Abstract
Objective
This systematic comparison between pre‐ and postnatal imaging findings and postnatal motor outcome assesses the reliability of MRI accuracy in the prognostication of the future long‐term (mean, 11.4 years) ambulatory status in a historic group of postnatally repaired myelomeningocele (MMC) cases.
Methods
A retrospective, single‐center study of 34 postnatally repaired MMC patients was performed. We used fetal and postnatal magnetic resonance imaging (MRI) to compare the fetal and postnatal radiological lesion level to each other and to the postnatal ambulatory level as a standard of reference and analyzed Chiari II malformation characteristics.
Results
In 13/15 (87%) and 29/31 (94%) cases, the functional level was equal to or better than the prenatal and postnatal radiological lesion level. A radiological lesion level agreement within two segments could be achieved in 13/15 (87%) patients. A worse than expected functional level occurred in cases with Myelocele (2/3 patients), coexistent crowding of the posterior fossa (2/3 patients) and/or abnormal white matter architecture, represented by callosal dysgenesis (1/3 patients). In all patients (2/2) with a radiological disagreement of more than two segments, segmentation disorders and scoliosis were observed.
Conclusion
Fetal and postnatal MRI are predictive of the long‐term ambulatory status in postnatally repaired MMC patients.
Key points
What's already known about this topic?
Fetal and postnatal magnetic resonance imaging (MRI) show a good correlation in identifying the level of the myelomeningocele (MMC) lesion.
Prenatal ultrasound (US) and fetal MRI show a comparable agreement, within two segments, in predicting the short‐term ambulatory status.
What does this study add?
Fetal and postnatal MRI have a good predictive value for the long‐term ambulatory status in patients with postnatal MMC closure.
MMC patients with worse than expected ambulatory status showed signs of vermian displacement and corpus callosum dysgenesis.
In MMC patients with spinal segmentation disorders and scoliosis, there was a major disagreement between fetal and postnatal MRI, specifically with regard to the MMC lesion level.
1. INTRODUCTION
The incidence of myelomeningocele (MMC) has decreased in the past several years due to an increase in folate acid supplementation.1, 2 Nevertheless, with a prevalence rate between 0.2 and eight per 1000 live births, MMC is the most common form of spinal dysraphism and is associated with Chiari II malformation.3, 4, 5, 6, 7, 8, 9, 10
For an appropriate treatment plan, an early prenatal diagnosis is of the utmost importance.11 As a result of the improvement in prenatal ultrasound (US) diagnostics, 88% of neural tube defects (NTD) in Europe are diagnosed during a median gestational age of 17 weeks.12 Consequently, the abortion rate in cases of NTD has increased in most European countries, especially of MMC patients who are not eligible for prenatal closure.12, 13, 14, 15, 16 These profound changes in evaluation and treatment measures have led to a decrease of postnatal MMC repairs. The legitimate concern of a poor ambulatory status, in particular, leads to substantial parental uncertainty with regard to the option against surgical treatment and for the termination of pregnancy. A better definition of the imaging‐based predictability of future motor outcome in MMC cases would help to reduce this uncertainty and strengthen parents’ confidence in this difficult decision. More recent studies have focused on the functional prediction value of prenatal sonography in postnatally repaired MMC patients. There is a wide variation in studies about the correlation between ambulatory status and radiological lesion level assigned by prenatal sonographic findings, with the neuromotor level reportedly equal to or better than the anatomic level in 52.9%‐91%.17, 18, 19, 20, 21, 22, 23 Many centers have implemented MRI as an complementary prenatal examination method in cases of a suspected NTD. Depending on the expertise and quality of fetal MRI and US, there is much variation with regard to the superiority of either technique.17, 24 Fetal MRI allows a complete examination of the neuroaxis by the second and third trimester, regardless of technique‐related limitations in transabdominal ultrasonography.25, 26, 27 When fetal sonography is compared to postnatal MRI for the identification of the MMC lesion level, a good correlation within one level could be observed in 82% of cases.28 By predicting the ambulatory status, prenatal US and fetal MRI showed an equal agreement, within two segments, in 79.4% of cases.29 However, the studies that analyzed the predictive value of fetal and postnatal MRI with regard to the ambulatory status of patients with postnatally repaired MMC lesions were limited by small patient groups or a short‐term follow‐up usually assessed within one week of postnatal MMC closure.17, 29, 30 Furthermore, other Chiari II malformation characteristics, such as corpus callosum (CC) deformities or vermian displacement, were not considered as additional factors that could potentially explain a poorer functional outcome than that based on the prediction according to the anatomical level alone.31, 32
In this single‐center, follow‐up study, we aimed to retrospectively assess fetal and postnatal MR examinations and correlate these to the ambulatory status of postnatally repaired MMC patients. The main goal of our study was to determine the anatomically predictive and functionally prognostic value of fetal and postnatal MRI in postnatally repaired MMC patients, with respect to the long‐term (mean follow‐up period of 11.4 years) ambulatory status as the standard of reference. By examining the entire neuroaxis using fetal, as well as postnatal MRI, we aimed to identify and neuroradiologically characterize factors that negatively affect the long‐term motor outcome of postnatally repaired MMC patients. This descriptive reference data, derived from a “historic” cohort may serve as an important benchmark for the comparison of long‐term outcomes in prenatal surgery trials.
2. MATERIALS AND METHODS
The study protocol was approved by the Ethics Committee of the Medical University of Vienna (EK 1318/2019).
2.1. Patients
Patients with open spina bifida who underwent a postnatal MMC closure at the Neurosurgery Department of the Medical University of Vienna between the years 2000 and 2015 were included in this study.
