Summary
Idelalisib (IDL) is an oral first‐in‐class phosphatidylinositol 3‐kinase delta (PI3Kδ) inhibitor approved for chronic lymphocytic leukaemia (CLL) alongside rituximab (R) since 2014. However, little data exist on routine practice. The RETRO‐idel was a protocol‐led, retrospective study of 110 patients [n = 27 front‐line (1L)] who received IDL‐R. The primary end‐point was clinical overall response rate (ORR). The median (range) follow‐up of the whole cohort was 30·2 (0·1–51·9) months. The median (range) age was 72 (48–89) years. Tumour protein p53‐disruption was common [100% 1L, 32·5% relapsed/refractory (R/R)]. The best ORR (intention‐to‐treat) was 88·2% (1L 96·3%, R/R 85·5%). Overall, the median event‐free survival (mEFS) was 20·3 months and time‐to‐next treatment was 29·2 months. The mEFS for 1L patients was 18·7 months and R/R patients was 21·7 months. The 3‐year overall survival was 56·1% (95% confidence interval 45·7–65·3). IDL was discontinued in 87·3% (n = 96). More patients discontinued due to adverse events in the front‐line setting (1L 63·0% vs. R/R 44·6%) and due to progressive disease in R/R patients (20·5% vs. 3·7% in 1L). Lower respiratory tract infection/pneumonia were reported in 34·5% (Grade ≥3, 19·1%), diarrhoea in 30·9% (Grade ≥3, 6·4%), and colitis in 9·1% (Grade ≥3, 5·5%). Overall, these data describe clear efficacy for IDL‐R in routine practice. No new safety signals were identified, although careful management of known toxicities is required.
Keywords: chronic lymphocytic leukaemia, B‐cell receptor inhibitor, idelalisib, PI3K, retrospective
Introduction
The B‐cell receptor (BCR) signalling pathway plays a key role in the pathogenesis of chronic lymphocytic leukaemia (CLL). Overexpression of phosphatidylinositol 3‐kinase delta isoform (PI3Kδ) is a characteristic feature of malignant lymphoid cells.1 Idelalisib (IDL) is an oral, selective, first‐in‐class, PI3Kδ inhibitor approved for CLL treatment.2 IDL in combination with rituximab (IDL‐R) demonstrated clear efficacy in a randomised clinical trial of heavily pre‐treated patients with CLL. When compared with rituximab monotherapy, a significant progression‐free survival (PFS) [hazard ratio (HR) 0·15; P < 0·001] and overall survival (OS) (HR 0·28; P = 0·02) advantage was seen with IDL‐R.3 Long‐term follow‐up has shown an ongoing PFS advantage.4 Novel agents targeting Bruton tyrosine kinase (BTK) such as ibrutinib and acalabrutinib or B‐cell lymphoma 2 such as venetoclax [± cluster of differentiation 20 (CD20) monoclonal antibody] are also highly effective oral options in relapsed/refractory (R/R) CLL5, 6, 7, 8, 9, 10, 11 and more recently in the front‐line setting.12, 13, 14 Although these agents are highly active outside of clinical trials, questions have been raised regarding the tolerability of ibrutinib in particular,15, 16 with intolerance the dominant cause of discontinuation in large USA and UK series.
Despite its clear efficacy, the utility of IDL‐R in CLL has been restricted by the agent’s safety prolife.17 Immune‐related adverse events (AEs) and infectious complications have the potential to limit the agent’s long‐term use. As a result, specific AE management guidance has been produced.18 Understanding which patients may benefit from IDL‐R and the outcomes of patients who must stop IDL due to AEs remains of clinical importance.
Since the approval of IDL‐R, there has been a relative paucity of published data evaluating its efficacy and safety outside of the clinical trial setting. A recent study of 294 Medicare beneficiaries noted increased rates of fatal infections and a shorter time on IDL‐R compared to 89 trial patients.19 A retrospective series of 682 patients explored the optimal sequencing of targeted agents in R/R CLL, but included only 62 (9%) patients treated with IDL‐R, limiting its conclusions regarding this agent’s utility.20 A recent large series evaluating options in patients previously exposed to venetoclax in R/R CLL evaluated only 17 PI3K inhibitor‐treated patients.21 The safety profile has also limited IDL‐R use in the front‐line setting,22, 23, 24 where it is also licensed for patients with 17p deletion or tumour protein p53 (TP53) mutation who have no other therapeutic options. To the authors’ knowledge, there are no published data available regarding the utility of front‐line IDL‐R in routine clinical practice and minimal data in R/R CLL.
In the present study, we aimed to evaluate the efficacy and safety of IDL‐R in patients with CLL in routine clinical practice in the UK and Ireland through a protocol‐led, retrospective, cohort study, RETRO‐idel (NCT03582098).
Methods
Data from 16 centres were collected using electronic case report forms from medical records by trained chart abstractors who were familiar with the management of CLL. Eligible patients included those who initiated IDL‐R as part of routine clinical management from September 2014 (following European approval) until 31/12/2017. We collected baseline characteristics including age, gender, prior therapies and TP53 mutation/17p deletion status.
