Abstract
Umbilical metastases form a clinical challenge, especially when they represent the first sign of malignant disease and the primary tumor is unknown. Our study aims to generate insight into the origin and timing of umbilical metastasis, as well as patient survival, using population‐based data. A nationwide review of pathology records of patients diagnosed with an umbilical metastasis between 1979 and 2015 was performed. Data was collected from the Nationwide Network and Registry of Histopathology and Cytopathology (PALGA) and the Netherlands Cancer Registry. Kaplan‐Meier analyses and log‐rank testing were used to estimate overall survival and a Cox proportional hazard model was used to determine multivariable hazard ratios. A total of 806 patients with an umbilical metastasis were included. There were 210 male (26.1%) and 596 female (73.9%) patients. Distribution of umbilical metastases was different between male and female patients due to the high incidence of umbilical metastases originating from the ovaries in females. They most frequently originated from the ovaries in female patients (38.8%) and from the colon in male patients (43.8%). In 18% of cases no primary tumor could be identified. Prognosis after diagnosis of an umbilical metastasis was dismal with a median survival of 7.9 months (95% confidence interval 6.7‐9.1). The origin of the primary tumor was an independent prognostic factor for overall survival. In conclusion, umbilical metastases relatively rare, mainly originating from intraabdominal primary tumors. Survival is dependent on the origin of the primary tumor and poor overall survival rates warrant early recognition.
Keywords: colorectal cancer, ovarian cancer, umilical metastasis
Short abstract
What's new?
Umbilical metastases are a rare consequence of malignant disease that pose unique clinical challenges. Very little is known about these metastases, especially regarding incidence and survival. This population‐based analysis of more than 800 patients in the Netherlands shows that the distribution in umbilical metastases differs between males and females. In females, metastases most commonly originated from the ovaries, while in males, the colon was most common. Umbilical metastases, however, were linked to a variety of primary tumors and were frequently diagnosed synchronously with the primary tumor. While prognosis was poor overall, survival was influenced by primary tumor origin.
Abbreviations
- IKNL
Netherlands Comprehensive Cancer Organization
- NCR
Netherlands Cancer Registry
- OS
overall survival
- PALGA
Nationwide Network and Registry of Histopathology and Cytopathology in the Netherlands
1. INTRODUCTION
Umbilical metastases represent a rare manifestation of cancer. They are traditionally referred to as Sister Mary Joseph's Nodules, named after the first person who noticed the association between this metastatic site and intraabdominal cancer.1 Umbilical metastases form a clinical challenge, especially when they are the first sign of malignant disease while the primary tumor remains unknown. Current knowledge regarding umbilical metastases is mostly derived from case reports or case series and representative large series are lacking.2 As a consequence the incidence of umbilical metastases is unclear, and it is unknown from which primary tumor site they arise most commonly.2 Umbilical metastases are generally associated with poor survival, although it is unknown to what extent this varies across different tumor types. Moreover, there is no insight into the interval between primary tumors and umbilical metastases and its impact on a patient's prognosis.
Our study aims to generate insight into the origin and timing of umbilical metastasis, as well as patient survival, using real‐world population‐based data. To this end, a data linkage was established between two national databases, one containing nationwide data on histopathology and cytopathology gathered by pathology laboratories, the other being a national cancer registry containing clinical data.
2. MATERIALS AND METHODS
2.1. Data linkage
All study data were obtained from the Netherlands Cancer Registry (NCR) and the Nationwide Network and Registry of Histopathology and Cytopathology in the Netherlands (PALGA).3 Cases were selected from PALGA on the basis of an umbilical malignancy histopathologically confirmed between July 1979 and January 2015. Founded in 1971, PALGA achieved nationwide coverage in 1990 and has since harbored all pathology results from the 46 pathology laboratories in the Netherlands, which are submitted on a daily basis.
