Abstract
Premise
Cunoniaceae are a family of shrubs and trees with 27 genera and ca. 335 species, mostly confined to tropical and wet temperate zones of the southern hemisphere. There are several known issues regarding generic limits, and the family also displays a number of intriguing long‐range disjunctions.
Methods
We performed a phylogenomic study using the universal Angiosperms353 probe set for targeted sequence capture. We sampled 37 species covering all genera in the Cunoniaceae, and those in the three closely related families of the crown Oxalidales (Brunelliaceae, Cephalotaceae, and Elaeocarpaceae). We also performed analyses for molecular dating and ancestral area reconstruction.
Results
We recovered the topology (Cunoniaceae, (Cephalotaceae, (Brunelliaceae, Elaeocarpaceae))) and a well‐resolved genus‐level phylogeny of Cunoniaceae with strongly supported clades corresponding to all previously recognized tribes. As previously suspected, the genera Ackama and Weinmannia were recovered as paraphyletic. Australasia was inferred as the likely ancestral area for the family.
Conclusions
The current distribution of Cunoniaceae is best explained by long‐distance dispersal with a few possible cases of Australasian–American vicariance events. Extinctions may have been important in determining the mostly Oceanian distribution of this family while some genera in the tribe Cunonieae and in New Caledonia have undergone recent bursts of diversification. New generic diagnoses, 80 new combinations, and one new name are provided for a recircumscribed Ackama (including Spiraeopsis), a much smaller Weinmannia (mostly New World), and a resurrected Pterophylla to accommodate Old World taxa previously in Weinmannia.
Keywords: Antarctica, Australia, Caldcluvia, Gondwana, Madagascar, relicts
Cunoniaceae are a medium‐sized family of flowering plants with 27 genera and ca. 335 species (Bradford et al., 2004) of shrubs and trees mostly confined to tropical (including montane) and wet temperate zones of the southern hemisphere. The greatest diversity of species and genera is found in Oceania, particularly New Guinea, eastern Australia, and New Caledonia. The family has a rich fossil record, especially in Australia (Barnes et al., 2001), but also in other areas where it is no longer extant, such as Antarctica (Poole et al., 2003), Sweden (Schönenberger et al., 2001), and Burma (Chambers et al., 2010). Cunoniaceae are now placed in the order Oxalidales (APG IV, 2016), along with six other families (Fig. 1). Huaceae (2 genera and 3 spp., tropical Africa) appears to be sister to the rest of the order (Soltis et al., 2011), which is composed of two well‐supported clades: Connaraceae + Oxalidaceae, and Brunelliaceae + Cephalotaceae + Cunoniaceae + Elaeocarpaceae (Wang et al., 2009; Soltis et al., 2011). The relationships within the latter clade, which could be referred to as crown Oxalidales or Elaeocarpineae Engler (1898, p. 148), remain unresolved, and therefore the sister group of Cunoniaceae is uncertain.
FIGURE 1.

One representative of each of the four crown families of Oxalidales, clockwise from top left: Cunonia capensis L. (Cunoniaceae), drawn by Matilda Smith and reproduced from Curtis’ Bot. Mag. vol. 139, tab. 8504 (1913); Cephalotus follicularis Labill. (Cephalotaceae), drawn by Louis‐Constantin Stroobant and reproduced from L. B. van Hoote, Flore des serres et des jardins de l’Europe, vol. 3, tab. 8 (1847); Brunellia sibundoya Cuatrec. (Brunelliaceae), artist unknown. Reproduced from the Project to digitize the drawings of the Royal Botanical Expedition of the New Kingdom of Granada (1783–1816), directed by José Celestino Mutis: www.rjb.csic.es/icones/mutis. Royal Botanic Garden‐CSIC; Elaeocarpus grandiflorus Sm. (Elaeocarpaceae), drawn by Walter Hood Fitch and reproduced from Curtis’ Bot. Mag. vol. 78, tab. 4680 (1852).
Hufford and Dickison (1992) conducted the first cladistic analysis of the family, using morphological and anatomical characters. A molecular phylogenetic analysis, based on plastid trnL and rbcL sequences, was later published, including 23 of the currently accepted genera in the family (Bradford and Barnes, 2001). This study confirmed the placement of Bauera Banks ex Andrews, Davidsonia F.Muell., and Eucryphia Cav. within Cunoniaceae, three genera that have often been placed in their own monotypic families. This study also enabled the delimitation of several monophyletic tribes: Caldcluvieae, Codieae, Cunonieae, Geissoieae, Schizomerieae, and Spiraeanthemeae, although the position of some other genera (e.g., Eucryphia, Acrophyllum Benth.) remained unresolved. This phylogenetic framework paved the way for subsequent, more narrowly targeted studies. In a phylogenetic analysis of the tribe Cunonieae (Cunonia L., Pancheria Brongn. & Gris, Vesselowskya Pamp., Weinmannia L.), Bradford (2002) found support for the division of Weinmannia, the largest genus of the family (ca. 150 species), into five monophyletic sections, while only recovering weak support for the monophyly of the entire genus. A new, monotypic genus from New Caledonia, Hooglandia McPherson & Lowry, was later discovered and described (McPherson and Lowry, 2004), with molecular phylogenetics indicating that it occupied an isolated position (Sweeney et al., 2004). A phylogenetic analysis of Spiraeanthemeae (Pillon et al., 2009a) suggested that two genera, Acsmithia Hoogland and Spiraeanthemum A.Gray, were not monophyletic and were therefore reunited. A later study of Geissoieae (Hopkins et al., 2013), including Geissois Labill., Pseudoweinmannia Engl., and the first published molecular data for Lamanonia Vell., led to the placement of the two Australian species of Geissois in the newly described Karrabina Rozefelds & H.C.Hopkins. Before the present work was initiated, no molecular data were available for the New Guinean endemics Aistopetalum Schltr. (2 spp.) and Opocunonia Schltr. (1 sp.), and in addition, questions regarding the monophyly and, hence, the limits of Ackama A.Cunn. and Weinmannia remained unclear (Y. Pillon, unpublished data).
Most phylogenetic studies of Cunoniaceae have been conducted with a handful of plastid (Bradford and Barnes, 2001) or nuclear (Pillon et al., 2009b) genes that lacked the power to resolve recalcitrant nodes. High‐throughput DNA sequencing permits data gathering from a large number of loci, thereby increasing significantly the resolution of phylogenetic studies. Plastome phylogenomic studies (e.g., Givnish et al., 2010; Drew et al., 2014; Li et al., 2019) have become common, but they rely on a number of loci that are tightly linked. Cytoplasmic gene flow implies that such approaches could be misleading (Rieseberg and Soltis, 1991), particularly between closely related species, but also even at higher ranks, e.g., between orders (Sun et al., 2015). Therefore, approaches using many unlinked nuclear loci are expected to yield more robust phylogenies. One such approach, targeted sequence capture, has now been made accessible to all angiosperm researchers through the development of a universal probe set, known as Angiosperms353 (Johnson et al., 2019). Also, targeted sequence capture employs short‐read sequencing, which permits the use of degraded genomic DNA, such as that typically found in and extracted from herbarium specimens, i.e., museomics (e.g., Zedane et al., 2016), which can be a good substitute for fresh material for species that are extinct or difficult to procure. The efficacy of target sequence capture using Angiosperms353 on DNA from herbarium specimens has been well demonstrated (Brewer et al., 2019).
The primary aims of this study were to (1) clarify the relationships between Cunoniaceae and its closest relatives in the crown Oxalidales (viz Brunelliaceae, Cephalotaceae, and Elaeocarpaceae), (2) produce a phylogenetic tree of Cunoniaceae based on a sampling of every genus, (3) investigate the limits of two particularly problematic genera: Ackama and Weinmannia, and (4) investigate the biogeographical history of this predominantly southern hemisphere group. Secondary goals were to ensure that Cunoniaceae genera for which we sampled several species were monophyletic and to make the necessary taxonomic and nomenclatural adjustments to genera and species on the basis of a strongly supported phylogeny that was further supported by morphological characters whenever possible.
MATERIALS AND METHODS
Sampling
The sampling included all 27 genera currently recognized within Cunoniaceae (following Bradford et al., 2004; Pillon et al., 2009a; Hopkins et al., 2013), as well as all of the genera of the related families: Brunelliaceae (monogeneric, Kubitzki, 2004), Cephalotaceae (monogeneric, Conran, 2004), and Elaeocarpaceae (12 genera, Coode, 2004), except Sloanea L. in the last family, for which sequencing was not successful (Appendix S1). Within Cunoniaceae, we sampled all five sections within Weinmannia (sect. Weinmannia, Fasciculatae, Inspersae, Leiospermum, Spicatae; sensu Bradford, 1998, 1998). To test generic monophyly and for biogeographic analysis, one species from each geographical area for those taxa with a disjunct distribution was included in the sampling: Cunonia (southern Africa and New Caledonia), Eucryphia (South America and Australia), Ackama (Australia and New Zealand), and Weinmannia sect. Weinmannia (Americas and Mascarenes). Two species of Connaraceae, Manotes expansa Sol. ex Planch. and Rourea calophylla (Gilg ex G. Schellenb.) Jongkind, and one of Oxalidaceae, Sarcotheca macrophylla Blume, were chosen as outgroup taxa. Accessions were sourced from the DNA & Tissue Bank at the Royal Botanic Gardens, Kew, or were selected from herbarium specimens.
DNA extraction, library preparation, hybridization, and sequencing
DNA was extracted using a modified CTAB protocol (Doyle and Doyle, 1987) and purified using Mag‐Bind TotalPure NGS (Omega Bio‐tek, Norcross, GA, USA). The quality and concentration of the DNA extracts were assessed using a 1.5% agarose gel (to evaluate average fragment size) and a Qubit 3.0 fluorometer (Life Technologies, Carlsbad, CA, USA). DNA extracts with fragment sizes above 350 bp were sheared using a Covaris M220 Focused‐ultrasonicatorTM with Covaris microTUBES AFA Fiber Pre‐Slit Snap‐Cap (Covaris, Woburn, MA, USA). Dual‐indexed libraries for Illumina sequencing were prepared using the DNA NEBNext UltraTM II Library Prep Kit using half the recommended volume, with Dual Index Primers Set 1, NEBNext Multiplex Oligos for Illumina (New England BioLabs, Ipswich, MA, USA). The quality of the resulting libraries was evaluated on an Agilent Technologies 4200 TapeStation System using High Sensitivity D1000 ScreenTape (Agilent Technologies, Santa Clara, CA, USA). Libraries were pooled (equimolar 1 μg per pool) and enriched using the Angiosperms353 probe kit (Catalog #308196; Johnson et al., 2019) following the manufacturer’s protocol v4 (4.0; http://www.arborbiosci.com/mybaits‐manual). Hybridizations were performed at 65°C for 28–32 h in a Hybex Microsample Incubator (SciGene, Sunnyvale, CA, USA) and using red Chill‐out Liquid Wax (Bio‐Rad, Hercules, California, USA) to prevent evaporation. Enriched products were amplified with KAPA HiFi 2X HotStart ReadyMix PCR Kit (Roche, Basel, Switzerland) for 10 cycles. PCR products were then cleaned using the QIAquick PCR purification kit (Qiagen, Hilden, Germany). Products were quantified with a Qubit 3.0 fluorometer, in some cases, reamplified a second time between 3 and 8 cycles. Final products were run on an Agilent Technologies 4200 TapeStation System using High Sensitivity D1000 ScreenTape to assess quality and average fragment size. Several pooled libraries were multiplexed, and sequencing was performed at the Royal Botanic Gardens, Kew on an Illumina MiSeq (Illumina San Diego, CA, USA) with v 3 reagent chemistry (2 × 300‐bp paired‐end reads) or at Macrogen (Takeley, UK) on an Illumina HiSeq to produce 2 × 150‐bp paired‐end reads.
Read mapping and sequence alignments
The reads of the sequencing output (.fastq) were trimmed using Trimmomatic (Bolger et al., 2014) to remove reads with a quality score below 30 and reads that had any 4‐bp window below 30, retaining reads with at least 36 bp (LEADING: 30 TRAILING: 230 SLIDING WINDOW:4:30 MINLEN:36). The MINLENGTH setting was also used with length set to 36 to remove shorter reads that might not be uniquely positionable against other sequences. Paired reads and combined unpaired reads were used to recover target sequences using HybPiper version 1.3 (Johnson et al., 2016) using a target file available at https://github.com/mossmatters/Angiosperms353. Reads were mapped to de‐gapped medoid sequences using BLASTX (Camacho et al., 2009), each gene was assembled de novo using SPAdes (Bankevich et al., 2012), and coding sequences were extracted using Exonerate (Slater and Birney, 2005). HybPiper was run using the BLAST option (Altschul et al., 1990) because it has been found to produce longer sequences (Murphy et al., 2020). Noncoding sequences (i.e., introns and untranslated regions [UTRs]) flanking the coding sequences were recovered using the script intronerate.py available with HybPiper. Gene matrices were aligned separately using MAFFT V7 (mafft‐7.419‐gcc_fc6.x86), with accuracy‐oriented methods (‐‐localpair ‐‐maxiterate 1000) and the option to generate reverse complement sequences to align them together with the remaining sequences based on 6‐mer counting (‐‐adjustdirectionaccurately). Matrices were subsequently trimmed using phyutility (https://github.com/blackrim/phyutility) to delete sites that were missing 80% data (‐clean 0.8). Gene trees from trimmed matrices were generated using IQtree V1.6.12 (Minh et al., 2020), using ultrafast bootstrap with partition models (Chernomor et al., 2016). In a first iteration, generated gene trees were evaluated using TreeShrink 1.3.1 (Mai and Mirarab, 2018) to identify and exclude branches that increased the diameter of each gene tree by more than 20% using centroid re‐rooting (‐b 20 ‐c). Each locus was then realigned, trimmed, and analyzed using IQtree with bipartition support assessed with 1000 UFBoot2 (Hoang et al., 2018) bootstrap replicates (‐bb 1000), while collapsing branches with support values below 10% (Mirarab, 2019 [Preprint]) using Newick Utilities 1.6 (Junier and Zdobnov, 2010). We additionally excluded genes that contained data for fewer than 25% of species. A species tree was constructed from the set of trees produced based on the supercontigs (exons + introns) individually produced with IQ‐Tree. Tree and extensive branch annotations were generated using ASTRAL‐II (Mirarab and Warnow, 2015) using alternative quartet topologies (‐t 2): indicating the local posterior probabilities of the percentage of quartets in gene.
Divergence time estimates
To limit the effects of rate and topology heterogeneity between genes on divergence time estimations and the computational times required for such analysis, we first selected a set of genes using Sortadate (Smith et al., 2018). Genes chosen were those that were at least 10% concordant (bipartition >0.1) with the species tree produced with ASTRAL, had a root‐to‐tip variation less than 0.003, and had a tree length exceeding 2.1. The threshold for the agreement with the species tree was based on those used in previous studies (e.g., Shee et al., 2020), while those for the root‐to‐tip variation and tree length were determined using the median of the values obtained with Sortadate for all gene trees.
