Abstract
Background
Vitiligo is a depigmentation disorder associated with genetic loss of melanocytes and decreased melanin synthesis. The current literature is conflicting in regard to vitiligo patients' risk of cutaneous malignant melanoma and keratinocyte cancer.
Objective
To investigate the risk of cutaneous malignant melanoma and keratinocyte cancer in vitiligo patients.
Methods
We conducted a population-based study, including 2,339 subjects with a first-time vitiligo diagnosis between 1994 and 2017 and 23,293 age- and sex-matched (1:10) controls. To address surveillance bias, we included 12,380 subjects with a first-time diagnosis of lichen planus.
Results
Age was the only significant factor for cutaneous malignant melanoma in comparison of vitiligo with controls and lichen planus (hazard ratio 1.04, 95% confidence interval [CI] 1.03-1.05; and hazard ratio 1.02, 95% CI 1.01-1.04, respectively). Similarly, age was a significant factor for keratinocyte cancer in comparison of vitiligo with controls and lichen planus (hazard ratio 1.07, 95% CI 1.06-1.07; and hazard ratio 1.06, 95% CI 1.05-1.07). Male sex was an additional factor for keratinocyte cancer in comparison of vitiligo with lichen planus (hazard ratio 1.38; 95% CI 1.09-1.75). Phototherapy did not increase the risk of receiving a diagnosis of cutaneous malignant melanoma or keratinocyte cancer in the vitiligo cohort.
Conclusion
We observed no significant difference in cutaneous malignant melanoma or keratinocyte cancer risk among vitiligo subjects. Phototherapy use was not associated with a higher skin cancer risk in vitiligo compared with other skin diseases.
Key words: basal cell, epidemiology, lichen planus, melanoma, squamous cell, vitiligo
Abbreviations used: CI, confidence interval; ICD-8, International Classification of Diseases, Eighth Revision; ICD-10, International Statistical Classification of Diseases and Related Health Problems, 10th Revision
Capsule Summary.
-
•
The literature is biased and conflicting in regard to vitiligo patients' risk of cutaneous malignant melanoma and keratinocyte cancer and whether phototherapy increases the risk.
-
•
Phototherapy use was not associated with a higher skin cancer risk in vitiligo compared with other skin diseases. We did not find evidence to support recommendations for changed skin cancer surveillance for patients with vitiligo.
Introduction
Vitiligo is a chronic idiopathic depigmentation disorder affecting approximately 1% of the population regardless of age, sex, and socioeconomic status.1 Loss of epidermal melanocytes is characteristic of vitiligo and generally presents itself in a localized segmental or universal form.2,3 Although vitiligo is among the most prevalent pigmentation disorders, its exact etiology is mostly unknown; however, an elevated frequency of comorbid autoimmune diseases has been observed in patients with vitiligo, advocating autoimmune etiology.4
Malignant melanoma is a cutaneous neoplasm originating from pathogenic melanocytes. In smaller cohorts of patients receiving a diagnosis of cutaneous malignant melanoma, subsequent hypopigmentation has proved a favorable prognostic sign.5,6 These findings suggest that hypopigmentation is an indicator of robust antimelanoma immunity.7 If an autoimmune response indeed causes vitiligo, the disorder could be a protective factor against the occurrence and progression of cutaneous malignant melanoma. Genes involved in melanin synthesis are further linked to vitiligo.8,9 Furthermore, genetic studies have found an inverse relationship between vitiligo and keratinocyte cancer.10,11 Elevated autoimmunity and loss of melanocytes may protect against cutaneous malignant melanoma; however, decreased melanin synthesis may increase the photodamage and risk of keratinocyte cancers or nonmelanoma skin cancers. Phototherapy is a conventional treatment for vitiligo; however, because of decreased melanin synthesis, concerns have been raised about a potential exacerbated carcinogenic effect in patients with vitiligo.12 Previous studies investigating the risk of cutaneous malignant melanoma and keratinocyte cancer in patients with vitiligo have found conflicting results, which may be due to challenges in finding appropriate control populations and to surveillance bias.13, 14, 15 Therefore, we aimed to assess the risk of skin cancer in patients with vitiligo while addressing relevant confounders and surveillance bias.
