Abstract
DNA damage is a constant stressor to the cell. Persistent damage to the DNA over time results in an increased risk of mutation and an accumulation of mutations with age. Loss of efficient DNA damage repair can lead to accelerated ageing phenotypes or an increased cancer risk, and the trade-off between cancer susceptibility and longevity is often driven by the cell's response to DNA damage. High levels of mutations in DNA repair mutants often leads to excessive cell death and stem cell exhaustion which may promote premature ageing. Stem cells themselves have distinct characteristics that enable them to retain low mutation rates. However, when mutations do arise, stem cell clonal expansion can also contribute to age-related tissue dysfunction as well as heightened cancer risk. In this review, we will highlight increasing DNA damage and mutation accumulation as hallmarks common to both ageing and cancer. We will propose that anti-ageing interventions might be cancer preventative and discuss the mechanisms through which they may act.
Keywords: Ageing, Cancer, Longevity, Lifespan, Healthspan, DNA damage, DNA repair, Stem cells, Chromatin, Mutation, Epigenetic
1. Types and sources of DNA damage
DNA is subject to constant assault, an estimated 70,000 lesions occur per day for a typical human cell [1]. This damage can originate from endogenous sources, such as reactive oxygen species (ROS), enzyme action and replication errors. Depurination, depyrimidination, single strand breaks (SSBs), 8-oxoG and cytosine deamination are the most common forms of DNA damage that arise spontaneously [1]. ROS causes direct modification to DNA bases by oxidation and results in conversion of guanine to 8oxoG. Depurination arises from spontaneous chemical reactions, typically hydrolysis, which breaks the labile glycosidic bonds between the DNA base and the deoxyribose creating an abasic site. These sites can result in mutations due to misincorporation by DNA polymerase or translesion DNA synthesis (TLS) [2]. Spontaneous, hydrolytic reactions such as deamination, can also cause mutations, for instance cytosine deamination converts it to uracil, while deaminated 5-methylcytosine becomes thymine. This process results in mutation accumulation over time and the mutational signature of 5-methyl-cytosine deamination shows a strong positive correlation with the age of when cancer is diagnosed [3]. During DNA replication the frequency of these spontaneous reactions increases due to the exposure of more vulnerable ssDNA [4]. Therefore, in scenarios where ssDNA is exposed for prolonged periods such as replication or transcriptional stress, the likelihood of damage occurring increases (see Fig. 1).
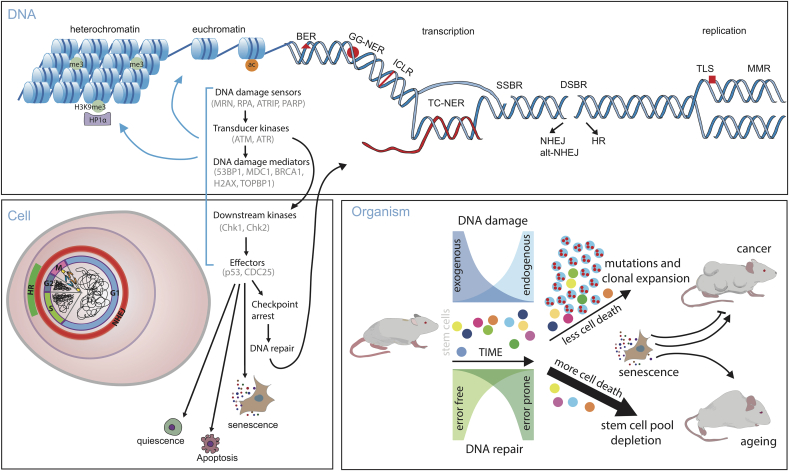
Fig. 1.
DNA damage and repair on DNA, cellular, and organismal level. DNA repair may differ if the damage is situated in the heterochromatin or euchromatin [5,6]. Heterochromatic regions have increased H3K9me3 mark, which is bound by HP1α (heterochromatin protein 1 alpha) [7]. Acetylation of histones in euchromatin increases chromatin availability [8]. Represented are different types of DNA repair, such as BER (base excision repair), GG-NER (global genomic nucleotide excision repair), ICLR (inter-strand crosslink repair), TC-NER (transcription coupled nucleotide excision repair), SSBR (single strand break repair), DSBR (double strand break repair), which can be repaired by HR (homologous recombination) or by either NHEJ (non-homologous end joining) or more mutagenic alt-NHEJ (alternative NHEJ) [9]. NHEJ is active throughout the cell cycle while HR is restricted to the late S and G2 phase. Replication errors are repaired by MMR (mismatch repair system) or are tolerated and bypassed by TLS (translesion synthesis repair). Transcription and replication make genome more vulnerable to damage and are associated with specialised types of repair. Upon DNA damage, damage sensors, such as PARP, mediate recruitment of transducer kinases ATM or ATR, whose activation leads to activation of DNA damage response to downstream proteins MDC1, BRCA1, 53BP1 and others. In presence of damage, ATM and ATR also activate Chk2 and Chk1, respectively [9]. CDC25 is one of the effector proteins that arrests cell cycle to allow damage repair [10]. p53 modifies transcription and thereby has a role in cell cycle arrest, apoptosis and senescence [11]. Mutations accumulate during ageing and are caused by different exposure to endogenous and exogenous factors, which produce DNA damage that can be repaired in error-free or error prone manner. Highly damaged cells are targeted for cell death, excess of which protects from cancer but depletes the stem cell pool and has pro-ageing effect [12,13]. Shown is clonal expansion of a mutated stem cell clone. If cell death is not induced in damaged and aberrant cells, then this increases chances clonal selection and expansion. Senescence is cancer protective but excess of prolonged senescence can promote both ageing and cancer via SASP (senescence associated secretory phenotype) [14].
Mitochondrial dysfunction and metabolic stress can result in increased production of reactive by-products such as ROS, lipid peroxides, oxidatively damaged proteins and aldehydes, of which lipid peroxidation may be particularly harmful due to its unique ability to propagate and amplify [15,16]. These reactive by-products, as well as endonuclease cleavage can cause endogenous SSBs and double strand breaks (DSBs) [16,17]. Another cause for SSBs and DSBs can be transcriptional stress due to abortive topoisomerase 2 action (TOP2CC cleavage complexes). The frequency of these events is increased in the presence of other lesions such as base cytotoxic modifications [18]. In addition, DNA damage lesions that are close in proximity, such as SSBs or closely spaced ongoing BER or NER on opposite strands, can further progress into DSB [19]. DSBs can also form when stalled replication forks collapse [20], further underlining how replication and transcriptional stress can contribute to endogenous DNA damage. The variety of causes for DSBs is important because, although this is the least frequent form of damage, DSBs are the most dangerous form of damage.
Multiple exogenous agents induce DNA damage, including UV light, ionising radiation, and chemical mutagens, such as polycyclic aromatic hydrocarbons present in tobacco smoke [[21], [22], [23]]. These agents cause chemical or physical modifications to the DNA often producing specific structures that are recognised by distinct repair enzymes. Interestingly, some cancer inducing chemicals do not seem to cause DNA mutations, but they induce selective constraint and expansion of existing clones instead, without generating specific mutation signatures [24]. Perhaps similarly, an “invisible” chemical or a metabolite, that does not leave fingerprints on DNA, could abet clonal expansion among hematopoietic stem cells (HSC) during ageing [25]. Another intriguing recent finding is that overexpression of some proteins, belonging to a variety of cellular functions, can also cause DNA damage and mutations [26].
Overall, DNA is continuously being damaged, often by unavoidable cellular metabolic processes. The challenge is to match a variety of different DNA lesions with the most adequate DNA repair enzymes and to coordinate the repair with other cellular activities.
2. How is DNA damage repaired? DNA damage repair pathways
Complex repair pathways have evolved to deal with the persistent problem of DNA damage [27]. They have been particularly well characterised in E. coli, yeast and mammals, and remarkable evolutionary conservation of repair enzymes have been observed between different organisms [28,9]. The repair pathway utilised depends on the type of damage incurred, the phase of the cell cycle and the availability of repair machinery. Below we describe DNA repair pathways, focusing mainly on mammalian systems.
Replication fidelity is preserved by the proofreading activity of polymerases [29], mismatch repair (MMR) [[30], [31], [32]], and regulation of the nucleotide pool's quality and quantity [33]. Lesions which block replicative DNA pol δ/ε can be bypassed using translational DNA synthesis (TLS) polymerases [[34], [35], [36]] or repaired by template switching (TS) [37,38]. Both TLS and TS, can be mutagenic and can be thought of as damage tolerance instead of repair.
Lesions that distort the DNA helix, such as bulky adducts or UV-light induced pyrimidine-dimers, are repaired by nucleotide-excision repair (NER). Lesions that do not alter the helical structure of DNA, such as 8-oxoG, uracil or an AP (apurinic/apyrimidinic) site, are repaired by base excision repair (BER). BER also acts upon SSBs. DSBs are repaired by homologous recombination (HR), non-homologous end joining (NHEJ) or alternative NHEJ (Alt-NHEJ), a microhomology-based pathway. Different repair pathways are predominant in different phases of the cell cycle, for example HR requires the presence of homologous or sister chromosomes and is therefore active during late S and G2 phase [39,40]. Pathway activity is determined by CDK activity which regulates the expression of the required repair factors. For example, CDK2 activity is required for the expression of CtIP which promotes end resection, a key step for the initiation of HR [41,42].
NHEJ is divided into classical NHEJ (c-NHEJ) and alternative NHEJ (Alt-NHEJ). HR is divided into gene conversion HR (GC-HR) and single strand annealing (SSA-HR) [42]. HR requires a plethora of repair enzymes and factors which are often shared with other repair pathways such as the Fanconi anaemia (FA) pathways [43] which repair interstrand cross links (ICLs) and template switching [44] which relies on the use of HR machinery e.g. BRCA2 and Rad51 [45].
The switch from NHEJ to HR is influenced by the complexity of the break site, by the availability of DNA repair components and the compaction of the chromatin [[46], [47], [48], [49]]. NHEJ is thought to be more erroneous than HR, however the cell repairs over 80% of DSBs by using NHEJ [46,[50], [51], [52]]. Even in G2 phase cells, NHEJ may be the first-choice pathway, as cells which lack DNA LigIV, a key component for NHEJ, not only exhibit a G1 phase repair defect but a G2 phase repair defect as well [46,48]. This may be due to the ease and speed of NHEJ. A subset of DSBs, around 15–20%, which cannot be repaired by NHEJ undergo end resection and repair by HR [46,53].
The choice between NHEJ and HR is also governed by levels of 53BP1. 53BP1 acts to restrain end resection whilst CtIP acts to promote it. However, 53BP1, along with its effector protein Shieldin [54,55] also determines whether HR is carried out by gene conversion (GC), typically thought of as an ‘error-free’ repair mechanism, or SSA which is a result of extensive end resection and is highly erroneous. In this way 53BP1 prevents excessive end resection and ensures the fidelity of HR [56]. Recent studies further indicate that HR can be mutagenic [40,57,58]. This is likely to be due to the involvement of translesion synthesis DNA polymerases such as Polζ [[59], [60], [61], [62]] and because the ssDNA generated in the repair process is more at risk of being damaged [63].
The repair of DNA must happen within the broader context of the nuclear landscape/architecture; therefore, chromatin dynamics and modifications are also key player in the repair. The DNA damage response invokes a large array of histone modifications, e.g. poly-ADP ribose (PAR) chains, γH2AX, H2A-Ubiquitination. These facilitate repair in multiple ways, for example, reducing local rate of transcription, opening up compacted chromatin regions or serving as recruitment or signalling platforms for repair enzymes/processes [56,[64], [65], [66], [67], [68]]. High levels of chromatin accessibility and transcription are associated with fewer base-pair substitutions perhaps due to more efficient MMR or TC-NER [[69], [70], [71]]. Mutation rate in euchromatic, early replicating regions of the DNA is reduced compared to late-replicating, heterochromatic regions [72]. This may be due to early regions having more time to detect and repair faults while late-replicating regions are often rich in repetitive sequences which are problematic for polymerases. Chromatin accessibly is not, however, a guarantee of more proficient repair, as despite the DNA being accessible in transcriptionally active regions, the presence of DNA-binding factors such as transcription factors can cause exclusion of repair proteins [73]. Transcriptionally active and accessible regions tend to accumulate genomic rearrangements [74] and mutational hot spots [75], including regulatory regions [76,77].
Heterochromatic DSBs are preferentially repaired by HR [48,49,78,79]. The co-localisation of γH2AX, 53BP1, and MDC1 is exclusive to areas of H3K9me3, a mark of condensed chromatin. This suggests the assembly of these factors is promoted in heterochromatic regions and may contribute to the preference of HR in heterochromatic regions [80]. Within heterochromatin, DSB repair may rely on a specific HR pathway that is dependent on ATM and involves Artemis, 53BP1, RNF168 and RNF8 [81]. Repair efficiency has also been correlated with the mobility of DSBs. Heterochromatic DSBs are often extruded to the periphery of the heterochromatic domain to undergo repair [5]. However, extrusion of DSBs also occurs for irreparable DSBs. They are pushed out to the nuclear periphery as a last resort to prevent interference with undamaged DNA [6]. Recently, heterochromatin, specifically H3K9me3 marks, have been associated with mechanosensing. Nuclear softening driven by loss of H3K9me3, protects the cell from DNA damage induced by mechanical stress [82].
The cell is equipped with a remarkable set of tools to repair DNA. However, it is essential that the least mutagenic repair complex gets priority access to its cognate lesion, and that these repair enzymes, capable of cutting, resecting, and ligating DNA, are firmly controlled to avoid mutations and chromosomal aberrations.
3. How does the cell respond to DNA damage? DNA damage detection, checkpoint arrest, and choice of cell fate
A key element in DNA repair is damage detection. Major detectors of single and double strand DNA breaks are PARP1 (poly(ADP-ribose) polymerase 1) and PARP2 (poly(ADP-ribose) polymerase 2) enzymes, which signal broken DNA by decorating adjacent histones with poly(ADP-ribose) chains, that at the same time relax chromatin and increase access of DNA repair proteins to the damage [66,83]. Often, PARP enzymes are aided by PARP complex accessory proteins, such as HPF1 (histone PARylation factor 1), which limits PARylation to serine residues, a typical post-translational modification of DNA repair proteins, rather than other residues such as glutamate and aspartate [[84], [85], [86], [87]]. PARylation is an early, brief event in DNA repair, which is terminated by removal of PAR chains by PARG and ARH3-mediated hydrolysis, once DDR factors are recruited to the lesion [66,83]. The importance of PARP enzymes in DNA repair is exploited in cancer therapy and PARP inhibitors have shown great success, specifically in HR-defective cancers [88,89]. A critical feature of PARP inhibitors is trapping PARP enzyme on broken DNA, which instead of initiating DNA repair generates further obstruction and damage and can lead to replication fork collapse. This creates an excess in the amount of substrates for recombination repair, which in HR-deficient cancer cells, such as BRCA1 mutated tumours, is limited to repair by lower-fidelity NHEJ, leading to chromosomal aberration such as radial chromosomes and selective death of tumour cells [88,89].
