analyses
The direct effect of TNFi on mSASSS was also significant in the sensitivity analysis allowing only a 6‐month window between imaging and clinical visits (Table 4). In this analysis, the interaction between ASDAS and TNFi at each visit was also significant (P = 0.062), reflecting a similar gradient of the strength of the association between ASDAS and mSASSS in those treated with TNFi at all visits (β = 0.08 [95% CI −0.09, 0.24]) and those never treated (β = 0.42 [95% CI 0.08, 0.75]) as in the main analysis. Of note, the direct effect remained significant after PS adjustment (β = −0.80 [95% CI –1.37, –0.22]). There was, however, no significant direct effect or indirect effect (data not shown) of TNFi on mSASSS when other definitions of exposure to TNFi were used (Table 4).
Table 4.
Sensitivity analyses of the association between exposure to TNFi and radiographic progression 2 years later (multivariable models)*
|
mSASSS, β (95% CI) |
|
|---|---|
| 6‐month window between imaging and the clinical visit | |
| Model 1 (adjusted for ASDAS) (n = 266) | −0.76 (−1.28, −0.25)† |
| Model 2 (adjusted for ASDAS) (n = 249) | −0.88 (−1.52, −0.23)† |
| Main analysis after PS adjustment | |
| Model 1 (PS population, no PS adjustment) | NA |
| Model 1 (PS population, PS adjusted) | NA |
| Model 2 (PS population, no PS adjustment) (n = 301) | −0.87 (−1.45, −0.28)† |
| Model 2 (PS population, PS adjusted) (n = 301) | −0.80 (−1.37, −0.22)† |
| Alternative definitions of exposure to TNFi | |
| Model 1 | |
| TNFi between visits (yes/no) (n = 300) | −0.37 (−0.86, 0.11) |
| Duration of TNFi between visits (years) (n = 300) | −0.25 (−0.52, 0.01) |
| Proportion of time between visits with TNFi (0–100%) (n = 300) | |
| Continuous variable (0–100%) | −0.51 (−1.04, 0.03) |
| Binary variable (>50 versus ≤50%) | −0.42 (−0.94, 0.11) |
| Long versus short continuous TNFi exposure (≥4 years versus <4 years) (n = 313) | −0.31 (−0.85, 0.22) |
| Model 2 | |
| TNFi between visits (yes/no) (n = 293) | −0.45 (−1.02, 0.11) |
| Duration of TNFi between visits (years) (n = 293) | −0.20 (−0.52, 0.11) |
| Proportion of time between visits with TNFi (0–100%) (n = 293) | −0.41 (−1.04, 0.22) |
| Proportion of time between visits with TNFi (>50% versus ≤50%) (n = 293) | −0.23 (−0.81, 0.35) |
| Continuous TNFi (≥4 years versus <4 years) (n = 306) | −0.33 (−0.89, 0.24) |
Model 1 was a multivariable multilevel longitudinal generalized estimating equation (GEE) model with individual modified Stoke Ankylosing Spondylitis Spine Score (mSASSS) per reader as the outcome, adjusted for the correlation of mSASSS within each reader. Model 2 was a multivariable longitudinal GEE model with the average of the 2 readers’ mSASSS scores as the outcome. Both models were adjusted for mSASSS at the start of the interval, Ankylosing Spondylitis Disease Activity Score (ASDAS), symptom duration (years), sex, HLA–B27, and the number of tumor necrosis factor inhibitors (TNFi) used before inclusion. 95% CI = 95% confidence interval; PS = propensity score; NA = not applicable.
Significant association.
DISCUSSION
In the present study of patients from both academic and community‐based practice, we have shown that, in patients with radiographic axial SpA followed up in daily clinical practice, treatment with TNFi slows spinal radiographic progression by mechanisms both dependent on and independent of their effect on inflammation as measured by the ASDAS. TNFi suppress the negative impact of systemic inflammation on radiographic progression (indirect effect), which supports a strategy of targeting ASDAS to retard structural progression. In addition, TNFi reduce progression independently of ASDAS inflammation, suggesting that either residual inflammation not captured by the ASDAS or other, unknown mechanisms also contribute to structural modification by TNFi.
