Abstract
The pathogenic model of hidradenitis suppurativa is in the midst of a paradigm shift away from a disorder of primary follicular occlusion to an autoinflammatory keratinization disease. Observational, experimental, and therapeutic evidence supports the concept of hidradenitis suppurativa as a primarily inflammatory disorder, a disorder of autoimmunity, or both, in contrast to the current prevailing paradigm of primary follicular occlusion. The lack of reliable and high-fidelity disease models has limited the available experimental and mechanistic evidence to support or refute one pathogenic model over another. This scholarly review synthesizes the existing clinical, histologic, and molecular data to evaluate the extant evidence supporting the autoinflammatory paradigm and further informing the molecular mechanisms of hidradenitis suppurativa pathogenesis. Follicular hyperkeratosis/occlusion and perifollicular inflammation coexist in histologic specimens, with interleukin 1α demonstrated to stimulate comedogenesis in the infundibulum. pH elevation in occluded body sites alters the microbiome and amplifies existing T-helper cell type 17 immunoresponses. Known metabolic comorbidities and smoking are known to upregulate interleukin 1α in follicular keratinocytes. Identified genetic variants may alter epidermal growth factor receptor signaling, leading to upregulated keratinocyte inflammatory responses. The process of follicular rupture and dermal tunnel formation can be explained as secondary responses to inflammatory activation of fibroblasts and epithelial-mesenchymal transition, with antibody production associated with inflammatory amplification in advanced disease. This review aims to reevaluate and integrate the current clinical, histologic, and molecular data into a pathogenic model of hidradenitis suppurativa. This is essential to advance our understanding of the disease and identify novel therapeutic targets and approaches.
Key words: acne inversa, autoinflammatory, hidradenitis suppurative, inflammation, mechanism, pathogenesis
Abbreviations used: EGFR, Epidermal growth factor receptor; IL, interleukin
Capsule Summary.
-
•
Hidradenitis suppurativa is known as a disorder of follicular occlusion, with inflammation being a primary manifestation of disease. Clinical, histologic, and molecular evidence suggests that inflammation is central to multiple aspects of disease, but this remains poorly integrated into the existing pathogenic paradigm.
-
•
Histologic and molecular evidence supports the concept of inflammation as the primary driver of disease activity in hidradenitis suppurativa. It enables explanation of observed events such as follicular rupture, tunnel formation, and systemic inflammation, which are poorly described in the follicular occlusion paradigm. Hidradenitis suppurativa is an autoinflammatory keratinization disease. Reframing our pathologic and clinical understanding in the context of this paradigm is vital to identify and implement novel therapeutic strategies for this burdensome disease.
Introduction
The pathogenic model of hidradenitis suppurativa is in the midst of a paradigm shift1 away from a disorder of (primary) follicular occlusion2 to an autoinflammatory keratinization disease.1 There is observational, experimental, and therapeutic evidence to support the concept of hidradenitis suppurativa as a primarily inflammatory disorder,1 a disorder of autoimmunity3 (in contrast to that primarily of follicular occlusion), or both (Fig 1); however, the lack of reliable disease models4,5 has limited experimental and mechanistic evidence to support or refute one pathogenic model over another (Fig 1). This review aims to reevaluate and integrate the current clinical, histologic, and molecular data into a pathogenic model of hidradenitis suppurativa. This is essential to advance our understanding of the disease6 and identify novel therapeutic targets.7,8
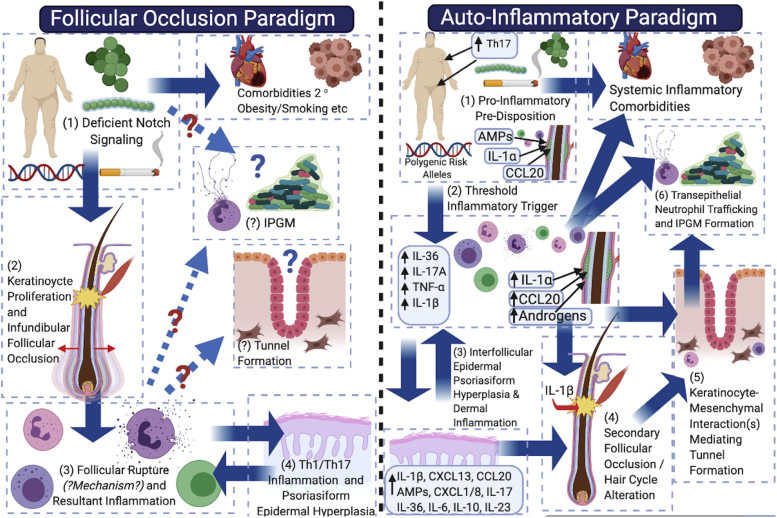
Fig 1.
Schematic representation and comparison of the follicular occlusion paradigm (left panel) and the autoinflammatory paradigm (right panel) in the pathogenesis of hidradenitis suppurativa. In the follicular occlusion paradigm, deficient Notch signaling (1) directly results in infundibular keratinocyte proliferation and follicular occlusion (2), leading to follicular dilatation, rupture, and resultant inflammation. One deficiency of this paradigm is the lack of hypothesized mechanisms by which rupture occurs and why deep follicular rupture occurs preferentially to expulsion of the comedo. The resultant T-helper cell 1/17 inflammatory axis (3) (4) then results in the observed inflammatory profile of disease; however, no clear mechanism is hypothesized for how tunnels form and how the infiltrative proliferative gelatinous mass results. The autoinflammatory paradigm (right panel) places inflammation as the primary driver of disease, with subclinical inflammation (1) developing as a result of disparate contributing factors on a background of topographic predisposition. Dermal inflammatory infiltrates (2) then drive secondary follicular occlusion (3 and 4), with resultant tunnel formation a consequence of keratinocyte-mesenchymal interactions (5) that mimic outer-root sheath keratinocyte downgrowth in follicular development in early anagen. Chemokine gradients in epithelialized tunnels then drive neutrophil trafficking to the lumen and formation of the infiltrative proliferative gelatinous mass (6).
