Abstract
Objective
Rimegepant is an orally administered small‐molecule calcitonin gene‐related peptide (CGRP) receptor antagonist, with demonstrated efficacy in the acute treatment of migraine. Recent estimates from a single‐arm trial (BHV3000‐201) have also shown evidence of long‐term preventive effects in monthly migraine days (MMDs) and health‐related quality of life (HRQoL). This study aimed to compare MMDs and HRQoL data for oral rimegepant to those obtained in placebo‐controlled trials for injectable anti‐CGRP monoclonal antibodies (mAbs) galcanezumab and erenumab.
Methods
Matching‐adjusted indirect comparisons (MAICs) were conducted using rimegepant subject‐level data and published aggregate‐level results from mAb trials. Rimegepant baseline characteristics were matched to the pooled subject characteristics from EVOLVE‐I/II (galcanezumab vs. placebo; n = 1773) and STRIVE (ereumab vs. placebo; n = 955) by reweighting the rimegepant subjects to more closely match the distributions observed in these trials. To align with inclusion criteria of the mAb trials, only the subset of rimegepant subjects with a history of 4–14 MMDs were included (n = 257). Weighted mean differences were used to calculate adjusted change in MMDs, Migraine Disability Assessment Test (MIDAS) score, and Migraine‐Specific Quality of Life Questionnaire version 2 (MSQv2) scores from baseline to week 12.
Results
When matched to the EVOLVE trials, rimegepant was superior to placebo with a mean difference in MMD change from baseline [95% confidence interval] of −1.16 [−1.80, −0.52] and was not statistically significantly different from galcanezumab 0.59 [−0.13, 1.32]. When matched to the STRIVE trial, rimegepant was superior to placebo −1.59 [−2.15, −1.03] and was not statistically significantly different from erenumab −0.06 [−0.61, 0.50]. Rimegepant showed superior MIDAS and MSQv2 results compared with placebo in both EVOLVE trials and in the STRIVE trial, no statistically significant differences from galcanezumab and erenumab regarding MIDAS, and favorable results compared with erenumab across all MSQv2 domains, while being generally similar to galcanezumab across all MSQv2 domains.
Conclusions
When adjustments were made to reflect baseline characteristics in published literature, supporting data from BHV3000‐201 suggest that rimegepant every other day is an effective therapy in reducing disability and MMDs and enhancing migraine‐specific HRQoL. These data support the preventive benefit observed in randomized trials of rimegepant and further validate its efficacy for both acute and preventive treatment of migraine.
Keywords: health‐related quality of life, matching‐adjusted indirect comparison, migraine, monthly migraine days
Abbreviations
- CFB
change from baseline
- CGRP
calcitonin gene‐related peptide
- CI
confidence interval
- DD
disease duration
- EF
emotional function
- EOD
every other day
- ESS
effective sample size
- HRQoL
health‐related quality of life
- mAbs
monoclonal antibodies
- MAIC
matching‐adjusted indirect comparison
- MIDAS
migraine disability assessment test
- MMDs
monthly migraine days
- MSQv2
Migraine‐Specific Quality of life Questionnaire version 2
- NICE
National Institute for Health and Care Excellence
- NR
not reported
- PRF
role function‐preventive
- PRN
as needed
- RCT
randomized controlled trial
- RRF
role function‐restrictive
- SD
standard deviation
INTRODUCTION
Migraine is a common debilitating neurological condition that affects over one billion people worldwide.1 In addition to severe headaches, patients with migraine may experience a range of symptoms including photophobia, phonophobia, nausea, and vomiting.2 Many individuals with migraine experience a diminished health‐related quality of life (HRQoL), reduction in workplace productivity, and limited enjoyment of social and leisure activities.3, 4, 5 Migraine management consists of acute therapies taken during an attack to provide symptomatic relief and preventive treatments taken regularly to reduce the number of monthly migraine days (MMDs) for patients with ≥4 MMDs.6 Historically, migraine therapies have been developed for either acute or preventive treatment, but not both.
Rimegepant is an orally administered small‐molecule calcitonin gene‐related peptide (CGRP) receptor antagonist (gepant) and the first to show efficacy in both acute and preventive treatment of migraine.2, 7, 8, 9 Currently, rimegepant has been approved by the FDA for an acute indication. However, given the pharmacokinetic properties of rimegepant—namely its half‐life of ~11 h—and its ability to inhibit CGRP signaling, it was postulated that treatment may also confer preventive benefits when taken regularly. This hypothesis has been confirmed in a recent Phase 2/3 clinical trial that evaluated the efficacy and safety of rimegepant administered every other day (EOD) for up to 12 weeks.9 Furthermore, the repeated use of rimegepant was not associated with increased risk of medication overuse headache,9, 10 which is a challenge for frequent use of other acute therapies including triptans and opioids.11, 12 Long‐term (1‐year) follow‐up from this trial is ongoing.
