Abstract
Background
The prevalence and health-related quality of life of skin disease have been understudied in adolescents.
Objective
To investigate the prevalence and relevant patient-reported outcomes of noncommunicable skin diseases in college students.
Methods
First-year college students from 5 universities in China were investigated in the cross-sectional study. Skin diseases were diagnosed by dermatologists in the field survey. Itch and pain, symptoms of depression and anxiety, and sleep quality were measured by validated tools.
Results
A total of 28,364 students consented to participate and completed the survey. The prevalence of acne, eczema, chronic urticaria, psoriasis, and vitiligo was 10.3%, 5.8%, 2.6%, 0.16%, and 0.23%, respectively. Eczema and chronic urticaria were associated with lower health utility estimates. Most diseases, but not psoriasis and vitiligo, were associated with the symptoms of depression and sleep disturbance. Itch intensity predicted other patient-reported outcome scores better in healthy controls than in individuals with skin diseases. Sex difference in the associations of skin diseases with patient-reported outcomes was not identified.
Limitations
Cross-sectional study design and limited generalizability to the nonstudent population.
Conclusion
Skin diseases are associated with moderately impaired emotional well-being, sleep quality, and quality of life, partly attributable to cutaneous symptoms.
Key words: college student, health-related quality of life, noncommunicable skin diseases, patient-reported outcomes
Abbreviation used: AOR, adjusted odds ratio
Capsule Summary.
-
•
This study provides estimates of the prevalence and health-related quality of life of noncommunicable skin diseases among college students in China.
-
•
Dermatologists and primary care physicians should pay attention to adverse mental and behavioral effects of skin diseases, in addition to visible skin lesions and cutaneous symptoms.
Introduction
Skin diseases affect a large number of people and are associated with a heavy burden of disease worldwide. They contributed to 1.79% of the global burden of disease measured in disability-adjusted life-years in 2013, according to the estimation by the Global Burden of Disease Study.1 The top skin diseases that contributed to disability-adjusted life-years (per 100,000 persons) were noncommunicable diseases: dermatitis (128.7), acne (96.7), urticaria (67.0), and psoriasis (66.8).1
The disability weights for noncommunicable skin diseases were mainly determined by the level of disfigurement and cutaneous symptoms. However, many skin diseases are associated with neglected but important aspects of impaired health-related quality of life. For example, itch occurring in skin diseases such as eczema has been associated with subsequent clinical outcomes such as sleep disturbances and psychological and behavioral disorders.2,3 It was reported that at least 30% of patients with skin diseases have significant psychological morbidities.4 The most common psychological symptoms observed in dermatologic patients are depression and anxiety.5,6 Except symptoms and disfigurement, the underlying mechanisms of inflammatory skin diseases, such as changes in cytokine levels, cardiometabolic profiles, and microbiome diversity, may also be involved in the development of sleep and mental disorders.7, 8, 9 Patient-reported outcomes, widely used as the end points in clinical and epidemiologic studies, capture the effects of diseases or interventions from the patient's perspective.10 However, the effects of skin disorders on these patient-reported outcomes show variations in the magnitude owing to differences in culture, setting, the tool of measurement, criteria of diagnosis, etc. The Berkson bias in hospital-based studies11,12 may further result in a biased estimation of the burden of disease.
Adolescents with skin diseases usually experience more pronounced effects in regard to self-esteem, stigmatization, bullying, and family and social relationships compared with their disease-free peers.13 However, there is a lack of community-based studies on the prevalence and health-related quality of life of skin diseases among adolescents in China. The current study aimed to investigate the prevalence of noncommunicable skin diseases and to describe the symptoms of itch and pain, sleep disturbance, depression and anxiety, and quality of life through a cross-sectional survey to provide population-based evidence for the estimation of prevalence and burden of skin diseases.
Methods
Study design and participants
This was a cross-sectional study. Data collected from 2017 to 2018 were used for analysis. In 2017, a university in Changsha, China, was selected for the pilot study.14,15 In 2018, 5 universities in different regions of China were selected in the main study (Supplemental Fig 1).16 The university included in the pilot study was also included in the main study. The first-year college students who consented to participate underwent a health examination and completed an online questionnaire survey immediately after their enrollment in the universities.