The primary end‐point was clinical overall response rate (ORR). Radiological response reassessments are only sporadically performed in the routine clinical management of CLL, unlike clinical trials where they are conducted at a protocol‐specified frequency. However, we did not wish to exclude patients without radiological reassessment and, therefore, investigator‐assessed clinical ORR was used as the primary end‐point. Secondary end‐points included event‐free survival (EFS), OS, PFS, time‐to‐next treatment (TTNT), duration of response (DOR) and safety [including serious AEs (SAE) and pre‐defined AEs of special interest (AESI)]. Analysis was based on the Full Analysis Set (FAS), which comprised all patients enrolled who received one or more dose(s) of IDL‐R in line with eligibility (Table SI). The minimum follow‐up period for all patients was 9 months (excluding those who died or were lost to follow‐up). All patients treated at contributing sites were included in the analysis between the defined collection dates. Pneumocystis jiroveci pneumonia (PJP) prophylaxis and cytomegalovirus (CMV) monitoring were routinely used at contributing sites following announcement of the IDL‐R safety signal in March 2016 until data censoring; however, specific data regarding CMV and PJP management was not otherwise collected outside of AE recording. Data collection started on 12/09/2018 and the database was locked in March 2019 for analysis.
Statistical analysis
The EFS, PFS, OS, TTNT, and DOR outcomes were estimated using the Kaplan–Meier method with appropriate censoring at the date of last data capture25 (Table SI). Intention‐to‐treat clinical ORR was estimated as the ratio of the number of patients who had documented clinical response and the number of patients in the FAS. The 95% confidence intervals (CIs) were based on the Clopper–Pearson method. For the analysis of EFS, patients were counted as having an event by Kaplan–Meier analysis if they progressed or died from any cause or initiated another line of therapy for CLL. For the analysis of PFS and DOR, censoring rules were as follows: (i) for patients without an event or next CLL treatment, these were censored at the data collection end date; (ii) for patients who started next CLL treatment, these were censored at the date of next treatment. For analysis of TTNT, patients who had not initiated any further CLL treatment were censored at data collection end date. Analyses were performed in Statistical Analysis System (SAS), version 9.4 (SAS Institute Inc., Cary, NC, USA). Radiological assessment was not routinely required at pre‐defined intervals given the nature of this non‐trial, population‐based data collection. Therefore, ORR was intentionally not divided into partial and complete responses. The study was conducted in accordance with the Declaration of Helsinki and received ethics approval (REC: 18/EM/0206).
Results
A total of 110 patients commenced IDL‐R between September 2014 and December 2017. Most patients (n = 88, 80·0%) were aged ≥65 years, with a median (range) age of 72 (48–89) years (Table I). Overall, patients received a median (range) of 1 (0–10) prior line and the median (range) time since initial CLL diagnosis was 6·6 (0·1–23·3) years (1·0 year for front‐line, 8·5 years for R/R). In all, 25% (27/110) of patients received IDL‐R as front‐line treatment; all front‐line patients had TP53 disruption (TP53 mutation and/or 17p deletion). Two‐thirds were male, with similar proportions in the front‐line and relapsed setting. For 83 R/R patients, the median (range) number of prior lines was 2 (1–10). Fludarabine, cyclophosphamide and rituximab (FCR) (n = 43), bendamustine‐rituximab (BR) (n = 25), chlorambucil (n = 19) and chlorambucil‐rituximab (n = 10) were the most common prior therapies (Table SII). Seven patients had received prior ibrutinib and no patients were previously exposed to venetoclax. Eight patients (7·3%) had a previous allogeneic haematopoietic stem‐cell transplant (allo‐HSCT). Eastern Cooperative Oncology Group Performance Status (ECOG PS) was not available or not assessed at baseline in 68·2%. Most patients with recorded ECOG PS data had a low score (ECOG PS 0, 15·5%).
Table I.
Baseline characteristics (full analysis set)
| Variables | Front‐line N = 27 | Relapsed N = 83 | All patients N = 110 |
|---|---|---|---|
| Age, years, median (range) | 71 (53–83) | 72 (48–89) | 72 (48–89) |
| Age categories, n (%) | |||
| <65 years | 7 (25·9) | 15 (18·1) | 22 (20) |
| ≥65 years | 20 (74·1) | 68 (81·9) | 88 (80) |
| Gender, n (%) | |||
| Male | 19 (70·4) | 55 (66·3) | 74 (67·3) |
| Female | 8 (29·6) | 28 (33·7) | 36 (32·7) |
| Number of prior lines of therapy, median (range) | NA | 2 (0–10) | 1 (0–10) |
| 17p deletion and/or TP53 mutation, n (%) | |||
| Yes | 27 (100) | 27 (32·5) | 54 (49·1) |
| No | 0 | 29 (34·9) | 29 (26·4) |
| Unknown/missing | 0 | 27 (32·5) | 27 (24·6) |
One patient assigned to the relapsed cohort (based on original electronic case report form response) was later found to have received no prior treatment for CLL and has subsequently been excluded from any post hoc analysis performed on this group. NA, not applicable.