The dual action of rimegepant (for both acute and preventive therapy) was initially observed by results from a long‐term, 1‐year, single‐arm safety trial (BHV3000‐201; NCT03266588), which explored the preventive effects of rimegepant in terms of MMD reduction and HRQoL measures.10, 13 BHV3000‐201 evaluated long term as needed (PRN) dosing (analogous to repeated acute treatments) as well as EOD dosing (analogous to treatment regimen in the pivotal prevention trial) in three enrollment groups further characterized by baseline MMDs (Group 1: 2–8 baseline MMDs receiving rimegepant PRN, Group 2: 9–14 baseline MMDs receiving rimegepant PRN, and Group 3: 4–14 baseline MMDs EOD plus PRN).10, 13 Interestingly, rimegepant showed evidence of MMD reduction for all three groups, demonstrating a mean reduction of 0.5 MMDs (2–8 PRN group), 2.9 MMDs (9–14 PRN group), and 3.3 MMDs (4–14 EOD/PRN group) over the trial duration.10, 13
Although this single‐arm trial (BHV3000‐201) did not allow for direct calculation of a rimegepant treatment effect relative to a randomized comparator, the benefits observed across treatment regimens and baseline MMD frequencies warrant further consideration. In particular, how rimegepant compares with currently available preventive CGRP antagonists (the injectable monoclonal antibodies [mAbs], which were developed specifically for migraine prevention) is unknown and may help contextualize the value of rimegepant in the current preventive landscape. In these circumstances, a matching‐adjusted indirect comparison (MAIC) can be used to compare the treatment effects indirectly across therapies that have not been studied head‐to‐head, while accounting for key differences in the trial populations.
The objective of this study was to compare MMD reduction and improvement in HRQoL (using the Migraine‐Specific Quality of Life Questionnaire version 2 [MSQv2] and Migraine Disability Assessment Test [MIDAS] questionnaire) in subjects receiving oral rimegepant in trial BHV3000‐201 with subjects being treated with injectable anti‐CGRP mAbs (galcanezumab and erenumab) while accounting for any differences in the trial populations via MAICs. Given the magnitude of effects observed in BHV3000‐201, and the relative similarities across trial populations, we hypothesized that after formally and quantitatively accounting for population differences, similar effects on MMDs and HRQoL would be estimated between rimegepant, galcanezumab, and erenumab.
METHODS
Motivation
The gold standard for obtaining relative treatment effects not directly compared in a randomized controlled trial (RCT) would be to conduct a network meta‐analysis using a collection of RCTs linked through common comparators. Given the single‐arm design of BHV3000‐201, an MAIC is an alternative option to adjust for population differences when comparing results across trials. MAICs use subject‐level data to balance baseline characteristics between trials, allowing for bias reduction in the estimation of relative treatment effect.14 The methodology used to perform the MAICs presented here was based on the National Institute for Health and Care Excellence (NICE) Decision Support Unit's latest guidelines for population‐adjusted indirect treatment comparisons.15
Evidence base
MAICs were conducted using rimegepant subject‐level data from BHV3000‐201 [NCT03266588] and published aggregate‐level results from mAb clinical trials (EVOLVE‐I/II [galcanezumab vs. placebo; NCT02614183, NCT02614196; n = 1773] and STRIVE [erenumab vs. placebo; NCT02456740; n = 955]). BHV3000‐201 was conducted in accordance with Good Clinical Practice guidelines as defined by the International Conference on Harmonisation and in accordance with all applicable local regulations. The protocol was approved by centralized and local Institutional Review Boards, and participants provided written informed consent before they were screened for the study.
The mAb trials had inclusion criteria which allowed only subjects with 4–14 MMDs on average across the 3 months prior to screening and during baseline to participate. Since BHV3000‐201 allowed for subject groups with a history of 2–8, 9–14, or 4–14 MMDs, in order to align with the inclusion criteria of the mAb trials, only the subset of rimegepant subjects with a history of 4–14 MMDs were included (n = 257). Furthermore, these were the subjects in BHV3000‐201 who were taking the EOD/PRN preventive regimen of rimegepant, which was most analogous with the pivotal prevention trial.9 All included trials for this analysis were deemed to have a generally low to mild risk of bias in terms of randomization, blinding, and outcome reporting.
