Abstract
Background
Previous analysis showed that the incidence rates of skin cancer in Singapore increased from 1968 to 2006, especially among older Chinese, and particularly for basal cell carcinoma (BCC).
Objective
We updated the skin cancer incidence rates and time trends among the Chinese, Malays, and Indians in Singapore.
Methods
We analyzed the skin cancer incidence from the nationwide Singapore Cancer Registry from 1968 to 2016.
Results
Among 12,692 cases diagnosed from 1968 to 2016, there were 8367 (65.9%) cases of BCC, 3598 (28.3%) of squamous cell carcinoma (SCC), and 727 (5.8%) of melanoma. The mean ages at skin cancer diagnosis were 72.7 years for SCC, 66.9 years for BCC, and 59.8 years for melanoma. Sun-exposed areas accounted for 81.3% of BCCs, 61.6% of SCCs, and 26.7% of melanomas. The age-standardized incidence rate of cutaneous BCC was highest in the Chinese and increased by 2.5 fold over the study period, followed by a lower rate and slower increase in the Malays, and the lowest rate among the Indians. The SCC and melanoma incidences remained low in all 3 ethnicities during this study period. The Chinese had the highest relative risk for developing any skin cancer (P < .0001) compared with those of the Malays and Indians. Most cases of skin cancer were diagnosed at age ≥60, with men showing a higher incidence of SCC compared with that of women.
Conclusion
Incidence rates of BCC have increased in Singapore, especially among the Chinese, in the past 5 decades. The SCC and melanoma incidence rates remained low and stable.
Key words: basal cell carcinoma, squamous cell carcinoma, melanoma, Singapore, Chinese
Abbreviations used: ASIR, age-standardized incidence rate; BCC, basal cell carcinoma; RR, relative risk; SCC, squamous cell carcinoma
Capsule Summary.
-
•
Basal cell carcinoma is the most common skin cancer in Singapore, followed by squamous cell carcinoma and melanoma.
-
•
Fairer-skinned Chinese have the highest relative risk for developing any skin cancer compared with those of darker-skinned Malays and Indians.
Introduction
Skin cancers are among the most commonly occurring malignancies worldwide. In a recent systematic review of the worldwide incidence of nonmelanoma skin cancer, the incidence for nonmelanoma skin cancer varied widely, with the highest rates in Australia (>1000 per 100,000 person-years for basal cell carcinoma [BCC]) and the lowest rates in parts of Africa (<1 per 100,000 person-years for BCC).1 This is in accordance with the difference in Fitzpatrick skin phototypes, a classification of skin type according to the amount of pigment in the skin, which in turn is inversely associated with the risk of skin cancer development.2 In Canada, BCC and squamous cell carcinoma (SCC) increased by 2.4% each year among adults aged >40 years from the 1970s to 2000.3 In the United Kingdom, BCC increased by 39% from 2000 to 2011 in all age groups >30 years.4 The incidence of melanoma also increased in most Western countries.5 In the United States, the incidence increased from 1992 to 2006 among the white-skinned population for all age groups.6 In Denmark, from 1985 to 2012, the incidence of invasive melanoma increased by 4.5% in men and by 4.3% in women.7
Singapore, an equatorial country with a multiethnic population of 5.7 million people, has one of the highest UV index scores in the world.8 In Singapore, skin cancer is consistently ranked among the top 10 cancers, being ranked sixth among men and seventh among women.9 Singapore is a multiracial country with ethnic Chinese (76.2% of the citizen population), Malays (15.0%), and ethnic Indians (7.4%). Chinese Singaporeans make up the majority of the population. Of note, these 3 major ethnic groups represent people with different Fitzpatrick's skin types ranging from III (Chinese) to VI (Indians).
The first study of skin cancers in Singapore reported that the overall rates of skin cancer in Singapore increased from 6.0/100,000 person-years in the early period of 1968 to 1972 to 8.9/100,000 person-years in the period from 1993 to 1997.10 This small increase after about 25 years was attributed to higher diagnosis rates because of improved health care in Singapore. Following that, an update of skin cancers in Singapore reported that the skin cancer incidence rates remained stable, ranging from 8.4 per 100,000 person-years from 1998 to 2002 and subsequently declining slightly to 7.4 per 100,000 person-years in the subsequent years from 2003 to 2006.11 The skin cancer incidence trend after 2006 remains uncertain.
