Abstract
Background
Although atopic dermatitis (AD) is becoming a pressing public health concern in the world, Madagascar is underrepresented in the AD literature.
Objective
We aimed to study the demographic and clinical pattern of AD in adult dermatology outpatients.
Methods
A cross-sectional study was conducted in the Department of Dermatology, University Hospital, Antananarivo, Madagascar. Patients >15 years old with a registered diagnosis of AD, from January 2010 to February 2019, were included. AD was diagnosed by a dermatologist according to Hanifin and Rajka criteria. The severity of AD was assessed using scoring atopic dermatitis (SCORAD).
Results
Forty-two cases of AD were included. The prevalence was 0.5%. The median patient age was 37 years. The age of onset of AD was before the age of 15 years in 38% of the patients and after the age of 15 years in 61.9% of the patients. There was a female preponderance (female to male ratio, 2:1), but no correlation was found between sex and the severity of AD. People living in urban areas were the most affected. According to SCORAD, 37 cases presented moderate AD and 2 cases presented severe AD.
Conclusion
The prevalence of AD in adult dermatology outpatients is still low, and moderate AD is the most frequent form, according to SCORAD.
Key words: adults, atopic dermatitis, epidemiology, low prevalence, moderate form
Abbreviations used: AD, atopic dermatitis; SCORAD, scoring atopic dermatitis
Capsule Summary.
-
•
The prevalence of atopic dermatitis (AD) in adult dermatology outpatients was 0.5% (95% confidence interval 0.37-0.69).
-
•
The onset of AD was after the age of 15 years in 61.9% of the patients, and moderate AD was the most frequent form.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disorder, characterized by intense itching and eczematous lesions.1 AD can present for the first time in adults (adult-onset AD),2 but 50% of childhood AD persists into adulthood and becomes a chronic, lifelong condition.3 Rates of AD prevalence in adults vary widely depending on geography, likely because of varied diagnostic criteria and international differences in clinical phenotype. According to studies of 21st century data for adults, the point prevalence of AD varied from 0.6% to 9.7%.4 Although atopic dermatitis is becoming a pressing public health concern, Madagascar is underrepresented in the AD literature. So, we aimed to describe the demographic and clinical pattern of AD in adult dermatology outpatients.
Methodology
A cross-sectional study was conducted based on a review of medical registries in the Department of Dermatology, University Hospital Joseph Raseta Befelatanana, Antananarivo, Madagascar, which is the only dermatologic specialist service in Madagascar. Patients aged 15 years and older, who required health care services from January 2010 to February 2019 and who had a registered diagnosis of AD, were included. The diagnosis of AD was made according to Hanifin and Rajka criteria.5 Age, sex, age of onset, associated comorbidities, personal and familial past medical history (particularly personal and familial atopy), and geographic origin were obtained. Clinical presentation of AD, topographic distribution of lesions, and body surface area involved were evaluated. The severity of AD was assessed by the dermatologist using the scoring atopic dermatitis (SCORAD) index (retrospective collection of data).
The study participants were informed about the study procedures, and written informed consent was obtained from all the patients before they were included in the study. Consent was obtained from all the patients before they were included in the study for data extraction from the clinical record for scientific purposes.
Data were collected using Microsoft Excel. Statistical analyses were processed by “logiciel R Epi info”. The Χ2 test was used to analyze the results, and P < .05 was considered statistically significant.
Results
Of 7875 patients >15 years old in the dermatologic registry from January 2010 to February 2019, 42 patients with AD were identified. The mean age ± standard deviation of patients with AD was 39.04 ± 16.30 years (min: 16 years; max: 69 years). The age of onset of AD was before the age of 15 years in 38.1% of patients and after the age of 15 years in 61.9% of patients. There was a female preponderance (female to male ratio, 2:1). No correlation was found between sex and the severity of AD (P = .12). Overall, 73.8% of the AD patients lived in urban regions (developed regions where pollution is more severe).
