Abstract
Aims
Diabetes is recognized as the leading cause of chronic kidney disease (CKD); however, the association of prediabetes with CKD remains unclear, in particular, the independent effect of prediabetes on proteinuria or estimated glomerular filtration rate (eGFR) has not been evaluated. This study aimed to investigate the associations of prediabetes with the proteinuria development and with eGFR decline separately in the Japanese general population without CKD.
Methods
Participants who underwent health check‐ups in 2014 and had adequate data after 2 years were retrospectively analysed. A total of 405,487 participants without CKD (eGFR, ≥60 ml min−1 1.73 m−2, with negative or trace urinary protein) at baseline were categorized according to fasting plasma glucose as having diabetes (≥126 mg/dl [7.0 mmol/l]), prediabetes (100–125 mg/dl [5.6–6.9 mmol/l]) or normal glucose level (˂100 mg/dl [5.6 mmol/l]). Logistic regression analysis was used to analyse the effects of prediabetes (vs. normal glucose level) on the proteinuria development (urinary protein of ≥1+) and eGFR decline (˂60 ml min−1 1.73 m−2) after 2 years.
Results
After 2 years, 7037 participants (1.7%) developed proteinuria alone, 19,015 (4.7%) presented eGFR decline alone and 636 (0.2%) showed both proteinuria and eGFR decline. Compared to normal glucose level and adjusting for prognostic factors, prediabetes was independently associated with the proteinuria development (odds ratio [OR] 1.233; 95% confidence interval [CI] 1.170–1.301], whereas prediabetes was not associated with eGFR decline (OR 0.981; 95% CI 0.947–1.017).
Conclusions
Prediabetes is associated with the proteinuria development but not with eGFR decline in the general population.
Keywords: epidemiology, nephropathy
What is already known?
A large meta‐analysis reported that prediabetes is moderately associated with chronic kidney disease (defined as the combination of proteinuria and low estimated glomerular filtration rate [eGFR]).
Previous cohort studies reported that prediabetes had no association with low eGFR alone.
Prediabetes may affect proteinuria development and eGFR decline in different ways.
What this study has found?
Prediabetes is associated with proteinuria development, but not with eGFR decline in the Japanese general population.
What are the clinical implications of the study?
Proteinuria in prediabetes might be an early detectable renal change.
1. INTRODUCTION
Chronic kidney disease (CKD) has become a serious public health concern as its prevalence is increasing worldwide and increases the risk for end‐stage renal disease (ESRD), cardiovascular disease (CVD) and mortality, requiring high medical expenses.1, 2, 3 To reduce the health and economic burden, identifying factors predisposing an individual to CKD is warranted. CKD is classified according to both albuminuria (or proteinuria) and estimated glomerular filtration rate (eGFR) stages, and the risk for all‐cause mortality, CVD mortality and ESRD in each category is reflected by the colour intensity in a heat map.4
Diabetes is well recognized as the leading cause of CKD in most countries, accounting for 30%–40% of CKD in the United States.5 Prediabetes is defined as an intermediate state of hyperglycaemia, in which blood glucose is higher than normal but lower than that in diabetes, and 34.5% of US adults, four times of the number of diabetes, are estimated to have prediabetes.6 Several studies reported that 20%–30% of individuals with newly diagnosed diabetes mellitus already have microalbuminuria,7, 8, 9 suggesting that renal damage already occurs before blood glucose reaches levels that define diabetes.
A large meta‐analysis reported that prediabetes is modestly associated with CKD risk.10 Conversely, majority of previous cohort studies reported that prediabetes was not associated with low eGFR alone.11, 12, 13 Both high albuminuria and low eGFR are associated with higher risk for mortality, CVD and ESRD with or without diabetes, and these two renal changes are considered to involve different pathophysiological mechanisms leading to the occurrence of CKD.14, 15, 16 Thus, investigating the associations of prediabetes with albuminuria development and low eGFR separately is important to determine whether prediabetes affects these two parameters of CKD in a different way.
In Japan, a mandatory occupational health check‐up system has been implemented to reduce the prevalence of lifestyle‐related diseases.17 The ‘Ningen Dock’ is a comprehensive health check‐up system providing a series of medical examinations using a standardized method throughout Japan, and >3 million people per year currently undergo the ‘Ningen Dock’. People of all ages, including many in their middle ages, experience the ‘Ningen Dock’ annually as a mandatory occupational health check‐up.