This study aimed to update the incidence rates and trends of BCC, SCC, and melanoma among the 3 major Asian ethnic groups (Chinese, Malay, and Indian) in Singapore from 1968 to 2016, using data from the Singapore nationwide cancer registry.
Methods
Study design
This was a descriptive study of skin cancer data obtained from the Singapore Cancer Registry, focusing on the 3 most common skin cancers in Singapore: BCC, SCC, and melanoma.
Source of data
The Singapore Cancer Registry was started in 1968 and is part of the National Registry of Diseases Office. It collects epidemiological and clinical data of all newly diagnosed cancer cases while tracking cancer deaths. It provides a comprehensive database for the study of cancer survival and allows a better understanding of the cancer trends in the country.12 Notification of all cases of cancers diagnosed or treated in Singapore is mandatory under the National Registry of Diseases Act 2007.13 These notifications are performed via the submission of hard or soft copies of cancer notification forms, pathology reports, laboratory results, hospital records, and death certificates.
Case definition
Skin cancer was defined according to the site as “C43” and “C44” according to the International Classification of Diseases, 10th revision (ICD-10) code.14 Skin cancer sites were categorized into sun-exposed sites (face and neck: ICD-10 codes for C43.0-C43.3, C44.0-C44.3) and sites that are usually not exposed to the sun (trunk and lower limbs: ICD-10 codes for C43.5, C43.7, C44.5, C44.7) for subgroup analysis. Melanoma was defined as having a morphology code of “8720-8790”. Skin cancer with a morphology code of “8050-8078” or “8083-8084” was classified as SCC and skin cancer coded as “8090-8098” was classified as BCC according to the International Classification of Diseases for Oncology, third edition (ICD-O-3) code.15
Statistical analysis
Direct standardization was used to compute the standardized incidence rate, which was an average of the age-specific rates weighted according to the relative age distribution of the arbitrary Segi population.16 We used generalized linear models with Poisson distribution weighted by the population to compute the relative risk and the 95% confidence intervals of skin cancer associated with age, ethnicity, and sex in the same model. All statistical analyses were performed using SAS ver. 9.4 (SAS Institute Inc). The study was approved by the Institutional Review Board at the National University of Singapore.
Results
A total of 14,441 cases of BCC, SCC, and melanoma were reported from 1968 to 2016. Of these, BCC comprised 65.9% (8367 cases), SCC 28.3% (3598 cases), and melanoma 5.8% (727 cases) (Table I). There were equal numbers of men and women for BCC (50% each), and there were slightly more men for SCC (59.1%) and melanoma (53.5%). SCC developed in older patients (mean age 72.7 years) compared with BCC (mean age 66.9 years) and melanoma (mean age 59.8 years). There was also an increase in the number of skin cancer cases reported in the past decade (2008-2016), with 42.3% of all BCC cases, 37.1% of all SCC cases, and 36.2% of all melanoma cases reported from 2008 to 2016. Sun-exposed areas (these areas included the lip, eyelid, ear, face, scalp and neck, and upper limbs) accounted for the majority of BCC cases (81.3%) and SCC cases (61.6%) but the minority of melanoma cases (26.7%).
Table I.
Characteristics of patients in Singapore with basal cell carcinoma (BCC), squamous cell carcinoma (SCC), and melanoma, from 1968 to 2016, by ethnicities
| Skin cancer type | All | Chinese | Malay | Indian |
|---|---|---|---|---|
| All skin cancers, No. (%) | 14,441 (100) | 12,264 (84.9) | 717 (5.0) | 357 (2.5) |
| Mean age (± SD), y | 66.4 (16.8) | 67.8 (16.4) | 60.5 (18.4) | 57.5 (18.8) |
| Men, No. (%) | 7663 (53.1) | 6233 (50.8) | 397 (55.4) | 210 (58.8) |
| Sun-exposed area, No. (%) | 9902 (68.6) | 8646 (70.5) | 435 (60.7) | 187 (52.4) |
| BCC | 8367 | 7039 (84.1) | 322 (3.9) | 127 (1.5) |
| Mean age (± SD), y | 66.9 (14.5) | 68.3 (14.2) | 65.6 (13.8) | 63.1 (14.9) |
| Men, No. (%) | 4180 (50.0) | 3309 (47.0) | 156 (48.5) | 64 (50.4) |
| Sun-exposed area, No. (%) | 6804 (81.3) | 5888 (83.7) | 277 (86.0) | 104 (81.9) |
| SCC | 3598 | 3212 (89.3) | 176 (4.9) | 105 (2.9) |
| Mean age (± SD), y | 72.7 (14.0) | 73.6 (13.8) | 65.5 (13.8) | 63.7 (13.7) |
| Men, No. (%) | 2126 (59.1) | 1860 (57.9) | 115 (65.3) | 67 (63.8) |
| Sun-exposed area, No. (%) | 2217 (61.6) | 2003 (62.4) | 95 (54.0) | 50 (47.6) |
| Melanoma | 727 | 555 (76.3) | 66 (9.1) | 20 (2.8) |
| Mean age (± SD), y | 59.8 (17.7) | 60.8 (17.9) | 60.2 (18.2) | 56.1 (11.5) |
| Men, No. (%) | 389 (53.5) | 271 (48.8) | 34 (51.5) | 16 (80.0) |
| Sun-exposed area, No. (%) | 194 (26.7) | 156 (28.1) | 15 (22.7) | 4 (20.0) |
BCC, Basal cell carcinoma; SCC, squamous cell carcinoma.
