Abstract
Aims
Over decades, left ventricular assist device (LVAD) technology has transitioned from less durable bulky pumps to smaller continuous‐flow pumps which have substantially improved long‐term outcomes and quality of life. Contemporary LVAD therapy is beleaguered by haemocompatibility‐related adverse events including thrombosis, stroke and bleeding. A fully magnetically levitated pump, the HeartMate 3 (HM3, Abbott, USA) LVAD, has been shown to be superior to the older HeartMate II (HMII, Abbott, USA) pump by improving haemocompatibility. Experience with the HM3 LVAD suggests near elimination of de‐novo pump thrombosis, a marked reduction in stroke rates, and only a modest decrease in bleeding complications. Since the advent of continuous‐flow LVAD therapy, patients have been prescribed a combination of aspirin and anticoagulation therapy on the presumption that platelet activation and perturbations to the haemostatic axis determine their necessity. Observational studies in patients implanted with the HM3 LVAD who suffer bleeding have suggested a signal of reduced subsequent bleeding events with withdrawal of aspirin. The notion of whether antiplatelet therapy can be avoided in an effort to reduce bleeding complications has now been advanced.
Methods
To evaluate this hypothesis and its clinical benefits, the Antiplatelet Removal and Hemocompatibility Events with the HeartMate 3 Pump (ARIES HM3) has been introduced as the first‐ever international prospective, randomized, double‐blind and placebo‐controlled, non‐inferiority trial in a patient population implanted with a LVAD.
Conclusion
This paper reviews the biological and clinical role of aspirin (100 mg) with LVADs and discusses the rationale and design of the ARIES HM3 trial.
Keywords: LVAD, Aspirin, Outcomes, Hemocompatibility, Bleeding, Advanced heart failure, Assist devices, Mechanical Circulatory Support
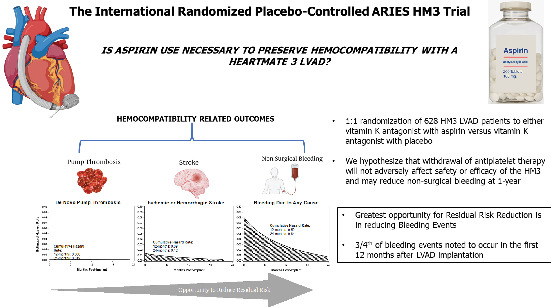
This figure incorporates the rationale for ARIES HM3 (Antiplatelet Removal and Hemocompatibility Events with the HeartMate 3 Pump), the principal study hypothesis and a summary of its design. LVAD, left ventricular assist device.
Introduction
Left ventricular assist devices (LVAD) are an established surgical treatment in patients with advanced heart failure refractory to optimal medical therapy. Over the past several decades, LVAD technology has transitioned from less durable larger, pulsatile‐designed pumps to smaller continuous flow pumps which reduce or eliminate a palpable pulse.1 These newer pumps have greatly improved long‐term outcomes while substantially improving quality of life. However, contemporary LVAD therapy is beleaguered by haemocompatibility‐related adverse events including thrombosis, stroke and bleeding.2, 3 A fully magnetically levitated pump, the HeartMate 3 (HM3, Abbott, USA) LVAD, has been shown to be superior to the HeartMate II (HMII, Abbott, USA) pump by demonstrating improved haemocompatibility.4, 5, 6 Experience with the HM3 LVAD has demonstrated near elimination of de‐novo pump thrombosis, a marked reduction in stroke rates, but only a modest decrease in bleeding complications, which remain burdensome.7, 8, 9 Thus, an opportunity exists to address the ongoing risk of bleeding, especially non‐surgical mucosal bleeding (e.g. gastrointestinal bleeding), a sequela of the un‐natural circulatory physiology with continuous‐flow LVADs.
Since the advent of continuous‐flow LVAD therapy, patients have been prescribed a combination of antiplatelet and anticoagulation therapy with vitamin K antagonists on the presumption that platelet activation and perturbations to the haemostatic axis determine their necessity. The greater haemocompatibility achieved with the HM3 has led to examination of different doses of antiplatelet use or evaluation of lower targets of anticoagulation in select patients.10 Observational analyses from within the MOMENTUM 3 pivotal trial have suggested that usual dose aspirin (325 mg daily) is no different in achieving a similar degree of haemocompatibility when compared with low‐dose aspirin (81 mg daily).11 This observation has advanced the notion that antiplatelet therapy could potentially be withdrawn from the backdrop of anticoagulation with vitamin K antagonists and such an approach may reduce bleeding complications. To evaluate this hypothesis and its clinical benefits, the Antiplatelet Removal and Hemocompatibility Events with the HeartMate 3 Pump (ARIES HM3) has been introduced as an international prospective, randomized, double‐blind and placebo‐controlled trial in a patient population implanted with the HM3 LVAD.
