Summary
Background
Knowledge of the cost of illness of inflammatory bowel disease (IBD) is essential for health policy makers worldwide.
Aim
To assess the cost of illness of IBD from the societal perspective taking into account time trends and geographical differences.
Methods
A systematic review of all population‐based studies on cost of illness of IBD published in Embase, Medline, Web of Science and Google Scholar. Methodology of included studies was assessed and costs were adjusted to 2018 US dollars.
Results
Study methodologies differed considerably, with large differences in perspective, valuation method and population. For prevalent Crohn's disease (CD) cases in the last ten years annual healthcare costs were in Asia $4417 (range $1230‐$31 161); Europe $12 439 ($7694‐$15 807) and North America $17 495 ($14 454‐$20 535). For ulcerative colitis (UC), these were $1606 ($309‐$14 572), $7224 ($3228‐$9779) and $13 559 ($13 559‐$13 559). The main cost driver was medication, the cost of which increased considerably between 1985 and 2018, while outpatient and inpatient costs remained stable. IBD had a negative impact on work productivity. Annual costs of absenteeism for CD and UC were in Asia (with presenteeism) $5638 ($5638‐$5638) and $4828 ($4828‐$4828); Europe $2660 ($641‐$5277) and $2394 ($651‐$5992); North America $752 ($307‐$1303) and $1443 ($85‐$2350).
Conclusion
IBD societal cost of illness is increasing, driven by growing costs of medication, and varies considerably between continents. While biologic therapy was expected to decrease inpatient costs by reducing hospitalisations and surgery, these costs have not declined.
Costs of IBD are increasing due to biologics and vary between continents.
Biologics do not reduce inpatient costs.

1. INTRODUCTION
Biologics and small molecules are increasingly used and are potent agents for the treatment of inflammatory bowel disease (IBD).1, 2, 3, 4, 5 As shown in randomised clinical trials, these drugs are efficacious in inducing clinical remission, improving work productivity,6, 7 and reducing hospitalisation and surgery rates.8, 9 They are expensive however, potentially costing over $10 000 per treatment year depending on country and access to biosimilars,10 and could increase the cost burden on the already strained healthcare systems worldwide.11, 12
Two systematic reviews were published on the costs of Crohn's disease (CD) and ulcerative colitis (UC) in Europe and North America in 2008 and 2010 respectively.13, 14 Since then, many new treatment modalities have become available, and numerous studies on the costs of IBD have been published. Reviews on the indirect costs of IBD and costs for the paediatric population have been published since,15, 16, 17, 18, 19 but no comprehensive review on the cost of illness of IBD has been carried out.
With the increasing incidence and prevalence of IBD outside the Western world,20 knowledge on the economic burden and cost drivers is essential for health policymakers worldwide. This requires a systematic assessment from a societal perspective, which includes the costs of healthcare, patients own financial contribution and work impairment, such as absenteeism and presenteeism.21
We aimed to estimate the global economic impact of IBD and therefore conducted this systematic review of the cost of illness of IBD from a societal perspective and determined cost drivers over a 30‐year time period (1985‐2018) in different geographical areas.
2. MATERIALS AND METHODS
2.1. Protocol and registration
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement.22 The review protocol was prospectively registered in PROSPERO under CRD42020158567.23
2.2. Eligibility criteria
Eligible for inclusion in the systematic review were observational studies published in peer‐reviewed journals on healthcare, productivity or patients costs of patients with CD, UC, IBD‐unclassified/indeterminate (IBD‐U), or a combination of these diseases. Studies on interventions and those reporting costs only for a subset of patients defined by phenotype or treatment were excluded, as they do not give a representative estimate of the cost‐of‐illness of IBD. According to the PRISMA statement, the eligibility criteria were published beforehand.23 During the conduct of the systematic review, the protocol was changed because a systematic review on cost of illness in children was published during the conduct of this systematic review.19 After the change, only studies on an adult population and studies on a combined population of both adults and children were eligible. Studies reporting costs for a population younger than 18 years of age were excluded. Studies in other languages than English were excluded, as were all conference abstracts and posters. No limit on publication date was applied.
2.3. Search and information sources
A systematic search to find relevant articles was performed in cooperation with an information specialist of the medical library in four databases: Embase, Medline (Ovid), Web of Science and Google Scholar. For Google Scholar, it was decided a priori to only include the first 200 results to limit the proportion of articles that are not formally published in journals.24 A single search string query was used to search the databases for reproducibility and adaptability (see File S1). For retrieval in Google Scholar, the programme Publish or Perish (version 7.19) was used. During the search, the queries were refined to only retrieve articles in English and to exclude animal studies and conference abstracts. The search was performed on 28 August 2019 and updated on 20 January 2020 and on 11 June 2020. This could affect the retrieved references as the amount or relevance ranking can differ due to either added or redacted index terms or due to a changed relevance ranking (as seen with Google Scholar). The retrieved studies were crosschecked with the reference lists of the prior published reviews to search for missing studies.13, 14, 15, 17, 18
2.4. Study selection
Two independent reviewers (RvL and EV) reviewed all studies found in the initial search on title and abstract. Subsequently, the studies that met all eligibility criteria were independently screened on full text by the two reviewers. In case of disagreement, the article was discussed until consensus was reached. These articles are marked with an asterisk in the results.
2.5. Data collection and items
The two aforementioned reviewers separately collected the data from the included studies using a case report form developed for this review. In case of disagreement, inconsistencies were corrected by jointly returning to the article in question. For each study, the following data were collected: cost sectors, cost components, method of quantifying resource use, method of valuating costs and results with a measure of spread (e.g., mean and standard deviation). Metadata were collected on general study aspects (title, authors, year of publication), disease population, perspective, currency and period and country of data collection.
If the perspective from which resource use and valuation was not made explicit by the study, it was determined from the study methodology. When the currency year was not stated, the final year of follow‐up was used. When cost data were collected for a period longer than a year and reported as a single estimate, we treated this estimate as representative for the middle of the period when graphing the data.
2.6. Bias
The main risk of bias lies at the level of individual studies because of the widely different methods used in these studies.18 Methodology and risk of bias were assessed by two reviewers (RvL and EV) independently using the tool published by Larg and Moss.25
2.7. Synthesis of results
Where cost data were not available per patient, the per patient cost was calculated from the total costs and patient numbers. To compare results, all costs were converted to 2018 United States (US) dollars using the gross domestic product (GDP) deflator (preferred measure to adjust for inflation from the societal perspective)26 and purchasing power parity (PPP) as defined by the World Bank.27 Where World Bank estimates were not available, GDP deflator and PPP as defined by the Organisation for Economic Co‐operation and Development (OECD) were used.28, 29 For Taiwan and Hong Kong, the GDP deflator published by their respective office for national statistics was used.30, 31 For Taiwan, PPP as defined by the International Monetary Fund was used.31
Because of heterogeneity between studies, no formal meta‐analysis was attempted, but descriptive summaries are given. All costs are reported as annual costs per patient per year. Where possible, healthcare costs were divided into three different categories: inpatient included the cost of hospital admissions and surgery; outpatient included the cost of physician or emergency room visits, outpatient diagnostics and outpatient surgery; and medication included costs for biologic and non‐biologic therapy. Work productivity costs were categorised as absenteeism (sick leave), presenteeism (reduced efficiency at work), early retirement or disability, unpaid time loss or caregiver productivity loss.
Studies that distinguished between CD and UC cohorts are reported under the respective disease category while studies that aggregated CD and UC patients are reported under IBD.
To assess changes in cost components over time (1985‐2018), only studies that reported means for all three components (inpatient, outpatient and medication) were included. If multiple studies reported a distribution of cost components for a given year, the average distribution of the studies was used. To determine the present‐day annual healthcare costs of prevalent IBD patients, all studies reporting on the mean CD or UC attributable healthcare costs from 2010 onward were used. The proportion of biologic users per study was compared with healthcare costs for all studies reporting mean healthcare costs and the proportion of patients on biologics. To compare productivity costs with healthcare costs, only studies that estimated mean healthcare costs and any category of mean productivity costs were included.