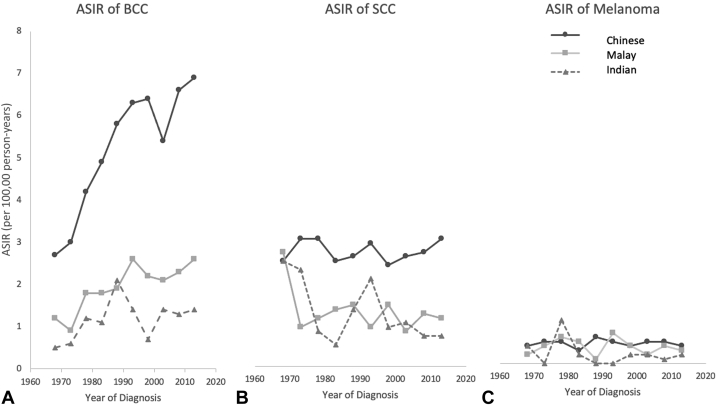
Table II shows the crude and age-standardized incidence rates (ASIRs) for skin cancers from 1968 up until 2016. Overall, the Chinese had the highest ASIRs for BCC and SCC, with Chinese men showing higher ASIRs than those of Chinese women for these 2 cancers. The ASIR for melanoma remained low and stable in all 3 ethnicities. The ASIRs for BCC among the 3 ethnicities from 1968 to 2016 showed the largest increase among the Chinese from 2.7 tumors per 100,000 person-years in 1968 to 6.9 tumors per 100,000 person-years in 2016 (an increase of 2.5 times). For the Malay, the ASIR increased approximately 2.2 times, from 1.2 tumors per 100,000 person-years in 1968 to 2.6 tumors per 100,000 person-years in 2016. The ASIR remained low among the Indians, increasing from 0.5 tumors per 100,000 person-years in 1968 to 1.4 tumors per 100,000 person-years in 2016. In all ethnicities, the ASIR for SCC was lower than the ASIR for BCC. The ASIR for SCC was stable among the Chinese, ranging between 2.4 and 2.9 tumors per 100,000 person-years throughout this period. The ASIRs for SCC in Malays and Indians showed a decline over the years. The ASIRs for melanoma remained low and stable in all ethnicities over the years (Fig 1).
Table II.