Biology of aspirin use with left ventricular assist device therapy
The role of using a combination of antiplatelet and anticoagulant therapy with LVAD implantation has not been adequately studied with respect to haemocompatibility‐related adverse events in patients implanted with the HM3 pump. The use of aspirin as the antiplatelet of choice is based on the presumed benefit of its unique action to not only influence antiplatelet pathways but to also exploit the important effects upon the inflammatory cascade.12 Aspirin, even in low doses, sufficiently and irreversibly acetylates serine 530 of cyclooxygenase (COX)‐1. This effect inhibits platelet generation of thromboxane A2 and results in an antiplatelet effect.13 Low doses of aspirin (75 mg) have been shown to inhibit innate immune‐mediated responses by preventing polymorphonuclear leucocytes and macrophage accumulation in response to tissue injury.14 Aspirin acetylates COX‐2 that is constitutively expressed in endothelial cells or up‐regulated in response to a local inflammatory stimulus. In vitro studies have shown that acetylated COX‐2 triggers 15‐epi‐lipoxin A4 synthesis, which facilitates the release of nitric oxide from endothelial cells that in turn negatively regulates leucocyte–endothelial cell interaction.14
These pharmacologic effects are uniquely suited to regulate thrombogenicity in the presence of continuous‐flow LVADs via two intersecting pathways (mechanical or inflammation) which may result in pro‐thrombotic events.15, 16 Continuous‐flow LVAD circulatory physiology induces effects that result in increased shear stress and promote platelet activation.15, 16, 17 Similarly, inflammation is propagated by up‐regulation of innate immune pathways, endothelial cell activation with consequent increase in nuclear factor‐kappa B, cytokines and integrins.15, 17 Thus, these observations have been used to support the argument for persistent use of aspirin in LVADs to reduce the risk of thromboembolic complications, although these pathophysiological observations have not been adequately translated into compelling clinical evidence for utility. The continued use of aspirin with LVADs as accepted therapy is based on the supposition that it may be necessary to adequately tackle platelet activation pathways in concert with the use of anticoagulant therapy to maintain the milieu from incurring severe thromboembolic complications such as ischaemic stroke or pump thrombosis, which can be debilitating, require pump replacement and may lead to death. Similarly, the negative consequences of aspirin use, especially in increasing bleeding complications, have not been adequately evaluated. This delicate balance between thrombosis and bleeding can ideally only be examined conclusively by conducting a well‐designed randomized controlled trial such as ARIES HM3. The magnetically levitated rotor within the HM3 LVAD is designed to reduce friction and, in combination with the wider pathways, is specifically suited to reduce platelet activation as a consequence of shear stress. Clinical evidence with this device has shown a tilt in the balance towards more bleeding‐related complications and these observations now allow for the question of aspirin use to be investigated in the context of a clinical trial.8
Clinical experience of aspirin use with left ventricular assist device therapy
With the use of continuous‐flow pumps, bleeding complications (especially gastrointestinal bleeding) were encountered frequently. Consequently, clinical attention focused on strategies to reduce bleeding in the hope that thrombosis would not be precipitated, by reducing the exposure to aspirin. Such studies have been generally limited to those subsets of patients who are suffering from bleeding as the primary medical complication experienced during LVAD support. Katz and colleagues18 conducted a multicentre observational study in 100 HMII LVAD implants in the United States, most of whom (82%) underwent a reduction in antithrombotic therapy due to clinician response to a bleeding event. Pharmacotherapy at the time of reduced antithrombotic therapy included warfarin only (38%), aspirin only (28%), or no antithrombotic agent (34%) [by 1 year, 57% were on warfarin alone]. In this analysis, freedom from ischemic stroke at 1 year was 93.8%, and freedom from device thrombosis was 92.7%. However, despite reduced antithrombotic therapy, a subsequent bleeding event occurred in 52% of patients. In a European experience, Netuka et al.19 reported 101 patients implanted with the HMII pump who were maintained off aspirin and on a vitamin K antagonist alone due to clinician preference (92%). At 2 years, freedom from bleeding was 81 ± 6% while freedom from ischaemic stroke, haemorrhagic stroke, and pump thrombosis was 96 ± 2%, 94 ± 3%, and 94 ± 3%, respectively. In these studies, neither indication‐dependent nor clinician preference‐dependent removal of aspirin resulted in an increase in thromboembolic events with the HMII LVAD. Encouraged by these observational experiences, the PREVENTion of non‐surgical bleeding by management of HeartMate II patients without antiplatelet therapy (PREVENT II) trial was launched but was unable to be completed and abandoned after enrolment of 65 evaluable patients of the originally intended 350 subjects.20 This was a randomized, placebo‐controlled trial of aspirin use or withdrawal with the HMII LVAD and was not completed due to the introduction of the HM3 pump which largely replaced the use the of the HMII device and futility in enrolment was evident. The trial was not stopped due to concern for thrombosis‐related complications. The limited trial experience did provide important insight with the demonstration that at 1 year more bleeding episodes occurred in the aspirin group while stroke rates were not different.
However, it is important to recognize that antithrombotic therapies including the role of aspirin may be dependent on the specific LVAD implanted, since removal of aspirin or use of a reduced dose has been associated with increased thrombotic complications. The HeartWare HVAD pump (Medtronic, Minneapolis, MN, USA) trial experience has suggested that aspirin in a dose of 325 mg daily may be required to maintain haemocompatibility as higher rates of pump thrombosis are seen when lower dose aspirin (81 mg) is used.21, 22 However, this has not been found to be the case with the HM3 LVAD when some investigators have tested lowered exposure to aspirin. Lim and colleagues23 evaluated 90 HM3 implants at their centre in whom 53 patients discontinued aspirin due to bleeding or clinician preference by 3 months and were then maintained on warfarin monotherapy [international normalized ratio (INR) 2–3]. There was no pump thrombosis or thromboembolism noted in the cohort with 82% survival at 2 years. In a separate experience, Consolo and colleagues24 reported on 30 patients with a HM3 implant, seven of whom were discharged on oral anticoagulation alone without aspirin due to a postoperative bleeding event or with a HAS‐BLED score ≥4. Over a median of 645 (431–802) days, none of the seven without aspirin suffered a bleeding event; whereas in the 23 patients treated with aspirin, nine episodes of bleeding were recorded (39%). No thrombotic (pump thrombosis) or thromboembolic complications (including stroke, transient ischaemic attack, cerebrovascular accident) were recorded irrespective of aspirin. Overall, these two small cohort studies23, 24 have not raised safety concerns for increased thrombotic complications in indication‐dependent removal of aspirin with the HM3 LVAD. However, these experiences should only be considered anecdotal due to the selection of patients and small number of such cases evaluated. Saeed and colleagues11 studied the effectiveness of two different doses of aspirin within the HM3 arm of the MOMENTUM 3 trial. In this exploratory analysis, these investigators compared usual‐dose (325 mg, n = 141) and low‐dose (81 mg, n = 180) aspirin with both groups using anticoagulation targeted to an INR of 2.0–3.0. At 2 years, a similar proportion of patients in the usual‐ and low‐dose groups (43.4% vs. 45.3%, P = 0.94) met the primary endpoint of survival free from haemocompatibility‐related adverse events (non‐surgical bleeding, pump thrombosis, stroke, and peripheral arterial thromboembolic events). There were also no differences in survival free from haemorrhagic (usual‐dose: 54.4% vs. low‐dose: 51.7%, P = 0.42) or thrombotic (usual‐dose: 76.8% vs. low‐dose: 75.7%, P = 0.92) events. It should be emphasized that most studies have altered aspirin or warfarin or both for indications of bleeding and only in highly selected patient populations. Whether such patients have an inherent propensity to suffer bleeding complications while others may represent distinct biological profiles in response to aspirin remains uncertain. Thus, it is important to examine the concept of aspirin use across a generalizable spectrum of patients including those equally prone to bleeding‐related adverse events or to thrombotic complications in an effort to appropriately assess the risk–benefit ratio of aspirin withdrawal.
Rationale for the ARIES HM3 trial
Haemocompatibility‐related adverse events, specifically bleeding events, with the HM3, while decreased in comparison to the HMII pump, remain burdensome.6 For the purposes of designing the ARIES HM3 clinical study, we went back to the HM3 arm of the MOMENTUM 3 pivotal trial and calculated the estimated cumulative hazard rates for device thrombosis, stroke and bleeding events at 12 and 24 months. Hazard rates to first event were calculated using kernel‐smoothed hazard function. With the HM3, pump thrombosis was a rare event. For stroke and bleeding, >75% of the cumulative hazard occurred within the first year of LVAD support (Figure 1). However, bleeding events provide the greatest opportunity for reduction in residual risk.
Figure 1.