Follicular occlusion is clinically and experimentally a product of inflammation rather than a cause
Follicular hyperkeratosis and comedogenesis coexist with perifollicular inflammation in hidradenitis suppurativa.9, 10, 11 Comedones are present in flexural and nonflexural inflamed and scarred tissues, as well as noninflamed tissues.9, 10, 11 Subclinical inflammation (observed in hidradenitis suppurativa)12,13 precedes comedogenesis in acne-prone skin,14, 15, 16, 17, 18, 19, 20 involving keratinocyte-derived proinflammatory mediators (lipoteichoic acid, CCL20, and interleukin [IL] 1α).18, 19, 20, 21, 22, 23, 24 Ex vivo studies of the follicular infundibum25 isolated in vitro are able to recapitulate the formation of comedones with addition of IL-1α and prevent formation with the addition of IL-1 receptor antagonist.25 It is acknowledged that the in vitro studies performed are based on highly sebaceous follicular units that have distinct differences from apocrine-bearing skin26; however, the similarities in immunologic milieu between sebaceous and apocrine skin in T-helper cell 17–associated mediators7,26 (central to inflammation in hidradenitis suppurativa)26 raises the possibility that these mechanisms are shared. Molecular and ex vivo evidence suggests comedo formation may be secondary to subclinical inflammation. These results may explain the diffuse scattering of comedones observed in hidradenitis suppurativa–prone areas, the presence of comedones in extraflexural sites, and their presence in previously inflamed (“burned-out”) tissue or sites distant from a follicular unit.9,10
Skin fold occlusion is associated with microbiome alterations and subsequent proinflammatory keratinocyte responses
From a clinical perspective, follicular occlusion may refer to anatomic sites of disease predilection (axillary, inguinal, and submammary folds).2 These areas demonstrate alterations in moisture, pH, and microbiological colonization2 (Fig 2), particularly in the setting of obesity.27, 28, 29 The follicular infundibulum is an immunologically active, microbially colonized site23,30,31 involved in the development of immune tolerance to commensal organisms.23,30,31 This differs substantially from other portions of the follicle (such as the bulb), which are considered immunologically privileged sites.32 Infundibular keratinocytes produce CCL20 and antimicrobial peptides under normal physiologic conditions23 (Fig 2). Increasing moisture decreases the pH of the stratum corneum,28,29 promoting the colonization and activity of hidradenitis suppurativa–associated microbionts (eg, Porphyromonas)33,34 (Fig 2). Other bacteria,35,36 yeasts,37 and associated proteins (including lipoteichoic acid) induce the release of preformed IL-1α in keratinocytes.38 Indirect evidence for the role of yeasts in inflammatory activity in hidradenitis suppurativa39 has been demonstrated in recent observational studies.39,40 Although the precise mechanisms of specific microbiological species and strains in hidradenitis suppurativa is ill defined, their functional role in producing an aberrant proinflammatory response (either directly or indirectly via keratinocytes) is consistent with observational studies identifying these microbionts in both early and advanced disease.34,35
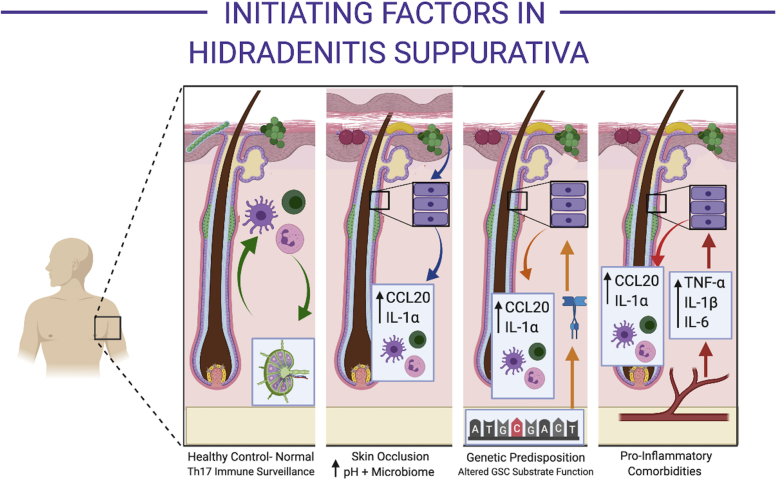
Fig 2.
Initiating factors in hidradenitis suppurativa. Normal control skin (first panel from the left) from hidradenitis suppurativa–associated cutaneous sites (eg, axilla) have normal colonizing microbionts (including within the follicular infundibulum), which are continuously monitored by circulating immune cells in homeostasis (circulating to and from regional lymph nodes (inset in first panel from the left). Known predisposing factors, including skin occlusion (second panel from left), predisposing genetic mutations (third panel from the left), and proinflammatory comorbidities such as obesity and insulin resistance, increase the inflammatory drive of infundibular keratinocytes (purple rectangular cells) via varied mechanisms. Skin occlusion (second panel from the left) alters the microbiological composition of the skin (red and yellow microbionts) via increases in cutaneous pH. These microbionts increase the production of CCL20 and interleukin (IL) 1α by infundibular keratinocytes. Genetic mutations in the γ-secretase complex are known to affect Notch signaling and also substrates including epidermal growth factor receptors, which are active in the follicular infundibulum. Dysregulation of EGFR signaling is known to increase CCL20 and IL-1α production by infundibular keratinocytes. Metabolic comorbidities produce increased levels of circulating tumor necrosis factor-α, IL-1β, and IL-6. These mediators stimulate CCL20 and IL-1α production.
Inflammation in hidradenitis suppurativa: evidence from existing studies
The inflammatory signature of established hidradenitis suppurativa has been well characterized in multiple histologic26,41 and molecular studies.26,42, 43, 44, 45 Similarities and parallels with psoriasis26 have been observed in lesional and perilesional hidradenitis suppurativa tissue,26,46 with lesional nodules demonstrating mixed inflammatory infiltrates comprising T cells, dendritic cells, plasma cells, neutrophils,47, 48, 49, 50 and monocytes.51 Chronic long-standing disease appears autoinflammatory52, 53, 54 and also demonstrates B-cell infiltrates,3,12 NETosis,3 and development of epithelialized tunnels.55 An issue with understanding the characteristics of inflammation in hidradenitis suppurativa is that the majority of specimens isolated for studies are from individuals with severe, long-standing disease.3,12,13 Hence, we have limited insight into the initiating events in early and mild hidradenitis suppurativa. Additionally, until recently there were no standardized, defined biopsy sites for investigational studies.56 Given that hidradenitis suppurativa is morphologically diverse, it would be erroneous to assume that a biopsy from one portion of tissue is representative of all the different epidermal (and deep dermal) morphologies present across the spectrum of hidradenitis suppurativa.56 Therefore, studies that do not define the severity, treatments, sites, and lesion types of biopsies should be interpreted with caution.41,42
The mechanisms of lesion development are unclear because perilesional inflammation is of the same character (albeit less intense) as nearby lesional inflammation42,43,57; however, lesional cytokine profiles are unable to be experimentally generated from the addition of IL-1α, IL-1β, or both to perilesional tissue.5,57 This raises the prospect that the process of inflammation in hidradenitis suppurativa is more complex than initially thought and that the inflammatory characteristics of perilesional tissue are distinct from those of lesional tissue.5