Methods
We conducted this study in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology recommendations.16 The study was approved by the Danish Data Protection Agency, which constitutes the necessary legal requirements for the study.17
We used the Danish National Patient Register,18 which comprises information on all inpatient and outpatient hospital visits in Denmark using International Classification of Diseases, Eighth Revision (ICD-8) until 1994, and after that the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). We retrieved tax-reported household income from Statistical Denmark19 and patient age, sex, and vital status from the Danish Civil Registration System.20
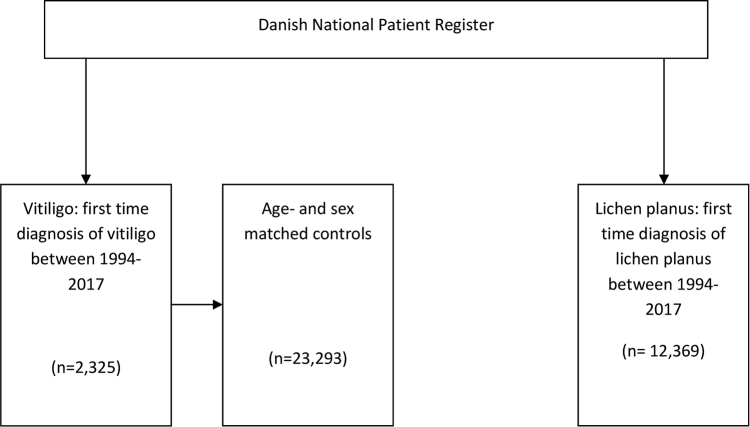
We identified all Danish patients alive and residing in Denmark with a first-time diagnosis of vitiligo (ICD-10 code L80) between January 1994 and January 2017. For the control group, we randomly matched 10 control subjects alive and residing in Denmark at the matching vitiligo subject inclusion date, and with the same sex and birth date. Control subjects had the same index date as the corresponding vitiligo subject.
To address potential surveillance bias caused by an increased frequency of dermatologist visits among patients with vitiligo, we included a second control group with lichen planus because these patients are also routinely treated by dermatologists and often treated with phototherapy. For the second control group, we identified all Danish patients alive and residing in Denmark with a first-time diagnosis of lichen planus (ICD-10 code L43) between January 1994 and January 2017.
We excluded all subjects with a history of vitiligo (ICD-8 code 70901), lichen planus (ICD-8 code 69709), or cutaneous malignant melanoma (ICD-8 code 172) from 1977 to 1994. We followed the subjects from the time of their diagnosis of vitiligo or lichen planus, or control group inclusion date, until January 2017, death, or the occurrence of cutaneous malignant melanoma, whichever came first. We did not exclude subjects with a history of keratinocyte cancer because the primary outcome was cutaneous malignant melanoma.
All statistical analysis was performed with Stata (version 14, StataCorp 2015, StataCorp LP, College Station, TX). The continuous baseline data are presented with mean ± standard deviation or median (range), depending on the distribution pattern. Categoric data are presented as number (percent). We compared the data between subjects with vitiligo and controls and subjects with vitiligo and lichen planus with unpaired t test, Mann-Whitney U test, or χ2 test, depending on the data type. Cox regression models were then performed to obtain hazard ratios for the risk of cutaneous malignant melanoma and keratinocyte cancer and adjusted for age, sex, annual income, and phototherapy sessions. Baseline variables (age and sex) were chosen for the Cox regression a priori and income and phototherapy sessions were chosen because of their significance level in the univariate analysis. The proportional hazards assumption was tested with the Schoenfeld residuals test and was found to be valid for both cutaneous malignant melanoma and keratinocyte cancer. Because vitiligo cannot clinically be distinguished from melanoma-associated leukoderma,21 we performed a sensitivity analysis and excluded all patients who received a diagnosis of melanoma less than 2 years after vitiligo diagnosis. A 2-tailed P < .05 was considered significant.
