Abstract
Objective
To evaluate the impact of fremanezumab on the severity and duration of remaining migraine attacks in patients with chronic migraine (CM) or episodic migraine (EM).
Background
Fremanezumab is a fully humanized monoclonal antibody (IgGΔa) that selectively targets calcitonin gene‐related peptide and is efficacious in reducing migraine frequency.
Methods
This exploratory post hoc analysis included data from three randomized, double‐blind, 12‐week, phase 3 studies (HALO CM, HALO EM, and FOCUS). In all three studies, patients with CM or EM were randomized 1:1:1 to receive subcutaneous quarterly fremanezumab (month 1/2/3: 675 mg/placebo/placebo), monthly fremanezumab (month 1/2/3: 675 mg [CM], 225 mg [EM]/225 mg/225 mg), or matched monthly placebo. Changes from baseline were evaluated in the proportion of headache days of at least moderate severity, peak severity of headache days, mean monthly headache hours (of any severity and at least moderate severity), and mean headache hours per headache day of any severity.
Results
A total of 2843 patients were randomized with 2823 patients included in the efficacy analyses across all studies (HALO CM, N = 1121; HALO EM, N = 865; FOCUS, N = 837). At study baseline, mean (standard deviation [SD]) monthly number of headache days rated moderate or severe in the quarterly fremanezumab, monthly fremanezumab, and placebo groups, respectively, were 13.2 (5.5), 12.8 (5.8), and 13.3 (5.8) in HALO CM; 7.2 (3.1), 6.8 (2.9), and 6.9 (3.1) in HALO EM; and 12.4 (5.8), 12.7 (5.8), and 12.8 (5.9) in FOCUS. Patients experienced significant least‐squares mean (LSM; 95% confidence interval) percent reductions from baseline in monthly number of headache days rated moderate or severe during the 12 weeks: HALO CM, quarterly fremanezumab, 34.5% (−39.8, −29.2) and monthly fremanezumab, 36.2% (−41.4, −31.0) vs. placebo, 19.6% (−20.0, −14.3); HALO EM, quarterly fremanezumab, 40.7% (−47.8, −33.5) and monthly fremanezumab, 43.4% (−50.4, −36.3) vs. placebo, 17.9% (−24.9, −11.0); and FOCUS, quarterly fremanezumab, 36.5% (−41.9, −31.1) and monthly fremanezumab, 38.6% (−44.0, −33.3) vs. placebo, 3.5% (−8.9, 1.8); all p < 0.0001. At study baseline, mean (SD) number of monthly headache hours rated moderate or severe in the quarterly fremanezumab, monthly fremanezumab, and placebo groups, respectively, were 66.4 (58.8), 68.0 (53.9), and 68.5 (57.0) in HALO CM; 33.3 (25.4), 31.7 (23.7), and 31.6 (23.2) in HALO EM; and 59.2 (54.7), 64.3 (65.2), and 65.9 (70.2) in FOCUS. Significant reductions were observed in LSM (standard error) number of monthly headache hours of at least moderate severity: HALO CM, quarterly fremanezumab, 24.4 (2.5) and monthly fremanezumab, 26.4 (2.3) vs. placebo, 14.1 (2.5); HALO EM, quarterly fremanezumab, 14.5 (1.4) and monthly fremanezumab, 15.5 (1.3) vs. placebo, 8.1 (1.3); and FOCUS, quarterly fremanezumab, 16.8 (3.0) and monthly fremanezumab, 18.3 (3.0) vs. placebo, 2.3 (3.0); all p < 0.001.
Conclusion
These analyses demonstrated that quarterly or monthly treatment with fremanezumab significantly reduced headache severity and duration in patients with CM or EM, including in patients with documented inadequate response to two to four prior migraine preventive medication classes.
