Abstract
Aims
Sodium–glucose co‐transporter 2 (SGLT2) inhibitors, originally developed as glucose‐lowering agents, have been shown to reduce heart failure hospitalizations in patients with type 2 diabetes without established heart failure, and in patients with heart failure with and without diabetes. Their role in patients with heart failure with preserved and mildly reduced ejection fraction remains unknown.
Methods
Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction Heart Failure (DELIVER) is an international, multicentre, parallel group, event‐driven, randomized, double‐blind trial in patients with chronic heart failure and left ventricular ejection fraction (LVEF) >40%, comparing the effect of dapagliflozin 10 mg once daily, vs. placebo, in addition to standard of care. Patients with or without diabetes, with signs and symptoms of heart failure, a LVEF >40%, elevation in natriuretic peptides and evidence of structural heart disease are eligible. The primary endpoint is time‐to‐first cardiovascular death or worsening heart failure event (heart failure hospitalization or urgent heart failure visit), and will be assessed in dual primary analyses – the full population and in those with LVEF <60%. The study is event‐driven and will target 1117 primary events. A total of 6263 patients have been randomized.
Conclusions
DELIVER will determine the efficacy and safety of the SGLT2 inhibitor dapagliflozin, added to conventional therapy, in patients with heart failure and preserved and mildly reduced ejection fraction.
Keywords: Heart failure with preserved ejection fraction, Sodium–glucose co‐transporter 2 inhibitors
Introduction
Sodium–glucose co‐transporter 2 (SGLT2) inhibitors, originally developed as glucose‐lowering agents, have been shown to reduce heart failure hospitalizations in patients with type 2 diabetes, including those without established heart failure.1, 2, 3 Moreover, in patients with heart failure and reduced left ventricular ejection fraction (LVEF ≤40%; HFrEF), including those with and without type 2 diabetes, both dapagliflozin and empagliflozin reduced cardiovascular death or heart failure events when added to standard therapy.4, 5 While the mechanisms by which SGLT2 inhibitors improve outcomes in heart failure continue to be investigated, they are postulated to include favourable effects on haemodynamics,6, 7 improvement in myocardial energetics and loading conditions, favourable effects on endothelial function and inflammation, and slowing of the progression of kidney disease.8 These effects may collectively underlie observed early and sustained improvements in filling pressures and ventricular remodeling.7, 9, 10, 11
Patients with preserved or mildly reduced ejection fraction (LVEF >40%) now represent the majority of those with heart failure, and experience a comparable burden of poor outcomes, such as death, hospitalizations and symptom burden, as those with LVEF ≤40%; yet suffer from dearth of effective therapies. Therefore, there is a large and urgent unmet clinical need for efficacious and safe treatments in this vulnerable patient group. Whether the benefits of SGLT2 inhibitors observed in HFrEF extend to patients with heart failure and LVEF >40% remains unknown. The benefit of dapagliflozin in DAPA‐HF was similar throughout the ejection fraction spectrum under 40%,12 and data from two trials of a combined SGLT1 and 2 inhibitor, including one that enrolled recently hospitalized patients with diabetes and heart failure, suggest potential benefits in people with LVEF >40%.13, 14 Nevertheless, most heart failure therapies that have proven effective in patients with LVEF <40% have been ineffective or significantly less effective in those with higher LVEF, with several studies showing some attenuation of benefit as LVEF rose into the normal range.15, 16, 17, 18 The heterogeneity of the heart failure with preserved ejection fraction (HFpEF) syndrome has emerged as a key hypothesis underlying the inability to identify treatments that reduce its morbidity and mortality. While HFrEF is also a heterogeneous disorder, it has proven to respond to therapies in a more homogeneous fashion, with multiple drug classes associated with improvements in morbidity and mortality. Many of these benefits appear to extend to heart failure with mildly reduced LVEF (40–50%, HFmrEF) but not for LVEF >50%. However, there is reason to believe that SGLT2 inhibitors may be beneficial in a broad spectrum of HFpEF despite the heterogeneous nature of the HFpEF syndrome. Congestion and impaired renal function are hallmarks of all types of heart failure, including HFpEF, and appear to be ameliorated by SGLT2 inhibitors. In addition, chronic kidney disease is a major risk factor for adverse outcomes in HFpEF; therefore, it is very possible that by improving kidney function, SGLT2 inhibitors may have beneficial effects across the range of LVEF. SGLT2 inhibitors also appear to improve diastolic function, reduce visceral fat (including epicardial fat), reduce arterial stiffness, and have favourable effects on endothelial function and inflammation, all of which are important mechanisms of HFpEF pathogenesis.
The Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction Heart Failure (DELIVER) trial is testing the hypothesis that the SGLT2 inhibitor dapagliflozin will reduce cardiovascular death and heart failure hospitalization in patients with heart failure with a LVEF >40% (HFpEF and HFmrEF ). The design of DELIVER takes into account many of the learnings from prior trials in heart failure with LVEF >40% and, along with DAPA‐HF, will provide evidence for the efficacy of dapagliflozin across the full spectrum of LVEF in patients with heart failure.