3. RESULTS
3.1. Study selection
The systematic search identified 7566 studies, of which 2729 were removed because of duplication. The remaining 4837 studies were screened on title and abstract and 4730 excluded. After full‐text screening, 64 of the remaining 107 studies were included in the systematic review.32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95 No missed studies were found in the reference lists of the other systematic reviews. For the study selection process and reasons for exclusion, see the PRISMA flowchart (Figure 1). Cohen's kappa for inclusion of a study was 0.88. The reduced kappa can be explained by one of the reviewers' learning curve. After comparing the decisions made by both reviewers, there was no disagreement on which studies should be included in the study.
FIGURE 1.

PRISMA flow diagram depicting study selection
3.2. Study characteristics
An overview of the study populations is presented in Table S1. A detailed overview of the study characteristics, methodologies, results and the summaries that each study contributed to can be found in File S2. Of the 64 studies, seven were performed in Asia, 29 in Europe, 26 in North America (United States and Canada) and two in Oceania. Most studies (54) used a prevalence‐based approach. Of these, five studies applied a top‐down costing method and the others a bottom‐up approach. All 11 incidence‐based studies used a bottom‐up costing method. One study reported costs for both a prevalent as well an incident cohort.70 Only three studies reported on the costs of IBD‐U (File S2).42, 47, 92
The studies covered a time period of more than 30 years (1985‐2018).82, 91 Study timeframes differed considerably. Some studies were cross‐sectional with a 1‐week recall period to determine costs, while others were longitudinal with a time period of up to 10 years. For most incidence‐based studies, costs in the first year after diagnosis could be calculated. Three incidence‐based studies reported the annual costs over a 10‐year period.67, 71, 77
The perspectives from which resource use and valuation were determined varied. Twenty studies used the third‐party payer perspective, using insurance charges or payments as costs. The employer perspective, focusing on the value of productivity foregone because of IBD, was used in seven studies. The healthcare system perspective, determining the impact of IBD on healthcare costs, was used in 15 studies. In addition, four studies took the government perspective, examining healthcare costs from the perspective of a government‐funded healthcare system and productivity costs. Seventeen studies adopted a societal perspective, estimating all costs no matter who incurred them. The perspective of one study remained unclear.40
IBD‐attributable costs were determined by 55 studies. This was done by either matching a control group, adjusting for differences between IBD‐ and non‐IBD cases in a regression analysis, or counting IBD‐attributable resource use. Nine studies reported all healthcare costs incurred by IBD patients.32, 44, 46, 47, 50, 53, 56, 68, 82 The methods to determine and value resource use and productivity losses differed considerably. Five studies reported median costs per patient, and one study reported mean healthcare costs but median productivity costs.35, 44, 47, 59, 68
3.3. Healthcare costs
The reported healthcare costs varied considerably between studies and geographical regions. The annual mean healthcare costs for prevalent and incident cases of IBD, CD and UC are summarised in Table 1. Changes in mean annual healthcare costs and component costs over the 30‐year time period for total IBD, CD and UC are presented in Figures 2, 3, 4.
TABLE 1.
Mean (range) annual healthcare costs per prevalent case for IBD, CD and UC in 2018 US dollars per continent
| Disease | Asia | Europe | North America | Oceania |
|---|---|---|---|---|
| IBD (prevalent) | ||||
| Healthcare costs | ||||
| N | 7 | 6 | 12 | 0 |
| Mean | $3333 | $5938 | $13 212 | — |
| Min‐Max | $587‐$18 355 | $1035‐$7894 | $2016‐$25 373 | — |
| IBD (incident) | ||||
| Healthcare costs | ||||
| N | 2 | 3 | 0 | 1 |
| Mean | $6254 | $4952 | — | $6484 |
| Min‐Max | $1779‐$10 728 | $3152‐$5870 | — | $6484‐$6484 |
| CD (prevalent) | ||||
| Healthcare costs | ||||
| N | 17 | 19 | 13 | 0 |
| Mean | $3755 | $10 484 | $11 725 | — |
| Min‐Max | $1230‐$31,161 | $5005‐$15 807 | $2343‐$21 107 | — |
| CD (incident) | ||||
| Healthcare costs | ||||
| N | 2 | 5 | 0 | 1 |
| Mean | $8692 | $8281 | — | $7663 |
| Min‐Max | $3047‐$14 336 | $4293‐$12 631 | — | $7663‐$7663 |
| UC (prevalent) | ||||
| Healthcare costs | ||||
| N | 28 | 17 | 11 | 0 |
| Mean | $1051 | $6529 | $8053 | — |
| Min‐Max | $281‐$14 572 | $2863‐$9779 | $917‐$13 559 | — |
| UC (prevalent) | ||||
| Healthcare Costs | ||||
| N | 2 | 5 | 1 | 1 |
| Mean | $4428 | $4979 | $14 598 | $4692 |
| Min‐Max | $1138‐$7718 | $2568‐$6567 | $14 598‐$14 598 | $4692‐$4692 |
Abbreviations: —, no studies done; CD, Crohn's disease; IBD, inflammatory bowel disease; N, number of observations; UC, ulcerative colitis.
FIGURE 2.

Mean annual healthcare costs per prevalent inflammatory bowel disease case in 2018 US dollars. Lines indicate longitudinal studies. Numbers indicate corresponding reference
FIGURE 3.

Mean annual healthcare costs per prevalent Crohn's disease case in 2018 US dollars. Lines indicate longitudinal studies. Numbers indicate corresponding reference
FIGURE 4.
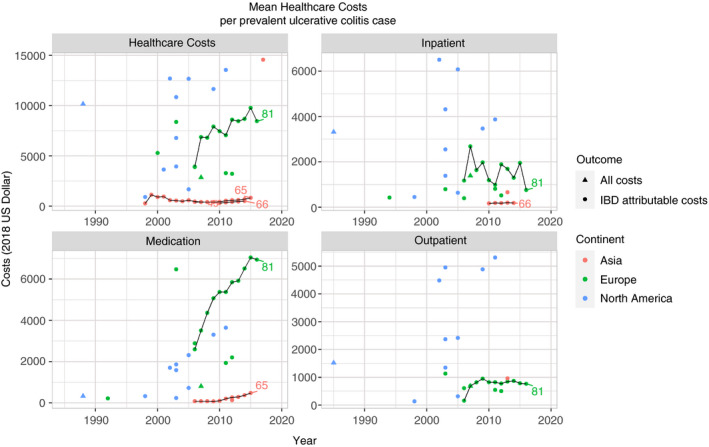
Mean annual healthcare costs per prevalent ulcerative colitis case in 2018 US dollars. Lines indicate longitudinal studies. Numbers indicate corresponding reference
The means of the reported mean annual healthcare costs for prevalent cases of IBD, CD and UC were between $1051 and $3755 in Asia; $5938 and $10 484 in Europe; and $8053 and $13 212 in North America. No studies from Oceania reported mean healthcare costs, but median healthcare costs were estimated to be between $3700 and $11 340 (File S2). Annual mean healthcare costs per prevalent case of IBD, CD and UC seem to be increasing in Asia, Europe and North America. Longitudinal studies from Europe and Asia show that this increase is mostly driven by an increase in medication costs.65, 81
Over the 30‐year period, cost drivers have shifted from inpatient to medication costs for IBD in general, CD and UC (Figure 5A‐C and Figures S1‐S3). This cost trend is primarily attributable to an increase in medication costs, while inpatient and outpatient costs were relatively stable during the same time period. This was seen in Europe and North America, as no studies from Asia or Oceania were eligible for this analysis. For the comparison between proportion of biologic users and mean healthcare costs per study, see Figures S4‐S6.
FIGURE 5.
