Abstract
Background
Recent studies have demonstrated that early division of the forehead flap (FHF) is possible if angiography is performed or a remnant of the pedicle is left behind. Whether or not careful selection of patients allows for complete division of the pedicle has not been studied.
Objective
To assess if careful selection of patients allows for early complete division of the FHF.
Methods
The exclusion criteria were trauma in the donor region, full-thickness defects, or a larger cartilage grafting. In the selected patients, complete division of the FHF pedicle was performed at early time points, when the pedicle was clinically engrafted (n = 12).
Results
The median age of the patients was 80 years ± 8. The average size of the wounds was 6.6 cm2 ± 4.0. The complete division of the pedicle was performed in 10 patients after 7 days, 1 patient after 8 days, and 1 patient after 11 days (median 7.4 days ± 1.1). One patient developed a wound infection, and 1 suffered from postoperative bleeding. The latter patient was the only 1 who required debulking in a third surgical procedure. No necrosis or flap failures were observed.
Limitations
Retrospective, single-center study.
Conclusion
Careful selection allows for complete early division of the pedicle of FHF.
Key words: basal cell carcinoma, dermatosurgery, interpolation flap, Mohs micrographic surgery, nasal reconstruction, nose interpolation flap, paramedian forehead flap, surgery
Abbreviation used: FHF, forehead flap
Capsule Summary.
-
•
The forehead flap is a valuable tool as a 2-stage procedure for reconstructing large defects of the nose.
-
•
In select patients, it is possible to divide the forehead flap after 1 week of engraftment using a 2-stage procedure.
Introduction
Reconstruction of the nose after skin tumor surgery is commonly required.1 Smaller defects can be closed with direct closure or local flaps.2, 3, 4, 5, 6 Larger defects can be a challenge to reconstruct as the stiff tissue of the nose makes it difficult to adequately mobilize the tissue. Skin grafting may be an option for these cases.2,7,8 However, cosmesis may suffer as skin mismatch is a common phenomenon after skin grafting.2
The forehead flap (FHF) is a well-established alternative, with good cosmesis, especially for reconstructing defects of the lower third of the nose.9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Different modifications of FHF allow it to be applied for a variety of nasal defects, including those that need cartilage grafting or lining of the nasal ala.9,16, 17, 18 It is a safe procedure, with only minor complication rates19 and can even be used in elderly patients.20 However, FHF is (at least) a 2-stage procedure, requiring engraftment of the FHF in the recipient's defect bed between the first and second surgical procedure. The division of the pedicle of FHF is generally recommended after 3-4 weeks.16, 17, 18 During this time period, the quality of life of patients is reduced as the pedicle requires changing of dressings several times per week; the pedicle makes it difficult to wear glasses. Further, the pedicle in the center of the face has a poor cosmetic outcome.
Recent studies have demonstrated that the division of the pedicle can be performed after 2 weeks, when intraoperative laser fluorescence angiography is performed.21,22 However, intraoperative laser fluorescence angiography might not be available in all centers. Others have reported that the division of the pedicle is possible after 1 week if a remnant of the pedicle is left for 2 weeks.23,24 However, this approach requires at least 3 surgeries as the remnant is removed in another surgical procedure.
As far as we know, no study has investigated if careful selection of patients allows the total division of the pedicle of FHF after 1 week of engraftment. As flaps engraft fast in the facial area, and FHF is a very well-vascularized flap,18,24, 25, 26, 27 we proposed that the division of FHF may be possible after 1 week of the engraftment of the flap in the recipient's bed. We studied 12 consecutive cases that had undergone early division of the pedicle of FHF by the senior author.
Methods
Study design
This is a retrospective study. Twelve consecutive patients who underwent a modified early takedown technique by the senior author between June 2016 and April 2019 were included in the study.
Exclusion criteria
The exclusion criteria for the patients were as follows: (i) full-thickness defects with the loss of mucosa, cartilage, and external soft tissue; (ii) defects that required cartilage grafting for more than 50% of the wound ground; and (iii) a previous trauma in the donor region.