Another essential control of DNA damage response is brought about by phosphoinositide 3-kinase (PI3K)-related kinases: ATM, ATR and DNA-PKcs. These kinases are recruited to DNA breaks by their corresponding interacting proteins, for instance NSB1 from the Mre11/Rad50/NSB1 (MRN) complex recruits ATM double strand breaks. DSBs are also recognised by the Ku80 protein thereby aiding DNA-PKcs access to DNA damage. ATRIP, bound to RPA, is activating ATR kinase once replication forks are stalled [90]. These kinases provide a critical signalling cascade that orchestrates and activates a variety of DNA repair proteins that are specialised for given lesions [90].
The DNA repair process is helped by the cell cycle checkpoint arrest. The DNA damage response must be rapid and occurs before transition to the next phase of the cell cycle. Induction of checkpoint arrest relies on phosphorylation events such as ATR/ATM kinases acting to phosphorylate Chk1/2. Whereas maintenance of the checkpoint, whilst repair is occurring, relies on slower mechanisms involving transcription and expression of p53 and p21 [91].
Repair networks are brought back to homeostasis by the action of phosphatases, such as Wip1 and PP2A, to inactivate the DDR (DNA damage response). Depending on the level of DNA damage, several cycles or oscillations of effector proteins such as p53 [91] occur until the cell manages to repair the damage or a threshold is reached at which the cell commits to apoptosis, senescence or alternative cell death mechanisms due to prolonged activation of the DDR. The choice of cell fate, such as senescence, apoptosis, and quiescence, is governed by a combination of several factors, including the degree and type of damage, the activity of the p53-p21 axis, growth and survival signalling through PTEN-PI3K-AKT-mTOR and MAPK, the cell type, and the environment. The type and level of DNA damage, the efficiency of repair and the cellular response to this damage dictates whether an organism becomes more prone to cancer (by preserving mutated cell) or exhibits accelerated ageing phenotypes (by excessively eliminating cells with genomic aberations).
4. Somatic mutations are common characteristics of both cancer and ageing
Here we will present data showing how mutation accumulation is a common characteristic of both cancer and ageing [92,12], and some recent findings showing that non-tumorigenic normal tissue has surprisingly high mutation burden [93]. Cancer is a disease initiated and fuelled by genetic mutations, with multiple ‘hits’ required to malignantly transform a cell [93,94] and failure of DNA repair mechanisms may result in mutations [95]. In lung and skin cancers, mutation rates are dramatically increased by exposure to tobacco smoke and UV light, respectively [3,95,96]. Mutation rates in normal, somatic cells (B and T cells, fibroblasts, retinal and intestinal epithelium) is reported to be in the order of 2–10 mutations per cell division [97]. However, the incidence of cancer cannot be explained by this rate alone as the number of driver mutations generated would be insufficient to cause cancer. Instead, clonal expansion and hyper-mutation have been proposed to increase both the number of cells at risk and account for the discrepancy between mutation frequencies and cancer rates [98,99]. The predicted tissue-specific risk factor for cancer was proposed to be largely determined by stem cell endogenous replication error rates as opposed to exposure to exogenous factors [100,101], although there are other factors at play [102,103]. The exact number of driver mutations required to cause cancer is still unknown, and this may depend on the type of cancer and type of mutations acquired. Recent studies have shown that tobacco smoking, despite inducing a high mutational burden in the lung epithelial cells, leaves a population of quiescent cells which escape the high levels of DNA damage. These ‘protected’ cells go on to repopulate the lungs in those who stop smoking [104]. This highlights the cellular heterogeneity of mutation and how the selective process of regeneration can impact the mutational landscape of whole tissues over time.
Beyond cancer, accumulation of somatic mutations is thought to play a key role in ageing. Since mutations accumulate during ageing, this likely explains why ageing is the major risk factor for cancer [12,13]. The accumulation of somatic mutations in normal tissues is not well understood, they occur spontaneously throughout life and in a tissue-dependent manner. In the skin of the eyelid of normal, healthy persons, thousands of point mutations have been acquired by middle age and approximately 30% of cells have at least one driver mutation [105]. Daily exposure to sun light will have increased the number of mutations in the skin, however in the oesophagus, hundreds of clones are still present per square centimetre of tissue and these somatic mutations accumulate with age of the donor [106]. Recent single cell genome analysis of liver cells revealed the differentiated cells (hepatocytes) harbour higher levels of mutations accumulated with age compared to the adult stem cells [107], indicating certain cell populations, namely stem cells, are protected to an extent. This accumulation of mutations and clonal expansions in aged persons may contribute to the significant increase in cancer risk with age, from 2% risk at the age of 40, to a 50% risk by the age of 80 [93]. The accumulation of mutations and clonal expansion may lead to tissue dysfunction while changes in the tissue environment, such as inflammation, may drive further clonal selection and expansion [[108], [109], [110]]. While other unknown mechanisms which constrain clonal expansion may contribute to protection against cancer with age [111].
Overall, this common hallmark of cancer and ageing suggests that more crosstalk between these fields is urgently needed for better and faster understanding of the underlying causes of mutation accumulation.
5. Insights about cancer and ageing from DNA repair and growth signaling pathway mutants
Interesting insights about ageing and cancer could be gained by examining phenotypes of different DNA repair mutants, some of which are pro-ageing while others pro-cancer. In addition, in recent years there is evidence that down-regulation of growth pathway signalling can impact cancer and as well as ageing.
Several human disorders characterised by accelerated ageing are caused by deficiencies in DNA repair pathways. They often exhibit a high cancer incidence [112,113]. Examples include Werner and Bloom syndromes, both caused by mutations in the RecQ type helicases [114]. When modelled in yeast and mice, pro-ageing and pro-cancer phenotypes are observed as well as an increase in mutation frequency [[114], [115], [116], [117], [118]]. Pro-ageing and pro-cancer phenotypes are also seen in mice deficient in other key DNA damage repair proteins such as ATM [119] and p53 [120] suggesting that a functional DNA damage repair system is required for both cancer protection and longevity. Several studies in animal and cell models, in which the amount of DNA damage and mutations has been altered by deactivating or over-activing DNA repair genes, result in accelerated or decelerated ageing, respectively [12,13]. Overexpression of DNA repair genes in Drosophila including loki (Chk2), mei-41 (ATR) and WRN amongst others extend lifespan [[121], [122], [123], [124]]. It should be noted however that overexpression of DNA repair enzymes led to either longer or shorter lifespan, depending on target tissue, level of expression and sex of the animals tested, therefore more investigation is needed to understand how DNA repair can be enhanced [123]. Given that the DNA repair process requires that numerous signalling proteins and repair enzymes act in concert, it is challenging to enhance DNA repair by overexpressing a single enzyme. More promising, albeit more pleiotropic, would be to alter some of the upstream regulatory pathways. For instance, enhanced capacity for DNA repair is reported in the long-lived Ames and Snell dwarf mice, in which IIS is reduced [125,126]. Ames dwarf mice also exhibit delayed accumulation of spontaneous mutations as do mice which are subject to caloric restriction, a regime which reduces IIS/mTOR signalling and extends lifespan [127]. Furthermore, in Snell dwarf and growth hormone receptor knock-out (GHR-KO) mice the downregulation of TORC1 activity was linked to upregulation of several proteins involved in DNA repair [128]. These data suggest that the down-regulation of IIS/mTOR signalling may promote longevity through upregulation of DNA repair pathways resulting in reduced age-associated mutation accumulation. Reduced cancer incidence is observed in the long-lived GHR-KO, Ames dwarf and Snell dwarf mice [129,130] as well as in mice treated with rapamycin [131,132]. However, other long-lived models, such as S6K1−/− mice do not show any difference in tumour incidence compared with controls [133] but they do show reduced incidence of other age-related pathologies. In yeast deletion of the S6K homologue (Sch 9) reduces genomic instability with age [134] and further deletion of homologues for TOR and Ras combined with the Sch 9 deletion produces a four-fold extension in lifespan with reduced age-related mutational frequency and genomic instability [135]. These studies suggest there is a strong link between the lifespan extending mechanisms of reduced IIS/mTOR signalling and genomic stability in old age which may explain the reduced cancer incidence often observed in long-lived animal models. Long-lived mutants are often also healthier, nevertheless it is important for future potential translational approaches that health improvements and healthspan, or the period of good health of individual mutants is also carefully characterised as well as longevity [136].
Interestingly, mouse models of accelerated-ageing syndromes which exhibit high levels of DNA damage (NER deficiency) also show attenuated IGF-1 signalling [137]. Initially this was puzzling because if IGF-1 signalling was reduced, why was lifespan not extended in these mice? It is now thought that cells respond to DNA damage by decreasing IGF-1 signalling to re-direct resources from growth to maintenance, therefore reducing IGF-1 signalling may act to enhance DNA damage repair in normal, healthy cells. For progeria-like syndromes however or DNA repair mutants the re-direction of resources is not enough, the high levels of DNA damage due to the deficiency in DNA repair ultimately leads to cell death, stem-cell functional decline and accelerated ageing [113,137,138]. However, when placing these NER deficient mice on caloric restriction, further downregulating IGF-1 signalling, this significantly extended their lifespan, health-span and increased genomic stability [139].
Comparative studies of several mammalian species have revealed positive correlations between DNA repair efficiency and lifespan [[140], [141], [142], [143]]. The long-lived naked mole rat upregulates several genes involved in DNA damage repair resulting in more efficient base-excision repair (BER), mismatch repair (MMR), double strand break (DSB) repair and upregulation of the tumour suppressor gene, TP53, promoting cancer resistance [[144], [145], [146], [147], [148], [149]]. Other mechanisms proposed for the naked mole rats cancer resistance include secretion of high molecular-mass hyaluronan which renders cells hypersensitive to contact inhibition, and these cells stop proliferating upon only a few cell-cell contacts [150].
p53 is a key player in the DNA damage response. In mice constant over-activation of p53 (p53+/mut) results in protection from cancer, likely due to heightened cell death but at the expense of a shorter lifespan [151], suggesting there is a trade-off between longevity and cancer protection, and that cancer protection can only be achieved at the expense of shorter lifespan. However, cancer protection and a normal lifespan is seen in super-p53 mice, which has an extra copy of p53 driven by the native promoter providing enhanced DNA repair capacity but only when required [152]. Interestingly, it is only when super-p53 mice have an additional copy of the tumour suppressor p19Arf that lifespan extension is achieved alongside cancer protection demonstrating that an anti-ageing and anti-cancer phenotype can be achieved, despite multiple examples of this trade-off [153]. The trade-off between pro-ageing but cancer protective mechanisms and anti-ageing yet pro-cancer phenotypes is often observed [[13], [154]] and may be explained by differing responses to DNA damage. A response which leads to excessive apoptosis or senescence provides protection against mutation and therefore cancer, but at the cost of a pro-ageing phenotype due to stem cell pool depletion or accumulation of senescent cells. It should be noted that evidence for stem cell pool depletion is primarily observed in DNA repair mutant animals or in normal cells and animals that have been exposed to stressors or exogenous agents. The evidence regarding stem cells being depleted under the normal ageing process is scarce [[155], [156], [157]], and it is more likely that during normal ageing stem cell functional decline occurs [158].
On the other hand, a lack of cell death following DNA damage may promote longevity, but favours the accumulation of mutations and build-up of pre-malignant cells, resulting in elevated cancer risk. This trade-off phenomenon is exemplified by comparing Cockayne Syndrome and Xeroderma Pigmentosum (XP) both of which are a result of deficiencies in NER. Cockayne Syndrome is due to mutations in the CSA (Ercc8) or CSB (Ercc6) genes involved in the first steps of transcription-coupled repair (TC-NER). When cells with TC-NER deficiency sustain DNA damage they die due to transcriptional stress. This results in an accelerated ageing phenotype because the stem cell pool is depleted, but no cancer arises as the damaged cells are eliminated before they accrue mutations. In Xeroderma pigmentosum, cells are deficient in global-genome nucleotide excision repair (GG-NER) due to mutation of the XPC gene. TC-NER is still functional in these patients and promotes cell survival which delays premature ageing. However, due to the lack of GG-NER, lesions occurring in the non-transcribed genomic regions or in the template strand of active regions frequently result in mutations during replication. Therefore cancer incidence is high in XP patients [159].
In summary, defects in DNA damage repair can result in an accumulation of mutations or increased cell death, promoting cancer or ageing, respectively. These phenotypes lie at two ends of the spectrum with several possible intermediary phenotypes. In the context of translational medicine, the most interesting and relevant mutants are those displaying both cancer resistance and delayed ageing, for example the previously mentioned super-p53/Arf mice [153]. Another example is the long-lived C. elegans daf-2 (insulin receptor) mutant which shows resistance to lethal germ-line tumours caused by gld-1 mutation [160]. The mechanism behind such resistance is thought to be due to increased apoptosis, in which the daf-2 mutant background imposes a metabolic strain on the organism which results in selective apoptosis of the heavily, metabolically-demanding tumour cells but the surrounding normal tissue is not affected.
Growing evidence indicates cellular senescence occurring with age is a result of DNA damage accumulation [[161], [162], [163]]. Accumulation of senescent cells often results in an adverse senescence associated secretory phenotype (SASP) and can create a pro-tumorigenic environment [14]. The clearance of senescent cells extends lifespan and delays the onset of cancer [[164], [165], [166], [167], [168], [169], [170]].
During normal ageing the function and efficiency of several DNA repair pathways are thought to decline with age [171] including the p53 response [172]. A lack of efficient DNA damage repair combined with reduced apoptosis, due to an inefficient p53 response, may contribute to mutation accumulation with age. Overall, these studies demonstrate a functional DNA damage response is required to preserve genomic integrity which is essential for both longevity and cancer protection [173]. IIS/mTOR signalling regulates ageing and may contribute to some aspects of DNA damage repair and genomic stability. However, whilst it is expected that protection from DNA mutations will reduce cancer incidence, a careful balance is required between tumour suppression and maintenance of functional stem cell pool to ensure a long-life.