In BHV3000‐201 regarding baseline characteristics, no subjects had missing data for any variable used in the matching. In terms of outcomes, six subjects had missing data regarding change in MMDs at 12 weeks, two subjects had missing MIDAS data, and three subjects had missing data on all three MSQv2 domains.
The characteristics of subjects included in the MAIC analysis are shown in Table 1. For galcanezumab, MMDs, MIDAS, and MSQv2 outcomes were pooled across the 120 and 240 mg arms in the EVOLVE‐I and EVOLVE‐II trials. Similarly, the placebo arms were pooled across the two trials. For erenumab, the 70 and 140 mg arms from the STRIVE trial were also pooled for all outcomes. Discontinuation rates were higher in EVOLVE‐I (18.1%) and EVOLVE‐II (14.1%) compared with STRIVE (10.2%) and the subset of subjects from BHV3000‐201 (5.4%).
TABLE 1.
Subject characteristics used in MAICs from included trials.
| Trial | BHV3000‐201 | EVOLVE‐I and EVOLVE‐II pooled (Galcanezumab vs Placebo) | STRIVE (Erenumab vs Placebo) |
|---|---|---|---|
| Treatment | Rimegepant (4‐14 MMDs subgroup) | All treatments pooled | All treatments pooled |
| N | 257 | 1773 | 955 |
| Age (years): mean (SD) | 40.4 (12.1) | 41.3 (11.3) | 40.9 (11.2) |
| Female: n (%) | 224 (87.2%) | 1500 (84.6%) | 814 (85.2%) |
| White ethnicity: n (%) | 216 (84.0%) | 1333 (75.2%) | 851 (89.1%) |
| Monthly migraine days at baseline: mean (SD) | 8.9 (3.7) | 9.1 (3.0) | 8.3 (2.5) |
| Disease duration (years): mean (SD) | 20.3 (12.9) | 20.3 (12.4) | NR |
| History of migraine with aura: n (%) | 74 (28.8%) | 938 (52.9%) | NR |
Abbreviations: MMDs, monthly migraine days; NR, not reported; SD, standard deviation.
The grey represents the BHV3000‐201 study. The greenish color represents the unmatched EVOLVE studies. The yellow represents the unmatched STRIVE study.
Endpoints
The endpoints of interest in this analysis were the change from baseline in MMDs, MIDAS, and MSQv2. Reduction in MMDs from baseline is a common measure used to show the efficacy of preventive migraine therapies. The MIDAS questionnaire is a patient‐reported outcome developed to measure and quantify the impact headache‐related disability has on a subject's life and captures information on disability in terms of number of days of missed work for pay, household work, social interactions, family interactions, and recreation.16 The MSQv2 questionnaire evaluates the impact of migraine on the subject's HRQoL over the past 4 weeks across three dimensions: role function‐restrictive (RRF), role function‐preventive (PRF), and emotional function (EF).17 Both mAbs and rimegepant (BHV3000‐201) have been shown to have benefits in both MMD reduction and HRQoL outcomes.18, 19
Statistical analyses
Rimegepant subjects were each weighted by matching their baseline characteristics to the pooled subject characteristics from the mAb clinical trials such that the distributions of characteristics were equivalent across both. Variables used for matching, given in Table 1, include age, percentage female, percentage White, MMDs at baseline, disease duration, and percentage of subjects with a history of aura. Note that a limitation of the comparison against the STRIVE trial is that we were unable to match using disease duration and percentage with aura as they were not reported. Age, MMDs at baseline, and disease duration were treated as continuous variables, whereas all others were count variables and were included as percentages in the model. Continuous variables were matched by both their mean and standard deviation (SD). For BHV3000‐201, frequencies, means, and SDs were all calculated after omitting missing data (only relevant for outcomes). For the EVOLVE and STRIVE trials, pooled frequencies, means, and SDs were extracted from the publications if available and were otherwise averaged across the arms of the respective trials. All analyses were conducted using R version V3.6.1.20
Subjects from BHV3000‐201 were weighted using a logistic propensity score model, which is a method used to attempt to mimic randomization between the two treatment arms in a comparison.15, 21 Using the objective and gradient functions as described in Phillippo et al.15 the logistic model was optimized by using the BFGS 22 method using the optim (stats package) function in R. Performance of the optimization method was assessed by whether the algorithm successfully converged (i.e., a minimum of the objective function was found). In cases of nonconvergence, variables were removed from the model one at a time until the convergence criteria were met. Weights were rescaled to be relative to the original subject weight, so that a weight of >1 carries more weight in the comparison and likewise a weight of <1 carries less weight.15 The effective sample size (ESS) is a function of these weights and is a measure of the remaining statistical power following matching. Matches with a small ESS are indicative of populations with little overlap and may result in unstable, unreliable estimates.23 Because it is not possible to match the population characteristics of both the EVOLVE and STRIVE trials simultaneously, separate MAICs were conducted. Note that no statistical power calculation was conducted prior to this study, and the sample sizes were based on data availability from these RCTs.