Similar to earlier studies, arterial hypertension, diabetes, smoking, and the use of blood thinners were not included in the exclusion criteria.23
Surgical procedure
After the surgeon decided that the tumor wound required coverage with a FHF, the patient was informed about this technique, and alternatives were discussed, if available. The FHF procedure was performed under tumescent local anesthesia [20 mL 2% lidocaine, 20 mL 1% ropivacaine, 0.5 mL 1:1000 adrenaline (10 lg/mL), and 460 mL Ringer's solution (0.1% tumescent local anesthesia solution)].
The technique is a modification of a recently published technique.20 In brief, a template of the size of the defect was drawn on the forehead, and the flap was drawn as a midline central artery FHF on the forehead. If functional or structural cartilage grafts were required, cartilage was taken from the conchal bowl and sutured into place with nonabsorbable sutures (4.0).
The flap was directly thinned with scissors in the distal two-thirds until all fat tissue was removed, and then it was fixed in place with both absorbable (4.0) and superficial (5.0) sutures.
The pedicle of the FHF was covered with a nonadhesive dressing and fixed with sutures.
When the flap was clinically engrafted (meaning that the flap's skin color was adjusted to the surrounding skin color), the pedicle was divided, and the remaining cranial part of the flap was also trimmed and fixed with sutures. Importantly, the skin sutures of the first surgery in the lower part of the flap were left in place to avoid further manipulation of the engrafting flap at this critical time point. A third surgery with trimming of the flap was required in 1 case. This patient had suffered from postoperative bleeding after the second surgery, and bulking of the flap had developed in the patient. In all the other patients, the procedure was only 2-staged.
Ethical standards
The study was approved by the local ethic committee of the Ärztekammer Sachsen-Anhalt (59-19).
Results
Division of the pedicle of the FHF is possible after 1 week
Twelve consecutive patients, who had undergone early division of the pedicle of the FHF, were included in this study (Table I). Seven patients were women, and 5 patients were men. The average age of the patients was 79.5 years ± 8.2 (range 60-90).
Table I.
Clinical and surgical data of 12 consecutive patients
| No. of patients | Diagnosis | Sex | Age (years) | Aesthetic nasal subunit | No. of aesthetic subunits | Size of the defect (cm2) | Pedicle division (days) | Complication | Third surgery required |
|---|---|---|---|---|---|---|---|---|---|
| 1 | BCC | M | 75 | Tip | 1 | 6.3 | 7 | None | No |
| 2 | BCC | M | 70 | Dorsum | 1 | 7.5 | 7 | Infection | No |
| 3 | BCC | F | 84 | Tip | 1 | 6.3 | 7 | None | No |
| 4 | BCC | F | 80 | Tip, Dorsum | 2 | 5.8 | 7 | None | No |
| 5 | BCC | F | 79 | Tip, ala | 2 | 9 | 7 | None | No |
| 6 | BCC | F | 78 | Dorsum and sidewalls | 3 | 18.1 | 7 | None | No |
| 7 | BCC | F | 87 | Tip | 1 | 6.5 | 7 | None | No |
| 8 | BCC | M | 60 | Sidewalls | 1 | 6.9 | 7 | Bleeding | Yes: debulking |
| 9 | BCC | M | 90 | Tip | 1 | 5.5 | 7 | None | No |
| 10 | BCC | M | 87 | Tip | 1 | 3.4 | 8 | None | No |
| 11 | BCC | F | 76 | Tip, ala | 2 | 6.3 | 11 | None | No |
| 12 | BCC | F | 87 | Ala | 1 | 7.3 | 7 | None | No |
| Average ± SD |
79.5 ±8.2 |
1.3 ±0.6 |
6.6 ±4.0 |
7.4 ±1.1 |
BCC, Basal cell carcinoma; F, female; M, male; SD, standard deviation.
In addition to the skin tumors, some of the patients suffered from cardiovascular diseases (8/12) or diabetes (5/12), some were smokers (1/12), or some were taking blood thinners (7/12). Anticoagulation treatment was not stopped in any case.
All the patients suffered from basal cell carcinomas. All the defects involved the lower third of the nose. The average defect size was 6.6 cm2 ± 4.0 and involved 1.2 aesthetic subunits ± 0.4. In all, 3/12 patients received cartilage grafts. However, no cartilage graft was larger than 50% of the wound bed.