6. Stem cells in ageing and cancer
Stem cells play a critical part in renewing our tissues, but repeated cell divisions makes stem cells vulnerable to both transformation and cell death, depending on the type of mutations they accumulate [25]. Mutant clonal expansion and selection of stem cells occurs within the intestine and hematopoietic system with age [[174], [175], [176], [177], [178]]. In the intestine of humans and mice, this leads to clonal dominance of single ISCs within crypts [[179], [180], [181], [182]]. Similarly, in human skin, there is also evidence of clonal dominance and prevalence of clones bearing mutations in NOTCH1, NOTCH2, TP53 and FAT1 [157]. Intriguingly, NOTCH1's fitness advantage is restricted to the normal oesophageal epithelium because this mutation is not overrepresented in oesophageal cancer [106,183].
Most evidence for the role of ISCs in ageing comes from the fruit fly, Drosophila. ISCs are regularly interspersed throughout the Drosophila gut, unlike mammalian ISCs which are located in crypts. Damage to the fly gut stimulates ISC proliferation to replace dead or dying cells [[184], [185], [186], [187], [188]]. At a young age this is a transient effect, and the stem cells return to quiescence but in aged guts ISC over-proliferation and an increased mis-differentiation is observed [184,189,190]. Preserving proliferative homeostasis in the Drosophila gut extends lifespan and reduces the incidence of hyperplasia with age [187,191,192] highlighting the importance of ISC quiescence in maintaining tissue integrity with age. These studies did not report directly on ISC genomic integrity, but DNA damage and somatic mutations do accumulate with age in Drosophila [[193], [194], [195]]. Genomic aberrations arising in the ISCs can drive gut neoplasia and dysplasia [25,196,197]. Therefore Drosophila, like humans, also exhibits an increased cancer risk with age [198]. In the crypts of mammalian intestines, the LGR5+ ISCs are highly proliferative [199], therefore unlike in Drosophila, quiescence is not important to maintain tissue integrity with age. Instead Wnt signalling appears to be the dominant factor regulating survival and regenerative capacity of ISCs in response to both DNA damage and ageing [[200], [201], [202], [203]].
Replication stress is a key source of endogenous DNA damage contributing to both ageing and cancer progression, particularly when stem cells are affected. Ageing Haematopoietic Stem Cells (HSCs) are particularly vulnerable to replication stress, which has been attributed to reduced levels of mini-chromosome maintenance (MCM) helicase components [158,204,205]. The old quiescent HSCs have the DNA damage marker γH2AX primarily concentrated in the nucleolus, which houses the rDNA genes required for ribosome biogenesis. rDNA genes have several features, such as multiple repeat clusters and high transcription rates, that make them particularly vulnerable to DNA damage [206]. Dephosphorylation of γH2AX seems to be hampered in the old quiescent HSCs because of cytoplasmic mislocalisation of PP4c phosphatase, all of which may lead to a decrease in ribosomal biogenesis and therefore limits the functionality of aged HSCs and their ability to regenerate the blood-cell lineage [158,207]. Mutant HSC clones accumulate with age and contribute significantly to the age-related risk of leukaemia in humans [177,178,208,209].
HSCs enter quiescence when not actively required, however in this state they attenuate their DNA repair responses resulting in an accumulation DNA damage with age. It is only upon re-entry into the cell cycle that DNA repair occurs in these aged HSCs [210]. Whilst cycling, cells are more likely to experience mutations arising from replication errors [211], yet may benefit from HR of DSBs during the S and G2 phases. Quiescent cells, on the other hand, may rely on NHEJ, but evidence shows a preference for classical NHEJ and active suppression of the more erroneous alt-NHEJ [212] indicating an attempt to keep mutation rates low.
Replication stress can shorten lifespan in mice. Loss of MCM2 promotes premature ageing [213] consistent with observations in murine HSCs [158,207]. ATR deficient mice exhibit dramatically reduced regenerative capacity and accelerated ageing [204,205] which is reflected in the human disease, Seckel syndrome [214]. Replication stress is also observed in another accelerated ageing syndrome, Ruijs-Aalfs syndrome [215]. Here, loss of Spartan results in destabilized replication forks that is further aggravated by a lack of translesion synthesis [[216], [217], [218]].
The attrition of functional HSCs with age may underlie common age-related dysfunctions such as a poor immune system/response and poor wound healing/regenerative capacity. However, in C. elegans the loss of MCM2 actually extends lifespan [219] and in long-lived daf-2 mutants’, MCM2 expression is decreased compared to wild-types [220]. The differences between these studies on the relationship between MCM2 levels and ageing may be explained by the fact that C. elegans is largely a post mitotic organism, meaning they do not suffer from high levels of replication stress except in the germline. Alternatively, deficiency in one repair pathway may perhaps cause compensatory upregulation of a different repair pathway, leading to genome protection.
In summary, stem cells can accumulate DNA damage and mutations, leading to clonal selection and expansion, with age in a tissue dependent manner. Although stem cells may possess a level of inherent protection against mutations [104,107], on a population level, this may be contributed to by a low threshold for apoptosis due to increased expression of pro-apoptotic proteins as well as mitochondrial priming [221].
7. Chromatin status in ageing and cancer
The role of epigenetic modification in facilitating access to DNA damage and in having a more direct role in DNA repair, has been increasingly recognised [[222], [223], [224]]. Here we will describe the intricate relationship between epigenome and DNA repair which ultimately affects ageing and cancer.
Several changes to histone expression and methylation are observed upon ageing which may affect chromatin structure [225]. Ageing organisms and senescent cells exhibit reduced levels of repressive heterochromatic marks including H3K9me3, H3K27me3, and H4K20me3 [[226], [227], [228], [229]] and an overall global loss and redistribution heterochromatin is a characteristic feature of ageing [230,231].
Levels of heterochromatin protein 1 (HP1α) are diminished in aged human cells and prematurely aged cells [232,233]. The level of H3K9me3 are reduced in both aged fibroblasts and fibroblasts isolated from patients with HGPS (Hutchinson-Gilford progeria syndrome), a premature ageing syndrome [234]. Cellular models of the accelerated ageing syndrome, Werner's, also report a global loss of H3K9me3 and an interaction with HP1α [235]. In C. elegans, lifespan is extended by inhibition of H3K27 demethylases but this lifespan extension could be mediated by the associated changes in IIS observed in these worms [236]. In Drosophila, a lack of functional HP1 reduces lifespan and overexpression of HP1 improves longevity [237] suggesting that increases heterochromatin do promote longevity in Drosophila. These studies suggest disorganisation and loss of heterochromatin promote ageing and maintenance of heterochromatin with age increases longevity.
Links between epigenetic modifications and lifespan were first highlighted in studies of yeast in which Sir2, an NAD + -dependent histone deacetylase, was overexpressed [238,239]. Additional evidence for the role of sirtuins in ageing comes from work on SIRT6. SIRT6 deficiency in mice results in premature ageing [240]. Overexpression of SIRT6 reduces genomic instability [241], increases DSB repair efficiency by both the HR and NHEJ pathways and extends lifespan [149,242]. Of the two activities associated with SIRT6, mono-ADP-ribosyl transferase and histone acetylase, the former is proposed to increase DSB repair efficiency via PARP1 activation [243]. The authors highlight how longevity across several species correlates with DSB repair efficiency and not with efficiency of other repair pathways such as NER. Instead NER efficiency shows correlation or coevolution with the level of sun exposure per species [149]. The deacetylase activity could still affect DNA damage via the level of chromatin compaction. A lack of deacetylase activity would relax the chromatin and potentially increase exposure to DNA damaging agents [244]. Since the histone deacetylase activity of sirtuns depends on NAD + levels, as does the activity of multiple DNA damage repair enzymes, including PARPs, it has been suggested that maintaining NAD + levels, which are known to decline with age, may protect the DNA and have anti-ageing effects [[245], [246], [247]].
Another epigenetic modification, DNA methylation, is also closely linked to ageing [248]. This has been exemplified through the recent advent of epigenetic ageing clocks used to predict biological age from the DNA methylome [[249], [250], [251]]. Well-known anti-aging interventions, such as dietary restriction, also have measurable effects on the epigenome [252]. Chromatin remodellers play key roles in DNA repair, genome stability and in preventing tumorigenesis [222,253,254]. Chromatin genes that are often mutated in cancer, for example H3.3 which is linked to paediatric glioblastoma [255,256], also have roles in lifespan regulation [257,258].
Overall, links between epigenetic modifications and ageing are complex due to the gene regulation that accompanies changes in chromatin packaging. However, it is clear that the regulation of the chromatin affects both DNA repair efficiency and subsequent mutation rate both of which contribute to ageing and cancer. Most studies indicate that maintenance of heterochromatin throughout ageing and efficient DSB repair promote longevity, yet how heterochromatin affects cancer risk/rate is not yet clear.
8. Summary and future prospects
Maintaining genome integrity is key for longevity and reduced cancer risk and we argue here that interventions that lower mutations are expected to improve ageing and delay cancer. It should be noted that in a controlled and limited way mutations can be beneficial. For instance, normal functioning of the adaptive immune system and somatic hypermutation process depend on mutations introduced by AID (activation-induced cytidine deaminase), which enables production of antibodies with greater antigen affinity [259]. In single cell organisms, such as bacteria, mutations enable survival in presence of antibiotics [260], and mutation frequency is sometimes adjusted depending on stressful conditions in the environment [261]. Despite mutations being essential for evolution, they are mostly detrimental, and repair of DNA damage must therefore occur effectively to prevent both mutations and excessive cell death, which would otherwise lead to increased cancer risk or accelerated ageing, respectively. The high mutational burden of normal, somatic cells was surprising [[105], [106]], as is the discovery that particular populations of stem cells may be protected from DNA damage and mutation [104,107]. Both studies highlight how clonal selection and expansion are key factors in understanding cancer risk and ageing and are of interest for potential therapeutic interventions. Stem cells are key in the balance between cancer and ageing, it will be interesting to see whether alterations to chromatin status or reductions in replication and transcription stresses are present in ‘protected’ stem cells [262] and how this can be used for improvement of health and disease prevention. For understanding of high mutational burden in normal cells it will be important to clarify which endogenous and exogenous molecules are causing mutations [2] and some indication can be provided by mutational signatures [23,93,111,263]. Many commonly used laboratory chemicals that damage DNA, and which helped uncover details about DNA repair, are not necessarily chemicals that our cells most commonly encounter and more work on mutation causing agents in humans are needed [16]. Another future challenge would be to develop treatments that could boost error-free DNA repair. However, this is challenging because most DNA repair enzymes work in complexes and overexpression of one of them will not result in balanced and improved DNA repair. Expanding our knowledge around the enhancement of select aspects of DNA repair capacity and the mechanisms which can confer protection against DNA damage infliction will be critical to simultaneously promote longevity and reduce cancer risk.
Credit author statement
Eleanor Rachel Stead: Conceptualization, Writing – original draft, Writing – review & editing, Visualization, Project administration, Funding acquisition, Ivana Bjedov: Conceptualization, Writing – original draft, Writing – review & editing, Visualization, Project administration, Funding acquisition.
Acknowledgment
IB acknowledges funding from ERC StG 311331, ERC PoC 842174, CRUK-UCL Centre Award [C416/A25145], Radiation Research Unit at the Cancer Research UK City of London Centre Award [C7893/A28990], Royal Society Research Grant (RSG/R1/180431) and the Bill Lyons foundation. ERS is grateful for MRC PhD studentship funding.
