ABSTRACT
Objective
Depression has been associated with metabolomic alterations. Depressive and anxiety disorders are often comorbid diagnoses and are suggested to share etiology. We investigated whether differential metabolomic alterations are present between anxiety and depressive disorders and which clinical characteristics of these disorders are related to metabolomic alterations.
Methods
Data were from the Netherlands Study of Depression and Anxiety (NESDA), including individuals with current comorbid anxiety and depressive disorders (N = 531), only a current depression (N = 304), only a current anxiety disorder (N = 548), remitted depressive and/or anxiety disorders (N = 897), and healthy controls (N = 634). Forty metabolites from a proton nuclear magnetic resonance lipid‐based metabolomics panel were analyzed. First, we examined differences in metabolites between disorder groups and healthy controls. Next, we assessed whether depression or anxiety clinical characteristics (severity and symptom duration) were associated with metabolites.
Results
As compared to healthy controls, seven metabolomic alterations were found in the group with only depression, reflecting an inflammatory (glycoprotein acetyls; Cohen's d = 0.12, p = 0.002) and atherogenic‐lipoprotein‐related (e.g., apolipoprotein B: Cohen's d = 0.08, p = 0.03, and VLDL cholesterol: Cohen's d = 0.08, p = 0.04) profile. The comorbid group showed an attenuated but similar pattern of deviations. No metabolomic alterations were found in the group with only anxiety disorders. The majority of metabolites associated with depression diagnosis were also associated with depression severity; no associations were found with anxiety severity or disease duration.
Conclusion
While substantial clinical overlap exists between depressive and anxiety disorders, this study suggests that altered inflammatory and atherogenic‐lipoprotein‐related metabolomic profiles are uniquely associated with depression rather than anxiety disorders.
Keywords: anxiety, depression, metabolomics
Significant outcomes
Despite the high clinical overlap between depression and anxiety, this study suggests that altered inflammatory and atherogenic‐lipoprotein‐related metabolomic profiles (e.g., increased glycoprotein acetyls and apolipoprotein B) are mainly associated with depression rather than anxiety disorders.
Limitations
The analyses carried out in this study are of a cross‐sectional nature, which precluded causal inference. Also, we lacked information about dietary habits, which may impact on circulating metabolite levels.
1. INTRODUCTION
Depression has been associated with adverse cardiometabolic health including cardiovascular disease (CVDs) and increased adiposity.1, 2, 3 Epidemiological studies found cross‐sectional associations and bidirectional relationships between depression and CVD (incidence). A recent large‐scale metabolomics meta‐analysis showed that depression is associated with a signature in circulating metabolites. Increased triglycerides and very‐low‐density lipoprotein (VLDL) cholesterol and decreased high‐density lipoprotein (HDL) cholesterol, acetate, and apolipoprotein A1 were associated with increased odds of lifetime depression.4 Associations became stronger when focusing on subjects reporting depression at the moment of metabolite assessment, suggesting that metabolomic alterations are more strongly associated with a current depressive state. Similar metabolomic profiles have been associated with BMI5 and incidence of CVD.6
Depression and anxiety are highly related. Depression frequently co‐occurs with anxiety: 50–60% of the patients with major depressive disorder have a history of anxiety disorders.7 These disorders share clinical manifestation of symptomatology and risk factors, including genetics.8, 9, 10, 11 Several twin studies showed overlapping genetic liabilities between major depression and anxiety disorders, with generalized anxiety disorder (GAD) having the largest genetic correlation (r = 0.86–1.0),12 which was confirmed by genome‐wide‐association studies.8, 10 Although less frequently studied, a higher anxiety disorder prevalence has been seen in CVD populations,13, 14 with some studies reporting a larger or additive effect of depression14 while others did not.15, 16 Also, prospectively, anxiety was found to increase the risk for CVD onset. A meta‐analysis of prospective cohort studies17 found increased CVD risk associated with anxiety (Hazard ratio = 1.52, 95%CI 1.36–1.71), comparable to the risk than that was earlier found for depression (Relative risk = 1.81, 95%CI 1.53–2.15).18 In contrast, Momen and colleagues19 found that this CVD association was stronger for anxiety. Regarding increased adiposity, the relationship with anxiety is less robust than for depression.20, 21, 22, 23 In summary, depression and anxiety show great similarities, but differences regarding somatic disorder comorbidities haven been observed.
Comprehensive metabolomic profiling in anxious individuals has not been reported yet. Some studies of individual lipid‐related measures reported associations of atherogenic lipid levels with anxiety disorder diagnosis or severity in the form of increased serum levels of triglycerides,24 cholesterol,25 or dyslipidemia,26 whereas others did not.27, 28, 29, 30 A previous study analyzing the current sample found that ω‐3‐polyunsaturated fatty acid (PUFA) levels were lowered in anxious individuals, but only when a comorbidity with depression was present.31 ω‐6 PUFA levels were not associated with diagnostic status of either disorders. Some studies analyzed individuals with a life‐time diagnosis of anxiety disorder and not exclusively with a current diagnosis,26, 28 and some only analyzed separate anxiety disorders and not anxiety disorders as a whole.25, 28 Furthermore, most studies in anxiety disorders analyzed single‐marker measures or several markers combined in one measure. Whether current anxiety disorder diagnoses are associated with a wider range of adverse metabolomic markers is still unknown. Also, whether dysregulated markers previously associated with depression4 are specific for depression or are common also in anxiety is unknown.
1.1. Aims of the study
The aim of this current study is twofold: First, to identify metabolomic differences across distinct groups of patients (those with only a current depressive disorder, only a current anxiety disorder, comorbid anxiety, and depressive disorders), remitted patients, and healthy controls. Second, we examined whether clinical characteristics of depression and anxiety (symptoms severity of anxious arousal, phobic avoidance, worry or depression, and duration of depressive or anxious symptoms) are associated with metabolomic markers.
2. METHODS
2.1. Sample
Participants were part of the Netherlands Study for Depression and Anxiety (NESDA), an ongoing longitudinal cohort study into the long‐term course and consequences of depressive and anxiety disorders. A description of the study rationale, design, and methods is given elsewhere.32 Briefly, the initial sample comprised 2,981 participants between the ages of 18 and 65 who were recruited between 2004 and 2007 and from the community (19.0%), primary care (54.0%), and specialized mental health care settings (27.0%). These participants were healthy controls or had a current or prior history of depressive and/or an anxiety disorder. Participants were not included when they could not speak Dutch fluently or had a primary other psychiatric diagnosis of, e.g., bipolar, psychotic, obsessive compulsive, or severe addictive disorder. Diagnoses of depression and anxiety disorders according to the Diagnostic and Statistical Manual of Mental Disorders (DSM)‐IV were established with the use of the Composite Interview Diagnostic Instrument (CIDI)—lifetime version 2.1.33 The Ethical Committee of all participating universities approved the NESDA project, and all participants provided written informed consent. Data collection included an extensive interview, blood collection, self‐reported questionnaires, and medical assessments. For the current study, we selected 2914 participants from the baseline assessment with data on metabolomics. This sample included 304 current purely depressed cases (i.e., diagnosis of major depressive disorder or dysthymia within the month prior to interview, but no anxiety disorder), 548 current purely anxious cases (i.e., diagnosis of generalized anxiety disorder, social anxiety disorder, panic disorder and/or agoraphobia within 1 month prior to interview, and no depressive disorder), 531 current comorbid cases (i.e., diagnoses of both a depressive disorder and anxiety disorder within 1 month prior to interview), 897 remitted individuals (i.e., lifetime—but no current—diagnosis of anxiety and/or depressive disorder), and 634 healthy controls (i.e., no lifetime psychiatric diagnosis). Among subjects with current anxiety disorders, there were 390 diagnoses of GAD, 540 of social anxiety disorder, 497 of agoraphobia, and 500 of panic disorder, many of which overlapping.