Divergence times were estimated using the Bayesian approach implemented in BEAST v1.10.4 (Suchard et al., 2018) on the CIPRES Science Gateway V. 3.3 (https://www.phylo.org/). We used the selected genes as independent partitions with their specific DNA substitution models and clock models, both unlinked. The topology of the tree was constrained to the relationships retrieved in the ASTRAL analysis. We used an uncorrelated relaxed clock with a lognormal distribution prior and a Yule process tree prior. Six fossil taxa were used for calibration using a normal distribution prior (Table 1). Five independent runs were conducted with 100 million generations, sampled every 2000 generations. Parameter convergence and appropriate effective sample sizes were verified in Tracer v1.7 (Rambaut et al., 2018). Tree files were combined in LogCombiner with a burnin of 25% (based on results visualized in Tracer), while the maximum credibility tree and associated posterior probabilities were computed in TreeAnnotator (Suchard et al., 2018). Diversification rates were calculated using equation 4 of Magallón and Sanderson (2001).
TABLE 1.
List of the six fossil taxa used as calibration points in the molecular dating analysis of Cunoniaceae and relatives. Ages are reported in millions of years. SD, standard deviation.
| Fossil taxa | Period | Position | Mean age, Myr (SD) | Reference |
|---|---|---|---|---|
| 1. Tropidogyne | Early Cretaceous (Upper Albian) | Crown node of Oxalidales | 100.5 (1.0) | Chambers et al. (2010) |
| 2. Lacinipetalum | Early Paleocene |
Stem node of tribe Schizomerieae |
66.0 (1.0) | Jud et al. (2021) |
| 3. Eucryphia | Late Paleocene | Stem node of Eucryphia | 66.0 (1.0) | Barnes et al. (2001) |
| 4. Codia | Middle Eocene‐Oligocene | Crown node of Codia and Callicoma | 47.8 (1.0) | Barnes et al. (2001) |
| 5. Vesselowskya | Early Oligocene | Stem node of Vesselowskya | 33.9 (1.0) | Barnes et al. (2001) |
| 6. Elaeocarpus | Early Oligocene | Crown node of Elaeocarpus | 33.9 (1.0) | Crayn et al. (2006) |
Biogeographical patterns
Ancestral range estimation for Cunoniaceae was performed using the dispersal–extinction cladogenesis (DEC) (Ree and Smith, 2008) and DEC+J models as implemented in the R package BIOGEOBEARS (Matzke, 2013). Geographic areas, as defined by Buerki et al. (2011), were used with the addition of Antarctica as one of the available regions because of its potential role in the past as a dispersal route for plants and animals (de la Estrella et al., 2017, 2019). The adopted area of delimitation approximates that of Buerki et al. (2009) and de la Estrella et al. (2019) with some modifications as follows: A, Africa; B, Madagascar (including Comoro and Mascarene Islands); C, Australia/New Guinea; D, New Caledonia; E, New Zealand; F, Americas; G, West Malesia (including India and Sri Lanka); H, Pacific Islands; I, Antarctica (see Appendix S2 for detailed definition). India and Sri Lanka were included within West Malesia to reduce the number of areas in our analysis, as only one sampled genus occurs in India (Elaeocarpus). Area assignments for each terminal are listed in Appendix S3. The biogeographical models proposed by Buerki et al. (2011) and de la Estrella et al. (2019) with five time slices reflecting the probability of area connectivity through time served as the basis for the development of a model tailored for Cunoniaceae (Appendix S2). Four of the time slices from de la Estrella et al. (2019) were employed here because the oldest one, 160–125 Ma, is older than the time spanned by the current analysis (i.e., 100.2 Ma). We performed these biogeographical analyses including and excluding Antarctica as an area to ascertain its impact on the estimation of the biogeographical patterns. The outgroup taxa were removed before these analyses.
RESULTS
Cunoniaceae were recovered as the sister group of a clade comprising (Brunelliaceae, (Cephalotaceae, Elaeocarpaceae)) (Fig. 2). The sister relationship of Cephalotaceae to the Brunelliaceae + Elaeocarpaceae clade is only moderately supported (local posterior probability, LPP = 0.97; quartet values q1 = 0.45, q2 = 0.31, q3 = 0.24). Within Cunoniaceae, most nodes are strongly supported (i.e., LPP = 1) with a few exceptions. Spiraenthemum, Hooglandia, Aistopetalum, and Bauera were the successive sister groups to the rest of the Cunoniaceae, although the placement of Bauera is only weakly supported (LPP = 0.36; q1 = 0.51, q2 = 0.27, q3 = 0.22).
FIGURE 2.
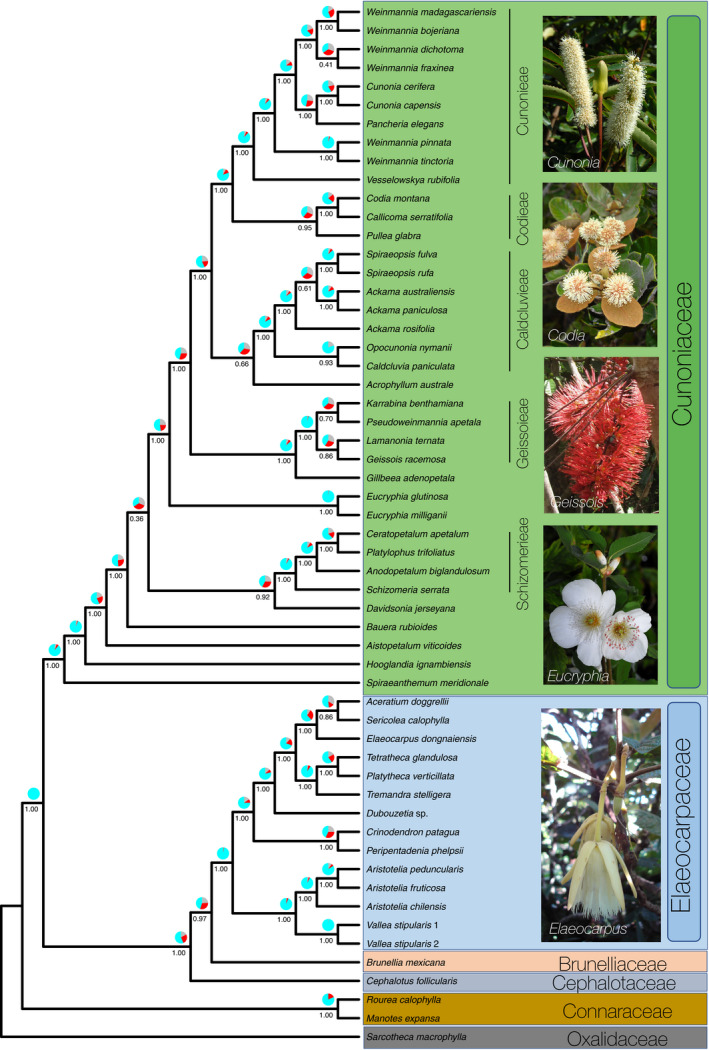
Genus‐level phylogenetic tree of Cunoniaceae and its relationships with the closely related families Brunelliaceae, Cephalotaceae, and Elaeocarpaceae. Tree based on the universal Angiosperms353 probe set for targeted sequence capture. Numbers below branches represent local posterior probability values, and pie charts indicate quartet support.
The tribes Caldcluvieae (Ackama, Caldcluvia D.Don, Opocunonia, Spiraeopsis Miq.), Codieae (Callicoma Andrews, Codia J.R.Forst. & G.Forst., Pullea Schltr.), Cunonieae (Cunonia, Pancheria, Vesselowskya, Weinmannia), Geissoieae (Geissois, Karrabina, Lamanonia, Pseudoweinmannia), Schizomerieae (Anodopetalum A.Cunn. ex Endl., Ceratopetalum Sm., Platylophus D.Don, Schizomeria D.Don) were all recovered as monophyletic with strong support (LPP = 1; except for tribe Codieae, LPP = 0.95). Davidsonia was the sister of tribe Schizomerieae, but this relationship was only moderately supported (LPP = 0.92; q1 = 0.42, q2 = 0.33, q3 = 0.26). Within tribe Schizomerieae, the relationships between the genera differed from the published studies based on two plastid genes. For instance, Anodopetalum was previously recovered as the sister group to Platylophus with strong support (Bradford and Barnes, 2001; Hopkins et al., 2013), while here Ceratopetalum is the sister group to Platylophus with strong support (LPP = 1).
A strongly supported (LPP = 1) “core Cunoniaceae” (sensu Bradford et al., 2004), including Acrophyllum, Eucryphia, Gillbeea F.Muell., and the tribes Caldcluvieae, Codieae, Cunonieae, and Geissoieae, was recovered as in previous studies (Bradford and Barnes, 2001; Hopkins et al., 2013). Relationships resolved within tribe Codieae agree with previous studies (Bradford and Barnes, 2001; Hopkins et al., 2013). The relationships between the four genera of tribe Geissoieae were here only moderately supported (Fig. 2), with Lamanonia and Geissois as sister groups (LPP = 0.86), and Karrabina and Pseudoweinmannia as sister groups (LPP = 0.70). Opocunonia was recovered in the tribe Caldcluvieae as the sister group to Caldcluvia (LPP = 0.93). In this tribe, the genus Ackama was paraphyletic with respect to Spiraeopsis Miq., with the Australian and New Zealand species of Ackama forming separate subclades, based on our sampling.
In Cunonieae, Weinmannia formed two distinct clades (Fig. 2). The species belonging to the four Old World sections (sect. Fasciculatae, Inspersae, Spicatae, and Leiospermum) formed a monophyletic group (LPP = 1) sister to Cunonia + Pancheria (LPP = 1). The fifth section, composed of taxa from the Americas and the Mascarenes (sect. Weinmannia), was sister to this assemblage. The two sections of Weinmannia endemic to Madagascar and the Comoros (Inspersae and Spicatae) formed a monophyletic group sister to section Leiospermum from the Pacific Islands. Several groups showing major geographic disjunctions were also recovered: Cunonia (southern Africa, New Caledonia), Eucryphia (South America, Australia), and Weinmannia sect. Weinmannia (Americas, Mascarenes) were all monophyletic. The other genera endemic to America (Caldcluvia, 1 sp., Lamanonia, 6 spp.) and South Africa (Platylophus, 1 sp.) all have their closest relatives in Oceania.
A set of 41 gene trees were selected based on their concordance, which was further reduced to include only those that comprised at least 48 of the 56 taxa (86%) included in this study. This resulted in the set of 14 genes totalling 51,614 characters that were used in the molecular dating analyses.
All parameters from the combined BEAST analyses reached convergence, except for a few for which the effective sample size was slightly under the generally accepted threshold of 100. Most age estimates have relatively small confidence intervals (Appendix S4), including the estimate for the crown node of Elaeocarpaceae (66.7 Ma) and the crown node of Cunoniaceae (88.6 Ma; Appendix S4). The DEC+J model was favored over the DEC model (p < 0.01), which is indicative of a greater role of vicariance in explaining the biogeographical patterns observed in the study group. The analyses with Antarctica included or not as an available area produced very similar results; only the results including Antarctica are presented hereafter (Fig. 3). The most likely ancestral area for the crown node of Cunoniaceae is a combination of Australia/New Guinea (area C) + New Caledonia (area D); the same ancestral area is assigned to the subsequent node in Cunoniaceae. The remainder of the earliest‐diverging nodes in Cunoniaceae are reconstructed as occurring in Australia/New Guinea with dispersal to other regions later in the history of the group. For Elaeocarpaceae, America is the most likely area assigned to the crown node of the family, with Australia/New Guinea the second most likely reconstruction. In the clade comprising the genera Vallea Mutis ex L.f. and Aristotelia L’Hér., America is the most likely ancestral area, while for the clade comprising the remainder of the family, Australia/New Guinea is favored.
FIGURE 3.
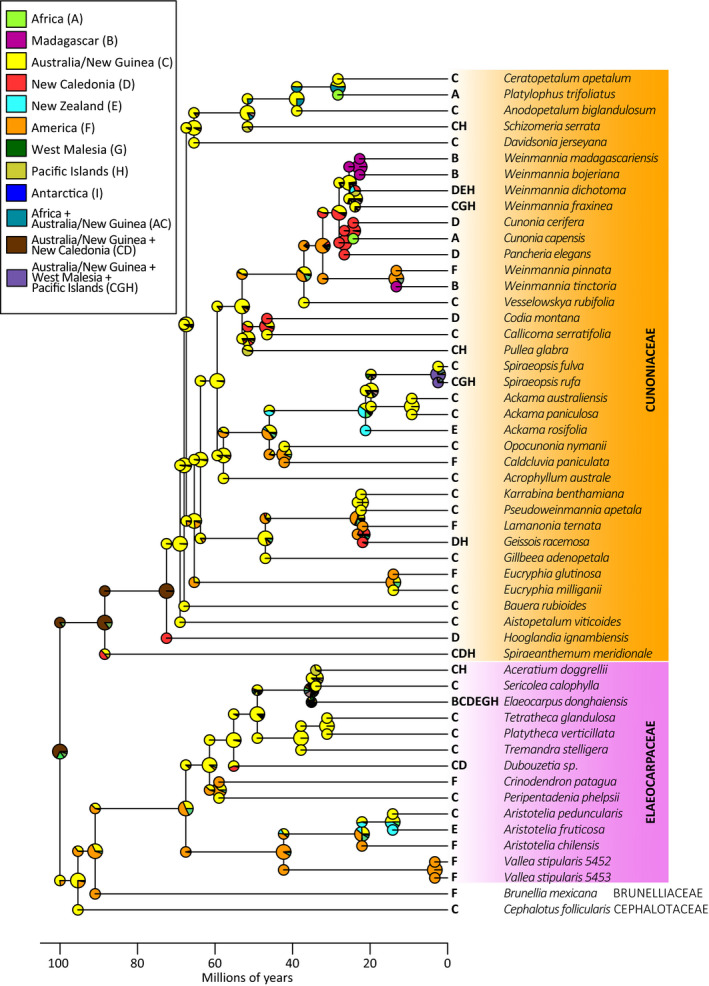
Ancestral area reconstruction in Cunoniaceae and related families using the DEC+J model.
DISCUSSION
This study resolved the phylogenetic relationships amongst the largest clade within Oxalidales as follows: (Cunoniaceae, (Cephalotaceae, (Brunelliaceae, Elaeocarpaceae))). It also confirmed that the monospecific Cephalotaceae (Albany pitcher plant) from southwestern Australia is nested in an otherwise entirely woody and mostly tropical clade. No obvious floral characters support this particular arrangement amongst the four families (Matthews and Endress, 2002). All four are predominantly found in the southern hemisphere, although Elaeocarpaceae (Hably et al., 2007; Manchester and Kvaček, 2009), like Cunoniaceae, has credible fossils in several regions of the northern hemisphere where it no longer occurs.
The monophyly of Cunoniaceae with its current limits is confirmed, and our analysis supports the inclusion of the families Baueraceae, Davidsoniaceae, and Eucryphiaceae within it (Angiosperm Phylogeny Group, 1998). We found support for the monophyly of all six of the tribes delineated by Bradford and Barnes (2001): Caldcluvieae, Codieae, Cunonieae, Geissoieae, Schizomerieae, and Spiraeanthemeae, the last being now monogeneric (Pillon, et al., 2009a). Assigning all genera to tribes would require the description of several new tribes (mostly monotypic) and will be dealt with elsewhere. The generic relationships recovered within Elaeocarpaceae agree with those of Crayn et al. (2006).