Patients were referred to fetal MRI in cases where an MMC was suspected by prenatal US examination. Postnatal MRI examinations were performed only in cases of ambulatory deterioration due to suspected tethered cord or CSF shunt malfunction. Fetal MR images were obtained in 16 patients (47%) and 32 patients (94%) underwent postnatal MRI. Fetal and postnatal MR images were evaluated, in consensus, by one neurosurgeon (T.C.) and one neuroradiologist (G.K.), both with more than 10 years‘ experience in pediatric neuroradiology and fetal MRI. The level of the MMC lesion was defined as the segmental level of the fetal neural placode and the postnatal tethered spinal cord. We defined the most cranial part of the lesion level as the border to the seemingly normal myelon segment, and also used the last completely intact vertebral body as a landmark (Figure 1). A myelon segment was defined as normal if a T2‐hyperintense CSF signal was visible around the myelon. A vertebral body was defined as completely intact only in cases of a complete posterior arch and spinous process. The vertebral bodies were counted beginning caudally or cranially, depending on the level of lesion. In lumbar lesions, vertebral bodies were counted beginning caudally using the promontory as landmark. In thoracic lesions, vertebral bodies were counted beginning cranially, using the foramen magnum as landmark. The method for counting the vertebral bodies did not differ intraindividually between fetal and postnatal MRI. The caudal part of the neural placode or tethered spinal cord was not described. Furthermore, we evaluated Chiari II malformation characteristics, particularly vermian displacement and the shape of the CC. As previously emphasized by Harvey Sarnat,33 herniation relates to an active displacement of an organ through a local tissue defect and since it is not clear if the vermis primarily develops in the spinal canal or is secondarily displaced, we used the term vermian displacement.34 For assessing vermian displacement the foramen magnum was used as reference as well.
FIGURE 1.
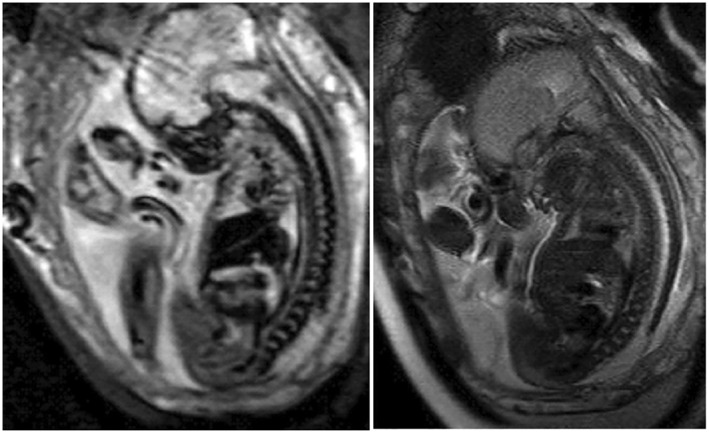
Segmental level of the neural placode. Patient with a Myelomeningocele and lesion level at L1 and fetal vermian displacement at C3. Functional level at L3‐L5. Left: EPI/T2* sequence. Right: T2‐weighted sequence
Due to the great heterogeneity in the nomenclature and the definition of callosal anomalies,35 we decided to use a modified classification system based on Edwards et al.36 and classified the morphology of the CC into four classes (Figure 2). Some showed an inconspicuous form and were thus defined as (1) “normal” (Figure 3). Due to an MMC‐associated hydrocephalus, some CC showed a thinned form and were specifically grouped as (2) “hydrocephalic” (Figure 4) rather than hypoplastic, as originally described by Edwards et al.36 The CC was defined as (3) “dysgenetic” in cases of “small” abnormally shaped or missing areas, especially in the region of the splenium and rostrum. A combination of group (2) and (3) was observed as well, and thus, were grouped as (4) “dysgenic + hydrocephalic”.
FIGURE 2.
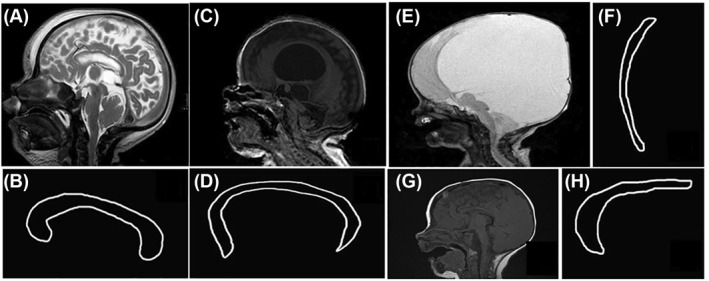
Corpus callosum (CC) morphology. A,B, Normal form of the CC. C‐ F, Hydrocephalic configuration of the CC. G, H, Dysgenetic CC: the splenium of the CC is absent and the proportions of the different callosal segments abnormal
FIGURE 3.
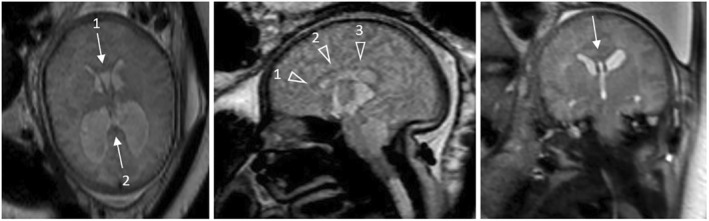
Fetal magnetic resonance (MR) images at 37 gestational weeks of a Myelomeningocele patient depicting the normal shape of the corpus callosum (CC). Left: Axial MR image shows a normal configuration of the CC at the genu (arrow 1) and splenium (arrow 2). Middle: Sagittal MR image shows a normal configuration of the genu (arrowhead 1), body (arrowhead 2) and isthmus (arrowhead 3) of the CC. Right: Coronal MR image shows a normal configuration of the body of the CC
FIGURE 4.
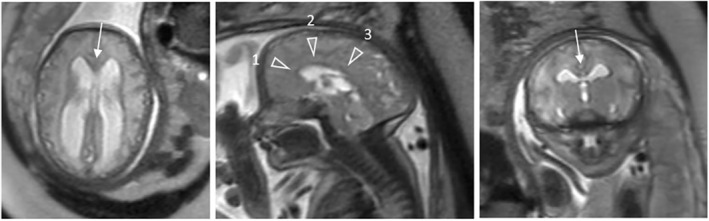
Fetal magnetic resonance (MR) images at 33 gestational weeks of a Myelomeningocele patient with a “hydrocephalic” configuration of the corpus callosum (CC) and functional lesion level of L3‐L5. Left: Axial MR image shows a thinned genu (arrow) and splenium of the CC. Middle: Sagittal MR image shows a thinned genu (arrowhead 1), body (arrowhead 2) and posterior segment (arrowhead 3) of the CC. Right: Coronal MR image shows a thinned body of the CC
Thirty infants (88%) were delivered via Caesarean section and four (12%) via vaginal birth between 31 + 3 and 40 + 1 week of estimated gestational age, respectively (Table 1).