2.2. Metabolomic biomarkers measurement
Plasma samples were obtained during the baseline interview in the morning (generally between 8.30 and 9.30 am) after an overnight fast, stored in the ethylenediaminetetraacetic acid (EDTA) detergent, and kept frozen at −80°C till assaying. Plasma samples were shipped in two batches (April and December 2014, further referred to as batch 1 and 2, respectively). Metabolomic profiles were measured using a proton nuclear magnetic resonance (NMR) platform (Nightingale Health Ltd., Helsinki, Finland).34 The Nightingale Health NMR metabolomics platform quantified 231 levels of metabolites and ratios. For the current analyses, we selected a total of 51 lipids, fatty acids, and low molecular weight metabolites, containing eight amino acids, two apolipoproteins, nine cholesterol measures, eight fatty acids, two fluid balance‐related measures, nine glycerides and phospholipids, three glycolysis‐related metabolites, one inflammation‐related metabolite, three ketone bodies, three lipoprotein particle sizes, and three total fatty acids and saturation measures, as classified by Nightingale Health Ltd. The NMR platform includes additional sub‐measures and ratios of these lipoprotein (i.e., 98 lipid composition and particle concentration measures of lipoprotein subclasses and 81 lipid and fatty acids ratios) that were not included in the current analyses because of redundancy of information. Table S1 presents lists of these 51 metabolites and the category to which these belong, as defined by Nightingale Health Ltd. The raw metabolite variables were prepared for analyses according to a standardized protocol suggested by the manufacturer and followed in earlier studies35: A value of 1 was added to each value, after which we applied a natural log transformation. Values varying >5 SD from the mean were set as missing.
Next, we examined whether metabolites could be sensitive for a batch effect. Metabolomics assessments were performed using the same platform in two separate batches. Samples in the two batches were drawn from subjects with slightly different proportions of diagnoses (with current comorbid and current depressed cases being more likely to be measured in batch 1 and remitted cases and current anxious cases being more likely to be measured in batch 2, see Table 1). The unequal distribution of diagnostic groups across batch can potentially induce spurious results or bias because of batch effect on the estimates of analyses examining differences in metabolites concentrations across diagnostic groups. We therefore determined which metabolites were potentially batch‐sensitive. As a substantial number of healthy controls were assessed in both batches (batch 1: n = 396, batch 2: n = 238), we examined which metabolites could vary because of a batch effect within controls by fitting linear models for each metabolite separately, with batch (second vs first) as the predictor while adjusting for sex, age, anxiety severity (as indexed by Beck Anxiety Inventory), and depression severity (as indexed by Inventory of Depressive Symptomatology) and corrected for multiple testing based on the Benjamini–Hochberg procedure36 (see Table S2 for results). We identified 11 metabolites that differed significantly at a false discovery rate (FDR) <5% between the two batches potentially sensitive for batch effect and excluded these from the main analyses. Thus, in the main text, we mainly report results for the remaining 40 metabolites, but for completeness analyses of the 11 batch‐sensitive metabolites are reported in the Supplement.
TABLE 1.
Sociodemographic and clinical sample characteristics (N = 2914)
| Healthy controls | Remitted depressive or anxiety disorder | Current pure anxiety disorder | Current pure depressive disorder | Current comorbid depressive and anxiety disorder | p | |
|---|---|---|---|---|---|---|
| N | 634 | 897 | 548 | 304 | 531 | |
| Sociodemographic variables | ||||||
| Age, mean (sd) | 41.3 (14.6) | 42.5 (13.0) | 41.6 (12.6) | 42.3 (12.5) | 41.9 (11.8) | 0.48 |
| Female, % | 61.8 | 69.7 | 67.0 | 64.1 | 67.4 | 0.02 |
| Years of education, mean (sd) | 12.8 (3.2) | 12.5 (3.2) | 12.1 (3.2) | 11.8 (3.2) | 11.0 (3.2) | <0.001 |
| Lifestyle and somatic health variables | ||||||
| BMI, mean (sd) | 25.1 (4.6) | 25.6 (4.8) | 25.2 (4.9) | 26.1 (5.2) | 26.4 (5.6) | <0.001 |
| Current smoker, % | 26.5 | 37.9 | 42.2 | 39.1 | 50.5 | <0.001 |
| Glasses of alcohol consumed/week, mean (sd) | 7.5 (9.5) | 6.8 (9.1) | 6.8 (10.0) | 6.8 (1.6) | 7.2 (12.0) | 0.70 |
| Total kiloMET‐minutes/week, mean (sd) | 4.9 (5.5) | 4.9 (5.3) | 4.9 (5.4) | 4.8 (6.8) | 4.3 (5.5) | 0.39 |
| Number of chronic somatic diseases, mean (sd)a | 0.7 (1.0) | 0.8 (1.0) | 0.9 (1.1) | 1.0 (1.2) | 1.1 (1.2) | <0.001 |
| Lipid lowering drug use, %b | 6.3 | 6.4 | 7.5 | 6.9 | 8.7 | 0.48 |
| Clinical characteristics of anxiety | ||||||
| BAI, mean (sd) | 4.0 (4.9) | 8.1 (6.9) | 16.1 (9.9) | 14.9 (9.6) | 22.9 (1.9) | <0.001 |
| FQ, mean (sd) | 12.0 (12.1) | 18.6 (14.7) | 33.5 (18.7) | 25.6 (18.0) | 41.5 (21.9) | <0.001 |
| PSWQ, mean (sd) | 20.5 (8.7) | 28.4 (10.1) | 34.9 (10.0) | 36.2 (9.9) | 41.4 (9.0) | <0.001 |
| Duration of anxious symptoms in percentage of time, mean (sd) | NA | 23.8 (25.3) | 46.4 (35.8) | 32.0 (28.7) | 56.4 (32.7) | NA |
| Generalized anxiety disorder, %c | 0.0 | 0.0 | 22.4 | 0.0 | 50.3 | NA |
| Social anxiety disorder, %c | 0.0 | 0.0 | 48.7 | 0.0 | 51.4 | NA |
| Panic disorder, %c | 0.0 | 0.0 | 45.4 | 0.0 | 47.3 | NA |
| Agoraphobia, %c | 0.0 | 0.0 | 48.9 | 0.0 | 43.1 | NA |
| Clinical characteristics of depression | ||||||
| IDS, mean (sd) | 8.6 (7.5) | 16.2 (9.7) | 23.8 (1.2) | 31.7 (1.6) | 37.8 (1.9) | <0.001 |
| Duration of depressive symptoms in percentage of time, mean (sd) | NA | 18.9 (19.8) | 25.1 (24.4) | 35.6 (28.4) | 48.2 (31.4) | NA |
| Blood sampling variables | ||||||
| Overnight fasting at time of blood draw, % | 97.8 | 94.1 | 95.6 | 95.7 | 94.4 | 0.01 |
| Assessed in batch 1, % | 62.5 | 24.9 | 33.2 | 93.4 | 94.7 | <0.001 |
p values are obtained from analyses of variance and chi‐square tests.
Includes somatic diseases including heart disease and diabetes, thyroid gland disease, arthritis or arthrosis, rheumatism, intestinal disorders, liver disease, cancer, neurological conditions, and allergies.
Consists of statins, fibrates, and nicotinic acids.
Diagnosed within one month prior to interview. Abbreviations: BAI, Beck Anxiety Inventory; FQ, Fear Questionnaire; IDS, Inventory of Depressive Symptomatology; MET, Metabolic Equivalent of Task; NA, not applicable; PSWQ, Penn State Worry Questionnaire. NA indicates a missing value because of missing data or because there was no test performed.
2.3. Clinical characteristics of depression and anxiety
Three different anxiety severity scores were assessed using three self‐report questionnaires. First, the Beck Anxiety Inventory (BAI) reflects the severity of anxious arousal symptoms as common in panic and generalized anxiety disorders, ranging from 0 (not severe) to 63 (severe).37 Second, the 11‐item Penn State Worry Questionnaire (PSWQ) on worry engagement was used to measure pathological worry.38 Sum score ranges from 11 (“not typical of me”) to 55 (“very typical of me”). Third, the Fear Questionnaire (FQ) was used to measure avoidance of situations because of phobia of blood/injuries, social phobia, and agoraphobia, resulting in a sum score of these three subscales ranging from 0 (no avoidance) to 120 (severe avoidance).39 Depression severity was assessed with the Inventory Depressive Symptomatology (IDS).40 This 30‐item questionnaire assesses the presence of all symptom domains of a major depressive episode in the past seven days ranging from 0 (not severe) to 84 (severe). For both depression and anxiety disorders, measures on duration (course) of psychopathology were assessed. The Life Chart Interview was used to determine the percentage of time in which symptoms relevant for the disorder were experienced in the past four years.41
2.4. Covariates
Sex, age, education level (continuous, in years), BMI (in kg/m2), and current smoking status (yes vs no) were assessed as part of the baseline interview in NESDA. Level of physical activity was calculated using the total Metabolic Equivalent of Task (MET) score derived from the International Physical Activity Questionnaire (IPAQ).42 The number of alcoholic drinks consumed per week was calculated based on the Alcohol Use Disorders Identification Test.43 The number of self‐reported chronic somatic diseases included heart diseases, diabetes mellitus, thyroid gland disease, arthritis or arthrosis, rheumatism, intestinal disorders, liver disease, cancer, neurological conditions and allergies. Medication use was based on drug container inspection of all medications used in the past month, classified according to the World Health Organization Anatomical Therapeutic Chemical (ATC) classification.44 The use of treatment for elevated lipid levels and reduced HDL cholesterol levels (ATC: C10) were documented. Use of antidepressant medication was assessed. We distinguished three classes of antidepressants: selective serotonin reuptake inhibitors (SSRI; ATC: N06AB), tricyclic antidepressants (TCA; ATC: N06AA), and other antidepressants (ATC: N06AX). Finally, overnight fasting status (yes/no) at time of blood withdrawal was documented.