The Caldcluvieae currently comprises four genera: Ackama, Caldcluvia, Opocunonia, and Spiraeopsis (Bradford et al., 2004). Although this tribe contains only 12 species, seven generic names have been published for them. The last complete species‐level revision was by Hoogland (1979), who treated the entire group as a single genus, Caldcluvia, but this broad generic concept has not been followed in most subsequent works (de Lange et al., 2002; Hopkins and Hoogland, 2002; Bradford et al., 2004; Heslewood and Wilson, 2013). Here we found that Ackama was paraphyletic with Spiraeopsis nested inside it, and so we sink Spiraeaopsis into Ackama, while Opocunonia and Caldcluvia s.s. remain as distinct, monotypic genera (see Appendix 1: Taxonomic treatment). Characters that unite Ackama s.l. and distinguish it from Caldcluvia s.s. and Opocunonia are the size and shape of the inflorescence, the size of the flowers and length of their pedicels, and the number and arrangement of the stipules.
Our data supported the paraphyly of the genus Weinmannia that was suspected by Bradford (2002) and Y. Pillon (unpublished data). The species of Weinmannia fell into two clades. The first, comprising sect. Weinnmannia, was represented in our analysis by one species from the Americas (W. pinnata L., the type of the genus) and one from the Mascarenes (W. tinctoria Sm.). The second clade includes the remaining species in our analysis, all from the Old World, representing sections Fasciculatae (Malesia and Pacific), Inspersae (Madagascar), Leiospermum (Pacific), and Spicatae (Madagascar + Comoros). We therefore propose splitting Weinmannia into two genera, Weinmannia s.s., which equates to sect. Weinmannia, and Pterophylla D.Don, which we re‐establish for the clade including the four other sections (see Appendix 1: Taxonomic treatment). These two groups differ in inflorescence architecture, corolla, and seeds.
This phylogenomic study provides insights into the enigmatic biogeography of Cunoniaceae, traditionally considered a “Gondwanan” family (Raven and Axelrod, 1974). Our ancestral area reconstruction indicates a combination of Australia/New Guinea (area C) + New Caledonia (area D) as the likely ancestral area for the family (Fig. 3). Indeed, the greatest number of extant species and genera are found in Oceania, and particularly in eastern Australia, New Guinea, and New Caledonia (Fig. 4). The three lineages in the basal grade (Spiraeanthemum, Hooglandia, Aistopetalum) are all restricted to Oceania. Few lineages are found outside Oceania and nearby Southeast Asia (Malesia), and they are all phylogenetically distantly related to one another. The divergence of the southern African Cunonia capensis L. (24.5 Ma), the South American Lamanonia (20.8 Ma) and Eucryphia (14.5 Ma), and the Malagasy Pterophylla (25.6 Ma) from their Oceanian relatives postdate land connections between Australia and their current ranges. Their distributions may therefore be explained by long‐distance dispersal. It is worth noting that the only major difference between the reconstructions including and excluding Antarctica concerns the ancestral area of the node subtending Platylophus and Ceratopetalum (27.9 Ma). It is reconstructed as Australia/New Guinea when Antarctica is not accounted for and as a combination of Africa and Australia/New Guinea when Antarctica is included (Fig. 3). This scenario could suggest that the ancestor of Platylophus would have dispersed to Africa from Australia/New Guinea via Antarctica. The older timing of divergence of the mostly American Weinmannia s.s. (32.3 Ma) and the American Caldcluvia (41.2 Ma) from their Oceanian relatives may also be compatible with colonization through Antarctica. Indeed, until the Eocene, Antarctica was either directly connected to, or separated by relatively narrow seas from, both South America and Australia (Scotese, 2004; Müller et al., 2016), increasing the possibility of biotic exchange between these two now distant landmasses. It is not precisely clear when these landmasses became irreversibly separated by sea barriers, but it was probably sometime in the Eocene, ca. 35–41 Ma (Stickley et al., 2004; Scher and Martin, 2006). The same “via‐Antarctica” scenario might also apply to two Elaeocarpaceae genera confined to South America: Crinodendron Molina (58.1 Ma) and Vallea (41.8 Ma). Nevertheless, the ancestral area reconstruction using the models proposed by de la Estrella et al. (2019) did not suggest Antarctica as an ancestral area for Cunoniaceae, although the family has a substantial fossil record there (Cantrill and Poole, 2012).
FIGURE 4.
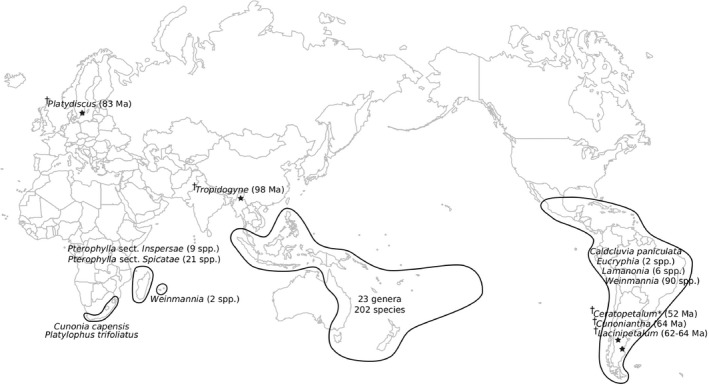
Distribution and diversity of Cunoniaceae according to the new taxonomic framework presented here. *Ceratopetalum is still extant in Australia, New Guinea, and New Britain. †Extinct genera (age of fossil deposit).
The parsimonious scenario of an Oceanian origin of the extant diversity of Cunoniaceae with repeated dispersal events to Africa, the Americas, and Madagascar, may however, be in conflict with the fossil record. Firstly, Cretaceous fossils from Sweden (Schönenberger et al., 2001) and Burma (Chambers et al., 2010; Poinar and Chambers, 2017, 2019) have been attributed to this predominantly southern hemisphere family although some authors have recently considered the fossil evidence for Cunoniaceae in the northern hemisphere to be equivocal (Carpenter and Rozefelds, 2021). However, the attribution of fossils from Greenland and North America (Manchester and Kvaček, 2009) and Italy (Hably et al., 2007) to the extant genus Sloanea (Elaeocarpaceae) is more difficult to challenge, and the two closely related families have very similar present‐day distributions. Secondly, the Southern Cone of South America appears to have been another significant center of diversity for the Cunoniaceae, with fossils described from the Paleocene and Eocene of Argentina. These comprise two extinct genera, Lacinipetalum Jud, Gandolfo, Iglesias & Wilf (Jud et al., 2018) and Cunoniantha Jud & Gandolfo (Jud and Gandolfo, 2021), as well as an extinct species of Ceratopetalum (Gandolfo and Hermsen, 2017), a genus now composed of nine extant species in Australia and New Guinea. In addition, fossils from the Oligocene of Tasmania have been placed in the genera Acsmithia Hoogland (=Spiraeanthemum), Callicoma, Schizomeria, and Vesselowskya, although these genera no longer occur on this island (Carpenter and Buchanan, 1993), and a fossil assigned to Codia was described from the middle Eocene to Oligocene of Western Australia (Barnes and Hill, 1999), although this genus is now confined to New Caledonia. Extinction has clearly been important in the history of the Cunoniaceae and this family has other features of relictual groups. Most genera have few species with a median of only three. Furthermore, the family is similar to several small families of the ANA grade (Amborellales, Nymphaeales, Austrobaileyales), Chloranthales and Magnoliids in being over‐represented in eastern Asia and Oceania, where supposed climatic stability may have played a key role in the survival of these ancient lineages (Morley, 2001; Buerki et al., 2014; Pouteau et al., 2015). Thus, the Australia/New Guinea + New Caledonia origin inferred here for Cunoniaceae may be the result of their greater persistence in these regions, compared to areas such as South America (Kooyman et al., 2014; Barreda et al., 2021).
One tribe, nevertheless, is remarkably successful at the present time: Cunonieae. It comprises two thirds of the species in the family and contains the four largest genera (Weinmannia s.s., Pterophylla, Pancheria, Cunonia). Crown Cunonieae (i.e., excluding Vesselowskya Pamp.) has a diversification rate of 0.316, 2.2 times higher than the rate for the entire family (0.144). The tribe also has the largest distribution, with major radiations in the Andes, Madagascar, and New Caledonia. The genus Pterophylla is widespread in the Pacific and has reached the remote islands of the Marquesas and Rapa Iti. Weinmannia s.s. has itself an enigmatic distribution, divided between the Americas and the Mascarenes. Although the present study included only a single species from each of these two areas, for which divergence is estimated at 13.4 Ma, the previous study by Bradford (2002) included two additional ones in a phylogenetic analysis and numerous others in his morphological study. He found that the temperate W. trichosperma Cav. (Southern Cone of South America) was resolved as sister to a tropical clade (Americas + Mascarenes), suggesting a dispersal from the Americas to the Mascarenes. This may be one of the most intriguing dispersal events known, and the disjunction is similar to one in the palm tribe Chamaedoreeae, which comprises four genera in the neotropics and Hyophorbe in the Mascarenes (Baker and Couvreur, 2013). The disjunction in Cunonia between South Africa and New Caledonia is similar to that in Dietes Salisb. ex Klatt (Iridaceae), which is found in Africa, including southern Africa, and on Lord Howe Island (Goldblatt, 1981), and is also probably due to transoceanic dispersal. It is not clear why Cunonieae diversified and dispersed more than other tribes. It has small winged or hairy seeds, features found in several other genera of the family.
Outside Cunonieae, all genera with more than 10 species occur in New Caledonia. The remarkable success of Cunoniaceae on this archipelago (particularly Geissois: 13 species, Codia: 15 spp., Cunonia: 24 spp., Pancheria 27 spp.) mirrors that of the relictual conifer family Araucariaceae, in which the largest (and recent, <20 Ma) radiations of Agathis Salis. and Araucaria Henkel & W.Hochst. are also in New Caledonia (Setoguchi et al., 1998; Kranitz et al., 2014). Both families have a marked bias toward ultramafic substrates, an important feature of New Caledonia, and one that has probably had major effects on the survival (or not) of immigrant taxa and their diversification on the island (Pillon et al., 2010; Isnard et al., 2016).
CONCLUSIONS
The phylogenomic approach using Angiosperms353 has allowed us to resolve relationships across Cunoniaceae and neighboring families of Oxalidales, as well as issues of generic delimitation relating to Ackama and Weinmannia. It is hoped that the classification obtained in this study will be stable in the future, requiring few changes in generic concepts. The resulting Cunoniaceae have a total of 27 genera and ca. 335 species (Table 2). This phylogenetic framework for Cunoniaceae and the related families Brunelliaceae, Cephalotaceae, and Elaeocarpaceae was used to produce a linear sequence of genera for herbarium arrangement (Appendix S5) following the rules of Trias‐Blais et al. (2015). Cunoniaceae has a mixture of relict features and recent radiations that will be better understood with further research on fossils and through species‐level phylogenies.
TABLE 2.
The 27 genera recognized within Cunoniaceae in this study, their tribal placement, number of species, distribution, and selected taxonomic references.
| Genus | Tribe | No. of species | Distribution | Selected taxonomic references |
|---|---|---|---|---|
| Ackama | Caldcluvieae | 10 | Australia, New Zealand, Malesia, Solomon Islands | Hopkins and Hoogland (2002); de Lange et al. (2002); Schönenberger et al. (2020); APNI (2020) |
| Acrophyllum | unplaced | 1 | Australia | Hoogland (1960, 1981) |
| Aistopetalum | unplaced | 2 | New Guinea | Hopkins and Hoogland (2002) |
| Anodopetalum | Schizomerieae | 1 | Tasmania | Barnes and Rozefelds (2000) |
| Bauera | unplaced | 4 | Australia | APNI (2020) |
| Caldcluvia | Caldcluvieae | 1 | South America | Hoogland (1979); Rodriguez et al. (2018) |
| Callicoma | Codieae | 1 | Australia | APNI (2020) |
| Ceratopetalum | Schizomerieae | 9 | Australia, New Guinea | Rozefelds and Barnes (2002) |
| Codia | Codieae | 15 | New Caledonia | Hopkins et al. (2014) |
| Cunonia | Cunonieae | 24 + 1 | New Caledonia + South Africa | Hopkins et al. (2014); Goldblatt and Manning (2000) |
| Davidsonia | unplaced | 3 | Australia | Harden and Williams (2000) |
| Eucryphia | unplaced | 5 + 2 | Australia + South America | Taylor and Hill (1996); Rodriguez et al. (2018) |
| Geissois | Geissoieae | 19 | New Caledonia, Fiji, Vanuatu, Solomon Islands | Hopkins (2006); Hopkins et al. (2014) |
| Gillbeea | unplaced | 3 | Australia, New Guinea | Rozefelds and Pellow (2000); Hopkins and Hoogland (2002) |
| Hooglandia | unplaced | 1 | New Caledonia | McPherson and Lowry (2004) |
| Karrabina | Geissoieae | 2 | Australia | Schimanski and Rozefelds (2002, as Geissois); Hopkins et al. (2013) |
| Lamanonia | Geissoieae | 6 | South America | Zickel and Leitão Filho (1993); Hopkins (2018a) |
| Opocunonia | Caldcluvieae | 1 | New Guinea | Hopkins and Hoogland (2002) |
| Pancheria | Cunonieae | 27 | New Caledonia | Hopkins et al. (2014) |
| Platylophus | Schizomerieae | 1 | South Africa | Goldblatt and Manning (2000) |
| Pseudoweinmannia | Geissoieae | 2 | Australia | Rozefelds and Pellow (2011) |
| Pterophylla | Cunonieae | 68 | Madagascar, Comoros, Malesia, Pacific Islands |
Madagacar and Comoros: Bradford (2001, 2001, 2001); Bradford and Miller (2001); Rogers (2017). Malesia and Pacific; Hopkins (1998, 1998, 1998); Hopkins and Bradford (1998, 1998); Hopkins and Florence (1998); Hopkins et al. (1998) |
| Pullea | Codieae | 3 | Australia, Malesia, Fiji | Hoogland (1979); Hopkins and Hoogland (2002) |
| Schizomeria | Schizomerieae | 9 | Australia, Malesia, Solomon Islands | Hopkins (2018b) |
| Spiraeanthemum | Spiraeanthemeae | 19 | Australia, New Guinea, Moluccas, Pacific Islands | Pillon et al. (2009a) |
| Vesselowskya | Cunonieae | 2 | Australia | Rozefelds et al. (2001) |
| Weinmannia | Cunonieae | 90 + 2 | Americas, Caribbean + Mascarenes | America: Bernardi (1961, 1963). No recent checklist available for all American taxa, but several regional treatmentsa. Mascarenes: Scott and Bosser (1997) |
Regional treatments for American Weinmannia include: Central America and Mexico (Morales, 2010, 2011), Venezuela (Bradford and Berry, 1998), Ecuador (Bradford, 1999; Harling, 1999), Peru (Zarucchi, 1993), Bolivia (Harling and Fuentes, 2014), Chile (Rodriguez et al., 2018), Southern Cone (Hopkins, 2008).
AUTHOR CONTRIBUTIONS
Yohan Pillon: Conceptualization (equal); Supervision (equal); Visualization (equal); Writing – original draft (equal); Writing – review & editing (equal). Helen C.F. Hopkins: Data curation (equal); Writing – original draft (equal); Writing – review & editing (equal). Olivier Maurin: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Visualization (equal); Writing – original draft (equal); Writing – review & editing (equal). Niroshini Epitawalage: Data curation (equal). Jason Bradford: Validation (equal); Writing – review & editing (equal). Zachary S Rogers: Investigation (equal); Validation (equal); Writing – original draft (equal); Writing – review & editing (equal). William J Baker: Conceptualization (equal); Funding acquisition (equal); Methodology (equal); Project administration (equal); Supervision (equal); Writing – review & editing (equal). Felix Forest: Conceptualization (equal); Formal analysis (equal); Funding acquisition (equal); Project administration (equal); Visualization (equal); Writing – original draft (equal); Writing – review & editing (equal).