Results
In total, we identified 38,012 subjects, including 2,339 with vitiligo, 12,380 with lichen planus, and 23,293 control subjects in Denmark between 1994 and 2017 (Supplemental Fig 1). Four thousand nine hundred eighty-two subjects died in the follow-up period (Table I). The vitiligo group was significantly younger and composed of more female patients compared with the lichen planus group (P < .001 for both). During the follow-up period, more people died in the lichen planus group compared with the vitiligo group (P < .001). Slightly more subjects in the vitiligo group were receiving public welfare compared with the controls, and subjects with vitiligo had a slightly higher annual income compared with the control group (P < .05, P < .001, and P < .001, respectively). The vitiligo group had a lower annual income, whereas fewer subjects were receiving public welfare compared with the lichen planus group (P < .001 and P < .001, respectively). Subjects with vitiligo had received a significantly higher number of phototherapy sessions compared with the matched controls and lichen planus groups (P < .001 and P < .001, respectively). Keratinocyte cancer was diagnosed more frequently in the lichen planus group compared with the vitiligo group (P < .001); however, there were no differences between the number of subjects with a keratinocyte cancer diagnosis between the vitiligo and control groups. There were no differences in cutaneous malignant melanoma occurrence between the vitiligo and control group and the vitiligo and lichen planus group (Figs 1 and 2). Subjects in the vitiligo group with phototherapy sessions (412 subjects, 17.72%) did not have a higher incidence of cutaneous malignant melanoma or keratinocyte cancer than vitiligo subjects without phototherapy sessions (1913, 82.28%).
Table I.
Study group demographics and skin cancer outcomes
| Characteristics | Total, n = 37,987 | Vitiligo, n = 2325 | Control, n = 23,293 | LP, n = 12,369 | Vitiligo vs control, P value | Vitiligo vs LP, P value |
|---|---|---|---|---|---|---|
| Age, mean ± SD, y | 44.02 ± 21.20 | 37.68 ± 20.79 | 37.67 ± 20.78 | 57.19 ± 15.14 | NS | .001 |
| Deaths, No. (%) | 4982 (13.12) | 248 (10.67) | 2312 (9.93) | 2422 (19.58) | NS | .001 |
| Follow-up time,∗ mean ± SD, mo | 126.21 ± 76.88 | 128.85 ± 77.31 | 136.29 ± 76.00 | 106.72 ± 74.70 | .001 | .001 |
| Time until CMM end point,† mean ± SD, mo | 125.86 ± 76.87 | 128.53 ± 77.43 | 135.99 ± 75.98 | 106.28 ± 74.67 | .001 | .001 |
| Time until KC end point,‡ mean ± SD, mo | 125.47 ± 76.84 | 128.12 ± 77.23 | 135.75 ± 75.93 | 105.59 ± 74.58 | .001 | .001 |
| Sex, No. (%) | ||||||
| Women | 22,855 (60.1) | 1366 (58.75) | 13,654 (58.62) | 7835 (63.34) | NS | .001 |
| Men | 15,132 (39.9) | 959 (41.25) | 9639 (41.38) | 4534 (36.66) | NS | .001 |
| Social status, No. (%) | ||||||
| Employed | 22,300 (58.70) | 1358 (58.41) | 13,957 (60.00) | 6967 (56.33) | NS | NS |
| Children (<15 y) | 1502 (3.95) | 135 (5.81) | 1334 (5.73) | 33 (0.27) | NS | .001 |
| Receiving public welfare | 10,040 (26.43) | 597 (25.68) | 3954 (23.57) | 3954 (31.97) | .05 | .001 |
| Self-employed | 3679 (9.68) | 202 (8.69) | 2222 (9.54) | 1255 (10.15) | NS | .05 |
| Other§ | 150 (0.39) | 12 (0.52) | 117 (0.5) | 21 (0.17) | NS | .001 |
| Missing | 316 (0.83) | 21 (0.90) | 156 (0.67) | 139 (1.12) | NS | NS |
| Annual income, mean ± SD, $ǁ | 24,920.02 ± 29,708.51 | 24,167.2 ± 27,487.28 | 23,377.35 ± 20,482.47 | 27,980.31 ± 42,056.62 | .05 | .001 |
| Missing income, No. (%) | 316 (0.83) | 21 (0.9) | 156 (0.67) | 139 (1.12) | NS | NS |
| No. of phototherapy sessions, mean ± SD | 0.91 ± 8.17 | 8.98 ± 25.87 | 0.05 ± 1.56 | 1.00 ± 7.82 | .001 | .001 |
| No. of subjects with CMM, No. (%) | 193 (0.51) | 9 (0.39) | 105 (0.45) | 79 (0.64) | NS | NS |
| No. of subjects with KC, No. (%) | 516 (1.36) | 26 (1.12) | 226 (0.97) | 264 (2.13) | NS | .001 |
CMM, Cutaneous malignant melanoma; KC, keratinocyte cancer; LP, lichen planus; No., number; NS, not significant; SD, standard deviation.
