To the Editor: Atopic dermatitis (AD) places a substantial financial burden on patients and society.1 Abrocitinib, a once-daily oral selective Janus kinase 1 inhibitor in development for moderate-to-severe AD, was effective and well tolerated in patients with moderate-to-severe AD as monotherapy (JADE MONO-2 [NCT03575871])2 or in combination with topical therapy (JADE COMPARE [NCT037204700]).3 However, the economic impact of abrocitinib in terms of direct health care costs and indirect costs remains unknown. This preliminary post hoc economic analysis examined short-term direct and indirect cost reductions, from health care payer and societal perspectives, respectively, associated with abrocitinib (200 mg and 100 mg) treatment versus dupilumab or placebo in patients with moderate-to-severe AD.
Outcomes included the number of physician visits in the past 3 months from JADE COMPARE and the overall work impairment (ie, absenteeism and presenteeism measured using the Work Productivity and Activity Impairment Questionnaire: Atopic Dermatitis, Version 2.0) from JADE MONO-2. Physician cost savings (annualized, mean per patient) were based on the reduction in physician visit frequency from baseline to week 16 multiplied by the physician visit unit cost ($265 [2016]).4 Indirect cost savings were estimated based on the reduction in the overall work impairment1 from baseline to week 12 and multiplied by the annual median wage in the United States ($49,348 [the first quarter of 2020]).5
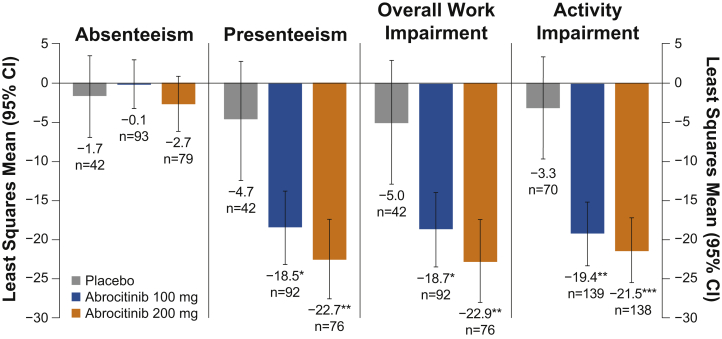
The demographic and baseline characteristics were similar among the patients in JADE COMPARE (n = 837)3 and JADE MONO-2 (n = 391).2 Of the included patients, 720 in JADE COMPARE (abrocitinib 200 mg, n = 196; abrocitinib 100 mg, n = 204; dupilumab injection 300 mg, n = 211; and placebo, n = 109) had physician visit data, and 347 patients in JADE MONO-2 (abrocitinib 200 mg, n = 138; abrocitinib 100 mg, n = 139; and placebo, n = 70) completed the Work Productivity and Activity Impairment-AD questionnaires. The patients treated with abrocitinib (200 mg and 100 mg), dupilumab, or placebo in JADE COMPARE reported similar decreases in the mean number of physician visits from the baseline (mean [SD], 2.8 [3.4], 3.0 [5.0], 2.8 [3.1], and 3.0 [3.4], respectively) to week 16 (mean [SD], 0.9 [2.3], 1.0 [2.3], 1.3 [2.8], and 1.6 [2.6], respectively; Table I). The patients treated with abrocitinib (200 mg or 100 mg) in JADE MONO-2 reported a greater reduction in overall work impairment at week 12 versus placebo (least squares mean change from baseline [95% CI], −22.9 [−28.2, −17.6] or −18.7 [−23.4, −14.0] versus −5.0 [−12.8, 2.8], respectively; Fig 1). The estimated annualized indirect/direct (total) cost reductions from baseline in patients who received abrocitinib 200 mg or abrocitinib 100 mg versus placebo were $11,301/$1636 ($12,937) and $9228/$1723 ($10,951), respectively.
Table I.
Physician visits in JADE COMPARE at week 16
| Study period | Abrocitinib 200 mg QD n = 226 |
Abrocitinib 100 mg QD n = 238 |
Dupilumab 300 mg Q2W n = 242 |
Placebo n = 131 |
|---|---|---|---|---|
| Baseline | ||||
| n | 224 | 237 | 236 | 129 |
| Mean (SD) | 2.8 (3.4) | 3.0 (5.0) | 2.8 (3.1) | 3.0 (3.4) |
| Week 16 | ||||
| n | 196 | 204 | 211 | 109 |
| Mean (SD) | 0.9 (2.3) | 1.0 (2.3) | 1.3 (2.8) | 1.6 (2.6) |
Allowed medicated topical therapies included low- or medium-potency topical corticosteroids, topical calcineurin inhibitors, and topical phosphodiesterase-4 inhibitors.