Trial design and methods
Overall study design and governance
DELIVER is an international, multicentre, parallel group, event‐driven, randomized, double‐blind trial in patients with chronic heart failure and LVEF >40%, comparing the effect of dapagliflozin 10 mg once daily, vs. placebo, in addition to standard of care. The overall study design is summarized in Figure 1. DELIVER was designed jointly by the academic steering committee in conjunction with the sponsor. The conduct of the trial is overseen by the academic executive committee and the sponsor in conjunction with national lead investigators. The trial is registered as ClinicalTrials.gov Identifier: NCT03619213.
Figure 1.
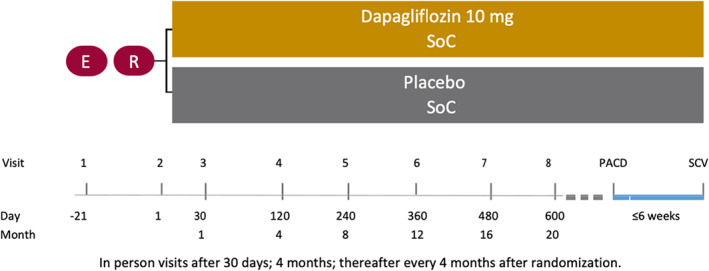
Study design of DELIVER. PACD, primary analysis censoring date; SCV, study closure visit; SoC, standard of care.
Patients
The eligibility criteria for DELIVER are summarized in Table 1. Briefly, patients with or without diabetes were required to be 40 years of age or older, with an LVEF >40% (documented by echocardiography or cardiac magnetic resonance imaging within the last 12 months prior to enrolment without a subsequent event that might lower LVEF), evidence of structural heart disease (either left atrial enlargement or left ventricular hypertrophy), and elevation in natriuretic peptides [N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) ≥300 pg/mL (≥600 pg/mL for patients in atrial fibrillation or flutter)]. Both ambulatory and hospitalized patients were eligible for enrolment.
Table 1.
Eligibility criteria for DELIVER
| Inclusion criteria |
|
| Exclusion criteria |
|
CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; LA, left atrial; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; SGLT2, sodium–glucose co‐transporter 2.
Key exclusion criteria included receiving an SGLT2 inhibitor within 4 weeks prior to randomization, or previous intolerance to SGLT2 inhibitors; type 1 diabetes; estimated glomerular filtration rate (eGFR) <25 mL/min/1.73 m2 at screening; systolic blood pressure ≥160 mmHg if not on three or more antihypertensive medications, or ≥180 mmHg regardless of number of medications; probable alternative diagnoses that might account for the patients' symptoms (e.g. anaemia, hypothyroidism, primary pulmonary hypertension, chronic thromboembolic disease, requirement for home oxygen therapy); uncorrected primary valvular disease; known infiltrative heart disease, including known or suspected amyloid heart disease; myo‐ or pericarditis; or hypertrophic cardiomyopathy.
Enrolment in DELIVER began on 27 August 2018 following approval by appropriate ethics boards, and written informed consent was obtained from all patients enrolled in DELIVER. The last patient was randomized on 18 January 2021. Patients are enrolled at 353 sites, in 20 countries in most major geographic regions (online supplementary Table S1). The study is being conducted in accordance with Good Clinical Practice and the Declaration of Helsinki.
Study procedures
Randomization and capping
Following informed consent and screening, and once a patient has fulfilled the criteria for randomization, all patients were centrally assigned to randomized investigational product (IP) using an interactive voice/web response system (IxRS). Randomization to IP was performed in balanced blocks to ensure approximate balance between the treatment groups (1:1). Randomization was stratified in the IxRS system based on whether the patient was or was not known to have type 2 diabetes at the time of randomization (based on either an established diagnosis or glycated haemoglobin ≥6.5% at enrolment). Patients were randomized in a 1:1 fashion to dapagliflozin 10 mg or matching placebo once daily. Several factors, including LVEF value, New York Heart Association (NYHA) class, ‘subacute’ status (randomized in‐hospital or within 30 days after discharge), and atrial fibrillation were monitored to enable capped randomization in the IxRS to avoid over or under‐representation of these patient subgroups in each country.
Concomitant medications
Patients are treated according to regional standard of care for all comorbidities, including diabetes and hypertension, with the exception of concomitant use of an SGLT2 inhibitor, which is not allowed by protocol.
Study visits and monitoring
Following randomization, study visits occur at or around days 30, 120, 240, 360, and 480 after randomization, and then every 120 days thereafter. Unscheduled visits can also be performed if considered appropriate in the opinion of the investigator. The full schedule of assessments is shown in online supplementary Table S2 . Treatment adherence is assessed by asking patients to return all unused investigational product and empty packages to the clinic at site visits, and non‐compliant patients are counselled on the importance of taking study medication.