Baseline demographics and skin cancer outcomes of the study population.
Time from inclusion until death or January 2017.
Time from inclusion until cutaneous malignant melanoma, death, or January 2017.
Time from inclusion until KC, death or January 2017.
Subjects not working, enrolled in education or right to public welfare.
US dollars were converted from Danish krones with a 100:14.95 conversion rate.
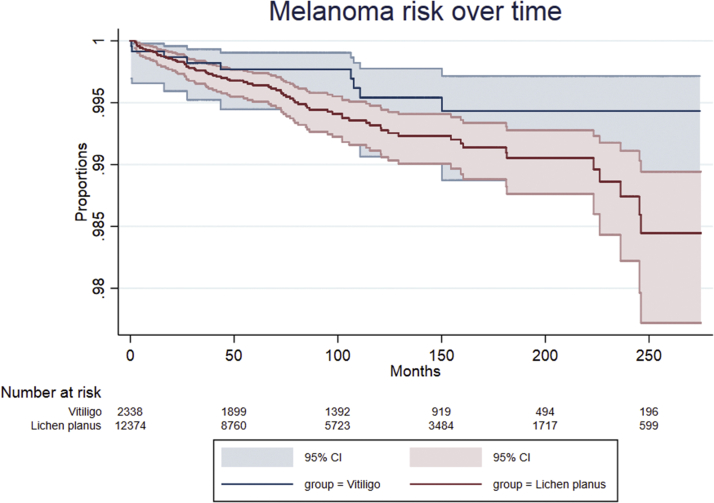
Fig 1.
Kaplan-Meier curve of melanoma risk over time stratified by vitiligo and control group. Melanoma risk over time and the subjects at risk at different points. Light blue and light red areas show the 95% confidence bands for each point. CI, Confidence interval.
Fig 2.
Kaplan Meier curve of melanoma risk over time stratified by vitiligo and lichen planus group. Melanoma risk over time and the subjects at risk at different points. Light blue and light red areas show the 95% confidence bands for each point. CI, Confidence interval.
In the multivariate cutaneous malignant melanoma analysis, age was the only significant factor for cutaneous malignant melanoma in both the vitiligo versus control and vitiligo versus lichen planus analysis (hazard ratio 1.04, 95% confidence interval [CI] 1.03-1.05, P < .001; and hazard ratio 1.02, 95% CI 1.01-1.04, P < .001, respectively) (Table II). Vitiligo diagnosis, sex, income, and phototherapy sessions were not significant risk factors.
Table II.
Multivariate cox regression of cutaneous malignant melanoma risk
| Variable | Hazard ratio | 95% Confidence interval | P value |
|---|---|---|---|
| Vitiligo vs controls | |||
| Vitiligo | 1.00 | 0.49–2.01 | NS |
| Age | 1.04 | 1.03–1.05 | <.001 |
| Men | 0.93 | 0.63–1.38 | NS |
| No. of phototherapy sessions | 0.98 | 0.93–1.04 | NS |
| Income | 1.00 | 1.00–1.00 | NS |
| Vitiligo vs lichen planus | NS | ||
| Vitiligo | 0.94 | 0.45–1.95 | NS |
| Age | 1.02 | 1.01–1.04 | <.05 |
| Men | 1.03 | 0.65–1.64 | NS |
| No. of phototherapy sessions | 0.89 | 0.74–1.08 | NS |
| Income | 1.00 | 1.00–1.00 | NS |
No, Number; NS, not significant.
The individual hazard ratio for each variable in the multivariate Cox regression analysis of cutaneous malignant melanoma risk in subjects with vitiligo versus controls and vitiligo versus lichen planus.
In the multivariate keratinocyte cancer analysis, age was the only significant factor for keratinocyte cancer (hazard ratio 1.07; 95% CI 1.06-1.07; P < .001) (Table III). When the vitiligo group was compared with the lichen planus group, age and male sex were the only significant factors for keratinocyte cancer (hazard ratio 1.06, 95% CI 1.05-1.07, P < .001; and hazard ratio 1.38, 95% CI 1.09-1.75, P < .05, respectively) (Table III). Vitiligo diagnosis, income, and phototherapy sessions were not significant risk factors. When we excluded in a sensitivity analysis all patients receiving a diagnosis of melanoma less than 2 years after the index date, we did not find any altered risk (data not shown).