References
- 1.Lindahl T., Barnes D.E. Repair of endogenous DNA damage. Cold Spring Harbor Symp. Quant. Biol. 2000;65:127–134. doi: 10.1101/sqb.2000.65.127. [DOI] [PubMed] [Google Scholar]
- 2.Tubbs A., Nussenzweig A. Endogenous DNA damage as a source of genomic instability in cancer. Cell. 2017;168(4):644–656. doi: 10.1016/j.cell.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A.J.R., Behjati S., Biankin A.V., Bignell G.R., Bolli N., Borg A., Børresen-Dale A.-L., Boyault S., Burkhardt B., Butler A.P., Caldas C., Davies H.R., Desmedt C., Eils R., Eyfjörd J.E., Foekens J.A., Greaves M., Hosoda F., Hutter B., Ilicic T., Imbeaud S., Imielinski M., Jäger N., Jones D.T.W., Jones D., Knappskog S., Kool M., Lakhani S.R., López-Otín C., Martin S., Munshi N.C., Nakamura H., Northcott P.A., Pajic M., Papaemmanuil E., Paradiso A., Pearson J.V., Puente X.S., Raine K., Ramakrishna M., Richardson A.L., Richter J., Rosenstiel P., Schlesner M., Schumacher T.N., Span P.N., Teague J.W., Totoki Y., Tutt A.N.J., Valdés-Mas R., van Buuren M.M., van ’t Veer L., Vincent-Salomon A., Waddell N., Yates L.R., Zucman-Rossi J., Andrew Futreal P., McDermott U., Lichter P., Meyerson M., Grimmond S.M., Siebert R., Campo E., Shibata T., Pfister S.M., Campbell P.J., Stratton M.R., Australian Pancreatic Cancer Genome I., Consortium I.B.C., Consortium I.M.-S., PedBrain I. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362(6422):709–715. doi: 10.1038/362709a0. [DOI] [PubMed] [Google Scholar]
- 5.Caridi C.P., D'Agostino C., Ryu T., Zapotoczny G., Delabaere L., Li X., Khodaverdian V.Y., Amaral N., Lin E., Rau A.R., Chiolo I. Nuclear F-actin and myosins drive relocalization of heterochromatic breaks. Nature. 2018;559(7712):54–60. doi: 10.1038/s41586-018-0242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marnef A., Legube G. Organizing DNA repair in the nucleus: DSBs hit the road. Curr. Opin. Cell Biol. 2017;46:1–8. doi: 10.1016/j.ceb.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Penagos-Puig A., Furlan-Magaril M. Heterochromatin as an important driver of genome organization. Front. Cell Dev. Biol. 2020;8:579137. doi: 10.3389/fcell.2020.579137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannister A.J., Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21(3):381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciccia A., Elledge S.J. The DNA damage response: making it safe to play with knives. Mol. Cell. 2010;40(2):179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sur S., Agrawal D.K. Phosphatases and kinases regulating CDC25 activity in the cell cycle: clinical implications of CDC25 overexpression and potential treatment strategies. Mol. Cell. Biochem. 2016;416(1–2):33–46. doi: 10.1007/s11010-016-2693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaiser A.M., Attardi L.D. Deconstructing networks of p53-mediated tumor suppression in vivo. Cell Death Differ. 2018;25(1):93–103. doi: 10.1038/cdd.2017.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkel T., Serrano M., Blasco M.A. The common biology of cancer and ageing. Nature. 2007;448(7155):767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 14.Coppé J.-P., Desprez P.-Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Ann. Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic. Biol. Med. 2009;47(5):469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 16.Garaycoechea J.I., Crossan G.P., Langevin F., Mulderrig L., Louzada S., Yang F., Guilbaud G., Park N., Roerink S., Nik-Zainal S., Stratton M.R., Patel K.J. Alcohol and endogenous aldehydes damage chromosomes and mutate stem cells. Nature. 2018;553(7687):171–177. doi: 10.1038/nature25154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizumoto A., Ohashi S., Hirohashi K., Amanuma Y., Matsuda T., Muto M. Molecular mechanisms of acetaldehyde-mediated carcinogenesis in squamous epithelium. Int. J. Mol. Sci. 2017;18(9):1943. doi: 10.3390/ijms18091943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pommier Y., Sun Y., Shar-yin N.H., Nitiss J.L. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell Biol. 2016;17(11):703. doi: 10.1038/nrm.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortez D. Preventing replication fork collapse to maintain genome integrity. DNA Repair. 2015;32:149–157. doi: 10.1016/j.dnarep.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halazonetis T.D., Gorgoulis V.G., Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319(5868):1352–1355. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- 21.Barnes J.L., Zubair M., John K., Poirier M.C., Martin F.L. Carcinogens and DNA damage. Biochem. Soc. Trans. 2018;46(5):1213–1224. doi: 10.1042/BST20180519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basu A.K. DNA damage, mutagenesis and cancer. Int. J. Mol. Sci. 2018;19(4):970. doi: 10.3390/ijms19040970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kucab J.E., Zou X., Morganella S., Joel M., Nanda A.S., Nagy E., Gomez C., Degasperi A., Harris R., Jackson S.P., Arlt V.M., Phillips D.H., Nik-Zainal S. A compendium of mutational signatures of environmental agents. Cell. 2019;177(4):821–836 e816. doi: 10.1016/j.cell.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riva L., Pandiri A.R., Li Y.R., Droop A., Hewinson J., Quail M.A., Iyer V., Shepherd R., Herbert R.A., Campbell P.J., Sills R.C., Alexandrov L.B., Balmain A., Adams D.J. The mutational signature profile of known and suspected human carcinogens in mice. Nat. Genet. 2020;52(11):1189–1197. doi: 10.1038/s41588-020-0692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al Zouabi L., Bardin A.J. Stem cell DNA damage and genome mutation in the context of aging and cancer initiation. Cold Spring Harb Perspect. Biol. 2020;12(10) doi: 10.1101/cshperspect.a036210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia J., Chiu L.Y., Nehring R.B., Bravo Nunez M.A., Mei Q., Perez M., Zhai Y., Fitzgerald D.M., Pribis J.P., Wang Y., Hu C.W., Powell R.T., LaBonte S.A., Jalali A., Matadamas Guzman M.L., Lentzsch A.M., Szafran A.T., Joshi M.C., Richters M., Gibson J.L., Frisch R.L., Hastings P.J., Bates D., Queitsch C., Hilsenbeck S.G., Coarfa C., Hu J.C., Siegele D.A., Scott K.L., Liang H., Mancini M.A., Herman C., Miller K.M., Rosenberg S.M. Bacteria-to-Human protein networks reveal origins of endogenous DNA damage. Cell. 2019;176(1–2):127–143. doi: 10.1016/j.cell.2018.12.008. e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoeijmakers J.H.J. DNA repair mechanisms. Maturitas. 2001;38(1):17–22. doi: 10.1016/s0378-5122(00)00188-2. [DOI] [PubMed] [Google Scholar]
- 28.Aravind L., Walker D.R., Koonin E.V. Conserved domains in DNA repair proteins and evolution of repair systems. Nucleic Acids Res. 1999;27(5):1223–1242. doi: 10.1093/nar/27.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunkel T.A., Bebenek K. DNA replication fidelity. Annu. Rev. Biochem. 2000;69:497–529. doi: 10.1146/annurev.biochem.69.1.497. [DOI] [PubMed] [Google Scholar]
- 30.Stojic L., Brun R., Jiricny J. Mismatch repair and DNA damage signalling. DNA Repair. 2004;3(8–9):1091–1101. doi: 10.1016/j.dnarep.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Kolodner R.D. A personal historical view of DNA mismatch repair with an emphasis on eukaryotic DNA mismatch repair. DNA Repair. 2016;38:3–13. doi: 10.1016/j.dnarep.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radman M. Mismatch repair earns Nobel Prize in Chemistry 2015 to Paul Modrich for a biochemical tour de force. DNA Repair. 2016;37:A22–A28. doi: 10.1016/j.dnarep.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Mathews C.K. DNA precursor metabolism and genomic stability. Faseb. J. 2006;20(9):1300–1314. doi: 10.1096/fj.06-5730rev. [DOI] [PubMed] [Google Scholar]
- 34.Friedberg E.C. Suffering in silence: the tolerance of DNA damage. Nat. Rev. Mol. Cell Biol. 2005;6(12):943–953. doi: 10.1038/nrm1781. [DOI] [PubMed] [Google Scholar]
- 35.Fuchs R.P. Tolerance of lesions in E. coli: chronological competition between translesion synthesis and damage avoidance. DNA Repair. 2016;44:51–58. doi: 10.1016/j.dnarep.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Yang W., Gao Y. Translesion and repair DNA polymerases: diverse structure and mechanism. Annu. Rev. Biochem. 2018;87:239–261. doi: 10.1146/annurev-biochem-062917-012405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berti M., Vindigni A. Replication stress: getting back on track. Nat. Struct. Mol. Biol. 2016;23(2):103–109. doi: 10.1038/nsmb.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lovett S.T. Template-switching during replication fork repair in bacteria. DNA Repair. 2017;56:118–128. doi: 10.1016/j.dnarep.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Branzei D., Foiani M. Regulation of DNA repair throughout the cell cycle. Nat. Rev. Mol. Cell Biol. 2008;9:297. doi: 10.1038/nrm2351. [DOI] [PubMed] [Google Scholar]
- 40.Her J., Bunting S.F. How cells ensure correct repair of DNA double-strand breaks. J. Biol. Chem. 2018;293(27):10502–10511. doi: 10.1074/jbc.TM118.000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buis J., Stoneham T., Spehalski E., Ferguson D.O. Mre11 regulates CtIP-dependent double-strand break repair by interaction with CDK2. Nat. Struct. Mol. Biol. 2012;19(2):246–252. doi: 10.1038/nsmb.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferretti L.P., Lafranchi L., Sartori A.A. Controlling DNA-end resection: a new task for CDKs. Front. Genet. 2013;4:99. doi: 10.3389/fgene.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michl J., Zimmer J., Tarsounas M. Interplay between Fanconi anemia and homologous recombination pathways in genome integrity. EMBO J. 2016;35(9):909–923. doi: 10.15252/embj.201693860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pilzecker B., Buoninfante O.A., Jacobs H. DNA damage tolerance in stem cells, ageing, mutagenesis, disease and cancer therapy. Nucleic Acids Res. 2019;47(14):7163–7181. doi: 10.1093/nar/gkz531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan M.R., Bernstein K.A. RAD-ical new insights into RAD51 regulation. Genes. 2018;9(12) doi: 10.3390/genes9120629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beucher A., Birraux J., Tchouandong L., Barton O., Shibata A., Conrad S., Goodarzi A.A., Krempler A., Jeggo P.A., Löbrich M. ATM and Artemis promote homologous recombination of radiation-induced DNA double-strand breaks in G2. EMBO J. 2009;28(21):3413–3427. doi: 10.1038/emboj.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neal J.A., Dang V., Douglas P., Wold M.S., Lees-Miller S.P., Meek K. Inhibition of homologous recombination by DNA-dependent protein kinase requires kinase activity, is titratable, and is modulated by autophosphorylation. Mol. Cell Biol. 2011;31(8):1719–1733. doi: 10.1128/MCB.01298-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shibata A., Conrad S., Birraux J., Geuting V., Barton O., Ismail A., Kakarougkas A., Meek K., Taucher‐Scholz G., Löbrich M. Factors determining DNA double‐strand break repair pathway choice in G2 phase. EMBO J. 2011;30(6):1079–1092. doi: 10.1038/emboj.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kakarougkas A., Ismail A., Klement K., Goodarzi A.A., Conrad S., Freire R., Shibata A., Lobrich M., Jeggo P.A. Opposing roles for 53BP1 during homologous recombination. Nucleic Acids Res. 2013;41(21):9719–9731. doi: 10.1093/nar/gkt729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mao Z., Bozzella M., Seluanov A., Gorbunova V. Comparison of nonhomologous end joining and homologous recombination in human cells. DNA Repair. 2008;7(10):1765–1771. doi: 10.1016/j.dnarep.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karanam K., Kafri R., Loewer A., Lahav G. Quantitative live cell imaging reveals a gradual shift between DNA repair mechanisms and a maximal use of HR in mid S phase. Mol. Cell. 2012;47(2):320–329. doi: 10.1016/j.molcel.2012.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shahar O., Ram E.R., Shimshoni E., Hareli S., Meshorer E., Goldberg M. Live imaging of induced and controlled DNA double-strand break formation reveals extremely low repair by homologous recombination in human cells. Oncogene. 2012;31(30):3495. doi: 10.1038/onc.2011.516. [DOI] [PubMed] [Google Scholar]
- 53.Ward J.F. Complexity of damage produced by ionizing radiation. Cold Spring Harbor Symp. Quant. Biol. 2000;65:377–382. doi: 10.1101/sqb.2000.65.377. [DOI] [PubMed] [Google Scholar]
- 54.Gupta R., Somyajit K., Narita T., Maskey E., Stanlie A., Kremer M., Typas D., Lammers M., Mailand N., Nussenzweig A., Lukas J., Choudhary C. DNA repair network analysis reveals shieldin as a key regulator of NHEJ and PARP inhibitor sensitivity. Cell. 2018;173(4):972–988. doi: 10.1016/j.cell.2018.03.050. e923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noordermeer S.M., Adam S., Setiaputra D., Barazas M., Pettitt S.J., Ling A.K., Olivieri M., Álvarez-Quilón A., Moatti N., Zimmermann M., Annunziato S., Krastev D.B., Song F., Brandsma I., Frankum J., Brough R., Sherker A., Landry S., Szilard R.K., Munro M.M., McEwan A., Goullet de Rugy T., Lin Z.-Y., Hart T., Moffat J., Gingras A.-C., Martin A., van Attikum H., Jonkers J., Lord C.J., Rottenberg S., Durocher D. The shieldin complex mediates 53BP1-dependent DNA repair. Nature. 2018;560(7716):117–121. doi: 10.1038/s41586-018-0340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ochs F., Somyajit K., Altmeyer M., Rask M.B., Lukas J., Lukas C. 53BP1 fosters fidelity of homology-directed DNA repair. Nat. Struct. Mol. Biol. 2016;23(8):714–721. doi: 10.1038/nsmb.3251. [DOI] [PubMed] [Google Scholar]