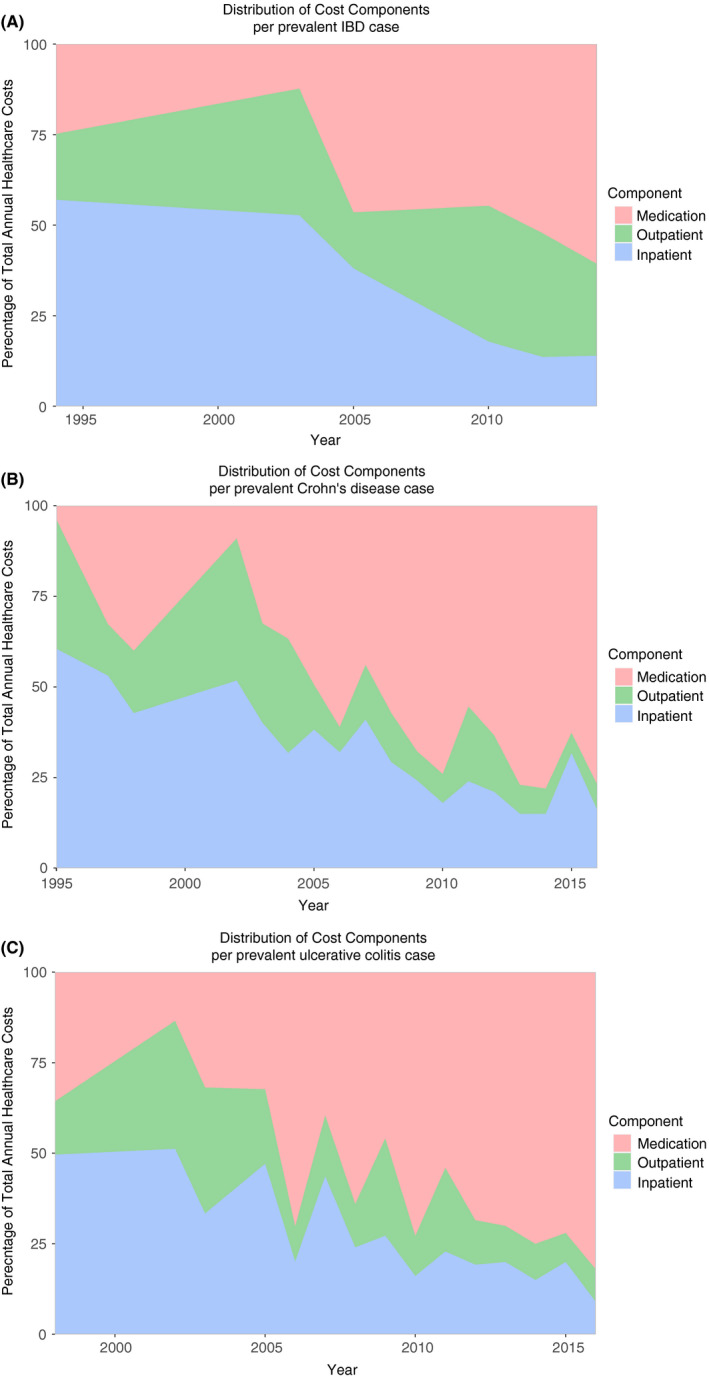
Distribution of healthcare cost components in proportions per prevalent (A) inflammatory bowel disease, (B) Crohn's disease and (C) ulcerative colitis cases in 2018 US dollars
The mean present‐day IBD‐attributable healthcare costs differed considerably between geographical areas (Table 2). In most cases, the primary cost driver was found to be medication, except for North America, where outpatient costs were the main cost driver.
TABLE 2.
Mean (range) annual IBD‐attributable healthcare costs and component cost per prevalent case for CD and UC in 2018 US dollars per continent for the period 2010‐2018
| Disease | Asia | Europe | North America |
|---|---|---|---|
| CD | |||
| Healthcare costs | |||
| N | 13 | 10 | 2 |
| Mean | $4417 | $12 439 | $17 495 |
| Min‐Max | $1230‐$31 161 | $7694‐$15 807 | $14 454‐$20 535 |
| Inpatient | |||
| N | 5 | 10 | 2 |
| Mean | $639 | $2487 | $4350 |
| Min‐Max | $623‐$664 | $1575‐$3503 | $3339‐$5360 |
| Outpatient | |||
| N | 0 | 10 | 2 |
| Mean | — | $890 | $6754 |
| Min‐Max | — | $403‐$1112 | $4496‐$9013 |
| Medication | |||
| N | 7 | 10 | 2 |
| Mean | $1777 | $9048 | $5796 |
| Min‐Max | $1342‐$2468 | $3788‐$12 013 | $5016‐$6577 |
| UC | |||
| Healthcare costs | |||
| N | 13 | 9 | 1 |
| Mean | $1606 | $7224 | $13 559 |
| Min‐Max | $309‐$14 572 | $3228‐$9779 | $13 559‐$13 559 |
| Inpatient | |||
| N | 5 | 9 | 1 |
| Mean | $187 | $1236 | $3874 |
| Min‐Max | $171‐$200 | $524‐$1956 | $3874‐$3874 |
| Outpatient | |||
| N | 0 | 9 | 1 |
| Mean | — | $746 | $5307 |
| Min‐Max | — | $499‐$868 | $5307‐$5307 |
| Medication | |||
| N | 7 | 9 | 1 |
| Mean | $268 | $5238 | $3643 |
| Min‐Max | $102‐$483 | $1936‐$7041 | $3643‐$3643 |
Costs do not sum as not all included studies reported all cost components separately.
Abbreviations: —, no studies done; CD, Crohn's disease; IBD, inflammatory bowel disease; N, number of observations; UC, ulcerative colitis.
Longitudinal data to assess changes in total and component costs for incident cases were not available for different time periods. Most studies reported annual healthcare costs for incident cases in the time period 2008‐2012,42, 47, 70, 71, 73, 76, 92, 93, 95 and other incidence‐based studies reported healthcare costs in the first ten years after diagnosis.67, 77
Mean annual IBD‐attributable costs of incident CD and UC cases in the first year after diagnosis were assessed in seven studies (Table 3).42, 70, 73, 76, 92, 93, 95 Three of these studies were conducted in Europe,42, 92, 93 of which two reported on different timeframes for the same patient population.42, 92 Costs reported in the more comprehensive of the two studies were used.92 Cost drivers differed per study but were mostly either inpatient or outpatient costs. Medication was the main cost driver for the study in Australia.
TABLE 3.
Healthcare costs in the first year after diagnosis for CD and UC in 2018 US dollars per incident case per study
| Disease | Study | Country | Time period | Total | Inpatient | Outpatient | Medication |
|---|---|---|---|---|---|---|---|
| CD | Lee70 | South Korea | 2010‐2015 | $3047 | — | — | $776 |
| Mak73 | Hong Kong | 2010‐2013 | $14 336 | $8445 | $3624 | $2268 | |
| Niewiadomski76 | Australia | 2007‐2008, 2010‐2013 | $7663 | $3447 | $1755 | $2462 | |
| Burisch 292 | Europe and Israel | 2010 | $6494 | $2077 | $2689 | $1753 | |
| Vadstrup93 | Denmark | 2003‐2016 | $12 631 | $6762 | $5387 | $483 | |
| UC | Lee70 | South Korea | 2010‐2015 | $1138 | — | — | $105 |
| Mak73 | Hong Kong | 2010‐2013 | $7718 | $2349 | $2745 | $2624 | |
| Niewiadomski76 | Australia | 2007‐2008, 2010‐2013 | $4692 | $1355 | $1529 | $1809 | |
| Burisch 292 | Europe and Israel | 2010 | $5675 | $2894 | $2157 | $681 | |
| Pilon95 | United States | 1999‐2017 | $14 598 | — | — | $3247 | |
| Vadstrup93 | Denmark | 2003‐2016 | $6026 | $2944 | $2404 | $678 |
Abbreviations: —, no reported; CD, Crohn's disease; UC, ulcerative colitis.
Costs for incident cases were found to be between $700 (+35%) and $1000 (+55%) higher than for prevalent cases.70 This was mostly because of the higher costs for diagnostics and surgery for the incident cases, notwithstanding a lower cost for biologic therapy for incident CD cases. Costs for incident cases peaked in the first year after diagnosis, and then quickly declined and stabilised after two years, driven by a decrease in hospitalisation, surgical and investigation costs. However, the cost decline is partially offset by increased costs for biologics each year after diagnosis.70, 71, 73, 77, 92, 93
3.4. Productivity costs
The annual mean productivity costs for prevalent and incident cases of IBD, CD and UC are summarised in Table 4. The comparison between healthcare costs and productivity costs can be found in Figures S7‐S9. Less than half of the studies (n = 25) reported on productivity costs and those studies mainly focused on absenteeism. Presenteeism was measured in most studies63, 64, 74, 75, 89, 90, 94 with the Work Productivity and Activity Impairment (WPAI) questionnaire, and one study58 developed their own survey to measure presenteeism. The productivity costs and components reported in the studies varied considerably. All studies used the human capital approach either implicitly or explicitly.