Responses and survival
For the entire cohort, the median (range) follow‐up was 30·2 (0·1–51·9) months and median (range) duration of exposure to IDL was 11·9 (0·1–47·2) months. Clinical ORR across all patients was 88·2% (97/110) (95% CI 80·6–93·6%) (Table IIA). Four (3·6%) patients had no documented response; the response data was missing for nine of 110 (8·1%) patients; these 13 patients were deemed non‐responders in an intention‐to‐treat fashion. The median for all patients was 20·3 months (95% CI 16·1–26·1). The median DOR was 32·8 months [95% CI 21·0–not reached (NR)], median PFS was 29·6 months (95% CI 25·9–NR), median OS was not reached (95% CI 31·5–NR) (Table IIB; Fig 1A,B; Figure S1), and median TTNT was 29·2 months (95% CI 25·4–42·8). Post hoc analysis demonstrated a numerically longer median EFS in patients aged <65 years (22·6 months, 95% CI 10·6–37·2) compared to patients aged ≥65 years (19·2 months, 95% CI 15·5–26·1), although this was not statistically significant (HR 0·97, 95% CI 0·57–1·67; P = 0·91). Detailed information on subsequent CLL treatments were not collected, but eight patients who stopped IDL‐R later proceeded to allo‐HSCT.
Table II.
Primary and secondary outcomes (full analysis set)
| (A) Overall response rate – primary endpoint (N = 110) | |||||
|---|---|---|---|---|---|
| Front‐line (N = 27) | Relapsed/refractory (N = 83) | Total (N = 110) | |||
| Best overall response, n (%) | |||||
| Yes | 26 (96·3) | 71 (85·5) | 97 (88·2) | ||
| No | 0 | 4 (4·8) | 4 (3·6) | ||
| Unknown | 1 (3·7) | 8 (9·6) | 9 (8·2) | ||
| Responder (Clinical overall response rate), n (%) | 26 (96·3) | 71 (85·5) | 97 (88·2) | ||
| 95% CI | 81·0–99·9 | 76·1–92·3 | 80·6–93·6 | ||
| (B) Median times in months and rates (%) for secondary outcomes (N = 110) | |||
|---|---|---|---|
| Front‐line (N = 27) | Relapsed/refractory (N = 83) | Total (N = 110) | |
| Overall survival (full analysis set) | |||
| Kaplan–Meier estimate of overall survival (months) | |||
| Median (95% CI) | NR (27·0–NR) | NR (26·5–NR) | NR (31·5–NR) |
| OS rate at 24 months (95% CI) | 77·0 (55·7–89·0) | 64·5 (53·0–73·8) | 67·6 (57·8–75·5) |
| OS rate at 36 months (95% CI) | 58·6 (36·1–75·5) | 55·5 (43·7–65·9) | 56·1 (45·7–65·3) |
| Front‐line (N = 27) | Relapsed/refractory (N = 83) | Total (N = 110) | |
|---|---|---|---|
| Event‐free survival (full analysis set) | |||
| Discontinued the study, n (%) | 10 (37·0) | 23 (27·7) | 33 (30·0) |
| Received other anti‐cancer therapy, n (%) | 10 (37·0) | 19 (22·9) | 29 (26·4) |
| Kaplan‐Meier estimate of event‐free survival (months) | |||
| Median (95% CI) | 18·7 (12·8–42·8) | 21·7 (15·5–26·1) | 20·3 (16·1–26·1) |
| EFS rate at 24 months (95% CI) | 43·9 (24·9–61·4) | 43·6 (32·7–54·1) | 43·7 (34·2–52·8) |
| EFS rate at 36 months (95% CI) | 39·9 (21·6–57·6) | 24·9 (15·5–35·5) | 28·9 (20·1–38·1) |
95% confidence interval (CI) for clinical overall response rate is based on Clopper–Pearson exact method. Patients without any response assessments are counted as ‘Unknown’.
Fig 1.
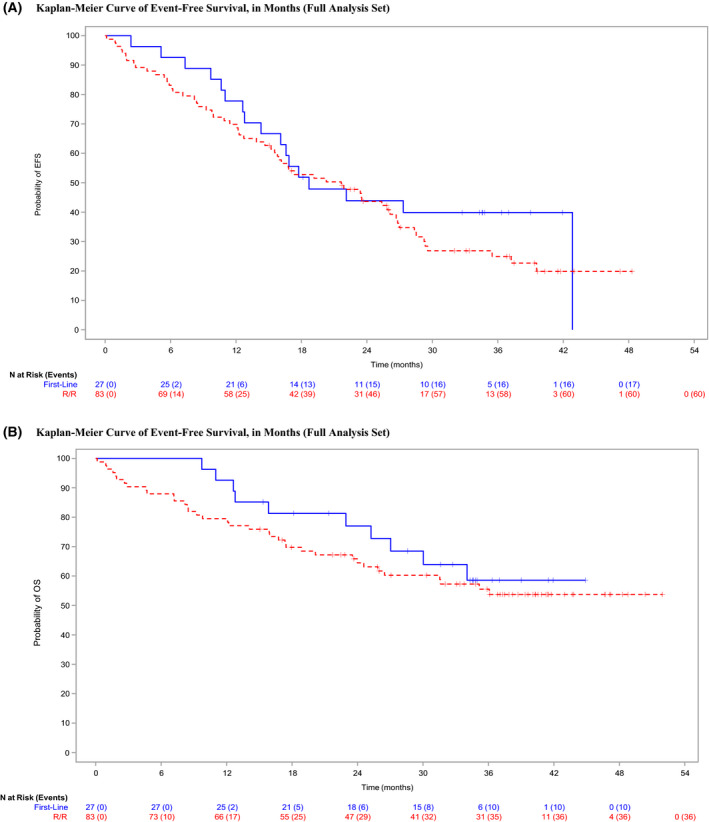
Secondary survival outcomes. (A) Kaplan–Meier curve of event‐free survival (EFS), in months (full analysis set). (B) Kaplan–Meier curve of overall survival (OS), in months (full analysis set). [Colour figure can be viewed at wileyonlinelibrary.com]
Comparison of frontline versus R/R patients
The median (range) age of the 27 front‐line patients was 71 (53–83) years and 70·4% were male. All had a TP53 mutation or 17p deletion. The median (range) follow‐up for was 31·6 (9·7–44·9) months. The clinical ORR was 96·3% (26/27) (95% CI 81·0–99·9%), which compared favourably with the clinical ORR observed in R/R patients (85·5% (71/83), 95% CI 76·1–92·3%). In total, 23 patients (85·2%) discontinued treatment; 17 due to AEs (63·0%), two by investigator discretion (7·4%), two died (7·4%), one due to progressive CLL (3·7%) and one by patient decision (3·7%). Of the 17 patients who discontinued IDL due to an AE, reasons for discontinuation were diarrhoea (five of 17, 18·5%), colitis (three of 17, 11·1%) and rash (two of 17, 7·4%).