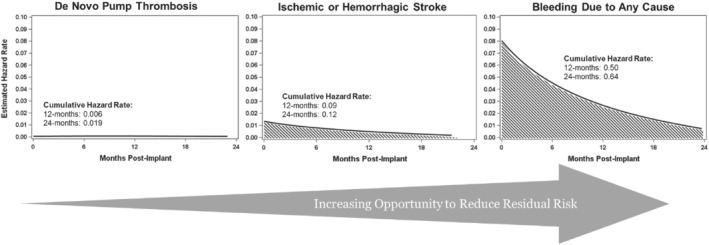
Residual risk of haemocompatibility‐related adverse effects with the HeartMate 3 (HM3) left ventricular assist device (LVAD). The figure depicts estimated hazard rates for device thrombosis, stroke and bleeding events in the HM3 arm of the MOMENTUM 3 pivotal trial.6 Hazard rates to first event are calculated using kernel‐smoothed hazard function. With the HM3, pump thrombosis has become a rare event. For stroke and bleeding, >75% of the cumulative hazard occurs within the first year of LVAD support, representing the greatest opportunity to reduce residual risk in HM3 patients.
The reduction in gastrointestinal bleeding observed in HM3 patients after cessation of aspirin is supportive but not confirmatory and this experience is limited to a handful of single‐centre studies as previously discussed.23, 24 In non‐LVAD situations, even low‐dose aspirin is associated with gastrointestinal bleeding when used for primary prevention of cardiovascular events.13, 25 In a 2016 meta‐analysis, low‐dose aspirin use (≤100 mg daily) was associated with an increased risk of major gastrointestinal bleeding of 58% [odds ratio (OR) 1.58, 95% confidence interval (CI) 1.29–1.95] and a non‐significant increase in haemorrhagic stroke of 27% (OR 1.27, 95% CI 0.96–1.68).26 In another analysis which included the three large clinical trials, low‐dose aspirin use (≤100 mg daily) increased the risk of intracranial haemorrhage [relative risk (RR) 1.37, 95% CI 1.13–1.66], compared with placebo.27 The greatest risk was for subdural or extradural haemorrhage (RR 1.53, 95% CI 1.08–2.18). Such trials have indicated that even low‐dose aspirin is associated with an increased risk of bleeding in those otherwise healthy individuals with a history of gastrointestinal bleeding or peptic ulcer disease, age >70 years, thrombocytopenia, coagulopathy, chronic kidney disease, and concurrent use of non‐steroidal anti‐inflammatory drugs, steroids, and anticoagulants.24 Several of these conditions exist in advanced heart failure patients implanted with LVADs and, when coupled with the unique risk of bleeding due to aberrations in von Willebrand factor (vWF) and predisposition to arteriovenous malformations, suggests that even low‐dose aspirin may not be without haemorrhagic risk.28, 29, 30, 31
Since the risk–benefit ratio for use of aspirin with LVAD therapy remains poorly studied, a strong rationale for the evaluation of its usefulness exists with the HM3 LVAD which has the least burden of thrombotic complications while having a bleeding risk that remains a persistent problem. Improved preservation of high molecular weight vWF multimers has been demonstrated with HM3 compared with HMII and is likely responsible for the decrease in observed bleeding events.30, 31 vWF plays a critical role in the haemostasis of mucosal surfaces such as the gastrointestinal tract and is important for binding platelets to damaged sub‐endothelium. Removing aspirin which impairs the platelet activation and interaction with vWF – a critical step in primary haemostasis – may overcome this hurdle by reducing bleeding and improving outcomes for patients with LVAD.
ARIES HM3 trial design
The ARIES HM3 clinical trial is an international, prospective, randomized, double‐blind, placebo‐controlled non‐inferiority clinical investigation of advanced heart failure patients treated with the HM3 with two different antithrombotic regimens: vitamin K antagonist with aspirin (100 mg) vs. vitamin K antagonist with placebo, with target INR between 2.0–3.0. We hypothesize that withdrawal of antiplatelet therapy from the antithrombotic regimen of HM3 pump patients will not adversely affect safety or efficacy of the HM3 and may reduce non‐surgical bleeding. The rationale and key study details are summarized in the Graphical Abstract. The clinical trial design is summarized in Table 1.
Table 1.
Clinical trial summary
| Antiplatelet Removal and Hemocompatibility Events with the HeartMate 3 Pump (ARIES HM3) | |
|---|---|
| Trial design | International, prospective, randomized, double‐blind, placebo‐controlled non‐inferiority clinical investigation of advanced heart failure patients treated with the HM3 with two different antithrombotic regimens:
|
| Objective | To study the safety and efficacy of an antiplatelet‐free antithrombotic regimen in patients with advanced heart failure treated with the HM3 left ventricular assist system. |
| Hypothesis | Withdrawal of antiplatelet therapy from the antithrombotic regimen of HM3 pump patients will not adversely affect safety or efficacy of the HM3 and may reduce non‐surgical bleeding. |
| Primary endpoint | The composite of survival free of any non‐surgical1 major haemocompatibility‐related adverse event2 at 1 year post‐implant.
|
| Secondary endpoints | Secondary endpoints, listed below, will be analysed separately to provide context to each of the components of the composite primary endpoint:
|
| Descriptive endpoints | Changes in the haemocompatibility score, rehospitalization, and economic cost implications. |
HM3, HeartMate 3.
ARIES HM3 is being conducted at up to 50 centres worldwide (in the United States, Canada, United Kingdom, Europe, Kazakhstan, and Australia). Patients will be considered enrolled upon consent (which will occur prior to implantation) but will start study participation upon randomization, which occurs 2–7 days post‐implant. Patients will have to meet all inclusion and no exclusion criteria (Table 2), as applicable, both at consent and upon randomization. Patients will be assessed prior to randomization by the investigational team to ensure no new comorbidities affecting equipoise have developed post‐implant. Reasons for screen failure of patients prior to consent or between consent and randomization will be captured and assessed.
Table 2.
Inclusion and exclusion criteria: evaluated at consent (enrolment) and randomization
| Inclusion criteria |
|
| Exclusion criteria |
|
HM3, HeartMate 3; LVAD, left ventricular assist device; MCS, mechanical circulatory support.
Patients will be randomized into the active and placebo arms of the study in a 1:1 ratio and followed until the final patient reaches 1 year of follow‐up. To safeguard study blinding, a third party, ALMAC Group (Craigavon, Northern Ireland), is responsible for randomization and re‐supply of drug through an automated web portal as well as overall treatment arm medication supply to sites and for inventory management. As discussed previously, there are no differences in outcomes in HM3 LVAD patients on low‐dose (81 mg) vs. usual‐dose (325 mg) aspirin.11 Of the major low‐dose formulations marketed internationally (75 mg, 81 mg, 100 mg), there is no evidence to suggest a clinically meaningful difference, therefore 100 mg was selected for practical reasons (logistics and potential regulatory implications).