TABLE 4.
Mean (range) productivity costs for IBD, CD and UC in 2018 US dollars per continent
| Continent | IBD | CD | CD (incident) | UC | UC (incident) |
|---|---|---|---|---|---|
| Asia | |||||
| Absenteeism + Presenteeism | |||||
| N | 1 | 1 | 0 | 1 | 0 |
| Mean | $4677 | $5638 | — | $4828 | — |
| Min‐Max | $4677‐$4677 | $5638‐$5638 | — | $4828‐$4828 | — |
| Europe | |||||
| Absenteeism + Presenteeism | |||||
| N | 2 | 3 | 0 | 3 | 0 |
| Mean | $7124 | $6485 | — | $6414 | — |
| Min‐Max | $4795‐$9452 | $4342‐$10 243 | — | $2925‐$11 619 | — |
| Absenteeism | |||||
| N | 3 | 6 | 1 | 7 | 1 |
| Mean | $1338 | $2660 | $1956 | $2394 | $1677 |
| Min‐Max | $698‐$2276 | $641‐$5277 | $1956‐$1956 | $651‐$5992 | $1677‐$1677 |
| Presenteeism | |||||
| N | 2 | 2 | 0 | 3 | 0 |
| Mean | $5636 | $3324 | — | $3828 | — |
| Min‐Max | $4097‐$7175 | $2420‐$4228 | — | $1944‐$5627 | — |
| Early retirement + Disability | |||||
| N | 2 | 3 | 1 | 2 | 1 |
| Mean | $1757 | $6661 | $5686 | $4478 | $3561 |
| Min‐Max | $1160‐$2354 | $2508‐$14 665 | $5686‐$5686 | $2126‐$6830 | $3561‐$3561 |
| North America | |||||
| Absenteeism + Presenteeism | |||||
| N | 1 | 0 | 0 | 0 | 0 |
| Mean | $20 074 | — | — | — | — |
| Min‐Max | $20 074‐$20 074 | — | — | — | — |
| Absenteeism | |||||
| N | 3 | 3 | 0 | 3 | 1 |
| Mean | $2074 | $752 | — | $1443 | $2266 |
| Min‐Max | $916‐$4310 | $307‐$1303 | — | $85‐$2350 | $2266‐$2266 |
| Presenteeism | |||||
| N | 1 | 0 | 0 | 0 | 0 |
| Mean | $15 764 | — | — | — | — |
| Min‐Max | $15 764‐$15 764 | — | — | — | — |
Abbreviations: —, no studies done; CD, Crohn's disease; IBD, inflammatory bowel disease; N, number of observations; UC, ulcerative colitis.
Costs were found to be highest in North America ($20 074), mostly driven by presenteeism.90 Only one study reported on absenteeism and presenteeism in Asia, and no studies were done in Oceania.89 Studies in Europe estimated a broader range of productivity costs, also reporting on early retirement or disability, loss of unpaid time and caregiver absenteeism. Annual costs of unpaid time loss in Europe for CD patients were estimated to be $3390 (range $866‐$5914). Annual caregiver productivity costs were estimated for both CD and UC patients in Europe and were respectively $468 ($98‐$837) and $83 ($83‐$83).
3.5. Patient costs
A large range of IBD‐attributable patient costs were reported, including diet, equipment, informational material, hygiene articles, alternative therapy, household support, patient activities, insurance deductible and over‐the‐counter (OTC) drug use. Only eight studies (all in Europe) reported on patient costs with mean annual costs of $582 for CD ($81‐$1927) and $497 ($181‐$1341) for UC.
4. DISCUSSION
4.1. Healthcare costs
Societal cost of illness of IBD appears to be increasing worldwide, with the highest costs in North America and the lowest costs in Asia. This increase seems to be mainly caused by increasing medication costs while inpatient and outpatient costs remained stable. While there are considerable differences in total costs per patient per geographical region, the increasing trend over time can be seen in all geographical regions.
Costs in Asia are reported to be considerably lower than those in Europe and North America. It is unclear whether this is caused by relatively lower uptake of biologics, as most studies in Asia did not report the proportion of patients on biologic therapy. The only study from Asia that looked at cost drivers was done in Korea and attributed these costs to biologics, as these costs rapidly increased over time and accounted for 48.8% and 68.8% of the total costs for UC and CD respectively in 2015.65 Higher costs were reported in the Iran study compared to all other studies reviewed.91 A possible explanation is the current economic instability in Iran, leading to biased estimates compared to other countries. Costs were not reported in the local currency and component costs added up to more than 200% of the total reported costs. The drivers of these high costs are unclear.
In Europe, cost trends were highest in Switzerland,81 while in the Netherlands and Serbia the annual cost per patient was considerably lower.68, 87, 88 This difference is most likely driven by differing uptake of biologic therapy with the proportion of patients on biologic therapy being the highest in Switzerland.
Healthcare costs reported in North America fluctuated widely, partly because of within‐region differences in study methods. While some studies used the Medical Expenditure Panel Survey (MEPS) to determine costs, most studies used health insurance databases, which contain only insured patients and thus might not adequately reflect the entire IBD population. Moreover, the case‐finding method used in the insurance‐based studies skews the patient population towards higher disease severity. As patients were identified using ICD‐9 or ICD‐10 claim codes for IBD with a claim‐free pre‐index period of 6‐12 months, only patients who used healthcare during the study period were identified, and those in remission were excluded.
The cost of biologics is also reflected in the present‐day mean IBD‐attributable healthcare costs, particularly in Europe, where medication costs were by far the most important cost driver. Most observations came from studies in the Netherlands and Switzerland, skewing the outcomes towards more affluent European countries. Studies in Asia showed a similar but less pronounced trend towards medication as the most important cost driver, but this analysis was hampered by a considerable number of studies not reporting all component costs. Present‐day costs for North America are derived from insurance‐based studies, which explain the relatively high costs because they were not representative for the population as a whole.
Few data were available on incident costs of IBD, CD and UC and differences in study methods, time periods and geographical areas hinder comparisons. Costs per patient in the first year after diagnosis were driven mostly by admissions, surgery and outpatient diagnostics, and decreased in subsequent years.
The increasing economic burden of biologic therapy is not an IBD‐specific problem. A recent systematic review on the cost of illness of rheumatoid arthritis also reported a shift in cost drivers from inpatient to medication costs.96 Direct comparison of annual costs per patient between diseases is hampered by the heterogeneity in included studies in both reviews, leading to broad ranges of costs.
Increasing use and costs for biologic therapy are probably driven by acceptance of a treat‐to‐target approach that has shown to improve outcomes.97, 98 The question remains however whether such an approach is also cost‐effective, as incremental cost‐effectiveness ratios of biologic therapy often exceeded $100 000 per quality‐adjusted life year.99
The introduction of biologic therapy was expected to improve disease control and reduce hospitalisations and surgery and consequently reduce inpatient costs.8, 9 Earlier reports claimed that healthcare costs did not increase, but cost components shifted from inpatient to medication.87 Our systematic review shows that while medication costs have increased, inpatient costs do not seem to be declining. These findings are in line with earlier studies that found little to no association between biologic use and hospitalisation or surgery rates at population level.100, 101, 102 The discrepancy between randomised controlled trials and real‐world evidence is cause for concern, as the market share of biologic agents is increasing,5 and the real‐world effect on healthcare use is doubtful. Future initiatives need to focus on increasing value by optimising the use of biologics in daily practice and consequently improve effectiveness and reduce costs.
4.2. Productivity costs
Productivity costs are a key IBD cost burden, with absenteeism and presenteeism being a substantial proportion of the total costs. Productivity losses can exceed healthcare costs when factors such as early retirement, disability and unpaid time loss are taken into account. Only a few studies assessed productivity costs and most of these studies only reported on absenteeism. Because few data are available, time trends in productivity costs and consequently the effect of the introduction of biologics on productivity costs could not be assessed. The high societal burden of productivity costs and uncertainty around these estimates call for research on IBD‐related productivity costs, especially presenteeism, early retirement, and unpaid time loss.
