To the Editor: Dupilumab has demonstrated good efficacy1 and effectiveness2,3 for adult patients with moderate to severe atopic dermatitis. However, it needs to be administered subcutaneously every 2 weeks. In Japan, self-injection of dupilumab has been available since May 2019. Without self-injection, patients need to visit physicians every 2 weeks; however, regular visits to a hospital or clinic are often hindered for various reasons. Self-injection at home can release patients from regular visits, which is expected to lead to higher compliance, but it has not been introduced in many clinics. In this study, we retrospectively investigated whether introduction of self-injection increases compliance to dupilumab administration.
Adult atopic dermatitis patients who were treated with dupilumab for more than 3 months before initiating self-injection (without self-injection) and were treated for more than 3 months after initiating self-injection (under self-injection) in our hospital as of January 31, 2020, were included in this study. Other inclusion criteria were the same as those described in our previous article.2 All patients received a 600-mg loading dose of dupilumab; then, starting 2 weeks later, 300 mg of dupilumab was scheduled to be administered subcutaneously every 2 weeks. The compliance rate was calculated as follows: the number of actual administrations divided by the number of scheduled administrations × 100%. We calculated the compliance rates before and after initiation of self-injection during the same periods for each patient, and then compared the compliance rates before and after introduction of self-injection with Wilcoxon's matched-pairs signed rank test.
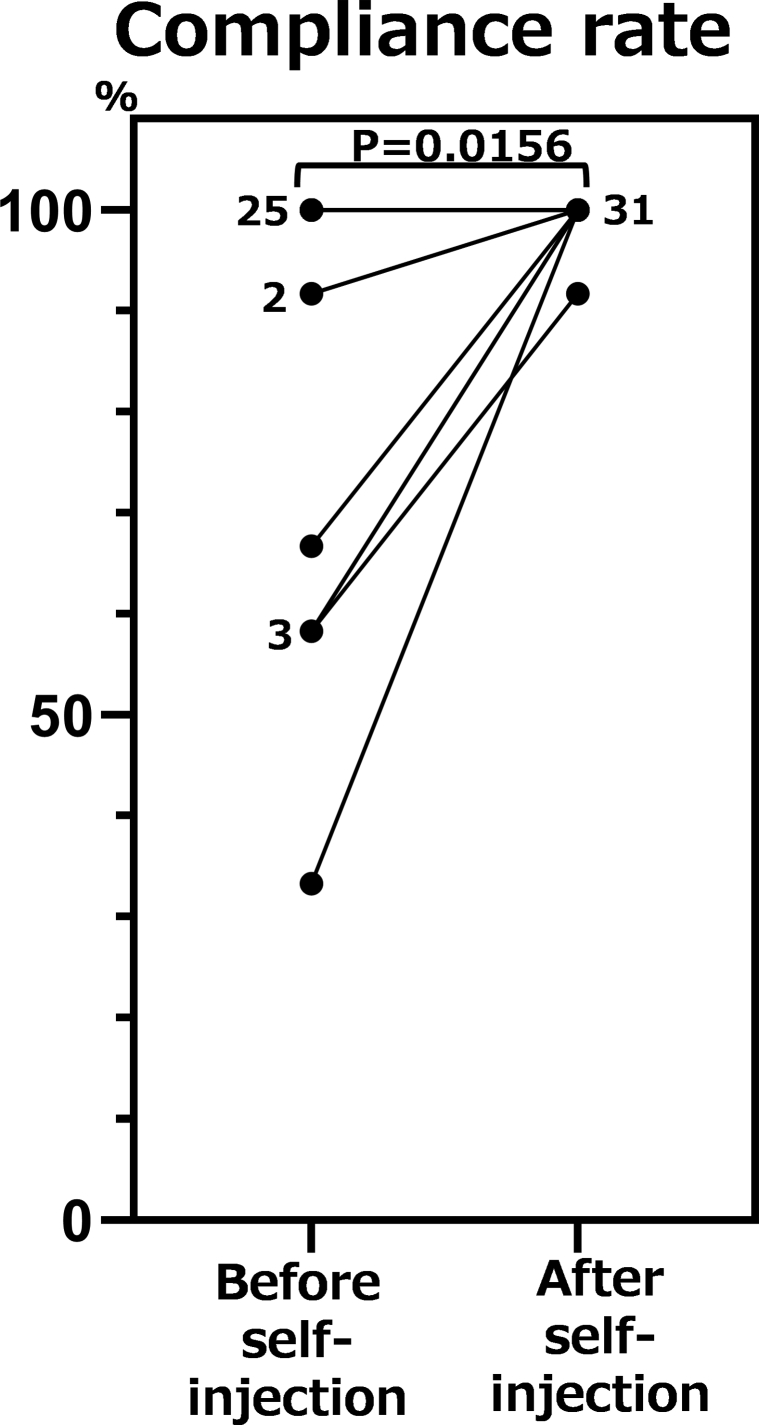
Data on 32 Japanese adult atopic dermatitis patients (28 men and 4 women) were analyzed. The mean age was 41.4 years (range 17-61 years), and the mean duration of atopic dermatitis at initiation of dupilumab was 38.9 years (standard deviation 10.4 years). The mean observed period, the period either before or after initiation of self-injection during which we calculated the compliance rate, was 5.4 ± 0.92 months. After introduction of self-injection, the compliance rate of administering dupilumab improved in 7 patients and it worsened in none. The reasons why they could not visit our hospital before initiating self-injection could be categorized into 2 groups. Five patients could not visit because of inconvenience such as business or personal reasons. For 2 patients, the regular visit to our hospital was interrupted because of problems related to health insurance. The compliance rate of administering dupilumab before introduction of self-injection was 92.4% ± 17.1%, and that after introduction of self-injection was 99.7% ± 1.47%, showing a significant difference (P = .02) (Fig 1). The percentage of patients with a compliance rate of 100% before introduction of self-injection was 78.1%, whereas that after introduction of self-injection was 96.9%.