Disease initiation is associated with systemic subclinical inflammation and dysregulated infundibular keratinocytes
Understanding of the initiating factors associated with the excessive and self-perpetuating perifollicular inflammation in hidradenitis suppurativa remains incomplete. Epidemiologic and clinical observations suggest that a number of systemic disorders (including insulin resistance, hormonal dysregulation, and obesity) may be associated with hidradenitis suppurativa58 and contribute to a proinflammatory state59,60 (Fig 2). In other inflammatory disorders, such as psoriasis,61 rheumatoid arthritis,62 and atherosclerosis,63 these factors have been found to be associated. However, the causation between disease and systemic inflammation is still a topic of contention.64
Guidelines65,66 and clinical evidence67,68 suggest weight loss, smoking cessation, and dietary counseling as an integral part of hidradenitis suppurativa management65,66 through suppression of inflammation.69, 70, 71 Smoking, via polycyclic aromatic hydrocarbons, can directly alter follicular keratinocyte differentiation, resulting in comedogenesis.72 It can also produce widespread methylation changes and systemic increases in IL-6, C-reactive protein, fibrinogen, and multiple members of the nuclear factor kappa-light-chain-enhancer of activated B cells family.71 Adipose tissue can produce proinflammatory signatures, including IL-6, IL-1β, and tumor necrosis factor-α in the setting of chronic nutrient excess.69,70 Additionally, adipokines can mediate both inflammation and the development of insulin resistance73 (Fig 2), which is also associated with hidradenitis suppurativa.58 Keratinocytes in the infra-infundibulum of the follicle express type 1 5-hydroxytestosterone,74 modulating infundibular keratinocyte differentiation programs both directly75 and via fibroblast activation and fibroblast-keratinocyte interactions, contributing to androgen-induced follicular changes.74
Overall, these associations suggest that a systemic proinflammatory state and localized infundibular keratinocyte dysregulation are potential predisposing factors to clinical disease. There are contradictory reports76 pertaining to the benefit of withdrawing these predisposing factors (eg, cessation of smoking, weight loss) during established disease. These findings appear contradictory only if one holds the assumption that the initiating and perpetuating factors of clinical disease in hidradenitis suppurativa are one and the same. As other authors have suggested,77 there may be unique factors contributing to each state (initiation of disease and perpetuation of disease); and our lack of data regarding early (subclinical) disease has not allowed us to appreciate this fact.77
T-helper cell 17 feed-forward inflammation is prominent in established disease
The T-helper cell 17 axis is strongly implicated in established self-perpetuating clinical disease26; however, the mechanisms leading to T-helper cell 17 feed-forward self-amplification in hidradenitis suppurativa are still unclear. It is assumed to be similar to the activation of the T-helper cell 17 axis in psoriasis,78 with the predisposition of the axillae and other areas of apocrine-gland-rich skin to a T-helper cell 17 immunoresponse, as demonstrated experimentally.25 There is well-documented evidence (largely from the psoriasis literature) regarding feed-forward mechanisms between IL-1β, IL-6, and tumor necrosis factor-α by IL-17,78,79 leading to further IL-1β, IL-6, and tumor necrosis factor-α production, as well as downstream activation of acute phase reactants and neutrophilic and complement-mediated inflammatory responses.78, 79, 80 This is further perpetuated through leucocyte-keratinocyte interactions,78, 79, 80 further amplifying antimicrobial peptide and chemokine production (including CXCL1 and CXCL8),81 leading to further inflammatory cell recruitment adjacent to IL-17–activated epidermal keratinocytes (Fig 3). Such inflammatory cell localization has been observed surrounding intrafollicular and interfollicular sites adjacent to epidermal keratinocytes in early histologic specimens of hidradenitis suppurativa,9, 10, 11,64 with evidence of early psoriasiform hyperplasia suggestive of IL-17–induced epidermal changes. Despite that the majority of translational work focuses on IL-17A (given the body of preexisting work based on psoriasis), significant elevations of other IL-17 isoforms, including IL-17C and IL-17F, are observed in hidradenitis suppurativa tissue,81,82 and these may be significant contributors to disease activity that are not targeted by anti-IL-17A therapies alone.
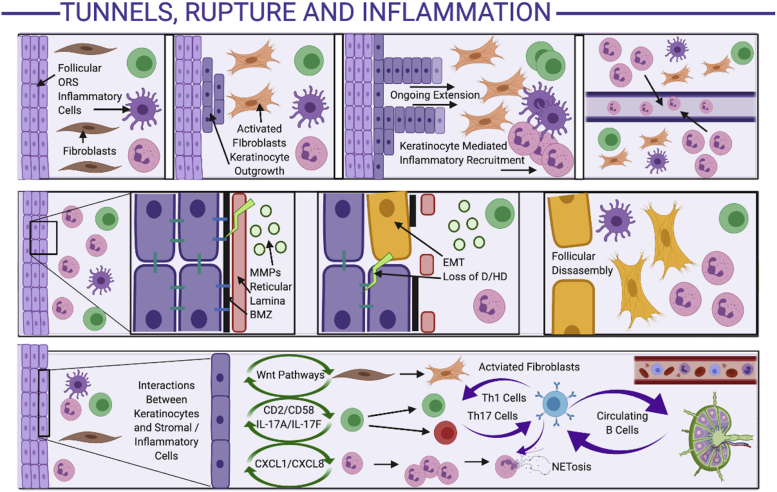
Fig 3.
Mechanisms of tunnel formation, follicular rupture, and perpetuation of inflammation in hidradenitis suppurativa. Development of tunnels (top panel): Inflammation adjacent to the follicular outer root sheath activates fibroblasts, with stromal-keratinocyte feedback resulting in keratinocyte outgrowth from the follicular wall. The ongoing keratinocyte outgrowth results in keratinocyte-mediated inflammatory cell recruitment, further amplifying the stromally mediated keratinocyte outgrowth in a positive-feedback loop. The inflammatory cells are attracted to the keratinocyte chemokine (CXCL1/CXCL8) gradient, resulting in migration into the lumen of the tunnels. Mechanisms of follicular rupture (middle panel): The inflammatory infiltrate is associated with high levels of matrix metalloproteinases, which degrade the reticular lamina. Keratinocyte-leucocyte cross talk activated epithelial-mesenchyme-transition mechanisms, leading to degradation of the basement membrane zone, loss of hemidesmosomes and desmosomes, and keratinocytes expressing mesenchymal cell surface markers (yellow keratinocytes). Eventually, the follicular wall is disassembled, replaced by mesenchymal cells and dense inflammatory infiltrates. Mechanisms of inflammatory amplification (bottom panel): Activated keratinocytes interact with inflammatory and stromal cells via various pathways to result in activated fibroblasts, T-helper cell types 1 and 17, and infiltration of dendritic cells and neutrophils. Circulating B cells (circulating to and from regional lymph nodes; far right of lower panel), activated by the high-interferon-mediated milieu, interact with multiple cell types to amplify existing inflammatory loops, as well as recirculate in the lymphatic and vascular system, contributing to systemic inflammation. D, Desmosome; HD, hemidesmosome; MMP, matrix metalloproteinase; ORS, outer root sheath.