This study aimed to investigate the relationship between prediabetes and CKD, focusing on the proteinuria development and eGFR decline separately, using the ‘Ningen Dock’ database including a large Japanese general population without CKD.
2. MATERIALS AND METHODS
2.1. Study design
Participants who underwent health check‐ups in 2014 and had adequate data for renal function after 2 years were retrospectively analysed. A total of 405,487 participants without CKD (eGFR, ≥60 ml min−1 1.73 m−2, with negative or trace urinary protein) at baseline were categorized according to the baseline diabetic status (diabetes, prediabetes or normal glucose level). Logistic regression analysis was performed to analyse the effects of prediabetes on the proteinuria development (urinary protein, ≥1+) and eGFR decline (˂60 ml min−1 1.73 m−2) after 2 years.
2.2. Population
The current study population consisted of adults aged ≥20 years who underwent health check‐ups in 2014 at 174 facilities that belong to Japan Society of Ningen Dock and had complete annual data in 2014. Participants with urinary protein scores of ≥1+, eGFR of ˂60 ml min−1 1.73 m−2 or with history of kidney diseases based on the interview at baseline (2014) were excluded. After exclusions, the analysis was limited to those who underwent health check‐ups after 2 years (2016) and had data on fasting plasma glucose (FPG), eGFR and urinary protein. Finally, participants without CKD at baseline were included for analysis (Figure 1).
FIGURE 1.
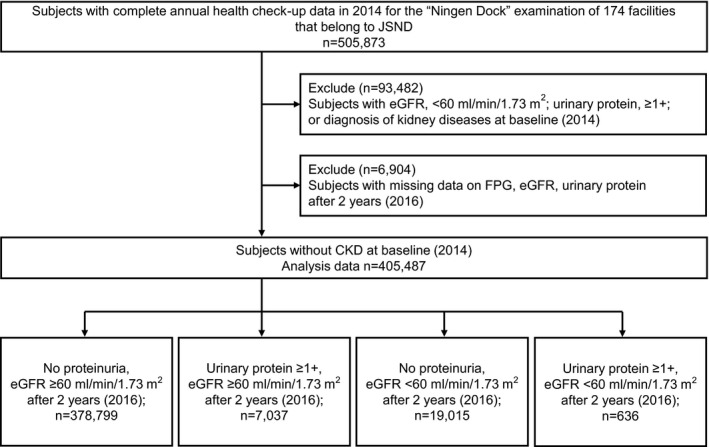
Flowchart of the study population. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; JSND, Japan Society of Ningen Dock
This study was conducted using the large‐scale database belonging to Japan Society of Ningen Dock with the support from ‘Research Committee for the Large‐scale Database of Japan Society of Ningen Dock’. This research was conducted in compliance with Declaration of Helsinki. This study was approved by the Ethics Committee of Japan Society of Ningen Dock (approval number JSND‐EC: 2017‐0015) and Ethical Committee of Comprehensive Health Science Center (approval date December 28, 2018). Participants’ data were collected and anonymized at each facility, and datasets were sent to Japan Society of Ningen Dock. Requirement for written informed consent was waived, given the retrospective and anonymized nature of the study.
2.3. Measurements
The database consists of demographic, physical and laboratory data including body mass index (BMI) [weight (kg)/height (m)2], waist circumference (WC), systolic and diastolic blood pressure (BP), FPG, glycated haemoglobin (HbA1c), triglycerides, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol, serum creatinine and urinary protein. After an overnight fasting, complete medical check‐up was performed in the morning, and blood samples and random urine samples were obtained at each facility. BP was measured after 5 minutes of quiet rest, and mean of two BP measurements at a visit was used. All participants were required to complete self‐administered, standardized questionnaires during health check‐ups to assess for the current intake of medications for diabetes, hypertension and hyperlipidaemia; lifestyle habits such as smoking status; daily exercise; eating habit; and history of cardiac disease, stroke and kidney disease. The quality of health check‐up from each facility was standardized as all facilities passed the functional evaluation conducted by the Japan Society of Ningen Dock. In these facilities, health assessments were performed by doctors, nurses, dietitians, laboratory technicians and radiologists who undergo regular training. Therefore, the bias in the measurements is considered to be minimized. Hypertension was defined as BP of ≥140/90 mmHg or use of antihypertensive medications according to the Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019).18 Dyslipidaemia was defined as triglyceride level of ≥150 mg/dl (1.7 mmol/L), HDL cholesterol of ˂40 mg/dl (1.0 mmol/L), LDL cholesterol of ≥140 mg/dl (3.6 mmol/L) or use of lipid‐lowering medication according to the Japan Atherosclerosis Society guideline.19
2.4. Definition of prediabetes and diabetes
Plasma glucose level was measured using the hexokinase method. Following the criteria of the American Diabetes Association (ADA),20 participants were classified as having diabetes if their FPG values were ≥126 mg/dl (7.0 mmol/L) or if they were taking glucose‐lowering medications at baseline. Prediabetes was defined as FPG of 100–125 mg/dl (5.6–6.9 mmol/L) without taking glucose‐lowering medications at baseline. Those with normal glucose level (NGL) had FPG values of ˂100 mg/dl (5.6 mmol/L) without taking glucose‐lowering drugs at baseline.