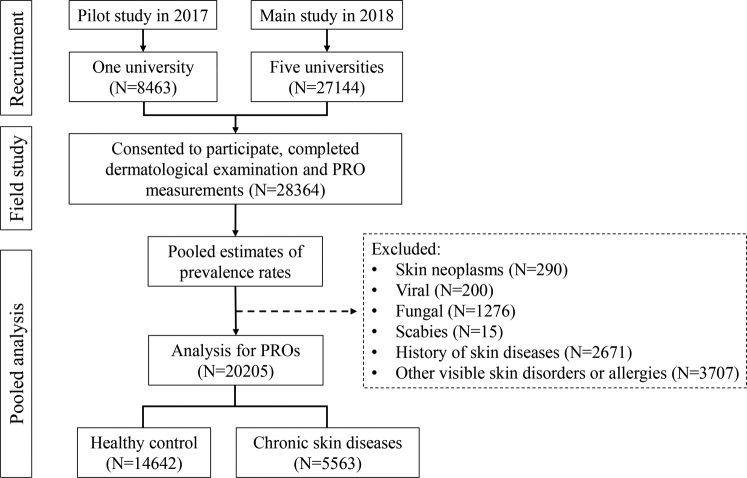
To describe the patient-reported outcomes, cases and controls were defined. The cases were defined as participants receiving a diagnosis of noncommunicable skin diseases in the field survey, including moderate to severe acne, eczema, chronic urticaria, psoriasis, vitiligo, and rosacea. The diseases were selected owing to either a heavy burden of disease or relatively high prevalence. A healthy control was defined as having no visible skin diseases (Fig 1).
Fig 1.
Flow chart for the inclusion of study participants. PRO, Patient-reported outcome.
Clinical evaluation
Diagnosis of skin diseases and disease history taking were performed by certified dermatologists during the dermatologic examination. Clinical manifestation, disease history, and family history of participants were inquired about, and physical examinations were conducted to diagnose all skin diseases. Moderate to severe acne was defined as grade 2 to 4 according to the grading system.17 Atopic dermatitis was diagnosed according to the guideline from the American Academy of Dermatology.18 Chronic urticaria was defined as having persistent or recurrent wheals for more than 6 weeks.19 Other skin diseases were diagnosed according to clinical features.
Certain cases were further evaluated after diagnosis. Psoriasis was evaluated with the Psoriasis Area and Severity Index. Vitiligo was evaluated with the Vitiligo Area Scoring Index. Atopic dermatitis was evaluated with the Scoring Atopic Dermatitis instrument.
Patient-reported outcome measures
Owing to the feasibility of a large-scale population-based investigation, validated short tools were used in our study. Itch and pain of the skin were measured by the numeric rating scale. Depression and anxiety were measured by the 2-item Patient Health Questionnaire and Generalized Anxiety Disorder–2 scale, respectively. Sleep quality was measured by the Pittsburgh Sleep Quality Index. Quality of life was measured by the generic tool EQ-5D-3L and mapped to health utility estimates according to the method proposed by Liu et al.20
Generalized Anxiety Disorder–2 and 2-item Patient Health Questionnaire scores were also dichotomized by the cutoff greater than or equal to 3, according to previous studies.21,22 Itch and pain numeric rating scale scores were dichotomized by the cutoff greater than or equal to 3 (moderate to severe).23 Pittsburgh Sleep Quality Index scores were dichotomized by the cutoff greater than or equal to 6, according to a previous validation study of US college students.24
Statistical analyses
Sex-specific prevalence was reported, and the prevalence rate was not standardized by age because most participants were aged approximately 18 years. Means and standard deviations of the patient-reported outcome scores were described and compared with the Dunnett t test. Mixed linear or generalized linear models (individual as level 1 and university as level 2) were used to deal with the intracluster correlations and estimate the associations of skin diseases with patient-reported outcome scores. The effect size was presented as mean difference for continuous data and adjusted odds ratio (AOR) for categoric data. All effect sizes were adjusted for level 1 covariates (age and sex) and level 2 random effects (university). Correlations among patient-reported outcome scores were analyzed with mixed models stratified by skin disease. Subgroup analysis was performed by sex. Sensitivity analysis was conducted by excluding data collected in the pilot study and using the cutoff greater than or equal to 7 for the numeric rating scale (severe itch). Statistical analyses were performed with SAS (version 9.4, SAS Institute, Inc, Cary, NC). P < .005 was considered statistically significant for multiple comparisons.
Results
A total of 8463 and 27,144 college students were newly enrolled in the universities in 2017 and 2018, respectively. Among them, 28,364 participants (response rate 79.7%) consented to participate and completed the field dermatologic examination and an online questionnaire. Moderate to severe acne (10.2%), eczema (5.8%), rosacea (3.0%), and chronic urticaria (2.6%) were the most common skin diseases among the participants (Table I). Significant sex differences were observed in the prevalence rates of acne (men > women), eczema (men < women), chronic spontaneous urticaria (men < women), and rosacea (men < women), according to the 95% uncertainty intervals.
Table I.