Table III.
Multivariate Cox regression of keratinocyte cancer risk
| Variable | Odds ratio | Confidence interval | P value |
|---|---|---|---|
| Vitiligo vs controls | |||
| Vitiligo | 1.4 | 0.9–2.1 | NS |
| Age | 1.07 | 1.06–1.07 | <.001 |
| Men | 1.10 | 0.85–1.42 | NS |
| No. of phototherapy sessions | 0.98 | 0.94–1.02 | NS |
| Income | 1.00 | 1.00–1.00 | NS |
| Vitiligo vs lichen planus | |||
| Vitiligo | 0.95 | 0.63–1.44 | NS |
| Age | 1.06 | 1.05–1.07 | <.001 |
| Men | 1.38 | 1.09 –1.75 | <.05 |
| No. of phototherapy sessions | 1.00 | 0.98–1.00 | NS |
| Income | 1.00 | 1.00–1.00 | NS |
No., Number; NS, not significant.
The individual odds ratio for each variable in the multivariate logistic regression analysis of keratinocyte cancer risk in subjects with vitiligo versus controls and vitiligo versus lichen planus.
Discussion
In this nationwide cohort study, we investigated skin cancer risk in subjects with vitiligo. We compared vitiligo subjects with matched controls and compared vitiligo subjects with subjects with lichen planus to address surveillance bias. We further adjusted for age, sex, income, and phototherapy sessions and found no altered risk of skin cancer in subjects with vitiligo. Age was the only predictor for cutaneous malignant melanoma. It was also the only risk factor for keratinocyte cancer compared with the control population. However, when the vitiligo and lichen planus populations were compared, age and sex were the only significant factors for keratinocyte cancer. We did not find phototherapy sessions to be a particularly greater risk factor for receiving a diagnosis of cutaneous malignant melanoma or keratinocyte cancer in vitiligo compared with other phototherapy indications.
Two other nationwide register studies have previously been published, both with Asian populations.15,22 In a large recent study, Kim et al15 compared vitiligo subjects in Korea with age- and sex-matched controls (1:20) and found an increased risk of cutaneous malignant melanoma and keratinocyte cancer in vitiligo patients. Li et al22 compared vitiligo subjects in Taiwan with estimated cancer incidences in the general Taiwanese population and found no statistically significant altered risk of cutaneous malignant melanoma or keratinocyte cancer. The findings of Kim et al15 also differ from the findings in white populations.23, 24, 25 Three studies in the white population with a control group exist.23, 24, 25 Two of these studies found lower incidences of cutaneous malignant melanoma and keratinocyte cancer in vitiligo patients23,24 and 1 found similar incidence of keratinocyte cancer in vitiligo patients.25 The discrepancy between the current literature may be due to challenges in finding proper control groups, adjusting for confounders, and surveillance bias of the vitiligo cohort. Further ethnic differences make comparisons between Asian and white populations difficult.26 In Asian cultures, the incidence of cutaneous malignant melanoma is low and, like vitiligo, the melanoma has a predilection for acral surfaces.15 As noted by Kim et al,15 their findings may therefore be subject to surveillance bias. In our study, we addressed surveillance bias, which has not previously been accounted for in the literature, to our knowledge. We further adjusted for age, sex, income, and phototherapy sessions and did not find vitiligo patients to have an increased risk of receiving a diagnosis of cutaneous malignant melanoma or keratinocyte cancer. Compared with previous studies, our study also had a considerably longer follow-up period. However, even longer follow-up time is needed to ascertain our findings because vitiligo is commonly diagnosed first in young adults,1,9 and cutaneous malignant melanoma in Denmark is more common in the elderly (>60 years).27 We did not find phototherapy sessions to be related to cutaneous malignant melanoma or keratinocyte cancer in vitiligo patients. This is in line with the only other study of whites that adjusted for phototherapy sessions24 and a small study of long-term phototherapy use in vitiligo patients.28 Our findings that cutaneous malignant melanoma is associated with increasing age and that keratinocyte cancer is associated with increased age and sex are overall in line with the literature.29,30
Although we addressed surveillance bias by comparing our vitiligo cohort with a lichen planus cohort, this study was not without limitations. Because this was a register-based study, we could not adjust for the subjects' skin type, sun exposure, or the extent and distribution of vitiligo, which are potential confounders for cutaneous malignant melanoma and keratinocyte cancer. However, this is a general issue in most register studies using administrative-claims-based data.15,22 We did not adjust for vitiligo-associated autoimmune diseases,31 which are potential confounders, and future studies should investigate whether there is a link to cutaneous malignant melanoma and keratinocyte cancer.32 We included fewer patients in our study compared with the 2 aforementioned studies largely owing to methodological differences.15,22,23 We restricted our observations to subjects with a first-time vitiligo diagnosis, whereas the other studies included all subjects with vitiligo, regardless of when they received a diagnosis. We further performed a sensitivity analysis by excluding all subjects receiving a diagnosis of melanoma less than 2 years after the index date and found no change in outcome. This is likely because our diagnosis was rooted in pathology reports and not a clinical diagnosis of melanoma. Last, our findings may not be generalizable because genetics and baseline ultraviolet exposure vary in other populations.