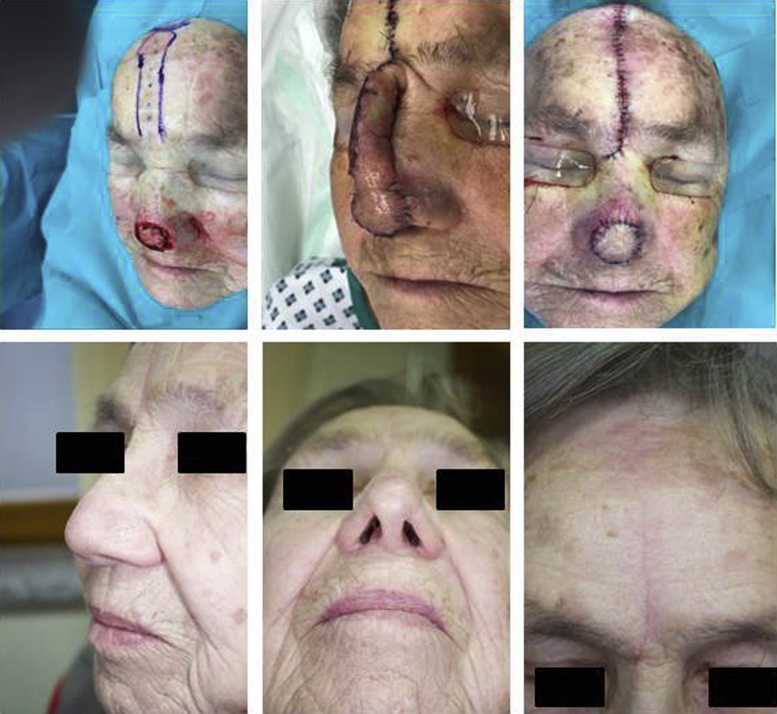
In 10 patients, division of the pedicle was performed after 7 days; in 1 patient, it was performed after 8 days; and in 1 patient; it was performed after 11 days of the engraftment (average 7.4 days ± 1.1). One patient (8%) suffered from a surgical site infection. This was successfully treated with oral antibiotics (cefuroxime). One patient (8%) suffered from postoperative bleeding after the second surgical procedure even though he was not taking blood thinners. Thus, trimming of the flap was required afterward in a third surgical procedure. All the other patients underwent only 2 surgeries for the reconstruction of the nose. None of the patients reported necrosis or flap failure (for examples, see Fig 1, Fig 2, Fig 3).
Fig 1.
Forehead flap (FHF). Example of a FHF with division of the pedicle after 7 days (patient 5 in Table I). The defect involved the aesthetic subunits of the left ala and tip. Cartilage grafting was required. However, the cartilage covered not more than 50% of the wound ground. Complete division of the pedicle was performed when the FHF had been clinically engrafted (middle of the upper row). The postoperative images in the lower row were taken after 6 months. FHF, Forehead flap.
Fig 2.
Forehead flap (FHF). Surgical example of a FHF with division of the pedicle after 7 days (patient 6 in Table I). The defect involved the aesthetic subunits of the dorsum and both sidewalls. Complete division of the pedicle was performed when the FHF had been clinically engrafted (image in the center). The postoperative images in the lower row were taken after 2 years. FHF, Forehead flap.
Fig 3.
Forehead flap (FHF). Example of a FHF with division of the pedicle after 7 days (patient 7 in Table I). The defect involved the aesthetic subunit of the nasal tip. Complete division of the pedicle was performed when the FHF had clinically been engrafted (image in the center). The postoperative images in the lower row were taken after 9 months. FHF, Forehead flap.