Using the weights generated from the propensity score model, weighted means for each outcome were calculated using the BHV3000‐201 individual subject‐level data. Then, using these weighted means and the reported values from the EVOLVE and STRIVE trials, treatment differences between rimegepant and placebo, galcanezumab, and erenumab were calculated for change in MMDs, MIDAS, and the three individual MSQv2 domain (RRF, PRF, and EF) scores from baseline to week 12. A clinically meaningful difference in MIDAS score is recognized as a decrease of 5 points,24 and clinically meaningful difference in MSQv2 domains are recommended as the following: RRF 3.2, PRF 4.6, EF 7.5.25 A p‐value of less than 0.05 coming from a two‐tailed test was the criterion used for statistical significance.
RESULTS
Subject matching, model performance, and effective sample size
All subject characteristics reported in BHV3000‐201, EVOLVE‐I/II, and STRIVE were used for matching to improve comparability in the populations (Table 2, Figure 1). When matched to both EVOLVE‐I/II and STRIVE, the optimization algorithm did achieve convergence with all available variables included.
TABLE 2.
Effective sample sizes and matched subject characteristics
| BHV3000‐201 (4‐14 MMDs subgroup) | BHV3000‐201 matched to: | ||
|---|---|---|---|
| EVOLVE‐I and EVOLVE‐II pooled (Galcanezumab vs Placebo) | STRIVE (Erenumab vs Placebo) | ||
| Effective sample size | 257 (original N) | 169 | 214 |
| Age (years): mean (SD) | 40.4 (12.1) | 41.3 (11.3) | 40.9 (11.2) |
| Female: % | 87.2% | 84.6% | 85.2% |
| White ethnicity: % | 84.0% | 75.2% | 89.1% |
| Monthly migraine days at baseline: mean (SD) | 8.9 (3.7) | 9.1 (3.0) | 8.3 (2.5) |
| Disease duration (years): mean (SD) | 20.3 (12.9) | 20.3 (12.4) | NR |
| History of migraine with aura: % | 28.8% | 52.9% | NR |
Abbreviations: MMDs, monthly migraine days; NR, not reported; SD, standard deviation.
The grey represents the BHV3000‐201 study. The purple represents the matched EVOLVE studies. The orange represents the matched STRIVE study.
FIGURE 1.

Matched and unmatched subject characteristics from analyzed trials. DD, disease duration; MMDs, monthly migraine days
Compared with an original sample size of 257, when matched to the pooled subject characteristics of the EVOLVE trials, the ESS for rimegepant was 169; whereas, rimegepant had a larger ESS of 214 when matched to STRIVE.
The relative BHV3000‐201 subject weights when matched to the EVOLVE‐I/II and STRIVE populations are shown in Figure 2. By design, the mean of the weights for each comparison was 1; whereas, the SD when matched to EVOLVE‐I/II was 0.73 and 0.45 when matched to STRIVE. Using the published aggregate baseline characteristics from EVOLVE‐I/II and STRIVE, the likelihood that each subject from BHV3000‐201 belonged to a similar trial population was determined via a weight for each subject. In general, the more similar a BHV300‐201 subject was to the external trial, the larger weight that subject would receive (i.e., upweighted). Conversely, outlying or dissimilar subjects would receive weights smaller than one (i.e., downweighted). When matched to the EVOLVE trials, there were more subjects with very large weights as well as more with weights less than 1, indicating the BHV3000‐201 and EVOLVE populations were more dissimilar compared with the BHV3000‐201 and STRIVE populations (which is indicative of the smaller ESS and larger SD of the weights). However, considering the ESSs were still relatively large compared with the original sample size, estimates compared with both trials were stable.
FIGURE 2.