2.5. Statistical analyses
Differences in sociodemographic and clinical variables across diagnostic groups were examined with χ2‐tests and analyses of variance (ANOVAs). All subsequent models were adjusted for the covariates age, sex, current smoking status, fasting status, years of education, number of somatic diseases, and BMI. For our first research aim, we first examined whether metabolomic biomarkers differed across diagnostic groups (healthy controls, remitted, current purely anxious, current purely depressed, and current comorbid cases) in analyses of covariance (ANCOVAs), with metabolite as the outcome and diagnostic group (as categorical variable, a five‐level factor) as the independent variable. We corrected for multiple testing across the 40 metabolites using the Benjamini–Hochberg procedure36 and considered an FDR of 5% as significant. Next, for the metabolomic markers identified as significantly different across diagnostic groups, we performed two additional analyses. First, to examine which of the patient groups exactly differed from controls (remitted, current purely anxious, current purely depressed, and current comorbid groups), we performed linear regression models, with metabolite as the outcome and used contrasts of diagnostic group (as categorical variable, a five‐level factor, with healthy controls as the reference group). Second, we broke down the heterogenous group of anxiety disorders according to specific diagnoses, to test their potential differential associations with metabolites. For this, within current anxious cases (current comorbid and current purely anxious groups combined, N = 1079), one linear model per metabolomic marker was fit, with each variable that specified the presence of current diagnoses (yes vs no) of GAD, social anxiety disorder, panic disorder and agoraphobia as independent, and metabolomic marker as dependent variables. Considering the high comorbidity rates between the anxiety disorders, we inspected the level of multicollinearity by calculating the variance inflation factor (VIF) for each independent variable in all models and considered 10 as a threshold for high multicollinearity.
For our second research aim, we examined whether major clinical characteristics of depression and anxiety were associated with the 40 metabolomic markers by performing a linear regression for each metabolite–characteristic combination, with metabolites as dependent and clinical characteristics as independent variables. Associations between metabolites and severity scores of anxiety and depression (i.e., BAI, FQ, PSWQ, and IDS scores) were examined within the whole sample (N = 2914) and duration of anxious symptoms and duration of depressive symptoms were examined within current anxious (N = 1079) and depressed cases (N = 835), respectively. Again, multiple testing correction was based on the Benjamini–Hochberg procedure.36 All statistical analyses were conducted with the use of R software version 3.6.0.45 Figures were produced using the packages forestplot, ggdendro, ggplot2, and ggrepel.46, 47, 48, 49
3. RESULTS
Table 1 presents sociodemographic, clinical, and metabolomic assessment characteristics of remitted, current purely depressed, current purely anxious, current comorbid, and healthy control groups. Compared to healthy controls, cases were more often female, current smokers, lower educated and had a higher average BMI and number of chronic somatic diseases. As expected, a trend of increasing scores of anxiety (i.e., BAI, FQ, and PSWQ) and depression severity (i.e., IDS) were seen across diagnostic groups, with current comorbid patients having the highest severity, followed by the groups with one current diagnosis. Among anxiety disorders, GAD had the highest comorbidity with depression (68.5%), followed by social anxiety disorder (50.6%), agoraphobia (50.2%), and panic disorder (46.1%).
3.1. Metabolomic markers across diagnostic groups
ANCOVAs were run to detect differences in metabolomic markers across diagnostic groups while adjusting for the covariates (i.e., age, sex, smoking status, fasting status, years of education, number of somatic diseases, and BMI). Of the forty examined markers, 13 differed significantly (FDR q < 0.05) across groups including the inflammatory marker glycoprotein acetyls (Gp, p = 1.15*10−16), two fatty acids measures (docosahexaenoic acid; DHA, p = 6.59*10−8 and total ω‐3 fatty acids, p = 7.29*10−7), three glycerides and phospholipids (serum total triglycerides, p = 2.04*10−7; triglycerides in VLDL, p = 9.02*10−7 and total phosphoglycerides, p = 8.82*10−3), one apolipoprotein (apolipoprotein B; ApoB, p = 3.19*10−5), one lipoprotein particle size (mean diameter for VLDL particles, p = 3.34*10−5), two cholesterol measures (VLDL cholesterol, p = 1.23*10−4 and remnant cholesterol, p = 3.64*10−3), one glycolysis‐related metabolite (glucose, p = 1.73*10−4), one ketone body (acetoacetate, p = 2.62*10−3), and one measure of total fatty acids and saturation (estimated degree of unsaturation, p = 6.65*10−3). Table 2 presents for each diagnostic group, the adjusted means of the 13 metabolites that differed across groups and the overall significance level for testing differences across groups. Table S3 presents results for all of the forty tested metabolites.
TABLE 2.
Adjusted means of metabolites that differed across diagnostic groups (at q<.05) in ANCOVAs
| Metabolites | Healthy controls | Remitted depressive or anxiety disorder | Current pure anxiety disorder | Current pure depressive disorder | Current comorbid depressive and anxiety disorder | p (ANCOVA) |
|---|---|---|---|---|---|---|
| Name (units) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| Glycoprotein acetyls (mmol/L) | 1.34 (1.33–1.36) | 1.33 (1.32–1.35) | 1.34 (1.32–1.36) | 1.38 (1.36–1.41) | 1.37 (1.35–1.39) | 1.15E−16 |
| Docosahexaenoic acid, 22:6 (mmol/L) | 0.14 (0.13–0.14) | 0.14 (0.13–0.14) | 0.14 (0.13–0.14) | 0.12 (0.12–0.13) | 0.12 (0.12–0.13) | 6.59E−08 |
| Serum total triglycerides (mmol/L) | 1.21 (1.16–1.27) | 1.18 (1.13–1.23) | 1.19 (1.13–1.24) | 1.28 (1.21–1.35) | 1.23 (1.18–1.29) | 2.04E−07 |
| Omega‐3 fatty acids (mmol/L) | 0.38 (0.37–0.40) | 0.38 (0.37–0.39) | 0.39 (0.37–0.40) | 0.36 (0.35–0.38) | 0.36 (0.35–0.37) | 7.29E−07 |
| Triglycerides in VLDL (mmol/L) | 0.85 (0.81–0.90) | 0.84 (0.79–0.88) | 0.84 (0.80–0.89) | 0.91 (0.85–0.97) | 0.87 (0.82–0.92) | 9.02E−07 |
| Apolipoprotein B (g/L) | 0.95 (0.93–0.98) | 0.95 (0.93–0.97) | 0.95 (0.93–0.97) | 0.98 (0.96–1.01) | 0.97 (0.95–0.99) | 3.19E−05 |
| Mean diameter for VLDL particles (nm) | 36.59 (36.46–36.72) | 36.56 (36.44–36.67) | 36.61 (36.47–36.74) | 36.73 (36.57–36.88) | 36.63 (36.50–36.76) | 3.34E−05 |
| VLDL cholesterol (mmol/L) | 0.86 (0.83–0.88) | 0.87 (0.84–0.89) | 0.86 (0.83–0.89) | 0.89 (0.86–0.93) | 0.87 (0.85–0.90) | 1.23E−04 |
| Glucose (mmol/L) | 4.35 (4.28–4.42) | 4.27 (4.21–4.33) | 4.32 (4.26–4.39) | 4.36 (4.28–4.44) | 4.35 (4.28–4.42) | 1.73E−04 |
| Acetoacetate (mmol/L) | 0.04 (0.04–0.05) | 0.04 (0.03–0.04) | 0.04 (0.04–0.04) | 0.04 (0.04–0.05) | 0.04 (0.04–0.05) | 2.62E−03 |
| Remnant cholesterol (mmol/L) | 1.57 (1.53–1.61) | 1.59 (1.55–1.63) | 1.58 (1.54–1.62) | 1.62 (1.57–1.67) | 1.60 (1.56–1.64) | 3.64E−03 |
| Estimated degree of unsaturation | 1.22 (1.21–1.23) | 1.22 (1.21–1.22) | 1.22 (1.21–1.23) | 1.22 (1.21–1.23) | 1.22 (1.21–1.22) | 6.65E−03 |
| Total phosphoglycerides (mmol/L) | 1.87 (1.83–1.91) | 1.89 (1.86–1.93) | 1.88 (1.84–1.92) | 1.85 (1.80–1.90) | 1.85 (1.81–1.89) | 8.82E−03 |
Means of the 13 metabolites variables that differed across groups at a significance level of false discovery rate <5% in ANCOVAs. The second to fifth column contain means adjusted for age, sex, smoking status, fasting status, years of education, number of somatic diseases, and BMI. The rightest column contains the raw p values obtained by ANCOVAs. Results of all forty tested metabolites can be found in Table S3.