Both treatment effects were tested in longitudinal models adjusted for time‐varying confounders. In these models, we evaluated whether TNFi treatment at the start of each interval influenced the mSASSS 2 years later during follow‐up, taking into account the presence of damage at the start of the interval (the autoregressive factor). This type of statistical model isolates the “within‐patient” effect and, as such, allows a longitudinal interpretation that best translates daily clinical practice and, in the absence of a proper randomized controlled trial (RCT), approximates causality when combined with PS adjustment. In addition, each model was fit using 2 types of techniques to handle the fact that the outcome (mSASSS) is reported by more than 1 reader. The individual‐reader multilevel model (model 1) increases the statistical power to detect subtle associations (25). The model with between‐reader agreement scores (model 2; e.g., average of 2 scores) renders results easier to interpret.
Within this analytical framework, we have found that treatment with TNFi over time modifies the longitudinal association between ASDAS and mSASSS as noted by the significant interaction between TNFi and ASDAS. This finding follows a previous study, performed in the same cohort (26), and another in an independent cohort (6), in which higher ASDAS was found to be longitudinally associated with an increase in mSASSS 2 years later. In the present study, the impact of ASDAS on mSASSS in patients who have been continuously treated with TNFi during the follow‐up was, on average, less than half compared to the impact in those who were never treated. This finding is consistent with recent well‐designed observational studies, suggesting that TNFi interferes with radiographic progression by decreasing inflammation as measured by the ASDAS (21, 22). Thus, even without a definite answer provided by an RCT to this highly clinically relevant question, enough evidence has accumulated to convincingly argue in favor of a positive effect of lowering ASDAS on spinal radiographic progression for the management of axial SpA.
In contrast to previous studies, however, we did not find that the reduction in ASDAS fully explained the beneficial effect of TNFi on structural progression (21, 22). There was also a significant direct effect. On average, patients treated with a TNFi had 0.9 mSASSS units less progression at the end of the interval compared to those not treated, independently of ASDAS. Similarly, patients treated with TNFi were 30% less likely to develop a new syndesmophyte 2 years later compared to those not treated. Different from the effect on mSASSS, the effect of TNFi on syndesmophyte formation was only significant in the model with individual‐reader data. As noted above, the higher statistical power yielded by this type of model compared to the model with agreement scores most likely explains the discrepancy. Of note, we did not find, in either model, a significant effect for alternative definitions of treatment with TNFi, most of which reflected the time receiving treatment. Although our data do not support the hypothesis that duration of exposure to TNFi influences its structural effects, the majority of patients had a maximum of 4 years of exposure, thus still with relatively limited follow‐up.
Differences in study design, patient characteristics, and analytical approaches might, at least partially, explain why, contrary to previous studies, we detected a direct effect of TNFi on mSASSS (21, 22). In addition, it should be noted that the “direct effect” may also reflect the effect of TNFi on inflammation detected on MRI, which might not be picked up by the ASDAS, or even “residual” inflammation not captured by any currently available measure. Also, between‐visit fluctuations in inflammation can also account for part of the unmeasured inflammatory burden. These observations might, to a certain degree, explain why it has previously been found that radiographic progression still occurs in patients with inactive disease according to the ASDAS (6), and that most new bone formation in the spine occurs in sites without previous evidence of inflammatory lesions on MRI (5, 8).
Keeping the above words of caution in mind, it is not unreasonable to hypothesize that at least part of the ASDAS‐independent effect seen in the present study goes beyond residual confounding. In fact, TNFi have been shown to have a wide range of biologic actions (27), some of which could interfere with processes other than those driving inflammatory activity. For example, several histopathologic studies have demonstrated granulation tissue in the subchondral bone marrow of several types of affected joints in radiographic axial SpA, such as the sacroiliac, manubriosternal, and facet joints, as well as in vertebral bodies (28, 29, 30, 31). Cells lining the granulation tissue express typical markers of osteoblasts, and the directed invasion of the granulation tissue into the subchondral bone and the colocalization of aberrant bone formation with this tissue support an instrumental role of this granulation tissue in the progressive joint remodeling and ankylosis in radiographic axial SpA (32). Within this tissue, osteoclasts have been located almost exclusively at the edges of the granulation tissue at the apical border facing the subchondral bone, suggesting that they facilitate the invasion of this tissue through the subchondral bone (28). TNF‐mediated osteoclast activation and bone erosion may therefore constitute a crucial early step in the development of structural damage that ultimately leads to ankylosis.