Discussion
FHF is a very secure method to reconstruct large and complex wounds of the lower third of the nose.19 The principle of reconstructing the nose with the skin of the forehead dates back to 700 BC.17 In recent decades, several modifications have been published,9, 10, 11, 12, 13, 14, 15, 16, 17, 18 which make FHF a workhorse to reconstruct large and complex nasal defects. However, the morbidity associated with the pedicle has been recognized for a long time.28,29 Twenty years ago, attempts were made to establish FHF as a single-stage procedure.28,29 The pedicle is directly implanted in the upper part of the nose, and instead, healthy tissue is removed. However, removal of the procerus muscle and venous congestion are the major problems of this procedure. Therefore, more recent studies have tried to shorten the time period between the first and second surgeries.20, 21, 22, 23, 24
As far as we know, Somoano et al were the first to discover that division of the pedicle is possible after 1 week if a remnant of the pedicle is left. This remnant is removed in the third surgical procedure. These data were confirmed by Kendler et al in 2014.24 In the last few years, more sophisticated approaches have been taken using indocyanine green angiography.20, 21, 22,30 These studies established that the complete division of the pedicle can be performed after 2 weeks, when indocyanine green angiography is performed. However, well-performed studies have shown that the artery does not need to be included in the flap, questioning if angiography is required in all cases.31 Despite this, until now, it was unclear if complete division of the pedicle is possible after 1 week in a select cohort of patients. Here, to our knowledge, we show for the first time that complete division of the pedicle of FHF is possible after 1 week, when the exclusion criteria are followed. The new modification of the old technique had only minor complications. This is in line with earlier studies that showed that FHF is a safe procedure. In a recent study of a large cohort of patients (n = 2175), the most common complications of this procedure were postoperative bleeding (1.4%) and postoperative infections (2.9%).19 Similarly, in our study, we observed postoperative bleeding in 1 patient and a surgical site infection in another patient. In another study, the most common complications were partial flap loss (6/53; 11.3%), donor site dehiscence (4/53; 7.5%), postoperative flap dehiscence (2/53; 3.8%), and surgical site infection (1/53; 1.9%).32 In our smaller cohort of patients, we did not observe any partial flap loss, necrosis, or donor site dehiscence. Similar observations were made by other groups, in which partial flap loss or necrosis were quite rare.20,33 The defect in the study by Rudolph et al was larger; this explains the higher rate of dehiscence of the donor region. One reason for the partial flap loss could be differences in the technique. Similar to the study by Somoano et al, thinning of the paddle of the flap was performed in our study. We agree with Somoano et al that this is most likely one of the clues that allows the early division of the pedicle. It is very likely that the metabolic demand is less if the skin is thinner. However, while Somoano et al removed the superficial stiches in the flap after 1 week, these were not removed in our study. We believe that nonmanipulation of the tip of the flap at this critical time period is another reason why engraftment can be successful. This may be more important when the upper part of the engrafted flap is, at the same time, thinned to fit into place and sutured into place.
Another reason for the low rate of flap necrosis is that FHF is a well-vascularized flap.18,25,26 Recent innovative imaging studies have well established that the vascular supply of FHF is very good after 2 weeks of engraftment.20, 21, 22,30 It will be interesting to use this new technique to determine the vascular supply of the flap after 1 week and assess if complete dissection of the FHF can be performed in all patients. In our study, complete division of the pedicle of the FHF was only performed in those patients who did not suffer from scarring in the donor region and whose defects were partial-thickness and did not require cartilage grafting for more than 50% of the wound region.
Overall, in the presented study, we show that it is possible to perform FHF as a 2-stage procedure in 1 week. It will be interesting to verify this in a multicenter, multidisciplinary study. New imaging techniques will help to study if this new modification of the FHF protocol will be possible for all patients.
Footnotes
Funding sources: Deutsche Forschungsgemeinschaft (DFG-funded Research Training Group 2099 “Hallmarks of Skin Cancer” [project number 259332240]).
Conflicts of interest: None disclosed.
IRB approval status: The study was approved by the Ärztekammer Sachsen-Anhalt (59-19).
Contributor Information
Moritz Felcht, Email: Moritz.felcht@umm.de.
Tino Wetzig, Email: t.wetzig@asklepios.com.