The median EFS for front‐line patients was 18·7 months (95% CI 12·8–42·8). The 12‐month EFS was 77·8 months (95% CI 57·1–89·3) and 24‐month EFS was 43·9 months (95% CI 24·9–61·4). The median EFS for the entire R/R cohort was 21·7 months (95% CI 15·5–26·1) and was influenced by the line of therapy. The median EFS for those who received IDL‐R in two or more lines was 20·3 months (95% CI 15·2–26·1) (front‐line reference: HR 1·32, 95% CI 0·77–2·26; P = 0·31). Likewise, those patients receiving IDL‐R third‐ or fourth‐line had an increasingly shorter median EFS of 17·3 months (95% CI 11·4–25·4) and 15·2 months (95% CI 5·7–23·5) respectively; HR 1·68 (95% CI 0·95–2·96; P = 0·07) and HR 2·13 (95% CI 1·13–4·04; P = 0·02) versus front‐line respectively. However, when comparing EFS across front‐line and R/R cohorts this difference was not statistically significant (HR 1·29, 95% CI 0·75–2·21; P = 0·36). The median PFS for the front‐line cohort was NR (95% CI 22·1–NR) and OS was NR (95% CI 27·0–NR). The median PFS and OS for the R/R cohort was 28·3 months (95% CI 23·5–39·5) and NR (95% CI 26·4–NR) respectively.
AEs and reasons for discontinuation
Across all patients, 96 patients (87%) discontinued IDL‐R. Causes of discontinuation were as follows; AEs [n = 54, 49·1% overall; 63% (17/27) in front‐line versus 47·0% (39/83) in R/R], progressive disease (n = 18, 16·4%), death and investigator discretion (each n = 11; 10·0%) and patient decision (n = 2; 1·8%) (Table SIII). During the observational period, 46 of 110 patients (41·8%) died, occurring in 37·0% (n = 10) of front‐line patients and 43·4% (n = 36) R/R patients. In all, 19 patients (17·3%) died of progressive disease and three (2·7%) from Richter’s transformation (RT). In all, 22 patients (20%) died of causes other than CLL/RT (Table SIVA). Cause of death was unknown in five patients (4·5%). The most common events leading to death in the FAS were disease progression (n = 6, 5·5%), pneumonia (n = 5, 4·5%), and CLL (n = 3, 2·7%). In the front‐line cohort, 10 patients had died by the data cut‐off (Table SIVB); five were related to disease progression (n = 4 CLL, n = 1 RT) and five were from other causes. AEs leading to death were generally similar between the front‐line and R/R groups.
Throughout the observation period, 73·6% experienced a SAE. AEs are detailed in Table IIIA,B (AESI Grade 1–2 and Grade 3–4 respectively) and Table SV (AEs Grade 1–2 and Grade 3–4 respectively). The most frequently reported SAEs in the FAS were pneumonia/lower respiratory tract infection (LRTI) (n = 30, 27·2%) and neutropenic sepsis (n = 10, 9·1%). In front‐line patients, the only SAE occurring in more than two patients was colitis (n = 5, 18·5%), and in R/R patients, the most common SAEs were pneumonia/LRTI (n = 26, 32·4%) and neutropenic sepsis (n = 8, 9·6%). In the FAS, 48 patients (43·6%) experienced SAEs assessed by the investigator as related to IDL. The most frequently reported treatment‐related SAEs were pneumonia/LRTI (n = 16, 14·6%), colitis and neutropenic sepsis (each n = 7, 6·4%), and diarrhoea and sepsis (each n = 6, 5·5%).
Table III.