TABLE 1.
Characteristics of the study patients
| Variable | Value |
|---|---|
| Total no. of patients | 34 (100%) |
| Sex | |
| M | 13 (38.2%) |
| F | 21 (61.8%) |
| Type of delivery | |
| Vaginal | 4 (11.8%) |
| C‐section | 30 (88.2%) |
| Gestational age at birth (in weeks) | 37.7 (31.4–40.1) |
| Time of MMC closure | |
| ≤24 h | 31 (91.2%) |
| ≤48 h | 3 (8.8%) |
| Gestational age at fetal MRI (in weeks) | 27.8 (19.4–37.0) |
| Gestational age at 2nd fetal MRI (in weeks) | 29.8 (22.4–35.4) |
| Age at 1st postnatal MRI (in d) | 75 (0–4087) |
| CSF Shunt | 27 (79.4%) |
Note: Values are expressed as numbers (%) or as median (range).
Abbreviations: CSF, cerebral spinal fluid; MMC, myelomeningocele; MRI, magnetic resonance imaging.
MMC repair was performed within 24 h in 31 (91%) and 48 h in three (9%) patients.
Follow‐up was conducted at the University Hospital of Vienna, an MMC‐specialized center, by a multidisciplinary team of pediatric neurosurgeons, neuroradiologists, neuropediatricians, orthopedists and urologists, with a mean follow‐up of 11.4 years (range, 3‐18 years). Five patients were between three to five years of age at the last follow‐up. Twenty‐nine patients were older than seven years.
2.2. Functional level
The functional level of all 34 MMC patients was assessed by a neuropediatrician based on patients' best ambulatory status during follow‐up and was classified into three groups (Table 2).
TABLE 2.
Functional level assessed by clinical neurological examination
| Functional lesion level | Mobility |
|---|---|
| Above or equal to L2 | Wheelchair |
| L3‐L5 | Supporting devices (walking frame, crutches, ankle/knee/hip orthosis) |
| Below or equal to S1 | Unassisted |
Those who were incapable of knee extension and/or hip flexion, and thus, were in need of a wheelchair, were assigned to group ≤L2.37 In cases of knee flexion and/or dorsal foot extension deficits, patients were assigned to group L3‐L5. These patients were ambulatory only with the help of various devices, such as a walking frame, crutches, or an ankle/knee/hip orthosis. Patients with a deficit in plantar flexion and/or who were able to walk without any supporting device were assigned to group ≥S1.38 In cases of an asymmetric functional neurological level, the worst functional level was used.
2.3. Fetal MRI
Fetal MRI examination was performed at the Department of Biomedical imaging and Image‐guided Therapy at the Medical University of Vienna, without any sedation.
The fetal MRI protocol consisted of T2‐TSE sequences in three orthogonal planes (TE = 100–140 ms, TR = variable, slice thickness = 3–4.4 mm, FOV = 230–290 mm), echoplanar sequences, and SSFPE/FIESTA/true‐FISP‐sequences (FOV = 260–300 mm, over continuous slices with 3 mm overlays).
In 16/34 MMC patients (47%), the fetal MRI examination was performed after transabdominal US showed a developmental anomaly. In 6/16 patients (38%), a second fetal MRI examination was performed. The first and second fetal MRI examinations were performed at a median estimated gestational age of 27 + 6 weeks (range, 19 + 3–37 weeks) and 29 + 6 weeks (range, 22 + 3–35 + 3 weeks), respectively.
2.4. Postnatal MRI
Postnatal MRI examinations were performed at the patients' local diagnostic center or at the Department of Biomedical imaging and Image‐guided Therapy at the Medical University of Vienna.
The postnatal MRI protocol consisted of T2‐TSE‐sequences in three orthogonal planes (TE = 50–140 ms, TR = variable, slice thickness = 2–6.5 mm, FOV = 180–1024 mm), T1‐sequences (TE = 2.1–23 ms, TR = 20–1800 ms, slice thickness = 0.9–6.5 mm, FOV = 128–1024 mm), and CISS sequences (TE = 2.4–4.4 ms, TR = 5.3–8.9 ms, slice thickness = 0.6–1 mm, FOV = 128–512 mm).
In 32/34 MMC patients (94%), a postnatal MRI examination was performed after closure of the MMC lesion. The first postnatal MRI examination was done within the first day and 11.3 years (median, 75 days) after delivery.
One spinal, one cranial, and two cranial + spinal MRI examinations were not performed postnatally, since functional neurological development either improved or did not deteriorate during clinical follow‐up. According to the surgical records, the MMC lesions were in the lumbosacral area; one patient had a functional level of L3‐L5, and two patients were classified into the ≥S1 functional group.
2.5. Statistical analysis
Data were analyzed using IBM SPSS Statistics for Windows version 26.0 (IBM, Armonk NY). Data are described as absolute frequencies and percentages. Due to the descriptive character of the study, no statistical analyses were performed.
3. Results
All of the 34 postnatally repaired MMC patients were assigned to one of the predefined functional level groups. Fetal MR images were available in 16 patients (47%). However, the image quality was limited in one case. Thus, fetal MR images of 15 patients were diagnostically used. Based on fetal MR images, MMC was diagnosed in nine of these patients and Myelocele in 5/15 patients. Myelocystocele was diagnosed in one patient who showed mild Chiari II malformation characteristics with no vermian displacement. However, according to the surgical report a neural placode was visible at birth and thus the lesion was defined as MMC.
Postnatal MRI examinations were done in 32/34 patients (94%). The postnatal segmental level of the tethered spinal cord was assigned to 31/34 patients (91%) since, in one patient, only a cranial MRI examination was performed. Of 34 patients, 27 (79%) received a CSF shunt.
There was one death at the age of 10 years, with pneumonia as the cause of death.
3.1. MMC lesion level
There were 8/34 (24%), 19/34 (56%), and 7/34 (21%) patients who had a functional level of ≤L2, L3‐L5, and ≥S1, respectively (Table 3). The functional level, as the reference standard, was compared with fetal and postnatal MR images. For better comparison, the radiological lesion level was organized into groups as well.