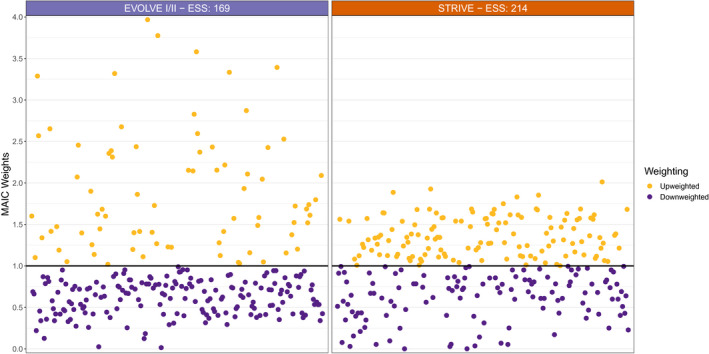
Weighted BHV3000‐201 subjects when population matched to EVOLVE and STRIVE trials, weights were rescaled to be relative to the original subject weight, so that a weight of >1 carries more weight in the comparison (upweighted) and likewise a weight of <1 carries less weight (downweighted). ESS, effective sample size; MAIC, matching‐adjusted indirect comparison
Treatment comparisons
For the outcome of change in MIDAS from baseline to 12 weeks, rimegepant was found to be consistently superior to placebo, and not statistically significantly different from both mAbs. When observing the individual MSQv2 domains, rimegepant was associated with greater improvement from baseline across all three domains compared with placebo and erenumab, was not statistically significantly different from galcanezumab in the PRF and EF MSQv2 domains, and was associated with less improvement compared with galcanezumab in the RRF domain. Finally, in terms of MMDs, rimegepant was favorably compared with placebo in both comparisons and not statistically significantly different compared with both mAbs. Figure 3 shows all unadjusted (triangles) and adjusted (squares) estimates across outcomes and comparisons. Purple indicates favorable results for rimegepant, whereas yellow indicates favorable results for erenumab or galcanezumab.
FIGURE 3.

Summary of all outcomes based on unadjusted and population adjusted estimates; differences in mean CFB (95% CI; p‐value). CFB, change from baseline; CI, confidence interval; EF, emotional function; mAb, monoclonal antibody; MIDAS, migraine disability assessment; MMDs, monthly migraine days; MSQv2, Migraine‐Specific Quality of Life Questionnaire version 2; PRF, role function‐preventive; RRF, role function‐restrictive
Rimegepant versus galcanezumab and placebo in EVOLVE‐I and EVOLVE‐II
When matched to the pooled EVOLVE trials, rimegepant was superior to pooled placebo with a mean difference in MIDAS change from baseline [95% confidence intervals] of −7.37 [−12.91, −1.83] and not statistically significantly different from pooled galcanezumab −0.09 [−6.01, 5.83]. For RRF, PRF, and EF, rimegepant also showed dominant results versus pooled placebo: 4.08 [0.92, 7.24], 4.76 [1.88, 7.63], 4.94 [1.30, 8.57], and generally similar results compared with galcanezumab: −3.88 [−7.40, −0.35], −1.45 [−4.66, 1.77] −3.01 [−7.01, 0.98]. Rimegepant was superior to pooled placebo with a mean difference in MMD change from baseline [95% confidence intervals] of −1.16 [−1.80, −0.52], and not statistically significantly different from pooled galcanezumab 0.59 [−0.13, 1.32].
While results were generally consistent pre‐ and postmatching, the treatment effect of rimegepant versus galcanezumab showed improvement after matching to the population of the EVOLVE trials.
Rimegepant versus erenumab and placebo in STRIVE
When matched to the STRIVE trial and looking at the change in the MIDAS score, rimegepant was superior to placebo −5.67 [−10.65, −0.69] and not statistically significantly different from pooled erenumab 1.75 [−3.24, 6.73]. For RRF, PRF, and EF, rimegepant also showed dominant results versus pooled placebo: 13.00 [9.94, 16.06], 9.29 [6.42, 12.15], 16.18 [12.61, 19.76] and erenumab: 7.22 [4.13, 10.31], 4.57 [1.55, 7.59], 10.52 [6.95, 14.09], respectively. For MMDs, rimegepant was superior to placebo −1.59 [−2.15, −1.03] and not statistically significantly different from pooled erenumab −0.06 [−0.61, 0.50].
DISCUSSION
Oral rimegepant taken EOD/PRN was found to be associated with a similar reduction in MMDs compared with the established mAb‐injectable therapies erenumab and galcanezumab, based on MAIC analyses. In addition, rimegepant demonstrated superior results compared with erenumab in terms of MSQv2, no statistically significant differences in terms of MIDAS, and no statistically significant differences compared with galcanezumab in these outcomes.