Adverse Events of Special Interest (>5% of patients) by Preferred Term (Full Analysis Set)
| (A) AESI (>5% of patients) of Grade 1–2 only | |||
|---|---|---|---|
| Front‐line (N = 27) | Relapsed/refractory (N = 83) | Total (N = 110) | |
| Number of subjects with TEAEs of special interest (any grade) | 26 (96.3%) | 73 (88.0%) | 99 (90.0%) |
| Number of subjects with event, per preferred term (of grade 1–2 only) | |||
| Diarrhoea | 9 (33.3%) | 18 (21.7%) | 27 (24.5%) |
| Neutropenia | 2 (7.4%) | 14 (16.9%) | 16 (14.5%) |
| Lower respiratory tract infection | 1 (3.7%) | 12 (14.5%) | 13 (11.8%) |
| Rash | 7 (25.9%) | 7 (8.4%) | 14 (12.7%) |
| Pneumonia | 0 (0%) | 3 (3.6%) | 3 (2.7%) |
| Colitis | 2 (7.4%) | 2 (2.4%) | 4 (3.6%) |
| Neutropenic sepsis | 1 (3.7%) | 3 (3.6%) | 4 (3.6%) |
| Alanine aminotransferase increased | 0 (0%) | 5 (6.0%) | 5 (4.5%) |
| Neutrophil count decreased | 1 (3.7%) | 5 (6.0%) | 6 (5.4%) |
| Sepsis | 0 | 2 (2.4%) | 2 (1.8%) |
| Febrile neutropenia | 0 | 2 (2.4%) | 2 (1.8%) |
| Urinary tract infection | 0 (0%) | 2 (2.4%) | 2 (1.8%) |
| Transaminases increased | 2 (7.4%) | 2 (2.4%) | 4 (3.6%) |
| Rash macular | 0 | 1 (1.2%) | 1 (0.9%) |
| (B) AESIs (>5% of patients) of Grade 3–4 only | |||
|---|---|---|---|
| Front‐line (N = 27) | Relapsed/refractory (N = 83) | Total (N = 110) | |
| Number of subjects with TEAEs of special interest (any grade) | 26 (96.3%) | 73 (88.0%) | 99 (90.0%) |
| Number of subjects with event, per preferred term (of Grade 3–4 only) | |||
| Pneumonia | 2 (7.4%) | 9 (10.8%) | 11 (10.0%) |
| Lower respiratory tract infection | 2 (7.4%) | 7 (8.4%) | 9 (8.2%) |
| Diarrhoea* | 1 (3.7%) | 6 (7.2%) | 7 (6.4%) |
| Neutropenia | 1 (3.7%) | 6 (7.2%) | 7 (6.4%) |
| Colitis* | 4 (14.8%) | 2 (2.4%) | 6 (5.5%) |
| Neutropenic sepsis | 1 (3.7%) | 4 (4.8%) | 5 (4.5%) |
| Sepsis* | 2 (7.4%) | 2 (2.4%) | 4 (3.6%) |
| Rash* | 2 (7.4%) | 1 (1.2%) | 3 (2.7%) |
| Febrile neutropenia* | 0 | 3 (3.6%) | 3 (2.7%) |
| Urinary tract infection* | 2 (7.4%) | 1 (1.2%) | 3 (2.7%) |
| Rash macular* | 1 (3.7%) | 1 (1.2%) | 2 (1.8%) |
| Alanine aminotransferase increased* | 1 (3.7%) | 0 | 1 (0.9%) |
| Neutrophil count decreased* | 0 | 0 | 0 |
| Transaminases increased* | 0 | 0 | 0 |
Treatment‐emergent adverse events (TEAEs) began on or after the idelalisib start date up to 30 days after permanent discontinuation of study drug or led to premature study drug discontinuation. Adverse events are coded according to MedDRA Version 21.1. Multiple occurrences of AE under a Preferred Term (PT) are counted once per patient. PTs are presented in decreasing order of the frequencies of Total column.
Grade 3 AESIs only, no Grade 4 AESIs observed.
The AESIs included diarrhoea without clinical suspicion of colitis, diarrhoea with clinical suspicion of or confirmed colitis, transaminase elevations/hepatotoxicity, pneumonitis/ organising pneumonia, neutropenia, rash/Stevens–Johnson syndrome/toxic epidermal necrolysis, and/or infections (PJP/CMV/bacterial/fungal/viral/other). In the FAS, the three most common AESIs were diarrhoea (n = 34, 30·9%), neutropenia (n = 23, 20·9%) and LRTI (n = 22, 20·0%). In front‐line patients, the three most common AESIs were diarrhoea (n = 10, 37·0%), rash (n = 9, 33·3%) and colitis (n = 6, 22·2%), and in R/R patients these were diarrhoea (n = 24, 28·9%), neutropenia (n = 20, 24·1) and LRTI (n = 19, 22·9%). Most patients (n = 92, 83·6%) received antibiotic prophylaxis during the observation period. A total of 37 patients experienced LRTIs and/or pneumonia. Of these, 32 patients (86·5%) received prophylaxis. Five front‐line patients experienced LRTIs and/or pneumonia, all of whom received prophylaxis. In all, 32 R/R patients experienced LRTIs and/or pneumonia, 27 of whom (84·4%) received prophylaxis.
Outcomes for patients stopping due to AEs (n = 54)
We assessed the clinical benefit of IDL beyond treatment duration observed in the 54 (49%) patients who prematurely discontinued IDL due to AEs within the overall FAS. The median (range) time‐on‐therapy for this cohort was 8·1 (0·4–39·2) months. The clinical ORR was 92·6% (95% CI 82·1–97·9) and the median TTNT was 25·4 months (95% CI 17·7–36·4). The median PFS and OS of this subgroup was NR (95% CI 35·5–NR) and NR (95% CI 34·0–NR) respectively. Of these 54 patients, 29 subsequently initiated a new line of therapy and five patients did so within 8 weeks of IDL discontinuation. A post hoc analysis was performed on the 49 patients who stopped IDL‐R due to AEs, but did not proceed to another line of therapy within 8 weeks of treatment discontinuation. The median (range) time‐on‐therapy for these 49 patients was 8 (0·4–39·2) months. The clinical ORR was 91·8% (95% CI 80·4–97·7) and the median TTNT was 26·1 months (95% CI 18·7–42·8). The median PFS and OS from the time of IDL‐R discontinuation was NR (95% CI 35·5–NR) and NR (95% CI 35·2–NR) respectively.