TABLE 3.
Functional lesion level of study patients and rate of shunted patients in relation to their functional level group
| Functional level | Patients (n = 34) | Shunted patients (n = 27) |
|---|---|---|
| Above or equal to L2 | 8 (23.5%) | 7/8 (87.5%) |
| L3‐L5 | 19 (55.9%) | 15/19 (78.9%) |
| Below or equal to S1 | 7 (20.6%) | 5/7 (71.4%) |
Note: Values are expressed as numbers (%).
3.1.1. Functional lesion level versus prenatal radiological lesion level
In 13/15 (87%) cases, the functional level was equal to or better than the prenatal lesion level as determined by fetal MRI. There were 4/15 (26.7%) patients who showed a better than expected ambulatory status. In two cases, the functional level was worse than expected and both cases were diagnosed with a Myelocele. In one case (case A), a prenatal genetic examination was performed, which showed a 4q‐17p translocation. This patient (case A) had an anatomical prenatal lesion level of L3‐L5 with a functional level of ≤L2. The second patient (case B) was diagnosed with a lesion level of ≥S1 and had a functional level of L3‐L5.
The fetal MR images of case A showed the last intact vertebral body and a normal myelon segment at L2 and the neural placode at L3. In the postnatal MR images at the age of 22 days, the lesion level was diagnosed at L4 and the normal myelon segment at L3. Prenatal vermian displacement was diagnosed at C3 and postnatally at C2. The CC showed a dysgenetic form on fetal and postnatal MR images. On the 12th day of life, the patient received a cerebral spinal fluid (CSF) shunt, and five months after birth, a craniocervical decompression was performed.
In case B (Figure 5), the fetal MR images showed the last intact vertebral body and normal myelon segment at L5 and the neural placode at S1. In the postnatal MR images at the age of four months, the lesion level was diagnosed at L4/5. Prenatal vermian displacement was diagnosed at C3/4 and postnatally at C0. The CC had a hydrocephalic form on fetal and postnatal MR images. Furthermore, one singular periventricular heterotopia was diagnosed on postnatal MRI examination, which could not be detected by fetal MRI at a gestational age of 22 + 3 weeks. The patient received a CSF shunt on the 18th day of life. On fetal and postnatal MR images, sacral segmentation defects and scoliosis could be observed. During follow‐up, two untetherings were performed due to neurological deterioration with no signs of CSF shunt malfunction. After each operation at the age of 1.5 and 4.8 years, the neurological function improved, and the patient was ambulatory with the help of a supporting device.
FIGURE 5.
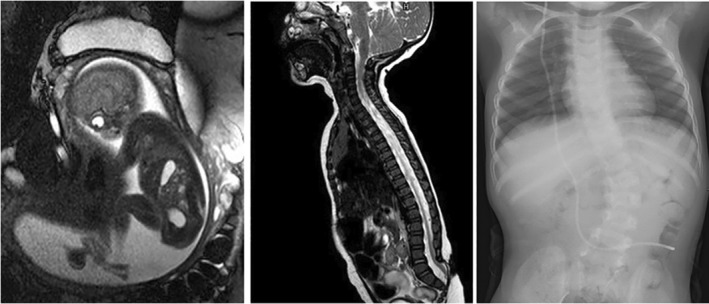
Fetal and postnatal magnetic resonance (MR) images and a radiograph of a myelocele patient with a functional lesion level of L3‐L5. Left: Fetal MR image at an estimated gestational age of 19 + 3 weeks indicates the position of the neural placode at level S1. Center: Four months after birth, the postnatal MR image shows the defect distally to the vertebral bodies L5 and tethering of the spinal cord. Right: The radiograph at the age of four years shows scoliosis and segmentation defects of the lumbar spine
3.1.2. Functional lesion level versus postnatal radiological lesion level
In 29/31 (94%) cases, the functional level was equal to or better than the postnatal radiological lesion level. There were 7/31 (22.6%) patients who showed a better than expected ambulatory status. The functional level was worse than expected in only two cases. Both patients with a postnatal radiological lesion level of L3‐L5 had a functional level of ≤L2.
Case A was already mentioned above. In the second case (case C) (Figure 6), the postnatal MR images showed the last intact vertebral body at L3 and a tethering of the spinal cord.
FIGURE 6.
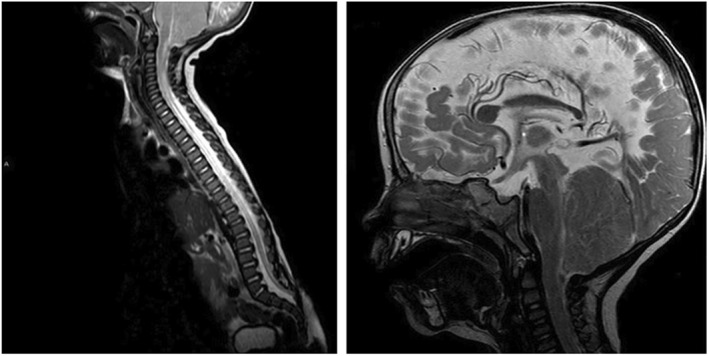
Postnatal magnetic resonance images of an myelomeningocele patient with a functional lesion level of ≤L2. Left: T2‐images three months after birth show the last intact vertebral body at L3 and tethering of the spinal cord. Right: At the age of 14 months, cranial T2‐images indicate crowding of the hypoplastic posterior fossa and obliterated subarachnoid spaces at the craniocervical junction
On the eighth day of life, the patient received a CSF shunt and, on the first postnatal MR images, a normal CC (circumscribed secondary defect to shunt placement at the genu/body transition ‐ Figure 6) and no vermian displacement with bulging of the atlantooccipital membrane could be observed.
After deterioration in hip flexion at the age of six months, MRI showed tethering of the spinal cord at L3/4 with a syringomyelia at C5. Based on the clinical deterioration with the concordant neuroradiological results, an untethering was performed. Eight months later, a neurological deterioration of the upper extremities and a progressive dysphagia led to a craniocervical decompression at the age of 1.2 years.