Rimegepant is currently approved in the United States for the acute treatment of migraine and is the first novel therapy to demonstrate efficacy for both acute and preventive therapy in phase 3 clinical trials.2, 8, 9 The current analysis helps to contextualize the additional supporting evidence from a single‐arm safety trial, in which a preventive benefit of rimegepant was observed.10 Although, at the time of writing, rimegepant is not approved for preventive use in migraine, this indirect comparison suggests no statistically significant differences in efficacy outcomes between rimegepant and the established anti‐CGRP mAbs in terms of both MMD reduction and improvement in HRQoL.
To date, migraine therapies have been targeted to either acute or preventive treatment. Of the novel CGRP antagonists, specifically, four mAbs are indicated for migraine prevention (erenumab, galcanezumab, fremanezumab, and eptinezumab),26, 27, 28, 29 two gepants are indicated for acute therapy (rimegepant and ubrogepant),2, 7, 30, 31 and two gepants have demonstrated efficacy for prevention (rimegepant and atogepant).9, 32 The dual therapy action of rimegepant, which has demonstrated both acute and preventive benefits, represents a paradigm shift for migraine treatments and their evaluation. A therapy with these characteristics may offer advantages for some patients, allowing for a simplified medication regimen, increased treatment flexibility, and greater patient autonomy. However, in the current landscape, which is built on the dichotomy of acute and preventive therapies, the adoption of an oral agent with dual therapy action could run into several obstacles, including those related to cost and reimbursement limits. This could hinder treatment flexibility at the patient level if, for example, only a limited number of doses are approved for reimbursement at any given time, and will require further consideration.
It is possible that the oral prevention dosing schedules of the gepants (daily for atogepant and EOD for rimegepant) could be associated with lower compliance in clinical practice compared with the once‐monthly mAbs. This could factor into their relative effectiveness; however, to date, this has not been studied and warrants future investigation. Nonetheless, rimegepant is associated with several favorable attributes relative to anti‐CGRP mAbs. For example, one study reported that half of headache patients prefer oral versus injectable treatments for prevention, largely due to familiarity with orals and an aversion to needles.33 The half‐life of anti‐CGRP mAbs (27–31 days compared with ~11 h for rimegepant) limits the ability for immediate cessation of treatment in the event of pregnancy, hypersensitivity reaction, or serious adverse events. Given that migraine disproportionately affects women of childbearing potential, there is particular value in a preventive treatment that provides this flexibility.34 Furthermore, MMDs have been shown to fluctuate over time (in both decreasing and increasing frequency),35 and the future ability to tailor treatment based on current disease status could be highly valuable to some patients. Other potential areas of unmet need with anti‐CGRP mAbs—which could be addressed by a novel therapy—are only now emerging with real‐world evidence of effectiveness and patient satisfaction. For example, a recent study of erenumab in chronic migraine patients suggests that there could be challenges with erenumab persistence and evidence of a potential wearing‐off effect toward the end of each treatment cycle, both of which warrant further investigation.36
In the absence of head‐to‐head comparisons between mAbs and rimegepant, the current analysis provides evidence of not statistically significant differences in terms of key efficacy outcomes and supports further investigation into rimegepant as a preventive migraine therapy. An unanchored population adjustment (i.e., population adjustment without a common comparator) is an established analytic option to incorporate data from single‐arm trials. An underlying assumption of an MAIC is that all relevant population characteristics are accounted for.15 A limitation of this analysis is that we were limited to the availability of published aggregate data for the competing trials, so it was not possible to adjust for variables that were not reported in trial publications. Important prognostic factors including migraine comorbidities and history of acute medication use were not accounted for in the propensity model,37, 38, 39 which may affect the validity of the findings. Another factor that we were unable to control for in the MAIC was the potential difference in expectation of treatment benefit between subjects in the double‐blind treatment trials (erenumab and galcanezumab) and those in the open‐label trial (rimegepant). If the expected treatment benefit was indeed greater in the open‐label trial, the results may be biased in favor of rimegepant. Future work that incorporates the results of the pivotal prevention trial for rimegepant will be of interest and would add to this growing body of knowledge.9
CONCLUSIONS
Oral rimegepant taken EOD/PRN was found to be superior to placebo and not statistically significantly different from injectable galcanezumab and erenumab in reducing MIDAS, superior to placebo and erenumab in improving MSQv2, generally similar to galcanezumab in improving MSQv2, and superior to placebo and not statistically significantly different from injectable galcanezumab and erenumab in reducing MMDs. When adjustments were made to reflect baseline characteristics in the published literature, current data suggest that rimegepant is an effective therapy in reducing disability and MMDs and enhancing migraine‐specific HRQoL. These data support the preventive benefit observed in randomized trials of rimegepant and further validate its efficacy for both acute and preventive treatment of migraine.