Discussion
To our knowledge, we report on the largest series of patients receiving IDL‐R in routine clinical practice in the literature. Our present findings corroborate those from interventional studies.3, 4, 22 The EFS reported was considerably shorter in patients in both the front‐line and R/R setting compared to PFS. This could be due to stopping IDL‐R for toxicity in either setting being counted as an event without documented disease progression. The difference between the front‐line EFS (24‐month, 43·9%) and PFS (24‐month, 67·6%) is particularly marked and serves to demonstrate the challenging toxicities that front‐line patients may face. The longer PFS observed in our present study across the whole cohort when compared to prospective trials in relapsed CLL likely reflects the fewer median number of prior therapies. The median TTNT was slightly shorter than the median PFS likely due to several patients discontinuing IDL‐R without progressive disease, but then continuing to a subsequent therapy.
We demonstrate that this combination is active in TP53 disrupted CLL and provides a treatment option for patients with TP53‐disrupted CLL in the front‐line and R/R setting. It is worth noting that, of those approximately two‐thirds of patients with available data on TP53 disruption, all patients treated with IDL‐R in the front‐line setting had a TP53 disruption compared to just 32·5% in R/R CLL. Our present findings are consistent with the recently described activity of IDL‐R in TP53‐disrupted B‐cell prolymphocytic leukaemia26, 27 and the outcomes of patients with CLL within trials of TP53‐disrupted CLL in the front‐line22 and relapsed setting.2, 3 Therapeutic activity has been noted in all these studies, but toxicity‐related discontinuation rates are consistently higher in younger patients and those receiving therapy in the front‐line setting.23, 24 We note that the duration of PFS and OS were similar for front‐line treated patients. This is explained by an examination of the causes of death. A total of 10 deaths occurred, including five from unrelated causes whilst CLL was in remission. The other five were related to disease progression in high‐risk (TP53 disrupted) patients treated in an era prior to widespread venetoclax availability.
The ASCEND trial (ClinicalTrials.gov NCT02970318)28 demonstrated a superior PFS for patients treated with acalabrutinib monotherapy over the investigator’s choice of bendamustine‐rituximab or IDL‐R in R/R CLL. In ASCEND,28 patients treated with IDL‐R had a much lower incidence of TP53 disruption (22% vs. 32%) and longer duration of IDL‐R therapy (16·5 vs. 11·9 months) but a shorter PFS (15·8 vs. 28·3 months) than seen in the R/R patient subgroup in our present study. It therefore appears that the patients in our present study despite having a higher frequency of TP53 disruption and shorter duration of therapy actually had a longer PFS. It is highly unusual for real‐world patients to survive better than those in studies, but this probably reflects that within the ASCEND study patients could cross over from the IDL‐R arm to acalabrutinib (23% crossed over), whereas when our present study was undertaken there were very limited suitable alternative therapies that meant patients simply had to stop IDL‐R if toxicities could not be managed. As such, some of the benefit seen in patients crossed over from the IDL‐R to acalabrutinib arm due to toxicity may well be attributable in part to the prior IDL‐R therapy. It is therefore recognised that in situations where a BTK inhibitor such as acalabrutinib is available for routine use in R/R CLL, this option may be preferable in certain patients without contraindication in those who are BCR inhibitor naïve. Ibrutinib and venetoclax are also both licensed and available therapeutic options in both settings, and although they represent effective and generally well tolerated options, there are some selected situations, such as patients with a significant cardiovascular history, anticoagulation, significant risk of tumour lysis or severe renal impairment, where IDL‐R may be preferred ahead of these novel agents.
A noteworthy observation in this report was that with a median duration of IDL therapy of only 11·9 months, the clinical benefit as measured by median PFS and TTNT was substantially longer at 29·6 and 29·2 months, respectively. Even when we conservatively looked at EFS, this benefit persisted (mEFS of 20·3 months). Likewise, when considering the 49 patients who had to stop IDL due to AEs and had not initiated a new line of therapy within 8 weeks of discontinuation, the median TTNT was 26·1 months despite a median of only 8 months of IDL‐R therapy. These data suggest that the beneficial effects of IDL extend considerably beyond the actual duration of treatment. The rationale behind this observation, as noted in our present study, may relate to T‐cell activation through PI3Kδ isoform inhibition leading to AEs, treatment discontinuation, and subsequent enhanced immune surveillance; however, the mechanisms for this phenomenon remain poorly understood and require further investigation. Whether these findings suggest that fixed‐duration therapy might provide benefit for patients receiving PI3Kδ inhibition beyond time of discontinuation is unclear and requires prospective evaluation.