Crude (CIR) and age-standardized (ASIR) incidence rates for BCC, SCC, and melanoma by sex and ethnicity, 1968-2016
| Skin cancer type | Ethnic group | No. | CIR (95%CI) | ASIR (95%CI) |
|---|---|---|---|---|
| BCC | ||||
| Men | Chinese | 3309 | 6.1 (5.9-6.3) | 6.1 (5.9-6.3) |
| Malay | 156 | 1.5 (1.3-1.8) | 2.1 (1.8-2.4) | |
| Indian | 64 | 1.1 (0.8-1.4) | 1.1 (0.8-1.4) | |
| Women | Chinese | 3730 | 6.8 (6.6-7.0) | 5.5 (5.3-5.7) |
| Malay | 166 | 1.7 (1.4-1.9) | 2.2 (1.9-2.5) | |
| Indian | 63 | 1.2 (0.9-1.5) | 1.5 (1.1-1.8) | |
| SCC | ||||
| Men | Chinese | 1860 | 3.4 (3.3-3.6) | 3.5 (3.3-3.6) |
| Malay | 115 | 1.1 (0.9-1.3) | 1.5 (1.3-1.8) | |
| Indian | 67 | 1.1 (0.9-1.4) | 1.1 (0.8-1.4) | |
| Women | Chinese | 1352 | 2.5 (2.3-2.6) | 1.9 (1.8-2.0) |
| Malay | 61 | 0.6 (0.5-0.8) | 0.8 (0.6-1.0) | |
| Indian | 38 | 0.7 (0.5-1.0) | 1.0 (0.8-1.2) | |
| Melanoma | ||||
| Men | Chinese | 271 | 0.5 (0.4-0.6) | 0.5 (0.4-0.5) |
| Malay | 34 | 0.3 (0.2-0.5) | 0.4 (0.3-0.6) | |
| Indian | 16 | 0.3 (0.2-0.4) | 0.2 (0.1-0.4) | |
| Women | Chinese | 284 | 0.5 (0.4-0.6) | 0.4 (0.4-0.5) |
| Malay | 32 | 0.3 (0.2-0.5) | 0.4 (0.3-0.5) | |
| Indian | 4 | 0.08 (0.02-0.2) | 0.08 (0.03-0.2) | |
ASIR, Age-standardized incidence rate; BCC, basal cell carcinoma; CI, confidence interval; CIR, crude incidence rate; SCC, squamous cell carcinoma.
Fig 1.
Trends in ASIRs of (A) BCC, (B) SCC, and (C) melanoma from 1968 to 2016 in the Chinese, Malay, and Indian population in Singapore. ASIR, Age-standardized incidence rate; BCC, basal cell carcinoma; SCC, squamous cell carcinoma.
Table III shows the relative risks (RRs) of skin cancers stratified by ethnicity, sex, and age. The Chinese, with the lightest Fitzpatrick skin phototype among the 3 ethnicities, had a much higher RR for all 3 types of skin cancers compared with those of the darker-skinned Malays and Indians. Men had 1.6 times the risk for SCC compared with women. (RR 1.60, P < .0001). There were no differences between men and women for the risks of BCC and melanoma. The RRs of skin cancers increased steeply with increasing age, with people aged ≥70 years having 14.8 times and 40.4 times the risk of BCC and SCC compared with those <40 years of age.
Table III.
Relative risks of BCC, SCC, and melanoma stratified by ethnicity, sex, and age
| Demographics | BCC |
SCC |
Melanoma |
|||
|---|---|---|---|---|---|---|
| Cases | RR∗ | Cases | RR | Cases | RR | |
| Ethnicities | ||||||
| Chinese | 7039 | 1.00 | 3212 | 1.00 | 555 | 1.00 |
| Malay | 322 | 0.05 | 176 | 0.08 | 66 | 0.12 |
| Indian | 127 | 0.02 | 105 | 0.05 | 20 | 0.03 |
| Sex | ||||||
| Women | 4187 | 1.00 | 1472 | 1.00 | 338 | 1.00 |
| Men | 4180 | 0.94 | 2126 | 1.60 | 389 | 0.90 |
| Age groups (y) | ||||||
| <40 | 342 | 1.00 | 64 | 1.00 | 111 | 1.00 |
| 40-49 | 822 | 2.23 | 177 | 2.58 | 100 | 0.84 |
| 50-59 | 1360 | 4.40 | 431 | 6.69 | 137 | 1.18 |
| 60-69 | 2009 | 6.92 | 712 | 11.51 | 152 | 1.43 |
| 70+ | 3826 | 14.77 | 2206 | 40.39 | 227 | 2.34 |
BCC, Basal cell carcinoma; RR, relative risk; SCC, squamous cell carcinoma.
P values for all RR were <.0001 (95% confidence intervals are not shown because the differences were beyond the second decimal places for the estimates).
Discussion
Our study showed that the incidence of cutaneous BCC had increased from 1968 to 2016 and was highest among the Chinese, followed by Malays, and lowest among Indians. The SCC and melanoma incidences remained low in all 3 ethnicities during this study period. The risks of skin cancers increased sharply with increasing age after 60 years, with men showing a higher incidence of SCC compared with that of women. Most BCC and SCC cases were located on sun-exposed areas.