The impact of IBD on societal productivity costs might be lower than indicated in the studies included in this systematic review. While all studies used the human capital approach to value productivity losses, there is a debate on whether the friction cost method should be used to determine productivity costs from the societal perspective.103, 104, 105 The human capital approach incorporates all productivity lost due to a disease over a patient's lifetime, while the friction cost method only counts the losses during the time period required to replace an incapacitated worker. The friction cost method leads to lower estimates because costs for long‐term disability are restricted to the friction cost period.106 This was also seen in rheumatoid arthritis, where cost‐effectiveness of biologic agents was related to the valuation method used.107 For IBD, little is known about the effect of biologic therapy on productivity costs.99 As healthcare costs have only been increasing, cost‐effectiveness of biologics for IBD treatment might depend on their effect on productivity costs. Because considerable value can be gained by reducing productivity costs, the effects of biologic therapy on productivity should be further investigated.
4.3. Patient costs
Patient costs from the societal perspective are relatively low compared to healthcare and productivity costs but might place a significant burden on patients. Because cost items are measured differently, a wide range of estimates are reported in the studies. Only eight studies in Europe estimated patient costs and contained little information on cost drivers, time trends or geographical differences.
4.4. Limitations
This systematic review gives a detailed overview of the societal cost of illness of IBD. The review included studies in countries worldwide and did not focus on Western countries.13, 14 However, some limitations hamper the interpretability of results. For instance, geographic, socio‐economic and political characteristics can affect costs. Differences in currency and purchasing power were resolved using the GDP deflator and PPP. However, considerable differences in healthcare systems, disease phenotype and other characteristics between countries and periods could have affected cost of illness comparisons.
There were considerable differences in populations and data sources in the studies. Even though the aim was to assess cost of illness of an IBD patient in clinical practice, some studies used different segments of the population. Moreover, how IBD cases were identified may have led to biased estimates. For instance, North American insurance‐based studies often identified cases using insurance claims with ICD‐9‐CM codes for CD or UC with a 6‐month pre‐index period free of these codes.37, 45, 52, 56, 57, 62 This method captures only active periods of treatment and can skew the estimates to a population with higher disease severity.
Not all studies included the same cost components, and valuation methods differed considerably. For example, North American studies often reported insurance claims as costs, possibly leading to higher estimates.36, 37, 45, 48, 52, 56, 57, 62, 84, 95 As most studies only reported costs and not resource use, it was not possible to determine if a difference in resource use explained cost differences or whether this was due to differing prices per resource. We aimed to reduce heterogeneity by stratifying studies based on disease and geographical area. These results should however be interpreted with caution because considerable differences in study methods remained. Further stratification on methodology, country or perspective would have led to non‐informative groups, as only one or two studies per group would have remained. Currency and purchasing power transformed estimates per study can be found in File S2 for more detailed insights.
As most studies only reported a mean and little other data on the distribution of their cost data, it was not possible to determine whether changes in costs were due to changes in the overall patient population or whether this was due to a (high‐cost) subpopulation of the patients.
4.5. Future
Most studies included in this systematic review reported costs from before 2015. The treatment landscape for IBD is changing rapidly, and new biologics and small molecules have been approved in the last few years. These might further increase costs as they are still covered under patent. Conversely, for both infliximab and adalimumab, biosimilars have been approved. These might play an important role in constraining costs in the future, generating comparable outcomes at lower costs.109, 110 As only a few studies reported on costs after 2015, it was not possible to evaluate what the effect was of new biologics and biosimilars on healthcare costs.
To validate the findings of this review and to gain a better insight in the cost of illness of IBD, future cost‐of‐illness studies should be either international or longitudinal, but preferably both. Because single‐country studies are often conducted according to national guidelines, this makes comparisons between studies difficult. International studies can standardise perspective, resource use quantification, and valuation and inform on salient differences between countries and continents. Cross‐sectional studies often differ in methodology through the years, impeding comparisons over time.
Currently, the number of international and longitudinal studies is lacking. No such studies on costs of prevalent IBD cases were found, and there were three reports from two European cohorts42, 77, 108 on costs of incident cases. The other longitudinal studies34, 44, 53, 65, 66, 81 in this review did report information on changes in costs over time of prevalent cases but were limited by their focus on a single country.
5. CONCLUSION
This comprehensive systematic review shows that the healthcare costs of IBD seem to be increasing on all continents, most likely driven by an increasing use of expensive medication. The decrease in inpatient costs that was expected with the introduction of biologic therapy was not seen in this review. To contain the rapidly growing costs, future initiatives should aim at optimising the use of biologic therapy in daily practice. Moreover, this review indicates that productivity costs possibly exceed healthcare costs and are a key societal cost driver for IBD. Because the cost‐effectiveness of biologic therapy might mainly rely on the effect it has on work impairment, further research on this relationship is required. Lastly, longitudinal and international cost‐of‐illness studies on IBD are essential to validate these findings and clarify the global cost burden of IBD.
AUTHORSHIP
Guarantor of the article: R.L. West and D. van Noord.
Author contributions: R.C.A. van Linschoten, D. van Noord and R.L. West designed the study. C.D. Niehot and R.C.A. van Linschoten performed the systematic search. R.C.A. van Linschoten and E. Visser analysed the data. R.C.A. van Linschoten, E. Visser, C.J. van der Woude, J.A. Hazelzet, D. van Noord and R.L. West interpreted the data. R.C.A. van Linschoten and E. Visser wrote the manuscript. All authors critically reviewed the manuscript and approved the final version of the manuscript for submission. R.L. West and D. van Noord share last authorship.
Supporting information
File S1
File S2
ACKNOWLEDGEMENTS
The authors would like to thank Helen West for language editing and proofreading of the manuscript.
Declaration of personal interests: Drs. van Linschoten, Drs. Visser, Prof. Dr. Hazelzet and Drs. Niehot have nothing to disclose. Prof. Dr. van der Woude reports personal fees from Abbvie and Celltrion, and grants from Pfizer and Janssen outside the submitted work. Dr. van Noord reports grants from AbbVie, Falk, Ferring, Janssen, MSD, Pfizer, and Takeda and personal fees from Takeda and Janssen outside the submitted work. Dr. West reports grants from AbbVie, Falk, Ferring, Janssen, MSD, Pfizer, and Takeda and personal fees from AbbVie, Janssen, and Pfizer outside the submitted work.
van Linschoten RCA, Visser E, Niehot CD, et al. Systematic review: societal cost of illness of inflammatory bowel disease is increasing due to biologics and varies between continents. Aliment Pharmacol Ther. 2021;54:234–248. 10.1111/apt.16445
The Handling Editor for this article was Professor Jonathan Rhodes, and this uncommissioned review was accepted for publication after full peer‐review.
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this published article and its supplementary information files.
REFERENCES
- 1.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711–721. [DOI] [PubMed] [Google Scholar]
- 2.Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381:1201–1214. [DOI] [PubMed] [Google Scholar]
- 3.Colombel J, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65. [DOI] [PubMed] [Google Scholar]
- 4.Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376:1723–1736. [DOI] [PubMed] [Google Scholar]
- 5.Yu H, MacIsaac D, Wong JJ, et al. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment Pharmacol Ther. 2018;47:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feagan BG, Reilly MC, Gerlier L, Brabant Y, Brown M, Schreiber S. Clinical trial: the effects of certolizumab pegol therapy on work productivity in patients with moderate‐to‐severe Crohn's disease in the PRECiSE 2 study. Aliment Pharmacol Ther. 2010;31:1276–1285. [DOI] [PubMed] [Google Scholar]
- 7.Lichtiger S, Binion DG, Wolf DC, et al. The CHOICE trial: adalimumab demonstrates safety, fistula healing, improved quality of life and increased work productivity in patients with Crohn's disease who failed prior infliximab therapy. Aliment Pharmacol Ther. 2010;32:1228–1239. [DOI] [PubMed] [Google Scholar]
- 8.Feagan BG, Sandborn WJ, Lazar A, et al. Adalimumab therapy is associated with reduced risk of hospitalization in patients with ulcerative colitis. Gastroenterology. 2014;146:110–118.e3. [DOI] [PubMed] [Google Scholar]
- 9.Costa J, Magro F, Caldeira D, Alarcao J, Sousa R, Vaz‐Carneiro A. Infliximab reduces hospitalizations and surgery interventions in patients with inflammatory bowel disease: a systematic review and meta‐analysis. Inflamm Bowel Dis. 2013;19:2098–2110. [DOI] [PubMed] [Google Scholar]
- 10.van der Valk ME. Costs of inflammatory bowel disease in the Netherlands: the COIN study (PhD thesis). Utrecht University, 2015.