In a sub‐analysis considering HbA1c levels, participants with HbA1c ≥ 48 mmol/mol (6.5%) at baseline were classified as having diabetes, in addition to the above definitions.
2.5. Estimated glomerular filtration rate and urinary protein
The serum creatinine level was measured with an enzymatic method using automated analysers. The eGFR was determined using the following equation proposed by the Japanese Society of Nephrology: eGFR (ml min−1 1.73 m−2) = 194 × age (years)−0.287 × serum creatinine (mg/dl)−1.094 (×0.739, if woman).21 This equation is reasonably accurate in estimating GFR for the Japanese populations as the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation or the Modification of Diet in Renal Disease equation leads to overestimation of GFR in Japanese populations. The eGFR decline was defined as ˂60 ml min−1 1.73 m−2.4
Urinary protein was detected via urine dipstick chemical analysis with protein errors of indicators, and the results were classified as negative, trace, 1+, 2+ and ≥3+. Dipstick urine tests can mainly detect albumin, and a finding of 1+ represents 30 mg/dl of albumin.22 Proteinuria was defined as dipstick urinary protein scores of ≥1+.23 In this study, proteinuria and eGFR decline were analysed individually to investigate whether prediabetes has different effects on these two outcomes.
2.6. Statistical analysis
To characterize the participants at baseline (2014), continuous data were presented as median (interquartile range) as all continuous data were non‐normally distributed. Categorical variables were reported as frequency (%).
Univariate and multivariate logistic regression analyses were performed to examine the relationship between the diabetic status and renal outcomes (proteinuria development or eGFR decline) after 2 years. Age, sex, BMI, eGFR, hypertension, dyslipidaemia, current smoking status, history of cardiac disease and stroke were used as covariates based on the guideline about chronic kidney disease (CKD)4 and data from previous reports.5, 23 Age, BMI and eGFR were used as continuous covariates. Results of the regression analyses were presented as estimated odds ratios (ORs) with corresponding 95% confidence intervals (CIs). p values of <0.05 were considered statistically significant. All analyses were performed using the SPSS Statistics v. 25 (IBM Corp.).
3. RESULTS
A total of 505,873 participants underwent complete annual health check‐ups in 2014. After excluding participants with eGFR of ˂60 ml min−1 1.73 m−2, urine protein of ≥1+ or kidney disease diagnosis at baseline and those without data on FPG, eGFR, urinary protein after 2 years, 405,487 participants were identified without CKD at baseline. Among them, 378,799 participants retained the kidney function, 7037 developed proteinuria (urine protein of ≥1+, with eGFR of ≥60 ml min−1 1.73 m−2), 19,015 presented eGFR decline (eGFR of ˂60 ml min−1 1.73 m−2, without proteinuria) and 636 showed both proteinuria and eGFR decline after 2 years (Figure 1).
Table 1 shows the participants’ baseline characteristics stratified according to the diabetic status. The prevalence of diabetes was 5.6%, and prediabetes was 28.8%. Participants with prediabetes and diabetes were older, more likely man, had a higher BMI, higher WC, higher systolic and diastolic BP, higher triglycerides, lower HDL cholesterol, and were more likely to have past history of cardiac disease and stroke and to be current smokers. Hypertension and dyslipidaemia were more common in individuals with prediabetes and diabetes accompanied by higher rate of using antihypertensive and lipid‐lowering medications.