In this study, we did not find subjects with vitiligo to have an altered risk of developing cutaneous malignant melanoma and keratinocyte cancer compared with subjects without vitiligo. Phototherapy sessions were not associated with increased risk of receiving a diagnosis of skin cancer in vitiligo patients compared with other phototherapy indications. We did not find evidence to support recommendations for a changed skin cancer surveillance for patients with vitiligo.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
This clinical trial was approved by the institutional review body.
Supplementary Data
Supplemental Fig 1.
References
- 1.Krüger C., Schallreuter K.U. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51(10):1206–1212. doi: 10.1111/j.1365-4632.2011.05377.x. [DOI] [PubMed] [Google Scholar]
- 2.Ezzedine K., Eleftheriadou V., Whitton M., van Geel N. Vitiligo. Lancet. 2015;386(9988):74–84. doi: 10.1016/S0140-6736(14)60763-7. [DOI] [PubMed] [Google Scholar]
- 3.Taïeb A., Picardo M., VETF Members The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res. 2007;20(1):27–35. doi: 10.1111/j.1600-0749.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- 4.Gill L., Zarbo A., Isedeh P., Jacobsen G., Lim H.W., Hamzavi I. Comorbid autoimmune diseases in patients with vitiligo: a cross-sectional study. J Am Acad Dermatol. 2016;74(2):295–302. doi: 10.1016/j.jaad.2015.08.063. [DOI] [PubMed] [Google Scholar]
- 5.Quaglino P., Marenco F., Osella-Abate S. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol. 2010;21(2):409–414. doi: 10.1093/annonc/mdp325. [DOI] [PubMed] [Google Scholar]
- 6.Bystryn J.C., Rigel D., Friedman R.J., Kopf A. Prognostic significance of hypopigmentation in malignant melanoma. Arch Dermatol. 1987;123(8):1053–1055. [PubMed] [Google Scholar]
- 7.Teulings H.-E., Limpens J., Jansen S.N. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33(7):773–781. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 8.Jin Y., Birlea S.A., Fain P.R. Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N Engl J Med. 2010;362(18):1686–1697. doi: 10.1056/NEJMoa0908547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alkhateeb A., Fain P.R., Thody A., Bennett D.C., Spritz R.A. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16(3):208–214. doi: 10.1034/j.1600-0749.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 10.Wu W., Amos C.I., Lee J.E., Wei Q., Sarin K.Y., Han J. Inverse relationship between vitiligo-related genes and skin cancer risk. J Invest Dermatol. 2018;138(9):2072–2075. doi: 10.1016/j.jid.2018.03.1511. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues M. Skin cancer risk (nonmelanoma skin cancers/melanoma) in vitiligo patients. Dermatol Clin. 2017;35(2):129–134. doi: 10.1016/j.det.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Taieb A., Alomar A., Böhm M. Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol. 2013;168(1):5–19. doi: 10.1111/j.1365-2133.2012.11197.x. [DOI] [PubMed] [Google Scholar]
- 13.Ban L., Labbouz S., Grindlay D., Batchelor J.M., Ratib S. Risk of skin cancer in people with vitiligo: a systematic review and meta-analysis. Br J Dermatol. 2018;179(4):971–972. doi: 10.1111/bjd.16703. [DOI] [PubMed] [Google Scholar]
- 14.Hammoud S., Kruis R., Sigurdsson V. Prediction of the occurrence of melanoma and non-melanoma skin cancer in patients with vitiligo. Acta Derm Venereol. 2016;96(1):106–107. doi: 10.2340/00015555-2179. [DOI] [PubMed] [Google Scholar]
- 15.Kim H.S., Kim H.J., Hong E.S. The incidence and survival of melanoma and nonmelanoma skin cancer in patients with vitiligo: a nationwide population-based matched cohort study in Korea. Br J Dermatol. 2020;182(4):907–915. doi: 10.1111/bjd.18247. [DOI] [PubMed] [Google Scholar]