3.2. Metabolomics marker comparisons between depressed and anxious groups and controls
Subsequent linear regression analyses compared the levels of these 13 metabolites in healthy controls vs. each diagnostic group, including the same covariates. As compared to controls, the highest number of significantly different markers was found for the current purely depressed group (n = 7), less were found for the current comorbid (n = 3) and remitted groups (n = 2), and none were found for the current purely anxious group. A graphical representation of the regression coefficients from these analyses is provided by the forest plot in Figure 1. Table S4 lists the regression coefficients, standard errors and p values of these analyses, and Cohen's d reflecting differences between healthy controls and each disorder group. Compared to the healthy control group, the current purely depressed group had lower levels of DHA (Cohen's d = −0.13, p = 6.85*10−4) and ω‐3 PUFA (Cohen's d = −0.10, p = 9.55*10−3), and higher levels of Gp (Cohen's d = 0.12, p = 1.58*10−3), ApoB (Cohen's d = 0.08, p = 3.45*10−2), VLDL cholesterol (Cohen's d = 0.08, p = 3.91*10−2), serum total triglycerides (Cohen's d = 0.12, p = 4.57*10−2), and triglycerides in VLDL (Cohen's d = 0.07, p = 4.67*10−2). The current comorbid group exposed altered levels of three metabolites, namely lower levels of DHA (Cohen's d = −0.15, p = 3.42*10−5), ω‐3 PUFA (Cohen's d = −0.14, p = 2.60*10−4), and higher Gp (Cohen's d = 0.08, p = 2.70*10−2), which were all also found to be related to the pure depression group. Figures S1–S3 present plots comparing the association estimates of the 13 metabolites for the three current affected groups vs controls. These plots showed a higher consistency between estimates for current comorbid status and a current purely depressed status (r = 0.98, 95%CI: 0.94; 1.00, p < 0.001), while low consistencies were observed between estimates of the current purely anxious group with those of the current comorbid group (r = −0.19, 95%CI: −0.67; 0.40, p = 0.54) and those of the current purely depressed group (r = −0.18, 95%CI: −0.67; 0.41, p = 0.55). Finally, the remitted group was associated with decreased values of two metabolites, namely acetoacetate (Cohen's d = −0.10, p = 1.54*10−2) and glucose (Cohen's d = −0.09, p = 1.07*10−2), which was not seen in the current affected groups. In summary, these group comparisons suggest that the current comorbid and current purely depressed group showed overlapping metabolomic profiles distinguishable from healthy controls, whereas the current purely anxious group and the remitted group were not substantially different from the controls.
FIGURE 1.
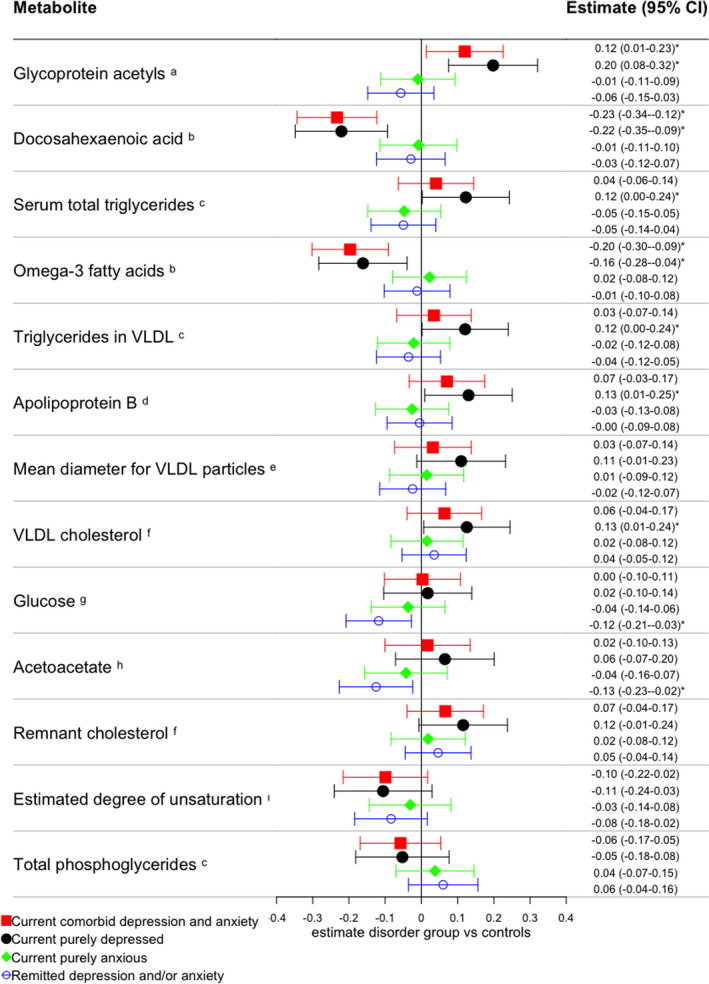
Comparisons between diagnosed groups and healthy controls of metabolites that differed across groups in ANCOVA. Forest plot presents estimated regression coefficients and 95% confidence intervals adjusted for age, sex, smoking status, fasting status, years of education, number of somatic diseases, and BMI, only for metabolites that were found to be significantly different across groups in ANCOVAs. Results were obtained from linear regressions in which group comparisons between each diagnosed group of depression and anxiety disorders and healthy controls were performed. *indicates that the disorder group differed from healthy controls at p < 0.05. The superscripts next to the metabolite indicate the metabolite category, as defined by Nightingale Health Ltd. aInflammation, bFatty acids, cglycerides and phospholipids, dapolipoproteins, elipoprotein and particle size, fcholesterol, gglycolysis related metabolites, hketone bodies, i total fatty acids and saturation measures. Metabolomic markers are ordered based on p values obtained from the ANCOVAs [Colour figure can be viewed at wileyonlinelibrary.com]
Tables S8 and S9 show results applied to the 11 metabolites with significantly different concentrations across batches. As compared to controls, several metabolites (i.e., histidine, sphingomyelins, phosphatidylcholine and other cholines, triglycerides in LDL, isoleucine, estimated description of fatty acid chain length, diacylglycerol, and mean diameter for LDL particles) were significantly lower in the remitted and pure anxiety group, while opposite effects in the purely depressed and comorbid groups. Although these findings could be affected by batch effects making interpretation less straightforward, they confirm again the division in metabolic profile in current depressed and comorbid cases from those with anxiety and remitted disorders.
3.3. Metabolomics marker comparisons across different types of anxiety disorders
In subsequent analyses focusing on 1079 subjects with current anxiety disorders, we examined whether specific diagnoses (i.e., GAD, social anxiety disorder, panic disorder, and agoraphobia) showed potential differential associations with the 13 metabolites that were previously identified. VIFs of the anxiety diagnosis variables in all 13 models were <1.5, indicating low levels of multicollinearity.50 The heatmap in Figure S4 presents estimated associations between each anxiety disorder diagnosis and the metabolites. Table S5 provides the regression coefficients, standard errors, and p values of these analyses. None of the specific diagnoses was associated with the metabolites after taking into account multiple testing. Of note, among the different disorders, the highest number of nominally significant associations was found for the GAD diagnosis, linked to metabolites (i.e., ApoB, VLDL cholesterol, remnant cholesterol, serum total triglycerides, and Gp) previously shown to be altered in the current purely depressed and current comorbid group. Table S10 contains estimated associations between each anxiety disorder diagnosis and the metabolites with significantly different concentrations across batches.