TABLE 1.
Baseline characteristics of study participants stratified according to the diabetic status
| Total | NGL | Prediabetes | Diabetes | |
|---|---|---|---|---|
| N = 405,487 | N = 265,708 (65.5%) | N = 116,951 (28.8%) | N = 22,828 (5.6%) | |
| Man (%) | 61.9 | 54.2 | 75.6 | 82.2 |
| Age (years) | 50 (43–57) | 48 (42–55) | 53 (46–59) | 57 (51–63) |
| BMI (kg/m2) | 22.6 (20.6–24.9) | 22.0 (20.1–24.1) | 23.6 (21.7–25.9) | 25.0 (22.7–27.8) |
| WC (cm) | 81.6 (75.5–87.7) | 79.8 (74.0–85.5) | 84.5 (79.2–90.2) | 88.3 (82.5–95.2) |
| FPG (mmol/L) | 5.28 (4.94–5.72) | 5.06 (4.83–5.28) | 5.83 (5.67–6.11) | 7.50 (7.00–8.44) |
| HbA1c (mmol/mol) | 37 (34–39) | 36 (33–38) | 38 (36–41) | 51 (45–57) |
| HbA1c (%) | 5.5 (5.3–5.7) | 5.4 (5.2–5.6) | 5.6 (5.4–5.9) | 6.8 (6.3–7.4) |
| Systolic BP (mmHg) | 117 (106–128) | 114 (104–125) | 122 (112–133) | 126 (116–137) |
| Diastolic BP (mmHg) | 74 (66–82) | 71 (64–80) | 78 (70–85) | 79 (71–86) |
| Antihypertensive medication (%) | 13.8 | 8.9 | 19.6 | 40.6 |
| Prevalence of hypertension (%) | 24.2 | 17.1 | 34.6 | 54.0 |
| Triglycerides (mmol/L) | 1.01 (0.71–1.46) | 0.92 (0.66–1.32) | 1.18 (0.84–1.69) | 1.30 (0.90–1.90) |
| HDL cholesterol (mmol/L) | 1.58 (1.32–1.89) | 1.63 (1.37–1.94) | 1.50 (1.27–1.81) | 1.37 (1.17–1.66) |
| LDL cholesterol (mmol/L) | 3.16 (2.64–3.68) | 3.11 (2.59–3.63) | 3.26 (2.77–3.81) | 3.08 (2.56–3.60) |
| Lipid‐lowering medication (%) | 10.0 | 6.8 | 12.6 | 34.2 |
| Prevalence of dyslipidaemia (%) | 46.1 | 39.6 | 56.4 | 68.8 |
| eGFR (ml min−1 1.73 m−2) | 77.3 (69.9–86.1) | 77.8 (71.4–86.7) | 76.0 (69.0–84.6) | 77.8 (69.8–87.6) |
| Past history of | ||||
| Cardiac disease (%) | 2.3 | 1.8 | 2.9 | 5.7 |
| Stroke (%) | 1.0 | 0.8 | 1.3 | 2.5 |
| Current smokers (%) | 21.8 | 21.1 | 22.6 | 26.2 |
Continuous variables are expressed as median (interquartile range).
Abbreviations: BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; NGL, normal glucose level; WC, waist circumference.
Table 2 shows the prevalence of proteinuria development (urine protein of ≥1+) and eGFR decline (eGFR ˂60 ml min−1 1.73 m−2) after 2 years according to the diabetic status. After 2 years, proteinuria development occurred in 3914 individuals (1.5%) with NGL, 2266 (1.9%) with prediabetes, and 857 (3.8%) with diabetes at baseline. Decline in eGFR occurred in 11,225 individuals (4.2%) with NGL, 6492 (5.6%) with prediabetes and 1298 (5.7%) with diabetes. Individuals who presented both proteinuria development and eGFR decline after 2 years were 287 (0.1%) with NGL, 250 (0.2%) with prediabetes and 99 (0.4%) with diabetes.
TABLE 2.