- 16.von Elm E., Altman D.G., Egger M. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 17.Thygesen L.C., Daasnes C., Thaulow I., Brønnum-Hansen H. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39(7 suppl):12–16. doi: 10.1177/1403494811399956. [DOI] [PubMed] [Google Scholar]
- 18.Lynge E., Sandegaard J.L., Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7 suppl):30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 19.Baadsgaard M., Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39(7 suppl):103–105. doi: 10.1177/1403494811405098. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt M., Pedersen L., Sørensen H.T. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 21.Lommerts J.E., Teulings H.-E., Ezzedine K. Melanoma-associated leukoderma and vitiligo cannot be differentiated based on blinded assessment by experts in the field. J Am Acad Dermatol. 2016;75(6):1198–1204. doi: 10.1016/j.jaad.2016.07.060. [DOI] [PubMed] [Google Scholar]
- 22.Li C.-Y., Dai Y.-X., Chen Y.-J. Cancer risks in vitiligo patients: a nationwide population-based study in Taiwan. Int J Environ Res Public Health. 2018;15(9):1847. doi: 10.3390/ijerph15091847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paradisi A., Tabolli S., Didona B., Sobrino L., Russo N., Abeni D. Markedly reduced incidence of melanoma and nonmelanoma skin cancer in a nonconcurrent cohort of 10,040 patients with vitiligo. J Am Acad Dermatol. 2014;71(6):1110–1116. doi: 10.1016/j.jaad.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 24.Teulings H.E., Overkamp M., Ceylan E. Decreased risk of melanoma and nonmelanoma skin cancer in patients with vitiligo: a survey among 1307 patients and their partners. Br J Dermatol. 2013;168(1):162–171. doi: 10.1111/bjd.12111. [DOI] [PubMed] [Google Scholar]
- 25.Hexsel C.L., Eide M.J., Johnson C.C. Incidence of nonmelanoma skin cancer in a cohort of patients with vitiligo. J Am Acad Dermatol. 2009;60(6):929–933. doi: 10.1016/j.jaad.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H.Y., Chay W.Y., Tang M.B., Chio M.T., Tan S.H. Melanoma: differences between Asian and Caucasian patients. Ann Acad Med Singapore. 2012;41(1):17–20. [PubMed] [Google Scholar]
- 27.Helvind N.M., Hölmich L.R., Smith S. Incidence of in situ and invasive melanoma in Denmark from 1985 through 2012. JAMA Dermatol. 2015;151(10):1087–1095. doi: 10.1001/jamadermatol.2015.1481. [DOI] [PubMed] [Google Scholar]
- 28.Park K.K., Murase J.E., Koo J. Long-term prognosis of vitiligo patients on narrowband UVB phototherapy. J Am Acad Dermatol. 2012;66(2):326–327. doi: 10.1016/j.jaad.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Wu S., Han J., Li W.-Q., Li T., Qureshi A.A. Basal-cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178(6):890–897. doi: 10.1093/aje/kwt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vuong K., McGeechan K., Armstrong B.K., Cust A.E. Risk prediction models for incident primary cutaneous melanoma. JAMA Dermatol. 2014;150(4):434–444. doi: 10.1001/jamadermatol.2013.8890. [DOI] [PubMed] [Google Scholar]
- 31.Dahir A.M., Thomsen S.F. Comorbidities in vitiligo: comprehensive review. Int J Dermatol. 2018;57(10):1157–1164. doi: 10.1111/ijd.14055. [DOI] [PubMed] [Google Scholar]
- 32.Scott F.I., Mamtani R., Brensinger C.M. Risk of nonmelanoma skin cancer associated with the use of immunosuppressant and biologic agents in patients with a history of autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol. 2016;152(2):164–172. doi: 10.1001/jamadermatol.2015.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]