Conceptualization: F.F., O.M., Y.P., W.J.B.; data curation: H.C.F.H., O.M., N.E.; formal analysis: F.F., O.M.; funding acquisition: F.F., W.J.B.; investigation: Z.R.; methodology: W.J.B.; project administration: F.F., W.J.B.; supervision: Y.P., W.J.B.; validation: J.B., Z.R.; visualization: F.F., O.M., Y.P.; writing original draft: F.F., O.M., Y.P., H.C.F.H., Z.R.; writing, review, and editing: F.F., H.C.F.H., J.B., O.M., Y.P., W.J.B., Z.R.
DATA AVAILABILTY STATEMENT
DNA sequences produced for this study are available in European Nucleotide Archive under umbrella project PRJEB35285; accessions numbers are provided in Appendix S1.
Supporting information
APPENDIX S1. List of sequenced material with full information on herbarium vouchers and data quality.
APPENDIX S2. Biogeographical model.
APPENDIX S3. Geographical area assignments for each terminal included in the biogeographical analysis.
APPENDIX S4. Dated tree of crown Oxalidales.
APPENDIX S5. Linear sequences of families and genera in the crown clade of Oxalidales for use in herbarium curation.
APPENDIX S6. The 12 currently recognized species in the Caldcluvieae, showing their distribution and historical and current generic placements.
Acknowledgments
We thank Rafaël Govaerts for advice regarding the termination of section names and Heather Lindon for other nomenclatural advice. We thank Julia Buckley, Craig Brough, and Tracy Wells, and Archivo del Real Jardín Botánico, CSIC for supplying images for Fig. 1 and for information about the artists, and Karine Gotty for editing the plate. This work was funded by grants from the Calleva Foundation and the Sackler Trust to the Plant and Fungal Tree of Life Project (PAFTOL) at the Royal Botanic Gardens, Kew. We thank Andrew Thornhill and an anonymous reviewer for critical comments on an earlier version of the manuscript.
APPENDIX 1. Taxonomic treatment
Here, we present a summary of the taxonomic history and morphology of the two groups of taxa whose re‐circumscription is justified above, plus conspectuses for Ackama and Pterophylla, which include the new combinations that are necessary. In these conspectuses, accepted taxa are listed with their homotypic synonyms, except for ca. 70 combinations in Windmannia P.Browne (nom. rej.) made by Kuntze (1891). Relevant heterotypic synonyms are provided for a few generic and sectional names. The year of publication is given for each name, but full publication details appear only with the basionym or replaced name in the case of a new combination or nomen novum, respectively.
Taxonomic history of the genera in Caldcluvieae
Because the genera and species in the Caldcluvieae have a convoluted taxonomic history, the generic placement of the species currently recognized here and in previous studies is compared in Appendix S6.
Caldcluvia was established in 1830, when it contained a single species, C. paniculata (Cav.) D.Don, from South America (Don, 1830). No other species have basionyms in this genus, which was not equated with any other genera until Hoogland (1979), who included all the species now in the tribe Caldcluiveae within this genus. Ackama was established by Cunningham (1839), based on a single species from New Zealand (A. rosifolia A.Cunn.), but others were subsequently added from Australia and New Zealand.
Miquel (1856) established Spiraeopsis, which initially included only S. celebica (Blume) Miq., based on the basionym Cunonia celebica Blume, from Sulawesi. This name also served as the basionym of Dirhynchosia celebica (Blume) Blume, the sole species in Dirhynchosia Blume, described in his privately published “Mélanges botaniques” (Blume, 1855). Due to the extremely rare print run of apparently only two copies, Stafleu and Cowan (1976) erroneously concluded that “Mélanges” had never in fact been printed, and until van Steenis (1986), it was generally accepted that the name Dirhynchosia was effectively published in a volume of Flora edited by Fürnrohr (1858). The fact that Dirhynchosia had been effectively published in 1855, the year before Spiraeopsis, was overlooked by Bradford and Barnes (2001), Bradford et al. (2004), and Hopkins and Hoogland (2002), which led to illegitimate combinations being made in Spiraeopsis.
Opocunonia and Stollaea Schltr. were described by Schlechter (1914) based on material from New Guinea; Stollaea contained one species (S. papuana Schltr.), whereas Opocunonia had three (O. nymanii (K.Schum.) Schltr., O. kanaiensis Schltr., O. trifoliata Schltr.), but all four names now apply to a single variable taxon, O. nymanii (Hopkins and Hoogland, 2002). Since Schlechter’s work, Opocunonia has never been considered to be synonymous with Ackama or Spiraeopsis, nor with Caldcluvia (except by Hoogland [1979]), although the basionym of O. nymanii was originally published in Ackama (Schumann and Lauterbach, 1905). In our study, Caldcluvia and Opocunonia are sister groups and together they are sister to Ackama + Spiraeopsis (all well‐supported clades).
The distinction between Ackama and Spiraeopsis (including Betchea) has not always been clear. Both Schlechter (1914) and Engler (1928) placed one of the Australian taxa in Ackama and the other in Betchea, and the basionym of S. papuana (Pulle) L.M.Perry is in Ackama. The synonymy of Betchea and Spiraeopsis is demonstrated by Engler’s (1928) placement of S. rufa (Schltr.) L.M.Perry and S. papuana, and/or some of their synonyms, in both genera simultaneously. Perry (1949) subsequently accepted Betchea as a synonym of Spiraeopsis. In summary, Ackama and Spiraeopsis have not always been clearly distinguished from each other but they have not generally been confused with either Opocunonia or Caldcluvia s.s.
Since our analysis shows Spiraeopsis is nested within Ackama, the options were to: (1) maintain Spiraeopsis as distinct and split Ackama into two, creating five genera in the Caldcluvieae; (2) include Spiraeopsis within Ackama, which would result in three genera in the tribe; or (3) combine Ackama and Spiraeopsis with Opocunonia and perhaps also with Caldcluvia s.s., further reducing the number of genera. We have chosen the second option, although this makes Ackama more heterogeneous than before, especially in its indumentum, fruits, and seeds (Table 3 and see below).
TABLE 3.
Comparison of selected morphological features of Ackama, Opocunonia, Spiraeopsis, and Caldcluvia s.s.
| Characters | Ackama (New Zealand) | Ackama (Australia) | Spiraeopsis | Opocunonia | Caldcluvia s.s. |
|---|---|---|---|---|---|
| Number of species | 2 | 2 | 5 | 1 | 1 |
| Leaf type | imparipinnate | imparipinnate | imparipinnate | imparipinnate or trifoliolate | simple |
|
Venation |
semi‐craspedodromous | semi‐craspedodromous | semi‐craspedodromous | semi‐craspedodromous to craspedodromous | craspedodromous |
|
Stipule position, number, margin |
interpetiolar, 1 pair per node, toothed in A. rosifolia | interpetiolar, 1 pair per node, not toothed | interpetiolar, 1 pair per node, not toothed | interpetiolar, 1 pair per node, usually stalked and bilobed, sometimes toothed |
lateral, 4 per node, sometimes asymmetric, toothed |
| Trichome type | simple | simple | simple and stellate | simple | simple |
| Presence of raised glands, peltate scales, or orbicular glands | yes | yes | yes | no (yes?) | no |
| Inflorescence size, shape a | large, triangular in outline | large, triangular in outline | large, triangular in outline | small, rounded or flattish in outline | small, rounded in outline |
| Pedicels (length) | ± absent | ± absent | 1–2.5 mm | 3–5 mm | 4–8 mm |
| Flower size b (length) | small (1.5 mm) | small (1–1.5 mm) | small (1.5–2 mm) | large (2–4 mm) | large (3–4 mm) |
| Petals | (4–) 5, oblanceolate‐elliptic | 4–6, oblanceolate‐elliptic | 4–5, oblanceolate‐elliptic | 5–6 (–7), ovate | 4 (–5?), oblanceolate‐elliptic |
| Carpel number | 2 (–3) | 2–4 | 2–5 | 2 | 2 |
| Replum in fruit | absent | absent |
present |
absent |
present |
| Sepals in fruit | persistent | persistent | persistent | persistent | caducous |
| Seed shape, pubescence | ovoid, pubescent all over | ovoid, pubescent all over | spindle‐shaped, glabrous | spindle‐shaped, glabrous | spindle‐shaped, glabrous |
Observations based on herbarium material at K and supplemented with data taken from literature: Godley (1979); Webb and Simson (1991); de Lange et al. (2002); Hopkins and Hoogland (2002).
Inflorescence size – large: typically 25–50 cm long though sometimes smaller in S. clemensiae L.M.Perry; small: typically 10–15 cm long.
Flower size – length measured from the base of the calyx to the tips of the lobes.
Morphology of Caldcluvieae
Characters shared by all members of the Caldcluvieae include valvate sepals, apparently bisexual flowers that are strongly protandrous in some, and loosely connate carpels that separate when dehiscing via a ventral split (Hoogland, 1979); axile placentae often thickened in fruit (Bradford, 1998, 1998) that either remain attached to the margins of the valves after dehiscence or result in the formation of a replum, i.e., strands of tissue that extend between the valves; tuft domatia on the underside of the leaves, usually in the axils of the secondary veins (more common in some species than others), and axillary, paniculate inflorescences (thyrses). However, other characters show a checkerboard pattern of occurrence amongst the species in the tribe (see Table 3).
One character that distinguished the species previously treated as Spiraeopsis from Ackama s.s. is the presence of stellate trichomes in the former, although both it and Ackama s.s. have structures that vary from raised “glands” (especially on the lower leaf surface in Ackama s.s.), to sessile, red or orange orbicular structures (often on the inflorescence axes), to peltate scales (on the leaves in Spiraeopsis).
Godley (1983) questioned Hoogland’s (1979) placement of Ackama rosifolia (New Zealand) in the same genus as Caldcluvia paniculata (Chile and Argentina) because the fruits of the latter have strands of tissue extending between the valves of the capsule after dehiscence. This structure is absent in A. rosifolia and the Australian species of Ackama, although Bradford (1998, 1998: 590, character 14) noted the underlying similarity of the thickened placentae in Caldcluvia s.s. and Ackama. A replum is often present in the fruits of the species previously in Spiraeopsis. Webb and Simpson (1991) noted that the seeds of A. rosifolia are ellipsoid and pubescent, whereas those of C. paniculata are spindle‐shaped and glabrous (as are those of species in Spiraeopsis).
Characters that support the inclusion of Spiraeopsis in Ackama and distinguish it from Caldcluvia s.s. are the size and shape of the inflorescence, the size of the flowers and length of their pedicels, and the number and arrangement of the stipules (Table 3). Opocunonia and Caldcluvia s.s. resemble each other in their inflorescence shape, the size of the flowers, and length of the pedicels, but are distinct in their inflorescences and stipules.
Ackama A.Cunn. in Ann. Nat. Hist. 2: 358 (1839). Type: Ackama rosifolia A. Cunn., as ‘rosaefolia’.
= Betchea Schltr. in Bot. Jahrb. Syst. 52: 146 (1914). Lectotype (Hutchinson, 1967): B. rufa Schltr.
= Dirhynchosia Blume in Mélanges Bot.: 6 (1855). Type: D. celebica (Blume) Blume.
= Spiraeopsis Miq. in Fl. Ned. Ind. 1: 719 (1856) syn. nov. Type: S. celebica (Blume) Miq.
Trees. Indumentum of simple hairs (all) and other structures varying from raised gland dots (especially on underside of foliage in some) to sessile, orbicular, red or orange structures (in some, especially on inflorescence axes) to peltate scales; stellate hairs sometimes present (spp. formerly in Spiraeopsis only, Malesia and Solomon Isl.). Stipules interpetiolar, one pair per node, margins toothed or entire. Leaves opposite and decussate, compound, imparipinnate, petiolate, rachis not winged; leaflet margins toothed; tuft domatia often present in axils of secondary veins. Inflorescence a many‐flowered, axillary, cone‐shaped thyrse, sometimes inserted in series, the lower axes opposite; floral maturation synchronous; flowers borne singly or in small fascicles. Flowers apparently bisexual but sometimes (often?) protandrous, small (calyx 1–2 mm from base to apex) and usually almost sessile; calyx lobes 4–5 (–6), valvate in bud; petals 4–5 (–6), oblanceolate‐elliptic; stamens 8 or 10 (12); disc annular and erect to almost lobed, the indentations corresponding to the bases of the filaments; ovary of 2–5 carpels, these fused or almost free (A. nubicola), each bearing a free stylodium. Capsule basipetally and septicidally dehiscent into 2–5 valves, margins of valves (remains of axile placentae) partly or entirely thickened, sometimes forming a replum (Malesia and Solomon Isl.); seeds ca. 4–16 per capsule, either spindle‐shaped and glabrous (Malesia and Solomon Isl.) or ellipsoid and pubescent (Australia and New Zealand).
Heterotypic synonymy for the taxa in Ackama can be found in Hoogland (1979, all taxa, including details of types), Hopkins and Hoogland (2002, Malesian taxa), and in the Australian Plant Name Index (APNI, 2020, Australian taxa).
Conspectus of Ackama
1. Ackama australiensis (Schltr.) C.T.White (1936) ≡ Betchea australiensis Schltr. (1914) ≡ Caldcluvia australiensis (Schltr.) Hoogland (1979).
2. Ackama brassii (L.M.Perry) Pillon & H.C.Hopkins comb. nov. ≡ Spiraeopsis brassii L.M.Perry in J. Arnold Arbor. 30: 147 (1949) ≡ Caldcluvia brassii (L.M.Perry) Hoogland (1979).
3. Ackama celebica (Blume) Pillon & H.C.Hopkins comb. nov. ≡ Cunonia celebica Blume in Bijdr. Fl. Ned. Ind.: 868 (1826) ≡ Dirhynchosia celebica (Blume) Blume (1855) ≡ Spiraeopsis celebica (Blume) Miq. (1856) ≡ Caldcluvia celebica (Blume) Hoogland (1979).
4. Ackama clemensiae (L.M.Perry) Pillon & H.C.Hopkins comb. nov. ≡ Spiraeopsis clemensiae L.M.Perry in J. Arnold Arbor. 30: 149 (1949) ≡ Caldcluvia clemensiae (L.M.Perry) Hoogland (1979).
5. Ackama fulva (Schltr.) Pillon & H.C.Hopkins comb. nov. ≡ Betchea fulva Schltr. in Bot. Jahrb. Syst. 52: 148 (1914) ≡ Spiraeopsis fulva (Schltr.) L.M.Perry (1949) ≡ Caldcluvia fulva (Schltr.) Hoogland (1979).
6. Ackama nubicola de Lange (2002).
7. Ackama paniculosa (F.Muell.) Heslewood (2013) ≡ Weinmannia paniculata F.Muell. (1860), nom. illeg. hom., non Cav. (1801) ≡ Weinmannia paniculosa F.Muell. (1861) ≡ Caldcluvia paniculosa (F.Muell.) Hoogland (1979).