According to the SCORAD index, severe AD was observed equally in patients living in urban areas as in those living in rural area (one patient with severe AD is living in an urban area and another patient lives in a rural area). Of the AD cases, 75% were seen in consultation during the winter (April and May). Pruritus was present in 37 (88%) cases. Personal atopy (allergic rhinitis and asthma) was present in 12 (60%) cases. A family history of atopy was noted in 13 (30.9%) cases, especially in those with a family history of AD in 4 (9.5%) cases.
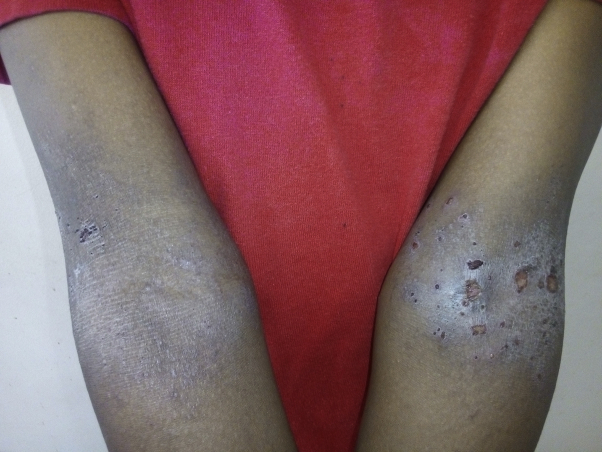
A papulovesicular lesion was present in 25 (59.5%) cases. Lichenified and impetiginized lesions were present in 10 (23.8%) and 5 (11.9%) cases, respectively. The distribution of the participants according to sociodemographic and clinical characteristics is shown in Table I. Skinfolds were the area most involved in adults with AD, in 21 (50%) cases. The face was affected in 15 (35.7%) cases and the trunk in 13 (30.9%) cases. The localization in the trunk was correlated with the mild form of AD according to the SCORAD index (P = .029). The topographic distribution of AD is shown in Table II. Photographs representing the topographic distribution of AD are shown in Figs 1 and 2.
Table I.
Distribution of participants according to sociodemographic and clinical characteristics
| Characteristics | N | Percentage (%) |
|---|---|---|
| Sex | ||
| Male | 14 | 33.3 |
| Female | 28 | 66.6 |
| Age group (years) | ||
| [15-25] | 12 | 28.5 |
| [25-35] | 9 | 21.9 |
| [35-45] | 4 | 9.7 |
| [45-55] | 6 | 14.6 |
| [55-65] | 10 | 24.3 |
| [65-75] | 1 | 2.4 |
| Mean age: 39.04 years | Minimum: 16 years | Maximum: 69 years |
| Median age: 37 years | ||
| Age of onset (years) | ||
| [0-15] | 16 | 38.1 |
| [15-30] | 11 | 26.1 |
| [30-70] | 15 | 35.7 |
| Geographical origin | ||
| Urban | 31 | 73.8 |
| Rural | 11 | 26.1 |
| Personal atopy | ||
| Allergic rhinitis | 10 | 21.1 |
| Food allergy | 6 | 16.5 |
| Asthma | 2 | 5.9 |
| Allergic conjunctivitis | 2 | 3.3 |
| Clinical presentation | ||
| Acute lesions (papulovesicular lesions) | 34 | 80.9 |
| Impetiginized lesion | 5 | 11.9 |
| Lichenification | 10 | 23.8 |
| Body surface area | ||
| <15% | 9 | 21.4 |
| 15%-40% | 27 | 64.3 |
| >40% | 6 | 14.3 |
Table II.
Topographic distribution of AD
| Topography | N | % |
|---|---|---|
| Face | 15 | 35.7 |
| Skinfolds | 21 | 50 |
| Upper portion of the limb | 14 | 33.3 |
| Lower portion of the limb | 13 | 30.9 |
| Trunk | 7 | 16.6 |
| Generalized | 1 | 2.3 |
AD, Atopic dermatitis.
Fig 1.