QD, Once daily; Q2W, every 2 weeks; SD, standard deviation.
Fig 1.
Least squares mean of change from baseline in Work Productivity and Activity Impairment Questionnaire: Atopic Dermatitis, Version 2.0, in JADE MONO-2 at week 12 for absenteeism, presenteeism, overall work impairment, and activity impairment.
Abrocitinib (200 mg or 100 mg) versus placebo: ∗P ≤ .05; ∗∗P < .001; ∗∗∗P < .0001.
Absenteeism is defined as the percentage of work time missed; presenteeism is defined as the percentage of impairment while working; overall work impairment is defined as the percentage of the overall work impairment; and activity impairment is defined as the percentage of activity impairment.
This preliminary economic analysis suggests that, although all patients in JADE COMPARE reported a similar decrease in physician visits, abrocitinib (200 mg and 100 mg) was associated with greater improvements in overall work impairment and associated costs versus placebo, as demonstrated in JADE MONO-2. The limitations of this economic analysis include not accounting for other health care costs and the extrapolation from short-term periods to annual costs, particularly since treatment nonresponders would likely be switched to another therapy in a real-world setting (thus, potentially improving their outcomes over the course of a full year). Future claims analyses can address these limitations; however, these findings represent a preliminary assessment of whether abrocitinib might provide a meaningful improvement in economic outcomes.
Conflicts of interest
Dr Gooderham has received grants, personal fees, honoraria, and/or nonfinancial support from Pfizer Inc, AbbVie, Amgen, Akros Pharma, Arcutis, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Dermira, Dermavant, Eli Lilly, Galderma, Janssen, Kyowa Kirin, LEO Pharma, MedImmune, Merck, Novartis, Roche, Sanofi Genzyme, Regeneron, Sun Pharma, UCB, and Bausch Health (Valeant). Dr Chu is an investigator for Pfizer Inc, AbbVie, Dermira, Eli Lilly, Novartis, Oneness Biotech, Regeneron, Roche, and Sanofi; a consultant for Pfizer Inc, AbbVie, Eli Lilly, Novartis, Roche, and Sanofi; a speaker for Pfizer Inc, AbbVie, Eli Lilly, Mylan, Novartis, Roche, and Sanofi; and is on advisory boards for Pfizer Inc, Mylan, Roche, and Sanofi. Drs Rojo, Valdez, Biswas, Cameron, Feeney, Encinas, Peeples-Lamirande, Cappelleri, and DiBonaventura and Ms Myers are employees and stockholders of Pfizer Inc.
Acknowledgments
Editorial/medical writing support under the guidance of the authors was provided by Susanna Bae, PharmD (ApotheCom, San Francisco, CA), and Gauri Saal, MA Economics (ApotheCom, London, United Kingdom). It was funded by Pfizer Inc, New York, NY, in accordance with the Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461-464).
Footnotes
Funding sources: This study was sponsored by Pfizer, Inc.
IRB approval status: Not applicable.
Data sharing: Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual deidentified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (ie, development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after the study completion. The deidentified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
References
- 1.Drucker A.M., Wang A.R., Li W.Q., Sevetson E., Block J.K., Qureshi A.A. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137(1):26–30. doi: 10.1016/j.jid.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg J.I., Simpson E.L., Thyssen J.P. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863–873. doi: 10.1001/jamadermatol.2020.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thaci T, Bieber T, Simpson EL, et al. A phase 3 study to investigate the efficacy and safety of abrocitinib and dupilumab in comparison with placebo in adults with moderate-to-severe atopic dermatitis. Paper presented at: 29th EADV Congress (Virtual); October 28-November 1, 2020. Poster P0207.
- 4.Machlin S.R., Mitchell E.M. Agency for Healthcare Research and Quality; 2018. Statistical brief #517: expenses for office-based physician visits by specialty and insurance type, 2016.https://meps.ahrq.gov/data_files/publications/st517/stat517.shtml Available at: [PubMed] [Google Scholar]
- 5.US Bureau of Labor Statistics . US Department of Labor; 2020. Usual weekly earnings of wage and salary workers. First quarter 2020.https://www.bls.gov/news.release/pdf/wkyeng.pdf [Google Scholar]