Prevalence of noncommunicable skin diseases in college students
| Skin disease | Prevalence per 1000 (95% UI) | Sex prevalence per 1000 (95% UI) |
|
|---|---|---|---|
| Women | Men | ||
| N | 28,364 | 13,185 | 15,179 |
| Moderate to severe acne | 103.1 (99.6–106.7) | 78.0 (73.5–82.6) | 124.9 (119.6–130.2) |
| Eczema | 58.3 (55.6–61.1) | 66.9 (62.6–71.2) | 50.9 (47.4–54.4) |
| Atopic dermatitis | 32.4 (30.3–34.4) | 37.2 (33.9–40.4) | 28.2 (25.6–30.8) |
| Chronic urticaria | 25.6 (23.8–27.5) | 27.0 (24.2–29.8) | 24.4 (22.0–26.9) |
| CSU | 14.9 (13.5–16.3) | 17.1 (14.9–19.3) | 13.0 (11.2–14.8) |
| CIU | 10.8 (9.6–12.0) | 9.9 (8.2–11.6) | 11.5 (9.8–13.2) |
| Psoriasis | 1.6 (1.1–2.0) | 1.7 (1.0–2.5) | 1.4 (0.8–2.1) |
| Vitiligo | 2.3 (1.7–2.8) | 1.6 (0.9–2.3) | 2.9 (2.0–3.8) |
| Rosacea | 30.0 (28.0–32.0) | 42.0 (38.6–45.4) | 19.6 (17.4–21.8) |
CIU, Chronic inducible urticaria; CSU, chronic spontaneous urticaria; UI, uncertainty interval.
After exclusion of participants with skin neoplasms, infectious skin diseases, skin diseases with acute episodes, and other irrelevant skin diseases, 14,642 healthy controls and 5563 case patients with noncommunicable skin diseases were included in the analysis for the patient-reported outcomes (Fig 1). The mean age was 18.4 ± 0.8 years, and 45% were women. The mixed linear model identified small intracluster correlations for the patient-reported outcome scores at the university level, varying from 0.9% to 2.9%. Patients with atopic dermatitis, psoriasis, and vitiligo received an assessment for the area and severity of skin lesions (Table II). All patients with psoriasis (Psoriasis Area and Severity Index score 2.4 ± 2.1; maximum 6.9) had mild cases, and all patients with atopic dermatitis (Scoring Atopic Dermatitis score 15.9 ± 8.4; maximum 26.7) and vitiligo (Vitiligo Area Scoring Index score 1.8 ± 2.5; maximum 10) had mild to moderate cases.
Table II.
Demographic characteristics and patient-reported outcomes of the participants by skin diseases
| Group | N | Age, years | Women, % | PRO measures score (mean ± SD) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Itch NRS | Pain NRS | PHQ-2 | GAD-2 | PSQI | Health utility | ||||
| Healthy controls | 14,642 | 18.4 ± 0.8 | 6614 (45.2) | 1.00 ± 1.36 | 0.31 ± 0.79 | 0.74 ± 1.11 | 0.79 ± 1.12 | 3.64 ± 2.72 | 0.97 ± 0.07 |
| Noncommunicable skin diseases∗ | 5563 | 18.3 ± 0.7 | 2527 (45.4) | 1.56 ± 1.83 | 0.44 ± 1.02 | 0.86 ± 1.21 | 0.92 ± 1.20 | 4.12 ± 2.62 | 0.97 ± 0.07 |
| Moderate to severe acne | 2902 | 18.3 ± 0.8 | 1019 (35.1) | 1.31 ± 1.60 | 0.39 ± 0.93 | 0.77 ± 1.13 | 0.82 ± 1.08 | 3.86 ± 2.62 | 0.97 ± 0.06 |
| Eczema | 1644 | 18.2 ± 0.7 | 876 (53.3) | 2.13 ± 2.18 | 0.57 ± 1.23 | 1.10 ± 1.40 | 1.18 ± 1.36 | 4.71 ± 2.61 | 0.96 ± 0.12 |
| Atopic dermatitis | 910 | 18.2 ± 0.7 | 486 (53.4) | 2.25 ± 2.3 | 0.52 ± 1.22 | 0.95 ± 1.25 | 1.02 ± 1.21 | 4.51 ± 2.53 | 0.97 ± 0.12 |
| Chronic urticaria | 716 | 18.2 ± 0.7 | 351 (49.0) | 1.98 ± 2.07 | 0.45 ± 0.97 | 0.85 ± 1.21 | 0.94 ± 1.23 | 4.04 ± 2.88 | 0.97 ± 0.14 |
| Chronic spontaneous urticaria | 414 | 18.3 ± 0.7 | 220 (53.1) | 2.07 ± 2.12 | 0.42 ± 0.89 | 0.93 ± 1.26 | 0.98 ± 1.25 | 4.23 ± 3.07 | 0.96 ± 0.14 |
| Chronic inducible urticaria | 302 | 18.3 ± 0.7 | 131 (43.4) | 1.87 ± 2.01 | 0.49 ± 1.07 | 0.74 ± 1.14 | 0.89 ± 1.21 | 3.78 ± 2.58 | 0.96 ± 0.06 |
| Psoriasis | 45 | 18.4 ± 1.0 | 23 (51.1) | 2.58 ± 2.74 | 1.02 ± 2.28 | 0.73 ± 1.12 | 1.00 ± 1.48 | 4.04 ± 2.66 | 0.97 ± 0.05 |
| Vitiligo | 64 | 18.4 ± 0.8 | 21 (32.8) | 1.32 ± 1.96 | 0.46 ± 0.82 | 0.86 ± 1.21 | 0.89 ± 1.16 | 3.84 ± 2.92 | 0.97 ± 0.06 |
| Rosacea | 851 | 18.2 ± 0.7 | 554 (65.1) | 1.32 ± 1.64 | 0.37 ± 0.90 | 0.90 ± 1.25 | 0.99 ± 1.23 | 4.33 ± 2.32 | 0.97 ± 0.06 |
GAD-2, Generalized Anxiety Disorder–2; NRS, numeric rating scale; PHQ, 2-item Patient Health Questionnaire; PRO, patient-reported outcome; PSQI, Pittsburgh Sleep Quality Index; SD, standard deviation.