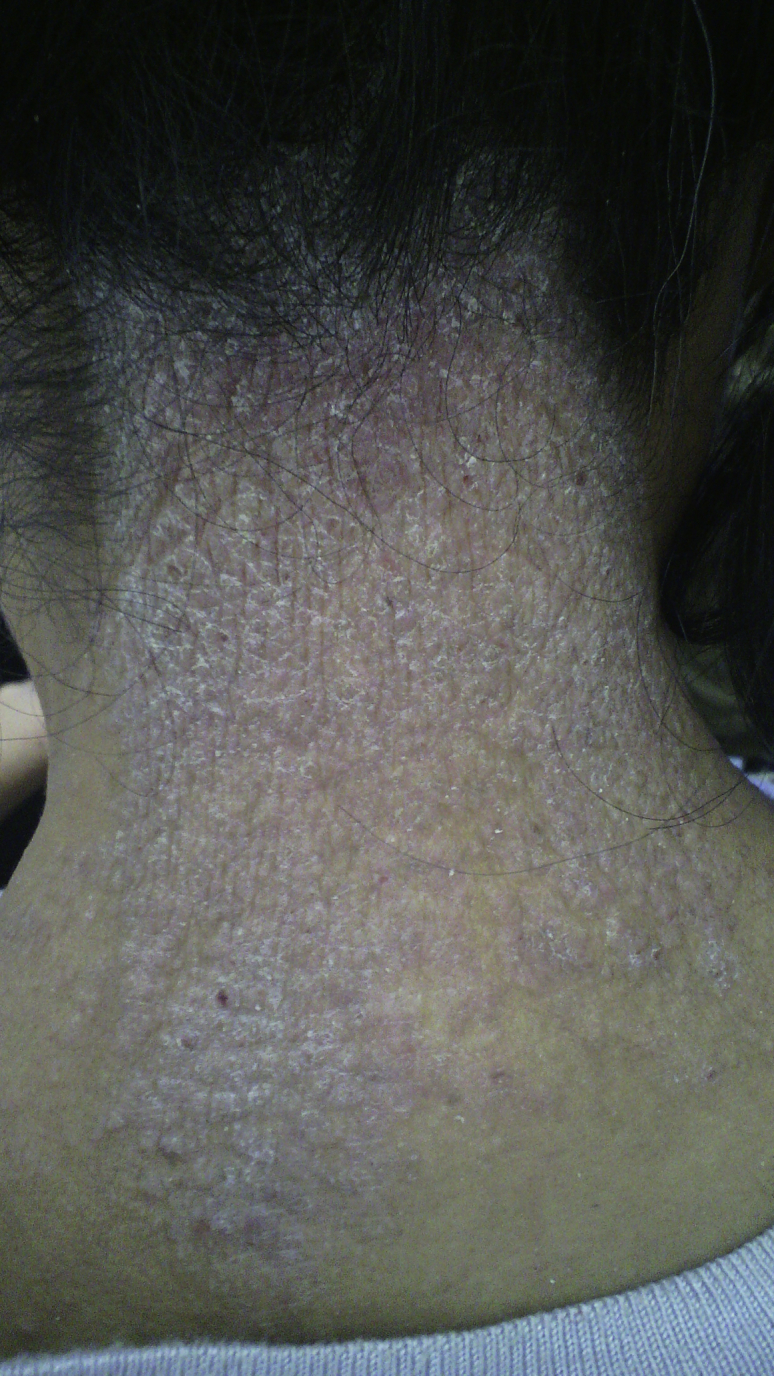
Atopic dermatitis on the neck.
Fig 2.
Atopic dermatitis inside the elbows.
According to the SCORAD index, a moderate extent of lesions was presented by 27 (64.28%) cases and extensive lesions (>40% of body surface area) by 6 (14.3%) cases. The mean SCORAD index ± standard deviation of patients with AD was 38.5 ± 7.4 (minimum: 20; maximum: 66). According to the SCORAD index, 37 (88%) cases presented moderate AD and 2 (4.8%) cases presented severe AD (Table III).
Table III.