3.4. Clinical characteristics of depression and anxiety
We examined whether clinical characteristics of depression (i.e., IDS and duration of depressive symptoms) and anxiety (i.e., BAI, FQ, PSWQ, and duration of anxious symptoms) were associated with the 40 metabolomic markers, within the entire sample. The heatmaps in Figures 2 and 3 show the covariate‐adjusted standardized effect sizes, obtained from separate linear models regressing metabolomic markers on severity scores and duration measures. Tables S6 and S7 present results for all 40 metabolites (regression coefficients, standard errors, p and q values). Five metabolomic markers were significantly associated with IDS scores, two markers with FQ, one with BAI, and none with PSWQ. We did not identify markers associated with duration of anxious symptoms or duration of depressive symptoms. Positive associations were found between IDS and levels of Gp (β = 0.09, p = 7.43*10−7), ApoB (β = 0.05, p = 6.17*10−3), and mean diameter of HDL particles (β = 0.05, p = 1.11*10−3), and inverse associations were found with levels of DHA (β = −0.08, p = 1.74*10−5) and ω‐3‐PUFA (β = −0.07, p = 2.09*10−4). Except for the mean diameter of HDL particles, these markers were also found to be different in the depressed cases as compared to controls (Table S3), indicating consistency across findings. On the other hand, serum total triglycerides, triglycerides in VLDL and VLDL cholesterol were associated with depressive diagnostic status, whereas the associations between these metabolites and IDS did not reach the significance threshold. As opposed to depression severity, no metabolites were associated with duration of depressive symptoms after correction for multiple testing, suggesting that a more chronic course of depression did not contribute significantly to the metabolomic profile.
FIGURE 2.
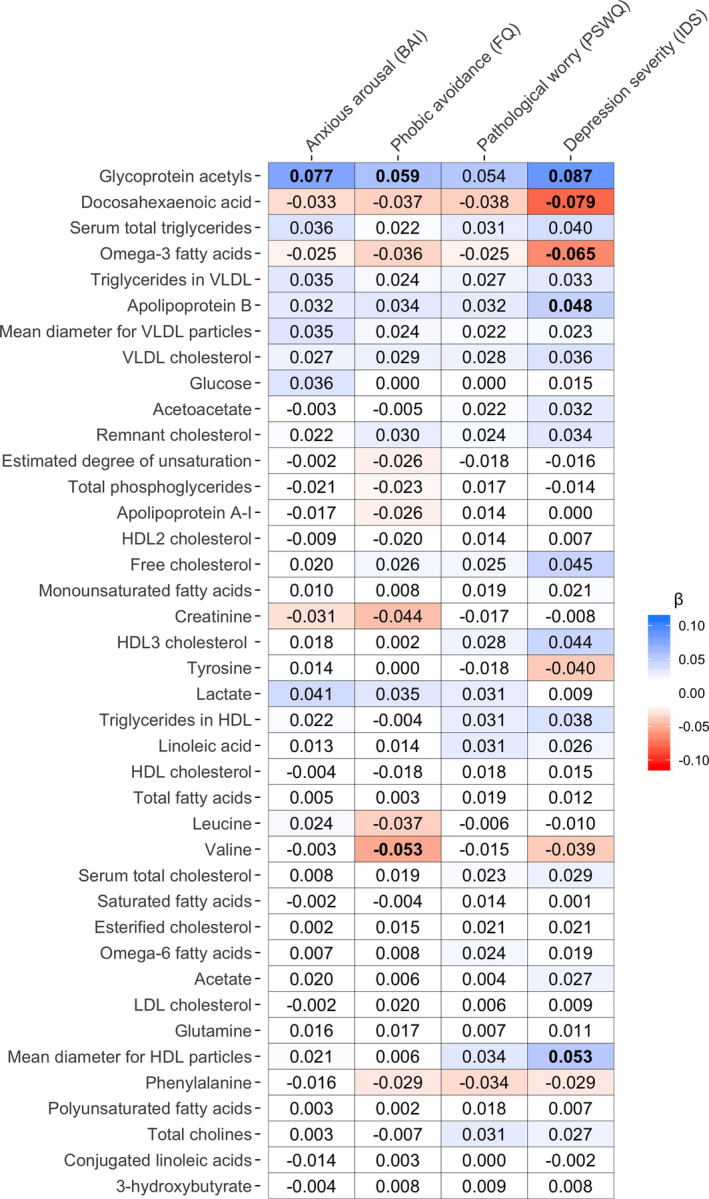
Heatmap of associations between metabolites and severity measures of anxiety and depression within entire sample. The heatmap shows the standardized effect sizes from linear models with severity scores of anxiety and depression as the predicting variable and metabolite variables as the outcome, including age, sex, education level, smoking status, fasting status, number of somatic diseases, and BMI as covariates. Bold values indicate significant associations at a false discovery rate <5%. Metabolomic markers are ordered based on p values obtained from the ANCOVAs [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
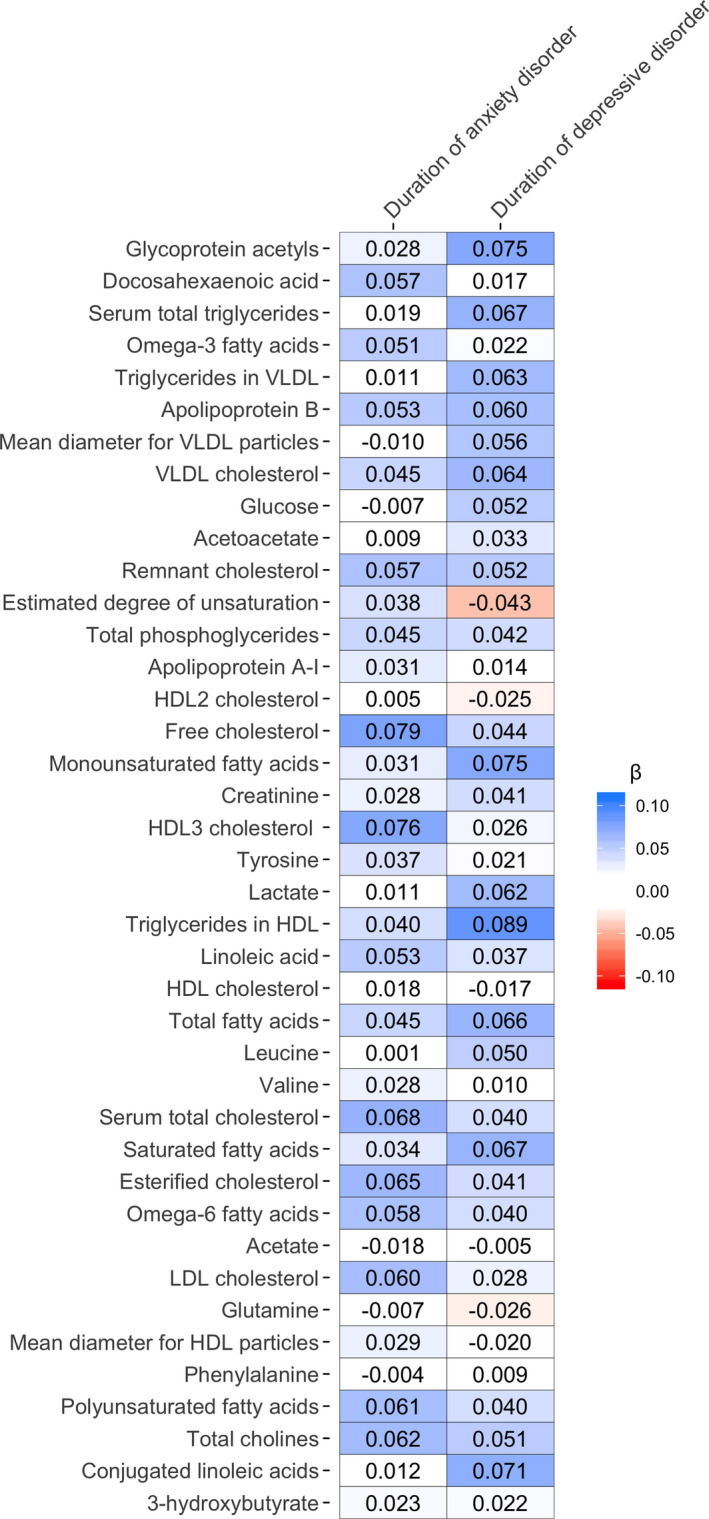
Heatmap of associations between metabolites and duration of symptoms of anxiety and depression within currently affected cases. The heatmap shows the standardized effect sizes obtained from linear models with duration of symptoms of anxiety and depression as the predicting variable and metabolite variables as the outcome, including age, sex, education level, smoking status, fasting status, number of somatic diseases, and BMI as covariates. Effect sizes of duration of anxious symptoms were calculated within cases with a current diagnosis of anxiety disorder (N = 1079), and effect sizes of duration of depressive symptoms were calculated within currently depressed cases (N = 835). No significant association (at a false discovery rate <0.05) was seen. Metabolomic markers are ordered based on p values obtained from the ANCOVAs [Colour figure can be viewed at wileyonlinelibrary.com]
The level of Gp (earlier linked to pure depression cases and comorbid cases) was found to be associated also with anxiety arousal severity (BAI: β = 0.08, p = 1.34*10−5) and with avoidance (FQ: β = 0.06, p = 6.45*10−4). Additionally, decreasing levels of valine were specifically associated with increasing levels of FQ (β = −0.05, p = 1.57*10−3).