This is a combined group of all the conditions listed beneath it (as subsets).
Compared with that of healthy controls, most skin diseases were associated with higher patient-reported outcome scores, with different effect sizes (Table I). Health utility estimates mapping from the EQ-5D-3L showed small variations across the groups. After adjustments for age and sex, all skin diseases except vitiligo were associated with stronger itch (Supplemental Fig 2). Psoriasis (mean difference 1.63; P < .001), eczema and atopic dermatitis (mean difference 1.12; P < .001), and chronic spontaneous urticaria (mean difference 1.06; P < .001) showed relatively strong itch intensity among the skin diseases (Supplemental Table I). Eczema and chronic urticaria were associated with higher 2-item Patient Health Questionnaire and Generalized Anxiety Disorder–2 scores. Unexpectedly, psoriasis and vitiligo were not significantly correlated with the symptoms of depression and anxiety. The effects of skin diseases on sleep disturbance were similar to those of depression and anxiety. Eczema and chronic inducible urticaria were significantly associated with lower health utility estimates.
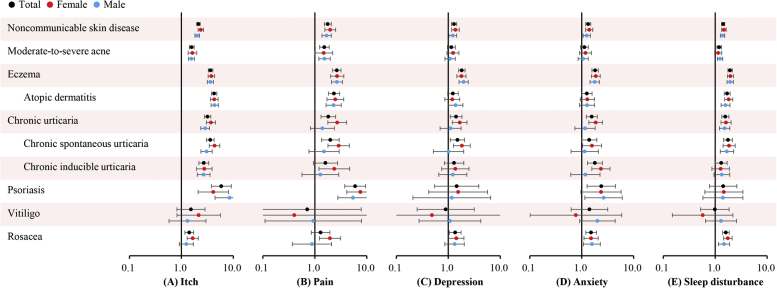
Patient-reported outcome scores were then categorized by clinical cutoffs (Fig 2 and Supplemental Table 2). Psoriasis and atopic dermatitis were associated with the highest risks of moderate to severe itch, with AORs of 5.87 (95% uncertainty interval 3.78-9.11; P < .001) and 4.29 (95% uncertainty interval 3.81-4.82; P < .001), respectively. Eczema was associated with the highest risk of depression (AOR 1.83; 95% uncertainty interval 1.58-2.11; P < .001), followed by chronic spontaneous urticaria (AOR 1.50; P = .012), and rosacea (AOR 1.36; P = .02). Psoriasis was associated with the highest risk of anxiety (AOR 2.38; 95% uncertainty interval 1.27-4.46), followed up eczema (AOR 1.81; P < .001), chronic inducible urticaria (AOR 1.80; P < .001), and rosacea (AOR 1.51; P < .001). The skin disorders were also associated with higher risks of sleep disturbance, and the magnitude was similar to that of depression.
Fig 2.
Adjusted odds ratios for patient-reported outcomes of noncommunicable skin diseases compared with that of healthy controls. A, Moderate to severe itch (numeric rating scale score ≥3). B, Moderate to severe pain (numeric rating scale score ≥3). C, Depression (2-item Patient Health Questionnaire score ≥3). D, Anxiety (Generalized Anxiety Disorder–2 score ≥3). E, Sleep disturbance (Pittsburgh Sleep Quality Index score ≥6).
Correlations of itch numeric rating scale score with anxiety, depression, and sleep disturbance were investigated with mixed models, stratified by disease (Table III). In general, itch numeric rating scale score predicted other patient-reported outcome scores better in healthy controls than in case patients with skin diseases. There was a lack of statistical associations of itch with other patient-reported outcomes among patients with vitiligo. The association of itch with depression was also insignificant in patients with psoriasis or chronic inducible urticaria.
Table III.