Prevalence of proteinuria development (urine protein of ≥1+) and eGFR decline (eGFR of ˂60 ml min−1 1.73 m−2) after 2 years according to the diabetic status
| Total | NGL | Prediabetes | Diabetes | |
|---|---|---|---|---|
| N = 405,487 | N = 265,708 | N = 116,951 | N = 22,828 | |
| Proteinuria ≥1+ after 2 years | 7037 (1.7%) | 3914 (1.5%) | 2266 (1.9%) | 857 (3.8%) |
| eGFR <60 ml min−1 1.73 m−2 after 2 years | 19,015 (4.7%) | 11,225 (4.2%) | 6492 (5.6%) | 1298 (5.7%) |
| Proteinuria ≥1+ and eGFR <60 ml min−1 1.73 m−2 after 2 years | 636 (0.2%) | 287 (0.1%) | 250 (0.2%) | 99 (0.4%) |
Abbreviations: eGFR, estimated glomerular filtration rate; NGL, normal glucose level.
Table 3 shows the logistic regression analysis for the proteinuria development (urine protein of ≥1+) after 2 years. Compared to NGL, prediabetes was significantly associated with the proteinuria development in the unadjusted model (OR, 1.369; 95% CI, 1.302–1.439) and adjusted model (OR, 1.233; 95% CI, 1.170–1.301). Table 4 shows the logistic regression analysis for eGFR decline (˂60 ml min−1 1.73 m−2) after 2 years. Compared to NGL, prediabetes was associated with eGFR decline in the unadjusted model (OR, 1.351; 95% CI, 1.310–1.393), but not in the adjusted model (OR, 0.981; 95% CI, 0.947–1.017).
TABLE 3.
Association of prediabetes with proteinuria development (urine protein of ≥1+) after 2 years
| Unadjusted model | Adjusted modela | |||
|---|---|---|---|---|
| OR (95% CI) | p‐value | OR (95% CI) | p‐value | |
| Diabetic status (at baseline) | ||||
| NGL | Reference | Reference | ||
| Prediabetes | 1.369 (1.302–1.439) | <0.001 | 1.233 (1.170–1.301) | <0.001 |
| Diabetes | 2.721 (2.533–2.923) | <0.001 | 2.241 (2.069–2.428) | <0.001 |
Abbreviations: CI, confidence intervals; NGL, normal glucose level; OR, odds ratio.
Adjusted for sex, age, BMI, eGFR, hypertension, dyslipidaemia, smoking, past history of cardiac disease and stroke at baseline.
TABLE 4.
Association of prediabetes with eGFR decline (eGFR of ˂60 ml min−1 1.73 m−2) after 2 years
| Unadjusted model | Adjusted modela | |||
|---|---|---|---|---|
| OR (95% CI) | p‐value | OR (95% CI) | p‐value | |
| Diabetic status (at baseline) | ||||
| NGL | Reference | Reference | ||
| Prediabetes | 1.351 (1.310–1.393) | <0.001 | 0.981 (0.947–1.017) | 0.294 |
| Diabetes | 1.439 (1.359–1.524) | <0.001 | 1.050 (0.982–1.123) | 1.050 |
Abbreviations: eGFR, estimated glomerular filtration rate; NGL, normal glucose level; OR, odds ratio; CI, confidence intervals.
Adjusted for sex, age, BMI, eGFR, hypertension, dyslipidaemia, smoking, past history of cardiac disease and stroke at baseline.
Another logistic regression analysis for the composite renal outcome (either proteinuria development or eGFR decline) was performed. Compared to NGL, prediabetes was statistically significantly associated with the composite renal outcome in the adjusted model (OR, 1.036; 95% CI, 1.006–1.067) (Table 5).
TABLE 5.
Association of prediabetes with the composite renal outcome [either proteinuria development (urine protein of ≥1+) or eGFR decline (˂60 ml min−1 1.73 m−2)] after 2 years
| Unadjusted model | Adjusted modela | |||
|---|---|---|---|---|
| OR (95% CI) | p‐value | OR (95% CI) | p‐value | |
| Diabetic status (at baseline) | ||||
| NGL | Reference | Reference | ||
| Prediabetes | 1.358 (1.318–1.391) | <0.001 | 1.036 (1.006–1.067) | 0.019 |
| Diabetes | 1.778 (1.697–1.862) | <0.001 | 1.362 (1.294–1.435) | <0.001 |
Abbreviations: CI, confidence intervals; eGFR, estimated glomerular filtration rate; NGL, normal glucose level; OR, odds ratio.
Adjusted for sex, age, BMI, eGFR, hypertension, dyslipidaemia, smoking, past history of cardiac disease and stroke at baseline.