Increasing skin cancer incidences have been recorded in European countries, the United States, and Australia.1,17,18 However, there is little information on the trends in skin cancer in Asia. In East Asia, a recent study in South Korea used data from the Korean nationwide cancer registry from 1999 to 2014 and reported that the incidences of cutaneous BCC, SCC, and melanoma had increased from 1999 to 2014.19 Our study showed that the incidences in Singapore resembled the rates reported in Japan, which also reported that while the age-adjusted incidence rate of BCC increased from the period 1976-1980 to 1986-1990, the rate of SCC and melanoma did not increase significantly.5,20 In contrast to the increasing incidence trends of SCC and melanoma in Western populations,1,6,7 the incidences of these 2 subtypes of skin cancer have been stable or shown decreasing trends in darker-skinned populations of Asian heritage.20 Our updated study findings are also in keeping with the previous low incidence rate of melanoma reported from 1967 to 2006 in Singapore.12,13 Importantly, this study confirmed the previous epidemiologic data of the propensity for melanomas to occur more commonly on non–sun-exposed skin in the darker-skinned population.20 Awareness of this unique distribution and the increased mortality of melanomas on non–sun-exposed skin in the darker-skinned population should be emphasized in Singapore to raise awareness among physicians and patients to examine non–sun-exposed areas during physical examination and self-examination.
Our study demonstrated that SCC was more common in men than in women in Singapore during the study period. Earlier epidemiologic studies reported that BCC and SCC were more common in men than in women.17 It is believed that lifestyle choices play a major role in this disparity between men and women. Historically, men tended to have occupations that required them to spend more time outdoors, and men were also less likely to use sun protection (protective headgear, body attire, umbrellas, and sunscreens)21,22 or participate in cancer screening than women.19 In addition, public awareness regarding skin cancers and treatment options remains relatively low in Asia.23, 24, 25
The global rise of skin cancer incidence could be related to increased exposure to UV light either via lifestyle habits or occupations,21 as well as occupational exposures to carcinogens such as coal tar.1,4 In Asia, exposure to Chinese proprietary medicines containing arsenic26 as well as consumption of drinking water containing inorganic arsenic27 could be additional risk factors. The higher prevalence of Chinese proprietary medicine use among the Chinese in Singapore compared with that in the other 2 minority groups could underlie the highest incidence of skin cancers in the Chinese population.28 A recent study conducted in a population-based Singapore Chinese cohort showed that higher fish intake was significantly associated with an increased risk of SCC in a dose-dependent manner.29 This could be related to the high arsenic levels in seafood,30 possibly because of contaminants in formulated feeding pellets.29 In fact, a study in Singapore reported that the mean daily intake of arsenic from seafood for a 60-kg person in Singapore reached 3.1 μg/kg body weight/d, which was above the recommendations by the Food and Agriculture Organization/World Health Organization.31
In our study, we reported that the lighter-skinned Chinese had the highest risk compared with those of the darker-skinned Malays and Indians for developing all 3 types of skin cancer. More effort could be made to raise the skin cancer awareness in this high-risk group that forms the majority of the Singapore population. Of note, the rising trend in BCC, consistent with global trends,1,4 is of concern. This could be partly attributed to the rapidly aging population in Singapore as well as increased awareness of BCC among family physicians with subsequent referrals to specialists for histologic confirmation of BCC. In Australia, which has the world's highest skin cancer incidence, primary prevention programs have shown success in reducing skin cancer rates.32 However, sustained efforts may be required for long-lasting skin cancer control.32,33 This should be balanced with other emerging health concerns, including the postulated benefits of vitamin D via sun exposure33 and the need for outdoor physical activity for a healthy lifestyle.34 Future campaigns should advise about sun protection without compromising the evidence-based advice for general good health.34
A potential limitation of this study is the underreporting of cases. Although the Singapore Cancer Registry actively seeks diagnosed cases, the possibility of some skin cancer cases being misdiagnosed or not notified cannot be completely excluded. This may lead to an underestimation of incidence rates and trends. Nonetheless, the nationwide Singapore Cancer Registry, which has been in place since 1968, has been shown to be comprehensive in its recording of cancer cases, and especially in the last decade when reporting became mandatory.
In conclusion, our study provided an update on the nationwide incidence of BCC, SCC, and melanoma in Singapore. While the incidence of BCC increased from 1968 to 2016, and especially so for the Chinese population, the SCC and melanoma incidences remained low during this period. More studies can be done in the future concerning carcinogen exposures and preventive measures such as sun protection, especially in Chinese men.