3.1.3. Radiological lesion level comparison
When the fetal and postnatal radiological lesion level were compared, an exact agreement could be achieved in 6/15 (40%) patients. An agreement within two segments could be observed in 13/15 (87%) patients. In one case (case D), the prenatal radiological lesion level was evaluated to be five segments more caudal. In another case (case E), the prenatal radiological lesion level was assumed to be three segments more cranial. Both patients had distinctive thoracolumbar segmentation defects, as well as scoliosis, and were classified into the functional group of ≤L2.
3.2. Chiari‐II‐malformation characteristics
3.2.1. Vermis
A vermian displacement between C1 and C6 could be observed by fetal MRI in 14/15 (93%) patients (Table 4 and Figure 7). All these patients received a CSF shunt implantation. All patients with Myelocele had a vermian displacement on fetal MRI. In one patient with no vermian displacement and no CSF‐shunt implantation, a Myelocystocele was diagnosed by fetal MRI. In 7/15 (46.7%) patients, the vermis prenatally appeared at the same cervical spinal level as on postnatal MR images. In 5/15 (33.3%) patients, the vermis “ascended” postnatally by one to four segments. In all of those five patients, MMC closure was performed within 24 h after birth (Table 4).
TABLE 4.
Chiari II malformation characteristics on fetal and postnatal MR images
| Chiari II Characteristics | Fetal MRI (n = 15) | Postnatal Cranial MRI (n = 31) | Shunted Patients with Postnatal MRI Scans (n = 27) |
|---|---|---|---|
| Vermian displacement | |||
| C0 | 1 (6.7%) | 9 (29%) | 4 (44.4%) |
| C1 | 1 (6.7%) | 2 (6.5%) | 2 (100%) |
| C2 | 2 (13.3%) | 3 (9.7%) | 2 (66.7%) |
| C3 | 6 (40%) | 6 (19.4%) | 6 (100%) |
| C4 | 3 (20%) | 8 (25.8%) | 8 (100%) |
| C5 | 0 | 2 (6.5%) | 2 (100%) |
| C6 | 2 (13.3%) | 1 (3.2%) | 1 (100%) |
| C7 | 0 | 0 | 0 |
| Corpus callosum | |||
| Normal | 2 (13.3%) | 8 (25.8%) | 6 (75%) |
| Dysgenetic | 4 (26.7%) | 8 (25.8%) | 7 (87.5%) |
| Hydrocephalic | 9 (60%) | 12 (38.7%) | 10 (83.3%) |
| Hydrocephalic + dysgenetic | 0 | 3 (9.7%) | 2 (66.7%) |
Note: Values are expressed as numbers (%) or as median (range). All fetal MRI patients needed a CSF shunt. In two patients with a CSF shunt, postnatal MRI scans could not be obtained.
Abbreviation: MRI, magnetic resonance imaging.
FIGURE 7.
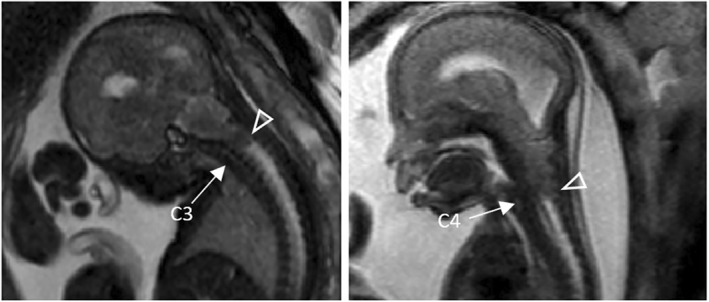
Left: Fetal magnetic resonance (MR) image of a Myelocele patient at an estimated gestational age of 27 weeks showing a vermian displacement (arrowhead) at C3. Right: Fetal MR image of a Myelocele patient at an estimated gestational age of 22 weeks showing vermian displacement (arrowhead) at C4. The exact level of displacement was determined by counting the T2‐weighted hyperintense intervertebral discs (arrow)
Of the 4/15 (26.7%) patients with a better than expected ambulatory status determined by fetal MRI, 2/4 (50%) had a vermian displacement at C3 and 1/4 (25%) at C6.
Of the 7/31 (22.6%) patients with a better than expected ambulatory status determined by postnatal MRI, vermian displacement at C1 (1/7, 14%), C3 (1/7, 14%), C4 (3/7, 43%), and C6 (1/7, 14%) could be observed. All of them received a CSF shunt. One patient (case F) with no vermian displacement and a prenatally normal and postnatally hydrocephalic CC did not receive a CSF shunt.
3.2.2. Corpus callosum
On fetal MR images, the CC appeared normal, hydrocephalic, and dysgenetic in 2/15 (13%), 9/15 (60%), and 4/15 (27%) patients, respectively. In only one case did a prenatal hydrocephalic CC appear dysgenetic on postnatal MR images (Table 4). In one prenatally normal (case F) and one dysgenetic CC, an additional hydrocephalic feature could be observed postnatally. One of these patients (case F) was diagnosed with a Myelocystocele by fetal MRI. Of the 4/15 (26.7%) patients with a better than expected ambulatory status determined by fetal MRI, a normal (1/4, 25%), dysgenetic (1/4, 25%), and hydrocephalic (2/4, 50%) configuration of the CC could be observed. Of the 7/31 (22.6%) patients with a better than expected ambulatory status determined by postnatal MRI, a normal (2/7, 29%), dysgenetic (1/7, 14%), hydrocephalic (2/7, 29%), and dysgenetic + hydrocephalic (2/7, 29%) configuration of the CC could be observed.
4. DISCUSSION
In this single‐center study, we retrospectively evaluated 34 postnatally repaired MMC patients with a mean follow‐up of 11.4 years (range, 3–18 years).
The neuroradiological lesion level showed a good correlation between fetal and postnatal MRI within two segments. Lesion level discrepancies of more than two segments occured in cases with scoliosis and segmentation disorders (Figure 8). With regard to the long‐term ambulatory status of postnatally repaired MMC patients, fetal and postnatal MRI showed a good predictability. Patients with a worse than predicted ambulatory status showed CC dysgenesis and significant vermian displacement.
FIGURE 8.