The NCR is hosted and maintained by the Netherlands Comprehensive Cancer Organization (IKNL), and includes nearly all (~95%) of primary malignancies diagnosed in the Netherlands since 1989. Recurrent or metastatic disease is not registered in the NCR. While PALGA serves as the main source of notification, case ascertainment and supplementation is provided by linkage with the central hospital discharge registry. Upon notification, specialized registrars gather data on patient and tumor characteristics by extracting information directly from hospital files. Follow‐up information on patients' vital status is obtained through linkage with the Municipal Personal Records Database, the most recent for the current study being performed in February 2019. Via a trusted third party the case selection from PALGA was linked to the entire NCR database in a prematch procedure involving only the available linkage variables. After the prematching run, record information from both PALGA and the NCR for potential matches was brought together for manual verification. Both parties performed a joint review to determine definitive matches, and the pseudonymized records were released for data analyses.
2.2. Data
Information in the PALGA database is recorded using standardized codes (based on the Systematized Nomenclature of Medicine, SNOMED). NCR data are coded according to international standards such as the ICD‐O topography codes for primary tumor sites, and the TNM system of the International Union Against Cancer (UICC) supplemented with the Extent of Disease code of the American Surveillance, Epidemiology and End Results (SEER) program for tumor stage. As both databases represent real‐world data, extracted information, for instance on tumor histology, reflects assessments made in routine practice, without central pathology review or other expert panels. Although the study only includes histologically proven umbilical metastases, included patients are expected to form a representative group given the accessibility of the umbilicus for histopathological diagnostics.
For every patient data on gender, age, date of umbilical metastasis, TNM stage and date of primary tumor were available. Data from both registries was complementary, there was no overlap. Information on cause of death or details on treatment of the umbilical metastasis were not available. Umbilical metastases that were found prior to or within 6 months after diagnosis of the primary tumor were considered synchronous. Metachronous metastases developed more than 6 months after diagnosis of the primary tumor.
2.3. Statistical analyses
Analysis of annual trends in incidence was performed from 1990 on, when nationwide coverage was reached for both PALGA and the NCR. To estimate the crude incidence rate of umbilical metastases for the period 1990 to 2014 data, the yearly incidence of cancer in the Netherlands was obtained from the NCR.4 All types of cancer (excluding hematological malignancies) were included to determine the annual incidence of cancer. The crude incidence rate equals the total number of umbilical metastases per year divided by the population at risk (patients diagnosed with a primary malignancy).
The χ 2‐square test was used to compare demographics and tumor characteristics between groups. In survival analyses, overall survival (OS) was defined as the interval between the date of umbilical metastasis (date of diagnosis for PALGA data) until the date of death or until last follow‐up (31 January 2018). Median follow up time since date of diagnosis of the primary tumor was 15.9 months. Patients who were alive at the end of follow‐up were censored in survival analyses. OS curves were generated according to the Kaplan‐Meier method. Multivariable analysis of overall survival was carried out using the Cox proportional hazard model including age, time of diagnosis of the umbilical metastasis, age at diagnosis of the umbilical metastasis, synchronous or metachronous timing of the metastasis and primary tumor origin. Covariates ware selected based upon availability of data. The log minus log plot has been used to assess whether the assumption of proportional hazards was reasonable. No significant violations were observed that demanded inclusion of a time‐dependent variable. Statistical analyses were performed with the software package SPSS 20.0 (SPSS Inc, Chicago, Illinois). All tests of significance were two‐tailed: differences at P‐values of less than .05 were considered to be significant.
3. RESULTS
A total of 806 patients with an umbilical metastasis were included. There were 210 male (26.1%) and 596 female (73.9%) patients. The median age at diagnosis of the umbilical metastasis was 68 years (range 20‐97). The median age at diagnosis of the primary tumor was 67 years (20‐95). Umbilical metastases were rarely the only metastatic site, with other histopathologically confirmed metastases in 71.3% of patients.
3.1. Incidence of umbilical metastases over time
The number of umbilical metastases varied between 17 and 43 cases annually. The crude incidence rate decreased over time from 4.4 per 10 000 cancer cases in 1990 to 1.7 per 10 000 in 2014 (Figure 1).
FIGURE 1.