Certain animal models suggest a role for TNF in the ankylosis of SpA. Bone morphogenetic proteins (BMPs) have been shown to play a role in the development of ankylosis in the ankylosing enthesitis model of SpA (33). Evaluation of synovial tissue obtained by arthroscopy from patients with SpA has demonstrated TNF‐mediated expression of BMPs in fibroblast‐like synoviocytes (FLS) (34). However, TNF blockade with the soluble TNF receptor etanercept did not ameliorate development of ankylosis in this model (35). A more recent study demonstrated that TNF did enhance osteoblastic differentiation of FLS derived from the synovial tissue of patients with SpA (36). A new animal model of SpA has been created based on selective overexpression of transmembrane TNF in mice which leads to axial and peripheral joint pathology reminiscent of human SpA with peripheral and axial synovitis, enthesitis, and osteitis (37). These mice displayed clear features of new bone formation in the inflamed peripheral joints as well as in the sacroiliac joint and spine. SpA‐like inflammation, but not osteoproliferation, was dependent on TNF receptor type I and mediated by stromal transmembrane TNF overexpression, while TNF receptor type II signaling contributed to pathologic new bone formation but was not essential for inflammation. Relative overexpression of transmembrane TNF compared to soluble TNF was also demonstrated in synovial tissue biopsy specimens from patients with active SpA versus active RA as control. These data support the premise that TNF drives distinct pathologies relevant to SpA which may be variably captured by clinical parameters of disease activity. Further research into the potential mechanisms that influence structural progression independently of inflammation could pave the way to new developments in the treatment of patients with axial SpA. Head‐to‐head trials comparing drugs with different modes of action could also offer some clues in the near future.
There are two additional important points concerning the direct effect. First, treatment with NSAIDs during follow‐up was not associated with the outcome, nor did it modify or confound the association between TNFi and mSASSS. The lack of a structural effect of concomitant treatment with NSAIDs has been noted before, including in one study in which the effect of the amount of exposure to NSAIDs was determined (21, 22). In only one study, which is currently available only in abstract form, was a positive additive effect of NSAIDs reported (38). Thus, available evidence mostly suggests that, among patients with radiographic axial SpA receiving TNFi, there is no (structural) benefit of adding NSAIDs. Whether or not such a benefit exists in TNFi‐naive patients is yet to be clarified (13, 14, 15). Second, the direct effect of TNFi on mSASSS was still present after adjustment for PS. With PS adjustment, we aimed to handle the absence of random treatment allocation and as such mitigate, to the extent possible, the possible effect of confounding by indication. A similar approach was used in a recent study that led to similar conclusions (39). Variables that precede, and influence, the decision to prescribe a TNFi and that also associate with radiographic progression were included and balanced among patients who were treated and those who were not treated at baseline. Even with imperfect balancing, the decrease in the effect from 0.9 to 0.8 after PS adjustment suggests that confounding by indication was indeed present and that it was, at least partially, handled by the PS.
Our study is not without limitations. First, residual confounding cannot be completely ruled out. However, this problem is common to all observational research in which an increase in external validity comes with a decrease in internal validity. Also, we did carefully consider and address confounding with a robust analytical approach aimed at minimizing its detrimental effects. Second, most patients included in the study had either one or two intervals with radiographs available. Thus, interpretation of our findings is limited as regards possible long‐term treatment effects. However, by using longitudinal data analysis we made the best use of the available data compared to the traditional completers’ analysis often undertaken when testing treatment effects. Finally, due to sample size restrictions, we could not evaluate the effect of each TNFi separately. However, there is currently no evidence to suggest that different types of TNFi might impact disease modification in a differential manner.