Severity of AD according to SCORAD
| SCORAD | N | Percentage (%) |
|---|---|---|
| <15 (Mild AD) | 3 | 7.1 |
| 15-40 (Moderate AD) | 37 | 88 |
| >40 (Severe AD) | 2 | 4.7 |
AD, Atopic dermatitis; SCORAD, scoring atopic dermatitis.
Discussion
Our study showed that the prevalence of AD in adult (age >15 years) dermatology outpatients was 0.5% (95% confidence interval 0.37-0.69). Our result was lower than those reported by previous studies. Patients with AD do not usually present to a doctor. Kim et al.6 in Korea found a prevalence of 2.6% in adult dermatology outpatients. In a Cameroon population-based study, Pefura-Yone et al7 in 2014 reported a prevalence of AD at 2.1%. A high prevalence was reported in a United States population-based study by Silverberg and Hanifin8 in 2013, which was 10.2%, and in Italy by Pesce et al9 in 2015, which was 8.1%. The prevalence varied according to the diagnostic criteria used, the study design, the study size, and the time period.
In our entire cohort, 50% of the patients lived in urban areas (developed regions where pollution is more severe). Concerning the AD patients, 31 adults lived in urban areas and 11 adults in rural areas. Our result was consistent with those reported by Velter et al,10 who found that 56% of AD patients lived in urban areas. Several authors showed a relationship between urbanization and the risk of allergic diseases, including AD. Patients living in urban areas were more affected by AD compared with the rural population; this can be linked to lifestyle and the environmental effect (pollution).11,12
The age of onset of AD was before the age of 15 years in 38.1% of the patients and after the age of 15 years in 61.9% of the patients; among these, 23.8% had an age of onset between 15 and 35 years. Our result was not consistent with the data reported by the National Health Interview Survey in 2012, which showed that 8% of adults presented childhood AD.3 Other studies reported a high prevalence of adult AD whose age of onset was during infancy, such as a Korean study6 that reported that 82% of adults presented childhood AD, and a study in Italy by Zeppa et al13 that reported that 52.4% of cases of AD started in infancy or early childhood. Our result was close to that reported by Hello et al,14 who found that one-third of patients had AD starting in early childhood. According to the literature, the severity and the age of onset > 2years are identified as risk factors the persistence of AD.10,15
Allergic rhinitis and asthma were the personal atopy most presented by patients (12 cases). Our result was consistent with those reported by Orfali et al16 in Brazil and Zeppa et al13 in Italy, which showed that respiratory atopy was the most common personal history. Thirteen (30.9%) cases had a familial history of atopy, especially a family history of AD in 4 (9.5%) cases. According to the literature, 70% of AD patients had a first-degree relative with atopy compared with 20%-35% of nonatopic subjects (people who don't have clinical manifestations mediated by IgE [AD, asthma, allergic conjunctivitis…..]).17
Concerning the severity of AD according to the SCORAD index, the AD was mild in 7.1% of patients, moderate in 88%, and severe in 4.7%. Our result was consistent with international data in 2018,18 which reported that most cases of AD in adults were moderate. However, Kim et al6 reported that AD was mild in 70.6% of Korean patients.
Even though our results were not representative of the general population in Madagascar (participants were from a single institution), they were a preliminary result of the prevalence of AD in our country. Our department is the unique dermatology center in Madagascar, where 6 dermatologists work, and there is no dermatologist in private practice.
Conclusion
The present study showed that the prevalence of AD in adult dermatology outpatients was still low and the severity was moderate in most of the cases (37 patients) according to the SCORAD index.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: All study procedures were performed in accordance with the Ethics Committee of University Hospital Joseph Raseta Befelatanana, Antananarivo, Madagascar.