Associations of itch with symptoms of anxiety and depression and sleep disturbance by skin diseases
| Group | Associations of itch NRS score with scores of other PROs (adjusted β, 95% UI)∗ |
||
|---|---|---|---|
| PHQ-2 | GAD-2 | PSQI | |
| Healthy controls | 0.18 (0.16, 0.19) | 0.18 (0.17, 0.19) | 0.50 (0.47, 0.53) |
| Noncommunicable skin diseases† | 0.15 (0.13, 0.17) | 0.15 (0.14, 0.17) | 0.38 (0.34, 0.42) |
| Moderate-to-severe acne | 0.15 (0.13, 0.18) | 0.17 (0.14, 0.19) | 0.43 (0.37, 0.49) |
| Eczema | 0.13 (0.10, 0.17) | 0.14 (0.11, 0.17) | 0.34 (0.28, 0.39) |
| Atopic dermatitis | 0.13 (0.10, 0.17) | 0.13 (0.09, 0.16) | 0.34 (0.26, 0.41) |
| Chronic urticaria | 0.14 (0.10, 0.18) | 0.12 (0.08, 0.17) | 0.36 (0.26, 0.46) |
| Chronic spontaneous urticaria | 0.18 (0.13, 0.24) | 0.14 (0.09, 0.20) | 0.38 (0.25, 0.52) |
| Chronic inducible urticaria | 0.06 (0.00, 0.13) | 0.09 (0.03, 0.16) | 0.31 (0.17, 46) |
| Psoriasis | 0.07 (–0.05, 0.20) | 0.18 (0.01, 0.35) | 0.41 (0.14, 0.70) |
| Vitiligo | 0.10 (–0.06, 0.27) | 0.03 (–0.13, 0.18) | 0.18 (–0.22, 0.57) |
| Rosacea | 0.22 (0.17, 0.27) | 0.21 (0.16, 0.26) | 0.36 (0.27, 0.46) |
GAD-2, Generalized Anxiety Disorder–2; NRS, numeric rating scale; PHQ, 2-item Patient Health Questionnaire; PRO, patient-reported outcome; PSQI, Pittsburgh Sleep Quality Index; UI, uncertainty interval.
Adjusted for level 1 covariates (age and sex) and level 2 random effects (university).
This is a combined group of all the conditions listed beneath it (as subsets).
Subgroup analysis identified no sex differences in patient-reported outcome values or risks because most 95% uncertainty intervals overlapped (Fig 2). Sensitivity analysis by excluding data collected in the pilot study identified no significant changes in results. Sensitivity analysis using the cutoff of itch numeric rating scale score greater than or equal to 7 found a stronger effect of noncommunicable skin diseases on severe itch (AOR 4.20; 95% uncertainty interval 3.14-5.62; P < .001) than on moderate to severe itch (AOR 2.13; 95% uncertainty interval 1.98-2.30; P < .001). However, the analysis for specific skin diseases was not fulfilled owing to misconvergence of the model.
Discussion
We investigated the prevalence and relevant patient-reported outcomes of noncommunicable skin diseases among first-year college students in China. Moderate to severe acne, eczema, rosacea, and chronic urticaria were the most common skin diseases. The symptoms of depression and anxiety and sleep disturbance were more common in patients with skin diseases compared with healthy controls, and these outcomes were in part explained by cutaneous symptoms, whereas variations were observed in psoriasis, vitiligo, inducible urticaria, and rosacea. Eczema and chronic urticaria showed small but significant decreases in health utility estimates mapping from the EQ-5D-3L. To our knowledge, this was the first community-based study that systematically investigated the prevalence and patient-reported outcomes of noncommunicable skin diseases in a representative sample of college students in China.
Eczema and urticaria were associated with a 0.0177 and 0.0116 decrease in health utility estimates, respectively. Similarly, in the Global Burden of Disease Study 2010, eczema (0.0391) and urticaria (0.0321) had the highest average disability weights except for decubitus ulcers.25 Inducible urticaria rather than spontaneous urticaria was found to be associated with lower utility estimates in our study. Inducible urticaria can be triggered by changes in physical environments, such as pressure, heat, cold, friction, and vibration. A known trigger for urticaria may limit a patient's daily activities such as sports, bathing, clothing, and sex. In contrast, spontaneous urticaria can occur with no identifiable causes. The unpredictability of spontaneous urticaria may impair the activity less as measured by the EQ-5D-3L. Studies also showed that the duration of disease was longer, disease control was poorer, and severe complaints were more frequent in patients with chronic inducible urticaria.26
A paradoxic finding is that psoriasis and vitiligo were not associated with depression. Because appearance plays an important role in adolescence, with a social emphasis for homogeneity in the context of China and many other countries, the visibility of skin lesions may impair self-esteem and further result in emotional and behavioral problems.27 This unexpected finding, however, is consistent with the finding of a case-control study by Bilgic et al28 that investigated 48 psoriatic patients and healthy controls younger than 18 years. The sample size and severity of the disease might be the reasons for the negative result. In our field study, most patients had mild psoriasis (mean Psoriasis Area and Severity Index score 2.4) or vitiligo (mean Vitiligo Area Scoring Index score 1.8) at presentation. Besides, only 45 psoriasis and 64 vitiligo cases were identified, and the large sample size of the control group would not further increase the power of the test.