For patients reaching 1 year of follow‐up prior to the final patient, they will continue to be followed as long as they remain on randomized treatment arm medication (placebo or aspirin), e.g. they have not transitioned to an open‐label therapy. Patients who have transitioned to an open‐label therapy will complete study follow‐up at 1 year. The study flow diagram is depicted in Figure 2. This study design feature will provide additional follow‐up in patients who remain on the treatment arm medication to potentially allow assessment of the impact of the treatment arm on cumulative adverse events in patients who have multiple or recurrent events, specifically bleeding. As part of site activation, each study team has been provided patient management guidance documents to achieve uniform clinical care practices with respect to stopping of study medication for bleeding events or haemocompatibility‐related adverse events as well as unblinding of therapy. Clinical management of vitamin K antagonists will not be altered and allowed as per site‐based standard of care to achieve an INR of 2–3. All INRs and lactate dehydrogenase measurements throughout the study will be collected. Management of anticoagulation will be strictly analysed and reported between the study arms.
Figure 2.
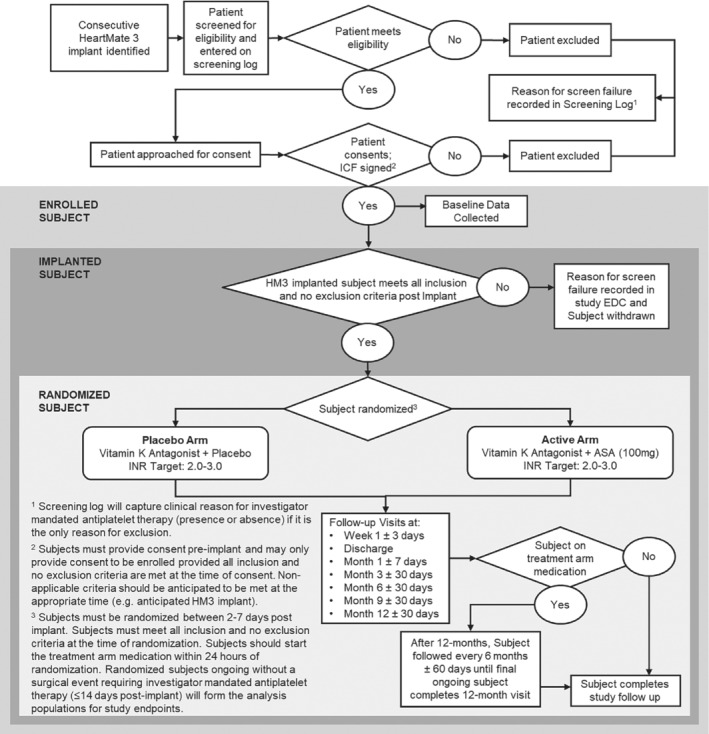
The ARIES HM3 study flow diagram. ASA, aspirin; EDC, electronic data capture; HM3, HeartMate 3; ICF, informed consent form; INR, international normalized ratio.
Study objectives and rationale for endpoints
The principal objective of this trial is to study the safety and efficacy of an aspirin‐free antithrombotic regimen in patients with advanced heart failure implanted with the HM3 LVAD. The primary endpoint for this study will be met if the placebo arm is non‐inferior to the aspirin arm in the composite of survival free of any non‐surgical (any event occurring >14 days post‐implant) major haemocompatibility‐related adverse event at 1 year post‐implant. The primary goal of the ARIES trial is to demonstrate that removal of aspirin from the anticoagulation regimen in HM3 patients does not adversely affect safety or efficacy and is therefore designed as a non‐inferiority study. Major haemocompatibility‐related adverse events include stroke, pump thrombosis (suspected or confirmed, including thrombosis within the pump or its inflow and outflow conduits), bleeding (including intracranial bleeds that do not meet the stroke definition), and arterial peripheral thromboembolism.8 Definitions for key adverse events, including major haemocompatibility‐related adverse events and events that could be adjudicated to them (haemolysis, neurologic dysfunction), are provided in online supplementary Appendix S1 . This study assesses the overall difference in the incidence of major haemocompatibility‐related adverse events between the two groups. The 14‐day blanking period was developed to ensure that surgical complications are not allocated to the study drug.
It is important to discuss the use of the primary endpoint which includes both thrombotic and bleeding events under the nomenclature of haemocompatibility.3 Such a composite endpoint is deemed important for an LVAD study due to the interdependence and inter‐relatedness of both thrombotic and haemorrhagic events, specifically LVAD patients are at risk for both types of events and prior events can influence the occurrence of subsequent events. Frequent changes to a patient's antithrombotic therapy that occur in the setting of haemocompatibility‐related adverse events increase the propensity toward opposing events. Specifically, treatment of a thrombotic event with additional antithrombotic intensity may result in a haemorrhagic event or vice versa. Thus, decoupling thrombotic and haemorrhagic events in a patient population predisposed to both is not possible. This can lead to difficulty in interpreting the results of clinical studies or, in the worst‐case scenario, result in a study with little or no interpretive value. As such, this study focuses on de‐novo LVAD implants and the first major events to avoid confounding by clinical management and prior to any modifications to the antithrombotic regimen, while encouraging investigators to maintain the randomized treatment arm therapy as long as clinically permissible.
Additional unpowered secondary endpoints include a breakdown of individual major haemocompatibility‐related adverse events by aetiology, specifically non‐surgical major haemorrhagic events and non‐surgical major thrombotic events, as well as assessment of survival, stroke, pump thrombosis, and bleeding. Bleeding will be assessed overall as well as within the subtypes of non‐surgical bleeding, moderate bleeding, severe bleeding, fatal bleeding, and gastrointestinal bleeding. These secondary endpoints will aid in contextualization of the primary endpoint results in terms of the contribution of (i) haemorrhagic vs. thrombotic events, and (ii) individual major haemocompatibility‐related adverse events. The definition for bleeding severity was inspired by using a modification of the INTERMACS bleeding adverse events definition which was in part based on the criteria proposed by the Academic Research Consortium.32 Explanted pumps in patients with suspected pump thrombosis will be returned to the sponsor for full analysis. This study will also assess changes in the haemocompatibility score, rehospitalization, and economic cost implications as a result of removal of antiplatelet therapy from the antithrombotic regimen as descriptive endpoints.8
Study blinding
All subjects, sites, Clinical Events Committee members, and sponsor personnel will remain blinded to treatment arm designation of individual patients as well as population level randomization data until the last ongoing study subject completes follow‐up (specifically, experiences an outcome or has final study visit) and all data have been adjudicated. To maintain blinding of the study, a third‐party vendor will be responsible for generation of the randomization schemes and randomization of patients. The Data and Safety Monitoring Board will be the only group with access to population level unblinded data, which is facilitated directly through the third‐party vendor and a statistician independent from the study team.