Study outcomes
Primary and other outcomes
The primary objective is to determine whether dapagliflozin is superior to placebo, when added to standard of care, in reducing the composite of worsening heart failure episodes (either unplanned hospitalization or urgent heart failure visit requiring intravenous therapy but not requiring a hospital admission) or cardiovascular death, analysed as time‐to‐first event (Table 2). The primary endpoint will be assessed in both the full population and in patients with LVEF <60% (dual primary analyses; see statistical analysis below). Secondary objectives are to determine whether dapagliflozin is superior to placebo in reducing the total number of heart failure events (hospitalization for heart failure or urgent heart failure visit) and cardiovascular death in (i) the full study population, and (ii) the sub‐population with LVEF <60%; in improving patient‐reported outcomes measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ) total symptom score (TSS); in reducing cardiovascular death; in reducing all‐cause mortality. Several exploratory endpoints will also be assessed (online supplementary Table S3 ): whether dapagliflozin compared with placebo will have an effect on slope of eGFR (assessed as between randomization and 1 and 2 years, respectively, and between a post‐randomization time point and 1 and 2 years, respectively); and assessment of benefits within the sub‐domains of the KCCQ, stratified by type 2 diabetes status at randomization, and adjusted for the baseline value.
Table 2.
Primary and secondary study objectives and endpoints
| Study objective | Corresponding endpoint |
|---|---|
| Primary objective | |
| To determine whether dapagliflozin is superior to placebo, when added to standard of care, in reducing the composite of CV death and HF events (hospitalization for HF or urgent HF visit) in patients with HF and preserved systolic function in (i) the full study population, and (ii) the sub‐population with LVEF <60% | Time to the first occurrencea of any of the components of this composite:1. CV death2. Hospitalization for HF3. Urgent HF visit (e.g. emergency department or outpatient visit) |
| Secondary objectives | |
| To determine whether dapagliflozin is superior to placebo in reducing the total number of HF events (hospitalization for HF or urgent HF visit) and CV death in (i) the full study population, and (ii) the sub‐population with LVEF <60% |
Total numberb of HF events (first and recurrent) and CV death |
| To determine whether dapagliflozin is superior to placebo in improving patient‐reported outcomes measured by KCCQ | Change from baseline in the total symptom score of the KCCQ at 8 months |
| To determine whether dapagliflozin is superior to placebo in reducing CV death | Time to the occurrence of CV death |
| To determine whether dapagliflozin is superior to placebo in reducing all‐cause mortality | Time to the occurrence of death from any cause |
| Safety objective | |
| To evaluate the safety and tolerability of dapagliflozin compared to placebo in patients with HFpEF | Serious AEs, AEs leading to treatment discontinuation, amputations, AEs leading to amputation and potential risk factor for AEs leading to amputations affecting the lower limbs |
AE, adverse event; CV, cardiovascular; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction.
Analysis using Cox regression stratified by type 2 diabetes at baseline.
Analysis performed using the semi‐parametric method of Lin, Wei, Yang and Ying (LWYY).
Endpoint adjudication
An independent Cardiovascular Endpoint Committee (CEC), blinded to treatment assignment, is categorizing all deaths and adjudicating non‐fatal cardiovascular events as possible endpoints based on the 2017 Cardiovascular and Stroke Endpoint Definitions for Clinical Trials developed by the Standardized Data Collection for Cardiovascular Trials Initiative.19 All potential episodes of possible ketoacidosis are also being adjudicated.
Accommodation for COVID‐19
As the COVID‐19 pandemic evolved during the course of the study, the trial has made several adaptations to ensure the quality and integrity of the data collected. These included as necessary conversion of in‐person visits to phone and virtual visits, remote data collection for patient‐reported outcomes, reporting of all COVID‐19 related adverse events and adjudication of COVID‐19 related hospitalizations and deaths.
Statistical considerations
Sample size considerations and statistical analyses
The primary objective of the study is to determine the superiority of dapagliflozin vs. placebo added to standard of care in reducing the composite of worsening heart failure events (hospitalization for heart failure or urgent heart failure visit) or cardiovascular death, measured as time‐to‐first occurrence of any of the components of the composite. Two hypotheses will be tested simultaneously (i.e. dual primary analyses) for the primary analysis: (i) in the full population and (ii) in the population with LVEF <60%. Alpha will be allocated to each primary test to ensure control of the overall type I error rate, with the exact alpha split determined prior to the interim analysis. The study protocol was modified on 12 November 2020 introducing the current dual primary analysis, in which cardiovascular death or worsening heart failure events will be evaluated in both the full study population (as original primary analysis) and the population with LVEF <60%. For the original analysis, 844 primary events were targeted to provide 90% power for the primary endpoint. To allow for testing the dual primary hypotheses, the target number of patients with a primary endpoint was subsequently increased to 1117 to provide adequate statistical power for each of the two dual primary analyses. The original targeted sample size of 4700 patients was increased to 6100 based on blinded monitoring of event accrual and the increase in target number of primary events. It is anticipated that at least 70% of the primary endpoint events (i.e. approximately 780 events) will be contributed by the LVEF <60% sub‐population. A total sample size of 6100 patients is anticipated to provide 93% power to detect a 20% relative risk reduction for the primary endpoint for a two‐sided nominal alpha of 0.024. Recruitment was completed on 21 January 2021 with a total of 6263 patients randomized. The anticipated median follow‐up will be 27 months. All analyses will be according to the intention‐to‐treat principle. Full details of all analyses will be provided in a statistical analysis plan prior to unblinding of the trial.