CONFLICT OF INTEREST
EP, KJ, and LP are employees of Broadstreet HEOR, which received funds from Biohaven for this work. RC, AT, LH, VC, and GL are employees of Biohaven. JM has been a speaker for Amgen/Aimovig, Ubrelvy/ubrogepant‐Allergan, and TEVA/Ajovy.
AUTHOR CONTRIBUTIONS
Study concept and design: Evan Popoff, Karissa Johnston, Linda Harris, Lauren Powell, Gilbert L’Italien. Acquisition of data: Robert Croop, Alexandra Thiry, Linda Harris, Vladimir Coric, Gilbert L’Italien. Analysis and interpretation of data: Evan Popoff, Karissa Johnston, Linda Harris, Lauren Powell, Gilbert L’Italien. Drafting of the manuscript: Evan Popoff, Karissa Johnston, Robert Croop, Alexandra Thiry, Linda Harris, Lauren Powell, Vladimir Coric, Gilbert L’Italien, James Moren. Revising it for intellectual content: Evan Popoff, Karissa Johnston, Linda Harris, Lauren Powell, Gilbert L’Italien, James Moren. Final approval of the completed manuscript: Evan Popoff, Karissa Johnston, Robert Croop, Alexandra Thiry, Linda Harris, Lauren Powell, Vladimir Coric, Gilbert L’Italien, James Moren.
Funding information
Biohaven Pharmaceuticals
REFERENCES
- 1.Stovner LJ, Nichols E, Steiner TJ, et al. Global, regional, and national burden of migraine and tension‐type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double‐blind, placebo‐controlled trial. Lancet. 2019;394:737‐745. [DOI] [PubMed] [Google Scholar]
- 3.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343‐349. [DOI] [PubMed] [Google Scholar]
- 4.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646‐657. [DOI] [PubMed] [Google Scholar]
- 5.Landy SH, Runken MC, Bell CF, Higbie RL, Haskins LS. Assessing the impact of migraine onset on work productivity. J Occup Environ Med. 2011;53:74‐81. [DOI] [PubMed] [Google Scholar]
- 6.American Headache Society . AHS consensus statement: the American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1‐18. [DOI] [PubMed] [Google Scholar]
- 7.Lipton RB, Croop R, Stock EG, et al. Rimegepant, an oral calcitonin gene‐related peptide receptor antagonist, for migraine. N Engl J Med. 2019;381:142‐149. [DOI] [PubMed] [Google Scholar]
- 8.Lipton RBCCM, Stock EG, Stock D, et al. Efficacy, safety, and tolerability of rimegepant 75 mg, an oral CGRP receptor antagonist, for the acute treatment of migraine: results from a double‐blind, randomized, placebo‐controlled trial, study 301. In: American Headache Society Meeting; 2018.
- 9.Croop R, Lipton RB, Kudrow D, et al. Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double‐blind, placebo‐controlled trial. Lancet. 2021;397:51‐60. [DOI] [PubMed] [Google Scholar]
- 10.Lipton RB, Berman G, Kudrow D, et al. Long‐term, open‐label safety study of rimegepant 75 mg for the treatment of migraine (study 201): interim analysis of safety and exploratory efficacy (IHC‐PO‐127). Cephalalgia. 2019;39(suppl):1‐337. [Google Scholar]
- 11.Diener H‐C, Dodick D, Evers S, et al. Pathophysiology, prevention, and treatment of medication overuse headache. Lancet Neurol. 2019;18:891‐902. [DOI] [PubMed] [Google Scholar]
- 12.Schwedt T, Alam A, Reed M, et al. Factors associated with acute medication overuse in people with migraine: results from the 2017 Migraine in America Symptoms and Treatment (MAST) Study. J Headache Pain. 2018;19:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGinley JS, L’Italien GJ, Thiry A, Croop R, Coric V, Lipton RB. Rimegepant 75 mg results in reductions in monthly migraine days: secondary analysis of a multicenter, open label long‐term safety study of rimegepant for the acute treatment of migraine (1793). Neurology. 2020;94(15 Suppl):1793. [Google Scholar]
- 14.Signorovitch JE, Sikirica V, Erder MH, et al. Matching‐adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15:940‐947. [DOI] [PubMed] [Google Scholar]
- 15.Phillippo D, Ades T, Dias S, Palmer S, Abrams KR, Welton N. NICE DSU Technical Support Document 18, vol. 18. Decision Support Unit, ScHARR, University of Sheffield: NICE Decision Support Unit; 2016. [Google Scholar]
- 16.Innovative Medical Research ; AstraZeneca Pharmaceuticals Ltd . The Migraine Disability Assessment Test. 2018; https://headaches.org/wp‐content/uploads/2018/02/MIDAS.pdf [Google Scholar]
- 17.Bagley CL, Rendas‐Baum R, Maglinte GA, et al. Validating Migraine‐Specific Quality of Life Questionnaire v2.1 in episodic and chronic migraine. Headache. 2012;52:409‐421. [DOI] [PubMed] [Google Scholar]