Sample size and power calculations
The MOMENTUM 3 trial database was used to derive a point estimate of 71% survival to 1 year free of any major haemocompatibility‐related adverse events in the aspirin treated arm. For the purposes of powering the study, it is assumed that in the absence of aspirin a 2% improvement in the composite endpoint will be noted, mainly due to the reduction of bleeding complications without a change in thromboembolic complications. Based on these assumptions, 220 patients will need to be randomized in each arm (440 total) to achieve 80% power to prove that the placebo group is non‐inferior to the aspirin group using a non‐inferiority margin of 10% with the Farrington–Manning risk difference approach to non‐inferiority at a one‐sided alpha = 0.025. To account for an expected 30% dropout rate associated with events occurring 2–14 days post‐implant, up to 628 patients will be randomized in the trial. To ensure the study will not be underpowered and avoid any bias inherent in an underpowered non‐inferiority trial, a pre‐specified adaptive interim analysis for sample size re‐estimation will be performed. Sample size calculations were performed using PASS 15 software.
Statistical analysis of the primary endpoint
The primary endpoint hypothesis is formally expressed as:
where πplacebo and πaspirin are the percentage of subjects who successfully achieve the composite endpoint in the placebo and aspirin groups and where ∆ is the non‐inferiority margin fixed at 10%. The primary endpoint will be assessed in the modified intention‐to‐treat (mITT) population.
The mITT population will include all randomized subjects except those who experience a surgical adverse event, defined as ≤14 days post‐implant, requiring investigator mandated antiplatelet therapy or who expire, are transplanted, or withdrawn within 14 days of implant. Patients transplanted after 14 days post‐implant will be included in the primary endpoint analysis up to the point of transplant. A patient will be considered a success if transplanted prior to 12 months post‐implant and without experiencing a haemocompatibility‐related adverse event. Non‐surgical major haemocompatibility‐related adverse events will only be analysed up to the transition to open label. Subjects will be analysed according to the treatment arm assigned at randomization.
The primary endpoint composite success rate will be calculated for each treatment arm based on the number of subjects who successfully meet the primary endpoint divided by the total number of subjects in the mITT population. The placebo arm will be considered non‐inferior to the aspirin arm if the lower boundary of the one‐sided 97.5% confidence limit of the risk difference in the composite success between treatment arms (placebo arm minus aspirin arm) is greater than the non‐inferiority margin (−10%).
The study will include a number of sensitivity analyses. First, a tipping point analysis will be performed to determine the effect of missing data on the primary endpoint. Second, if it is determined the time in therapeutic range for anticoagulation based on INR calculations is different between treatment arms, a sensitivity analysis will be performed to determine the effect on the primary endpoint. Third, a sensitivity analysis will be performed to determine differences in the time to the first event between treatment arms. Finally, a sensitivity analysis will be performed on haemocompatibility‐related adverse events that occur after a subject has been transitioned to open label.
Aspirin response testing
All patients randomized in the trial from the Unites States receiving the treatment arm medication will have their response to aspirin assessed by serum thromboxane B2 testing.33, 34 Testing will be performed by a core lab. To retain the study blind, sites will not receive the results of the test. Samples will be collected and processed by the sites at baseline, 3, 6, and 12 months post‐implant. Additional antiplatelet testing or platelet function testing, beyond the core lab test, will not be allowed while patients are on the treatment arm medication, as it may result in un‐blinding of the subject or the investigator.
Device position sub‐study
A sub‐study focused on device positioning will be conducted at up to 10 sites participating in the ARIES HM3 study. Previous studies have demonstrated that inflow cannula malposition, which occurs (i) due to incorrect surgical placement, or (ii) as a consequence of device migration, is associated with significant adverse events including pump thrombosis, stroke and persistent heart failure due to the inability to provide adequate left ventricular unloading and device flow.35 Specific surgical configurations have not been studied in depth with the HM3 LVAD, and their influence on haemocompatibility‐related events associated with LVAD therapy remains poorly understood. The high shear stress haemodynamic environment associated with LVADs may be exacerbated by malposition of the LVAD inflow cannula.36 Anecdotal evidence suggests that surgical implantation of the inflow cannula at different angles with respect to the apical ventricular axis influences LVAD thrombosis.36 Whether these conformational alterations also influence haemocompatibility with the HM3 LVAD has not been previously studied, amplifying the importance of this sub‐study.
Optimal positioning of the inflow cannula is oriented to the orifice of the mitral valve. This sub‐study utilizes a simple technique to identify a more anatomical reference point to assess HM3 inflow cannula position. A radiopaque surgical marker (i.e. surgical clips) will be placed on the anterior surface of the aortic root below the sino‐tubular ridge of the aorta just above the ostia of the right coronary artery, which reduces the likelihood of interference with the coronary artery. The surgical clips used in this study are standard to use in cardiac surgery for both haemostasis and as radiopaque markers. They have been used for the development of this sub‐study in four LVAD implants with a cumulative follow up of 245 days post‐implant at a single centre without safety concerns (unpublished data provided by Francis D. Pagani, MD, PhD). Placement of the clips will be under direct visualization and will not prolong the implant procedure. Participation in this sub‐study is not expected to present appreciable additional risk. This sub‐study will not affect the main objectives of the ARIES HM3 clinical trial.
The hypothesis of this sub‐study is to develop and validate this marker to serve as a consistent anatomical landmark to allow for accurate evaluation of the HM3 inflow cannula position at the time of implant and subsequently, over time. We hypothesize that differences in inflow cannula positions and their changes over time may correlate with clinical outcomes.
Discussion of unique study features
There are several unique features of this study, which have been implemented to conclusively establish the utility of aspirin in HM3 LVAD patients. These features include:
Patients are enrolled upon consent (pre‐implant) but randomized at 2–7 days post‐implant. This study is designed to enrol patients internationally to provide a broad and representative sample of HM3 LVAD patients. International enrolment is important as regional variations in LVAD management practice and, possibly, differences in risk of adverse events have been demonstrated.37 Therefore, the ARIES HM3 trial must conform to multiple regulatory frameworks. ISO standards require that patients be enrolled at the time of consent, however, scientifically, our primary focus in this study is on randomized patients. Therefore, this study will enrol enough patients to randomize 628 patients. This 2–7 day window from implant to randomization importantly allows for sufficient time for investigators to assess the patients' status post‐implant and the development of any exclusion criteria prior to committing to randomization.