In summary, the present study informs the rheumatology community by addressing the question of whether or not TNFi inhibit radiographic progression in axial SpA and if this effect is mediated solely by their effects on inflammation, as measured by the ASDAS, or whether additional mechanisms may be relevant. Our data further stress the potential impact of treatment strategies targeting the suppression of ASDAS in the management of axial SpA. In addition, we hypothesize that our finding of a direct effect of TNFi on radiographic progression suggests that these agents could also influence cells and pathways not directly linked to inflammation, such as osteoclasts. A better understanding of these mechanisms might open avenues to further treatment strategies that might finally lead to effective disease modification in axial SpA.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Sepriano had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Sepriano, Ramiro, Chiowchanwisawakit, van der Heijde, Landewé, Maksymowych.
Acquisition of data
Maksymowych.
Analysis and interpretation of data
Sepriano, Ramiro, Wichuk, Chiowchanwisawakit, Paschke, van der Heijde, Landewé, Maksymowych.
Supporting information
Supplementary Material
Acknowledgments
The authors sincerely thank the staff and all patients of the ALBERTA FORCAST study.
The Follow Up Research Cohort in Ankylosing Spondylitis Treatment (FORCAST) was supported by an unrestricted grant from AbbVie. Dr. Sepriano’s work was supported by a doctoral grant from the Fundação para a Ciência e Tecnologia (grant SFRH/BD/108246/2015).
Dr. Sepriano has received consulting fees, speaking fees, and/or honoraria from MSD, UCB, and Novartis (less than $10,000 each). Dr. Ramiro has received consulting fees, speaking fees, and/or honoraria from AbbVie, Eli Lilly, MSD, Novartis, UCB, and Sanofi (less than $10,000 each) and a research grant from MSD. Dr. Chiowchanwisawakit has received speaking fees from Novartis, Zuellig Pharma, Pfizer, and Janssen (less than $10,000 each) and a research grant from Pfizer. Dr. van der Heijde has received consulting fees from AbbVie, Amgen, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Cyxone, Daiichi, Eisai, Eli Lilly, Galápagos, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda, and UCB (less than $10,000 each) and is the director of Imaging Rheumatology BV. Dr. Landewé has received consulting fees from AbbVie, Bristol Myers Squibb, Celgene, Eli Lilly, Galápagos, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer, Roche, and UCB (less than $10,000 each) and is the director of Rheumatology Consultancy BV. Dr. Maksymowych has received consulting fees from AbbVie, Boehringer, Celgene, Galápagos, Janssen, Eli Lilly, Novartis, Pfizer, and UCB (less than $10,000 each), research support from AbbVie, Novartis, and Pfizer, and is the chief medical officer of CARE Arthritis. No other disclosures relevant to this article were reported.
References
- 1.Lukas C, Landewé R, Sieper J, Dougados M, Davis J, Braun J, et al. Development of an ASAS‐endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:18–24. [DOI] [PubMed] [Google Scholar]
- 2.Maksymowych WP. The role of imaging in the diagnosis and management of axial spondyloarthritis [review]. Nat Rev Rheumatol 2019;15:657–72. [DOI] [PubMed] [Google Scholar]
- 3.Machado P, Landewé R, Braun J, Hermann KG, Baraliakos X, Baker D, et al. A stratified model for health outcomes in ankylosing spondylitis. Ann Rheum Dis 2011;70:1758–64. [DOI] [PubMed] [Google Scholar]
- 4.Creemers MC, Franssen MJ, van ’t Hof MA, Gribnau FW, van de Putte LB, van Riel PL. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 2005;64:127–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van der Heijde D, Machado P, Braun J, Hermann KG, Baraliakos X, Hsu B, et al. MRI inflammation at the vertebral unit only marginally predicts new syndesmophyte formation: a multilevel analysis in patients with ankylosing spondylitis. Ann Rheum Dis 2012;71:369–73. [DOI] [PubMed] [Google Scholar]