Crude incidence rate of umbilical metastases per 10 000 cancer patients compared to the total number patients diagnosed with any type of cancer (dashed line)
3.2. Timing of umbilical metastases
Detection of the umbilical metastasis was synchronous with the primary tumor in 67.7% of cases (N = 546) and metachronous 32.3% of patients (N = 260). The interval between primary tumor and umbilical metastasis was distributed differently according to the primary tumor site (Figure 2 and Figure S1 for results stratified by sex). An umbilical metastasis was rarely diagnosed more than 5 years after the primary tumor, except in breast cancer patients, in whom 37.5% (N = 12) of umbilical metastases was diagnosed more than 5 years after the primary tumor. Umbilical metastases originating from the ovaries were diagnosed synchronously in 77.5% (N = 179) of cases, in colon cancer patients in 59.4% (N = 107) of cases. Umbilical metastases from rectal cancer were more commonly metachronous (84.6%; N = 11). In endometrial and gastric cancer, metastases were metachronous in 60% (N = 27) and 64.9% (N = 24), respectively.
FIGURE 2.
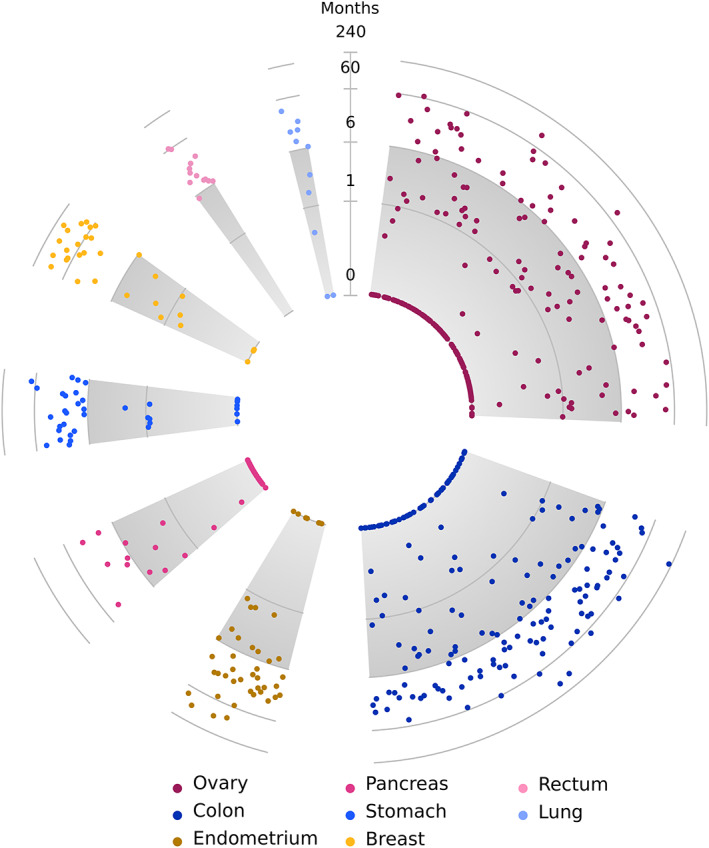
Timing of umbilical metastasis. Timing from date of diagnosis of the primary tumor to diagnosis of the umbilical metastasis, by origin of primary tumor (log2 scale). Primary tumor sites are only included if N ≥ 10 and if the primary tumor was known. The gray area marks the 6‐months interval (synchronous metastasis) [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Origin of umbilical metastases
The distribution of umbilical metastases was different between male and female patients, which was mainly caused by the high incidence of umbilical metastases originating from the female reproductive system. In female patients the most common origin of umbilical metastases were the ovaries (38.8%), followed by the group in which the primary tumor could not be identified (17.4%, Table S1 and Figure 3). Umbilical metastases with a colorectal origin were diagnosed in 16.1% of patients. In male patients the colorectum was the most common origin for umbilical metastases (46.2%), followed by unknown primary (18.6%). Umbilical metastases with an unknown primary tumor were most commonly adenocarcinoma not otherwise specified (53.8%) or mucinous adenocarcinoma (19.6%). In tumors originating from the colorectum, the majority was adenocarcinoma not otherwise specified (67.8%). Mucinous adenocarcinoma was found in 28.2% of cases, signet‐ring cell carcinoma in 1.0% and other subtypes in 2.9%. Primary colorectal tumors were pT3 or pT4 in 45.1% and 25.9% of patients, respectively, and lymph nodes metastases with the primary tumor were found in 61.6% of patients. In tumors originating from the ovaries, serous adenocarcinoma was found in 51.5%, adenocarcinoma not otherwise specified was found in 30.7%, mucinous adenocarcinoma in 7.4%, endometrioid carcinoma in 6.5% and other subtypes in 3.9%.