The correlation analysis for patient-reported outcome measurements showed that itch intensity predicted symptoms of anxiety and depression and sleep disturbance better in healthy controls than in patients with skin diseases. This indicates that although cutaneous symptoms could be an intermediator that links skin disorders and adverse psychological outcomes, disfigurement or underlying inflammatory mechanisms might be additional pathways to psychological comorbidities. Paradoxically, there was a lack of association of itch with depression in patients with psoriasis, inducible urticaria, or vitiligo. This indicates that, although they reported relatively strong itch, they presented less depressiveness. We therefore propose a resilience hypothesis for this. Psoriasis and inducible urticaria developed in childhood and adolescence can be viewed as continued trauma owing to the long course (in our sample, the course of psoriasis was 0.2-9 years, and the mean course was 2.5 years) and recurrent (or inducible) nature, whereas successful adaptation to this adversity results in continued normative or even enhanced development and well-being. A study showed that psoriatic patients had a significantly higher prevalence of childhood trauma and a lower resilience level compared with healthy controls.29 But our study participants had just enrolled in the universities owing to their excellent performance on the college entrance examination; this can be viewed as a sign of successful adaptation. In this regard, survivor bias might have been introduced.
A primary limitation of this study is the limited generalizability among adolescents who do not attend university. The burden of skin diseases in nonstudents of similar age may be different from that in students, particularly given potential socioeconomic differences. According to recent Chinese national statistics, the male-to-female ratio was 1.18 among people aged 15 to 19 years,30 and in our study, the ratio was 1.15 (15,179:13,185). This indicates that the proportion of sex distribution in our study participants was similar to that in the general population of similar age. Second, the sample size was small for skin diseases with a relatively low prevalence rate, such as psoriasis and vitiligo. This resulted in insufficient power of hypothesis tests. Third, owing to the limitation of a cross-sectional study, we could not confirm that these outcomes were attributable to skin diseases. Reverse causal relationships are possible. Last, the Generalized Anxiety Disorder–7 or the 9-item Patient Health Questionnaire was not used to evaluate for the symptoms of anxiety and depression. Nevertheless, previous studies have showed that both the Generalized Anxiety Disorder–2 and 2-item Patient Health Questionnaire had high sensitivity and specificity in screening for generalized anxiety disorder and major depressive disorder, respectively.21,22
The study also has strengths. First, this was a study among a group of college students who experienced similar social transition and comparable exposure to physical environments. In contrast, hospital-based studies have apparent selection bias because patients have a strong willingness to seek help owing to intolerable symptoms, impaired function, and decreased quality of life. As a result, the impairment in health-related quality of life is generally overestimated in hospital patients. Community samples provide a more representative estimation of health-related quality of life in public health settings. Second, the skin diseases were ascertained by dermatologists from the top hospitals of our study sites. Even in population-based studies such as the National Health and Nutrition Examination Survey, skin diseases were reported by the participants. Self-reports may result in misclassification bias, especially among participants with limited literacy. Third, measurements were performed with validated generic tools that enable comparisons across different skin diseases and with healthy controls. Unfortunately, the EQ-5D-3L showed a strong ceiling effect because most skin diseases did not affect function and daily activity among our study participants.
In summary, the study provides estimates of prevalence and health-related quality of life of skin diseases in college students in China. The data obtained from this representative sample of college students provides evidence for studies of disease burden. The clinical implication is that dermatologists and primary care physicians should pay attention to the adverse mental and behavioral effects of skin diseases, in addition to visible skin lesions and cutaneous symptoms.
Acknowledgments
The authors would like to thank the following dermatologists and investigators who participated in the field survey.
Central South University: Lei Cai, Duling Cao, Qin Cao, Chao Chen, Liping Chen, Menglin Chen, Mengting Chen, Xiang Chen, Qing Deng, Xin Gao, Yihuan Gong, Jia Guo, Yeye Guo, Rui Hu, Xin Hu, Chuchu Huang, Huining Huang, Kai Huang, Xiaoyan Huang, Yuzhou Huang, Danrong Jing, Xinwei Kuang, Li Lei, Jia Li, Jiaorui Li, Jie Li, Keke Li, Peiyao Li, Yajia Li, Yayun Li, Yangfan Li, Dan Liu, Dihui Liu, Fangfen Liu, Nian Liu, Panoan Liu, Runqiu Liu, Hui Lu, Wenhua Lu, Yan Luo, Zhongling Luo, Manyun Mao, Mengping Mao, Yuyan Ouyang, Shiyao Pei, Qunshi Qin, Ke Sha, Lirong Tan, Ling Tang, Ni Tang, Yan Tang, Ben Wang, Yaling Wang, Tianhao Wu, Yun Xie, Siyu Yan, Sha Yan, Bei Yan, Xizhao Yang, Lin Ye, Hu Yuan, Taolin Yu, Yan Yuan, Yi Yu, Rui Zhai, Jianghua Zhang, Jianglin Zhang, Mi Zhang, Xingyu Zhang, Zhibao Zhang, Yaqian Zhao, Kuangbiao Zhong, Lei Zhou, Youyou Zhou, Zhe Zhou, and Susi Zhu.