Temporal attribution of adverse events to the surgical procedure. Events occurring within 14 days of the implant of the HM3 LVAD are generally attributed to the implant procedure. This provides sufficient time for the effect of any pre‐implant aspirin use to wash out in those randomized to placebo. Furthermore, the perioperative period is dynamic and adverse events are largely driven by the implant procedure itself rather than the individual effect of aspirin, even if the relationship to the implant procedure may not always be readily apparent. For example, perioperative epistaxis may be caused by trauma from a nasogastric tube abrading the mucus membrane. By creating a uniform, temporally based cutoff for surgical events, we will pragmatically attribute relevant events to the effect of the treatment arm.
Generalizability of the patients included in randomization. Traditionally, the degree of equipoise to conduct a study is based on specific clinical scenarios and is a principal determinant of generalizability of use of the study findings. In patients implanted with an LVAD, the changes in circulatory biology (including but not limited to the development of an acquired von Willebrand syndrome) as well as the use of systemic anticoagulation with warfarin create a new pathophysiological state influencing haematological adverse outcomes. In this situation, evidence from a different prior circulatory state cannot be applied to the appropriateness of routine aspirin use in the unique setting after LVAD implantation. Therefore, there should be broad equipoise to enrol a generalizable study population reflecting the general LVAD patient population, including ischaemic heart failure patients. As an example, some may express concern that removing aspirin from those patients with underlying coronary artery disease, especially a recent coronary event or presence of a prior intra‐coronary stent may not be in accordance with traditionally advocated guidelines. We would emphasize that once a LVAD is implanted, the physiological conditions change significantly rendering many such prior convictions less viable. First, the role of a patent coronary vessel is reduced in the condition where the LVAD unloads the ventricle and drastically decreases myocardial oxygen demand. Second, alterations that change the rheology of blood elements as described above by the development of an acquired vWF disease may decrease the propensity for intravascular thrombosis. Third, alterations in the manner of flow result in a change in coronary blood flow with a continuous‐flow LVAD.38 In an animal experimental study, LVAD support caused a decrease in systolic and peak systolic flow of all three coronary vessels but increased diastolic right coronary artery flow.38 Therefore, we believe that the clinical setting of an LVAD implantation is one where an absolute indication for use of aspirin does not exist in most scenarios. However, if the investigators believe that antiplatelet therapy is warranted, they may choose to decline patient entry. The steering committee has discussed potential scenarios with investigators to ensure enrolment of a representative and generalizable population. As an example, we believe that most patients with underlying coronary arterial disease or a diagnosis of ischaemic cardiomyopathy can be enrolled in the trial due to the reasons articulated earlier. However, we also agree that some clinical scenarios may not be appropriate for enrolment, which include a recent acute coronary syndrome requiring a drug‐eluting stent implantation within 30 days. Similarly, if the investigators believe that coronary flow to the right ventricular system may be jeopardized (e.g. a drug‐eluting stent implanted into the proximal right coronary artery within 6 months), and withdrawal of antiplatelet therapy could negatively influence the function of the right ventricle which is unsupported, such a patient should not be included in the trial. We intend to document the reason for screen failures of any patient receiving an LVAD at participating centres to analyse and understand their clinical decision‐making.
Over‐the counter aspirin use. Use of over‐the‐counter aspirin containing products must be avoided by patients in this study. Aspirin and aspirin‐like compounds are very common ingredients in medicines including those for pain relief and common illness such as the cold. We have generated country‐specific aspirin‐containing products flyers and mandated training to all patients at follow‐up visits to help patients consistently avoid these medications.
Platelet function assays and maintenance of the study blind. Platelet function tests can break (or be perceived to break) the study blind for an individual patient. These tests will be avoided while patients are actively taking the treatment arm medication. Aspirin responsiveness tests include TEG PlateletMapping, VerifyNow, Aggregometry, and others but not tests which do not assess platelet activity, such as platelet count. The efficacy of aspirin in the LVAD population has not been well studied. Therefore, as part of this study, a core lab has been established to assay serum thromboxane B2 in all US‐based patients.
Study status and summary
The first patient was randomized on 17 July 2020. As of 25 June 2021, 46 of 50 sites are active and open for enrolment in the study and 210 of the expected 628 patients have been randomized. We anticipate completion of the study randomization by mid‐year 2022 and conclusion of 1‐year follow‐up in the last patient randomized by 2023.
Highlights
LVAD therapy is plagued by haemocompatibility‐related adverse events including thrombosis, stroke and bleeding.
Aspirin is used in combination with vitamin K antagonists to prevent LVAD thrombosis but whether both therapies are required remains uncertain.
The ARIES HM3 trial is an international, randomized controlled trial to test the hypothesis that aspirin may be removed safely from the antithrombotic regimen with the HM3 LVAD.
Reducing bleeding complications while preserving haemocompatibility will enhance outcomes with the HM3 LVAD and lead to greater cost‐effectiveness of this therapy.
Funding
ARIES HM3 (NCT04069156) is funded by Abbott (Chicago, IL).
Conflict of interest: M.R.M. reports payments made to his institution from Abbott for consulting; consultant fees from Medtronic, Janssen, Mesoblast, Portola, Bayer, Triple Gene, and Baim Institute for Clinical Research. Scientific Advisory Board Member for NuPulseCV, Leviticus and FineHeart. D.L.C. is an employee of Abbott, the sponsor of the study. F.G. reports honoraria from Abbott, Bayer, Pfizer, Alnylam, Boehringer Ingelheim Amgen, Pharmacosmos and Idorisa for consulting, and speaker fees from Novartis, AstraZeneca, Orion Pharma, and Vifor. U.P.J. reports honoraria from Abbott. J.N.K. reports consulting fees from Abbott. I.N. reports grants, personal fees and non‐financial support from Abbott during the conduct of the study; grants, personal fees and non‐financial support from CARMAT SA outside of the submitted work; non‐financial support and other from LeviticusCardio Ltd. outside of the submitted work; personal fees and non‐financial support from Evaheart Inc. outside the submitted work. N.U. reports consulting fees from Abbott and Medtronic and is on the scientific advisory board of Leviticus Cardio Ltd. J.M.C. reports honoraria from Abbott, Bristol‐Myers Squibb, Pfizer, Portola, Takeda and research funding to the institution from CSL Behring. P.S. is an employee of Abbott, the sponsor of the study. G.H. is an employee of Abbott, the sponsor of the study. F.D.P. reports that he is on the scientific advisory board of FineHeart, Data Safety and Monitoring Board for CARMAT SA and the NHLBI PumpKIN Trial. Additionally, he is a task force chair for the STS INTERMACS.
Supporting information
Appendix S1. Supporting Information.