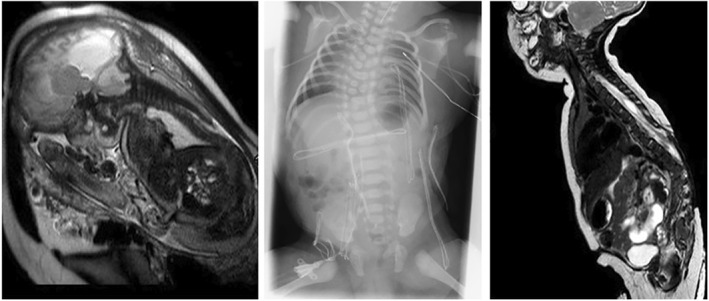
Fetal and postnatal magnetic resonance (MR) images and a radiograph of an myelomeningocele patient with a functional lesion level above L2. Left: Fetal MR image at an estimated gestational age of 36 + 4 weeks shows the neural placode at Th12. Center: Two months after birth, the radiograph shows scoliosis and multisegmental thoracic vertebral body segmentation defects. Right: Two months after birth, the postnatal MR image shows the tethered spinal cord at Th7
Similar results have been shown by other studies, where the predictability of the ambulatory status was examined using different prenatal neuroimaging examinations, such as US or fetal MRI, with good correlation between the prenatal lesion level and the ambulatory status of MMC patients.18, 19, 20, 25, 29 The most recent study was conducted by Sherrod et al., in which a comparison between fetal MR images and functional neurological level showed a correlation within one level in 79.4%.29 This study was limited by the short follow‐up, with a functional assessment within one week of postnatal MMC repair. The duration of follow‐up and the motor level classification were in contrast to our study, as each patient was assigned an exact motor level. We believe that, by consulting parents about the long‐term ambulatory status, it is more plausible to classify the ambulatory status of MMC patients taking into consideration the ambulatory helping device, for example, wheelchair, crutches, or without aid. However, disregarding the differences between our study and the study by Sherrod et al., we could confirm their results, showing that the ambulatory status was equal to or better than predicted in 29/31 (94%) and in 13/15 (87%) cases by postnatal and fetal MRI, respectively.
With regard to the three patients with a worse than predicted ambulatory status, all received a CSF shunt due to hydrocephalus and showed signs of vermian displacement, CC dysgenesis, massive thoracolumbar scoliosis, as well as segmentation disorders. As reported by Kollias et al.,19 this complete spectrum of abnormalities may explain the inadequate predictions of ambulatory status. Vermian displacement, in particular, may have a certain influence on the ambulatory status of patients with MMC and especially Myelocele.39 Posterior fossa crowding, which leads to parenchymal compression and to primary motor neuron lesions, results in a worse than expected ambulatory status.40 Furthermore, according to Nagaraj et al., patients with Myelocele are associated with severe Chiari II malformations.39 This was also supported by our results, which showed a vermian displacement in all of our Myelocele patients, of which two had a worse than predicted ambulatory status. All of our patients with a vermian displacement diagnosed by fetal MRI received a CSF shunt and were ambulatory with a supporting device (≤L2 or L3‐L5).
The only patient (case F) with no vermian displacement on fetal MR images did not receive a CSF shunt and was ambulatory without a supporting device (≥S1), even though the pre‐ and postnatal radiological lesion level was diagnosed at L3‐L5. In this patient, a Myelocystocele with mild Chiari II malformation characteristics was diagnosed by fetal MRI. However, since a neural placode was observed at birth, this spinal cord malformation was defined as MMC with no vermian displacement.
More recent studies suggest that the morphological configuration of the CC may play a significant role in controlling gait symmetry.31, 32 Thus, callosal alterations in MMC patients may reflect a more widespread white matter abnormality,41, 42 and consequently, cause ambulatory impairments.31, 32 On the basis of the aforementioned studies, our data indicate that the morphology of the CC could also have an additional influence on ambulatory status. Recently, Kunpalin et al. has shown that CC morphology could be assessed by prenatal US with 71.7% of fetuses with spina bifida showing CC abnormalities.43 Our MMC patients showed different CC forms, with 8/31 (25.8%) and 4/15 (26.7%) of the patients showing “dysgenetic” forms. In one patient (case A) with a worse than expected ambulatory status, the CC appeared dysgenetic on fetal and postnatal MR images. However, in 1/4 (prenatally) and 1/7 (postnatally) evaluated cases, a callosal dysgenesis was present, despite their better long‐term ambulatory function, as predicted by their prenatally or postnatally described anatomical lesion levels.
Usually, a normally shaped CC and normally positioned vermis in MMC cases are associated with a postnatal motor development that is better than predicted by the anatomical spinal lesion level alone. However, vermian displacement and callosal dysgenesis are present in up to 20% (prenatally) and 3% (prenatally) of cases with better than expected development, and thus, are not necessarily associated with a worse outcome. Finally, our data further indirectly point toward a potential beneficial effect of prenatal surgery, as the reversal of vermian displacement may prevent an aggravation of defective motor function due to early brainstem decompression.
In summary, CC morphology and vermian displacement40 need to be considered when ambulatory prediction is made by fetal MRI. Hence, to prevent overly optimistic assumptions of the ambulatory status in postnatally repaired patients, it is imperative to consider those alterations when consulting MMC‐affected families.
Early reversal of posterior fossa crowding by fetal surgery may be especially beneficial in these patients. Due to the high accuracy of fetal MRI in assessing the level of vermian displacement, this imaging method appears to be ideally suited to identify this subgroup of MMC patients.
5. STRENGTHS AND LIMITATIONS
This study is limited by the fact that not all of our cases systematically underwent fetal MRI at the time of data collection. In this regard, some information was missing.
Since US is a widely accessible method for prenatal examination purposes, it would have been interesting to compare our fetal MRI results with well‐structured and systematically assessed ultrasound data. However, due to the retrospective design of our study, the original ultrasound images were not accessible in most of our patients.
Furthermore, as data from a single center were included in this study, the small sample size allowed the use of only descriptive statistics.