- 11.World Health Organization . Global health expenditure database 2019, 2019. http://apps.who.int/nha/database/Select/Indicators/en
- 12.Organisation for Economic Co‐operation and Development . Health spending (indicator). 2019, 2019. https://data.oecd.org/healthres/health‐spending.htm
- 13.Yu AP, Cabanilla LA, Wu EQ, Mulani PM, Chao J. The costs of Crohn's disease in the United States and other Western countries: a systematic review. Curr Med Res Opin. 2008;24:319–328. [DOI] [PubMed] [Google Scholar]
- 14.Cohen RD, Yu AP, Wu EQ, Xie J, Mulani PM, Chao J. Systematic review: the costs of ulcerative colitis in Western countries. Aliment Pharmacol Ther. 2010;31:693–707. [DOI] [PubMed] [Google Scholar]
- 15.Busch K, da Silva SA, Holton M, Rabacow FM, Khalili H, Ludvigsson JF. Sick leave and disability pension in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2014;8:1362–1377. [DOI] [PubMed] [Google Scholar]
- 16.Constantin J, Atanasov P, Wirth D, Borsi A. Indirect costs associated with ulcerative colitis: a systematic literature review of real‐world data. BMC Gastroenterol. 2019;19:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawalec P. Indirect costs of inflammatory bowel diseases: Crohn's disease and ulcerative colitis. A systematic review. Arch Med Sci. 2016;12:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawalec P, Malinowski KP. Indirect health costs in ulcerative colitis and Crohn's disease: a systematic review and meta‐analysis. Expert Rev Pharmacoecon Outcomes Res. 2015;15:253–266. [DOI] [PubMed] [Google Scholar]
- 19.El‐Matary W, Kuenzig ME, Singh H, et al. Disease‐associated costs in children with inflammatory bowel disease: a systematic review. Inflamm Bowel Dis. 2020;26:206–215. [DOI] [PubMed] [Google Scholar]
- 20.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population‐based studies. Lancet. 2017;390:2769–2778. [DOI] [PubMed] [Google Scholar]
- 21.Byford S, Raftery J. Perspectives in economic evaluation. BMJ. 1998;316:1529–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DGGroup P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visser E, van Linschoten RCA, West RL, van Noord D, Hazelzet JA, van der Woude CJ. Cost of illness of inflammatory bowel disease: a systematic review, 2020. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=158567 [DOI] [PMC free article] [PubMed]
- 24.Haddaway NR, Collins AM, Coughlin D, Kirk S. The role of google scholar in evidence reviews and its applicability to grey literature searching. PLoS One. 2015;10:e0138237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larg A, Moss JR. Cost‐of‐illness studies: a guide to critical evaluation. Pharmacoeconomics. 2011;29:653–671. [DOI] [PubMed] [Google Scholar]
- 26.Dunn A, Grosse SD, Zuvekas SH. Adjusting health expenditures for inflation: a review of measures for health services research in the United States. Health Serv Res. 2018;53:175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The World Bank . World Development Indicators. 2020; https://databank.worldbank.org/reports.aspx?source=2&series=PPP,PA.NUS.PPPC.RF,PX.REX.REER,NY.GDP.DEFL.ZS,FP.CPI.TOTL#
- 28.Organisation for Economic Co‐operation and Development . Purchasing power parities (PPP). 2019; https://data.oecd.org/conversion/purchasing‐power‐parities‐ppp.htm
- 29.Organisation for Economic Co‐operation and Development . GDP deflators, forecast growth. 2019. https://stats.oecd.org/Index.aspx?QueryId=61354
- 30.National Statistics Republic of China (Taiwan) . Statistical Tables. 2020; https://eng.stat.gov.tw/ct.asp?xItem=37408&CtNode=5347&mp=5
- 31.Census and Statistics Department The Government of the Hong Kong Special Administrative Region. Table 030: Gross Domestic Product (GDP), implicit price deflator of GDP and per capita GDP. 2020 https://www.censtatd.gov.hk/hkstat/sub/sp250.jsp?tableID=030&ID=0&productType=8
- 32.Aldeguer X, Sicras‐Mainar A. Costs of ulcerative colitis from a societal perspective in a regional health care area in Spain: a database study. Gastroenterol Hepatol. 2016;39:9–19. [DOI] [PubMed] [Google Scholar]
- 33.Bahler C, Schoepfer AM, Vavricka SR, Brungger B, Reich O. Chronic comorbidities associated with inflammatory bowel disease: prevalence and impact on healthcare costs in Switzerland. Eur J Gastroenterol Hepatol. 2017;29:916–925. [DOI] [PubMed] [Google Scholar]
- 34.Bahler C, Vavricka SR, Schoepfer AM, Brungger B, Reich O. Trends in prevalence, mortality, health care utilization and health care costs of Swiss IBD patients: a claims data based study of the years 2010, 2012 and 2014. BMC Gastroenterol. 2017;17:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bassi A, Dodd S, Williamson P, Bodger K. Cost of illness of inflammatory bowel disease in the UK: a single centre retrospective study. Gut. 2004;53:1471–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernstein CN, Longobardi T, Finlayson G, Blanchard JF. Direct medical cost of managing IBD patients: a Canadian population‐based study. Inflamm Bowel Dis. 2012;18:1498–1508. [DOI] [PubMed] [Google Scholar]
- 37.Bickston SJ, Waters HC, Dabbous O, Tang B, Rahman MI. Administrative claims analysis of all‐cause annual costs of care and resource utilization by age category for ulcerative colitis patients. J Manag Care Pharm. 2008;14:352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blomqvist P, Ekbom A. Inflammatory bowel diseases: health care and costs in Sweden in 1994. Scand J Gastroenterol. 1997;32:1134–1139. [DOI] [PubMed] [Google Scholar]
- 39.Blomqvist P, Feltelius N, Löfberg R, Ekbom A. A 10‐year survey of inflammatory bowel diseases—drug therapy, costs and adverse reactions. Aliment Pharmacol Ther. 2001;15:475–481. [DOI] [PubMed] [Google Scholar]
- 40.Blumenstein I, Bock H, Weber C, et al. Health care and cost of medication for inflammatory bowel disease in the Rhein‐Main region, Germany: a multicenter, prospective, internet‐based study. Inflamm Bowel Dis. 2008;14:53–60. [DOI] [PubMed] [Google Scholar]
- 41.Bounthavong M, Li M, Watanabe JH. An evaluation of health care expenditures in Crohn's disease using the United States Medical Expenditure Panel Survey from 2003 to 2013. Res Social Adm Pharm. 2017;13:530–538. [DOI] [PubMed] [Google Scholar]
- 42.Burisch J, Vardi H, Pedersen N, et al. Costs and resource utilization for diagnosis and treatment during the initial year in a European inflammatory bowel disease inception cohort: an ECCO‐EpiCom Study. Inflamm Bowel Dis. 2015;21:121–131. [DOI] [PubMed] [Google Scholar]
- 43.Chen CY, Lee KT, Charles Tzu‐Chi L, Lai WT, Huang YB. Epidemiology and disease burden of ulcerative colitis in Taiwan: a nationwide population‐based study. Value Health Reg Issues. 2013;2:127–134. [DOI] [PubMed] [Google Scholar]
- 44.Click B, Lopez R, Arrigain S, Schold J, Regueiro M, Rizk M. Shifting cost‐drivers of health care expenditures in inflammatory bowel disease. Inflamm Bowel Dis. 2020;26:1268–1275. [DOI] [PubMed] [Google Scholar]
- 45.Cohen R, Skup M, Ozbay AB, et al. Direct and indirect healthcare resource utilization and costs associated with ulcerative colitis in a privately‐insured employed population in the US. J Med Econ. 2015;18:447–456. [DOI] [PubMed] [Google Scholar]