FIGURE 3.
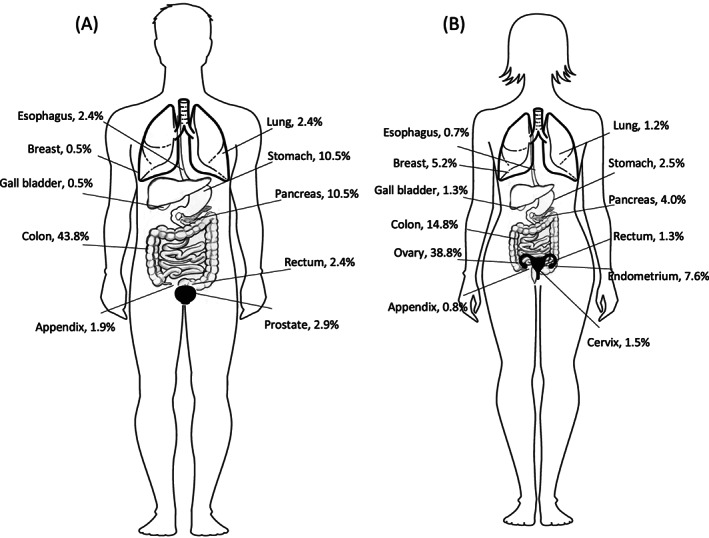
Distribution of umbilical metastases according to the primary tumor location, for (A) male and (B) female patients
3.4. Survival
Patients with umbilical metastases had a dismal prognosis, with a median OS of 7.9 months (95% CI 6.7‐9.1) from the date of diagnosis of the umbilical metastasis. Univariable survival analysis demonstrated that the worst survival was seen in patients in whom the primary tumor was unknown (median survival 2.7 months, 95% CI 1.97‐3.50) and in patients with a primary tumor originating from the pancreas (median survival 3.3 months, 95% CI 1.88‐4.79), Figure 4. Univariable survival analyses according to the origin of the primary tumor stratified by sex are available in Figure S3. There was no relation between the interval between the primary tumor and diagnosis of the umbilical metastasis and OS after the diagnosis of the umbilical metastasis (Figure 5). Results from the multivariable analysis showed that survival was not associated with the year of diagnosis of the umbilical metastasis nor with the synchronous or metachronous occurrence of the umbilical metastasis. It confirmed that the origin of the primary tumor was an independent prognostic factor for OS (Table 1). Moreover, gender was demonstrated to be an independent prognostic factor for OS, as well as age at diagnosis of the umbilical metastasis. Heterogeneity in the relationship between origin and survival by sex, age or calendar time was assessed, but no interaction was demonstrated.
FIGURE 4.