References
- 1.Weidinger S., Novak N. Atopic dermatitis. Lancet. 2016;387(10023):1109–1122. doi: 10.1016/S0140-6736(15)00149-X. [DOI] [PubMed] [Google Scholar]
- 2.Ozkaya E. Adult-onset atopic dermatitis. J Am Acad Dermatol. 2005;52(4):579–582. doi: 10.1016/j.jaad.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 3.Margolis J.S., Abuabara K., Bilker W., Hoffstad O., Margolis D.J. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 2014;150(6):593–600. doi: 10.1001/jamadermatol.2013.10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bylund S., von Kobyletzki L.B., Svalstedt M., Svensson A. Prevalence and incidence of atopic dermatitis: a systematic review. Acta Derm Venereol. 2020;100(12):adv00160. doi: 10.2340/00015555-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanifin J.M., Rajka G. Diagnostic features of atopic dermatitis. Acta Dermato-Venereologica. 1980;92(suppl):44–47. [Google Scholar]
- 6.Kim M.J., Kang T.W., Cho E.A. Prevalence of atopic dermatitis among Korean adults visiting health service center of the Catholic Medical Center in Seoul metropolitan area, Korea. J Korean Med Sci. 2010;25(12):1828–1830. doi: 10.3346/jkms.2010.25.12.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pefura-Yone E.W., Diffo Sonkoue L., Balkissou A.D., Djenabou A. Prévalence et déterminants de la dermatite atopique chez les adultes en population générale dans un pays d'Afrique sub-saharienne. Rev Fr Allergol. 2017;57(3):240–241. [Google Scholar]
- 8.Silverberg J., Hanifin J. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132(5):1132–1138. doi: 10.1016/j.jaci.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 9.Pesce G., Marcon A., Carosso A. Adult eczema in Italy: prevalence and associations with environmental factors. J Eur Acad Dermatol Venereol. 2015;29(6):1180–1187. doi: 10.1111/jdv.12784. [DOI] [PubMed] [Google Scholar]
- 10.Velter C., Lenormand C., Kluger N. Dermatite atopique et maladies inflammatoires. Ann Dermatol Venereol. 2018;145:IS3–IS24. doi: 10.1016/S0151-9638(18)30384-3. [DOI] [PubMed] [Google Scholar]
- 11.Filipiak B., Heinrich J., Schafer T., Ring J., Wichmann H.E. Farming, rural lifestyle and atopy in adults from southern Germany—results from the Monica/KORA study Augsburg. Clin Exp Allergy. 2001;31(12):1829–1838. doi: 10.1046/j.1365-2222.2001.01246.x. [DOI] [PubMed] [Google Scholar]
- 12.Yemaneberhan H., Flohr C., Lewis S.A. Prevalence and associated factors of atopic dermatitis symptoms in rural and urban Ethiopia. Clin Exp Allergy. 2004;34(5):779–785. doi: 10.1111/j.1365-2222.2004.1946.x. [DOI] [PubMed] [Google Scholar]
- 13.Zeppa L., Bellini V., Lisi P. Atopic dermatitis in adults. Dermatitis. 2011;22(1):40–46. [PubMed] [Google Scholar]
- 14.Hello M., Aubert H., Bernier C., Néel H., Barbarot S. Atopic dermatitis of the adult. Article in French. Rev Med Interne. 2016;37(2):91–99. doi: 10.1016/j.revmed.2015.10.345. [DOI] [PubMed] [Google Scholar]
- 15.Kluger N. Dermatite atopique: données épidémiologiques, passage au traitement systémique. Ann Dermatol Venereol. 2017;144:IIS21–IIS25. doi: 10.1016/S0151-9638(17)31051-7. [DOI] [PubMed] [Google Scholar]
- 16.Orfali R.L., Shimizu M.M., Takaoka R. Atopic dermatitis in adults: clinical and epidemiological considerations. Rev Assoc Med Bras (1992) 2013;59(3):270–275. doi: 10.1016/j.ramb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Taïeb A. Dermatite atopique. In: Saurat J.-H., Grosshans E., Laugier P., Lachappelle J.-M., editors. Dermatologie et Infection Sexuellement Transmissible. 5th éd. Masson; 2008. [Google Scholar]
- 18.Barbarot S., Auziere S., Gadkari A. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73(6):1284–1293. doi: 10.1111/all.13401. [DOI] [PubMed] [Google Scholar]