8. Ackama papuana Pulle (1912) ≡ Betchea papuana (Pulle) Schltr. (1914) ≡ Spiraeopsis papuana (Pulle) L.M.Perry (1949) ≡ Caldcluvia papuana (Pulle) Hoogland (1979).
9. Ackama rosifolia A.Cunn. (1839) as ‘rosaefolia’ ≡ Caldcluvia rosifolia (A. Cunn.) Hoogland (1979).
10. Ackama rufa (Schltr.) Pillon & H.C.Hopkins comb. nov. ≡ Betchea rufa Schltr. in Bot. Jahrb. Syst. 52: 148 (1914) ≡ Spiraeopsis rufa (Schltr.) L.M.Perry (1949) ≡ Caldcluvia rufa (Schltr.) Hoogland (1979).
Taxonomy of Weinmannia s.l.
Among the genera in tribe Cunonieae, Cunonia and Weinmannia were both described by Linnaeus and have always been considered quite distinct from each other. Although superficially similar, they differ by a number of characters (Hopkins et al., 2014): stipules (spoon‐shaped vs. triangular, or reniform vs. salverform), hairs on the seeds (absent/present), seed wing (present/absent), floral disk (adnate to/free from ovary), fruit dehiscence (acropetal/basipetal). The genera Vesselowskya and Pancheria were described later and differ from each other and from Weinmannia and Cunonia in characters such as digitate leaves, dioecy (considered derived within Weinmannia) and trimerous flowers in Vesselowskya (Rozefelds et al., 2001), and whorled simple, trifoliolate or imparipinnate leaves, dioecy, and 3–4‐merous flowers in capitate inflorescences in Pancheria (Hopkins et al., 2009). As mentioned above, the species of Weinmannia in its traditional sense fall into two clades. The first clade, sect. Weinmannia, includes the species from the Americas and the Mascarenes. The second clade includes the remaining species, all from the Old World: sections Fasciculatae (Malesia and Pacific), Inspersae (Madagascar), Leiospermum (Pacific), and Spicatae (Madagascar + Comoros).
Three names must be considered when seeking a new generic name for Old World Weinmannia: Arnoldia Blume (1826), Leiospermum D.Don (1830), and Pterophylla D.Don (1830). Arnoldia Blume (1826) is a later homonym of Arnoldia Cass. (1824), which is itself a synonym of Dimorphotheca Vaill. ex Moench (Asteraceae). Leiospermum and Pterophylla were published simultaneously and so have equal priority. However, Leiospermum D.Don has a later illegitimate homonym, Leiospermum Wall. (1832). We therefore choose the generic name Pterophylla D.Don for the species of Weinmannia from the Old World (excluding the Mascarenes) to avoid unnecessary homonymic confusion.
Morphology of Weinmannia s.l.
Comparison of the descriptions for Weinmannia sect. Weinmannia (hereafter Weinmannia s.s.) and the clade formed by the four other sections (hereafter Pterophylla) shows that their similarities are quite marked and several characters that define each genus are not entirely diagnostic (see character matrix in Bradford, 1998, 1998). The most obvious difference between the two groups is the architecture of the inflorescence. In Weinmannia s.s., each inflorescence is formed by two opposite racemes or sometimes spikes (sometimes referred to as pseudoracemes and pseudospikes because the flowers are borne in fascicles), which are inserted directly in the axils of the most distal pair of leaves on a stem; lower pairs of leaves do not subtend racemes or spikes. This morphology contrasts with Pterophylla (and other genera in Cunonieae such as Cunonia or Pancheria), in which the inflorescence is usually composed of complex units, termed inflorescence modules (IMs) by Bradford (1998, 2002), each consisting of a number of racemes or spikes, the internode to which they are attached, and any associated buds. These IMs can be either axillary at the distal end of a shoot (usually with an apical bud between them), or terminal, or a combination of the two states. A few exceptions occur in sect. Spicatae (see diagnosis) where the inflorescence consists of simple spikes, similar to those in Weinmannia s.s., but usually in the taxa in sect. Spicatae, the spikes are inserted at several nodes along a stem and are not confined to the most distal leaf axils, and thus what Bradford (1998, 1998) termed the total inflorescence (TI) encompasses multiple nodes of the stem in these taxa. The architecture of the inflorescence in Weinmannia s.l. was analyzed in detail by Bradford (1998, 2001), and this approach was extended to include all genera in the tribe Cunonieae by Bradford (2002). When looking at the evolution of the inflorescence architecture for the entire tribe, it appears that the structure of Weinmannia s.s. has a unique, derived set of traits that is diagnostic on most herbarium specimens; namely, the TI is reduced to a single node with racemes (not IMs) born directly in the axils of leaves or of reduced leaves.
Seed characters are only partly diagnostic. The seeds are straight (i.e., ellipsoid) and typically comose in the sections that now form Pterophylla, whereas in Weinmannia s.s. (as sect. Weinmannia) they are curved (reniform) with the hairs sparsely and widely distributed (Bradford, 1998, 1998). However, species descriptions for some New World taxa state that the seeds are ellipsoid, and the hairs are dispersed in a few species of Pterophylla. Another largely diagnostic character is the corolla, which commonly but not universally falls off as a “cap” of four petals soon after anthesis in the New World species (Weinmannia s.s.), whereas in Pterophylla, it appears never to be caducous.
Bradford’s (1998, 1998) morphological data matrix, based on examination of both Mascarenes species of Weinmannia and numerous American ones, also demonstrated that these two geographically separated groups within Weinmannia s.s. are not identical in all major characters, although overall, a greater range of variation occurs in Pterophylla than in Weinmannia s.s.
Pterophylla D.Don in Edinburgh New Philos. J. 9: 93 (1830). Type: Pterophylla fraxinea D.Don
Trees or shrubs, hermaphroditic, dioecious or polygamodioecious. Indumentum of simple hairs. Stipules interpetiolar, one pair per node. Leaves opposite and decussate (rarely whorled, P. commersonii, Madagascar), simple, trifoliolate or imparipinnate (rarely unifoliolate), petiolate or ± sessile (especially in some simple‐leaves spp.); in compound leaves, petiole and rachis unwinged or narrowly winged; leaflet margins toothed (rarely ± entire, e.g., P. mammea, Madagascar); domatia in axils of secondary veins absent. Inflorescence of racemes or sometimes spikes (pseudoracemes and pseudospikes when flowers in fascicles), these usually arranged in complex groups with a sterile basal peduncular segment, the groups either axillary or terminal or a combination (rarely spikes borne singly, directly in leaf axils, often of several successive pairs of leaves, e.g., P. comorensis, Comoros; P. baehniana, P. lucens, P. minutiflora, all Madagascar, sect. Spicatae); flowers inserted on axes either singly or in fascicles, each flower or fascicle subtended by a bract; floral maturation synchronous. Flowers bisexual or unisexual by early suppression of one sex, pedicellate or sometimes sessile; calyx lobes 4–5 (mostly 5 in Madagascar and New Caledonia, mostly 4 in the remaining species), imbricate in bud; petals 4–5, ± elliptic, membranous, persistent; stamens 8 or 10 (5 in P. sanguisugarum, Madagascar); disc segmented, ribbed or membranous; ovary of 2 (–3) fused carpels, each with a free stylodium. Capsule basipetally and septicidally dehiscent into 2 (–3) valves, central column often present, calyx persistent or caducous; seeds numerous, ellipsoid, usually comose or occasionally hairs widely distributed, either densely or sparsely so.
Conspectus of Pterophylla
Pterophylla sect. Pterophylla
≡ Weinmannia sect. Fasciculatae Bernardi ex Hoogland & H.C.Hopkins in Adansonia, sér. 3, 20: 21 (1998), as ‘Fasciculata’.
= Arnoldia Blume in Bijdr. Fl. Ned. Ind.15: 868 (1826), nom. illeg. hom., non Cass. (1824). Lectotype (Hopkins and Hoogland, 2002): A. heterophylla Blume.
This section was treated as Weinmannia sect. Fasciculatae in Hopkins and Bradford (1998, 1998), Hopkins (1998, 1998, 1998), and Hopkins et al. (1998), where a description of the section and details of types and heterotypic synonyms can be found (see also Hopkins and Hoogland, 2002).
1. Pterophylla aphanoneura (Airy Shaw) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia aphanoneura Airy Shaw in Bull. Misc. Inform. Kew 1940: 260 (1940).
2. Pterophylla celebica (Koord.) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia celebica Koord. in Meded. Lands Plantentuin 19: 640 [& 450] (1898).
3. Pterophylla clemensiae (Steenis) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia clemensiae Steenis in J. Bot. 72: 3 (1934).
4. Pterophylla coodei (H.C.Hopkins) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia coodei H.C.Hopkins in Adansonia, sér. 3, 20: 52, f. 4A–D (1998).
5. Pterophylla descombesiana (Bernardi) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia descombesiana Bernardi in Bot. Jahrb. Syst. 83: 186 in key, 190, f. 33 (1964).
6. Pterophylla devogelii (H.C.Hopkins) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia devogelii H.C.Hopkins in Adansonia, sér. 3, 20: 48, f. 1 (1998).
7. Pterophylla exigua (A.C.Sm.) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia exigua A.C.Sm. in J. Arnold Arbor. 33: 137 (1952).
8. Pterophylla eymana (H.C.Hopkins) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia eymana H.C.Hopkins in Adansonia, sér. 3, 20: 50, f. 3E–H, J (1998), as ‘eymaeana’.
9. Pterophylla fraxinea D .Don (1830) ≡ Weinmannia fraxinea (D.Don) Miq. (1856). The type of the genus and thus also the autonymic section.
10. Pterophylla furfuracea (H.C.Hopkins) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia furfuracea H.C.Hopkins in Adansonia, sér. 3, 20: 49, f. 3A–D (1998).
11. Pterophylla hooglandii (H.C.Hopkins & J.Bradford) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia hooglandii H.C.Hopkins & J.Bradford in Adansonia, sér. 3, 20: 37, f. 11 (1998).
12. Pterophylla hutchinsonii (Merr.) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia hutchinsonii Merr. in Philipp. J. Sci., C. 2: 275 (1907).
13. Pterophylla lucida (Merr.) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia lucida Merr. in Philipp. J. Sci., C. 10: 7 (1915).
14. Pterophylla luzoniensis (S.Vidal) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia luzoniensis S.Vidal in Révis. Pl. Vasc. Filip.: 125 (1886).
15. Pterophylla macgillivrayi (Seem.) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia macgillivrayi Seem. in Fl. Vit. [Seemann] 1: 109 (1866).
16. Pterophylla negrosensis (Elmer) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia negrosensis Elmer in Leafl. Philipp. Bot. 2: 577 (1909).
17. Pterophylla pullei (Schltr.) Pillon & H.C.Hopkins comb. nov. ≡ Weimannia pullei Schltr. in Bot. Jahrb. Syst. 52: 164 (1914).
18. Pterophylla richii (A.Gray) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia richii A Gray in U.S. Expl. Exped., Phan. 15: 675, Atlas t. 85 (1854).
19. Pterophylla urdanetensis (Elmer) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia urdanetensis Elmer in Leafl. Philipp. Bot. 7: 2608 (1915).
20. Pterophylla ysabelensis (L.M.Perry) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia ysabelensis L.M.Perry in J. Arnold Arbor. 30: 162 (1949).
Pterophyllasect. Leiospermum (D.Don) Pillon & H.C.Hopkins comb. nov. ≡ Leiospermum D.Don in Edinburgh New Philos. J. 9: 91 (1830) ≡ Weinmannia sect. Leiospermum (D.Don) Engl. in Nat. Pflanzenfam. III, 2a: 101 (1891). Lectotype (Hopkins and Bradford, 1998, 1998): Leiospermum racemosum (L. f.) D.Don.
= Weinmannia sect. Racemosae Bernardi in Bot. Jahrb. Syst. 83: 132, 185 (1964).
With the exception of species from Samoa, Rarotonga, and New Zealand, this section was revised as Weinmannia sect. Leiospermum in Hopkins and Bradford (1998, 1998), Hopkins et al. (1998), and Hopkins and Florence (1998), where a description of the section and details of types and heterotypic synonyms can be found. Minor updates including new taxa have been published by Hopkins and Pillon (2011, New Caledonia), Lorence and Wagner (2011, Marquesas Isl.); see also Hopkins and Hoogland (2002, Malesia), Hopkins et al. (2014, New Caledonia), and Sykes (2016, Cook Isl.) for regional treatments.
New combinations are unnecessary for Weinmannia manuana Christoph. and W. rarotongensis Hemsl. because they are considered to be synonyms of W. samoensis A.Gray (Hopkins et al., 1998; Sykes, 2016), and W. spiraeoides A.Gray is omitted on account of its doubtful status (Bernardi, 1964). Names of accepted taxa in New Zealand are taken from Schönberger et al. (2020).
1. Pterophylla affinis (A.Gray) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia affinis A.Gray, U.S. Expl. Exped., Phan. 15: 674 (1854).
2. Pterophylla croftii (H.C.Hopkins) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia croftii H.C.Hopkins, Adansonia, sér. 3, 20: 76, f. 4 (1998).
3. Pterophylla denhamii (Seem.) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia denhamii Seem., Fl. Vit. [Seemann] 1: 109 (1866), as ‘denhami’.
4a. Pterophylla dichotoma (Brongn. & Gris) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia dichotoma Brongn. & Gris, Bull. Soc. Bot. France 9: 73 (1862).
4b. Pterophylla dichotoma var. monticola (Däniker) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia monticola Däniker, Vierteljahrsschr. Naturf. Ges. Zürich 76: 165 (1931) ≡ Weinmannia dichotoma var. monticola (Däniker) H.C.Hopkins & Pillon (2012).
5a. Pterophylla marquesana (F.Br.) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia marquesana F.Br., Bull. Bernice P. Bishop Mus. 130: 99 (1935).
5b. Pterophylla marquesana var. angustifolia (Lorence & W.L. Wagner) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia marquesana var. angustifolia Lorence & W.L.Wagner, PhytoKeys 4: 62 (2011).
5c. Pterophylla marquesana var. myrsinites (Fosberg & Sachet) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia parviflora var. myrsinites Fosberg & Sachet, Micronesica 8: 45 (1972) ≡ Weinmannia marquesana var. myrsinites (Fosberg & Sachet) H.C.Hopkins & J.Florence (1998).
6. Pterophylla ouaiemensis (Guillaumin & Virot) Pillon & H.C.Hopkins comb. nov. ≡ Cunonia ouaiemensis Guillaumin & Virot, Mem. Mus. Natl. Hist. Nat., B, Bot. 4: 28 (1953) ≡ Weinmannia ouaiemensis (Guillaumin & Virot) Hoogland (1998).
7. Pterophylla paitensis (Schltr.) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia paitensis Schltr., Bot. Jahrb. Syst. 39: 124 (1906).
8. Pterophylla parviflora (G.Forst.) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia parviflora G.Forst., Fl. Ins. Austr.: 29 (1786), as ‘paruiflora’.
9. Pterophylla purpurea (L.M.Perry) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia purpurea L.M.Perry, J. Arnold Arbor. 30: 159 (1949).
10. Pterophylla racemosa (L.f.) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia racemosa L.f., Suppl. Pl.: 227 (1782) ≡ Leiospermum racemosum D.Don (1830).
11. Pterophylla raiateensis (J.W.Moore) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia raiateensis J.W.Moore, Bull. Bernice P. Bishop Mus. 102: 28 (1933).