Methods of statistical analysis
A closed testing procedure including a pre‐specified hierarchical ordering of the primary and secondary endpoints will be utilized. Statistical significance will be assessed in two branches in the pre‐specified order of the endpoints and populations as specified in Figure 2. Following the dual assessment of the primary endpoint (time‐to‐first event for the composite endpoint of cardiovascular death and worsening heart failure event) in both the full population, and in those with LVEF <60%, subsequent testing will occur in two parallel paths as shown. If the null hypothesis is rejected in the full population for the primary analysis analysed using a Cox proportional hazards model, then testing will occur for recurrent events in the full population using the LWYY method, followed by comparison of change from baseline to 8 months in the KCCQ‐TSS using the rank ANCOVA method (to test difference in distribution) and the win ratio test (to estimate treatment effect), followed by comparison of time to cardiovascular death, followed by time to all‐cause death in Cox regression analyses. If the null hypothesis is rejected in the sub‐population with LVEF <60%, total and recurrent events will be assessed first in the sub‐population and subsequently in the full population, with allocation of alpha for each successive positive test. If all null hypotheses are rejected within a particular branch, the alpha is recycled to the other branch where the hypotheses will be tested with full alpha.
Figure 2.
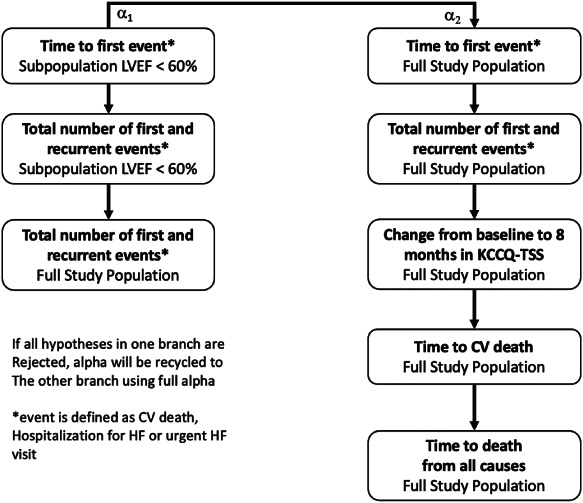
Hierarchical testing scheme for primary and secondary endpoints. CV, cardiovascular; HF, heart failure; KCCQ‐TSS, Kansas City Cardiomyopathy Questionnaire total symptom score; LVEF, left ventricular ejection fraction.
Analysis of secondary endpoints
Sub‐domains of the KCCQ‐TSS (symptom frequency and symptom burden) and overall symptom score (OSS) will be analysed with rank ANCOVA and win ratio in the same manner as for TSS. Descriptive statistics of scores and changes from baseline will be presented for all scores.
Subgroup analyses
The following pre‐specified sub‐groups of interest will be assessed for the dual primary endpoints: age at vs. below, vs. above, the median; sex; race; geographic region; NYHA class (II vs. III/IV) at enrolment; LVEF category at enrolment (41–49% vs. 50–59%, and ≥60%); NT‐proBNP at enrolment (at or below, vs. above the median); randomization in hospital or within 30 days of discharge vs. others; eGFR at enrolment (<60 vs. ≥60 mL/min/1.73 m2); body mass index at enrolment (<30 vs. ≥30 kg/m2); diabetes status at enrolment; systolic blood pressure at randomization (at or below vs. above the median); atrial fibrillation or flutter vs. other rhythms at enrolment. The effect of treatment will also be assessed as a function of LVEF and glycated haemoglobin examined as continuous variables.
In addition to the within trial analyses, we have pre‐specified prior to the unblinding of DAPA‐HF that data from both dapagliflozin heart failure trials, DAPA‐HF and DELIVER, will be pooled and assessed in a patient‐level meta‐analysis to assess the effect of dapagliflozin across the full spectrum of heart failure.
Data monitoring committee and interim analysis
A data safety monitoring committee is overseeing the trial and will undertake one interim analysis when approximately 67% of the target number of primary endpoints are reached, where the primary composite endpoint will be tested in the full study population at a significance level of 0.2%. If the null hypothesis is rejected, superiority of dapagliflozin to placebo on cardiovascular death will be tested at the same significance level.