We also performed a sub‐analysis when participants with HbA1c ≥ 48 mmol/mol (6.5%) at baseline were classified as having diabetes. Logistic regression analysis in the adjusted model showed that prediabetes was associated with proteinuria development and the composite renal outcome, whereas it was not associated with eGFR decline as compared to NGL (Tables S1‐S3). In addition, we performed a population sub‐analysis with the same diabetic status both at baseline (2014) and after 2 years (2016) to eliminate the effects of changes in diabetic status >2 years. Logistic regression analysis in the adjusted model revealed that the group with maintained prediabetes was associated with proteinuria development and composite renal outcome, whereas it was not associated with eGFR decline compared with the group that maintained NGL (Tables S4‐S6).
4. DISCUSSION
This is the largest study that investigated the association of prediabetes with proteinuria development and eGFR decline separately among 405,487 individuals, and clearly demonstrated that prediabetes was independently associated with the proteinuria development but not with eGFR decline after 2 years.
While most previous studies defined CKD as the combination of albuminuria (or proteinuria) and low eGFR,10, 24, 25, 26 we investigated the association of prediabetes with the proteinuria development and with eGFR decline separately in this study. Our data demonstrated that prediabetes was independently associated with the proteinuria development; however, prediabetes when adjusted for prognostic factors was not associated with eGFR decline. We also showed that prediabetes was associated with the composite renal outcome (defined as the combination of proteinuria and eGFR decline). These results are consistent with a previous meta‐analysis (mean duration of follow‐up, 6 years) showing that prediabetes is moderately associated with CKD (defined as the combination of albuminuria [or proteinuria] and low eGFR),10 as well as with majority of cohort studies (duration of follow‐up: 3.8–8 years) reporting that prediabetes is not associated with low eGFR alone.11, 12, 13 People with prediabetes present with hyperfiltration.27 This might be one of the reasons why eGFR decline was not observed in individuals with prediabetes after 2 years.
A recent study, a secondary analysis of the Systolic Blood Pressure Intervention Trial (SPRINT), investigated the association of impaired fasting glucose with albuminuria and worsening eGFR, and reported that impaired fasting glucose was not associated with the albuminuria development or worsening eGFR.28 In the study, all participants had hypertension and at least one additional CVD risk factor at baseline, which are factors leading to the albuminuria development. Thus, prediabetes may not be an independent additional risk factor for the albuminuria development among participants who are already at high risk for it. Our study population consisted of participants undergoing a health check‐up with lower rate of hypertension and lower risk for CVD. Thus, prediabetes is suggested to be associated with the proteinuria development among patients in the general population and that proteinuria development is an earlier detectable renal change in patients with prediabetes.
Whether pathological changes occur in the kidney in patients with prediabetes remains unclear. A recent study with kidney biopsies reported that increasing albuminuria was significantly associated with increased glomerular basement membrane width, mesangial fractional volume and mean glomerular volume, whereas GFR decline did not reflect such kidney lesions.29 In the study, it was concluded that albuminuria level was more informative for predicting structural changes in the kidneys during the earlier stage of diabetic nephropathy when GFR is >60 ml/min. Our results showed that proteinuria development, not eGFR decline, was an early clinical renal change in prediabetes. Thus, it is possible that renal pathological changes in the earlier stage of diabetic nephropathy also occur in patients with prediabetes, given that prediabetes and diabetes are considered as the continuum from prehyperglycaemia to hyperglycaemia. However, further pathological studies are needed to prove this hypothesis.