The role of B cells, despite their dominance, remains unclear
Long-standing and severe disease may have a unique inflammatory profile compared with milder or less established forms of hidradenitis suppurativa. Histologic and transcriptomic studies44,45 have identified a high level of B-cell3 and plasma-cell12,13 signatures, complement (specifically C5a) activation,47, 48, 49, 50 and extensive tissue remodeling via matrix metalloproteinases with subsequent destruction of follicular and glandular structures in the dermis.2,11 The role and characteristics of B cells in mild to moderate hidradenitis suppurativa are unclear.83 The presence of B cells and plasma cells in skin and blood3,12,13 suggests the possibility that some component of severe or long-standing hidradenitis suppurativa may be an autoimmune or antibody-mediated disorder. However, to date no product has been definitively identified as an autoimmune target for the disease.83 B cells are present in other chronic inflammatory disorders without known autoimmune targets, including psoriasis and atopic dermatitis.84 In these conditions, they are thought to be bystanders (secondary to combined B-cell and T-cell chemoattractants such as CXCL13 or CCL20) or secondary amplifiers of T-cell–mediated inflammation.83 (Fig 3). Byrd et al3 demonstrated that antibodies to citrullinated peptides contribute to the development of neutrophil extracellular traps in advanced disease, with parallels to B-cell and neutrophil extracellular traps in rheumatoid arthritis.3 Case reports of rituximab ameliorating hidradenitis suppurativa disease activity are known,83 but overall, the role of B cells as bystanders, amplifiers of existing inflammation, or central pathogenic players is unclear and requires further investigation.83
Genetic variants in hidradenitis suppurativa may act via EGFR-associated pathways linking follicles, T-helper cell 17−mediated inflammation, and drug-induced disease
A minority of patients with familial and spontaneous hidradenitis suppurativa have been identified with GSC mutations.85 The precise mechanism of action of GSC mutations in the pathogenesis of hidradenitis suppurativa is unclear.86 The GSC complex cleaves more than 70 different substrates involved in cell cycle and inflammation, including epidermal growth factor receptor (EGFR), IL-1, tumor necrosis factor-α, and Notch.86 Notch is proposed as the unifying motif in hidradenitis suppurativa pathogenesis via associations with keratinocyte proliferation,87 smoking, and sequence variants in GSC.88,89 However, Notch dysregulation is also present in multiple other inflammatory dermatoses,90 arguing against a unique role in hidradenitis suppurativa. In silico evidence86 has identified ERbb4 and Tie1 as differentially expressed GSC substrates that distinguish the transcriptome of hidradenitis suppurativa from familial Alzheimer disease and other inflammatory skin diseases.90 These components of the EGFR pathway (active in the follicular infundibulum23) are associated with SOX9 and Wnt signaling linked with hair cycle progression, IL-17A production23,91 (through shared downstream Act1 activity), and epithelial cell fate,91 all mechanisms identified in transcriptomic analysis of hidradenitis suppurativa tissues.44,77 GSC knockdown results in IL-36α production,92 alterations in EGFR signaling,93 and increased sensitivity to interferon-mediated proinflammatory pathways92 (Fig 2). POFUT-1 (identified in cases of Dowling-Degos disease associated with hidradenitis suppurativa94,95) is a fucosyltransferase that is active on multiple substrates, including Notch and EGFR,96 and is important for posttranslational modification of receptors.96 This suggests a role for EGFR signaling in hidradenitis suppurativa, supported by reports of hidradenitis suppurativa associated with use of EGFR antagonists in oncology.97
The evidence and proposed mechanisms for follicular rupture
Follicular rupture is proposed as the primary mechanism by which follicular occlusion leads to dermal inflammation in hidradenitis suppurativa, but the molecular mechanisms remain unclear.2 Observational studies demonstrate the coexistence of dense perifollicular and intrafollicular inflammation and discontinuities in follicular epithelium in affected tissues9,10,64 (Fig 3). Long-standing disease demonstrates a noticeable absence of follicular and adnexal structures,98 consistent with profound dermal inflammation. A reduction in the thickness of the fibroreticular lamina surrounding follicles and sebaceous glands99 has been observed. Occluded follicles in other conditions (such as epidermal inclusion cysts100) are testament to the potential size intrafollicular collections may progress to before rupture. However, the early presence of inflammation in hidradenitis suppurativa lesions suggests an inflammation-related mechanism101 that is well documented to disassemble the basement membrane zone as part of the wound-healing process.102 Epithelial-mesenchymal transition pathways103 are part of the normal wound-healing response and have been identified in transcriptomic analysis of hidradenitis suppurativa tissues.84,104 It may also explain the presence of keratin-staining cells in the dermis of hidradenitis suppurativa sections41 (via keratinocytes undergoing epithelial-mesenchymal transition but still expressing keratin proteins), the destruction of follicular and adnexal structures in advanced disease,98 and the development of dermal tunnels103 (Fig 3). Similar inductions in epithelial-mesenchymal transition-associated signaling pathways are observed in malignancy and wound healing and contribute to the metastatic potential of cancer and long-standing wounds.105 Hence, the concept of follicular rupture may be more appropriately described as a process of “follicular disassembly” (Fig 3) induced by the chronic inflammatory changes via epithelial-mesenchymal transition and aberrant extracellular remodeling wound-healing programs.103
Dermal tunnels are active inflammatory structures and their development is orchestrated by dermal inflammation
Dermal tunnels in hidradenitis suppurativa are unique structures comprising stratified squamous epithelia that recapitulate the structure of the overlying epidermis and produce active inflammatory mediators.106 This is in contrast to other tunnel-like structures in chronic inflammatory conditions such as fistulizing Crohn's disease, which do not recapitulate mucosal structures with the same degree of fidelity.107 The mechanisms leading to tunnel formation are unclear; however, it is hypothesized that these tunnels derive from the aberrant keratinocyte outgrowth from the outer root sheath of the follicle103 (Fig 3). Tunnels do not extend into the subcutaneous tissues or fistulize with other hollow organs (except in the context of coexistent inflammatory bowel disease), suggesting an association with signaling from the dermis.103 This parallels the development of the hair follicle and early anagen downgrowth in the hair cycle,105,108 which are mediated via platelet derived growth factor α-derived signaling from the dermal condensate.105 Platelet derived growth factor α-mediated signaling has also been identified in transcriptomic data from hidradenitis suppurativa–associated fibroblasts.103 Given that these fibroblast-derived signals are secondary to inflammation-mediated epigenetic modifications,103 it is plausible to assume that the development of tunnels is an inflammation-driven process. However, once these tunnels are established, the CXCL1/8 gradient established across the epithelia106 (including tunnels) results in transepithelial neutrophil trafficking and neutrophil extracellular trap formation in tunnel lumen.3 This results in development of the infiltrative proliferative gelatinous mass109 and biofilm formation in hidradenitis suppurativa tunnels110 (Fig 3). This in turn drives further inflammatory recruitment surrounding these established tunnels, leading to the ongoing cycle of severe intractable inflammation and drainage.
Conclusions
The available histologic and molecular evidence suggests inflammation is a central component to the pathogenesis of hidradenitis suppurativa. Placing inflammation as the primary driver of disease provides a scaffold for testable hypotheses regarding polygenic risk loci for the development of hidradenitis suppurativa, drug-induced causes of hidradenitis suppurativa, the development of dermal tunnels, and the inflammatory proliferative gelatinous mass, which are currently poorly integrated into the follicular occlusion model of hidradenitis suppurativa (Fig 1). More mechanistic and translational investigations are needed to further evaluate the role of genetics and B cells in hidradenitis suppurativa, as well as provide mechanistic evidence about the development of follicular rupture and tunnel formation. Such basic cellular and molecular investigations are vital to develop our understanding of the disease. Realigning the pathogenic paradigm with the molecular evidence is essential to enable the identification and exploration of novel targets, interventions, and therapeutics for this chronic debilitating disease.
Footnotes
Funding sources: Supported by grant UL1 TR001866 from the National Center for Advancing Translational Sciences, National Institutes of Health Clinical and Translational Science Award program.
Conflicts of interest: None disclosed.