- 57.Hicks W.M., Kim M., Haber J.E. Increased mutagenesis and unique mutation signature associated with mitotic gene conversion. Science. 2010;329(5987):82–85. doi: 10.1126/science.1191125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodgers K., McVey M. Error-prone repair of DNA double-strand breaks. J. Cell. Physiol. 2016;231(1):15–24. doi: 10.1002/jcp.25053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J., Holzschu D.L., Sugiyama T. PCNA is efficiently loaded on the DNA recombination intermediate to modulate polymerase delta, eta, and zeta activities. Proc. Natl. Acad. Sci. U. S. A. 2013;110(19):7672–7677. doi: 10.1073/pnas.1222241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sebesta M., Burkovics P., Juhasz S., Zhang S., Szabo J.E., Lee M.Y., Haracska L., Krejci L. Role of PCNA and TLS polymerases in D-loop extension during homologous recombination in humans. DNA Repair. 2013;12(9):691–698. doi: 10.1016/j.dnarep.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakofsky C.J., Ayyar S., Deem A.K., Chung W.H., Ira G., Malkova A. Translesion polymerases drive microhomology-mediated break-induced replication leading to complex chromosomal rearrangements. Mol. Cell. 2015;60(6):860–872. doi: 10.1016/j.molcel.2015.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McVey M., Khodaverdian V.Y., Meyer D., Cerqueira P.G., Heyer W.D. Eukaryotic DNA polymerases in homologous recombination. Annu. Rev. Genet. 2016;50:393–421. doi: 10.1146/annurev-genet-120215-035243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y., Gordenin D.A., Resnick M.A. A single-strand specific lesion drives MMS-induced hyper-mutability at a double-strand break in yeast. DNA Repair. 2010;9(8):914–921. doi: 10.1016/j.dnarep.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mattiroli F., Joseph H.A., Vissers Willem, van Dijk J., Ikpa P., Citterio E., Vermeulen W., Jurgen A Marteijn, Sixma Titia K. RNF168 ubiquitinates K13-15 on H2A/H2AX to drive DNA damage signaling. Cell. 2012;150(6):1182–1195. doi: 10.1016/j.cell.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 65.Cao L.-L., Shen C., Zhu W.-G. Histone modifications in DNA damage response. Sci. China Life Sci. 2016;59(3):257–270. doi: 10.1007/s11427-016-5011-z. [DOI] [PubMed] [Google Scholar]
- 66.Palazzo L., Ahel I. PARPs in genome stability and signal transduction: implications for cancer therapy. Biochem. Soc. Trans. 2018;46(6):1681–1695. doi: 10.1042/BST20180418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van H.T., Santos M.A. Histone modifications and the DNA double-strand break response. Cell Cycle. 2018;17(21–22):2399–2410. doi: 10.1080/15384101.2018.1542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meisenberg C., Pinder S.I., Hopkins S.R., Wooller S.K., Benstead-Hume G., Pearl F.M.G., Jeggo P.A., Downs J.A. Repression of transcription at DNA breaks requires cohesin throughout interphase and prevents genome instability. Mol. Cell. 2019;73(2):212–223. doi: 10.1016/j.molcel.2018.11.001. e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng C.L., Wang N.J., Chung J., Moslehi H., Sanborn J.Z., Hur J.S., Collisson E.A., Vemula S.S., Naujokas A., Chiotti K.E., Cheng J.B., Fassihi H., Blumberg A.J., Bailey C.V., Fudem G.M., Mihm C.V., Cunningham B.B., Neuhaus I.M., Liao W., Oh D.H., Cleaver J.E., LeBoit P.E., Costello J.F., Lehmann A.R., Gray J.W., Spellman P.T., Arron S.T., Huh N., Purdom E., Cho R.J. Transcription Restores DNA Repair to Heterochromatin, Determining Regional Mutation Rates in Cancer Genomes. Cell Rep. 2014;9(4):1228–1234. doi: 10.1016/j.celrep.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Supek F., Lehner B. Differential DNA mismatch repair underlies mutation rate variation across the human genome. Nature. 2015;521(7550):81–84. doi: 10.1038/nature14173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haradhvala N.J., Polak P., Stojanov P., Covington K.R., Shinbrot E., Hess J.M., Rheinbay E., Kim J., Maruvka Y.E., Braunstein L.Z., Kamburov A., Hanawalt P.C., Wheeler D.A., Koren A., Lawrence M.S., Getz G. Mutational strand asymmetries in cancer genomes reveal mechanisms of DNA damage and repair. Cell. 2016;164(3):538–549. doi: 10.1016/j.cell.2015.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stamatoyannopoulos J.A., Adzhubei I., Thurman R.E., Kryukov G.V., Mirkin S.M., Sunyaev S.R. Human mutation rate associated with DNA replication timing. Nat. Genet. 2009;41(4):393–395. doi: 10.1038/ng.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sabarinathan R., Mularoni L., Deu-Pons J., Gonzalez-Perez A., López-Bigas N. Nucleotide excision repair is impaired by binding of transcription factors to DNA. Nature. 2016;532:264. doi: 10.1038/nature17661. [DOI] [PubMed] [Google Scholar]
- 74.Morganella S., Alexandrov L.B., Glodzik D., Zou X., Davies H., Staaf J., Sieuwerts A.M., Brinkman A.B., Martin S., Ramakrishna M., Butler A., Kim H.-Y., Borg Å., Sotiriou C., Futreal P.A., Campbell P.J., Span P.N., Van Laere S., Lakhani S.R., Eyfjord J.E., Thompson A.M., Stunnenberg H.G., van de Vijver M.J., Martens J.W.M., Børresen-Dale A.-L., Richardson A.L., Kong G., Thomas G., Sale J., Rada C., Stratton M.R., Birney E., Nik-Zainal S. The topography of mutational processes in breast cancer genomes. Nat. Commun. 2016;7 doi: 10.1038/ncomms11383. 11383-11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lodato M.A., Woodworth M.B., Lee S., Evrony G.D., Mehta B.K., Karger A., Lee S., Chittenden T.W., D'Gama A.M., Cai X., Luquette L.J., Lee E., Park P.J., Walsh C.A. Somatic mutation in single human neurons tracks developmental and transcriptional history. Science (New York, N.Y.) 2015;350(6256):94–98. doi: 10.1126/science.aab1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aguilera A. The connection between transcription and genomic instability. EMBO J. 2002;21(3):195–201. doi: 10.1093/emboj/21.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perera D., Poulos R.C., Shah A., Beck D., Pimanda J.E., Wong J.W.H. Differential DNA repair underlies mutation hotspots at active promoters in cancer genomes. Nature. 2016;532:259. doi: 10.1038/nature17437. [DOI] [PubMed] [Google Scholar]
- 78.Goodarzi A.A., Jeggo P., Lobrich M. The influence of heterochromatin on DNA double strand break repair: getting the strong, silent type to relax. DNA Repair. 2010;9(12):1273–1282. doi: 10.1016/j.dnarep.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 79.Goodarzi A.A., Kurka T., Jeggo P.A. KAP-1 phosphorylation regulates CHD3 nucleosome remodeling during the DNA double-strand break response. Nat. Struct. Mol. Biol. 2011;18(7):831. doi: 10.1038/nsmb.2077. [DOI] [PubMed] [Google Scholar]
- 80.Rübe C.E., Lorat Y., Schuler N., Schanz S., Wennemuth G., Rübe C. DNA repair in the context of chromatin: new molecular insights by the nanoscale detection of DNA repair complexes using transmission electron microscopy. DNA Repair. 2011;10(4):427–437. doi: 10.1016/j.dnarep.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 81.Goodarzi A.A., Noon A.T., Deckbar D., Ziv Y., Shiloh Y., Löbrich M., Jeggo P.A. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol. Cell. 2008;31(2):167–177. doi: 10.1016/j.molcel.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 82.Nava M.M., Miroshnikova Y.A., Biggs L.C., Whitefield D.B., Metge F., Boucas J., Vihinen H., Jokitalo E., Li X., García Arcos J.M., Hoffmann B., Merkel R., Niessen C.M., Dahl K.N., Wickström S.A. Cell; 2020. Heterochromatin-Driven Nuclear Softening Protects the Genome against Mechanical Stress-Induced Damage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Langelier M.F., Eisemann T., Riccio A.A., Pascal J.M. PARP family enzymes: regulation and catalysis of the poly(ADP-ribose) posttranslational modification. Curr. Opin. Struct. Biol. 2018;53:187–198. doi: 10.1016/j.sbi.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonfiglio J.J., Fontana P., Zhang Q., Colby T., Gibbs-Seymour I., Atanassov I., Bartlett E., Zaja R., Ahel I., Matic I. Serine ADP-ribosylation depends on HPF1. Mol. Cell. 2017;65(5):932–940. doi: 10.1016/j.molcel.2017.01.003. e936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Palazzo L., Leidecker O., Prokhorova E., Dauben H., Matic I., Ahel I. Serine is the major residue for ADP-ribosylation upon DNA damage. Elife. 2018;7 doi: 10.7554/eLife.34334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bilokapic S., Suskiewicz M.J., Ahel I., Halic M. Bridging of DNA breaks activates PARP2-HPF1 to modify chromatin. Nature. 2020;585(7826):609–613. doi: 10.1038/s41586-020-2725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suskiewicz M.J., Zobel F., Ogden T.E.H., Fontana P., Ariza A., Yang J.C., Zhu K., Bracken L., Hawthorne W.J., Ahel D., Neuhaus D., Ahel I. HPF1 completes the PARP active site for DNA damage-induced ADP-ribosylation. Nature. 2020;579(7800):598–602. doi: 10.1038/s41586-020-2013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.D'Andrea A.D. Mechanisms of PARP inhibitor sensitivity and resistance. DNA Repair. 2018;71:172–176. doi: 10.1016/j.dnarep.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 89.Slade D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020;1;34(5–6):360–394. doi: 10.1101/gad.334516.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blackford A.N., Jackson S.P. ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response. Mol. Cell. 2017;66(6):801–817. doi: 10.1016/j.molcel.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 91.Medema R.H., Macůrek L. Checkpoint control and cancer. Oncogene. 2011;31:2601. doi: 10.1038/onc.2011.451. [DOI] [PubMed] [Google Scholar]
- 92.Hanahan D., Weinberg Robert A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 93.Martincorena I., Campbell P.J. Somatic mutation in cancer and normal cells. Science. 2015;349(6255):1483–1489. doi: 10.1126/science.aab4082. [DOI] [PubMed] [Google Scholar]
- 94.Armitage P., Doll R. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br. J. Canc. 1954;8(1):1–12. doi: 10.1038/bjc.1954.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Friedberg E.C., Walker G.C., Siede W., Wood R.D. American Society for Microbiology Press; 2005. DNA Repair and Mutagenesis. [Google Scholar]
- 96.Stephens P.J., McBride D.J., Lin M.-L., Varela I., Pleasance E.D., Simpson J.T., Stebbings L.A., Leroy C., Edkins S., Mudie L.J., Greenman C.D., Jia M., Latimer C., Teague J.W., Lau K.W., Burton J., Quail M.A., Swerdlow H., Churcher C., Natrajan R., Sieuwerts A.M., Martens J.W.M., Silver D.P., Langerød A., Russnes H.E.G., Foekens J.A., Reis-Filho J.S., van 't Veer L., Richardson A.L., Børresen-Dale A.-L., Campbell P.J., Futreal P.A., Stratton M.R. Complex landscapes of somatic rearrangement in human breast cancer genomes. Nature. 2009;462(7276):1005–1010. doi: 10.1038/nature08645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lynch M. Rate, molecular spectrum, and consequences of human mutation. Proc. Natl. Acad. Sci. Unit. States Am. 2010;107(3):961–968. doi: 10.1073/pnas.0912629107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tomlinson I.P., Novelli M.R., Bodmer W.F. The mutation rate and cancer. Proceed. Nat. Acad. Sci. United States of America. 1996;93(25):14800–14803. doi: 10.1073/pnas.93.25.14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Loeb L.A., Loeb K.R., Anderson J.P. Multiple mutations and cancer. Proceed. Nat. Acad. Sci. United States of America. 2003;100(3):776–781. doi: 10.1073/pnas.0334858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tomasetti C., Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science (New York, N.Y.) 2015;347(6217):78–81. doi: 10.1126/science.1260825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tomasetti C., Li L., Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science (New York, N.Y.) 2017;355(6331):1330–1334. doi: 10.1126/science.aaf9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu S., Powers S., Zhu W., Hannun Y.A. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2016;529(7584):43–47. doi: 10.1038/nature16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nowak M.A., Waclaw B. Genes, environment, and “bad luck”. Science. 2017;355(6331):1266–1267. doi: 10.1126/science.aam9746. [DOI] [PubMed] [Google Scholar]
- 104.Yoshida K., Gowers K.H.C., Lee-Six H., Chandrasekharan D.P., Coorens T., Maughan E.F., Beal K., Menzies A., Millar F.R., Anderson E., Clarke S.E., Pennycuick A., Thakrar R.M., Butler C.R., Kakiuchi N., Hirano T., Hynds R.E., Stratton M.R., Martincorena I., Janes S.M., Campbell P.J. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature. 2020;578(7794):266–272. doi: 10.1038/s41586-020-1961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martincorena I., Roshan A., Gerstung M., Ellis P., Van Loo P., McLaren S., Wedge D.C., Fullam A., Alexandrov L.B., Tubio J.M., Stebbings L., Menzies A., Widaa S., Stratton M.R., Jones P.H., Campbell P.J. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science (New York, N.Y.) 2015;348(6237):880–886. doi: 10.1126/science.aaa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Martincorena I., Fowler J.C., Wabik A., Lawson A.R.J., Abascal F., Hall M.W.J., Cagan A., Murai K., Mahbubani K., Stratton M.R., Fitzgerald R.C., Handford P.A., Campbell P.J., Saeb-Parsy K., Jones P.H. Somatic mutant clones colonize the human esophagus with age. Science. 2018;362(6417):911–917. doi: 10.1126/science.aau3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brazhnik K., Sun S., Alani O., Kinkhabwala M., Wolkoff A.W., Maslov A.Y., Dong X., Vijg J. Single-cell analysis reveals different age-related somatic mutation profiles between stem and differentiated cells in human liver. Sci. Adv. 2020;6(5) doi: 10.1126/sciadv.aax2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Greaves M., Maley C.C. Clonal evolution in cancer. Nature. 2012;481(7381):306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Parikh N., Shuck R.L., Gagea M., Shen L., Donehower L.A. Enhanced inflammation and attenuated tumor suppressor pathways are associated with oncogene-induced lung tumors in aged mice. Aging Cell. 2018;17(1) doi: 10.1111/acel.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Laconi E., Marongiu F., DeGregori J. Cancer as a disease of old age: changing mutational and microenvironmental landscapes. Br. J. Canc. 2020;122(7):943–952. doi: 10.1038/s41416-019-0721-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martincorena I. Somatic mutation and clonal expansions in human tissues. Genome Med. 2019;11(1):35. doi: 10.1186/s13073-019-0648-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Garinis G.A., van der Horst G.T.J., Vijg J., Hoeijmakers J.H.J. DNA damage and ageing: new-age ideas for an age-old problem. Nat. Cell Biol. 2008;10(11):1241–1247. doi: 10.1038/ncb1108-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schumacher B., Garinis G.A., Hoeijmakers J.H.J. Age to survive: DNA damage and aging. Trends Genet. 2008;24(2):77–85. doi: 10.1016/j.tig.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 114.Chu W.K., Hickson I.D. RecQ helicases: multifunctional genome caretakers. Nat. Rev. Canc. 2009;9:644. doi: 10.1038/nrc2682. [DOI] [PubMed] [Google Scholar]