The strength of our study is the long‐term follow‐up at a single center, where all fetal MRI and functional neurological examinations were conducted, and the use of a “historic” cohort that received high‐quality prenatal MRI and a high level of postnatal multidisciplinary care. This offers an important benchmark for prenatal surgery trials, of which long‐term follow‐up data is soon expected. Moreover, our data may serve as a reference in counseling families affected by MMC.
6. CONCLUSIONS
Fetal and postnatal MRI showed a good agreement in determining the radiological MMC lesion level. However, major disagreement between both examinations could be observed in patients with segmentation disorders and scoliosis; thus, a prediction of the radiological lesion level by either of these examinations is less reliable in these cases.
Despite the fact that postnatal MRI is the gold standard in predicting the functional level of MMC patients, similar results were provided by fetal MRI. Both examinations have a good predictive value for the long‐term ambulatory status in MMC patients. “Outliers” could be detected that showed patients with a worse or better than expected ambulatory status. The absence of vermian displacement is generally associated with a better outcome than that predicted by the assessment of the anatomical lesion level alone. The presence of vermian displacement and callosal dysgenesis negatively impact the long‐term ambulatory status in some, but not all, cases.
ACKNOWLEDGMENTS
None
Khalaveh F, Seidl R, Czech T, et al. Myelomeningocele–Chiari II malformation–Neurological predictability based on fetal and postnatal magnetic resonance imaging. Prenatal Diagnosis. 2021;41(8):922–932. 10.1002/pd.5987
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Prevention of neural tube defects: results of the medical research council vitamin study. MRC vitamin study research group. Lancet. 1991;338(8760):131‐137. [PubMed] [Google Scholar]
- 2.Czeizel AE, Dudas I. Prevention of the first occurrence of neural‐tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327(26):1832‐1835. [DOI] [PubMed] [Google Scholar]
- 3.Bowman RM, Boshnjaku V, McLone DG. The changing incidence of myelomeningocele and its impact on pediatric neurosurgery: a review from the children's memorial hospital. Childs Nerv Syst. 2009;25(7):801‐806. [DOI] [PubMed] [Google Scholar]
- 4.Canfield MA, Annegers JF, Brender JD, Cooper SP, Greenberg F. Hispanic origin and neural tube defects in Houston/Harris County, Texas. II. Risk factors. Am J Epidemiol. 1996;143(1):12‐24. [DOI] [PubMed] [Google Scholar]
- 5.Chan A, Robertson EF, Haan EA, Keane RJ, Ranieri E, Carney A. Prevalence of neural tube defects in South Australia, 1966‐91: effectiveness and impact of prenatal diagnosis. BMJ. 1993;307(6906):703‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frey L, Hauser WA. Epidemiology of neural tube defects. Epilepsia. 2003;44(3):4‐13. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues AB, Krebs VL, Matushita H, de Carvalho, , WB. Short‐term prognostic factors in myelomeningocele patients. Childs Nerv Syst. 2016;32(4):675‐680. [DOI] [PubMed] [Google Scholar]
- 8.Saitsu H, Yamada S, Uwabe C, et al. Development of the posterior neural tube in human embryos. Anat Embryol. 2004;209(2):107‐117. [DOI] [PubMed] [Google Scholar]
- 9.Zheng XY, Song XM, Chen G, et al. [Epidemiology of birth defects in high‐prevalence areas of China]. Zhonghua Liuxingbingxue Zazhi. 2007;28(1):5‐9. [PubMed] [Google Scholar]
- 10.Albright AL, Adelson PD, Pollack IF. Principles and Practice of Pediatric Neurosurgery. Thieme; 2008. [Google Scholar]
- 11.Miller E, Ben‐Sira L, Constantini S, et al. Impact of prenatal magnetic resonance imaging on postnatal neurosurgical treatment. J Neurosurg. 2006;105(3):203‐209. [DOI] [PubMed] [Google Scholar]
- 12.Boyd PA, Devigan C, Khoshnood B, Loane M, Garne E, Dolk H. Survey of prenatal screening policies in Europe for structural malformations and chromosome anomalies, and their impact on detection and termination rates for neural tube defects and Down's syndrome. BJOG. 2008;115(6):689‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miskin M, Rothberg R, Rudd NL, Benzie RJ, Shime J. Arthrogryposis multiplex congenita‐‐prenatal assessment with diagnostic ultrasound and fetoscopy. J Pediatr. 1979;95(3):463‐464. [DOI] [PubMed] [Google Scholar]
- 14.Pooh RK. Neurosonoembryology by three‐dimensional ultrasound. Semin Fetal Neonatal Med. 2012;17(5):261‐268. [DOI] [PubMed] [Google Scholar]
- 15.Trigubo D, Negri M, Salvatico RM, Leguizamón G. The role of intrauterine magnetic resonance in the management of myelomenigocele. Childs Nerv Syst. 2017;33(7):1107‐1111. [DOI] [PubMed] [Google Scholar]
- 16.Carreras E, Maroto A, Illescas T, et al. Prenatal ultrasound evaluation of segmental level of neurological lesion in fetuses with myelomeningocele: development of a new technique. Ultrasound Obstet Gynecol. 2016;47(2):162‐167. [DOI] [PubMed] [Google Scholar]
- 17.Appasamy M, Roberts D, Pilling D, Buxton N. Antenatal ultrasound and magnetic resonance imaging in localizing the level of lesion in spina bifida and correlation with postnatal outcome. Ultrasound Obstet Gynecol. 2006;27(5):530‐536. [DOI] [PubMed] [Google Scholar]
- 18.Coniglio SJ, Anderson SM, FergusonJE, 2nd. Functional motor outcome in children with myelomeningocele: correlation with anatomic level on prenatal ultrasound. Dev Med Child Neurol. 1996;38(8):675‐680. [DOI] [PubMed] [Google Scholar]