References
- 1.Akiyama M., Takeichi T., McGrath J.A., Sugiura K. Autoinflammatory keratinization diseases. J Allergy Clin Immunol. 2017;140:1545–1547. doi: 10.1016/j.jaci.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Vossen A.R.J.V., van der Zee H.H., Prens E.P. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi: 10.3389/fimmu.2018.02965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrd A.S., Carmona-Rivera C., O'Neil L.J. Neutrophil extracellular traps, B cells, and type I interferons contribute to immune dysregulation in hidradenitis suppurativa. Sci Transl Med. 2019;11(508):eaav5908. doi: 10.1126/scitranslmed.aav5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van der zee H.H., Laman J.D. Prens EP. Can animal skin diseases or current transgenic mice serve as a model for hidradenitis suppurativa? Dermatology. 2012;225(1):9–13. doi: 10.1159/000339773. [DOI] [PubMed] [Google Scholar]
- 5.Frew J.W., Piguet V. Ex-vivo models and interpretation of mechanistic studies in hidradenitis suppurativa. J Invest Dermatol. 2020;140(7):1323–1326. doi: 10.1016/j.jid.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Frew J.W. Commentary: hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2019;10:302. doi: 10.3389/fimmu.2019.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czarnowicki T., He H., Krueger J.G., Guttman-Yassky E. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143(1):1–11. doi: 10.1016/j.jaci.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Frew J.W., Marzano A., Wolk K. Identifying novel therapeutic targets in hidradenitis suppurativa. J Invest Dermatol. 2020 doi: 10.1016/j.jid.2020.06.019. (In Press) [DOI] [PubMed] [Google Scholar]
- 9.Von Laffert M., Stadie V., Wohlrab J., Marsch W.C. Hidradenitis suppurativa/acne inversa: bilocated epithelial hyperplasia with very different sequelae. Br J Dermatol. 2011;164(2):367–371. doi: 10.1111/j.1365-2133.2010.10034.x. [DOI] [PubMed] [Google Scholar]
- 10.von Laffert M., Helmbold P., Wohlrab J., Fiedler E., Stadie V., Marsch W.C. Hidradenitis suppurativa (acne inversa): early inflammatory events at terminal follicles and at interfollicular epidermis. Exp Dermatol. 2010;19(6):533–537. doi: 10.1111/j.1600-0625.2009.00915.x. [DOI] [PubMed] [Google Scholar]
- 11.Zouboulis C.C., Nogueira de Costa A., Fimmel S., Zouboulis K.C. Apocrine glands are bystanders in hidradenitis suppurativa and their involvement in gender specific. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16264. [DOI] [PubMed] [Google Scholar]
- 12.Musilova J., Moran B., Sweeney C.M. Enrichment of plasma cells in the peripheral blood and skin of patients with hidradenitis suppurativa. J Invest Dermatol. 2020;140(5):1091–1094.e2. doi: 10.1016/j.jid.2019.08.453. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman L.K., Tomalin L.E., Schultz G. Integrating the skin and blood transcriptomes and serum proteome in hidradenitis suppurativa reveals complement dysregulation and a plasma cell signature. PLoS One. 2018;13(9):e0203672. doi: 10.1371/journal.pone.0203672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacarubba F., Dall'Oglio F., Musumeci M.L., Nasca M.R., Micali G. Secondary comedones in a case of acne conglobata correlate with double-ended pseudocomedones in hidradenitis suppurativa. Acta Dermatol Venereol. 2017;97(8):969–970. doi: 10.2340/00015555-2664. [DOI] [PubMed] [Google Scholar]
- 15.Boer J., Jemed G.B. Mechanical stress and the development of pseudo-comedones and tunnels in hidradenitis suppurativa/acne inversa. Exp Dermatol. 2016;25(5):396–397. doi: 10.1111/exd.12926. [DOI] [PubMed] [Google Scholar]
- 16.Lacarrubba F., Musumeci M.L., Nasca M.R.W., Verzi A.E., Fiorentini F., Micali G. Double-ended pseudocomedones in hidradenitis suppurativa: clinical, dermoscopic, and histopathological correlation. Acta Derm Venereol. 2017;97(6):763–764. doi: 10.2340/00015555-2601. [DOI] [PubMed] [Google Scholar]
- 17.Higgins R., Pink A., Hunger R., Yawalkar N., Navarini A.A. Generalized comedones, acne, and hidradenitis suppurativa in a patient with an FGFR2 missense mutation. Front Med (Lausanne) 2017;4:16. doi: 10.3389/fmed.2017.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fontao F., von Engelbrechten M., Seilaz C., Sorg O., Saurat J.H. Microcomedones in non-lesional acne prone skin new orientations on comedogenesis and its prevention. J Eur Acad Dermatol Venereol. 2020;34(2):357–364. doi: 10.1111/jdv.15926. [DOI] [PubMed] [Google Scholar]
- 19.Capitanio B., Lora V., Ludovici M. Modulation of sebum oxidation and interleukin-1α levels associates with clinical improvement of mild comedonal acne. J Eur Acad Dermatol Venereol. 2014;28(12):1792–1797. doi: 10.1111/jdv.12431. [DOI] [PubMed] [Google Scholar]
- 20.Antiga E., Verdelli A., Bonciani D., Bonciolini V., Caproni M., Fabbri P. Acne: a new model of immune-mediated chronic inflammatory skin disease. G Ital Dermatol Venereol. 2015;150(2):247–254. [PubMed] [Google Scholar]
- 21.Ingham E., Eady A., Goodwin C.E., Cove J.H., Cunliffe W.J. Pro-inflammatory levels of interleukin-1 alpha-like bioactivity are present in the majority of open comedones in acne vulgaris. J Invest Dermatol. 1992;98(6):895–901. doi: 10.1111/1523-1747.ep12460324. [DOI] [PubMed] [Google Scholar]
- 22.Jeremy A.H.T., Holland D.B., Roberts S.G., Thomson K.F., Cunliffe W.J. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121(1):20–27. doi: 10.1046/j.1523-1747.2003.12321.x. [DOI] [PubMed] [Google Scholar]
- 23.Schneider M.R., Paus R. Deciphering the functions of the hair follicle infundibulum in skin physiology and disease. Cell Tissue Res. 2014;358(3):697–704. doi: 10.1007/s00441-014-1999-1. [DOI] [PubMed] [Google Scholar]
- 24.Guy R., Green M.R., Kealey T. Modeling acne in vitro. J Invest Dermatol. 1996;106(1):176–182. doi: 10.1111/1523-1747.ep12329907. [DOI] [PubMed] [Google Scholar]
- 25.Jenei A., Dajnoki Z., Medgyesi B., Gáspár K., Béke G., Kinyó Á. Apocrine gland–rich skin has a non-inflammatory IL-17–related immune milieu, that turns to inflammatory IL-17–mediated disease in hidradenitis suppurativa. J Invest Dermatol. 2019;139(4):964–968. doi: 10.1016/j.jid.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Melnik B.C., John S.M., Chen W., Plewig G. T helper 17 cell/regulatory T-cell imbalance in hidradenitis suppurativa.acne inversa: the link to hair follicle dissection, obesity, smoking and autoimmune comorbidities. Br J Dermatol. 2018;179(2):260–272. doi: 10.1111/bjd.16561. [DOI] [PubMed] [Google Scholar]