References
- 1.Lobeck A., Weiss C., Orouji A. Single center analysis of the dermatosurgical patient cohort of a tumor center in Germany. Hautarzt. 2017;68(5):377–384. doi: 10.1007/s00105-017-3951-2. [DOI] [PubMed] [Google Scholar]
- 2.Haneke E. Surgical treatment of defects on the tip of the nose. Dermatol Surg. 1998;24(7):711–717. doi: 10.1111/j.1524-4725.1998.tb04238.x. [DOI] [PubMed] [Google Scholar]
- 3.Cook J.L. Reconstructive utility of the bilobed flap: lessons from flap successes and failures. Dermatol Surg. 2005;31:1024–1033. doi: 10.1111/j.1524-4725.2005.31827. [DOI] [PubMed] [Google Scholar]
- 4.Eberlin K.R., Nguyen B., Karia P.S., Carter J.B., Liang C.A., Schmults C.D. The Z-advancement flap for reconstruction of lateral nasal tip and medial alar defects. Dermatol Surg. 2014;40(2):101–109. doi: 10.1111/dsu.12409. [DOI] [PubMed] [Google Scholar]
- 5.Felcht M. The caudolaterally inserted transposition flap and its variations for reconstruction of defects of the nasal ala. J Dtsch Dermatol Ges. 2017;15(10):981–987. doi: 10.1111/ddg.13318. [DOI] [PubMed] [Google Scholar]
- 6.Benecke J., Olsavszky V., Schaarschmidt M.L., Bauer C., Koch P.S., Felcht M. Reconstruction of defects of the proximal nasal sidewall using the procerus perforator flap. J Dtsch Dermatol Ges. 2019;17(2):210–213. doi: 10.1111/ddg.13752. [DOI] [PubMed] [Google Scholar]
- 7.Riml S., Wallner H., Larcher L., Amann U., Kompatscher P. Aesthetic improvements of skin grafts in nasal tip reconstruction. Aesthetic Plast Surg. 2011;35(4):475–479. doi: 10.1007/s00266-010-9639-y. [DOI] [PubMed] [Google Scholar]
- 8.Moratin K., Koch P.S., Benecke J. Reconstruction of nasal defects with dermal skin substitutes-a retrospective study of 36 defects. J Cutan Med Surg. 2019;23(4):413–420. doi: 10.1177/1203475419852060. [DOI] [PubMed] [Google Scholar]
- 9.Burget G.C., Menick F.J. Nasal support and lining: the marriage of beauty and blood supply. Plast Reconstr Surg. 1989;84(2):189–202. doi: 10.1097/00006534-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Zitelli J.A., Fazio M.J. Reconstruction of the nose with local flaps. J Dermatol Surg Oncol. 1991;17(2):184–189. doi: 10.1111/j.1524-4725.1991.tb01613.x. [DOI] [PubMed] [Google Scholar]
- 11.Driscoll B.P., Baker S.R. Reconstruction of nasal alar defects. Arch Facial Plast Surg. 2001;3(2):91–99. doi: 10.1001/archfaci.3.2.91. [DOI] [PubMed] [Google Scholar]
- 12.Menick F.J. A 10-year experience in nasal reconstruction with the three-stage forehead flap. Plast Reconstr Surg. 2002;109(6):1839–1855. doi: 10.1097/00006534-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Jackson I.T. Midline forehead flaps in nasal reconstruction. Eur J Plast Surg. 2004;27(3):105–113. [Google Scholar]
- 14.Brodland D.G. Paramedian forehead flap reconstruction for nasal defects. Dermatol Surg. 2005;31:1046–1052. doi: 10.1111/j.1524-4725.2005.31829. [DOI] [PubMed] [Google Scholar]
- 15.Burget G.C., Walton R.L. Optimal use of microvascular free flaps, cartilage grafts, and a paramedian forehead flap for aesthetic reconstruction of the nose and adjacent facial units. Plast Reconstr Surg. 2007;120(5):1171–1207. doi: 10.1097/01.prs.0000254362.53706.91. [DOI] [PubMed] [Google Scholar]