Tables S11 and S12 present results for the potentially batch‐sensitive metabolites. Depression severity was significantly associated with nine metabolites, several of which showed consistent significant association with BAI (n = 6), followed by PSWQ (n = 4) and FQ (n = 3).
4. DISCUSSION
In a large cohort well‐characterized in terms of psychiatric status, the present study examined the association of circulating lipid‐related metabolomic markers and the presence of anxiety and depressive disorders and their clinical characteristics. The findings showed that alterations, reflecting an inflammatory and atherogenic metabolomic profile, were mainly related to the presence and severity of depression. These associations were more state‐like than trait‐like, as no associations with life‐chart duration were found. We did not identify metabolomic markers consistently associated with an anxiety disorder diagnosis nor with anxiety clinical features. The depression‐specific metabolomic profile we identified concerns increased glycoprotein acetyls and some measures related to the ApoB lipidome (i.e., measures of lipoprotein particles containing apolipoprotein B, namely chylomicrons, chylomicrons remnant, VLDL, IDL, and LDL51) and decreased ω‐3 fatty acids. These alterations in individual metabolites (i.e., glycoprotein acetyls, ApoB, and ω‐3 fatty acids) have been associated with increased inflammation and atherosclerosis.52, 53, 54, 55, 56, 57 Overall, despite large clinical overlap between depression and anxiety, we identified a metabolomic profile unique for depression.
The present findings support the hypothesis that the inflammatory and atherogenic metabolomic profile found is specific for depression's pathophysiology and not related to anxiety. This could be explained by several mechanisms, such as immunometabolic disturbances, CVD‐related risk factors, and lifestyle. Firstly, immunometabolic disturbances in the form of adiposity and inflammatory marker levels have been more prominently associated with depression than with anxiety,20, 21, 22, 23, 58, 59, 60 which was also confirmed before in NESDA.61, 62, 63 Mendelian randomization analyses suggested that increased BMI or body fat was unidirectionally causal for depression liability,10, 64, 65 whereas increased BMI has been causally associated with lower liabilities of exposing anxiety‐related phenotypes.66 As increased adiposity has been associated with a similar metabolomic pattern as found in this study,5, 67 and the metabolomic profile related to depression is suggested to have proinflammatory properties52, 53; it could be that the metabolomic profile identified in this study is part of the causal relationship between increased adiposity, inflammation, and depression. We partially adjusted for adiposity through inclusion of BMI as a covariate in all analyses, so BMI does not fully explain the depression‐specific metabolomic profile. However, it could still be that immunometabolic disturbances, among which adiposity, underlie the differences in metabolomic profiles between depression and anxiety. Secondly, it could be that the mechanisms that underlie the relationship of CVD with depression is partly different from those with anxiety, which explains our results. The depression‐specific profile identified here have properties promoting atherosclerosis, which is the main cause of CVD.53, 68 The atherosclerotic properties of the metabolomic profile could be of lower importance in the association between CVD and anxiety. Anxiety's component pathological worry69 has been particularly associated with adverse cardiac health, mainly by activation of the sympathetic nervous system causing decreased heart rate variability, increased levels of circulating catecholamines, and increased blood pressure.70 This explanation is corroborated by preliminary findings from a recent study that suggests that, despite the great overlap in genetic liabilities of depression and anxiety, genomic regions that were specific for depressive symptoms were enriched for gene sets related to hypertriglyceridemia and regions specific for anxiety symptoms were enriched for multiple gene sets related to blood pressure.11 Thirdly, the observed discrepancy in metabolomic profile between depression and anxiety could also be a result of differences in lifestyle. Both depression and anxiety are associated with unhealthy lifestyle habits, such as smoking, physical inactivity, and an unhealthy dietary pattern,71 which, in turn, have been associated with an inflammatory and atherogenic metabolomic profile as well.55, 72, 73, 74 However, it has been shown that an overall cluster of unhealthy lifestyle behaviors is more strongly associated with depression than with anxiety.75
It is unlikely that the discrepant metabolomic profile of depression and anxiety is explained by use of medication, such as antidepressants or lipid lowering drugs. In the present sample, antidepressants are used by the current purely depressed (37.8%) and current purely anxious (31.2%) groups in similar proportions. Earlier research shows that potential detrimental effects of antidepressants on dyslipidemia is evident mainly for tricyclic antidepressants,4, 27 but only 3% of NESDA respondents used these medications. Also the frequency of lipid lowering drug use was equal in the different diagnostic groups. So, it is unlikely that the use of these medications explain the systematic differences in the wider metabolomic profile observed in the present study between depression and anxiety.
Our results indicate that the metabolomic profile is predominantly linked to the current and not remitted state of depression, in other words more state‐ than trait‐like. The majority of metabolites associated with a continuous measure of depression severity were also associated with a current depression diagnosis and some metabolites being only associated to a current depression diagnosis. It may therefore be that the metabolomic alterations are especially present when the clinical threshold has reached. No markers were associated with the duration of depressive symptoms during the prior 4 years, suggesting that a more chronic course of depression did not contribute significantly to the metabolomic profile of current depressed individuals. This is in line with a previous study of NESDA, which did not find longitudinal associations between ω‐3‐PUFAs and depression.76 This study provided evidence for a cross‐sectional association between metabolites and depression. It may be worthwhile to monitor the (cardio‐)metabolic health of depressed individuals, whereas this may be of lesser importance for anxious individuals. Longitudinal studies may provide further insights on prospective associations between the metabolomic profile and future cardiometabolic health, and metabolites and depression risk in seemingly healthy individuals.
Previous publications utilizing NESDA’s metabolomics data focused on the association between two markers (i.e., ω‐3 and ω‐6 PUFA) with depression and anxiety,31, 76 and the association of depression (not anxiety) with metabolites in a harmonized meta‐analysis of nine cohorts, including NESDA.4 The current study extends these previous studies by investigating the differences between depression and anxiety and their clinical characteristics of a large set of metabolites.
Major limitations and strengths should be noted. The cross‐sectional nature precluded causal inference. We lacked information about dietary habits, which may impact on circulating metabolite levels.77, 78 Strengths of the current study were the naturalistic cohort design, the structured diagnostic assessment procedure, the large sample size with broad metabolomics profiling, and the variety of well‐characterized clinical characteristics of depression and anxiety.
To conclude, despite large clinical overlap, an inflammatory and atherogenic metabolomic profile may be uniquely associated with the presence of depression, and not with anxiety. The metabolomic profile could be a distinguishing feature for these highly correlated, but nonidentical emotional states.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/acps.13310.
Supporting information
Fig S1‐S4
Table S1‐S12
ACKNOWLEDGMENTS
The infrastructure for the NESDA study (www.nesda.nl) is funded through the Geestkracht program of the Netherlands Organisation for Health Research and Development (ZonMw, grant number 10‐000‐1002) and financial contributions by participating universities and mental health care organizations (VU University Medical Center, GGZ inGeest, Leiden University Medical Center, Leiden University, GGZ Rivierduinen, University Medical Center Groningen, University of Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Rob Giel Onderzoekscentrum).