- 27.Grice E.A., Segre J.A. The skin microbiome. Nat Rev Microbiol. 2011;9(4):244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grice E.A., Kong H.H., Conlan S., Deming C.B., Davis J., Young A.C. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324(5931):1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grice E.A. The skin microbiome: potential for novel diagnostic and therapeutic approached to cutaneous disease. Semin Cutan Med Surg. 2014;33(2):98–103. doi: 10.12788/j.sder.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polak-Witka K., Rudnicka L., Bume-Peytavi U., Vogt A. The role of the microbiome in scalp hair follicle biology and disease. Exp Dermatol. 2019;29(3):286–294. doi: 10.1111/exd.13935. [DOI] [PubMed] [Google Scholar]
- 31.Scharschmidt T.C., Vasquez K.S., Pauli M.L. Commensal microbes and hair follicle morphogenesis coordinately drive treg migration into neonatal skin cell host. Microbe. 2017;21(4):467–477. doi: 10.1016/j.chom.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paus R., Bulfone-Paus S., Bertolini M. Hair follicle immune privilege revisited: the key to alopecia areata management. J Invest Dermatol. 2018;19(1):S12–S17. doi: 10.1016/j.jisp.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Ring H.C., Thorsen J., Saunte D.M., Lilje B., Bay L., Riis P.T. The follicular skin microbiome in patients with hidradenitis suppurativa and healthy controls. JAMA Dermatol. 2017;153(9):897–905. doi: 10.1001/jamadermatol.2017.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ring H.C., Sigsgaard V., Thorsen J. The microbiome of tunnels in hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2019;33(9):1775–1780. doi: 10.1111/jdv.15597. [DOI] [PubMed] [Google Scholar]
- 35.Brauweiler A.M., Goleva E., Leung D.Y.M. Staphylococcus aureus lipoteichoic acid damaged the epidermal barrier through an IL-1 mediated pathway. J Invest Dermatol. 2019;139(8):1753–1761.e4. doi: 10.1016/j.jid.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin M., Pirouz A., Kim M.H., Krutzik S.R., Garbán H.J., Kim J. Propionibacterium acnes induces IL-1β secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014;134(2):381–388. doi: 10.1038/jid.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altmeier S., Toska A., Sparber F., Teijeira A., Halin C., Gut-Landmann S.L. IL-1 coordinates the neutrophil response to C albicans in the oral mucosa. PLoS Pathog. 2016;12(9):e1005882. doi: 10.1371/journal.ppat.1005882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanmiguel J.C., Olaru F., Li J., Mohr E., Jensen L.E. Interleukin-1 regulated keratinocyte expression of T cell targeting chemokines through interleukin-1 receptor associated kinase (IRAK-1) dependent and independent pathways Cell. Signal. 2009;21(5):685–694. doi: 10.1016/j.cellsig.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frew J.W. ASCA antibodies in hidradenitis suppurativa: more than a gut feeling. J Allerg Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 40.Assan F., Gottlieb J., Tubach F. Anti-Saccharomyces cerevisiae IgG and IgA antibodies are associated with systemic inflammation and advanced disease in hidradenitis suppurativa. J Allerg Clin Immunol. 2020 doi: 10.1016/j.jaci.2020.01.045. [DOI] [PubMed] [Google Scholar]
- 41.Frew J.W., Hawkes J.E., Krueger J.G. A systematic review and critical evaluation of immunohistochemical associations in hidradenitis suppurativa. F100Res. 2018;7:1923. doi: 10.12688/f1000research.17268.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frew J.W., Hawkes J.E., Krueger J.G. A systematic review and critical evaluation of inflammatory cytokine associations in hidradenitis suppurativa. F100Res. 2018;7:1930. doi: 10.12688/f1000research.17267.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vossen A.R.J.V., Ardon C.B., van der Zee H.H., Lubberts E., Prens E.P. The anti-inflammatory potency of biologics targeting tumour necrosis factor-alpha, interleukin (IL)-17A, IL-12/23 and CD20 in hidradenitis suppurativa: an ex vivo study. Br J Dermatol. 2019;181(2):314–323. doi: 10.1111/bjd.17641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zouboulis C.C., Nogueira da Costa A., Makrantonaki E., Hou X.X., Almansouri D., Dudley J.T. Alterations in innate immunity and epithelial cell differentiation are the molecular pillars of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2020;34(4):846–861. doi: 10.1111/jdv.16147. [DOI] [PubMed] [Google Scholar]
- 45.Shanmugan V.K., Jones D., McNish S., Bendall M.L., Crandall K.A. Transcriptome patterns in hidradenitis suppurativa: support for the role of antimicrobial peptides and interferon pathways in disease pathogenesis. Clin Exp Dermatol. 2019;44(8):882–892. doi: 10.1111/ced.13959. [DOI] [PubMed] [Google Scholar]
- 46.Wolk K., Warszawska K., Hoeflich C. Deficiency of IL-22 contributes to a chronic inflammatory disease: pathogenetic mechanisms in acne inversa. J Immunol. 2011;186(2):1228–1239. doi: 10.4049/jimmunol.0903907. [DOI] [PubMed] [Google Scholar]
- 47.Ghias M.H., Hyde M.J., Tomalin L.E. Role of the complement pathway in inflammatory skin diseases: a focus on hidradenitis suppurativa. J Invest Dermatol. 2020;140(3):531–536.e1. doi: 10.1016/j.jid.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Grand D., Navrazhina K., Frew J.W. Integrating complement into the molecular pathogenesis of hidradenitis suppurativa. Exp Dermatol. 2020;29(1):86–92. doi: 10.1111/exd.14056. [DOI] [PubMed] [Google Scholar]
- 49.Kanni T., Zenker O., Habel M., Riedemann N., Giamarellos-Bourboulis E.J. Complement activation in hidradenitis suppurativa: a new pathway of pathogenesis? Br J Dermatol. 2018;179(2):413–419. doi: 10.1111/bjd.16428. [DOI] [PubMed] [Google Scholar]
- 50.Giamarellos-Bouboulis E.J., Argyropoulou M., Kanni T. Clinical efficacy of complement C5a inhibition by IFX-1 in hidradenitis suppurativa: an open-label single-arm trial in patients not eligible for adalimumab. Br J Dermatol. 2020 doi: 10.1111/bjd.18877. [DOI] [PubMed] [Google Scholar]
- 51.Shah A., Alhusayen R., Amini-Nik S. The critical role of macrophages in the pathogenesis of hidradenitis suppurativa. Inflamm Res. 2017;66(11):931–945. doi: 10.1007/s00011-017-1074-y. [DOI] [PubMed] [Google Scholar]
- 52.Frings V.G., Sennefelder H., Presser D., Goebeler M., Schmidt M. Altered NOX expression does not seem to account for epidermal NLRP3 inflammasome activation in hidradenitis suppurativa. Br J Dermatol. 2019;181(2):391–392. doi: 10.1111/bjd.17647. [DOI] [PubMed] [Google Scholar]
- 53.Lima A.L., Karl I., Giner T. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174(3):514–521. doi: 10.1111/bjd.14214. [DOI] [PubMed] [Google Scholar]
- 54.Marzano A.V., Borghi A., Meroni P.L., Cugno M. Pyoderma gangrenosum and its syndromic forms: evidence for a link with autoinflammation. Br J Dermatol. 2016;175(5):882–891. doi: 10.1111/bjd.14691. [DOI] [PubMed] [Google Scholar]