- 19.Kollias SS, Goldstein RB, Cogen PH, Filly RA. Prenatally detected myelomeningoceles: sonographic accuracy in estimation of the spinal level. Radiology. 1992;185(1):109‐112. [DOI] [PubMed] [Google Scholar]
- 20.BiggioJR, Jr, Owen J, Wenstrom KD, Oakes WJ. Can prenatal ultrasound findings predict ambulatory status in fetuses with open spina bifida? Am J Obstet Gynecol. 2001;185(5):1016‐1020. [DOI] [PubMed] [Google Scholar]
- 21.Peralta CF, Bunduki V, Plese JP, Figueiredo EG, Miguelez J, Zugaib M. Association between prenatal sonographic findings and post‐natal outcomes in 30 cases of isolated spina bifida aperta. Prenat Diagn. 2003;23(4):311‐314. [DOI] [PubMed] [Google Scholar]
- 22.Van Der Vossen, S Pistorius LR, Mulder EJ, et al. Role of prenatal ultrasound in predicting survival and mental and motor functioning in children with spina bifida. Ultrasound Obstet Gynecol. 2009;34(3):253‐258. [DOI] [PubMed] [Google Scholar]
- 23.Rintoul NE, Sutton LN, Hubbard AM, et al. A new look at myelomeningoceles: functional level, vertebral level, shunting, and the implications for fetal intervention. Pediatrics. 2002;109(3):409‐413. [DOI] [PubMed] [Google Scholar]
- 24.Levine D, Barnes PD, Madsen JR, Abbott J, Mehta T, Edelman RR. Central nervous system abnormalities assessed with prenatal magnetic resonance imaging. Obstet Gynecol. 1999;94(6):1011‐1019. [DOI] [PubMed] [Google Scholar]
- 25.Aaronson OS, Hernanz‐Schulman M, Bruner JP, Reed GW, Tulipan NB. Myelomeningocele: prenatal evaluation‐‐comparison between transabdominal US and MR imaging. Radiology. 2003;227(3):839‐843. [DOI] [PubMed] [Google Scholar]
- 26.Egloff A, Bulas D. Magnetic resonance imaging evaluation of fetal neural tube defects. Semin Ultrasound CT MR. 2015;36(6):487‐500. [DOI] [PubMed] [Google Scholar]
- 27.Saleem SN, Said AH, Abdel‐Raouf M, et al. Fetal MRI in the evaluation of fetuses referred for sonographically suspected neural tube defects (NTDs): impact on diagnosis and management decision. Neuroradiology. 2009;51(11):761‐772. [DOI] [PubMed] [Google Scholar]
- 28.Nagaraj UD, Bierbrauer KS, Stevenson CB, et al. Spinal imaging findings of open spinal dysraphisms on fetal and postnatal MRI. AJNR Am J Neuroradiol. 2018;39(10):1947‐1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherrod BA, Ho WS, Hedlund A, Kennedy A, Ostrander B, Bollo RJ. A comparison of the accuracy of fetal MRI and prenatal ultrasonography at predicting lesion level and perinatal motor outcome in patients with myelomeningocele. Neurosurg Focus. 2019;47(4):E4. [DOI] [PubMed] [Google Scholar]
- 30.Chao TT, Dashe JS, Adams RC, Keefover‐Hicks A, McIntire DD, Twickler DM. Fetal spine findings on MRI and associated outcomes in children with open neural tube defects. AJR Am J Roentgenol. 2011;197(5):W956‐W961. [DOI] [PubMed] [Google Scholar]
- 31.Richmond SB, Fling BW. Transcallosal control of bilateral actions. Exerc Sport Sci Rev. 2019;47(4):251‐257. [DOI] [PubMed] [Google Scholar]
- 32.Brodoefel H, Ramachandran R, Pantol G, et al. Association between linear measurements of corpus callosum and gait in the elderly. Eur Radiol. 2013;23(8):2252‐2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarnat HB. Regional ependymal upregulation of vimentin in Chiari II malformation, aqueductal stenosis, and hydromyelia. Pediatr Dev Pathol. 2004;7(1):48‐60. [DOI] [PubMed] [Google Scholar]
- 34.Sarnat HB. Disorders of segmentation of the neural tube: Chiari malformations. Handb Clin Neurol. 2008;87:89‐103. [DOI] [PubMed] [Google Scholar]
- 35.Mahallati H, Sotiriadis A, Celestin C, et al. Heterogeneity in defining fetal callosal pathology: a systematic review. Ultrasound Obstet Gynecol. 2020. 10.1002/uog.22179 [DOI] [PubMed] [Google Scholar]
- 36.Edwards TJ, Sherr EH, Barkovich AJ, et al. Clinical, genetic and imaging findings identify new causes for corpus callosum development syndromes. Brain. 2014;137(Pt 6):1579‐1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cochrane DD, Wilson RD, Steinbok P, et al. Prenatal spinal evaluation and functional outcome of patients born with myelomeningocele: information for improved prenatal counselling and outcome prediction. Fetal Diagn Ther. 1996;11(3):159‐168. [DOI] [PubMed] [Google Scholar]
- 38.McDonald CM, Jaffe KM, Mosca VS, et al. Ambulatory outcome of children with myelomeningocele: effect of lower‐extremity muscle strength. Dev Med Child Neurol. 1991;33(6):482‐490. [DOI] [PubMed] [Google Scholar]
- 39.Nagaraj UD, Bierbrauer KS, Stevenson CB, et al. Myelomeningocele versus Myelocele on fetal MR images: are there differences in brain findings? AJR Am J Roentgenol. 2018;211(6):1376‐1380. [DOI] [PubMed] [Google Scholar]
- 40.Woitek R, Prayer D, Weber M, et al. Fetal diffusion tensor quantification of brainstem pathology in Chiari II malformation. Eur Radiol. 2016;26(5):1274‐1283. [DOI] [PubMed] [Google Scholar]
- 41.Ou X, Glasier CM, Snow JH. Diffusion tensor imaging evaluation of white matter in adolescents with myelomeningocele and Chiari II malformation. Pediatr Radiol. 2011;41(11):1407‐1415. [DOI] [PubMed] [Google Scholar]
- 42.Miller E, Widjaja E, Blaser S, Dennis M, Raybaud C. The old and the new: supratentorial MR findings in Chiari II malformation. Childs Nerv Syst. 2008;24(5):563‐575. [DOI] [PubMed] [Google Scholar]
- 43.Kunpalin Y, Deprest J, Papastefanou I, et al. Incidence and patterns of abnormal corpus callosum in fetuses with isolated spina bifida aperta. Prenat Diagn. 2021. 10.1002/pd.5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