- 115.Luo G., Santoro I.M., McDaniel L.D., Nishijima I., Mills M., Youssoufian H., Vogel H., Schultz R.A., Bradley A. Cancer predisposition caused by elevated mitotic recombination in Bloom mice. Nat. Genet. 2000;26(4):424–429. doi: 10.1038/82548. [DOI] [PubMed] [Google Scholar]
- 116.Goss K.H., Risinger M.A., Kordich J.J., Sanz M.M., Straughen J.E., Slovek L.E., Capobianco A.J., German J., Boivin G.P., Groden J. "Enhanced tumor formation in mice heterozygous for <em>Blm</em> mutation. Science. 2002;297(5589):2051. doi: 10.1126/science.1074340. [DOI] [PubMed] [Google Scholar]
- 117.Chang S., Multani A.S., Cabrera N.G., Naylor M.L., Laud P., Lombard D., Pathak S., Guarente L., DePinho R.A. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nat. Genet. 2004;36(8):877–882. doi: 10.1038/ng1389. [DOI] [PubMed] [Google Scholar]
- 118.Madia F., Gattazzo C., Wei M., Fabrizio P., Burhans W.C., Weinberger M., Galbani A., Smith J.R., Nguyen C., Huey S., Comai L., Longo V.D. "Longevity mutation in <em>SCH9</em> prevents recombination errors and premature genomic instability in a Werner/Bloom model system. J. Cell Biol. 2008;180(1):67. doi: 10.1083/jcb.200707154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shiloh Y., Kastan M.B. vol. 83. Academic Press; 2001. pp. 209–254. (ATM: Genome Stability, Neuronal Development, and Cancer Cross Paths. Advances in Cancer Research). [DOI] [PubMed] [Google Scholar]
- 120.Donehower L.A. The p53-deficient mouse: a model for basic and applied cancer studies. Semin. Canc. Biol. 1996;7(5):269–278. doi: 10.1006/scbi.1996.0035. [DOI] [PubMed] [Google Scholar]
- 121.Symphorien S., Woodruff R.C. Effect of DNA repair on aging of transgenic Drosophila melanogaster: I. mei-41 locus. J. Gerontol.: Ser. A. 2003;58(9):B782–B787. doi: 10.1093/gerona/58.9.b782. [DOI] [PubMed] [Google Scholar]
- 122.Shaposhnikov M., Moskalev A., Plyusnina E. Effect of PARP-1 overexpression and pharmacological inhibition of NF-kB on the lifespan of Drosophila melanogaster. Adv. Gerontol. 2011;24(3):405–419. [PubMed] [Google Scholar]
- 123.Shaposhnikov M., Proshkina E., Shilova L., Zhavoronkov A., Moskalev A. Lifespan and stress resistance in Drosophila with overexpressed DNA repair genes. Sci. Rep. 2015;5:15299. doi: 10.1038/srep15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Garschall K., Dellago H., Gáliková M., Schosserer M., Flatt T., Grillari J. Ubiquitous overexpression of the DNA repair factor dPrp19 reduces DNA damage and extends Drosophila life span. NPJ Aging Mech. Dis. 2017;3 doi: 10.1038/s41514-017-0005-z. 5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Salmon A.B., Murakami S., Bartke A., Kopchick J., Yasumura K., Miller R.A. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am. J. Physiol. Endocrinol. Metabol. 2005;289(1):E23–E29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- 126.Page M.M., Salmon A.B., Leiser S.F., Robb E.L., Brown M.F., Miller R.A., Stuart J.A. Mechanisms of stress resistance in Snell dwarf mouse fibroblasts: enhanced antioxidant and DNA base excision repair capacity, but no differences in mitochondrial metabolism. Free Radic. Biol. Med. 2009;46(8):1109–1118. doi: 10.1016/j.freeradbiomed.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Garcia A.M., Busuttil R.A., Calder R.B., Dollé M.E.T., Diaz V., McMahan C.A., Bartke A., Nelson J., Reddick R., Vijg J. Effect of Ames dwarfism and caloric restriction on spontaneous DNA mutation frequency in different mouse tissues. Mech. Age. Develop. 2008;129(9):528–533. doi: 10.1016/j.mad.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dominick G., Bowman J., Li X., Miller R.A., Garcia G.G. mTOR regulates the expression of DNA damage response enzymes in long-lived Snell dwarf, GHRKO, and PAPPA-KO mice. Aging Cell. 2017;16(1):52–60. doi: 10.1111/acel.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alderman J.M., Flurkey K., Brooks N.L., Naik S.B., Gutierrez J.M., Srinivas U., Ziara K.B., Jing L., Boysen G., Bronson R., Klebanov S., Chen X., Swenberg J.A., Stridsberg M., Parker C.E., Harrison D.E., Combs T.P. Neuroendocrine inhibition of glucose production and resistance to cancer in dwarf mice. Exp. Gerontol. 2009;44(1–2):26–33. doi: 10.1016/j.exger.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ikeno Y., Hubbard G.B., Lee S., Cortez L.A., Lew C.M., Webb C.R., Berryman D.E., List E.O., Kopchick J.J., Bartke A. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009;64(5):522–529. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Anisimov V.N., Zabezhinski M.A., Popovich I.G., Piskunova T.S., Semenchenko A.V., Tyndyk M.L., Yurova M.N., Antoch M.P., Blagosklonny M.V. Rapamycin extends maximal lifespan in cancer-prone mice. Am. J. Pathol. 2010;176(5):2092–2097. doi: 10.2353/ajpath.2010.091050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Anisimov V.N., Zabezhinski M.A., Popovich I.G., Piskunova T.S., Semenchenko A.V., Tyndyk M.L., Yurova M.N., Rosenfeld S.V., Blagosklonny M.V. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10(24):4230–4236. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- 133.Selman C., Tullet J.M.A., Wieser D., Irvine E., Lingard S.J., Choudhury A.I., Claret M., Al-Qassab H., Carmignac D., Ramadani F., Woods A., Robinson I.C.A., Schuster E., Batterham R.L., Kozma S.C., Thomas G., Carling D., Okkenhaug K., Thornton J.M., Partridge L., Gems D., Withers D.J. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science (New York, N.Y.) 2009;326(5949):140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wei M., Fabrizio P., Madia F., Hu J., Ge H., Li L.M., Longo V.D. Tor1/Sch9-regulated carbon source substitution is as effective as calorie restriction in life span extension. PLoS Genet. 2009;5(5) doi: 10.1371/journal.pgen.1000467. e1000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Guevara-Aguirre J., Balasubramanian P., Guevara-Aguirre M., Wei M., Madia F., Cheng C.-W., Hwang D., Martin-Montalvo A., Saavedra J., Ingles S., de Cabo R., Cohen P., Longo V.D. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med. 2011;3(70) doi: 10.1126/scitranslmed.3001845. 70ra13-70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hansen M., Kennedy B.K. Does longer lifespan mean longer healthspan? Trends Cell Biol. 2016;26(8):565–568. doi: 10.1016/j.tcb.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Niedernhofer L.J., Garinis G.A., Raams A., Lalai A.S., Robinson A.R., Appeldoorn E., Odijk H., Oostendorp R., Ahmad A., van Leeuwen W., Theil A.F., Vermeulen W., van der Horst G.T.J., Meinecke P., Kleijer W.J., Vijg J., Jaspers N.G.J., Hoeijmakers J.H.J. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006;444(7122):1038–1043. doi: 10.1038/nature05456. [DOI] [PubMed] [Google Scholar]
- 138.Schumacher B., Hoeijmakers J.H., Garinis G.A. Sealing the gap between nuclear DNA damage and longevity. Mol. Cell. Endocrinol. 2009;299(1):112–117. doi: 10.1016/j.mce.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 139.Vermeij W.P., Dollé M.E.T., Reiling E., Jaarsma D., Payan-Gomez C., Bombardieri C.R., Wu H., Roks A.J.M., Botter S.M., van der Eerden B.C., Youssef S.A., Kuiper R.V., Nagarajah B., van Oostrom C.T., Brandt R.M.C., Barnhoorn S., Imholz S., Pennings J.L.A., de Bruin A., Gyenis Á., Pothof J., Vijg J., van Steeg H., Hoeijmakers J.H.J. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature. 2016;537(7620):427–431. doi: 10.1038/nature19329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hart R.W., Setlow R.B. Correlation between deoxyribonucleic acid excision-repair and life-span in a number of mammalian species. Proceed. Nat. Acad. Sci. United States of America. 1974;71(6):2169–2173. doi: 10.1073/pnas.71.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Grube K., Bürkle A. Poly(ADP-ribose) polymerase activity in mononuclear leukocytes of 13 mammalian species correlates with species-specific life span. Proceed. Nat. Acad. Sci. United States of America. 1992;89(24):11759–11763. doi: 10.1073/pnas.89.24.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lorenzini A., Johnson F.B., Oliver A., Tresini M., Smith J.S., Hdeib M., Sell C., Cristofalo V.J., Stamato T.D. Significant correlation of species longevity with DNA double strand break recognition but not with telomere length. Mech. Age. Develop. 2009;130(11–12):784–792. doi: 10.1016/j.mad.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ma S., Upneja A., Galecki A., Tsai Y.-M., Burant C.F., Raskind S., Zhang Q., Zhang Z.D., Seluanov A., Gorbunova V., Clish C.B., Miller R.A., Gladyshev V.N. Cell culture-based profiling across mammals reveals DNA repair and metabolism as determinants of species longevity. eLife. 2016;5 doi: 10.7554/eLife.19130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Seluanov A., Hine C., Azpurua J., Feigenson M., Bozzella M., Mao Z., Catania K.C., Gorbunova V. Hypersensitivity to contact inhibition provides a clue to cancer resistance of naked mole-rat. Proc. Natl. Acad. Sci. Unit. States Am. 2009;106(46):19352. doi: 10.1073/pnas.0905252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liang S., Mele J., Wu Y., Buffenstein R., Hornsby P.J. Resistance to experimental tumorigenesis in cells of a long-lived mammal, the naked mole-rat (Heterocephalus glaber) Aging Cell. 2010;9(4):626–635. doi: 10.1111/j.1474-9726.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.MacRae S.L., Croken M.M., Calder R.B., Aliper A., Milholland B., White R.R., Zhavoronkov A., Gladyshev V.N., Seluanov A., Gorbunova V., Zhang Z.D., Vijg J. DNA repair in species with extreme lifespan differences. Aging. 2015;7(12):1171–1184. doi: 10.18632/aging.100866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Delaney M.A., Ward J.M., Walsh T.F., Chinnadurai S.K., Kerns K., Kinsel M.J., Treuting P.M. Initial case reports of cancer in naked mole-rats (Heterocephalus glaber) Veterin. Pathol. 2016;53(3):691–696. doi: 10.1177/0300985816630796. [DOI] [PubMed] [Google Scholar]
- 148.Tian X., Seluanov A., Gorbunova V. Molecular mechanisms determining lifespan in short- and long-lived species. Trends Endocrinol. Metabol. 2017;28(10):722–734. doi: 10.1016/j.tem.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tian X., Firsanov D., Zhang Z., Cheng Y., Luo L., Tombline G., Tan R., Simon M., Henderson S., Steffan J., Goldfarb A., Tam J., Zheng K., Cornwell A., Johnson A., Yang J.-N., Mao Z., Manta B., Dang W., Zhang Z., Vijg J., Wolfe A., Moody K., Kennedy B.K., Bohmann D., Gladyshev V.N., Seluanov A., Gorbunova V. SIRT6 is responsible for more efficient DNA double-strand break repair in long-lived species. Cell. 2019;177(3):622–638. doi: 10.1016/j.cell.2019.03.043. e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tian X., Azpurua J., Hine C., Vaidya A., Myakishev-Rempel M., Ablaeva J., Mao Z., Nevo E., Gorbunova V., Seluanov A. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature. 2013;499(7458):346–349. doi: 10.1038/nature12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tyner S.D., Venkatachalam S., Choi J., Jones S., Ghebranious N., Igelmann H., Lu X., Soron G., Cooper B., Brayton C., Hee Park S., Thompson T., Karsenty G., Bradley A., Donehower L.A. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415(6867):45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 152.García-Cao I., García-Cao M., Martín-Caballero J., Criado L.M., Klatt P., Flores J.M., Weill J.-C., Blasco M.A., Serrano M. Super p53" mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 2002;21(22):6225–6235. doi: 10.1093/emboj/cdf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Matheu A., Maraver A., Klatt P., Flores I., Garcia-Cao I., Borras C., Flores J.M., Viña J., Blasco M.A., Serrano M. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448:375. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- 154.Campisi J. Cellular senescence and apoptosis: how cellular responses might influence aging phenotypes. Exp. Gerontol. 2003;38(1):5–11. doi: 10.1016/s0531-5565(02)00152-3. [DOI] [PubMed] [Google Scholar]
- 155.Inomata K., Aoto T., Binh N.T., Okamoto N., Tanimura S., Wakayama T., Iseki S., Hara E., Masunaga T., Shimizu H., Nishimura E.K. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell. 2009;137(6):1088–1099. doi: 10.1016/j.cell.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 156.Matsumura H., Mohri Y., Binh N.T., Morinaga H., Fukuda M., Ito M., Kurata S., Hoeijmakers J., Nishimura E.K. Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science. 2016;351(6273):aad4395. doi: 10.1126/science.aad4395. [DOI] [PubMed] [Google Scholar]
- 157.Liu N., Matsumura H., Kato T., Ichinose S., Takada A., Namiki T., Asakawa K., Morinaga H., Mohri Y., De Arcangelis A., Geroges-Labouesse E., Nanba D., Nishimura E.K. Stem cell competition orchestrates skin homeostasis and ageing. Nature. 2019;568(7752):344–350. doi: 10.1038/s41586-019-1085-7. [DOI] [PubMed] [Google Scholar]
- 158.Flach J., Bakker S.T., Mohrin M., Conroy P.C., Pietras E.M., Reynaud D., Alvarez S., Diolaiti M.E., Ugarte F., Forsberg E.C., Le Beau M.M., Stohr B.A., Méndez J., Morrison C.G., Passegué E. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature. 2014;512(7513):198–202. doi: 10.1038/nature13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Marteijn J.A., Lans H., Vermeulen W., Hoeijmakers J.H.J. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol. 2014;15:465. doi: 10.1038/nrm3822. [DOI] [PubMed] [Google Scholar]
- 160.Pinkston J.M., Garigan D., Hansen M., Kenyon C. "Mutations that increase the life span of <em>C. elegans</em> inhibit tumor growth. Science. 2006;313(5789):971. doi: 10.1126/science.1121908. [DOI] [PubMed] [Google Scholar]
- 161.Kirkwood T.B.L. Understanding the odd science of aging. Cell. 2005;120(4):437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 162.White R.R., Vijg J. Do DNA double-strand breaks drive aging? Mol. Cell. 2016;63(5):729–738. doi: 10.1016/j.molcel.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Niedernhofer L.J., Gurkar A.U., Wang Y., Vijg J., Hoeijmakers J.H.J., Robbins P.D. Nuclear genomic instability and aging. Annu. Rev. Biochem. 2018;87(1):295–322. doi: 10.1146/annurev-biochem-062917-012239. [DOI] [PubMed] [Google Scholar]