- 55.Kurzen H., Jung E.G., Hartschuh W., Moll I., Franke W.W., Moll R. Forms of epithelial differentiation of draining sinus in acne inversa (hidradenitis suppurativa) Br J Dermatol. 1999;141(2):231–239. doi: 10.1046/j.1365-2133.1999.02970.x. [DOI] [PubMed] [Google Scholar]
- 56.Frew J.W., Navrazhina K., Byrd A.S. Defining lesional, perilesional and unaffected skin in hidradenitis suppurativa: proposed recommendations for clinical trials and translational research studies. Br J Dermatol. 2019;181(6):1339–1341. doi: 10.1111/bjd.18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vossen A.R.J.V., van Straalen K.R., Florencia E.F., Prens E.P. Lesional inflammatory profile in hidradenitis suppurativa is not solely driven by IL-1. J Invest Dermatol. 2020;140(7):1463–1466.e2. doi: 10.1016/j.jid.2020.01.023. [DOI] [PubMed] [Google Scholar]
- 58.Porter M.L., Kimball A.B. Comorbidities of hidradenitis suppurativa. Semin Cutan Med Surg. 2017;36(2):55–57. doi: 10.12788/j.sder.2017.018. [DOI] [PubMed] [Google Scholar]
- 59.Jimenez-Gallo D., de la Varga-Martinez R., Ossorio-Garcia L., Albarran-Planelles C., Rodriguez C., Linares-Barrios M. The clinical significance of increased serum proinflammatory cytokines, C-reactive protein, and erythrocyte sedimentation rate in patients with hidradenitis suppurativa. Mediators Inflamm. 2017;2017:2450401. doi: 10.1155/2017/2450401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanni T., Tzanetakou V., Savva A. Compartmentalized cytokine responses in hidradenitis suppurativa. PLoS One. 2015;10(6):e0130522. doi: 10.1371/journal.pone.0130522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jensen P., Skov L. Psoriasis and obesity. Dermatology. 2016;232(6):633–639. doi: 10.1159/000455840. [DOI] [PubMed] [Google Scholar]
- 62.Romano S., Salustri E., Ruscitti P., Carubbi F., Penco M., Giacomelli R. Cardiovascular and metabolic comorbidities in rheumatoid arthritis. Curr Rheumatol Rep. 2018;20(12):81. doi: 10.1007/s11926-018-0790-9. [DOI] [PubMed] [Google Scholar]
- 63.Raggi O.P., Genest J., Giles J.T. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis. 2018;276:98–108. doi: 10.1016/j.atherosclerosis.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 64.Jemec G.B., Hansen U. Histology of hidradenitis suppurativa. J Am Acad Dermatol. 1996;34(6):994–999. doi: 10.1016/s0190-9622(96)90277-7. [DOI] [PubMed] [Google Scholar]
- 65.Alikhan A., Sayed C., Alavi A. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II topical, intralesional and systemic medical management. J Am Acad Dermatol. 2019;81(1):91–101. doi: 10.1016/j.jaad.2019.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zouboulis C.C., Desai N., Emtestam L. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29(4):619–644. doi: 10.1111/jdv.12966. [DOI] [PubMed] [Google Scholar]
- 67.Simonart T. Hidradenitis suppurativa and smoking. J Am Acad Dermatol. 2010;62(1):149–150. doi: 10.1016/j.jaad.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 68.Gallagher C., Kirthi S., Burke T., O'Shea D., Tobin A.M. Remission of hidradenitis suppurativa after bariatric surgery. JAAD Case Rep. 2017;3(5):436–437. doi: 10.1016/j.jdcr.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reily S.M., Saltiel A.R. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 70.Deng T., Lyon C.J., Bergin S., Caliguri M.A., Hsueh W.A. Obesity, inflammation and cancer. Annu Rev Pathol. 2016;11:421–449. doi: 10.1146/annurev-pathol-012615-044359. [DOI] [PubMed] [Google Scholar]
- 71.Gonçalves R.B., Coletta R.D., Silvério K.G. Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res. 2011;60(5):409–424. doi: 10.1007/s00011-011-0308-7. [DOI] [PubMed] [Google Scholar]
- 72.Patterson A.T., Tian F.T., Elston D.M., Kaffenberger B.H. Occluded cigarette smoke exposure causing localized chloracne-like comedones. Dermatology. 2015;231:322–325. doi: 10.1159/000439046. [DOI] [PubMed] [Google Scholar]
- 73.Kwon H., Pessin J.E. Adipokines mediate inflammation and insulin resistance. Front Endocrinol. 2013;4:71. doi: 10.3389/fendo.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumtomrut C., Yamauchi T., Koike S., Aiba S., Yamasaki K. Androgens modulate keratinocyte differentiation indirectly through enhancing growth factor production from dermal fibroblasts. J Dermatol Sci. 2019;93(3):150–158. doi: 10.1016/j.jdermsci.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 75.Guy R., Kealey T. Modelling the infundibulum in acne. Dermatology. 1998;196(1):32–37. doi: 10.1159/000017862. [DOI] [PubMed] [Google Scholar]
- 76.Sivanand A., Gulliver W.P., Josan C.K., Alhusayen R., Fleming P.J. Weight loss and dietary interventions for hidradenitis suppurativa: a systematic review. J Cutan Med Surg. 2020;24(1):64–72. doi: 10.1177/1203475419874412. [DOI] [PubMed] [Google Scholar]
- 77.Hoffman L.K., Ghias M.H., Lowes M.A. Pathophysiology of hidradenitis suppurativa. Semin Cutan Med Surg. 2017;36(2):47–54. doi: 10.12788/j.sder.2017.017. [DOI] [PubMed] [Google Scholar]
- 78.Ogura H., Murakami M., Okuyama Y. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29:628–636. doi: 10.1016/j.immuni.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 79.Veldhoen M. Interleukin 17 is a chief orchestrator of immunity. Nat Immunol. 2017;18:612–621. doi: 10.1038/ni.3742. [DOI] [PubMed] [Google Scholar]
- 80.Ramirez-Carrozzi V., Sambandam A., Luis E. IL-17C regulates the innate immune functions of epithelial cells in an autocrine manner. Nat Immunol. 2011;12:1159–1166. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- 81.Navrazhina K., Frew J.W., Krueger J.G. Interleukin 17C is elevated in lesional tissue of hidradenitis suppurativa. Br J Dermatol. 2020;182(4):1045–1047. doi: 10.1111/bjd.18556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Witte-Handel E., Wolk K., Tsausi A. The IL-1 pathway is hyperactive in hidradenitis suppurativa and contributed to skin infiltration and destruction. J Invest Dermatol. 2019;139(6):1294–1305. doi: 10.1016/j.jid.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 83.Frew J.W., Grand D., Navrazhina K., Krueger J.G. Beyond antibodies: B cells in hidradenitis suppurativa: bystanders, contributors or therapeutic targets? Exp Dermatol. 2020;29(5):509–515. doi: 10.1111/exd.14092. [DOI] [PubMed] [Google Scholar]
- 84.Gauntner T.D. Hormonal, stem cell and Notch signaling as possible mechanisms of disease in hidradenitis suppurativa: a systems-level transcriptomic analysis. Br J Dermatol. 2018;180(1):203–204. doi: 10.1111/bjd.17093. [DOI] [PubMed] [Google Scholar]