The safety profile of IDL‐R was consistent with the clinical trial experience and no new safety signals were identified. Notable immune‐related and infective AEs were seen. Although the infection rate reported was similar to recent reports, many patients within the present study commenced IDL before the AE profile, especially infective risk, was reported and more fully understood. As discussed, recent monitoring and anti‐infective published guidance18 now drives the supportive management in clinical practice. All patients receiving IDL‐R should receive PJP prophylaxis and undergo regular CMV testing during and after treatment.
Our present study has several limitations. Although our data were collected from representative UK centres, retrospective data collection has inherent biases of patient level data collection, AE reporting and response assessment. Recognising this, we attempted where possible to mitigate against such biases by applying established criteria and focussing on the most objective parameters. We recognise the potential for non‐uniform follow‐up and the lack of scheduled, protocol‐derived radiological reassessment within this study. This is not unique to this routine practice real‐world data set, although we acknowledge has the theoretical potential to affect PFS and influence indirect comparison with PFS from clinical trials. As such, TTNT was intentionally collected to provide a robust end‐point without additional potential bias. We recognise that the clinical and prognostic data presented are somewhat limited, not uniformly collected, and responses were not confirmed centrally or using International Workshop on CLL (iwCLL) criteria; these are well known limitations of real‐world studies.
In summary, we demonstrate that IDL‐R remains an efficacious treatment option in CLL with demonstrable activity in routine clinical practice. Careful management of the now well documented AEs is required in all patients, with close attention paid to those patients receiving the combination in the front‐line setting (TP53 mutated/17p deleted) and those of younger age. We demonstrate that although treatment is often limited by the safety prolife of IDL, a considerable minority of patients benefit from durable remissions for a clinically meaningful time‐period following treatment cessation.
Author contributions
Conception and design of study: Nishanthan Rajakumaraswamy, Harry W. Smith and Toby A. Eyre. Collection and assembly of data: Nishanthan Rajakumaraswamy and Toby A. Eyre co‐ordinated the collection of national data alongside Gilead‐funded Contract Research Organisation (CRO) support (ICON). Toby A. Eyre, Gavin Preston, Huseini Kagdi, Amin Islam, Toby Nicholson and Christopher Fegan all managed patients in the study and were involved in collection and assembly of the data. Data analysis and interpretation: Nishanthan Rajakumaraswamy, Harry W. Smith, Toby A. Eyre, Christopher Fegan, Adam P. Cursley, Heribert Ramroth, Guan Xing and Lin Gu were involved in data analysis and interpretation. Guan Xing and Lin Gu performed the statistical analysis. Manuscript writing: Toby A. Eyre and Harry W. Smith wrote the manuscript, which all authors critically reviewed. Final approval of manuscript: All authors were involved in research design, or the acquisition, analysis or interpretation of data, critically revising the manuscript and the final approval.
Conflict of interest
Toby A. Eyre: Gilead: Consultancy, Research Support, Honoraria; Loxo: Consultancy, Beigene: Consultancy, Research Support, AstraZeneca: Honoraria, Consultancy, Research Support Roche: Honoraria; Abbvie: Honoraria, Conference Sponsorship; Janssen: Honoraria, Conference Sponsorship; Takeda: Conference Sponsorship. Gavin Preston: None. Huseini Kagdi: None. Amin Islam: Gilead; Honoraria, Conference Sponsorship. Toby Nicholson: Pfizer; Conference Sponsorship; Takeda; Conference Sponsorship. Harry W. Smith: Gilead: Employee. Adam P. Cursley: Gilead: Employee. Heribert Ramroth: Gilead: Employee. Guan Xing: Gilead: Employee. Lin Gu: Gilead: Employee. Nishanthan Rajakumaraswamy: Gilead: Employee. Christopher Fegan: Gilead: Honoraria; Roche: Honoraria; Janssen: Honoraria; Abbvie: Consultancy, Conference Sponsorship.
Supporting information
Fig S1. Secondary survival outcome [progression‐free survival (PFS)]. Kaplan–Meier curve of PFS, in months (full analysis set).
Table SI. GS‐UK‐312‐4639: study design and secondary endpoints.
Table SII. Prior therapies given for relapsed patients with chronic lymphocytic leukaemia (CLL) (full analysis set).
Table SIII. Reasons for discontinuation: investigator and patient reasons.
Table SIV. Causes of death for reasons other than chronic lymphocytic leukaemia (CLL).
Table SV. Adverse events (AEs) by grade (>5% patients) in any group (full analysis set).
Acknowledgements
This study was fully funded by Gilead Sciences Ltd. Toby A. Eyre recognises support from the Oxford Biomedical Research Centre. Views expressed are those of the authors and not necessarily those of the NHS or of Gilead Sciences Ltd. The authors would like to thank all the patients who were represented in this retrospective data collection and subsequent analysis, as well as all healthcare professionals from across the 16 centres within UK and Ireland who contributed towards the data collection.