- 164.Baker D.J., Wijshake T., Tchkonia T., LeBrasseur N.K., Childs B.G., van de Sluis B., Kirkland J.L., van Deursen J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Muñoz-Espín D., Serrano M. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014;15:482. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 166.van Deursen J.M. The role of senescent cells in ageing. Nature. 2014;509(7501):439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Baker D.J., Childs B.G., Durik M., Wijers M.E., Sieben C.J., Zhong J., Saltness R.A., Jeganathan K.B., Verzosa G.C., Pezeshki A., Khazaie K., Miller J.D., van Deursen J.M. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chang J., Wang Y., Shao L., Laberge R.-M., Demaria M., Campisi J., Janakiraman K., Sharpless N.E., Ding S., Feng W., Luo Y., Wang X., Aykin-Burns N., Krager K., Ponnappan U., Hauer-Jensen M., Meng A., Zhou D. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016;22(1):78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Roos C.M., Zhang B., Palmer A.K., Ogrodnik M.B., Pirtskhalava T., Thalji N.M., Hagler M., Jurk D., Smith L.A., Casaclang-Verzosa G., Zhu Y., Schafer M.J., Tchkonia T., Kirkland J.L., Miller J.D. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15(5):973–977. doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McHugh D., Gil J. Senescence and aging: causes, consequences, and therapeutic avenues. J. Cell Biol. 2018;217(1):65–77. doi: 10.1083/jcb.201708092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gorbunova V., Seluanov A., Mao Z., Hine C. Changes in DNA repair during aging. Nucleic Acids Res. 2007;35(22):7466–7474. doi: 10.1093/nar/gkm756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Feng Z., Hu W., Teresky A.K., Hernando E., Cordon-Cardo C., Levine A.J. Declining p53 function in the aging process: a possible mechanism for the increased tumor incidence in older populations. Proceed. Nat. Acad. Sci. United States of America. 2007;104(42):16633–16638. doi: 10.1073/pnas.0708043104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lombard D.B., Chua K.F., Mostoslavsky R., Franco S., Gostissa M., Alt F.W. DNA repair, genome stability, and aging. Cell. 2005;120(4):497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 174.Greaves L.C., Preston S.L., Tadrous P.J., Taylor R.W., Barron M.J., Oukrif D., Leedham S.J., Deheragoda M., Sasieni P., Novelli M.R., Jankowski J.A.Z., Turnbull D.M., Wright N.A., McDonald S.A.C. Mitochondrial DNA mutations are established in human colonic stem cells, and mutated clones expand by crypt fission. Proceed. Nat. Acad. Sci. United States of America. 2006;103(3):714–719. doi: 10.1073/pnas.0505903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Busque L., Patel J.P., Figueroa M.E., Vasanthakumar A., Provost S., Hamilou Z., Mollica L., Li J., Viale A., Heguy A., Hassimi M., Socci N., Bhatt P.K., Gonen M., Mason C.E., Melnick A., Godley L.A., Brennan C.W., Abdel-Wahab O., Levine R.L. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat. Genet. 2012;44(11):1179–1181. doi: 10.1038/ng.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hsieh J.C.F., Van Den Berg D., Kang H., Hsieh C.-L., Lieber M.R. Large chromosome deletions, duplications, and gene conversion events accumulate with age in normal human colon crypts. Aging Cell. 2013;12(2):269–279. doi: 10.1111/acel.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Genovese G., Kähler A.K., Handsaker R.E., Lindberg J., Rose S.A., Bakhoum S.F., Chambert K., Mick E., Neale B.M., Fromer M., Purcell S.M., Svantesson O., Landén M., Höglund M., Lehmann S., Gabriel S.B., Moran J.L., Lander E.S., Sullivan P.F., Sklar P., Grönberg H., Hultman C.M., McCarroll S.A. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 2014;371(26):2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jaiswal S., Fontanillas P., Flannick J., Manning A., Grauman P.V., Mar B.G., Lindsley R.C., Mermel C.H., Burtt N., Chavez A., Higgins J.M., Moltchanov V., Kuo F.C., Kluk M.J., Henderson B., Kinnunen L., Koistinen H.A., Ladenvall C., Getz G., Correa A., Banahan B.F., Gabriel S., Kathiresan S., Stringham H.M., McCarthy M.I., Boehnke M., Tuomilehto J., Haiman C., Groop L., Atzmon G., Wilson J.G., Neuberg D., Altshuler D., Ebert B.L. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014;371(26):2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kim K.-M., Shibata D. Methylation reveals a niche: stem cell succession in human colon crypts. Oncogene. 2002;21(35):5441–5449. doi: 10.1038/sj.onc.1205604. [DOI] [PubMed] [Google Scholar]
- 180.Lopez-Garcia C., Klein A.M., Simons B.D., Winton D.J. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 2010;330(6005):822–825. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- 181.Snippert H.J., Van Der Flier L.G., Sato T., Van Es J.H., Van Den Born M., Kroon-Veenboer C., Barker N., Klein A.M., Van Rheenen J., Simons B.D. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143(1):134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 182.Nicholson A.M., Olpe C., Hoyle A., Thorsen A.S., Rus T., Colombe M., Brunton-Sim R., Kemp R., Marks K., Quirke P., Malhotra S., Ten Hoopen R., Ibrahim A., Lindskog C., Myers M.B., Parsons B., Tavare S., Wilkinson M., Morrissey E., Winton D.J. Fixation and spread of somatic mutations in adult human colonic epithelium. Cell Stem Cell. 2018;22(6):909–918. doi: 10.1016/j.stem.2018.04.020. e908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yokoyama A., Kakiuchi N., Yoshizato T., Nannya Y., Suzuki H., Takeuchi Y., Shiozawa Y., Sato Y., Aoki K., Kim S.K., Fujii Y., Yoshida K., Kataoka K., Nakagawa M.M., Inoue Y., Hirano T., Shiraishi Y., Chiba K., Tanaka H., Sanada M., Nishikawa Y., Amanuma Y., Ohashi S., Aoyama I., Horimatsu T., Miyamoto S., Tsunoda S., Sakai Y., Narahara M., Brown J.B., Sato Y., Sawada G., Mimori K., Minamiguchi S., Haga H., Seno H., Miyano S., Makishima H., Muto M., Ogawa S. Age-related remodelling of oesophageal epithelia by mutated cancer drivers. Nature. 2019;565(7739):312–317. doi: 10.1038/s41586-018-0811-x. [DOI] [PubMed] [Google Scholar]
- 184.Biteau B., Hochmuth C.E., Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3(4):442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Amcheslavsky A., Jiang J., Ip Y.T. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4(1):49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Buchon N., Broderick N.A., Chakrabarti S., Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Gen. Develop. 2009;23(19):2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Biteau B., Karpac J., Supoyo S., Degennaro M., Lehmann R., Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010;6(10) doi: 10.1371/journal.pgen.1001159. e1001159-e1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Biteau B., Hochmuth C.E., Jasper H. Maintaining tissue homeostasis: dynamic control of somatic stem cell activity. Cell Stem Cell. 2011;9(5):402–411. doi: 10.1016/j.stem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Choi N.-H., Kim J.-G., Yang D.-J., Kim Y.-S., Yoo M.-A. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell. 2008;7(3):318–334. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Patel P.H., Dutta D., Edgar B.A. Niche appropriation by Drosophila intestinal stem cell tumours. Nat. Cell Biol. 2015;17(9):1182–1192. doi: 10.1038/ncb3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen H., Zheng X., Zheng Y. Age-associated loss of lamin-B leads to systemic inflammation and gut hyperplasia. Cell. 2014;159(4):829–843. doi: 10.1016/j.cell.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li H., Qi Y., Jasper H. Preventing age-related decline of gut compartmentalization limits microbiota dysbiosis and extends lifespan. Cell Host Microbe. 2016;19(2):240–253. doi: 10.1016/j.chom.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Garcia A.M., Calder R.B., Dolle M.E., Lundell M., Kapahi P., Vijg J. Age- and temperature-dependent somatic mutation accumulation in Drosophila melanogaster. PLoS Genet. 2010;6(5) doi: 10.1371/journal.pgen.1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Park J.S., Lee S.H., Na H.J., Pyo J.H., Kim Y.S., Yoo M.A. Age- and oxidative stress-induced DNA damage in Drosophila intestinal stem cells as marked by Gamma-H2AX. Exp. Gerontol. 2012;47(5):401–405. doi: 10.1016/j.exger.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 195.Kauppila T.E.S., Bratic A., Jensen M.B., Baggio F., Partridge L., Jasper H., Gronke S., Larsson N.G. Mutations of mitochondrial DNA are not major contributors to aging of fruit flies. Proc. Natl. Acad. Sci. U. S. A. 2018;115(41):E9620–E9629. doi: 10.1073/pnas.1721683115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Siudeja K., Nassari S., Gervais L., Skorski P., Lameiras S., Stolfa D., Zande M., Bernard V., Rio Frio T., Bardin A.J. Frequent somatic mutation in adult intestinal stem cells drives neoplasia and genetic mosaicism during aging. Cell Stem Cell. 2015;17(6):663–674. doi: 10.1016/j.stem.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Resende L.P., Monteiro A., Bras R., Lopes T., Sunkel C.E. Aneuploidy in intestinal stem cells promotes gut dysplasia in Drosophila. J. Cell Biol. 2018;217(11):3930–3946. doi: 10.1083/jcb.201804205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Milholland B., Auton A., Suh Y., Vijg J. Age-related somatic mutations in the cancer genome. Oncotarget. 2015;6(28):24627–24635. doi: 10.18632/oncotarget.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Barker N., Van Es J.H., Kuipers J., Kujala P., Van Den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 200.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154(2):274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 201.Tao S., Tang D., Morita Y., Sperka T., Omrani O., Lechel A., Sakk V., Kraus J., Kestler H.A., Kühl M., Rudolph K.L. Wnt activity and basal niche position sensitize intestinal stem and progenitor cells to DNA damage. EMBO J. 2015;34(5):624–640. doi: 10.15252/embj.201490700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Nalapareddy K., Nattamai K.J., Kumar R.S., Karns R., Wikenheiser-Brokamp K.A., Sampson L.L., Mahe M.M., Sundaram N., Yacyshyn M.-B., Yacyshyn B., Helmrath M.A., Zheng Y., Geiger H. Canonical wnt signaling ameliorates aging of intestinal stem cells. Cell Rep. 2017;18(11):2608–2621. doi: 10.1016/j.celrep.2017.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pentinmikko N., Iqbal S., Mana M., Andersson S., Cognetta A.B., Suciu R.M., Roper J., Luopajärvi K., Markelin E., Gopalakrishnan S., Smolander O.-P., Naranjo S., Saarinen T., Juuti A., Pietiläinen K., Auvinen P., Ristimäki A., Gupta N., Tammela T., Jacks T., Sabatini D.M., Cravatt B.F., Yilmaz Ö.H., Katajisto P. Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature. 2019;571(7765):398–402. doi: 10.1038/s41586-019-1383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ruzankina Y., Pinzon-Guzman C., Asare A., Ong T., Pontano L., Cotsarelis G., Zediak V.P., Velez M., Bhandoola A., Brown E.J. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1(1):113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Murga M., Bunting S., Montaña M.F., Soria R., Mulero F., Cañamero M., Lee Y., McKinnon P.J., Nussenzweig A., Fernandez-Capetillo O. A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging. Nat. Genet. 2009;41(8):891–898. doi: 10.1038/ng.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lindström M.S., Jurada D., Bursac S., Orsolic I., Bartek J., Volarevic S. Nucleolus as an emerging hub in maintenance of genome stability and cancer pathogenesis. Oncogene. 2018;37(18):2351–2366. doi: 10.1038/s41388-017-0121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bartek J., Hodny Z. Old blood stem cells feel the stress. Nature. 2014;512:140. doi: 10.1038/nature13652. [DOI] [PubMed] [Google Scholar]
- 208.Shlush L.I., Zandi S., Mitchell A., Chen W.C., Brandwein J.M., Gupta V., Kennedy J.A., Schimmer A.D., Schuh A.C., Yee K.W., McLeod J.L., Doedens M., Medeiros J.J.F., Marke R., Kim H.J., Lee K., McPherson J.D., Hudson T.J., Consortium H.P.-L.G.P., Brown A.M.K., Yousif F., Trinh Q.M., Stein L.D., Minden M.D., Wang J.C.Y., Dick J.E. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506(7488):328–333. doi: 10.1038/nature13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Xie M., Lu C., Wang J., McLellan M.D., Johnson K.J., Wendl M.C., McMichael J.F., Schmidt H.K., Yellapantula V., Miller C.A., Ozenberger B.A., Welch J.S., Link D.C., Walter M.J., Mardis E.R., Dipersio J.F., Chen F., Wilson R.K., Ley T.J., Ding L. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med. 2014;20(12):1472–1478. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Beerman I., Seita J., Inlay Matthew A., Weissman Irving L., Rossi Derrick J. Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell. 2014;15(1):37–50. doi: 10.1016/j.stem.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Walter D., Lier A., Geiselhart A., Thalheimer F.B., Huntscha S., Sobotta M.C., Moehrle B., Brocks D., Bayindir I., Kaschutnig P., Muedder K., Klein C., Jauch A., Schroeder T., Geiger H., Dick T.P., Holland-Letz T., Schmezer P., Lane S.W., Rieger M.A., Essers M.A.G., Williams D.A., Trumpp A., Milsom M.D. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature. 2015;520:549. doi: 10.1038/nature14131. [DOI] [PubMed] [Google Scholar]
- 212.Mohrin M., Bourke E., Alexander D., Warr M.R., Barry-Holson K., Le Beau M.M., Morrison C.G., Passegué E. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7(2):174–185. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Pruitt S.C., Bailey K.J., Freeland A. Reduced Mcm2 expression results in severe stem/progenitor cell deficiency and cancer. Stem Cell. 2007;25(12):3121–3132. doi: 10.1634/stemcells.2007-0483. [DOI] [PubMed] [Google Scholar]
- 214.O'Driscoll M., Ruiz-Perez V.L., Woods C.G., Jeggo P.A., Goodship J.A. A splicing mutation affecting expression of ataxia–telangiectasia and Rad3–related protein (ATR) results in Seckel syndrome. Nat. Genet. 2003;33(4):497–501. doi: 10.1038/ng1129. [DOI] [PubMed] [Google Scholar]