Discussion
Heart failure with LVEF >40% (HFpEF and HFmrEF) represents a large group of patients without clear guideline‐directed therapy with great unmet need. SGLT2 inhibitors are the first treatments being tested for heart failure with LVEF >40% that are not neurohormonal modulators. This might give this class an advantage over previously tested agents for both efficacy and safety. While vasodilator‐type agents clearly benefit patients with HFrEF, peripheral vasodilatation may provide less benefit and may be associated with more hypotension in patients with higher LVEF. On the other hand, several of the mechanisms by which SGLT2 inhibitors have been postulated to be beneficial in HFrEF would be expected to be similarly beneficial in patients with heart failure and higher LVEF, such as improvements in filling pressures and ventricular remodelling,9, 10, 11 and kidney benefits.8 Furthermore, SGLT2 inhibitors have been shown to improve diastolic function in patients with diabetes and LVEF ≥50%,20 reduce obesity and attenuate epicardial fat accumulation or its secretion of deleterious adipokines,21 as well as improve endothelial function and reduce inflammation – mechanistic factors particularly associated with heart failure in the setting of higher LVEF. Indeed, we observed no heterogeneity in the treatment response to dapagliflozin based on LVEF in patients with HFrEF, and more recent data from SOLOIST‐WHF and SCORED suggest that therapy with sotagliflozin benefited recently hospitalized patients with HFpEF.13, 14
Clinical trials in HFpEF have been challenging for several reasons, including difficulties in ensuring enrolment of the appropriate patients who truly have the clinical syndrome of heart failure, and because of the phenotypic, biological and likely therapeutic heterogeneity of the disease. All prior outcomes trials in this population to date have fallen short of demonstrating a convincing therapeutic benefit on their primary endpoint. Most recently, the PARAGON‐HF trial, which compared sacubitril/valsartan to valsartan, narrowly missed statistical significance for the primary endpoint of total heart failure hospitalizations and cardiovascular death.17 However, the data from PARAGON‐HF suggested that patients with LVEF at or below the pre‐specified median of 57% had a greater treatment benefit than those with higher LVEF, and this finding was confirmed when LVEF was assessed continuously. This pattern of declining benefit with increasing LVEF has also been observed in two other clinical trials, TOPCAT which compared spironolactone to placebo, and CHARM‐Preserved which compared candesartan to placebo.15, 16 Whether declining benefit with increasing LVEF is unique to these prior treatment approaches that utilized neurohormonal modulators or is in fact a general characteristic of patients with heart failure with LVEF >40%, is unknown. That prior HFpEF trials have shown that treatment effect with a broad range of therapies declined with increasing LVEF provided the rationale for the dual primary analysis incorporated into DELIVER.
The design of DELIVER is unique in several ways. First, DELIVER was designed to complement DAPA‐HF which assessed the efficacy of dapagliflozin in patients with HFrEF. The results of both studies will be pooled to assess the effects of dapagliflozin across the spectrum of ejection fraction. The entry criteria reflected the contemporary view that patients with heart failure should have both elevation of natriuretic peptides and evidence of structural heart disease (Table 3).17, 22, 23, 24, 25, 26 In contrast to the PARAGON‐HF and TOPCAT trials which were restricted to patients with LVEF ≥45% who had never had LVEF <40%, DELIVER is enrolling patients with LVEF >40%, and is allowing patients with previous LVEF ≤40%. This will allow for a wide range of patients with mildly reduced ejection fraction. Second, the primary outcome will incorporate both heart failure hospitalizations and urgent heart failure visits. Urgent heart failure visits, requiring evidence of intravenous diuretic therapy, have been a component of the primary endpoint of several prior heart failure trials, including DAPA‐HF, and have proven to be both prognostically similar to heart failure hospitalizations and similarly discriminative of treatment effects in several trials.27, 28 Moreover, with increasing needs for outpatient management of worsening heart failure due to changes in healthcare care delivery and patient preferences, urgent heart failure visits are logical for inclusion in the primary endpoint.
Table 3.