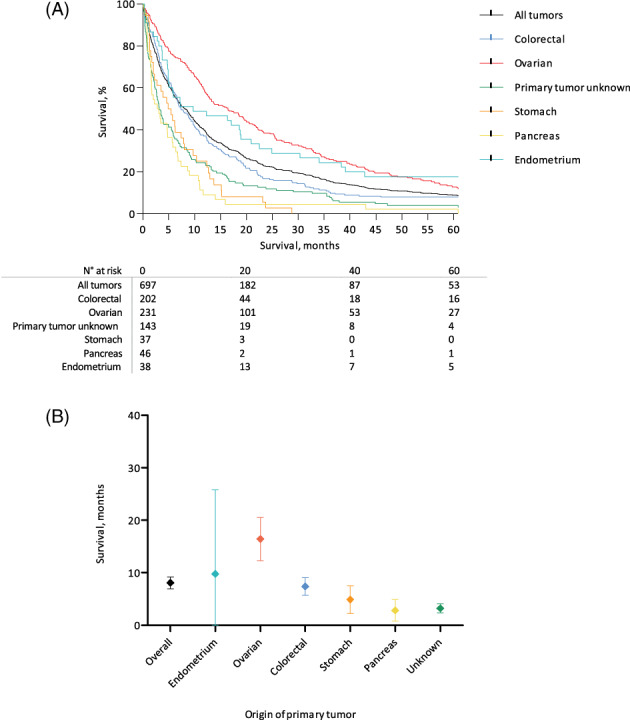
A, Overall survival rates for umbilical metastases according to the location of primary tumor from the date of diagnosis of the umbilical metastasis. B, Median overall survival rates with 95% confidence intervals according to origin of primary tumor from the date of diagnosis of the umbilical metastasis [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 5.

Interval between diagnosis of primary tumor and diagnosis of umbilical metastasis, related to survival. Every line represents a single patient. Data are grouped according to the origin of the primary tumor. Only deceased patients (N = 675) were included for analysis
TABLE 1.
Multivariable survival analysis using Cox model
| Variable | No. of patients | Hazard ratio | 95% confidence interval |
|---|---|---|---|
| Age at diagnosisa | |||
| <60 | 178 | 1 | |
| 60‐74 | 290 | 1.26 | 1.03‐1.54 |
| ≥75 | 229 | 1.73 | 1.40‐2.14 |
| Sex | |||
| Male | 184 | 1 | |
| Female | 513 | 0.73 | 0.60‐0.90 |
| Period of diagnosisa | |||
| 1979‐1990 | 64 | 1 | |
| 1991‐2000 | 271 | 1.13 | 0.85‐1.50 |
| 2001‐2015 | 362 | 0.85 | 0.64‐1.12 |
| Timing of umbilical metastasis | |||
| Synchronous | 502 | 1 | |
| Metachronous | 195 | 0.89 | 0.82‐1.19 |
| Primary tumor origin | |||
| Colorectal | 202 | 1 | |
| Ovarian | 231 | 0.83 | 0.66‐1.04 |
| Unknown | 143 | 1.54 | 1.22‐1.95 |
| Stomach | 37 | 1.99 | 1.38‐2.85 |
| Pancreas | 46 | 2.21 | 1.58‐3.09 |
| Endometrium | 38 | 0.79 | 0.54‐1.15 |
of umbilical metastasis.
4. DISCUSSION
Although rare, umbilical metastases can indicate an early sign of cancer. This is the first study that generates insight into umbilical metastases using population‐based data. It shows that umbilical metastases may develop from a wide variety of primary tumors, after various intervals and demonstrates that umbilical metastases are associated with an abysmal prognosis.
In rare oncological settings, population‐based studies are crucial to collect a sufficiently large number of patients to perform reliable analyses. Using data from two nationwide registries we were able to collect over 800 individual cases of umbilical metastases with detailed clinical and histological data. This allowed to generate a real‐world insight into the various aspects of umbilical metastases.
Over the included time period of more than 25 years, there was a decrease in crude incidence rate, despite an increase in the total number of cancer patients. An explanation for this decrease cannot be given based on the data from our study. In the Netherlands, the incidence rate of stage IV colorectal cancer and stage III/IV ovarian cancer remained stable over the past 20 years.5, 6 In the early days of laparoscopic surgery an association between umbilical metastases and laparoscopic surgery was suggested based on case reports given the frequent use of the subumbilical area for surgical port placement and specimen extraction.7 After our study, the introduction of laparoscopic surgery during this time frame did not seem to have caused an increase in the incidence of umbilical metastases.