Fig 1.
Comparison of compliance rates before and after introduction of self-injection of dupilumab in patients with atopic dermatitis treated with dupilumab. The number beside each dot represents the number of patients whose compliance rate was the same.
Patient self-injection is associated with a wide range of benefits, including increased flexibility in the time and place of injection administration,4,5 which may have contributed to higher compliance. Furthermore, self-injection results in reduced cost to both the patient and health care system, reduced travel time, and reduced caregiver burden. These findings suggest that self-injection may be associated with improved compliance and therefore may be a better option for many patients.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Kamata has received honoraria for lectures from Sanofi. Dr Tada has received a grant for research outside this work from Sanofi. Drs Ito, Uchida, Nagata, Fukaya, Hayashi, Fukuyasu, Tanaka, Ishikawa, and Ohnishi have no conflicts of interest to declare.
References
- 1.Simpson E.L., Bieber T., Guttman-Yassky E. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 2.Uchida H., Kamata M., Mizukawa I. Real-world effectiveness and safety of dupilumab for the treatment of atopic dermatitis in Japanese patients: a single-centre retrospective study. Br J Dermatol. 2019;181:1083–1085. doi: 10.1111/bjd.18163. [DOI] [PubMed] [Google Scholar]
- 3.Uchida H., Kamata M., Kato A. One-year real-world clinical effectiveness, safety and laboratory safety of dupilumab in Japanese adult patients with atopic dermatitis: a single-center retrospective study. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.05.102. [DOI] [PubMed] [Google Scholar]
- 4.Keininger D., Coteur G. Assessment of self-injection experience in patients with rheumatoid arthritis: psychometric validation of the Self-Injection Assessment Questionnaire (SIAQ) Health Qual Life Outcomes. 2011;9:2. doi: 10.1186/1477-7525-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van den Bemt B.J.F., Gettings L., Domanska B., Bruggraber R., Mountian I., Kristensen L.E. A portfolio of biologic self-injection devices in rheumatology: how patient involvement in device design can improve treatment experience. Drug Deliv. 2019;26:384–392. doi: 10.1080/10717544.2019.1587043. [DOI] [PMC free article] [PubMed] [Google Scholar]