Regarding the strengths of our study, a large general population was analysed throughout Japan, including a large number of participants with prediabetes or NGL. In addition, the data quality control was rigorous and standardized as all facilities passed the functional evaluation conducted by the Japan Society of Ningen Dock. However, several limitations still exist in this study. First, as the diagnosis of NGL, prediabetes and diabetes was based on a single assessment of FPG, there is a limit to the accuracy of the classification. For example, some participants defined to have prediabetes may have FPG levels reaching the diagnostic threshold of diabetes the following day. However, we also performed analyses when participants with HbA1c ≥ 48 mmol/mol (6.5%) at baseline were classified as having diabetes, and the results were consistent with those of the main analyses. Second, this study only included individuals who had health check‐up data after 2 years. Of 412,391 participants who had health check‐up data and did not present with CKD at baseline, 6904 were excluded due to the lack of data after 2 years. However, this number was relatively smaller than that of individuals who were included in the analysis (n = 405,487). In addition, individuals who did not have data after 2 years (n = 6904) commonly included those who had retired, transferred or changed jobs. Therefore, it was unlikely that selection bias affected the results on kidney function after 2 years. Third, the urine dipstick test was used to evaluate proteinuria in this study. It was not quantitative and false‐positive results are possible under certain conditions, such as highly concentrated or alkaline urine.30 However, previous studies considered urine dipsticks ≥1+ as proteinuria development,2, 15, 23 and urine dipstick tests are widely used in the health check‐up and are more economical for screening. Fourth, since the details of antihypertensive medications were unknown in this study, a potential effect on proteinuria development of these medications (e.g. renin–angiotensin–aldosterone system blockade) was not considered. Fifth, as the study population consisted of Japanese people, in which ethnicity is limited, confirmation in other ethnic groups is needed. Finally, the long‐term effect of prediabetes on renal outcomes was not examined because of the short follow‐up period.
In conclusion, prediabetes was found to be associated with the proteinuria development but not with eGFR decline in the general population without CKD after 2 years. Proteinuria in prediabetes might be an earlier detectable renal change, and the screening for prediabetes and evaluation of proteinuria are important for the prevention of CKD. Future prospective studies examining whether lifestyle interventions for prediabetes is effective for the improvement of proteinuria are needed to prove the importance of evaluating proteinuria in prediabetes.
CONFLICT OF INTEREST
None declared.
AUTHORS’ CONTRIBUTIONS
MF and KT conceptualized the study and designed the analysis plan. KK, TW and YS contributed to the data construction. MF and FK performed all the statistical analyses. MF drafted the manuscript. All co‐authors, MF, KT, KK, TW, YS, FK, TO, MG and HA, contributed to the acquisition, analysis or interpretation of data; provided critical revision of the manuscript for important intellectual content and contributed to the discussion; and approve the final version of this manuscript. MF is the guarantor of this work.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We thank all the people at the facilities, who kindly provided the data for individuals receiving annual health examinations. This study was conducted by Research Committee for the Large‐scale Database of Japan Society of Ningen Dock. Japan Society of Ningen Dock financially supported this study in database construction, but they were not involved in this research activity.
Furukawa M, Onoue T, Kato K, et al. Prediabetes is associated with proteinuria development but not with glomerular filtration rate decline: A longitudinal observational study. Diabet Med. 2021;38:e14607. 10.1111/dme.14607
Funding information
The Japan Society of Ningen Dock financially supported this study in terms of database construction. However, they were not involved in the research activity.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1.Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238‐1252. [DOI] [PubMed] [Google Scholar]
- 2.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all‐cause and cardiovascular mortality in general population cohorts: a collaborative meta‐analysis. Lancet. 2010;375:2073‐2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hallan SI, Matsushita K, Sang Y, et al. Age and association of kidney measures with mortality and end‐stage renal disease. JAMA. 2012;308:2349‐2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.KDIGO . Chapter 2: definition, identification, and prediction of CKD progression. Kidney Int Suppl. 2013;3:63‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165‐180. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020. [Google Scholar]