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1.Wium‐Andersen MK, Wium‐Andersen IK, Prescott EIB, Overvad K, Jørgensen MB, Osler M. An attempt to explain the bidirectional association between ischaemic heart disease, stroke and depression: a cohort and meta‐analytic approach. Br J Psychiatry. 2019;1–8. 10.1192/bjp.2019.130. [DOI] [PubMed] [Google Scholar]
- 2.Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatric Psychiatry 2007;22(7):613‐626. [DOI] [PubMed] [Google Scholar]
- 3.Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BWJH. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. 2019;24(1):18‐33. 10.1038/s41380-018-0017-5. [DOI] [PubMed] [Google Scholar]
- 4.Bot M, Milaneschi Y, Al‐Shehri T, et al. Metabolomics profile in depression: a pooled analysis of 230 metabolic markers in 5283 cases with depression and 10,145 controls. Biol Psychiatry. 2020;87(5):409‐418. 10.1016/j.biopsych.2019.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Würtz P, Wang Q, Kangas AJ, et al. Metabolic signatures of adiposity in young adults: Mendelian randomization analysis and effects of weight change. PLoS Med. 2014;11(12):e1001765. 10.1371/journal.pmed.1001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes MV, Millwood IY, Kartsonaki C, et al. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J Am Coll Cardiol. 2018;71(6):620‐632. 10.1016/j.jacc.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufman J, Charney D. Comorbidity of mood and anxiety disorders. Depression Anxiety. 2000;12(S1):69‐76. [DOI] [PubMed] [Google Scholar]
- 8.Levey DF, Gelernter J, Polimanti R, et al. Reproducible genetic risk loci for anxiety: results from approximately 200,000 participants in the million veteran program. Am J Psychiatry. 2020;177(3):223‐232. 10.1176/appi.ajp.2019.19030256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otowa T, Hek K, Lee M, et al. Meta‐analysis of genome‐wide association studies of anxiety disorders. Mol Psychiatry. 2016;21(10):1391‐1399. 10.1038/mp.2015.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wray NR, Ripke S, Mattheisen M, et al. Genome‐wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50(5):668‐681. 10.1038/s41588-018-0090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorp JG, Campos AI, Grotzinger AD, et al. 2020. 10.1101/2020.04.08.20057653 [DOI]
- 12.Middeldorp CM, Cath DC, Van Dyck R, Boomsma DI. The co‐morbidity of anxiety and depression in the perspective of genetic epidemiology. A review of twin and family studies. Psychol Med. 2005;35(5):611‐624. 10.1017/s003329170400412x. [DOI] [PubMed] [Google Scholar]
- 13.Tully PJ, Harrison NJ, Cheung P, Cosh S. Anxiety and cardiovascular disease risk: a review. Curr Cardiol Rep. 2016;18(12):120. 10.1007/s11886-016-0800-3. [DOI] [PubMed] [Google Scholar]
- 14.Vogelzangs N, Seldenrijk A, Beekman AT, van Hout HP, de Jonge P, Penninx BW. Cardiovascular disease in persons with depressive and anxiety disorders. J Affect Disord. 2010;125(1–3):241‐248. 10.1016/j.jad.2010.02.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivanovs R, Kivite A, Ziedonis D, Mintale I, Vrublevska J, Rancans E. Association of depression and anxiety with cardiovascular co‐morbidity in a primary care population in Latvia: a cross‐sectional study. BMC Public Health. 2018;18(1):328. 10.1186/s12889-018-5238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallagher D, O'Regan C, Savva GM, Cronin H, Lawlor BA, Kenny RA. Depression, anxiety and cardiovascular disease: which symptoms are associated with increased risk in community dwelling older adults? J Affect Disord. 2012;142(1–3):132‐138. 10.1016/j.jad.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Batelaan NM, Seldenrijk A, Bot M, van Balkom AJ, Penninx BWJH. Anxiety and new onset of cardiovascular disease: critical review and meta‐analysis. Br J Psychiatry. 2016;208(3):223‐231. 10.1192/bjp.bp.114.156554. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta‐analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27(23):2763‐2774. 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 19.Momen NC, Plana‐Ripoll O, Agerbo E, et al. Association between mental disorders and subsequent medical conditions. N Engl J Med. 2020;382(18):1721‐1731. 10.1056/NEJMoa1915784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajan TM, Menon V. Psychiatric disorders and obesity: A review of association studies. J Postgrad Med. 2017;63(3):182‐190. 10.4103/jpgm.JPGM_712_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomes AP, Soares ALG, Menezes AMB, et al. Adiposity, depression and anxiety: interrelationship and possible mediators. Rev Saude Publica. 2019;53:103. 10.11606/S1518-8787.2019053001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickering RP, Goldstein RB, Hasin DS, et al. Temporal relationships between overweight and obesity and DSM‐IV substance use, mood, and anxiety disorders: results from a prospective study, the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2011;72(11):1494‐1502. 10.4088/JCP.10m06077gry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjørngaard JH, Carslake D, Lund Nilsen TI, et al. Association of body mass index with depression, anxiety and suicide‐an instrumental variable analysis of the HUNT study. PLoS One. 2015;10(7):e0131708. 10.1371/journal.pone.0131708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hildrum B, Mykletun A, Midthjell K, Ismail K, Dahl AA. No association of depression and anxiety with the metabolic syndrome: the Norwegian HUNT study. Acta Psychiatr Scand. 2009;120(1):14‐22. 10.1111/j.1600-0447.2008.01315.x. [DOI] [PubMed] [Google Scholar]
- 25.Papakostas GI, Öngür D, Iosifescu DV, Mischoulon D, Fava M. Cholesterol in mood and anxiety disorders: review of the literature and new hypotheses. Eur Neuropsychopharmacol. 2004;14(2):135‐142. 10.1016/s0924-977x(03)00099-3. [DOI] [PubMed] [Google Scholar]
- 26.Kinley DJ, Lowry H, Katz C, Jacobi F, Jassal DS, Sareen J. Depression and anxiety disorders and the link to physician diagnosed cardiac disease and metabolic risk factors. Gen Hosp Psychiatry. 2015;37(4):288‐293. 10.1016/j.genhosppsych.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 27.van Reedt Dortland AK, Giltay EJ, van Veen T, Zitman FG, Penninx BWJH. Metabolic syndrome abnormalities are associated with severity of anxiety and depression and with tricyclic antidepressant use. Acta Psychiatr Scand. 2010;122(1):30‐39. 10.1111/j.1600-0447.2010.01565.x. [DOI] [PubMed] [Google Scholar]
- 28.Glaus J, Vandeleur C, Gholam‐Rezaee M, et al. Atypical depression and alcohol misuse are related to the cardiovascular risk in the general population. Acta Psychiatr Scand. 2013;128(4):282‐293. 10.1111/acps.12057. [DOI] [PubMed] [Google Scholar]
- 29.Holt RI, Phillips DI, Jameson KA, et al. The relationship between depression, anxiety and cardiovascular disease: findings from the Hertfordshire Cohort Study. J Affect Disord. 2013;150(1):84‐90. 10.1016/j.jad.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross AC, Kaizer AM, Ryder JR, et al. Relationships of anxiety and depression with cardiovascular health in youth with normal weight to severe obesity. J Pediatr. 2018;199:85‐91. 10.1016/j.jpeds.2018.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thesing CS, Bot M, Milaneschi Y, Giltay EJ, Penninx B. Omega‐3 and omega‐6 fatty acid levels in depressive and anxiety disorders. Psychoneuroendocrinology. 2018;87:53‐62. 10.1016/j.psyneuen.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Penninx BWJH, Beekman AT, Smit JH, et al. The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. Int J Methods Psychiatric Res. 2008;17(3):121‐140. 10.1002/mpr.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization . Composite International Diagnostic Interview, Core Version 2.1: Interviewer’s manual. Sydney, Australia: World Health Organization; 1997. [Google Scholar]
- 34.Soininen P, Kangas AJ, Würtz P, Suna T, Ala‐Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet. 2015;8(1):192‐206. 10.1161/CIRCGENETICS.114.000216. [DOI] [PubMed] [Google Scholar]
- 35.Onderwater GLJ, Ligthart L, Bot M, et al. Large‐scale plasma metabolome analysis reveals alterations in HDL metabolism in migraine. Neurology. 2019;92(16):e1899‐e1911. 10.1212/WNL.0000000000007313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289‐300. [Google Scholar]
- 37.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893. [DOI] [PubMed] [Google Scholar]
- 38.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the penn state worry questionnaire. Behav Res Ther. 1990;28(6):487‐495. 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 39.Marks I, Mathews A. Brief standard self‐rating for phobic patients. Behav Res Ther. 1979;17(3):263‐267. [DOI] [PubMed] [Google Scholar]