Comparison of DELIVER and other trials in heart failure with left ventricular ejection fraction >40%
| CHARM‐Preserved22 | PEP‐CHF23 | I‐PRESERVE24 | TOPCAT25 | PARAGON‐HF17 | EMPEROR‐Preserved26 | DELIVER | |
|---|---|---|---|---|---|---|---|
| Patients, n | 3023 | 850 | 4128 | 3445 | 4800 | 5988 | 6200 |
| Treatment arms | Candesartan vs. placebo | Perindopril vs. placebo | Irbesartan vs. placebo | Spironolactone vs. placebo | Sacubitril/valsartan vs. valsartan | Empagliflozin vs. placebo | Dapagliflozin vs. placebo |
| Key inclusion criteria | NYHA class II–IV, prior CV hospitalization | Clinical diagnosis of DHF with ≥signs/symptoms of HF, ≥2 of the following: LAE/LVH/impaired left ventricular filling/AF | NYHA class II–IV + any corroborating evidence (e.g. HF sign), LVH or LAE considered optional corroborating evidence, HFH required unless in NYHA class III–IV | ≥1 HF symptom + ≥1 HF sign, elevated NP or HFH | NYHA class II–IV, elevated NT‐proBNP (adjusted for AF and higher if no recent HF hospitalization), structural heart disease (LAE or LVH) | NYHA class II–IV, elevated NT‐proBNP | NYHA class II–IV, elevated NT‐proBNP (adjusted for AF), structural heart disease (LAE or LVH) |
| LVEF cutpoint | >40% | >40% | ≥45% | ≥45% | ≥45% | >40% | >40% |
| Endpoint | First of either CV death or HFH | First of either all‐cause death of HFH | First of either all‐cause death or hospitalization for a CV cause | First of either CV death, HFH, or RSD | CV death and total HFH (first and recurrent) | CV death or HFH | CV death or HFH either in the full population or in patients with LVEF <60% |
AF, atrial fibrillation CV, cardiovascular; DHF, diastolic heart failure; HF, heart failure; HFH, heart failure hospitalization; LAE, left atrial enlargement; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; NP, natriuretic peptide; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association.
While prior studies in this population with neurohormonal modulators have noted decline in efficacy with increasing LVEF, the unique mechanism of action of SGLT2 inhibitors and data from DECLARE‐TIMI 58 and DAPA‐CKD suggest the potential for beneficial effects across the spectrum of ejection fraction.3, 29
Nevertheless, the incorporation in DELIVER of dual‐primary analyses with appropriate protection against multiple comparisons allows for the possibility of differential effect by LVEF, while preserving adequate statistical power to assess both the overall study population.
While the primary outcomes in DELIVER are based on time‐to‐first event for cardiovascular death or heart failure event, which includes both heart failure hospitalizations and urgent heart failure visits, testing hierarchy for secondary endpoints allows for assessment of first and recurrent events in both the sub‐population of patients with LVEF <60% and then the full population (if the null hypothesis for the sub‐population is rejected) for the primary outcome. Recurrent events have provided more statistical power in some, but not all, trials in heart failure,30, 31 and may be especially helpful in trials of HFpEF where there are relatively few cardiovascular deaths relative to heart failure events. Furthermore, if the null hypotheses are rejected for each of these endpoints (primary for LVEF <60%, recurrent events for LVEF <60%, and recurrent events for the full population), the full population can then be re‐tested with full alpha. This unique approach takes into account the uncertainty regarding which population will have the greatest benefit and which testing method (time to first vs. recurrent events) would be most successful in this population and with this therapy. The specific order of the hierarchy, with assessment of KCCQ‐TSS above cardiovascular and all‐cause mortality, is based on the importance of the endpoints to the population, the relatively low number of deaths in this population, and the low overall likelihood of benefit for fatal endpoints.
There are both similarities and differences between DELIVER and EMPEROR‐Preserved,32 the other ongoing SGLT2 inhibitor outcome trial in a similar population. Both studies have similar entry criteria, including patients with heart failure and LVEF >40%, requirement for elevation in natriuretic peptides, and evidence for structural heart disease (although patients could be enrolled in EMPEROR‐Preserved without evidence of structural heart disease if they had a heart failure hospitalization within 12 months). The primary differences are that the dual primary endpoints in DELIVER allow for the possibility of testing the primary efficacy hypothesis in both the full population and in the subset of patients with LVEF <60%, an innovation that takes into account the results of prior trials. In addition, other trials, including EMPUSLE (NCT04157751)33 and DAPA ACT HF‐TIMI 68 (NCT04363697) are testing SGLT2 inhibitors in acute and stabilized hospitalized heart failure patients.
In summary, DELIVER will determine whether dapagliflozin compared with placebo will reduce the risk of cardiovascular death or worsening heart failure in patients with HFpEF or HFmrEF. DELIVER will provide complementary information to DAPA‐HF, which studied the adjacent population with HFrEF. The design of DELIVER takes into account the collective experience from prior trials in a patient population with great unmet need.
Funding
The DELIVER study was funded by AstraZeneca.
Conflict of interest: All authors have received research funding in the form of grants to institution from AstraZeneca and/or consulting to AstraZeneca.
Supporting information
Table S1. List of countries participating in DELIVER.
Table S2. Schedule of assessments.
Table S3. Exploratory objectives.