12. Pterophylla rapensis (F.Br.) Pillon & H.C.Hopkins comb. nov. ≡ Weimannia rapensis F.Br., Bull. Bernice P. Bishop Mus. 130: 100 (1935).
13. Pterophylla samoensis (A.Gray) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia samoensis A.Gray, U.S. Expl. Exped., Phan. 15: 677 (1854).
14. Pterophylla serrata (Brongn. & Gris) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia serrata Brongn. & Gris, Bull. Soc. Bot. France 9: 73 (1862).
15a. Pterophylla sylvicola (Sol. ex A.Cunn.) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia sylvicola Sol. ex A.Cunn., Ann. Nat. Hist. 2: 357 (1839).
15b. Pterophylla sylvicola var. betulina (A.Cunn.) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia sylvicola var. betulina (A.Cunn.) Hook.f. (1852) ≡ Weinmannia betulina A. Cunn., Ann. Nat. Hist. 2: 357 (1839).
16. Pterophylla tremuloides (H.C.Hopkins & J.Florence) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia tremuloides H.C.Hopkins & J.Florence, Adansonia, sér. 3, 20: 123 (1998).
17. Pterophylla vescoi (Drake) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia vescoi Drake, Ill. Fl. Ins. Pacif.: 35, pl. 13 (1886).
18. Pterophylla vitiensis (Seem.) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia vitiensis Seem., Fl. Vit. [Seemann] 1: 110 (1866).
Pterophyllasect. Spicatae(Bernardi ex J.Bradford) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia sect. Spicatae Bernardi ex J.Bradford in Ann. Missouri Bot. Gard. 89: 503 (2002). Type: Weinmannia bojeriana Tul.
= Ornithrophus Bojer ex Engl. in Linnaea 36: 636 (1870), nom. inval.
Our placement of Malagasy taxa into sections Spicatae and Inspersae follows Bradford (2001, 2001, 2001), who subsequently provided validating types and diagnoses for both sections (Bradford, 2002), which had originally been proposed by Bernardi (1964). Information on types of older accepted names and their heterotypic synonyms can be found in Bernardi (1964, 1965). The only non‐Malagasy species in either section is Pterophylla comorensis, a Comoran endemic (sect. Spicatae). Keys to species groups of both sections were provided by Bradford (2001, 2001, 2001), with some revisions as a result of new species described (see Rogers and Bradford, 2004). Taxonomic summaries of all Malagasy taxa including distributions and voucher specimens were given by Rogers (2017). New combinations are not required for two binomials that are based on material from Madagascar: Weinmannia trigyna Baker [≡ Homalium trigynum (Baker) Sleumer], and W. rhodoxylon Tul., which is a dubious name that can only be linked to two type sheets of unidentifiable juvenile material (Bernardi, 1964).
1. Pterophylla arguta (Bernardi) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia arguta (Bernardi) J.Bradford (2001, 2001, 2001) ≡ Weinmannia hildebrandtii var. arguta Bernardi, Fl. Madagasc. Fam. 93: 30 (1965).
2. Pterophylla baehniana (Bernardi) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia baehniana Bernardi, Ber. Schweiz. Bot. Ges. 74: 260, f. 1 (1964).
3. Pterophylla bernardii J.Bradford & Z.S.Rogers nom. nov. Replaced synonym: Weinmannia venosa J.Bradford, Adansonia, sér. 3, 23: 233, f. 6 (2001), nom. illeg. hom., non W. venosa Knowles & Westc. (1838) [= Acrophyllum australe (A.Cunn.) Hoogland]. The new epithet for this species is chosen to honor Luciano Bernardi (1920–2001), the Italian botanist who revised the genus Weinmannia, completely in Latin, in the 1960s while working at the Conservatoire et Jardin botaniques Genève.
4. Pterophylla bojeriana (Tul.) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia bojeriana Tul., Ann. Sci. Nat., Bot. sér. 4, 8: 155 (1857).
5. Pterophylla bradfordii (I.M.Turner) Z.S.Rogers comb. nov. ≡ Weinmannia bradfordii I.M.Turner, Ann. Bot. Fenn. 51: 309 (2014) ≡ Weinmannia integrifolia J.Bradford, Adansonia, sér. 3, 23: 225, f. 2 (2001), nom. illeg. hom., non W. integrifolia Lesq., Rep. U.S. Geol. Surv. Territ. 8: 178 (1883).
6. Pterophylla comorensis (Tul). J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia comorensis Tul., Ann. Sci. Nat., Bot. sér. 4, 8: 153 (1857).
7. Pterophylla decora (Tul.) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia decora Tul., Ann. Sci. Nat., Bot. sér. 4, 8: 154 (1857).
8. Pterophylla eriocarpa (Tul.) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia eriocarpa Tul., Ann. Sci. Nat., Bot. sér. 4, 8: 156 (1857).
9. Pterophylla hildebrandtii (Baill.) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia hildebrandtii Baill., Bull. Mens. Soc. Linn. Paris 1: 475 (1885).
10. Pterophylla humbertiana (Bernardi) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia humbertiana Bernardi, Bot. Jahrb. Syst. 83: 132 in key, 139, f. 5 (1964).
11a. Pterophylla humblotii (Baill.) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia humblotii Baill., Bull. Mens. Soc. Linn. Paris 1: 475 (1885).
11b. Pterophylla humblotii var. anceps (Bernardi) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia humblotii var. anceps Bernardi, Fl. Madagasc. Fam. 93: 41, f. 7 nos. 1–6 (1965).
12. Pterophylla icacifolia (Bernardi) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia icacifolia (Bernardi) Bernardi (1964) ≡ Weinmannia bojeriana var. icacifolia Bernardi, Bot. Jahrb. Syst. 83: 134 in key, 135, f. 1 (1964).
13. Pterophylla lucens (Baker) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia lucens Baker, J.Bot. 20: 70 (1882).
14. Pterophylla magnifica (J.Bradford & Z.S.Rogers) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia magnifica J.Bradford & Z.S.Rogers, Adansonia, sér. 3, 26: 85, f. 1 (2004).
15. Pterophylla mammea (Bernardi) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia mammea Bernardi, Bot. Jahrb. Syst. 83: 133 in key, 141, f. 8 (1964).
16. Pterophylla marojejyensis (J.S.Mill. & J.Bradford) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia marojejyensis J.S.Mill. & J.Bradford, Adansonia, sér. 3, 23: 227, f. 3 (2001).
17. Pterophylla minutiflora (Baker) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia minutiflora Baker, J. Linn. Soc., Bot. 21: 339 (1884).
18. Pterophylla pauciflora (J.Bradford) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia pauciflora J.Bradford, Adansonia, sér. 3, 23: 229, f. 4 (2001).
19. Pterophylla rakotomalazana (J.Bradford) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia rakotomalazana J.Bradford, Adansonia, sér. 3, 23: 229, f. 5 (2001).
20. Pterophylla sanguisugarum (Bernardi) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia sanguisugarum Bernardi, Candollea 24: 85 (1969).
21. Pterophylla stenostachya (Baker) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia stenostachya Baker, Bull. Misc. Inform. Kew 1895: 103 (1895).
Pterophyllasect. Inspersae (Bernardi ex J.Bradford) Pillon & H.C.Hopkins comb. nov. ≡ Weinmannia sect. Inspersae Bernardi ex J.Bradford in Ann. Missouri Bot. Gard. 89: 503 (2002). Type: Weinmannia madagascariensis DC. ex Ser.
1. Pterophylla aggregata (Z.S.Rogers & J.Bradford) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia aggregata Z.S.Rogers & J.Bradford, Adansonia, sér. 3, 26: 86, f. 1 (2004).
2. Pterophylla commersonii (Bernardi) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia commersonii Bernardi, Bot. Jahrb. Syst. 83: 144, f. 9 (1964).
3. Pterophylla henricorum (Bernardi) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia henricorum Bernardi, Bot. Jahrb. Syst. 83: 144 in key, 146, f. 10 (1964).
4. Pterophylla hepaticarum (Bernardi) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia hepaticarum Bernardi, Bot. Jahrb. Syst. 83: 143 in key, 147, f. 11 (1964).
5. Pterophylla louveliana (Bernardi) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia louveliana Bernardi, Bot. Jahrb. Syst. 83: 143 in key, 149, f. 12 (1964).
6. Pterophylla lowryana (J.Bradford) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia lowryana J.Bradford, Adansonia, sér. 3, 23: 223, f. 1 (2001).
7a. Pterophylla madagascariensis (DC. ex Ser.) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia madagascariensis DC. ex Ser., Prodr. [DC.] 4: 9 (1830).
7b. Pterophylla madagascariensis var. aniba (Bernardi) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia madagascariensis var. aniba Bernardi, Bot. Jahrb. Syst. 83: 144 in key, 152 (1964).
8. Pterophylla rutenbergii (Engl.) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia rutenbergii Engl., Abh. Naturwiss. Vereins Bremen 7: 16 (1880).
9. Pterophylla venusta (Bernardi) J.Bradford & Z.S.Rogers comb. nov. ≡ Weinmannia venusta Bernardi, Bot. Jahrb. Syst. 83: 156, f. 16 (1964).
WeinmanniaL. in Syst. Nat., ed. 10, 2: 997, 1005, 1367 (1759), nom. cons. Type: Weinmannia pinnata L.
≡ Weinmannia sect. Weinmannia
= Windmannia P. Browne in Civ. Nat. Hist. Jamaica: 212 (1756), nom. rej.
Trees or shrubs (rarely hemi‐epiphytes and strangling), hermaphroditic or dioecious
Indumentum of simple hairs. Stipules interpetiolar, one pair per node. Leaves opposite and decussate, simple (or unifoliolate?) and sometimes auriculate, trifoliolate or imparipinnate, petiolate or rarely sessile; in compound leaves, petiole and rachis commonly winged, sometimes quite broadly so (occasionally rachis not winged, e.g., W. trianaea Wedd.); leaflet margins toothed; domatia in axils of secondary veins absent. Inflorescence generally of simple racemes or occasionally spikes (strictly pseudoracemes and pseudospikes, see discussion), these inserted singly (not in groups on a sterile peduncular segment) in the axils of the most distal pair of leaves on a stem only (rarely at more than one node, W. condorensis Z.S.Rogers) and leaves subtending racemes or spikes sometimes reduced, caducous or persistent; (rarely axis of raceme irregularly branched, W. cogolloi J.F.Morales; rarely racemes short and dense, umbelliform, ovoid or globular, e.g., W. cochensis Hieron.; rarely racemes much reduced with only 2–8 flowers, W. bradfordiana Z.S.Rogers, or 1–2 flowers, W. condorensis); flowers inserted on axes in fascicles, each fascicle subtended by a bract; floral maturation synchronous. Flowers bisexual (most) or unisexual by late suppression of one sex (Mascarenes), pedicellate or occasionally ± sessile; calyx lobes 4 (–5), imbricate in bud; petals 4 (–5), elliptic, membranous, often caducous and falling as a cap; stamens 8 (10); disc annular with an entire margin, ribbed with alternating longitudinal costae of varying thickness; ovary of 2 fused carpels, each with a free stylodium. Capsule, basipetally and septicidally dehiscent into 2 valves, central column often present, calyx persistent; seeds numerous, kidney‐shaped (or sometimes ellipsoid?), pubescent, the hairs usually sparsely and widely distributed.
The recircumscribed genus Weinmannia s.s. now comprises ca. 90 species in Central and South America and the Caribbean (see Table 2) and with two additional disjunct species in the Mascarenes.
Pillon, Y., Hopkins H. C. F., Maurin O., Epitawalage N., Bradford J., Rogers Z. S., Baker W. J., and Forest F.. 2021. Phylogenomics and biogeography of Cunoniaceae (Oxalidales) with complete generic sampling and taxonomic realignments. American Journal of Botany 108(7): 1181–1200.