- 85.Duchatelet S., Miskintye S., Delage M. Low prevalence of gamma-secretase complex gene mutations in a large cohort of predominantly Caucasian patients with hidradenitis suppurativa. J Invest Dermatol. 2020 doi: 10.1016/j.jid.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 86.Frew J.W., Navrazhina K. In silico analysis of gamma-secretase-complex mutations in hidradenitis suppurativa demonstrates disease-specific substrate recognition and cleavage alterations. Front Med. 2019;6:206. doi: 10.3389/fmed.2019.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiao X., He Y., Li C., Zhang X., Xu H., Wang B. Nicastrin mutations in familial acne inversa impact keratinocyte proliferation and differentiation through the Notch and phosphoinositide 3-kinase/AKT signaling pathways. Br J Dermatol. 2016;174(3):522–532. doi: 10.1111/bjd.14223. [DOI] [PubMed] [Google Scholar]
- 88.Melnik B.C., Plewig G. Impaired Notch signaling: the unifying mechanism explaining the pathogenesis of hidradenitis suppurativa (acne inversa) Br J Dermatol. 2013;168(4):876–878. doi: 10.1111/bjd.12068. [DOI] [PubMed] [Google Scholar]
- 89.Melnik B.C., Plewig G. Impaired Notch-MKP-1 signaling in hidradenitis suppurativa: an approach to pathogenesis by evidence from translational biology. Exp Dermatol. 2013;22(3):172–177. doi: 10.1111/exd.12098. [DOI] [PubMed] [Google Scholar]
- 90.Frew J.W., Navrazhina K. No evidence that impaired Notch signaling differentiates hidradenitis suppurativa from other inflammatory skin diseases. Br J Dermatol. 2020;182(4):1042–1043. doi: 10.1111/bjd.18593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.He Y., Li C., Xu H. AKT-dependent hyperproliferation of keratinocytes in familial hidradenitis suppurativa with a NCSTN mutation: a potential role of defective miR-100-5p. Br J Dermatol. 2020;182(2):500–502. doi: 10.1111/bjd.18460. [DOI] [PubMed] [Google Scholar]
- 92.Cao L., Morales-Heil D.J., Roberson E.D.O. Nicastrin haploinsufficiency alters expression of type 1 interferon stimulated genes: the relationship to familial hidradenitis suppurativa. Clin Exp Dermatol. 2019;44(4):e118–e125. doi: 10.1111/ced.13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang J., Wang L., Zhang X. Keratin 5-Cre driven deletion of NCSTN in acne inversa-like mouse model leads to markedly increased IL36q and SPRR2 expression. Front Med. 2020;14(3):305–317. doi: 10.1007/s11684-019-0722-8. [DOI] [PubMed] [Google Scholar]
- 94.Gonzalez-Villanueva I., Guiterrez M., Hispan P., Betloch I., Pascual J.C. Novel POFUT1 mutation associated with hidradenitis suppurativa–Dowling-Degos disease firm up a role for Notch signaling in the pathogenesis of this disorder. Br J Dermatol. 2018;178(4):984–986. doi: 10.1111/bjd.16264. [DOI] [PubMed] [Google Scholar]
- 95.Jfri A.H., O'Brien E., Litvinov I. Hidradenitis suppurativa: comprehensive review of predisposing genetic mutations and changes. J Cutan Med Surg. 2019;23(5):519–527. doi: 10.1177/1203475419852049. [DOI] [PubMed] [Google Scholar]
- 96.Liu Y., Yen H., Chen C.Y. Sialylation and fucosylation of epidermal growth factor receptor suppresses its dimerization and activation in lung cancer cells. Proc Natl Acad Sci U S A. 2011;108(28):11322–11373. doi: 10.1073/pnas.1107385108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Frew J.W., Vekic D.A., Woods J.A., Cains G.D. Drug-associated hidradenitis suppurativa: a systematic review of case reports. J Am Acad Dermatol. 2018;78(1):217–219. doi: 10.1016/j.jaad.2017.08.046. [DOI] [PubMed] [Google Scholar]
- 98.Rongioletti F. Histopathology. In: Micali G., editor. Hidradenitis Suppurativa: A Diagnostic Atlas. Wiley; New York: 2017. [Google Scholar]
- 99.Danby F.W., Jemec G.B., Marsch WCh, von Laffert M. Preliminary findings suggest hidradenitis suppurativa may be due to defective follicular support. Br J Dermatol. 2013;168(5):1034–1039. doi: 10.1111/bjd.12233. [DOI] [PubMed] [Google Scholar]
- 100.Hoang V.T., Trinh C.T., Nguyen C.H., Chansomphou V., Chansomphou V., Tran T.T.T. Overview of epidermoid cyst. Eur J Radiol Open. 2019;6:291–301. doi: 10.1016/j.ejro.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mozeika E., Pilmane M., Nürnberg B.M. Tumour necrosis factor-alpha and matrix metalloproteinase-2 are expressed strongly in hidradenitis suppurativa. Acta Derm Venereol. 2013;93(3):301–304. doi: 10.2340/00015555-1492. [DOI] [PubMed] [Google Scholar]
- 102.Chuang Y.H., Dean D., Allen J., Dawber R., Wojnarowska F. Comparison between the expression of basement membrane zone antigens of human interfollicular epidermis and anagen hair follicle using indirect immunofluorescence. Br J Dermatol. 2003;149(2):274–281. doi: 10.1046/j.1365-2133.2003.05468.x. [DOI] [PubMed] [Google Scholar]
- 103.Frew J.W., Navrazhina K., Marohn M., Lu P.C., Krueger J.G. Contribution of fibroblasts to tunnel formation and inflammation in hidradenitis suppurativa/acne inversa. Exp Dermatol. 2019;28(8):886–891. doi: 10.1111/exd.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Coates M., Mariottoni P., Cocoran D.L. The skin transcriptome in hidradenitis suppurativa uncovers an antimicrobial and sweat gland gene signature which has distinct overlap with wounded skin. PLoS One. 2019;14(5):e0216249. doi: 10.1371/journal.pone.0216249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ge Y., Fuchs E. Stretching the limits: from homeostasis to stem cell plasticity in wound healing and cancer. Nat Rev Genet. 2018;19:311–325. doi: 10.1038/nrg.2018.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Navrazhina K., Frew J.W., Sullivan-Whelan M., Gilleadeau P., Garcet S., Krueger J.G. Epithelialized dermal tunnels drive “feed-forward” inflammation and transepithelial neutrophil migration in hidradenitis suppurativa. J Allerg Clin Immunol. 2020 [Google Scholar]
- 107.Scharl M., Rogler G. Pathophysiology of fistula formation in Crohn's disease World. J Gastrointest Pathophysiol. 2014;5(3):205–212. doi: 10.4291/wjgp.v5.i3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oh J.W., Kloepper J., Langan E.A. A guide to studying human hair follicle cycling in vivo. J Invest Dermatol. 2016;136(1):34–44. doi: 10.1038/JID.2015.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kidacki M., Cong Z., Flamm A., Helm K., Danby F.W., Nelson A.M. Invasive proliferative gelatinous mass of hidradenitis suppurativa contains distinct inflammatory components. Br J Dermatol. 2019;181(1):192–193. doi: 10.1111/bjd.17541. [DOI] [PubMed] [Google Scholar]
- 110.Ring H.C., Bay L., Nilsson M. Bacterial biofilm in chronic lesions of hidradenitis suppurativa. Br J Dermatol. 2017;176(4):993–1000. doi: 10.1111/bjd.15007. [DOI] [PubMed] [Google Scholar]