- 215.Lessel D., Vaz B., Halder S., Lockhart P.J., Marinovic-Terzic I., Lopez-Mosqueda J., Philipp M., Sim J.C.H., Smith K.R., Oehler J., Cabrera E., Freire R., Pope K., Nahid A., Norris F., Leventer R.J., Delatycki M.B., Barbi G., von Ameln S., Högel J., Degoricija M., Fertig R., Burkhalter M.D., Hofmann K., Thiele H., Altmüller J., Nürnberg G., Nürnberg P., Bahlo M., Martin G.M., Aalfs C.M., Oshima J., Terzic J., Amor D.J., Dikic I., Ramadan K., Kubisch C. Mutations in SPRTN cause early onset hepatocellular carcinoma, genomic instability and progeroid features. Nat. Genet. 2014;46(11):1239–1244. doi: 10.1038/ng.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Maskey R.S., Kim M.S., Baker D.J., Childs B., Malureanu L.A., Jeganathan K.B., Machida Y., van Deursen J.M., Machida Y.J. Spartan deficiency causes genomic instability and progeroid phenotypes. Nat. Commun. 2014;5:5744. doi: 10.1038/ncomms6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lopez-Mosqueda J., Maddi K., Prgomet S., Kalayil S., Marinovic-Terzic I., Terzic J., Dikic I. SPRTN is a mammalian DNA-binding metalloprotease that resolves DNA-protein crosslinks. eLife. 2016;5 doi: 10.7554/eLife.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Vaz B., Popovic M., Newman J.A., Fielden J., Aitkenhead H., Halder S., Singh A.N., Vendrell I., Fischer R., Torrecilla I., Drobnitzky N., Freire R., Amor D.J., Lockhart P.J., Kessler B.M., McKenna G.W., Gileadi O., Ramadan K. Metalloprotease SPRTN/DVC1 orchestrates replication-coupled DNA-protein crosslink repair. Mol. Cell. 2016;64(4):704–719. doi: 10.1016/j.molcel.2016.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Curran S.P., Ruvkun G. Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet. 2007;3(4) doi: 10.1371/journal.pgen.0030056. e56-e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Halaschek-Wiener J., Khattra J.S., McKay S., Pouzyrev A., Stott J.M., Yang G.S., Holt R.A., Jones S.J.M., Marra M.A., Brooks-Wilson A.R., Riddle D.L. Analysis of long-lived C. elegans daf-2 mutants using serial analysis of gene expression. Genome Res. 2005;15(5):603–615. doi: 10.1101/gr.3274805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liu J.C., Lerou P.H., Lahav G. Stem cells: balancing resistance and sensitivity to DNA damage. Trends Cell Biol. 2014;24(5):268–274. doi: 10.1016/j.tcb.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jeggo P.A., Downs J.A., Gasser S.M. Chromatin modifiers and remodellers in DNA repair and signalling. Phil. Trans. Roy. Soc. Lond. B Biol. Sci. 2017;372(1731):20160279. doi: 10.1098/rstb.2016.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ferrand J., Plessier A., Polo S.E. Control of the chromatin response to DNA damage: histone proteins pull the strings. Semin. Cell Dev. Biol. 2020 doi: 10.1016/j.semcdb.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 224.Sulkowski P.L., Oeck S., Dow J., Economos N.G., Mirfakhraie L., Liu Y., Noronha K., Bao X., Li J., Shuch B.M., King M.C., Bindra R.S., Glazer P.M. Oncometabolites suppress DNA repair by disrupting local chromatin signalling. Nature. 2020;582(7813):586–591. doi: 10.1038/s41586-020-2363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Booth Lauren N., Brunet A. The aging epigenome. Mol. Cell. 2016;62(5):728–744. doi: 10.1016/j.molcel.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.O'Sullivan R.J., Kubicek S., Schreiber S.L., Karlseder J. Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nat. Struct. Mol. Biol. 2010;17(10):1218–1225. doi: 10.1038/nsmb.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sidler C., Wóycicki R., Ilnytskyy Y., Metz G., Kovalchuk I., Kovalchuk O. Immunosenescence is associated with altered gene expression and epigenetic regulation in primary and secondary immune organs. Front. Genet. 2013;4 doi: 10.3389/fgene.2013.00211. 211-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.McCauley B.S., Dang W. Histone methylation and aging: lessons learned from model systems. Biochim. Biophys. Acta. 2014;1839(12):1454–1462. doi: 10.1016/j.bbagrm.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sidler C., Kovalchuk O., Kovalchuk I. Epigenetic regulation of cellular senescence and aging. Front. Genet. 2017;8 doi: 10.3389/fgene.2017.00138. 138-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Oberdoerffer P., Sinclair D.A. The role of nuclear architecture in genomic instability and ageing. Nat. Rev. Mol. Cell Biol. 2007;8:692. doi: 10.1038/nrm2238. [DOI] [PubMed] [Google Scholar]
- 231.Tsurumi A., Li W.X. Global heterochromatin loss: a unifying theory of aging? Epigenetics. 2012;7(7):680–688. doi: 10.4161/epi.20540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Pegoraro G., Kubben N., Wickert U., Göhler H., Hoffmann K., Misteli T. Ageing-related chromatin defects through loss of the NURD complex. Nat. Cell Biol. 2009;11(10):1261–1267. doi: 10.1038/ncb1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Pollina E.A., Brunet A. Epigenetic regulation of aging stem cells. Oncogene. 2011;30:3105. doi: 10.1038/onc.2011.45. [DOI] [PubMed] [Google Scholar]
- 234.Scaffidi P., Misteli T. Lamin A-dependent nuclear defects in human aging. Science (New York, N.Y.) 2006;312(5776):1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhang W., Li J., Suzuki K., Qu J., Wang P., Zhou J., Liu X., Ren R., Xu X., Ocampo A., Yuan T., Yang J., Li Y., Shi L., Guan D., Pan H., Duan S., Ding Z., Li M., Yi F., Bai R., Wang Y., Chen C., Yang F., Li X., Wang Z., Aizawa E., Goebl A., Soligalla R.D., Reddy P., Esteban C.R., Tang F., Liu G.-H., Belmonte J.C.I. A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science. 2015;348(6239):1160. doi: 10.1126/science.aaa1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jin C., Li J., Green Christopher D., Yu X., Tang X., Han D., Xian B., Wang D., Huang X., Cao X., Yan Z., Hou L., Liu J., Shukeir N., Khaitovich P., Chen Charlie D., Zhang H., Jenuwein T., Han J.-Dong J. Histone demethylase UTX-1 regulates C. elegans life span by targeting the insulin/IGF-1 signaling pathway. Cell Metabol. 2011;14(2):161–172. doi: 10.1016/j.cmet.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 237.Larson K., Yan S.-J., Tsurumi A., Liu J., Zhou J., Gaur K., Guo D., Eickbush T.H., Li W.X. Heterochromatin formation promotes longevity and represses ribosomal RNA synthesis. PLoS Genet. 2012;8(1) doi: 10.1371/journal.pgen.1002473. e1002473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kaeberlein M., McVey M., Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Gen. Develop. 1999;13(19):2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Dang W., Steffen K.K., Perry R., Dorsey J.A., Johnson F.B., Shilatifard A., Kaeberlein M., Kennedy B.K., Berger S.L. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature. 2009;459:802. doi: 10.1038/nature08085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Mostoslavsky R., Chua K.F., Lombard D.B., Pang W.W., Fischer M.R., Gellon L., Liu P., Mostoslavsky G., Franco S., Murphy M.M., Mills K.D., Patel P., Hsu J.T., Hong A.L., Ford E., Cheng H.-L., Kennedy C., Nunez N., Bronson R., Frendewey D., Auerbach W., Valenzuela D., Karow M., Hottiger M.O., Hursting S., Barrett J.C., Guarente L., Mulligan R., Demple B., Yancopoulos G.D., Alt F.W. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124(2):315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 241.Toiber D., Erdel F., Bouazoune K., Silberman D.M., Zhong L., Mulligan P., Sebastian C., Cosentino C., Martinez-Pastor B., Giacosa S., D'Urso A., Näär A.M., Kingston R., Rippe K., Mostoslavsky R. SIRT6 recruits SNF2H to DNA break sites, preventing genomic instability through chromatin remodeling. Mol. Cell. 2013;51(4):454–468. doi: 10.1016/j.molcel.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kanfi Y., Naiman S., Amir G., Peshti V., Zinman G., Nahum L., Bar-Joseph Z., Cohen H.Y. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 243.Mao Z., Hine C., Tian X., Van Meter M., Au M., Vaidya A., Seluanov A., Gorbunova V. SIRT6 promotes DNA repair under stress by activating PARP1. Science (New York, N.Y.) 2011;332(6036):1443–1446. doi: 10.1126/science.1202723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ermolaeva M., Neri F., Ori A., Rudolph K.L. Cellular and epigenetic drivers of stem cell ageing. Nat. Rev. Mol. Cell Biol. 2018;19(9):594–610. doi: 10.1038/s41580-018-0020-3. [DOI] [PubMed] [Google Scholar]
- 245.Imai S.-i., Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24(8):464–471. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Verdin E. "NAD+ in aging, metabolism, and neurodegeneration. Science. 2015;350(6265):1208. doi: 10.1126/science.aac4854. [DOI] [PubMed] [Google Scholar]
- 247.Li J., Bonkowski M.S., Moniot S., Zhang D., Hubbard B.P., Ling A.J.Y., Rajman L.A., Qin B., Lou Z., Gorbunova V., Aravind L., Steegborn C., Sinclair D.A. "A conserved NAD+ binding pocket that regulates protein-protein interactions during aging. Science. 2017;355(6331):1312. doi: 10.1126/science.aad8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Petkovich D.A., Podolskiy D.I., Lobanov A.V., Lee S.-G., Miller R.A., Gladyshev V.N. Using DNA methylation profiling to evaluate biological age and longevity interventions. Cell Metabol. 2017;25(4):954–960. doi: 10.1016/j.cmet.2017.03.016. e956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Field A.E., Robertson N.A., Wang T., Havas A., Ideker T., Adams P.D. DNA methylation clocks in aging: categories, causes, and consequences. Mol. Cell. 2018;71(6):882–895. doi: 10.1016/j.molcel.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Horvath S., Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018;19(6):371–384. doi: 10.1038/s41576-018-0004-3. [DOI] [PubMed] [Google Scholar]
- 251.Bell C.G., Lowe R., Adams P.D., Baccarelli A.A., Beck S., Bell J.T., Christensen B.C., Gladyshev V.N., Heijmans B.T., Horvath S., Ideker T., Issa J.-P.J., Kelsey K.T., Marioni R.E., Reik W., Relton C.L., Schalkwyk L.C., Teschendorff A.E., Wagner W., Zhang K., Rakyan V.K. DNA methylation aging clocks: challenges and recommendations. Genome Biol. 2019;20(1) doi: 10.1186/s13059-019-1824-y. 249-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hahn O., Grönke S., Stubbs T.M., Ficz G., Hendrich O., Krueger F., Andrews S., Zhang Q., Wakelam M.J., Beyer A., Reik W., Partridge L. Dietary restriction protects from age-associated DNA methylation and induces epigenetic reprogramming of lipid metabolism. Genome Biol. 2017;18(1) doi: 10.1186/s13059-017-1187-1. 56-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Jeggo P.A., Downs J.A. Roles of chromatin remodellers in DNA double strand break repair. Exp. Cell Res. 2014;329(1):69–77. doi: 10.1016/j.yexcr.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 254.Brownlee P.M., Meisenberg C., Downs J.A. The SWI/SNF chromatin remodelling complex: its role in maintaining genome stability and preventing tumourigenesis. DNA Repair. 2015;32:127–133. doi: 10.1016/j.dnarep.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 255.Schwartzentruber J., Korshunov A., Liu X.-Y., Jones D.T.W., Pfaff E., Jacob K., Sturm D., Fontebasso A.M., Quang D.-A.K., Tönjes M., Hovestadt V., Albrecht S., Kool M., Nantel A., Konermann C., Lindroth A., Jäger N., Rausch T., Ryzhova M., Korbel J.O., Hielscher T., Hauser P., Garami M., Klekner A., Bognar L., Ebinger M., Schuhmann M.U., Scheurlen W., Pekrun A., Frühwald M.C., Roggendorf W., Kramm C., Dürken M., Atkinson J., Lepage P., Montpetit A., Zakrzewska M., Zakrzewski K., Liberski P.P., Dong Z., Siegel P., Kulozik A.E., Zapatka M., Guha A., Malkin D., Felsberg J., Reifenberger G., von Deimling A., Ichimura K., Collins V.P., Witt H., Milde T., Witt O., Zhang C., Castelo-Branco P., Lichter P., Faury D., Tabori U., Plass C., Majewski J., Pfister S.M., Jabado N. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 256.Bjerke L., Mackay A., Nandhabalan M., Burford A., Jury A., Popov S., Bax D.A., Carvalho D., Taylor K.R., Vinci M., Bajrami I., McGonnell I.M., Lord C.J., Reis R.M., Hargrave D., Ashworth A., Workman P., Jones C. Histone H3.3. mutations drive pediatric glioblastoma through upregulation of MYCN. Canc. Discov. 2013;3(5):512–519. doi: 10.1158/2159-8290.CD-12-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Piazzesi A., Papić D., Bertan F., Salomoni P., Nicotera P., Bano D. Replication-independent histone variant H3.3 controls animal lifespan through the regulation of pro-longevity transcriptional programs. Cell Rep. 2016;17(4):987–996. doi: 10.1016/j.celrep.2016.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Bano D., Piazzesi A., Salomoni P., Nicotera P. The histone variant H3.3 claims its place in the crowded scene of epigenetics. Aging. 2017;9(3):602–614. doi: 10.18632/aging.101194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hwang J.K., Alt F.W., Yeap L.S. Related mechanisms of antibody somatic hypermutation and class switch recombination. Microbiol. Spectr. 2015;3(1) doi: 10.1128/microbiolspec.MDNA3-0037-2014. MDNA3-0037-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Mao E.F., Lane L., Lee J., Miller J.H. Proliferation of mutators in A cell population. J. Bacteriol. 1997;179(2):417–422. doi: 10.1128/jb.179.2.417-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Bjedov I., Tenaillon O., Gerard B., Souza V., Denamur E., Radman M., Taddei F., Matic I. Stress-induced mutagenesis in bacteria. Science. 2003;300(5624):1404–1409. doi: 10.1126/science.1082240. [DOI] [PubMed] [Google Scholar]
- 262.Teixeira V.H., Pipinikas C.P., Pennycuick A., Lee-Six H., Chandrasekharan D., Beane J., Morris T.J., Karpathakis A., Feber A., Breeze C.E., Ntolios P., Hynds R.E., Falzon M., Capitanio A., Carroll B., Durrenberger P.F., Hardavella G., Brown J.M., Lynch A.G., Farmery H., Paul D.S., Chambers R.C., McGranahan N., Navani N., Thakrar R.M., Swanton C., Beck S., George P.J., Spira A., Campbell P.J., Thirlwell C., Janes S.M. Deciphering the genomic, epigenomic, and transcriptomic landscapes of pre-invasive lung cancer lesions. Nat. Med. 2019;25(3):517–525. doi: 10.1038/s41591-018-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Riddiford N., Siudeja S., van den Beek M., Boumard B., Bardin A.J. Evolution and genomic signatures of spontaneous somatic mutation in Drosophila intestinal stem cells. bioRxiv. 2020 doi: 10.1101/2020.07.20.188979. [DOI] [PMC free article] [PubMed] [Google Scholar]