Huazhong University of Science and Technology: Xiangjie An, Siqi Da, Yaqi Dong, Yangxue Fu, Lixie Gao, Han Han, Biling Jiang, Jiajia Lan, Jun Li, Xiaonan Li, Yan Li, Liquan Liu, Yuchen Lou, Pu Meng, Yingli Nie, Gong Rao, Shanshan Sha, Xingyu Su, Huinan Suo, Rongying Wang, Jun Xie, Yuanxiang Yi, Jia Zhang, Qiao Zhang, Li Zhu, and Yanming Zhu.
Xiamen University: Zhiming Cai, Lina Chen, Xiaozhu Fu, Hongjun Jiang, Guihua Luo, Jianbing Xiahou, and Binxiang Zheng.
People's Hospital of Xinjiang Uygur Autonomous Region: Jianxia Chen, Xiaomin Chen, Xinqi Chen, Li Dai, Yanyan Feng, Fanhe Jiang, Lan Jin, Qingyu Ma, Qun Shi, Hongbo Tang, Fang Wang, Zhen Wang, Xiujuan Wu, Kunjie Zhang, and Yu Zhang.
Xinjiang Medical University: Huagui Li, Jianguang Li, and Lei Shi.
Inner Mongolia Medical University: Wei Wang, Rina Wu, Hongjun Xing, and Baogui Yang.
Footnotes
Funding sources: Supported by National Key Research and Development Project of China Precision Medicine Initiative grant 2016YFC0900802.
Conflicts of interest: None disclosed.
Reprints not available from the author(s).
IRB approval status: Reviewed and approved by the institutional research ethics boards of Xiangya Hospital, Central South University, Changsha, China (201709993).
Contributor Information
Yi Xiao, Email: xiaoyixy@csu.edu.cn.
Xiang Chen, Email: chenxiangck@126.com.
Supplementary data
References
- 1.Karimkhani C., Dellavalle R.P., Coffeng L.E. Global skin disease morbidity and mortality: an update from the Global Burden of Disease Study 2013. JAMA Dermatol. 2017;153(5):406–412. doi: 10.1001/jamadermatol.2016.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garg N., Silverberg J.I. Association between childhood allergic disease, psychological comorbidity, and injury requiring medical attention. Ann Allergy Asthma Immunol. 2014;112(6):525–532. doi: 10.1016/j.anai.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Silverberg J.I., Garg N.K., Paller A.S., Fishbein A.B., Zee P.C. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135(1):56–66. doi: 10.1038/jid.2014.325. [DOI] [PubMed] [Google Scholar]
- 4.Picardi A., Abeni D., Melchi C.F., Puddu P., Pasquini P. Psychiatric morbidity in dermatological outpatients: an issue to be recognized. Br J Dermatol. 2000;143(5):983–991. doi: 10.1046/j.1365-2133.2000.03831.x. [DOI] [PubMed] [Google Scholar]
- 5.Bao Q.Y., Chen L.N., Lu Z.Y. Association between eczema and risk of depression: a systematic review and meta-analysis of 188,495 participants. J Affect Disord. 2018;238:458–464. doi: 10.1016/j.jad.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Kuo C.L., Chen C.Y., Huang H.L. Increased risk of major depression subsequent to a first-attack and non-infection caused urticaria in adolescence: a nationwide population-based study. BMC Pediatr. 2014;14:181. doi: 10.1186/1471-2431-14-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiecolt-Glaser J.K., Derry H.M., Fagundes C.P. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075–1091. doi: 10.1176/appi.ajp.2015.15020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irwin M.R., Olmstead R., Carroll J.E. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan L.R., Zhao S., Zhu W. The Akkermansia muciniphila is a gut microbiota signature in psoriasis. Exp Dermatol. 2018;27(2):144–149. doi: 10.1111/exd.13463. [DOI] [PubMed] [Google Scholar]
- 10.Shen M.X., Chen X. Minimally important change: approach to interpretation of patient-reported outcomes. Br J Dermatol. 2019 doi: 10.1111/bjd.18711. [DOI] [PubMed] [Google Scholar]
- 11.Balieva F., Kupfer J., Lien L. The burden of common skin diseases assessed with the EQ5D: a European multicentre study in 13 countries. Br J Dermatol. 2017;176(5):1170–1178. doi: 10.1111/bjd.15280. [DOI] [PubMed] [Google Scholar]
- 12.Dalgard F.J., Gieler U., Tomas-Aragones L. The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J Invest Dermatol. 2015;135(4):984–991. doi: 10.1038/jid.2014.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez J., Cunningham K., Perlmutter J., Gottlieb A. Systematic review of health-related quality of life in adolescents with psoriasis. Dermatology. 2016;232(5):541–549. doi: 10.1159/000450826. [DOI] [PubMed] [Google Scholar]