LITERATURE CITED
- Altschul, S. F., Gish W., Miller W., Myers E. W., and Lipman D. J.. 1990. Basic local alignment search tool. Journal of Molecular Biology 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Angiosperm Phylogeny Group . 1998. An ordinal classification for the families of flowering plants. Annals of the Missouri Botanical Garden 85: 531–553. [Google Scholar]
- APG IV [Angiosperm Phylogeny Group IV] . 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society 181: 1–20. [Google Scholar]
- APNI . 2020. Australian plant name index. IBIS database, Centre for Australian National Botanic Gardens, Biodiversity Research, Canberra, Australia. Website: http://www.cpbr.gov.au/cgi‐bin/apni. [Google Scholar]
- Baker, W. J., and Couvreur T. L. P.. 2013. Global biogeography and diversification of palms sheds light on the evolution of tropical lineages. I. Historical biogeography. Journal of Biogeography 40: 274–285. [Google Scholar]
- Bankevich, A., Nurk S., Antipov D., Gurevich A. A., Dvorkin M., Kulikov A. S., Lesin V. M., et al. 2012. SPAdes: a new genome assembly algorithm and its applications to single‐cell sequencing. Journal of Computational Biology 19: 455–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, R. W., and Hill R. S.. 1999. Macrofossils of Callicoma and Codia (Cunoniaceae) from Australian Cainozoic sediments. Australian Systematic Botany 12: 647–670. [Google Scholar]
- Barnes, R. W., Hill R. S., and Bradford J. C.. 2001. The history of Cunoniaceae in Australia from macrofossil evidence. Australian Journal of Botany 49: 301–320. [Google Scholar]
- Barnes, R. W., and Rozefelds A. C.. 2000. Comparative morphology of Anodopetalum (Cunoniaceae). Australian Systematic Botany 13: 267–282. [Google Scholar]
- Barreda, V. D., Palazzesi L., Pujana R. R., Panti C., Tapia M. J., Fernández D. A., and Noetinger S.. 2021. The Gondwanan heritage of the Eocene‐Miocene Patagonian floras. Journal of South American Earth Sciences 107: 103022. [Google Scholar]
- Bernardi, L.1965. Cunoniaceae. InHumbert H. [ed.], Flore de Madagascar et des Comores, vol. 93. Muséum national d’Histoire naturelle, Paris, France. [Google Scholar]
- Bernardi, L.1961. Revisio generis Weinmanniae. Pars I: sectio Weinmanniae. Candollea 17: 123–189. [Google Scholar]
- Bernardi, L.1963. Revisio generis Weinmanniae. Pars II: sectio Simplicifoliae. Candollea 18: 285–334. [Google Scholar]
- Bernardi, L.1964. Revisio generis Weinmanniae. Pars III: sectiones III‐IV‐V‐VI (veteris orbis). Botanische Jahrbücher fur Systematik, Pflanzengeschichte und Pflanzengeographie 83: 126–184. [Google Scholar]
- Blume, C. L.1855. Mélanges botaniques. Leiden, Netherlands. [Google Scholar]
- Bolger, A. M., Lohse M., and Usadel B.. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford, J. C.1998. A cladistic analysis of species groups in Weinmannia (Cunoniaceae) based on morphology and inflorescence structure. Annals of the Missouri Botanical Garden 85: 565–593. [Google Scholar]
- Bradford, J. C.1999. Cunoniaceae. InJørgensen P. M. and S.León‐Yánez [eds.], Catalogue of the vascular plants of Ecuador, 419–420. Monographs in Systematic Botany from the Missouri Botanical Garden 75. Missouri Botanical Garden Press, St. Louis, MO, USA. [Google Scholar]
- Bradford, J. C.2002. Molecular phylogenetics and morphological evolution in Cunonieae (Cunoniaceae). Annals of the Missouri Botanical Garden 89: 491–503. [Google Scholar]
- Bradford, J. C.2001. The application of a cladistic analysis to the classification and identification of Weinmannia (Cunoniaceae) in Madagascar and the Comoro Islands. Adansonia 23: 237–246. [Google Scholar]
- Bradford, J. C., and Barnes R. W.. 2001. Phylogenetics and classification of Cunoniaceae (Oxalidales) using chloroplast DNA sequences and morphology. Systematic Botany 26: 354–385. [Google Scholar]
- Bradford, J. C., and Berry P. E.. 1998. Cunoniaceae. InSteyermark J., Berry P. E., Holst B. K., and K.Yatskievych [eds.], Flora of the Venezuelan Guyana, vol. 4, 462–469. Missouri Botanical Garden Press, St. Louis, MO, USA. [Google Scholar]
- Bradford, J. C., Hopkins H. C. F., and Barnes R. W.. 2004. Cunoniaceae. InKubitzki K. [ed.], The families and genera of vascular plants, vol. VI, 91–111. Springer, Berlin, Germany. [Google Scholar]
- Bradford, J. C., and Miller J. S.. 2001. New taxa and nomenclatural notes on the flora of the Marojejy massif. Madagascar. V. Cunoniaceae: Weinmannia . Adansonia 23: 219–236. [Google Scholar]
- Brewer, G. E., Clarkson J. J., Maurin O., Zuntini A. R., Barber V., Bellot S., Biggs N., et al. 2019. Factors affecting targeted sequencing of 353 nuclear genes from herbarium specimens spanning the diversity of angiosperms. Frontiers in Plant Science 10: 1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerki, S., Forest F., Acedevo‐Rodriguez P., Callmander M. W., Nylander J. A. A., Harrington M. G., Sanmartín I., et al. 2009. Plastid and nuclear DNA markers reveal intricate relationships at subfamilal and tribal levels in the soapberry family (Sapindaceae). Molecular Phylogenetics and Evolution 51: 238–258. [DOI] [PubMed] [Google Scholar]
- Buerki, S., Forest F., and Alvarez N.. 2014. Proto‐South‐East Asia as a trigger of early angiosperm diversification. Botanical Journal of the Linnean Society 174: 326–333. [Google Scholar]
- Buerki, S., Forest F., Alvarez N., Nylander J. A. A., Arrigo N., and Sanmartín I.. 2011. An evaluation of new parsimony‐based versus parametric inference methods in biogeography: a case study using the globally distributed plant family Sapindaceae. Journal of Biogeography 38: 531–550. [Google Scholar]
- Camacho, C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., and Madden T. L.. 2009. BLAST+: architecture and applications. BMC Bioinformatics 10: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrill, D. J., and Poole I.. 2012. The vegetation of Antarctica through geological time. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Carpenter, R. J., and Buchanan A. D.. 1993. Oligocene leaves, fruit and flowers of the Cunoniaceae from Cethana, Tasmania. Australian Systematic Botany 6: 91–109. [Google Scholar]
- Carpenter, R. J., and Rozefelds A. C.. 2021. Gondwanan or global? A commentary on: ‘Fossil evidence from South America for the diversification of Cunoniaceae by the earliest Palaeocene’. Annals of Botany 127: iii–v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers, K. L., Poinar G. Jr, and Buckley R.. 2010. Tropidogyne, a new genus of early Cretaceous eudicots (Angiospermae) from Burmese amber. Novon 20: 23–29. [Google Scholar]
- Chernomor, O., A. von Haeseler , and Minh B. Q.. 2016. Terrace aware data structure for phylogenomic inference from supermatrices. Systematic Biology 65: 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conran, J. G.2004. Cephalotaceae. InKubitzki K. [ed.], The families and genera of vascular plants, vol. VI, 65–68. Springer, Berlin, Germany. [Google Scholar]
- Coode, M. J. E.2004. Elaeocarpaceae. InKubitzki K. [ed.], The families and genera of vascular plants, vol. VI, 135–144. Springer, Berlin, Germany. [Google Scholar]
- Crayn, D. M., Rossetto M., and Maynard D. J.. 2006. Molecular phylogeny and dating reveals an oligo‐miocene radiation of dry‐adapted shrubs (former Tremandraceae) from rainforest tree progenitors (Elaeocarpaceae) in Australia. American Journal of Botany 93: 1328–1342. [DOI] [PubMed] [Google Scholar]
- Cunningham, A.1839. XXXI. Florae insularum novae zelandiae precursor; or a specimen of the botany of the islands of New Zealand. Annals of Natural History 3: 244–250. [Google Scholar]
- de la Estrella, M. de la , Buerki S., Vasconcelos T., Lucas E. J., and Forest F.. 2019. The role of Antarctica in biogeographical reconstruction: a point of view. International Journal of Plant Sciences 180: 63–71. [Google Scholar]
- de la Estrella, M. , Forest F., Wieringa J. J., Fougère‐Danezan M., and Bruneau A.. 2017. Insights on the evolutionary origin of Detarioideae, a clade of ecologically dominant tropical African trees. New Phytologist 214: 1722–1735. [DOI] [PubMed] [Google Scholar]
- de Lange, P. J. , Gardner R. O., and Riddell K. A.. 2002. Ackama nubicola (Cunoniaceae) a new species from western Northland, North Island, New Zealand. New Zealand Journal of Botany 40: 525–534. [Google Scholar]
- Don, D.1830. A monograph of the family of plants called Cunoniaceae. Edinburgh New Philosophical Journal 9: 84–96. [Google Scholar]
- Doyle, J. J., and Doyle J. L.. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19: 11–15. [Google Scholar]
- Drew, B. T., Ruhfel B. R., Smith S. A., Moore M. J., Briggs B. G., Gitzendanner M. A., Soltis P. S., and Soltis D. E.. 2014. Another look at the root of the angiosperms reveals a familiar tale. Systematic Biology 63: 368–382. [DOI] [PubMed] [Google Scholar]
- Engler, A.1898. Syllabus der Pflanzenfamilien. Verlag von Gebrüder Borntraeger, Berlin, Germany. [Google Scholar]
- Engler, A.1928. Cunoniaceae. InEngler A. and Prantl K. [eds.], Die natürlichen Pflanzenfamilien, 2nd ed., 18a, 229–262. Engelmann, Leipzig, Germany. [Google Scholar]
- Fürnrohr, A. E.1858. Literatur. C.L. Blume, Mélanges botaniques. Leyden I. 1. Aug. 1855. II. 1. Septbr. 1855. Flora 41: 254–256. [Google Scholar]
- Gandolfo, M. A., and Hermsen E. J.. 2017. Ceratopetalum (Cunoniaceae) fruits of Australasian affinity from the early Eocene Laguna del Hunco flora, Patagonia, Argentina. Annals of Botany 119: 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givnish, T. J., Ames M., McNeal J. R., McKain M. R., Steele P. R., dePamphilis C. W., Graham S. W., et al. 2010. Assembling the tree of life of monocotyledons: plastome sequence phylogeny and evolution of Poales. Annals of the Missouri Botanical Garden 97: 584–616. [Google Scholar]
- Godley, E. J.1979. Flower biology in New Zealand. New Zealand Journal of Botany 17: 441–466. [Google Scholar]
- Godley, E. J.1983. The fruit in Ackama, Caldcluvia, and Weinmannia (Cunoniaceae). New Zealand Journal of Botany 21: 455–456. [Google Scholar]
- Goldblatt, P.1981. Systematics, phylogeny and evolution of Dietes (Iridaceae). Annals of the Missouri Botanical Garden 68: 132–153. [Google Scholar]
- Goldblatt, P., and Manning J.. 2000. Cape plants: a conspectus of the Cape flora of South Africa. National Botanical Institute, Pretoria, South Africa; Missouri Botanical Garden Press, St. Louis, MO, USA. [Google Scholar]
- Hably, L., Tamás J., and Cioppi E.. 2007. Sloanea peolai n. comb. A new European record of Sloanea (Elaeocarpaceae) in the Italian Oligocene. Review of Palaeobotany and Palynology 146: 18–28. [Google Scholar]
- Harden, G. J., and Williams J. B.. 2000. A revision of Davidsonia (Cunoniaceae). Telopea 8: 413–428. [Google Scholar]
- Harling, G.1999. Cunoniaceae. Flora of Ecuador no. 61. University of Goteborg & Section for Botany, Riksmuseum, Stockholm. [Google Scholar]
- Harling, G., and Fuentes A.. 2014. Cunoniaceae. InJørgensen P. M., Nee M. H., and Beck S. G. [eds.], El catálogo de las plantas vasculares de Bolivia, 540–542. Monographs in Systematic Botany 127. Missouri Botanical Garden Press, St. Louis, MO, USA. [Google Scholar]
- Heslewood, M. M., and Wilson P. G.. 2013. A new combination in Ackama (Cunoniaceae). Telopea 15: 5–7. [Google Scholar]
- Hoang, D. T., Chernomor O., A. von Haeseler , Minh B. Q., and Vinh L. S.. 2018. UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogland, R. D.1960. Studies in the Cunoniaceae. I. The genera Ceratopetalum, Gillbeea, Aistopetalum and Calycomis . Australian Journal of Botany 8: 318–341. [Google Scholar]
- Hoogland, R. D.1979. Studies in the Cunoniaceae. II. The genera Caldcluvia, Pullea, Acsmithia and Spiraeanthemum . Blumea 25: 481–505. [Google Scholar]
- Hoogland, R. D.1981. Studies in the Cunoniaceae. III. Additional notes on Ceratopetalum and Acrophyllum . Brunonia 4: 213–216. [Google Scholar]
- Hopkins, H. C. F.1998. A revision of Weinmannia (Cunoniaceae) in Malesia and the Pacific. 2. Sulawesi and the Philippines. Adansonia 20: 43–66. [Google Scholar]
- Hopkins, H. C. F.2008. Cunoniaceae. InZuloaga F. O., Morrone O., and Belgrano M. J. [eds.], Catálogo de las plantas vasculares del Cono Sur, 1982–1983. Missouri Botanical Garden Press, St. Louis, MO, USA. [Google Scholar]
- Hopkins, H. C. F.2018a. Names and types relating to the South American genus Lamanonia (Cunoniaceae) and its synonyms, the identity of L. speciosa, and an account of the little‐known L. ulei . Kew Bulletin 73: 10. [Google Scholar]
- Hopkins, H. C. F.2006. Nomenclature and typification in Geissois (Cunoniaceae) in the South‐West Pacific. Adansonia 28: 311–327. [Google Scholar]
- Hopkins, H. C. F.2018b. The taxonomy and morphology of Schizomeria (Cunoniaceae) in New Guinea, the Moluccas and the Solomon Islands, with notes on seed dispersal and uses throughout the genus. Kew Bulletin 73: 11. [Google Scholar]
- Hopkins, H. C. F., and Bradford J. C.. 1998. A revision of Weinmannia (Cunoniaceae) in Malesia and the Pacific. 1. Introduction and an account of the species of Western Malesia, the Lesser Sunda Islands and the Moluccas. Adansonia 20: 5–41. [Google Scholar]
- Hopkins, H. C. F., and Florence J.. 1998. A revision of Weinmannia (Cunoniaceae) in Malesia and the Pacific. 4. The Society. Marquesas and Austral Islands. Adansonia 20: 107–130. [Google Scholar]
- Hopkins, H. C. F., and Hoogland R. D.. 2002. Cunoniaceae. InNoteboom H. P. [ed.], Flora malesiana, series 1, vol. 16, 53–165. Nationaal Herbarium Nederland, Leiden, Netherlands. [Google Scholar]
- Hopkins, H. C. F., Hoogland R. D., and Bradford J. C.. 1998. A revision of Weinmannia (Cunoniaceae) in Malesia and the Pacific. 3. New Guinea, Solomon Islands, Vanuatu and Fiji, with notes on the species of Samoa, Rarotonga, New Caledonia and New Zealand. Adansonia 20: 67–106. [Google Scholar]
- Hopkins, H. C. F., and Pillon Y.. 2011. Further new endemic taxa of Cunoniaceae from New Caledonia. Kew Bulletin 66: 405–423. [Google Scholar]
- Hopkins, H. C. F., Pillon Y., and Bradford J. C.. 2009. The endemic genus Pancheria (Cunoniaceae) in New Caledonia: notes on morphology and the description of three new species. Kew Bulletin 64: 429–446. [Google Scholar]
- Hopkins, H. C. F., Pillon Y., and Hoogland R. D.. 2014. Cunoniaceae: flore de la Nouvelle‐Calédonie, vol. 26. Muséum national d’Histoire naturelle / IRD, Paris, France. [Google Scholar]
- Hopkins, H. C. F., Rozefelds A. C., and Pillon Y.. 2013. Karrabina gen. nov. (Cunoniaceae), for the Australian species previously placed in Geissois, and a synopsis of genera in the tribe Geissoieae. Australian Systematic Botany 26: 167–185. [Google Scholar]
- Hufford, L., and Dickison W. C.. 1992. A phylogenetic analysis of Cunoniaceae. Systematic Botany 17: 181–200. [Google Scholar]
- Hutchinson, J.1967. The genera of flowering plants (Angiospermae). Oxford University Press, London, UK. [Google Scholar]
- Isnard, S., L’Huillier L., Rigault F., and Jaffré T.. 2016. How did the ultramafic soils shape the flora of the New Caledonian hotspot? Plant and Soil 403: 53–76. [Google Scholar]