- 18.Lambru G, Hill B, Murphy M, Tylova I, Andreou AP. A prospective real‐world analysis of erenumab in refractory chronic migraine. J Headache Pain. 2020;21:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo A, Silvestro M, Scotto di Clemente F, et al. Multidimensional assessment of the effects of erenumab in chronic migraine patients with previous unsuccessful preventive treatments: a comprehensive real‐world experience. J Headache Pain. 2020;21:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Core Team . R: A Language and Environment for Statistical Computing [computer program]. Version 3.6.1. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 21.Beal SJ, Kupzyk KA. An introduction to propensity scores: what, when, and how. J Early Adolescence. 2014;34:66‐92. [Google Scholar]
- 22.Broyden CG. The convergence of a class of double‐rank minimization algorithms: 2. The new algorithm. IMA J Appl Math. 1970;6:222‐231. [Google Scholar]
- 23.Bourdin A, Husereau D, Molinari N, et al. Matching‐adjusted indirect comparison of benralizumab versus interleukin‐5 inhibitors for the treatment of severe asthma: a systematic review. Eur Respir J. 2018;52:1801393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buse DC, Lipton RB, Hallström Y, et al. Migraine‐related disability, impact, and health‐related quality of life among patients with episodic migraine receiving preventive treatment with erenumab. Cephalalgia. 2018;38:1622‐1631. [DOI] [PubMed] [Google Scholar]
- 25.Haywood KL, Mars TS, Potter R, Patel S, Matharu M, Underwood M. Assessing the impact of headaches and the outcomes of treatment: a systematic review of patient‐reported outcome measures (PROMs). Cephalalgia. 2018;38:1374‐1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashina M, Saper J, Cady R, et al. Eptinezumab in episodic migraine: a randomized, double‐blind, placebo‐controlled study (PROMISE‐1). Cephalalgia. 2020;40:241‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;319:1999‐2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goadsby PJ, Uwe R, Hallstrom Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123‐2132. [DOI] [PubMed] [Google Scholar]
- 29.Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE‐1 randomized clinical trial. JAMA Neurol. 2018;75:1080‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the treatment of migraine. N Engl J Med. 2019;381:2230‐2241. [DOI] [PubMed] [Google Scholar]
- 31.Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II randomized clinical trial. JAMA. 2019;322:1887‐1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goadsby PJ, Dodick DW, Ailani J, et al. Safety, tolerability, and efficacy of orally administered atogepant for the prevention of episodic migraine in adults: a double‐blind, randomised phase 2b/3 trial. Lancet Neurol. 2020;19:727‐737. [DOI] [PubMed] [Google Scholar]
- 33.Mitsikostas DD, Belesioti I, Arvaniti C, et al. Patients’ preferences for headache acute and preventive treatment. J Headache Pain. 2017;18:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burch RC, Loder S, Loder E, Smitherman TA. The prevalence and burden of migraine and severe headache in the United States: updated statistics from government health surveillance studies. Headache. 2015;55:21‐34. [DOI] [PubMed] [Google Scholar]
- 35.Serrano D, Lipton R, Scher A, et al. Fluctuations in episodic and chronic migraine status over the course of 1 year: implications for diagnosis, treatment and clinical trial design. J Headache Pain. 2017;18:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robblee J, Devick KL, Mendez N, Potter J, Slonaker J, Starling AJ. Real‐world patient experience with erenumab for the preventive treatment of migraine. Headache. 2020;60:2014‐2025. [DOI] [PubMed] [Google Scholar]
- 37.Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population‐based study. Headache. 2008;48:1157‐1168. [DOI] [PubMed] [Google Scholar]
- 38.Lipton R, Fanning K, Buse D, et al. Migraine progression in subgroups of migraine based on comorbidities: results of the CaMEO Study. Neurology. 2019;93:e2224‐e2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipton RB. Tracing transformation: chronic migraine classification, progression, and epidemiology. Neurology. 2009;72(5 Suppl 1):S3‐S7. [DOI] [PubMed] [Google Scholar]