Conflict of interest
None disclosed.
Acknowledgments
Data for this study was provided by the Singapore Cancer Registry.
Footnotes
Funding sources: None.
IRB approval status: Approved by the Institutional Review Board at the National University of Singapore.
References
- 1.Lomas A., Leonardi-Bee J., Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 2.Gogia R., Binstock M., Hirose R., Boscardin W.J., Chren M.-M., Arron S.T. Fitzpatrick skin phototype is an independent predictor of squamous cell carcinoma risk after solid organ transplantation. J Am Acad Dermatol. 2013;68(4):585–591. doi: 10.1016/j.jaad.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demers A.A., Nugent Z., Mihalcioiu C., Wiseman M.C., Kliewer E.V. Trends of nonmelanoma skin cancer from 1960 through 2000 in a Canadian population. J Am Acad Dermatol. 2005;53(2):320–328. doi: 10.1016/j.jaad.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 4.Reinau D., Surber C., Jick S.S., Meier C.R. Epidemiology of basal cell carcinoma in the United Kingdom: incidence, lifestyle factors, and comorbidities. Br J Cancer. 2014;111(1):203–206. doi: 10.1038/bjc.2014.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ichihashi M., Naruse K., Harada S. Trends in nonmelanoma skin cancer in Japan. In: Garbe C., Schmitz S., Orfanos C.E., editors. Skin Cancer: Basic Science, Clinical Research and Treatment. Springer; 1995. pp. 263–273. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A., Saraiya M., Patel P. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S17–S25.e1. doi: 10.1016/j.jaad.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 7.Helvind N.M., Hölmich L.R., Smith S. Incidence of in situ and invasive melanoma in Denmark from 1985 through 2012: a national database study of 24,059 melanoma cases. JAMA Dermatol. 2015;151(10):1087–1095. doi: 10.1001/jamadermatol.2015.1481. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization Radiation: the ultraviolet (UV) index. https://www.who.int/uv/intersunprogramme/activities/uv_index/en/index3.html Available at:
- 9.Teo M.C.C., Soo K.C. Cancer trends and incidences in Singapore. Jpn J Clin Oncol. 2013;43(3):219–224. doi: 10.1093/jjco/hys230. [DOI] [PubMed] [Google Scholar]
- 10.National Registry of Diseases ACT (Chapter 201B) https://sso.agc.gov.sg/Act/NRDA2007 Accessed January 1, 2021.
- 11.Sng J., Koh D., Siong W.C., Choo T.B. Skin cancer trends among Asians living in Singapore from 1968 to 2006. J Am Acad Dermatol. 2009;61(3):426–432. doi: 10.1016/j.jaad.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Lee H.P. Monitoring cancer incidence and risk factors in Singapore. Ann Acad Med Singap. 1990;19(2):133–138. [PubMed] [Google Scholar]
- 13.National Registry of Diseases Office. https://www.nrdo.gov.sg/publications/cancer Accessed January 1, 2021.
- 14.World Health Organization . Vol. 2. World Health Organization; 2004. (International Statistical Classification of Diseases and Related Health Problems: Instruction Manual). [Google Scholar]
- 15.World Health Organization (2013) International classification of diseases for oncology (ICD-O) – 3rd edition. https://apps.who.int/iris/handle/10665/96612 Accessed January 1, 2021.