References
- 1.Sidhu K, Lam PH, Mehra MR. Evolving trends in mechanical circulatory support: clinical development of a fully magnetically levitated durable ventricular assist device. Trends Cardiovasc Med 2020;30:223–229. [DOI] [PubMed] [Google Scholar]
- 2.Pinney SP, Anyanwu AC, Lala A, Teuteberg JJ, Uriel N, Mehra MR. Left ventricular assist devices for lifelong support. J Am Coll Cardiol 2017;69:2845–2861. [DOI] [PubMed] [Google Scholar]
- 3.Mehra MR. The burden of haemocompatibility with left ventricular assist systems: a complex weave. Eur Heart J 2019;40:673–677. [DOI] [PubMed] [Google Scholar]
- 4.Mehra MR, Naka Y, Uriel N, Goldstein DJ, Cleveland JC Jr, Colombo PC, Walsh MN, Milano CA, Patel CB, Jorde UP, Pagani FD, Aaronson KD, Dean DA, McCants K, Itoh A, Ewald GA, Horstmanshof D, Long JW, Salerno C; MOMENTUM 3 Investigators . A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med 2017;376:440–450. [DOI] [PubMed] [Google Scholar]
- 5.Mehra MR, Goldstein DJ, Uriel N, Cleveland JC Jr, Yuzefpolskaya M, Salerno C, Walsh MN, Milano CA, Patel CB, Ewald GA, Itoh A, Dean D, Krishnamoorthy A, Cotts WG, Tatooles AJ, Jorde UP, Bruckner BA, Estep JD, Jeevanandam V, Sayer G, Horstmanshof D, Long JW, Gulati S, Skipper ER, O'Connell JB, Heatley G, Sood P, Naka Y; MOMENTUM 3 Investigators . Two‐year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med 2018;378:1386–1395. [DOI] [PubMed] [Google Scholar]
- 6.Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, Ransom J, Ewald GA, Itoh A, Raval NY, Silvestry SC, Cogswell R, John R, Bhimaraj A, Bruckner BA, Lowes BD, Um JY, Jeevanandam V, Sayer G, Mangi AA, Molina EJ, Sheikh F, Aaronson K, Pagani FD, Cotts WG, Tatooles AJ, Babu A, Chomsky D, Katz JN, Tessmann PB, Dean D, Krishnamoorthy A, Chuang J, Topuria I, Sood P, Goldstein DJ; MOMENTUM 3 Investigators . A fully magnetically levitated left ventricular assist device – final report. N Engl J Med 2019;380:1618–1627. [DOI] [PubMed] [Google Scholar]
- 7.Colombo PC, Mehra MR, Goldstein DJ, Estep JD, Salerno C, Jorde UP, Cowger JA, Cleveland JC Jr, Uriel N, Sayer G, Skipper ER, Downey FX, Ono M, Hooker R Jr, Anyanwu AC, Givertz MM, Mahr C, Topuria I, Somo SI, Crandall DL, Horstmanshof DA. Comprehensive analysis of stroke in the long‐term cohort of the MOMENTUM 3 study. Circulation 2019;139:155–168. [DOI] [PubMed] [Google Scholar]
- 8.Uriel N, Colombo PC, Cleveland JC, Long JW, Salerno C, Goldstein DJ, Patel CB, Ewald GA, Tatooles AJ, Silvestry SC, John R, Caldeira C, Jeevanandam V, Boyle AJ, Sundareswaran KS, Sood P, Mehra MR. Hemocompatibility‐related outcomes in the MOMENTUM 3 trial at 6 months: a randomized controlled study of a fully magnetically levitated pump in advanced heart failure. Circulation 2017;135:2003–2012. [DOI] [PubMed] [Google Scholar]
- 9.Zimpfer D, Gustafsson F, Potapov E, Pya Y, Schmitto J, Berchtold‐Herz M, Morshuis M, Shaw SM, Saeed D, Lavee J, Heatley G, Gazzola C, Garbade J. Two‐year outcome after implantation of a full magnetically levitated left ventricular assist device: results from the ELEVATE registry. Eur Heart J 2020;41:3801–3809. [DOI] [PubMed] [Google Scholar]
- 10.Netuka I, Ivák P, Tučanová Z, Gregor S, Szárszoi O, Sood P, Crandall D, Rimsans J, Connors JM, Mehra MR. Evaluation of low‐intensity anti‐coagulation with a fully magnetically levitated centrifugal‐flow circulatory pump – the MAGENTUM 1 study. J Heart Lung Transplant 2018;37:579–586. [DOI] [PubMed] [Google Scholar]
- 11.Saeed O, Colombo PC, Mehra MR, Uriel N, Goldstein DJ, Cleveland J, Connors JM, Najjar SS, Mokadam NA, Bansal A, Crandall DL, Sood P, Jorde UP. Effect of aspirin dose on hemocompatibility‐related outcomes with a magnetically levitated left ventricular assist device: an analysis from the MOMENTUM 3 study. J Heart Lung Transplant 2020;39:518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montinari MR, Minelli S, De Caterina R. The first 3500 years of aspirin history from its roots – a concise summary. Vascul Pharmacol 2019;113:1–8. [DOI] [PubMed] [Google Scholar]
- 13.Patrono C, Baigent C. Role of aspirin in primary prevention of cardiovascular disease. Nat Rev Cardiol 2019;16:675–686. [DOI] [PubMed] [Google Scholar]
- 14.Morris T, Stables M, Hobbs A, de Souza P, Colville‐Nash P, Warner T, Newson J, Bellingan G, Gilroy DW. Effects of low‐dose aspirin on acute inflammatory responses in humans. J Immunol 2009;183:2089–2096. [DOI] [PubMed] [Google Scholar]
- 15.Apostoli A, Bianchi V, Bono N, Dimasi A, Ammann KR, Moiia YR, Montisci A, Sheriff J, Bluestein D, Fiore GB, Pappalardo F, Candiani G, Redaelli A, Slepian MJ, Consolo F. Prothrombotic activity of cytokine‐activated endothelial cells and shear‐activated platelets in the setting of ventricular assist device support. J Heart Lung Transplant 2019;38:658–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Consolo F, Sferrazza G, Motolone G, Contri R, Valerio L, Lembo R, Pozzi L, Della Valle P, De Bonis M, Zangrillo A, Fiore GB, Redaelli A, Slepian MJ, Pappalardo F. Platelet activation is a preoperative risk factor for the development of thromboembolic complications in patients with continuous‐flow left ventricular assist device. Eur J Heart Fail 2018;20:792–800. [DOI] [PubMed] [Google Scholar]
- 17.Shah P, Tantry US, Bliden KP, Gurbel PA. Bleeding and thrombosis associated with ventricular assist device therapy. J Heart Lung Transplant 2017;36:1164–1173. [DOI] [PubMed] [Google Scholar]
- 18.Katz JN, Adamson RM, John R, Tatooles A, Sundareswaran K, Kallel F, Farrar DJ, Jorde UP; TRACE study . Safety of reduced anti‐thrombotic strategies in HeartMate II patients: a one‐year analysis of the US‐TRACE study. J Heart Lung Transplant 2015;34:1542–1548. [DOI] [PubMed] [Google Scholar]