- 46.Cohen RD, Waters HC, Tang B, Rahman MI. Effects of fistula on healthcare costs and utilization for patients with Crohn's disease treated in a managed care environment. Inflamm Bowel Dis. 2008;14:1707–1714. [DOI] [PubMed] [Google Scholar]
- 47.Colombara F, Martinato M, Girardin G, Gregori D. Higher levels of knowledge reduce health care costs in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:615–622. [DOI] [PubMed] [Google Scholar]
- 48.Dunn A, Whitmire B, Batch A, Fernando L, Rittmueller L. High spending growth rates for key diseases in 2000–14 were driven by technology and demographic factors. Health Aff (Millwood). 2018;37:915–924. [DOI] [PubMed] [Google Scholar]
- 49.Ebinger M, Leidl R, Thomas S, et al. Cost of outpatient care in patients with inflammatory bowel disease in a German University Hospital. J Gastroenterol Hepatol. 2004;19:192–199. [DOI] [PubMed] [Google Scholar]
- 50.Feagan BG, Vreeland MG, Larson LR, Bala MB. Annual cost of care for Crohn’s disease: a payor perspective. Am J Gastroenterol. 2000;95:1955–1960. [DOI] [PubMed] [Google Scholar]
- 51.Ganz ML, Sugarman R, Wang R, Hansen BB, Hakan‐Bloch J. The economic and health‐related impact of Crohn's disease in the United States: evidence from a nationally representative survey. Inflamm Bowel Dis. 2016;22:1032–1041. [DOI] [PubMed] [Google Scholar]
- 52.Gibson TB, Ng E, Ozminkowski RJ, et al. The direct and indirect cost burden of Crohn's disease and ulcerative colitis. J Occup Environ Med. 2008;50:1261–1272. [DOI] [PubMed] [Google Scholar]
- 53.Gleason PP, Alexander GC, Starner CI, et al. Health plan utilization and costs of specialty drugs within 4 chronic conditions. J Manag Care Pharm. 2013;19:542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gunnarsson C, Chen J, Rizzo JA, Ladapo JA, Lofland JH. Direct health care insurer and out‐of‐pocket expenditures of inflammatory bowel disease: evidence from a US national survey. Dig Dis Sci. 2012;57:3080–3091. [DOI] [PubMed] [Google Scholar]
- 55.Gunnarsson C, Chen J, Rizzo JA, Ladapo JA, Naim A, Lofland JH. The employee absenteeism costs of inflammatory bowel disease: evidence from US National Survey Data. J Occup Environ Med. 2013;55:393–401. [DOI] [PubMed] [Google Scholar]
- 56.Hay JW, Hay AR. Inflammatory bowel disease: costs‐of‐illness. J Clin Gastroenterol. 1992;14:309–317. [DOI] [PubMed] [Google Scholar]
- 57.Hillson E, Dybicz S, Waters HC, et al. Health care expenditures in ulcerative colitis: the perspective of a self‐insured employer. J Occup Environ Med. 2008;50:969–977. [DOI] [PubMed] [Google Scholar]
- 58.Holko P, Kawalec P, Mossakowska M, Pilc A. Health‐related quality of life impairment and indirect cost of Crohn's disease: a self‐report study in Poland. PLoS One. 2016;11:e0168586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jackson B, Con D, Ma R, Gorelik A, Liew D, De Cruz P. Health care costs associated with Australian tertiary inflammatory bowel disease care. Scand J Gastroenterol. 2017;52:851–856. [DOI] [PubMed] [Google Scholar]
- 60.Juan J, Estiarte R, Colomé E, Artés M, Jiménez FJ, Alonso J. Burden of illness of Crohn’s disease in Spain. Dig Liver Dis. 2003;35:853–861. [DOI] [PubMed] [Google Scholar]
- 61.Kappelman MD, Rifas–Shiman SL, Porter CQ, et al. Direct health care costs of Crohn's disease and ulcerative colitis in US children and adults. Gastroenterology. 2008;135:1907–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karve S, Candrilli S, Kappelman MD, Tolleson‐Rinehart S, Tennis P, Andrews E. Healthcare utilization and comorbidity burden among children and young adults in the United States with systemic lupus erythematosus or inflammatory bowel disease. J Pediatr. 2012;161:662–670.e2. [DOI] [PubMed] [Google Scholar]
- 63.Kawalec P, Stawowczyk E. Relationship between physician‐based assessment of disease activity, quality of life, and costs of ulcerative colitis in Poland. Prz Gastroenterol. 2018;13:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawalec P, Stawowczyk E, Mossakowska M, Pilc A. Disease activity, quality of life, and indirect costs of ulcerative colitis in Poland. Prz Gastroenterol. 2017;12:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim J‐W, Lee CK, Lee JK, et al. Long‐term evolution of direct healthcare costs for inflammatory bowel diseases: a population‐based study (2006–2015). Scand J Gastroenterol. 2019;54:419–426. [DOI] [PubMed] [Google Scholar]
- 66.Kim JW, Lee CK, Rhee SY, Oh CH, Shim JJ, Kim HJ. Trends in health‐care costs and utilization for inflammatory bowel disease from 2010 to 2014 in Korea: a nationwide population‐based study. J Gastroenterol Hepatol. 2018;33:847–854. [DOI] [PubMed] [Google Scholar]
- 67.Kohn A, Fano V, Monterubbianesi R, et al. Surgical and nonsurgical hospitalization rates and charges for patients with ulcerative colitis in Italy: a 10‐year cohort study. Dig Liver Dis. 2012;44:369–374. [DOI] [PubMed] [Google Scholar]
- 68.Kostic M, Djakovic L, Sujic R, Godman B, Jankovic SM. Inflammatory bowel diseases (Crohn s disease and ulcerative colitis): cost of treatment in Serbia and the implications. Appl Health Econ Health Policy. 2017;15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Le DV, Gupte R, Gabriel MH, Vaidya V. Inflammatory bowel disease: cost‐driving factors and impact of cost sharing on outpatient resource utilization. J Pharm Health Serv Res. 2018;9:301–307. [Google Scholar]
- 70.Lee J, Im JP, Han K, et al. Changes in direct healthcare costs before and after the diagnosis of inflammatory bowel disease: a nationwide population‐based study. Gut Liv. 2020;14:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lo B, Vind I, Vester‐Andersen MK, Bendtsen F, Burisch J. Direct and indirect costs of inflammatory bowel disease: ten years of follow‐up in a Danish population‐based inception cohort. J Crohns Colitis. 2020;14:53–63. [DOI] [PubMed] [Google Scholar]
- 72.Longobardi T, Jacobs P, Wu L, Bernstein CN. Work losses related to inflammatory bowel disease in Canada: results from a national population health survey. Am J Gastroenterol. 2003;98:844–849. [DOI] [PubMed] [Google Scholar]
- 73.Mak LY, Ng SC, Wong IOL, et al. Direct health‐care cost utilization in Hong Kong inflammatory bowel disease patients in the initial 2 years following diagnosis. J Gastroenterol Hepatol. 2018;33:141–149. [DOI] [PubMed] [Google Scholar]
- 74.Mesterton J, Jonsson L, Almer SH, Befrits R, Friis‐Liby I, Lindgren S. Resource use and societal costs for Crohn's disease in Sweden. Inflamm Bowel Dis. 2009;15:1882–1890. [DOI] [PubMed] [Google Scholar]
- 75.Michael MD, Bálint A, Lovász BD, et al. Work disability and productivity loss in patients with inflammatory bowel diseases in Hungary in the era of biologics. Eur J Health Econ. 2014;15(Suppl 1):S121–128. [DOI] [PubMed] [Google Scholar]
- 76.Niewiadomski O, Studd C, Hair C, et al. Health care cost analysis in a population‐based inception cohort of inflammatory bowel disease patients in the first year of diagnosis. J Crohns Colitis. 2015;9:988–996. [DOI] [PubMed] [Google Scholar]
- 77.Odes S, Vardi H, Friger M, et al. Cost analysis and cost determinants in a European inflammatory bowel disease inception cohort with 10 years of follow‐up evaluation. Gastroenterology. 2006;131:719–728. [DOI] [PubMed] [Google Scholar]