- 7.Davis TM, Stratton IM, Fox CJ, Holman RR, Turner RC. U.K. Prospective Diabetes Study 22. Effect of age at diagnosis on diabetic tissue damage during the first 6 years of NIDDM. Diabetes Care. 1997;20:1435‐1441. [DOI] [PubMed] [Google Scholar]
- 8.Kohler KA, McClellan WM, Ziemer DC, Kleinbaum DG, Boring JR. Risk factors for microalbuminuria in black Americans with newly diagnosed type 2 diabetes. Am J Kidney Dis. 2000;36:903‐913. [DOI] [PubMed] [Google Scholar]
- 9.Spijkerman AM, Dekker JM, Nijpels G, et al. Microvascular complications at time of diagnosis of type 2 diabetes are similar among diabetic patients detected by targeted screening and patients newly diagnosed in general practice: the hoorn screening study. Diabetes Care. 2003;26:2604‐2608. [DOI] [PubMed] [Google Scholar]
- 10.Echouffo‐Tcheugui JB, Narayan KM, Weisman D, Golden SH, Jaar BG. Association between prediabetes and risk of chronic kidney disease: a systematic review and meta‐analysis. Diabet Med. 2016;33:1615‐1624. [DOI] [PubMed] [Google Scholar]
- 11.Fox CS, Larson MG, Leip EP, Meigs JB, Wilson PW, Levy D. Glycemic status and development of kidney disease: the Framingham Heart Study. Diabetes Care. 2005;28:2436‐2440. [DOI] [PubMed] [Google Scholar]
- 12.Ryu S, Chang Y, Woo H‐Y, et al. Time‐dependent association between metabolic syndrome and risk of CKD in Korean men without hypertension or diabetes. Am J Kidney Dis. 2009;53:59‐69. [DOI] [PubMed] [Google Scholar]
- 13.Schottker B, Brenner H, Koenig W, Muller H, Rothenbacher D. Prognostic association of HbA1c and fasting plasma glucose with reduced kidney function in subjects with and without diabetes mellitus. Results from a population‐based cohort study from Germany. Prev Med. 2013;57:596‐600. [DOI] [PubMed] [Google Scholar]
- 14.Yamanouchi M, Furuichi K, Hoshino J, et al. Nonproteinuric versus proteinuric phenotypes in diabetic kidney disease: a propensity score‐matched analysis of a nationwide. Biopsy‐based cohort study. Diabetes Care. 2019;42:891‐902. [DOI] [PubMed] [Google Scholar]
- 15.Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end‐stage renal disease in individuals with and without diabetes: a meta‐analysis. Lancet. 2012;380:1662‐1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimizu M, Furuichi K, Yokoyama H, et al. Kidney lesions in diabetic patients with normoalbuminuric renal insufficiency. Clin Exp Nephrol. 2014;18:305‐312. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda N, Saito E, Kondo N, et al. What has made the population of Japan healthy? Lancet. 2011;378:1094‐1105. [DOI] [PubMed] [Google Scholar]
- 18.Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42:1235‐1481. [DOI] [PubMed] [Google Scholar]
- 19.Kinoshita M, Yokote K, Arai H, et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb. 2018;25:846‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes‐2019. Diabetes Care. 2019;42:S13‐S28. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982‐992. [DOI] [PubMed] [Google Scholar]
- 22.Orita Y, Gejou F, Ito Y, et al. Report on evaluation of clinical laboratory examination of GFR and urinary protein. [Translated from Japanese] Japanese J Nephrol. 2001;43:1‐19. [PubMed] [Google Scholar]
- 23.National Kidney Foundation . K/DOQI clinical practice guidelines for chronic kidney disease evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1‐266. [PubMed] [Google Scholar]
- 24.Lucove J, Vupputuri S, Heiss G, North K, Russell M. Metabolic syndrome and the development of CKD in American Indians: the Strong Heart Study. Am J Kidney Dis. 2008;51:21‐28. [DOI] [PubMed] [Google Scholar]
- 25.Jadhakhan F, Marshall T, Ryan R, Gill P. Risk of chronic kidney disease in young adults with impaired glucose tolerance/impaired fasting glucose: a retrospective cohort study using electronic primary care records. BMC Nephrol. 2018;19:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tozawa M, Iseki C, Tokashiki K, et al. Metabolic syndrome and risk of developing chronic kidney disease in Japanese adults. Hypertens Res. 2007;30:937‐943. [DOI] [PubMed] [Google Scholar]
- 27.Okada R, Yasuda Y, Tsushita K, Wakai K, Hamajima N, Matsuo S. Glomerular hyperfiltration in prediabetes and prehypertension. Nephrol Dial Transplant. 2012;27:1821‐1825. [DOI] [PubMed] [Google Scholar]
- 28.Vieira MB, Neves JS, Leitao L, et al. Impaired fasting glucose and chronic kidney disease, albuminuria, or worsening kidney function: a secondary analysis of the SPRINT. J Clin Endocrinol Metab. 2019;104:4024‐4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Looker HC, Mauer M, Saulnier P‐J, et al. Changes in albuminuria but not GFR are associated with early changes in kidney structure in type 2 diabetes. J Am Soc Nephrol. 2019;30:1049‐1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White SL, Yu R, Craig JC, Polkinghorne KR, Atkins RC, Chadban SJ. Diagnostic accuracy of urine dipsticks for detection of albuminuria in the general community. Am J Kidney Dis. 2011;58:19‐28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