- 40.Rush A, Gullion C, Basco M, Jarrett R, Trivedi M. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychol Med. 1996;26(3):477‐486. [DOI] [PubMed] [Google Scholar]
- 41.Lyketsos CG, Nestadt G, Cwi J, Heithoff K. The Life Chart Interview: A standardized method to describe the course of psychopathology. Int J Methods Psychiatric Res. 1994;4(3):143‐155. [Google Scholar]
- 42.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12‐country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381‐1395. 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 43.Saunders JB, Aasland OG, Babor TF, De la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption‐II. Addiction. 1993;88(6):791‐804. [DOI] [PubMed] [Google Scholar]
- 44.WHO Collaborating Centre for Drug Statistics Methodology . Guidelines for ATC classification and DDD assignment 2008. Oslo, Norway: WHO Collaborating Centre for Drug Statistics Methodology; 2007. [Google Scholar]
- 45.R: A language and environment for statistical computing . R Foundation for Statistical Computing. 2019. https://www.R‐project.org/ [Google Scholar]
- 46.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer‐Verlag; 2016. [Google Scholar]
- 47.ggrepel: Automatically Position Non‐Overlapping Text Labels with ‘ggplot2'. R package version 0.8.2. 2020. https://CRAN.R‐project.org/package=ggrepel [Google Scholar]
- 48.forestplot: Advanced Forest Plot Using ‘grid’ Graphics. R package version 1.10. 2020. https://CRAN.R‐project.org/package=forestplot [Google Scholar]
- 49.ggdendro: Create Dendrograms and Tree Diagrams Using ‘ggplot2'. R package version 0.1.21. 2020. https://CRAN.R‐project.org/package=ggdendro [Google Scholar]
- 50.Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol. 2010;1(1):3‐14. 10.1111/j.2041-210X.2009.00001.x. [DOI] [Google Scholar]
- 51.Berg JM, Tymoczko JL, Stryer L. Biochemistry, 5th edn. New York, NY: W. H. Freeman and Company; 2002. [Google Scholar]
- 52.Faraj M, Messier L, Bastard JP, et al. Apolipoprotein B: a predictor of inflammatory status in postmenopausal overweight and obese women. Diabetologia. 2006;49(7):1637‐1646. 10.1007/s00125-006-0259-7. [DOI] [PubMed] [Google Scholar]
- 53.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12(3):204‐212. 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 54.Connelly MA, Otvos JD, Shalaurova I, Playford MP, Mehta NN. GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk. J Transl Med. 2017;15(1):219. 10.1186/s12967-017-1321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuertes‐Martín R, Correig X, Vallvé JC, Amigó N. Human Serum/Plasma Glycoprotein Analysis by (1)H‐NMR, an emerging method of inflammatory assessment. J Clin Med. 2020;9(2). 10.3390/jcm9020354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shapiro MD, Fazio S. Apolipoprotein B‐containing lipoproteins and atherosclerotic cardiovascular disease. F1000Res. 2017;6:134. 10.12688/f1000research.9845.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calder PC. Omega‐3 fatty acids and inflammatory processes. Nutrients. 2010;2(3):355‐374. 10.3390/nu2030355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duivis HE, Vogelzangs N, Kupper N, de Jonge P, Penninx BWJH. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: findings from the Netherlands Study of Depression and Anxiety (NESDA). Psychoneuroendocrinology. 2013;38(9):1573‐1585. 10.1016/j.psyneuen.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Salim S, Chugh G, Asghar M. Inflammation in anxiety. Adv Protein Chem Struct Biol. 2012;88:1‐25. 10.1016/B978-0-12-398314-5.00001-5. [DOI] [PubMed] [Google Scholar]
- 60.Köhler CA, Freitas TH, Maes M, et al. Peripheral cytokine and chemokine alterations in depression: a meta‐analysis of 82 studies. Acta Psychiatr Scand. 2017;135(5):373‐387. 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- 61.de Wit LM, Fokkema M, van Straten A, Lamers F, Cuijpers P, Penninx BWJH. Depressive and anxiety disorders and the association with obesity, physical, and social activities. Depress Anxiety. 2010;27(11):1057‐1065. 10.1002/da.20738. [DOI] [PubMed] [Google Scholar]
- 62.Vogelzangs N, Beekman AT, de Jonge P, Penninx BW. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 2013;3:e249. 10.1038/tp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vogelzangs N, Duivis HE, Beekman AT, et al. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl Psychiatry. 2012;2:e79. 10.1038/tp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Speed MS, Jefsen OH, Børglum AD, Speed D, Østergaard SD. Investigating the association between body fat and depression via Mendelian randomization. Transl Psychiatry. 2019;9(1):184. 10.1038/s41398-019-0516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van den Broek N, Treur JL, Larsen JK, Verhagen M, Verweij KJH, Vink JM. Causal associations between body mass index and mental health: a Mendelian randomisation study. J Epidemiol Community Health. 2018;72(8):708‐710. 10.1136/jech-2017-210000. [DOI] [PubMed] [Google Scholar]
- 66.Millard LAC, Davies NM, Tilling K, Gaunt TR, Davey SG. Searching for the causal effects of body mass index in over 300 000 participants in UK Biobank, using Mendelian randomization. PLoS Genet. 2019;15(2):e1007951. 10.1371/journal.pgen.1007951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neeland IJ, Boone SC, Mook‐Kanamori DO, et al. Metabolomics profiling of visceral adipose tissue: results from MESA and the NEO Study. J Am Heart Assoc. 2019;8(9):e010810. 10.1161/JAHA.118.010810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Borén J, Williams KJ. The central role of arterial retention of cholesterol‐rich apolipoprotein‐B‐containing lipoproteins in the pathogenesis of atherosclerosis. Curr Opin Lipidol. 2016;27(5):473‐483. 10.1097/mol.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 69.Beard C, Millner AJ, Forgeard MJ, et al. Network analysis of depression and anxiety symptom relationships in a psychiatric sample. Psychol Med. 2016;46(16):3359‐3369. 10.1017/S0033291716002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huffman JC, Celano CM, Januzzi JL. The relationship between depression, anxiety, and cardiovascular outcomes in patients with acute coronary syndromes. Neuropsychiatr Dis Treat. 2010;6:123‐136. 10.2147/ndt.s6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saneei P, Esmaillzadeh A, Keshteli AH, et al. Combined healthy lifestyle is inversely associated with psychological disorders among adults. PLoS One. 2016;11(1):e0146888. 10.1371/journal.pone.0146888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pang Y, Kartsonaki C, Du H, et al. Physical activity, sedentary leisure time, circulating metabolic markers, and risk of major vascular diseases. Circ Genom Precis Med. 2019;12(9):386‐396. 10.1161/CIRCGEN.118.002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Slagter SN, van Vliet‐Ostaptchouk JV, Vonk JM, et al. Associations between smoking, components of metabolic syndrome and lipoprotein particle size. BMC Med. 2013;11:195. 10.1186/1741-7015-11-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kianoush S, Bittencourt MS, Lotufo PA, et al. Association between smoking and serum GlycA and High‐Sensitivity C‐Reactive Protein Levels: The Multi‐Ethnic Study of Atherosclerosis (MESA) and Brazilian Longitudinal Study of Adult Health (ELSA‐Brasil). J Am Heart Assoc. 2017;6(8): 10.1161/JAHA.117.006545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bonnet F, Irving K, Terra JL, Nony P, Berthezene F, Moulin P. Anxiety and depression are associated with unhealthy lifestyle in patients at risk of cardiovascular disease. Atherosclerosis. 2005;178(2):339‐344. 10.1016/j.atherosclerosis.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 76.Thesing CS, Bot M, Milaneschi Y, Giltay EJ, Penninx BWJH. Bidirectional longitudinal associations of omega‐3 polyunsaturated fatty acid plasma levels with depressive disorders. J Psychiatr Res. 2020;124:1‐8. 10.1016/j.jpsychires.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 77.Akbaraly T, Würtz P, Singh‐Manoux A, et al. Association of circulating metabolites with healthy diet and risk of cardiovascular disease: analysis of two cohort studies. Sci Rep. 2018;8(1):8620. 10.1038/s41598-018-26441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bogl LH, Pietiläinen KH, Rissanen A, et al. Association between habitual dietary intake and lipoprotein subclass profile in healthy young adults. Nutr Metab Cardiovasc Dis. 2013;23(11):1071‐1078. 10.1016/j.numecd.2012.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S4
Table S1‐S12
Data Availability Statement
Research data are not shared.