- 6.Ramiro S, van der Heijde D, van Tubergen A, Stolwijk C, Dougados M, van den Bosch F, et al. Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12‐year longitudinal data from the OASIS cohort. Ann Rheum Dis 2014;73:1455–61. [DOI] [PubMed] [Google Scholar]
- 7.Poddubnyy D, Protopopov M, Haibel H, Braun J, Rudwaleit M, Sieper J. High disease activity according to the Ankylosing Spondylitis Disease Activity Score is associated with accelerated radiographic spinal progression in patients with early axial spondyloarthritis: results from the GErman SPondyloarthritis Inception Cohort. Ann Rheum Dis 2016;75:2114–8. [DOI] [PubMed] [Google Scholar]
- 8.Machado PM, Baraliakos X, van der Heijde D, Braun J, Landewé R. MRI vertebral corner inflammation followed by fat deposition is the strongest contributor to the development of new bone at the same vertebral corner: a multilevel longitudinal analysis in patients with ankylosing spondylitis. Ann Rheum Dis 2016;75:1486–93. [DOI] [PubMed] [Google Scholar]
- 9.Baraliakos X, Listing J, Rudwaleit M, Sieper J, Braun J. The relationship between inflammation and new bone formation in patients with ankylosing spondylitis. Arthritis Res Ther 2008;10:R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haroon N, Inman RD, Learch TJ, Weisman MH, Lee M, Rahbar MH, et al. The impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum 2013;65:2645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sepriano A, Regel A, van der Heijde D, Braun J, Baraliakos X, Landewé R, et al. Efficacy and safety of biological and targeted‐synthetic DMARDs: a systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open 2017;3:e000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van der Heijde D, Ramiro S, Landewé R, Baraliakos X, van den Bosch F, Sepriano A, et al. 2016 update of the ASAS‐EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–91. [DOI] [PubMed] [Google Scholar]
- 13.Wanders A, van der Heijde D, Landewé R, Béhier JM, Calin A, Olivieri I, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum 2005;52:1756–65. [DOI] [PubMed] [Google Scholar]
- 14.Kroon F, Landewé R, Dougados M, van der Heijde D. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis 2012;71:1623–9. [DOI] [PubMed] [Google Scholar]
- 15.Sieper J, Listing J, Poddubnyy D, Song IH, Hermann KG, Callhoff J, et al. Effect of continuous versus on‐demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomised multicentre trial (ENRADAS). Ann Rheum Dis 2016;75:1438–43. [DOI] [PubMed] [Google Scholar]
- 16.Van der Heijde D, Landewé R. Inhibition of spinal bone formation in AS: 10 years after comparing adalimumab to OASIS [editorial]. Arthritis Res Ther 2019;21:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boers N, Michielsens CA, van der Heijde D, den Broeder AA, Welsing PM. The effect of tumour necrosis factor inhibitors on radiographic progression in axial spondyloarthritis: a systematic literature review. Rheumatology (Oxford) 2019;58:1907–22. [DOI] [PubMed] [Google Scholar]
- 18.Karmacharya P, Duarte‐Garcia A, Dubreuil M, Murad MH, Shahukhal R, Shrestha P, et al. Effect of therapy on radiographic progression in axial spondyloarthritis: a systematic review and meta‐analysis. Arthritis Rheumatol 2020;72:733–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baraliakos X, Gensler LS, D’Angelo S, Iannone F, Favalli EG, de Peyrecave N, et al. Biologic therapy and spinal radiographic progression in patients with axial spondyloarthritis: a structured literature review. Ther Adv Musculoskelet Dis 2020;12:1759720X20906040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dougados M, Baeten D. Spondyloarthritis. Lancet 2011;377:2127–37. [DOI] [PubMed] [Google Scholar]
- 21.Molnar C, Scherer A, Baraliakos X, de Hooge M, Micheroli R, Exer P, et al. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss Clinical Quality Management cohort. Ann Rheum Dis 2018;77:63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JW, Kim MJ, Lee JS, Ha YJ, Park JK, Kang EH, et al. Impact of tumor necrosis factor inhibitor versus nonsteroidal antiinflammatory drug treatment on radiographic progression in early ankylosing spondylitis: its relationship to inflammation control during treatment. Arthritis Rheumatol 2019;71:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis: a proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- 24.Ramiro S, Stolwijk C, van Tubergen A, van der Heijde D, Dougados M, van den Bosch F, et al. Evolution of radiographic damage in ankylosing spondylitis: a 12 year prospective follow‐up of the OASIS study. Ann Rheum Dis 2015;74:52–9. [DOI] [PubMed] [Google Scholar]