References
- 1.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA‐REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 2.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 3.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 4.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 5.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 6.Serenelli M, Böhm M, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, DeMets DL, Bengtsson O, Sjöstrand M, Langkilde AM, Anand IS, Chiang CE, Chopra VK, de Boer RA, Diez M, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Verma S, Docherty KF, Jhund PS, McMurray JJV. Effect of dapagliflozin according to baseline systolic blood pressure in the Dapagliflozin And Prevention of Adverse outcomes in Heart Failure trial (DAPA‐HF). Eur Heart J 2020;41:3402–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nassif ME, Qintar M, Windsor SL, Jermyn R, Shavelle DM, Tang F, Lamba S, Bhatt K, Brush J, Civitello A, Gordon R, Jonsson O, Lampert B, Pelzel J, Kosiborod M. Empagliflozin effects on pulmonary artery pressure in patients with heart failure: results from the EMBRACE‐HF trial. Circulation 2021;143:1673–1686. [DOI] [PubMed] [Google Scholar]
- 8.Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, Mahaffey KW, Charytan DM, Wheeler DC, Arnott C, Bompoint S, Levin A, Jardine MJ. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2019;7:845–854. [DOI] [PubMed] [Google Scholar]
- 9.Santos‐Gallego CG, Vargas‐Delgado AP, Requena‐Ibanez JA, Garcia‐Ropero A, Mancini D, Pinney S, Macaluso F, Sartori S, Roque M, Sabatel‐Perez F, Rodriguez‐Cordero A, Zafar MU, Fergus I, Atallah‐Lajam F, Contreras JP, Varley C, Moreno PR, Abascal VM, Lala A, Tamler R, Sanz J, Fuster V, Badimon JJ; EMPA‐TROPISM (ATRU‐4) Investigators . Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 2021;77:243–255. [DOI] [PubMed] [Google Scholar]
- 10.Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, Berry C, Chong V, Coyle L, Docherty KF, Dreisbach JG, Labinjoh C, Lang NN, Lennie V, McConnachie A, Murphy CL, Petrie CJ, Petrie JR, Speirits IA, Sourbron S, Welsh P, Woodward R, Radjenovic A, Mark PB, McMurray JJV, Jhund PS, Petrie MC, Sattar N. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR‐DM‐HF). Circulation 2021;143:516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omar M, Jensen J, Ali M, Frederiksen PH, Kistorp C, Videbæk L, Poulsen MK, Tuxen CD, Möller S, Gustafsson F, Køber L, Schou M, Møller JE. Associations of empagliflozin with left ventricular volumes, mass, and function in patients with heart failure and reduced ejection fraction: a substudy of the Empire HF randomized clinical trial. JAMA Cardiol 2021. Jan 6. 10.1001/jamacardio.2020.6827 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dewan P, Solomon SD, Jhund PS, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, DeMets DL, Sabatine MS, Bengtsson O, Sjöstrand M, Langkilde AM, Anand IS, Bělohlávek J, Chopra VK, Dukát A, Kitakaze M, Merkely B, O'Meara E, Schou M, Vinh PN, McMurray JJV; DAPA‐HF Investigators and Committees . Efficacy and safety of sodium‐glucose co‐transporter 2 inhibition according to left ventricular ejection fraction in DAPA‐HF. Eur J Heart Fail 2020;22:1247–1258. [DOI] [PubMed] [Google Scholar]
- 13.Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B; SOLOIST‐WHF Trial Investigators . Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117–128. [DOI] [PubMed] [Google Scholar]
- 14.Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Inzucchi SE, Kosiborod MN, Cherney DZI, Dwyer JP, Scirica BM, Bailey CJ, Díaz R, Ray KK, Udell JA, Lopes RD, Lapuerta P, Steg PG; SCORED Investigators . Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med 2021;384:129–139. [DOI] [PubMed] [Google Scholar]
- 15.Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, O'Meara E, Shah SJ, McKinlay S, Fleg JL, Sopko G, Pitt B, Pfeffer MA; TOPCAT Investigators . Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J 2016;37:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lund LH, Claggett B, Liu J, Lam CS, Jhund PS, Rosano GM, Swedberg K, Yusuf S, Granger CB, Pfeffer MA, McMurray JJV, Solomon SD. Heart failure with mid‐range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail 2018;20:1230–1239. [DOI] [PubMed] [Google Scholar]
- 17.Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin‐Colet J, Cleland J, Düngen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP; PARAGON‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609–1620. [DOI] [PubMed] [Google Scholar]
- 18.Solomon SD, Vaduganathan M, Claggett B, Packer M, Zile M, Swedberg K, Rouleau J, Pfeffer M, Desai A, Lund LH, Kober L, Anand I, Sweitzer N, Linssen G, Merkely B, Luis Arango J, Vinereanu D, Chen CH, Senni M, Sibulo A, Boytsov S, Shi V, Rizkala A, Lefkowitz M, McMurray JJV. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation 2020;141:352–361. [DOI] [PubMed] [Google Scholar]