- 16.Menick F.J. Nasal reconstruction with a forehead flap. Clin Plast Surg. 2009;36(3):443–459. doi: 10.1016/j.cps.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Correa B.J., Weathers W.M., Wolfswinkel E.M., Thornton J.F. The forehead flap: the gold standard of nasal soft tissue reconstruction. Semin Plast Surg. 2013;27(2):96–103. doi: 10.1055/s-0033-1351231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jellinek N.J., Nguyen T.H., Albertini J.G. Paramedian forehead flap: advances, procedural nuances, and variations in technique. Dermatol Surg. 2014;40:S30–S42. doi: 10.1097/DSS.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 19.Chen C.L., Most S.P., Branham G.H., Spataro E.A. Postoperative complications of paramedian forehead flap reconstruction. JAMA Facial Plast Surg. 2019;21(4):298–304. doi: 10.1001/jamafacial.2018.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kendler M., Averbeck M., Wetzig T. Reconstruction of nasal defects with forehead flaps in patients older than 75 years of age. J Eur Acad Dermatol Venereol. 2014;28(5):662–666. doi: 10.1111/jdv.12114. [DOI] [PubMed] [Google Scholar]
- 21.Surowitz J.B., Most S.P. Use of laser-assisted indocyanine green angiography for early division of the forehead flap pedicle. JAMA Facial Plast Surg. 2015;17(3):209–214. doi: 10.1001/jamafacial.2015.0171. [DOI] [PubMed] [Google Scholar]
- 22.Abdelwahab M., Kandathil C.K., Most S.P., Spataro E.A. Utility of indocyanine green angiography to identify clinical factors associated with perfusion of paramedian forehead flaps during nasal reconstruction surgery. JAMA Facial Plast Surg. 2019;21(3):206–212. doi: 10.1001/jamafacial.2018.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Somoano B., Kampp J., Gladstone H.B. Accelerated takedown of the paramedian forehead flap at 1 week: indications, technique, and improving patient quality of life. J Am Acad Dermatol. 2011;65(1):97–105. doi: 10.1016/j.jaad.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Kendler M., Simon J.C., Grunewald S., Voth H. Early takedown of paramedian forehead flap. J Dtsch Dermatol Ges. 2014;12(10):924–926. doi: 10.1111/ddg.12427. [DOI] [PubMed] [Google Scholar]
- 25.Shumrick K.A., Smith T.L. The anatomic basis for the design of forehead flaps in nasal reconstruction. Arch Otolaryngol Head Neck Surg. 1992;118(4):373–379. doi: 10.1001/archotol.1992.01880040031006. [DOI] [PubMed] [Google Scholar]
- 26.Kleintjes W.G. Forehead anatomy: arterial variations and venous link of the midline forehead flap. J Plast Reconstr Aesthet Surg. 2007;60(6):593–606. doi: 10.1016/j.bjps.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Abdelwahab M., Spataro E.A., Kandathil C.K., Most S.P. Neovascularization perfusion of melolabial flaps using intraoperative indocyanine green angiography. JAMA Facial Plast Surg. 2019;21(3):230–236. doi: 10.1001/jamafacial.2018.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park S.S. The single-stage forehead flap in nasal reconstruction: an alternative with advantages. Arch Facial Plast Surg. 2002;4(1):32–36. doi: 10.1001/archfaci.4.1.32. [DOI] [PubMed] [Google Scholar]
- 29.Fudem G.M., Montilla R.D., Vaughn C.J. Single-stage forehead flap in nasal reconstruction. Ann Plast Surg. 2010;64(5):645–648. doi: 10.1097/SAP.0b013e3181c925fc. [DOI] [PubMed] [Google Scholar]
- 30.Shah A., Au A. Case report: laser-assisted indocyanine green evaluation of paramedian forehead flap perfusion prior to pedicle division. Eplasty. 2013;13:e8. [PMC free article] [PubMed] [Google Scholar]
- 31.Stigall L., Bramlette T.B., Zitelli J.A., Brodland D.G. The paramedian forehead flap: a clinical and microanatomic study. Dermatol Surg. 2016;42(6):764–771. doi: 10.1097/DSS.0000000000000722. [DOI] [PubMed] [Google Scholar]
- 32.Rudolph M.A., Walker N.J., Rebowe R.E., Marks M.W. Broadening application and insights into cross-paramedian forehead flap over a 19-year period. J Plast Reconstr Aesthet Surg. 2019;72(5):763–770. doi: 10.1016/j.bjps.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Faris C., van der Eerden P., Vuyk H. The midline central artery forehead flap: a valid alternative to supratrochlear-based forehead flaps. JAMA Facial Plast Surg. 2015;17(1):16–22. doi: 10.1001/jamafacial.2014.738. [DOI] [PubMed] [Google Scholar]