- Johnson, M. G., Gardner E. M., Liu Y., Medina R., Goffinet B., Shaw A. J., Zerega N. J. C., and Wickett N. J.. 2016. HybPiper: extracting coding sequence and introns for phylogenetics from high‐throughput sequencing reads using target enrichment. Applications in Plant Sciences 4: 1600016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, M. G., Pokorny L., Dodsworth S., Botigué L. R., Cowan R. S., Devault A., Eiserhardt W. L., et al. 2019. A universal probe set for targeted sequencing of 353 nuclear genes from any flowering plant designed using k‐medoids clustering. Systematic Biology 68: 594–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jud, N. A., and Gandolfo M. A.. 2021. Fossil evidence from South America for the diversification of Cunoniaceae by the earliest Palaeocene. Annals of Botany 127: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jud, N. A., Gandolfo M. A., Iglesias A., and Wilf P.. 2018. Fossil flowers from the early Palaeocene of Patagonia, Argentina, with affinity to Schizomerieae (Cunoniaceae). Annals of Botany 121: 431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junier, T., and Zdobnov E. M.. 2010. The Newick utilities: high‐throughput phylogenetic tree processing in the UNIX shell. Bioinformatics 26: 1669–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooyman, R. M., Wilf P., Barreda V. D., Carpenter R. J., Jordan G. J., Sniderman J. M. K., Allen A., et al. 2014. Paleo‐Antarctic rainforest into the modern Old World tropics: the rich past and threatened future of the “southern wet forest survivors”. American Journal of Botany 101: 2121–2135. [DOI] [PubMed] [Google Scholar]
- Kranitz, M. L., Biffin E., Clark A., Hollingsworth M. L., Ruhsam M., Gardner M. F., Thomas P., et al. 2014. Evolutionary diversification of New Caledonian Araucaria . PloS ONE 9: e110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitzki, K.2004. Brunelliaceae. InKubitzki K. [ed.], The families and genera of vascular plants, vol. VI, 26–28. Springer, Berlin, Germany. [Google Scholar]
- Kuntze, O.1891. Revisio generum plantarum. Arthur Felix, Leipzig, Germany. [Google Scholar]
- Li, M.‐M., Wang D.‐E., Zhang L., Kang M.‐H., Lu Z.‐Q., Zhu R.‐B., Mao X.‐X., et al. 2019. Intergeneric relationships within the family Salicaceae s.l. based on plastid phylogenomics. International Journal of Molecular Sciences 20: 3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorence, D. H., and Wagner W. L.. 2011. Weinmannia marquesana var. angustifolia (Cunoniaceae), a new variety from the Marquesas Islands. PhytoKeys 4: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magallón, S., and Sanderson M. J.. 2001. Absolute diversification rates in angiosperm clades. Evolution 55: 1762–1780. [DOI] [PubMed] [Google Scholar]
- Mai, U., and Mirarab S.. 2018. TreeShrink: fast and accurate detection of outlier long branches in collections of phylogenetic trees. BMC Genomics 19: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchester, S. R., and Kvaček Z.. 2009. Fruits of Sloanea (Elaeocarpaceae) in the Paleogene of North America and Greenland. International Journal of Plant Sciences 170: 941–950. [Google Scholar]
- Matthews, M. L., and Endress P. K.. 2002. Comparative floral structure and systematics in Oxalidales (Oxalidaceae, Connaraceae, Brunelliaceae, Cephalotaceae, Cunoniaceae, Elaeocarpaceae, Tremandraceae). Botanical Journal of the Linnean Society 140: 321–381. [Google Scholar]
- Matzke, N. J.2013. Probabilistic historical biogeography: new models for founder‐event speciation, imperfect detection, and fossils allow improved accuracy and model‐testing. Frontiers of Biogeography 5: 242–248. [Google Scholar]
- McPherson, G., and Lowry P. P.. 2004. Hooglandia, a newly discovered genus of Cunoniaceae from New Caledonia. Annals of the Missouri Botanical Garden 91: 260–265. [Google Scholar]
- Minh, B. Q., Schmidt H. A., Chernomor O., Schrempf D., Woodhams M. D., A. von Haeseler , and Lanfear R.. 2020. IQ‐TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37: 1530–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel, F. A. W.1856. Flora van Nederlandsch Indië. C.G. van der Post, Amsterdam, Netherlands. [Google Scholar]
- Mirarab, S.2019. Species tree estimation using ASTRAL: practical considerations. arXiv:1904.03826 [Preprint]. [Google Scholar]
- Mirarab, S., and Warnow T.. 2015. ASTRAL‐II: coalescent‐based species tree estimation with many hundreds of taxa and thousands of genes. Bioinformatics 31: i44–i52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales, J. F.2011. Cunoniaceae. InDavidse G., Sousa Sánchez M., Knapp S., and Chiang Cabrera F. [eds.], Flora mesoamericana [online]. Missouri Botanial Garden, St. Louis, MO, USA. Website; http://www.tropicos.org/docs/meso/cunoniaceae.pdf [accessed 1 December 2020]. [Google Scholar]
- Morales, J. F.2010. Sinopsis del género Weinmannia (Cunoniaceae) en México y Centroamérica. Anales del Jardín Botánico de Madrid 67: 137–155. [Google Scholar]
- Morley, R. J.2001. Why are there so many primitive angiosperms in the rain forests of Asia‐Australasia?InMetcalfe I., Smith J. M. B., Morwood M., and Davidson I. [eds.], Faunal and floral migrations and evolution in SE Asia‐Australasia, 185–199. A.A. Balkema, Lisse, Netherlands. [Google Scholar]
- Müller, R. D., Seton M., Zahirovic S., Williams S. E., Matthews K. J., Wright N. M., Shephard G. E., et al. 2016. Ocean basin evolution and global‐scale plate reorganization events since Pangea breakup. Annual Review of Earth and Planetary Sciences 44: 107–138. [Google Scholar]
- Murphy, B., Forest F., Barraclough T., Rosindell J., Bellot S., Cowan R., Golos M., et al. 2020. A phylogenomic analysis of Nepenthes (Nepenthaceae). Molecular Phylogenetics and Evolution 144: 106668. [DOI] [PubMed] [Google Scholar]
- Perry, L. M.1949. Plantae Archboldianae, XIX. Journal of the Arnold Arboretum 30: 139–165. [Google Scholar]
- Pillon, Y., Hopkins H. C. F., Munzinger J., and Chase M. W.. 2009a. A molecular and morphological survey of generic limits of Acsmithia and Spiraeanthemum (Cunoniaceae). Systematic Botany 34: 141–148. [Google Scholar]
- Pillon, Y., Munzinger J., Amir H., Hopkins H. C. F., and Chase M. W.. 2009b. Reticulate evolution on a mosaic of soils: diversification of the New Caledonian endemic genus Codia (Cunoniaceae). Molecular Ecology 18: 2263–2275. [DOI] [PubMed] [Google Scholar]
- Pillon, Y., Munzinger J., Amir H., and Lebrun M.. 2010. Ultramafic soils and species sorting in the flora of New Caledonia. Journal of Ecology 98: 1108–1116. [Google Scholar]
- Poinar, G. O., and Chambers K. L.. 2017. Tropidogyne pentaptera, sp. nov., a new mid‐Cretaceous fossil angiosperm flower in Burmese amber. Palaeodiversity 10: 135–140. [Google Scholar]
- Poinar, G. O., and Chambers K. L.. 2019. Tropidogyne lobodisca sp. nov., a third species of the genus from mid‐Cretaceous Myanmar amber. Journal of the Botanical Research Institute of Texas 13: 461–466. [Google Scholar]
- Poole, I., Mennega A. M. W., and Cantrill D. J.. 2003. Valdivian ecosystems in the late Cretaceous and early Tertiary of Antarctica: further evidence from myrtaceous and eucryphiaceous fossil wood. Review of Palaeobotany and Palynology 124: 9–27. [Google Scholar]
- Pouteau, R., Trueba S., Feild T. S., and Isnard S.. 2015. New Caledonia: a Pleistocene refugium for rain forest lineages of relict angiosperms. Journal of Biogeography 42: 2062–2077. [Google Scholar]
- Rambaut, A., Drummond A. J., Xie D., Baele G., and Suchard M. A.. 2018. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67: 901–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven, P. H., and Axelrod D. I.. 1974. Angiosperm biogeography and past continental movements. Annals of the Missouri Botanical Garden 61: 539–673. [Google Scholar]
- Ree, R. H., and Smith S. A.. 2008. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Systematic Biology 57: 4–14. [DOI] [PubMed] [Google Scholar]
- Rieseberg, L. H., and Soltis D. E.. 1991. Phylogenetic consequences of cytoplasmic gene flow in plants. Evolutionary Trends in Plants 5: 65–84. [Google Scholar]
- Rodriguez, R., Marticorena C., Alarcón D., Baeza C., Cavieres L., Finot V. L., Fuentes N., et al. 2018. Catálogo de las plantas vasculares de Chile. Gayana Botánica 75: 1–430. [Google Scholar]
- Rogers, Z. S.2017. Weinmannia in catalogue of the vascular plants of Madagascar. Missouri Botanical Garden, St. Louis, USA and Antananarivo, Madagascar. Website: http://www.efloras.org/madagascar [accessed 01 December 2020]. [Google Scholar]
- Rogers, Z. S., and Bradford J. C.. 2004. Weinmannia magnifica and W. aggregata (Cunoniaceae): two distinctive new species from Madagascar. Adansonia 26: 83–91. [Google Scholar]
- Rozefelds, A. C., and Barnes R. W.. 2002. The systematic and biogeographical relationships of Ceratopetalum (Cunoniaceae) in Australia and New Guinea. International Journal of Plant Sciences 163: 651–673. [Google Scholar]
- Rozefelds, A. C., Barnes R. W., and Pellow B.. 2001. A new species and comparative morphology of Vesselowskya (Cunoniaceae). Australian Systematic Botany 14: 175–192. [Google Scholar]
- Rozefelds, A. C., and Pellow B.. 2000. A new species of Gillbeea (Cunoniaceae) from north‐eastern Queensland, Australia. Nordic Journal of Botany 20: 435–442. [Google Scholar]
- Rozefelds, A. C., and Pellow B.. 2011. A taxonomic revision of Pseudoweinmannia Engl. (Cunoniaceae: Geissoieae). Austrobaileya 8: 252–266. [Google Scholar]
- Scher, H. D., and Martin E. E.. 2006. Timing and climatic consequences of the opening of Drake passage. Science 312: 428–430. [DOI] [PubMed] [Google Scholar]
- Schimanski, L. J., and Rozefelds A. C.. 2002. Comparative morphology of the Australian species of Geissois (Cunoniaceae). Australian Systematic Botany 15: 221–236. [Google Scholar]
- Schlechter, F. R. R.1914. Die Cunoniaceae Papuasiens. Botanische Jahrbücher für Systematik Pflanzengeschichte und Pflanzengeographie 52: 139–166. [Google Scholar]
- Schönberger, I., Wilton A., Boardman K., Breitwieser I., P. de Lange , B. de Pauw , Ford K., et al. 2020. Checklist of the New Zealand flora — seed plants. Manaaki Whenua ‐ Landcare Research, Lincoln, NZ. Website: 10.26065/s3gg-v336. [DOI] [Google Scholar]
- Schönenberger, J., Friis E. M., Matthews M. L., and Endress P. K.. 2001. Cunoniaceae in the Cretaceous of Europe: evidence from fossil flowers. Annals of Botany 88: 423–437. [Google Scholar]
- Schumann, K., and Lauterbach K.. 1905. Nachträge zur Flora der deutschen Schutzgebiete in der Südsee: mit Ausschluss Samoa’s und der Karolinen. Gebrïuder Borntraeger, Leipzig, Germany. [Google Scholar]
- Scotese, C. R.2004. A continental drift flipbook. Journal of Geology 112: 792–741. [Google Scholar]
- Scott, A. J., and Bosser J.. 1997. Cunoniacées. InBosser J., Ferguson I. K., and C.Soopramanien [eds.], Flore des Mascareignes, 86: 81–89. IRD éditions, Paris, France. [Google Scholar]
- Setoguchi, H., Ohsawa P., Pintaud J. C., Jaffré T., and Veillon J. M.. 1998. Phylogenetic relationship of Araucariaceae inferred from rbcL gene sequences. American Journal of Botany 85: 1507–1516. [PubMed] [Google Scholar]
- Shee, Z. Q., Frodin D. G., Cámara‐Leret R., and Pokorny L.. 2020. Reconstructing the complex evolutionary history of the Papuasian Schefflera radiation through herbariomics. Frontiers in Plant Science 11: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater, G., and Birney E.. 2005. Automated generation of heuristics for biological sequence comparison. BMC Bioinformatics 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. A., Brown J. W., and Walker J. F.. 2018. So many genes, so little time: A practical approach to divergence‐time estimation in the genomic era. PLoS One 13: e0197433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis, D. E., Smith S. A., Cellinese N., Wurdack K. J., Tank D. C., Brockington S. F., Refulio‐Rodriguez N. W. J. B., et al. 2011. Angiosperm phylogeny: 17 genes, 640 taxa. American Journal of Botany 98: 704–730. [DOI] [PubMed] [Google Scholar]
- Stafleu, F. A., and Cowan R. S.. 1976. Taxonomic literature 2, vol. l, A–G. Regnum Vegetabile, vol. 94. Bohn, Scheltema & Holkema, Utrecht, Netherlands. [Google Scholar]
- Stickley, C. E., Brinkhuis H., Schellenberg S. A., Sluijs A., Röhl U., Fuller M., Grauert M., et al. 2004. Timing and nature of the deepening of the Tasmanian Gateway. Paleoceanography 19: PA4027. [Google Scholar]
- Suchard, M. A., Lemey P., Baele G., Ayres D. L., Drummond A. J., and Rambaut A.. 2018. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evolution 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, M., Soltis D. E., Soltis P. S., Zhu X., Burleigh J. G., and Chen Z.. 2015. Deep phylogenetic incongruence in the angiosperm clade Rosidae. Molecular Phylogenetics and Evolution 83: 156–166. [DOI] [PubMed] [Google Scholar]
- Sweeney, P. W., Bradford J. C., and Lowry P. P.. 2004. Phylogenetic position of the New Caledonian endemic genus Hooglandia (Cunoniaceae) as determined by maximum parsimony analysis of chloroplast DNA. Annals of the Missouri Botanical Garden 91: 266–274. [Google Scholar]
- Sykes, W. R.2016. Flora of the Cook Islands. National Tropical Botanical Garden, Kalaheo, Kaua’i, Hawai’i. [Google Scholar]
- Taylor, F., and Hill R. S.. 1996. A phylogenetic analysis of the Eucryphiaceae. Australian Systematic Botany 9: 735–748. [Google Scholar]
- Trias‐Blasi, A., Baker W. J., Haigh A. L., Simpson D. A., Weber O., and Wilkin P.. 2015. A genus‐level phylogenetic linear sequence of monocots. Taxon 64: 552–581. [Google Scholar]
- van Steenis, C. G. G. J. 1986. Blume’s ‘Mélanges botaniques’ effectively published in 1855. Taxon 35: 272–285 [facsimile: pp. 274–285]. [Google Scholar]
- Wang, H., Moore M. J., Soltis P. S., Bell C. D., Brockington S. F., Alexandre R., Davis C. C., et al. 2009. Rosid radiation and the rapid rise of angiosperm‐dominated forest. Proceedings of the National Academy of Sciences, USA 106: 3853–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, C. J., and Simpson M. J. A.. 1991. Seed morphology in relation to taxonomy in New Zealand species of Weinmannia, Ackama, and the related South American Caldcluvia paniculata (Cunoniaceae). New Zealand Journal of Botany 29: 451–453. [Google Scholar]
- Zarucchi, J.1993. Cunoniaceae. InBrako L. and Zarucchi J. [eds.], Catalogue of the flowering plants and gymnosperms of Peru. Monographs in Systematic Botany from the Missouri Botanical Garden 45, 384–387. Missouri Botanical Garden Press, St. Louis, MO, USA. [Google Scholar]
- Zedane, L., Hong‐Wa C., Murienne J., Jeziorski C., Baldwin B. G., and Besnard G.. 2016. Museomics illuminate the history of an extinct, paleoendemic plant lineage (Hesperelaea, Oleaceae) known from an 1875 collection from Guadalupe Island, Mexico: biogeographic history of Hesperelaea . Biological Journal of the Linnean Society 117: 44–57. [Google Scholar]
- Zickel, C. S., and H. F. de Leitão Filho . 1993. Revisão taxonômica de Lamanonia Vell. (Cunoniaceae). Revista Brasileira de Botânica 16: 73–91. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1. List of sequenced material with full information on herbarium vouchers and data quality.
APPENDIX S2. Biogeographical model.
APPENDIX S3. Geographical area assignments for each terminal included in the biogeographical analysis.
APPENDIX S4. Dated tree of crown Oxalidales.
APPENDIX S5. Linear sequences of families and genera in the crown clade of Oxalidales for use in herbarium curation.
APPENDIX S6. The 12 currently recognized species in the Caldcluvieae, showing their distribution and historical and current generic placements.