- 19.Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, Solomon SD, Marler JR, Teerlink JR, Farb A, Morrow DA, Targum SL, Sila CA, Hai MTT, Jaff MR, Joffe HV, Cutlip DE, Desai AS, Lewis EF, Gibson CM, Landray MJ, Lincoff AM, White CJ, Brooks SS, Rosenfield K, Domanski MJ, Lansky AJ, McMurray JJV, Tcheng JE, Steinhubl SR, Burton P, Mauri L, O'Connor CM, Pfeffer MA, Hung HMJ, Stockbridge NL, Chaitman BR, Temple RJ; Standardized Data Collection for Cardiovascular Trials Initiative (SCTI) . 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation 2018;137:961–972. [DOI] [PubMed] [Google Scholar]
- 20.Shim CY, Seo J, Cho I, Lee CJ, Cho IJ, Lhagvasuren P, Kang SM, Ha JW, Han G, Jang Y, Hong GR. Randomized, controlled trial to evaluate the effect of dapagliflozin on left ventricular diastolic function in patients with type 2 diabetes mellitus: the IDDIA trial. Circulation 2021;143:510–512. [DOI] [PubMed] [Google Scholar]
- 21.Díaz‐Rodríguez E, Agra RM, Fernández ÁL, Adrio B, García‐Caballero T, González‐Juanatey JR, Eiras S. Effects of dapagliflozin on human epicardial adipose tissue: modulation of insulin resistance, inflammatory chemokine production, and differentiation ability. Cardiovasc Res 2018;114:336–346. [DOI] [PubMed] [Google Scholar]
- 22.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J; CHARM Investigators and Committees . Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐Preserved Trial. Lancet 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- 23.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J; PEP‐CHF Investigators . The perindopril in Elderly People with Chronic Heart Failure (PEP‐CHF) study. Eur Heart J 2006;27:2338–2345. [DOI] [PubMed] [Google Scholar]
- 24.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A; I‐PRESERVE Investigators . Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456–2467. [DOI] [PubMed] [Google Scholar]
- 25.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM; TOPCAT Investigators . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 26.Anker SD, Butler J, Filippatos G, Shahzeb Khan M, Ferreira JP, Bocchi E, Böhm M, Brunner‐La Rocca HP, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Gomez‐Mesa JE, Janssens S, Januzzi JL, Gonzalez‐Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Seronde MF, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M; EMPEROR‐Preserved Trial Committees and Investigators . Baseline characteristics of patients with heart failure with preserved ejection fraction in the EMPEROR‐Preserved trial. Eur J Heart Fail 2020;22:2383–2392. [DOI] [PubMed] [Google Scholar]
- 27.Skali H, Dwyer EM, Goldstein R, Haigney M, Krone R, Kukin M, Lichstein E, McNitt S, Moss AJ, Pfeffer MA, Solomon SD. Prognosis and response to therapy of first inpatient and outpatient heart failure event in a heart failure clinical trial: MADIT‐CRT. Eur J Heart Fail 2014;16:560–565. [DOI] [PubMed] [Google Scholar]
- 28.Okumura N, Jhund PS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K, Zile MR, Solomon SD, Packer M, McMurray JJ; PARADIGM‐HF Investigators and Committees . Importance of clinical worsening of heart failure treated in the outpatient setting: evidence from the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial (PARADIGM‐HF). Circulation 2016;133:2254–2262. [DOI] [PubMed] [Google Scholar]
- 29.Heerspink HJL, Stefánsson BV, Correa‐Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA‐CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446. [DOI] [PubMed] [Google Scholar]
- 30.Rogers JK, Pocock SJ, McMurray JJ, Granger CB, Michelson EL, Östergren J, Pfeffer MA, Solomon SD, Swedberg K, Yusuf S. Analysing recurrent hospitalizations in heart failure: a review of statistical methodology, with application to CHARM‐Preserved. Eur J Heart Fail 2014;16:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponikowski P, Kirwan BA, Anker SD, McDonagh T, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Göhring UM, Keren A, Khintibidze I, Kragten H, Martinez FA, Metra M, Milicic D, Nicolau JC, Ohlsson M, Parkhomenko A, Pascual‐Figal DA, Ruschitzka F, Sim D, Skouri H, van der Meer P, Lewis BS, Comin‐Colet J, von Haehling S, Cohen‐Solal A, Danchin N, Doehner W, Dargie HJ, Motro M, Butler J, Friede T, Jensen KH, Pocock S, Jankowska EA; AFFIRM‐AHF investigators . Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double‐blind, randomised, controlled trial. Lancet 2020;396:1895–1904. [DOI] [PubMed] [Google Scholar]
- 32.Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Zannad F, Packer M; EMPEROR‐Preserved Trial Committees and Investigators . Evaluation of the effects of sodium‐glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR‐Preserved trial. Eur J Heart Fail 2019;21:1279–1287. [DOI] [PubMed] [Google Scholar]
- 33.Tromp J, Ponikowski P, Salsali A, Angermann CE, Biegus J, Blatchford J, Collins SP, Ferreira JP, Grauer C, Kosiborod M, Nassif ME, Psotka MA, Brueckmann M, Teerlink JR, Voors AA. Sodium‐glucose co‐transporter 2 inhibition in patients hospitalized for acute decompensated heart failure: rationale for and design of the EMPULSE trial. Eur J Heart Fail 2021;23:826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of countries participating in DELIVER.
Table S2. Schedule of assessments.
Table S3. Exploratory objectives.