- 19.Netuka I, Litzler PY, Berchtold‐Herz M, Flecher E, Zimpfer D, Damme L, Sundareswaran KS, Farrar DJ, Schmitto JD; EU TRACE Investigators . Outcomes in HeartMate II patients with no antiplatelet therapy: 2‐year results from the European TRACE study. Ann Thorac Surg 2017;103:1262–1268. [DOI] [PubMed] [Google Scholar]
- 20.Jorde UP, Katz JN, Colombo PC, Stulak J, Saeed O, Egnaczyk G, Haeusslein E, McCann P, Crandall D, Franke A, Adamson R; PREVENT II Study Investigators . PREVENTion of non‐surgical bleeding by management of HeartMate II patients without anti‐platelet therapy (PREVENT II) trial. J Heart Lung Transplant 2020;39:838–840. [DOI] [PubMed] [Google Scholar]
- 21.Slaughter MS, Pagani FD, McGee EC, Birks EJ, Cotts WG, Gregoric I, Howard Frazier O, Icenogle T, Najjar SS, Boyce SW, Acker MA, John R, Hathaway DR, Najarian KB, Aaronson KD; HeartWare Bridge to Transplant ADVANCE Trial Investigators . HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2013;32:675–683. [DOI] [PubMed] [Google Scholar]
- 22.Najjar SS, Slaughter MS, Pagani FD, Starling RC, McGee EC, Eckman P, Tatooles AJ, Moazami N, Kormos RL, Hathaway DR, Najarian KB, Bhat G, Aaronson KD, Boyce SW; HVAD Bridge to Transplant ADVANCE Trial Investigators . An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2014;33:23–34. [DOI] [PubMed] [Google Scholar]
- 23.Lim HS, Ranasinghe A, Chue C, Mascaro J. Two‐year outcome of warfarin monotherapy in HeartMate 3 left ventricular assist device: a single‐center experience. J Heart Lung Transplant 2020;39:1149–1151. [DOI] [PubMed] [Google Scholar]
- 24.Consolo F, Raimondi Lucchetti M, Tramontin C, Lapenna E, Pappalardo F. Do we need aspirin in HeartMate 3 patients? Eur J Heart Fail 2019;21:815–817. [DOI] [PubMed] [Google Scholar]
- 25.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;74:e177–e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitlock EP, Burda BU, Williams SB, Guirguis‐Blake JM, Evans CV. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2016;164:826–835. [DOI] [PubMed] [Google Scholar]
- 27.Huang WY, Saver JL, Wu YL, Lin CJ, Lee M, Ovbiagele B. Frequency of intracranial hemorrhage with low‐dose aspirin in individuals without symptomatic cardiovascular disease: a systematic review and meta‐analysis. JAMA Neurol 2019;76:906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birschmann I, Dittrich M, Eller T, Wiegmann B, Reininger AJ, Budde U, Strüber M. Ambient hemolysis and activation of coagulation is different between HeartMate II and HeartWare left ventricular assist devices. J Heart Lung Transplant 2014;33:80–87. [DOI] [PubMed] [Google Scholar]
- 29.Klovaite J, Gustafsson F, Mortensen SA, Sander K, Nielsen LB. Severely impaired von Willebrand factor‐dependent platelet aggregation in patients with a continuous‐flow left ventricular assist device (HeartMate II). J Am Coll Cardiol 2009;53:2162–2167. [DOI] [PubMed] [Google Scholar]
- 30.Netuka I, Kvasnička T, Kvasnička J, Hrachovinová I, Ivák P, Mareček F, Bílková J, Malíková I, Jančová M, Malý J, Sood P, Sundareswaran KS, Connors JM, Mehra MR. Evaluation of von Willebrand factor with a fully magnetically levitated centrifugal continuous‐flow left ventricular assist device in advanced heart failure. J Heart Lung Transplant 2016;35:860–867. [DOI] [PubMed] [Google Scholar]
- 31.Bansal A, Uriel N, Colombo PC, Narisetty K, Long JW, Bhimaraj A, Cleveland JC Jr, Goldstein DJ, Stulak JM, Najjar SS, Lanfear DE, Adler ED, Dembitsky WP, Somo SI, Crandall DL, Chen D, Connors JM, Mehra MR. Effects of a fully magnetically levitated centrifugal‐flow or axial‐flow left ventricular assist device on von Willebrand factor: a prospective multicenter clinical trial. J Heart Lung Transplant 2019;38:806–816. [DOI] [PubMed] [Google Scholar]
- 32.Kormos RL, Antonides CFJ, Goldstein DJ, Cowger JA, Starling RC, Kirklin JK, Rame JE, Rosenthal D, Mooney ML, Caliskan K, Messe SR, Teuteberg JJ, Mohacsi P, Slaughter MS, Potapov EV, Rao V, Schima H, Stehlik J, Joseph S, Koenig SC, Pagani FD. Updated definitions of adverse events for trials and registries of mechanical circulatory support: a consensus statement of the Mechanical Circulatory Support Academic Research Consortium. J Heart Lung Transplant 2020;39:735–750. [DOI] [PubMed] [Google Scholar]
- 33.Fritsma GA, Ens GE, Alvord MA, Carroll AA, Jensen R. Monitoring the antiplatelet action of aspirin. JAAPA 2001;14:57–58, 61–62. [PubMed] [Google Scholar]
- 34.Kidson‐Gerber G, Weaver J, Gemmell R, Prasan AM, Chong BH. Serum thromboxane B2 compared to five other platelet function tests for the evaluation of aspirin effect in stable cardiovascular disease. Heart Lung Circ 2010;19:234–242. [DOI] [PubMed] [Google Scholar]
- 35.Chivukula VK, Beckman JA, Prisco AR, Dardas T, Lin S, Smith JW, Mokadam NA, Aliseda A, Mahr C. Left ventricular assist device inflow cannula angle and thrombosis risk. Circ Heart Fail 2018;11:e004325. [DOI] [PubMed] [Google Scholar]
- 36.Bhama JK, Bansal A. Left ventricular assist device inflow cannula position may contribute to the development of HeartMate II left ventricular assist device pump thrombosis. Ochsner J 2018;18:131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirza KK, Xie R, Cowger J, Kirklin JK, Meyns B, Gustafsson F, Shaw SM, Goldstein DJ. Comparative analysis of regional outcomes and adverse events after continuous‐flow left ventricular assist device implantation: an IMACS analysis. J Heart Lung Transplant 2020;39:904–914. [DOI] [PubMed] [Google Scholar]
- 38.Ootaki Y, Kamohara K, Akiyama M, Zahr F, Kopcak MW Jr, Dessoffy R, Fukamachi K. Phasic coronary blood flow pattern during a continuous flow left ventricular assist support. Eur J Cardiothorac Surg 2005;28:711–716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