In over half of patients the umbilical deposit was found synchronously with the primary tumor. This was especially seen in patients with an intraabdominal origin of the primary tumor such as colon or ovarian cancer. These two origins accounted for 50% of umbilical metastases. Extraabdominal origins were rare, although breast cancer accounted for 5.2% of cases in female patients. The pathways behind the development of umbilical metastases are not well defined and several hypotheses suggesting either hematogenous or peritoneal spread have been proposed. The first hypothesis suggests that umbilical metastases may develop through peritoneal seeding in the presence of other peritoneal metastases. This is supported by the finding that 38.6% and 30.0% of patients had pathologically confirmed metastatic deposits in either the peritoneum or the omentum (data not shown). Moreover, the lower rate of peritoneal metastases in rectal cancer patients compared to colon cancer patients is indicative for this mechanism of spread.8 Another hypothesis suggests that umbilical metastases may occur via venous spread or through lymphatic ducts, that are well represented in the umbilical area through an anastomotic plexus.2, 9, 10 This has been strengthened by the occurrence of umbilical metastases in absence of other peritoneal metastases and by the occurrence of umbilical metastases from an extraabdominal origin. Furthermore, it has been hypothesized that embryonal remnants such as the vitelline duct, the urachus or median umbilical ligament and falciform ligament may be involved in metastatic spread to the umbilicus, serving as a guidance to the umbilical.2, 11, 12
Survival after the diagnosis of an umbilical metastasis was abysmal, with a median survival of less than 8 months. A relation between the timing of the umbilical metastasis and overall survival after the diagnosis of the umbilical metastasis could not be identified. This is most likely caused by the heterogeneity of patients with metastatic disease. The origin of the primary tumor was an independent prognostic factor for OS. Patients in whom the primary tumor was unknown had a meager median survival of 2.7 months, which is in line with the poor survival reported in cancer of unknown primary.13 Patients with an umbilical metastasis from ovarian cancer had the best overall survival compared to tumors from another origin. Since we were not able to determine the therapy given for the umbilical metastases, it should be noted that this may have influenced outcomes and may limit interpretation of the data. Furthermore, findings from the current study cannot determine the optimal treatment strategy for patients with umbilical metastases, given the common presence of more extensive metastatic disease.
Although our study represents the largest cohort of patients with umbilical metastases, there are some limitations that should be addressed. Firstly, it was not possible to determine the origin of the umbilical metastasis in 141 patients. This is in accordance with previous findings and underlines the clinical challenge of umbilical metastasis as the first sign of malignancy.10 Moreover, pathological specimens were not reviewed and diagnostic heterogeneity may have resulted in misclassification, which could not be corrected for. Selection of patients through a pathology registry may have led to inclusion bias. Since only patients with a histopathologically confirmed diagnosis of umbilical metastases were included in our study it is not inconceivable that patients with advanced metastatic disease, including an umbilical (not histopathologically proven) metastasis have not been included in our study. However, given the rarity of this finding together with the easy accessibility for histopathological diagnostics we consider the risk for this bias rather low.
Umbilical metastases are a rare form of metastatic disease, predominantly originating from intraabdominal primary tumors. In many cases, an umbilical metastasis is one of the first manifestations of malignant disease, although they may develop after more than 5 years after the primary tumor, in particular in cases with an extraabdominal origin. Survival is dependent on the origin of the primary tumor and warrants early recognition.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
Our study was conducted in compliance with national guidelines such as the Code of Conduct for the Use of Data in Health Research as issued by the Foundation Federation of Dutch Medical Scientific Societies (Federa), as well as the institutional regulations of PALGA and the NCR. All data were handled according to the General Data Protection Regulation. Consent for the design, data abstraction process from the NCR and storage protocols was obtained from the supervisory committee of the NCR.
Supporting information
Figure S1 Timing from date of diagnosis of the primary tumor to diagnosis of the umbilical metastasis, by origin of primary tumor (log2 scale). Primary tumor sites are only included if N ≥ 10 and if the primary tumor was known. The gray area marks the 6‐months interval (synchronous metastasis). Data are stratified according to sex. (M), male; (F) female.