- 16.Curado M.-P., Edwards B., Shin H.R., editors. IX. IARC Press; 2007. (Cancer Incidence in Five Continents). [Google Scholar]
- 17.Scrivener Y., Grosshans E., Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147(1):41–47. doi: 10.1046/j.1365-2133.2002.04804.x. [DOI] [PubMed] [Google Scholar]
- 18.Trakatelli M., Ulrich C., Del Marmol V., Euvrard S., Stockfleth E., Abeni D. Epidemiology of nonmelanoma skin cancer (NMSC) in Europe: accurate and comparable data are needed for effective public health monitoring and interventions. Br J Dermatol. 2007;156(suppl 3):1–7. doi: 10.1111/j.1365-2133.2007.07861.x. [DOI] [PubMed] [Google Scholar]
- 19.Suh M., Choi K.S., Park B. Trends in cancer screening rates among Korean men and women: results of the Korean National Cancer Screening Survey, 2004-2013. Cancer Res Treat. 2016;48(1):1–10. doi: 10.4143/crt.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishihara K., Saida T., Otsuka F., Yamazaki N., Prognosis and Statistical Investigation Committee of the Japanese Skin Cancer Society Statistical profiles of malignant melanoma and other skin cancers in Japan: 2007 update. Int J Clin Oncol. 2008;13(1):33–41. doi: 10.1007/s10147-007-0751-1. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy E.M., Ethridge K.P., Wagner R.F., Jr. Beach holiday sunburn: the sunscreen paradox and gender differences. Cutis. 1999;64(1):37–42. [PubMed] [Google Scholar]
- 22.Gawkrodger D.J. Occupational skin cancers. Occup Med (Lond) 2004;54(7):458–463. doi: 10.1093/occmed/kqh098. [DOI] [PubMed] [Google Scholar]
- 23.Kwan Z., Yong S.S., Robinson S. Analysis of Internet searches using Google Trends to measure interest in sun protection and skin cancer in selected South-East Asian populations. Photodermatol Photoimmunol Photomed. 2020;36(2):83–89. doi: 10.1111/phpp.12510. [DOI] [PubMed] [Google Scholar]
- 24.Cheng S., Lian S., Hao Y. Sun-exposure knowledge and protection behavior in a North Chinese population: a questionnaire-based study. Photodermatol Photoimmunol Photomed. 2010;26(4):177–181. doi: 10.1111/j.1600-0781.2010.00513.x. [DOI] [PubMed] [Google Scholar]
- 25.Yan S., Xu F., Yang C. Demographic differences in sun protection beliefs and behavior: a community-based study in Shanghai, China. Int J Environ Res Public Health. 2015;12(3):3232–3245. doi: 10.3390/ijerph120303232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martena M.J., Van Der Wielen J.C.A., Rietjens I.M.C.M., Klerx W.N.M., De Groot H.N., Konings E.J.M. Monitoring of mercury, arsenic, and lead in traditional Asian herbal preparations on the Dutch market and estimation of associated risks. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2010;27(2):190–205. doi: 10.1080/02652030903207235. [DOI] [PubMed] [Google Scholar]
- 27.Knobeloch L.M., Zierold K.M., Anderson H.A. Association of arsenic-contaminated drinking-water with prevalence of skin cancer in Wisconsin’s Fox River Valley. J Health Popul Nutr. 2006;24(2):206–213. [PubMed] [Google Scholar]
- 28.Lim M.K., Sadarangani P., Chan H.L., Heng J.Y. Complementary and alternative medicine use in multiracial Singapore. Complement Ther Med. 2005;13(1):16–24. doi: 10.1016/j.ctim.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Oh C.C., Jin A.Z., Yuan J.M., Koh W.P. Fish intake and risk of nonmelanoma skin cancer in a Chinese population: the Singapore Chinese Health Study. Clin Exp Dermatol. 2020;45(4):461–463. doi: 10.1111/ced.14112. [DOI] [PubMed] [Google Scholar]
- 30.Li W., Wei C., Zhang C., Van Hulle M., Cornelis R., Zhang X. A survey of arsenic species in Chinese seafood. Food Chem Toxicol. 2003;41(8):1103–1110. doi: 10.1016/s0278-6915(03)00063-2. [DOI] [PubMed] [Google Scholar]
- 31.Bayen S., Koroleva E., Lee H.K., Obbard J.P. Persistent organic pollutants and heavy metals in typical seafoods consumed in Singapore. J Toxicol Environ Health A. 2005;68(3):151–166. doi: 10.1080/15287390590890437. [DOI] [PubMed] [Google Scholar]
- 32.Iannacone M.R., Green A.C. Towards skin cancer prevention and early detection: evolution of skin cancer awareness campaigns in Australia. Melanoma Manag. 2014;1(1):75–84. doi: 10.2217/mmt.14.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janda M., Kimlin M.G., Whiteman D.C., Aitken J.F., Neale R.E. Sun protection messages, vitamin D and skin cancer: out of the frying pan and into the fire? Med J Aust. 2007;186(2):52–54. doi: 10.5694/j.1326-5377.2007.tb00798.x. [DOI] [PubMed] [Google Scholar]
- 34.Jardine A., Bright M., Knight L., Perina H., Vardon P., Harper C. Does physical activity increase the risk of unsafe sun exposure? Health Promot J Aust. 2012;23(1):52–57. doi: 10.1071/he12052. [DOI] [PubMed] [Google Scholar]