- 25.Sepriano A, Ramiro S, van der Heijde D, Dougados M, Claudepierre P, Feydy A, et al. Integrated longitudinal analysis does not compromise precision and reduces bias in the study of imaging outcomes: a comparative 5‐year analysis in the DESIR cohort. Semin Arthritis Rheum 2020;50:1394–9. [DOI] [PubMed] [Google Scholar]
- 26.Sepriano A, Ramiro S, Wichuk S, Chiowchanwisawakit P, Paschke J, van der Heijde D, et al. Disease activity is associated with spinal radiographic progression in axial spondyloarthritis independently of exposure to tumour necrosis factor inhibitors [letter]. Rheumatology (Oxford) 2020;60:461–2. [DOI] [PubMed] [Google Scholar]
- 27.Sedger LM, McDermott MF. TNF and TNF‐receptors: from mediators of cell death and inflammation to therapeutic giants– past, present and future [review]. Cytokine Growth Factor Rev 2014;25:453–72. [DOI] [PubMed] [Google Scholar]
- 28.Bleil J, Maier R, Hempfing A, Schlichting U, Appel H, Sieper J, et al. Histomorphologic and histomorphometric characteristics of zygapophyseal joint remodeling in ankylosing spondylitis. Arthritis Rheumatol 2014;66:1745–54. [DOI] [PubMed] [Google Scholar]
- 29.Bollow M, Fischer T, Reisshauer H, Backhaus M, Sieper J, Hamm B, et al. Quantitative analyses of sacroiliac biopsies in spondyloarthropathies: T cells and macrophages predominate in early and active sacroiliitis–cellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann Rheum Dis 2000;59:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cruickshank B. Histopathology of diarthrodial joints in ankylosing spondylitis. Ann Rheum Dis 1951;10:393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong Y, Zheng N, Chen SB, Xiao ZY, Wu MY, Liu Y, et al. Ten years’ experience with needle biopsy in the early diagnosis of sacroiliitis. Arthritis Rheum 2012;64:1399–406. [DOI] [PubMed] [Google Scholar]
- 32.Bleil J, Maier R, Hempfing A, Sieper J, Appel H, Syrbe U. Granulation tissue eroding the subchondral bone also promotes new bone formation in ankylosing spondylitis. Arthritis Rheumatol 2016;68:2456–65. [DOI] [PubMed] [Google Scholar]
- 33.Lories RJ, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest 2005;115:1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lories RJ, Derese I, Ceuppens JL, Luyten FP. Bone morphogenetic proteins 2 and 6, expressed in arthritic synovium, are regulated by proinflammatory cytokines and differentially modulate fibroblast‐like synoviocyte apoptosis. Arthritis Rheum 2003;48:2807–18. [DOI] [PubMed] [Google Scholar]
- 35.Lories RJ, Derese I, de Bari C, Luyten FP. Evidence for uncoupling of inflammation and joint remodeling in a mouse model of spondylarthritis. Arthritis Rheum 2007;56:489–97. [DOI] [PubMed] [Google Scholar]
- 36.Van Tok MN, van Duivenvoorde LM, Kramer I, Ingold P, Pfister S, Roth L, et al. Interleukin‐17A inhibition diminishes inflammation and new bone formation in experimental spondyloarthritis. Arthritis Rheumatol 2019;71:612–25. [DOI] [PubMed] [Google Scholar]
- 37.Kaaij MH, van Tok MN, Blijdorp IC, Ambarus CA, Stock M, Pots D, et al. Transmembrane TNF drives osteoproliferative joint inflammation reminiscent of human spondyloarthritis. J Exp Med 2020;217:e20200288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gensler LS, Gianfrancesco M, Weisman MH, Brown MA, Lee M, Learch T, et al. Combined effects of tumour necrosis factor inhibitors and NSAIDs on radiographic progression in ankylosing spondylitis [abstract]. Ann Rheum Dis 2018;77Suppl 2:148. [Google Scholar]
- 39.Koo BS, Oh JS, Park SY, Shin JH, Ahn GY, Lee S, et al. Tumour necrosis factor inhibitors slow radiographic progression in patients with ankylosing spondylitis: 18‐year real‐world evidence. Ann Rheum Dis 2020;79:1327–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