Figure S2 (A) Overall survival rates and (B) median overall survival rates with 95% confidence intervals for umbilical metastases according to the location of primary tumor from the date of diagnosis of the umbilical metastasis in male patients. (C) Overall survival rates and (D) medial overall survival rates with 95% confidence intervals for umbilical metastases according to the location of primary tumor from the date of diagnosis of the umbilical metastasis in female patients.
Table S1 Distribution of umbilical metastases according to the primary tumor location in male and female patients.
ACKNOWLEDGEMENT
This research received no specific grant from any funding agency in the public, commercial or nonprofit sector.
Hugen N, Kanne H, Simmer F, et al. Umbilical metastases: Real‐world data shows abysmal outcome. Int. J. Cancer. 2021;149(6):1266–1273. 10.1002/ijc.33684
DATA AVAILABILITY STATEMENT
Data is available upon request at the corresponding author.
REFERENCES
- 1.Storer H. Cases illustrative of obstetric disease. Boston Med Surg J. 1864;70:5. [Google Scholar]
- 2.Dubreuil A, Dompmartin A, Barjot P, et al. Umbilical metastasis or Sister Mary Joseph's nodule. Int J Dermatol. 1998;37:7‐13. [DOI] [PubMed] [Google Scholar]
- 3.Casparie M, Tiebosch AT, Burger G, et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29:19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Netherlands Cancer Registry. Accessed January 10, 2020.
- 5.Brouwer NPM, Bos A, Lemmens V, et al. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int J Cancer. 2018;143:2758‐2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Altena AM, Karim‐Kos HE, de Vries E, et al. Trends in therapy and survival of advanced stage epithelial ovarian cancer patients in The Netherlands. Gynecol Oncol. 2012;125:649‐654. [DOI] [PubMed] [Google Scholar]
- 7.Karayiannakis AJ, Knight MJ. Umbilical port metastasis from gallbladder carcinoma after laparoscopic cholecystectomy. Eur J Surg Oncol. 1997;23:186‐187. [DOI] [PubMed] [Google Scholar]
- 8.Hugen N, Nagtegaal ID. Distinct metastatic patterns in colorectal cancer patients based on primary tumour location. Eur J Cancer. 2017;75:3‐4. [DOI] [PubMed] [Google Scholar]
- 9.Hori T, Okada N, Nakauchi M, et al. Hematogenous umbilical metastasis from colon cancer treated by palliative single‐incision laparoscopic surgery. World J Gastrointest Surg. 2013;5:272‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabriele R, Conte M, Egidi F, et al. Umbilical metastases: current viewpoint. World J Surg Oncol. 2005;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powell FC, Cooper AJ, Massa MC, et al. Sister Mary Joseph's nodule: a clinical and histologic study. J Am Acad Dermatol. 1984;10:610‐615. [DOI] [PubMed] [Google Scholar]
- 12.Goodheart RS, Cooke CT, Tan E, et al. Sister Mary Joseph's nodule. Med J Aust. 1986;145:477‐478. [DOI] [PubMed] [Google Scholar]
- 13.Schroten‐Loef C, Verhoeven RHA, de Hingh I, et al. Unknown primary carcinoma in The Netherlands: decrease in incidence and survival times remain poor between 2000 and 2012. Eur J Cancer. 2018;101:77‐86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Timing from date of diagnosis of the primary tumor to diagnosis of the umbilical metastasis, by origin of primary tumor (log2 scale). Primary tumor sites are only included if N ≥ 10 and if the primary tumor was known. The gray area marks the 6‐months interval (synchronous metastasis). Data are stratified according to sex. (M), male; (F) female.
Figure S2 (A) Overall survival rates and (B) median overall survival rates with 95% confidence intervals for umbilical metastases according to the location of primary tumor from the date of diagnosis of the umbilical metastasis in male patients. (C) Overall survival rates and (D) medial overall survival rates with 95% confidence intervals for umbilical metastases according to the location of primary tumor from the date of diagnosis of the umbilical metastasis in female patients.
Table S1 Distribution of umbilical metastases according to the primary tumor location in male and female patients.
Data Availability Statement
Data is available upon request at the corresponding author.