- 78.Park KT, Colletti RB, Rubin DT, Sharma BK, Thompson A, Krueger A. Health insurance paid costs and drivers of costs for patients with Crohn's disease in the United States. Am J Gastroenterol. 2016;111:15–23. [DOI] [PubMed] [Google Scholar]
- 79.Park KT, Ehrlich OG, Allen JI, et al. The cost of inflammatory bowel disease: an initiative from the Crohn's & Colitis Foundation. Inflamm Bowel Dis. 2020;26:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park MD, Bhattacharya J, Park K. Differences in healthcare expenditures for inflammatory bowel disease by insurance status, income, and clinical care setting. PeerJ. 2014;2:e587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pillai N, Dusheiko M, Maillard MH, et al. The evolution of health care utilisation and costs for inflammatory bowel disease over ten years. J Crohns Colitis. 2019;13:744–754. [DOI] [PubMed] [Google Scholar]
- 82.Pinchbeck BR, Kirdeikis J, Thomson ABR. Economic impact of inflammatory bowel disease in Alberta. J Gastroenterol Hepatol. 1988;2:53–56. [Google Scholar]
- 83.Prenzler A, Bokemeyer B, von der Schulenburg JM, Mittendorf T. Health care costs and their predictors of inflammatory bowel diseases in Germany. Eur J Health Econ. 2011;12:273–283. [DOI] [PubMed] [Google Scholar]
- 84.Sandler RS, Everhart JE, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–1511. [DOI] [PubMed] [Google Scholar]
- 85.Severs M, Petersen RE, Siersema PD, Mangen MJ, Oldenburg B. Self‐reported health care utilization of patients with inflammatory bowel disease correlates perfectly with medical records. Inflamm Bowel Dis. 2016;22:688–693. [DOI] [PubMed] [Google Scholar]
- 86.Stark R, König HH, Leidl R. Costs of inflammatory bowel disease in Germany. Pharmacoeconomics. 2006;24:797–814. [DOI] [PubMed] [Google Scholar]
- 87.van der Valk ME, Mangen M‐J, Leenders M, et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti‐TNFα therapy: results from the COIN study. Gut. 2014;63:72–79. [DOI] [PubMed] [Google Scholar]
- 88.van der Valk ME, Mangen M‐J, Severs M, et al. Evolution of costs of inflammatory bowel disease over two years of follow‐up. PLoS One. 2016;11:e0142481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yamabe K, Liebert R, Flores N, Pashos CL. Health‐related quality of life outcomes and economic burden of inflammatory bowel disease in Japan. Clinicoecon Outcomes Res. 2019;11:221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zand A, van Deen WK, Inserra EK, et al. Presenteeism in inflammatory bowel diseases: a hidden problem with significant economic impact. Inflamm Bowel Dis. 2015;21:1623–1630. [DOI] [PubMed] [Google Scholar]
- 91.Balaii H, Olfatifar M, Narab SO, Hosseini AA, Salehi AS, Shahrokh S. Estimation the direct cost of inflammatory bowel disease in Iranian patients; the one‐year follow‐up. Gastroenterol Hepatol Bed Bench. 2019;12:S87–S93. [PMC free article] [PubMed] [Google Scholar]
- 92.Burisch J, Vardi H, Schwartz D, et al. Health‐care costs of inflammatory bowel disease in a pan‐European, community‐based, inception cohort during 5 years of follow‐up: a population‐based study. Lancet Gastroenterol Hepatol. 2020;5:454–464. [DOI] [PubMed] [Google Scholar]
- 93.Vadstrup K, Alulis S, Borsi A, et al. Societal costs attributable to Crohn’s disease and ulcerative colitis within the first 5 years after diagnosis: a Danish nationwide cost‐of‐illness study 2002–2016. Scand J Gastroenterol. 2020;55:41–46. [DOI] [PubMed] [Google Scholar]
- 94.van Gennep S, Evers SW, Rietdijk ST, et al. High disease burden drives indirect costs in employed inflammatory bowel disease patients: the WORK‐IBD study. Inflamm Bowel Dis. 2021;27:352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pilon D, Ding Z, Muser E, et al. Long‐term direct and indirect costs of ulcerative colitis in a privately‐insured United States population. Curr Med Res Opin. 2020;36:1285–1294. [DOI] [PubMed] [Google Scholar]
- 96.Hsieh PH, Wu O, Geue C, McIntosh E, McInnes IB, Siebert S. Economic burden of rheumatoid arthritis: a systematic review of literature in biologic era. Ann Rheum Dis. 2020;79:771–777. [DOI] [PubMed] [Google Scholar]
- 97.Peyrin‐Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat‐to‐target. Am J Gastroenterol. 2015;110:1324–1338. [DOI] [PubMed] [Google Scholar]
- 98.Colombel J‐F, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390:2779–2789. [DOI] [PubMed] [Google Scholar]
- 99.Pillai N, Dusheiko M, Burnand B, Pittet V. A systematic review of cost‐effectiveness studies comparing conventional, biological and surgical interventions for inflammatory bowel disease. PLoS One. 2017;12:e0185500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jeuring SFG, Bours PHA, Zeegers MP, et al. Disease outcome of ulcerative colitis in an era of changing treatment strategies: results from the Dutch population‐based IBDSL cohort. J Crohns Colitis. 2015;9:837–845. [DOI] [PubMed] [Google Scholar]
- 101.Jeuring SFG, van den Heuvel TRA, Liu LYL, et al. Improvements in the long‐term outcome of Crohn's disease over the past two decades and the relation to changes in medical management: results from the population‐based IBDSL cohort. Am J Gastroenterol. 2017;112:325–336. [DOI] [PubMed] [Google Scholar]
- 102.Murthy SK, Begum J, Benchimol EI, et al. Introduction of anti‐TNF therapy has not yielded expected declines in hospitalisation and intestinal resection rates in inflammatory bowel diseases: a population‐based interrupted time series study. Gut. 2020;69:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koopmanschap MA, Rutten FF, van Ineveld BM, Van Roijen L. The friction cost method for measuring indirect costs of disease. J Health Econ. 1995;14:171–189. [DOI] [PubMed] [Google Scholar]
- 104.Krol M, Brouwer W, Rutten F. Productivity costs in economic evaluations: past, present, future. Pharmacoeconomics. 2013;31:537–549. [DOI] [PubMed] [Google Scholar]
- 105.Nyman JA. Productivity costs revisited: toward a new US policy. Health Econ. 2012;21:1387–1401. [Google Scholar]
- 106.Pike J, Grosse SD. Friction cost estimates of productivity costs in cost‐of‐illness studies in comparison with human capital estimates: a review. Appl Health Econ Health Policy. 2018;16:765–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.van den Hout WB. The value of productivity: human‐capital versus friction‐cost method. Ann Rheum Dis. 2010;69(Suppl 1):i89–91. [DOI] [PubMed] [Google Scholar]
- 108.Fiorino G, Lytras T, Younge L, et al. Quality of care standards in inflammatory bowel diseases: a European Crohn's and Colitis Organisation (ECCO) position paper. J Crohns Colitis. 2020;14:1037–1048. [DOI] [PubMed] [Google Scholar]
- 109.Komaki Y, Yamada A, Komaki F, Micic D, Ido A, Sakuraba A. Systematic review with meta‐analysis: the efficacy and safety of CT‐P13, a biosimilar of anti‐tumour necrosis factor‐alpha agent (infliximab), in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;45:1043–1057. [DOI] [PubMed] [Google Scholar]
- 110.Meyer A, Rudant J, Drouin J, Coste J, Carbonnel F, Weill A. The effectiveness and safety of infliximab compared with biosimilar CT‐P13, in 3112 patients with ulcerative colitis. Aliment Pharmacol Ther. 2019;50:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1
File S2
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.