- 14.Huang X., Zhang J., Li J. Daily intake of soft drinks and moderate-to-severe acne vulgaris in Chinese adolescents. J Pediatr. 2019;204:256–262.e3. doi: 10.1016/j.jpeds.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 15.Xiao Y., Huang X., Jing D. The prevalence of atopic dermatitis and chronic spontaneous urticaria are associated with parental socioeconomic status in adolescents in China. Acta Derm Venereol. 2019;99(3):321–326. doi: 10.2340/00015555-3104. [DOI] [PubMed] [Google Scholar]
- 16.Wu T., Su J., Zhao S., Chen X., Shen M. Development and assessment of a brief tool to measure melanoma-related health literacy and attitude among adolescents. J Cancer Educ. 2019 doi: 10.1007/s13187-019-01541-2. [DOI] [PubMed] [Google Scholar]
- 17.Witkowski J.A., Parish L.C. The assessment of acne: an evaluation of grading and lesion counting in the measurement of acne. Clin Dermatol. 2004;22(5):394–397. doi: 10.1016/j.clindermatol.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Eichenfield L.F., Tom W.L., Chamlin S.L. Guidelines of care for the management of atopic dermatitis section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338–351. doi: 10.1016/j.jaad.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuberbier T., Aberer W., Asero R. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73(7):1393–1414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 20.Liu G.G., Wu H., Li M., Gao C., Luo N. Chinese time trade-off values for EQ-5D health states. Value Health. 2014;17(5):597–604. doi: 10.1016/j.jval.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z.W., Yu Y., Hu M., Liu H.M., Zhou L., Xiao S.Y. PHQ-9 and PHQ-2 for screening depression in Chinese rural elderly. PLoS One. 2016;11(3):e0151042. doi: 10.1371/journal.pone.0151042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plummer F., Manea L., Trepel D., McMillan D. Screening for anxiety disorders with the GAD-7 and GAD-2: a systematic review and diagnostic metaanalysis. Gen Hosp Psychiatry. 2016;39:24–31. doi: 10.1016/j.genhosppsych.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Reich A., Chatzigeorkidis E., Zeidler C. Tailoring the cut-off values of the visual analogue scale and numeric rating scale in itch assessment. Acta Derm Venereol. 2017;97(6):759–760. doi: 10.2340/00015555-2642. [DOI] [PubMed] [Google Scholar]
- 24.Dietch J.R., Taylor D.J., Sethi K., Kelly K., Bramoweth A.D., Roane B.M. Psychometric evaluation of the PSQI in US college students. J Clin Sleep Med. 2016;12(8):1121–1129. doi: 10.5664/jcsm.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization . Department of Health Statistics and Information Systems, WHO; Geneva, Switzerland: 2013. WHO methods and data sources for global burden of disease estimates 2000-2011 (global health estimates technical paper WHO/HIS/HSI/GHE/2013.4) [Google Scholar]
- 26.Kocaturk E., Kiziltac U., Can P. Validation of the Turkish version of the Urticaria Control Test: correlation with other tools and comparison between spontaneous and inducible chronic urticaria. World Allergy Organ J. 2019;12(1):100009. doi: 10.1016/j.waojou.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofmann S.G., Anu Asnaani M.A., Hinton D.E. Cultural aspects in social anxiety and social anxiety disorder. Depress Anxiety. 2010;27(12):1117–1127. doi: 10.1002/da.20759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bilgic A., Bilgic Ö., Akış H.K., Eskioğlu F., Kılıç E.Z. Psychiatric symptoms and health-related quality of life in children and adolescents with psoriasis. Pediatr Dermatol. 2010;27(6):614–617. doi: 10.1111/j.1525-1470.2010.01195.x. [DOI] [PubMed] [Google Scholar]
- 29.Crosta M.L., De Simone C., Di Pietro S. Childhood trauma and resilience in psoriatic patients: a preliminary report. J Psychosom Res. 2018;106:25–28. doi: 10.1016/j.jpsychores.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 30.National Bureau of Statistics of China . China Statistics Press; Beijing, China: 2019. China Statistical Yearbook 2019. Section 2-9: Population by Age and Sex. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.