References
- 1.Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, et al. The phosphoinositide 3′‐kinase delta inhibitor, CAL‐101, inhibits B‐cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;228:3603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner‐Johnston ND, et al. Idelalisib, an inhibitor of phosphatidylinositol 3‐kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123:3390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;270:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharman JP, Coutre SE, Furman RR, Cheson BD, Pagel JM, Hillmen P, et al. Final results of a randomized, phase III study of rituximab with or without idelalisib followed by open‐label idelalisib in patients with relapsed chronic lymphocytic leukemia. J Clin Oncol. 2019;37:1391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D’Rozario J, Assouline S, et al. Venetoclax‐rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378:1107–20. [DOI] [PubMed] [Google Scholar]
- 6.Stilgenbauer S, Eichhorst B, Schetelig J, Coutre S, Seymour JF, Munir T, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open‐label, phase 2 study. Lancet Oncol. 2016;17:768–78. [DOI] [PubMed] [Google Scholar]
- 7.Byrd JC, Harrington B, O’Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP‐196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eyre TA, Kirkwood AA, Gohill S, Follows G, Walewska R, Walter H, et al. Efficacy of venetoclax monotherapy in patients with relapsed chronic lymphocytic leukaemia in the post‐BCR inhibitor setting: a UK wide analysis. Br J Haematol. 2019;185:656–69. [DOI] [PubMed] [Google Scholar]
- 10.Eyre TA, Roeker LE, Fox CP, Gohill SH, Walewska R, Walter HS, et al. The efficacy and safety of venetoclax therapy in elderly patients with relapsed, refractory chronic lymphocytic leukaemia. Br J Haematol. 2020;188:918–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mato AR, Thompson M, Allan JN, Brander DM, Pagel JM, Ujjani CS, et al. Real world outcomes and management strategies for venetoclax‐treated chronic lymphocytic leukemia patients in the United States. Haematologica. 2018;103:1511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shanafelt TD, Wang XV, Kay NE, Hanson CA, O’Brien S, Barrientos J, et al. Ibrutinib‐rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381:432–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woyach JA, Ruppert AS, Heerema NA, Zhao W, Booth AM, Ding W, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379:2517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer K, Al‐Sawaf O, Bahlo J, Fink AM, Tandon M, Dixon M, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380:2225–36. [DOI] [PubMed] [Google Scholar]
- 15.Mato AR, Nabhan C, Thompson MC, Lamanna N, Brander DM, Hill B, et al. Toxicities and outcomes of 616 ibrutinib‐treated patients in the united states: a real‐world analysis. Haematologica. 2018;103:874–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.UK CLL Forum . Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica. 2016;101:1563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gopal AK, Kahl BS, de Vos S, Wagner‐Johnston ND, Schuster SJ, Jurczak WJ, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coutré SE, Barrientos JC, Brown JR, de Vos S, Furman RR, Keating MJ, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma. 2015;56:2779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bird ST, Tian F, Flowers N, Przepiorka D, Wang R, Jung TH, et al. Idelalisib for treatment of relapsed follicular lymphoma and chronic lymphocytic leukemia: a comparison of treatment outcomes in clinical trial participants vs medicare beneficiaries. JAMA Oncol. 2020;6:248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mato AR, Hill BT, Lamanna N, Barr PM, Ujjani CS, Brander DM, et al. Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multi‐center study of 683 patients. Ann Oncol. 2017;28:1050–6. [DOI] [PubMed] [Google Scholar]
- 21.Mato AR, Roeker LE, Jacobs R, Hill BT, Lamanna N, Brander D, et al. Assessment of the efficacy of therapies following venetoclax discontinuation in CLL reveals BTK inhibition as an effective strategy. Clin Cancer Res. 2020;26:3589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Brien SM, Lamanna N, Kipps TJ, Flinn I, Zelenetz AD, Burger JA, et al. A phase 2 study of idelalisib plus rituximab in treatment‐naïve older patients with chronic lymphocytic leukemia. Blood. 2015;126:2686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lampson BL, Kim HT, Davids MS, Abramson JS, Freedman AS, Jacobson CA, et al. Efficacy results of a phase 2 trial of first‐line idelalisib plus ofatumumab in chronic lymphocytic leukemia. Blood Adv. 2019;3:1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lampson BL, Kasar SN, Matos TR, Morgan EA, Rassenti L, Davids MS, et al. Idelalisib given front‐line for treatment of chronic lymphocytic leukemia causes frequent immune‐mediated hepatotoxicity. Blood. 2016;128:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 26.Eyre TA, Fox CP, Shankara P, Went R, Schuh AH. Idelalisib‐rituximab induces clinical remissions in patients with TP53 disrupted B cell prolymphocytic leukaemia. Br J Haematol. 2017;177:486–91. [DOI] [PubMed] [Google Scholar]
- 27.Eyre TA, Fox CP, Boden A, Bloor A, Dungawalla M, Shankara P, et al. Idelalisib‐rituximab induces durable remissions in TP53 disrupted B‐PLL but results in significant toxicity: updated results of the UK‐wide compassionate use programme. Br J Haematol. 2019;184:667–71. [DOI] [PubMed] [Google Scholar]
- 28.Ghia P, Pluta A, Wach M, Lysak D, Kozak T, Simkovic M, et al. ASCEND: phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38:2849–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Secondary survival outcome [progression‐free survival (PFS)]. Kaplan–Meier curve of PFS, in months (full analysis set).
Table SI. GS‐UK‐312‐4639: study design and secondary endpoints.
Table SII. Prior therapies given for relapsed patients with chronic lymphocytic leukaemia (CLL) (full analysis set).
Table SIII. Reasons for discontinuation: investigator and patient reasons.
Table SIV. Causes of death for reasons other than chronic lymphocytic leukaemia (CLL).
Table SV. Adverse events (AEs) by grade (>5% patients) in any group (full analysis set).