Keywords: anti‐calcitonin gene‐related peptide, fremanezumab, headache severity, migraine
Abbreviations
- CM
chronic migraine
- EM
episodic migraine
- ICHD‐3
International Classification of Headache Disorders, 3rd edition
- MRMM
mixed‐effects repeated‐measures analysis model
- LSM
least‐squares mean
- LSMD
least‐squares mean difference
- SE
standard error
INTRODUCTION
Approximately 40% of individuals with migraine could benefit from preventive therapy, but less than 15% of those individuals currently use preventive treatments.1 The primary goals of preventive migraine therapy are to reduce migraine frequency, severity, and duration; to improve responsiveness to treatment of acute attacks; and to improve function and reduce disability.2, 3, 4, 5 In the past, attempts to design migraine‐specific preventive therapies that targeted the underlying pathophysiology ultimately failed due to severe safety concerns from these medications.6 Traditionally recommended nonspecific preventive medications were limited by slow onset of action, poor adherence, inadequate efficacy, and poor tolerability.6 This led to a need for effective, safe, and well‐tolerated preventive therapies that specifically target the pathophysiology of migraine. The approvals of several monoclonal antibodies targeting the calcitonin gene‐related peptide pathway over the past several years has allowed a new opportunity for clinicians to reduce migraine frequency with good efficacy and limited tolerability or safety concerns.6 However, in addition to reductions in migraine days and attack frequency, a good preventive medication should also show benefit in decreasing the severity and duration of the remaining migraine attacks.7
Fremanezumab is a fully humanized monoclonal antibody (IgGΔa) that selectively targets calcitonin gene‐related peptide.8, 9, 10 The efficacy and safety of fremanezumab has been studied in three double‐blind, randomized, phase 3 studies (HALO CM, HALO EM, and FOCUS) in patients with chronic migraine (CM) or episodic migraine (EM), including those with difficult‐to‐treat migraine, based on inadequate response to multiple prior migraine preventive medications.8, 9, 10 Across these three studies, the frequency of migraine days was reduced in patients treated with fremanezumab versus placebo.9, 10 In addition to reduction in migraine frequency, benefits of successful treatment include a significant decrease in attack duration and severity.4, 7 It has been observed that 90% of patients with migraine have moderate to severe pain, and 75% of patients have reduced functional ability during their attacks.1 Therefore, it is crucial to understand the impact that migraine preventive treatments may have on headache severity and duration to guide clinical decision making when selecting a preventive medication. The analyses reported herein contain results from the pivotal HALO CM, HALO EM, and FOCUS studies regarding the effect of fremanezumab on headache severity and duration. We hypothesized that fremanezumab treatment would be associated with significant reductions in headache severity and duration in patients with migraine.
METHODS
Study design
This was an analysis of predefined exploratory as well as post hoc endpoints and in three international, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group, phase 3 trials in patients with CM and EM. HALO CM (ClinicalTrials.gov Identifier: NCT02621931) included patients with CM, HALO EM (NCT02629861) included patients with EM, and FOCUS (NCT03308968) included patients with CM or EM who had a documented inadequate response to two to four classes of prior migraine preventive medications. Detailed methods and study designs for the HALO studies and FOCUS have been previously reported8, 9, 10 and are briefly summarized here.
Patient population
HALO CM and HALO EM
Patients eligible for the HALO studies included adults (18–70 years of age) with a history of migraine (International Classification of Headache Disorders, 3rd edition [ICHD‐3] beta criteria) for ≥12 months prior to screening. In HALO CM, CM was defined as headache on ≥15 days, with ≥8 days fulfilling ICHD‐3 beta criteria for migraine, probable migraine, or use of triptan or ergot medications.8 In HALO EM, EM was defined as headache on 6–14 days, with ≥4 days fulfilling ICHD‐3 beta criteria for migraine, probable migraine, or use of triptan or ergot medications.9 Patients were excluded in both trials for use of onabotulinumtoxinA in the 4 months before screening, use of opioids or barbiturates on >4 days per month, use of interventions or devices for migraine in the 2 months before screening, or previous failure to ≥2 medication clusters after ≥3 months of treatment (divalproex sodium or sodium valproate; flunarizine and pizotifen; amitriptyline, nortriptyline, venlafaxine, and duloxetine; atenolol, nadolol, metoprolol, propranolol, and timolol).8, 9 A subset of patients was permitted use of one preventive migraine medication if the dosing was stable from ≥2 months before the pretreatment period to the end of the treatment period.9, 10
FOCUS
Eligible patients for the FOCUS study included adults (18–70 years of age) with a diagnosis of migraine (onset ≤50 years of age) and a history of migraine for ≥12 months prior to screening.10 Eligible patients also had documented inadequate response within the past 10 years to two to four of the following classes of prior migraine preventive medications: beta‐blockers, anticonvulsants, tricyclic antidepressants, calcium channel blockers, onabotulinumtoxinA, and valproic acid. An inadequate response was generally documented in the patient's medical record and defined by no clinically meaningful improvement (per the treating physician's judgment) after 3 months of stably dosed treatment, discontinuation due to poor tolerability, or contraindication or unsuitability of treatment for the patient.10
Standard protocol approvals, registrations, and patient consents
All three studies were conducted in accordance with their respective study protocols and the International Conference for Harmonisation guidelines for Good Clinical Practice, the Declaration of Helsinki, and relevant national and local regulations. The study protocols were approved by the appropriate ethics committees and institutional review boards. Participants provided written informed consent prior to performing any study procedure or assessment.
Study procedures
All three studies consisted of a screening visit; a 28‐day pretreatment period; a 12‐week double‐blind, placebo‐controlled treatment period; and a final evaluation at week 12. Enrolled patients were randomized 1:1:1 to receive subcutaneous quarterly fremanezumab (month 1/2/3: 675 mg/placebo/placebo), monthly fremanezumab (month 1/2/3: 675 mg [CM], 225 mg [EM]/225 mg/225 mg), or matched monthly placebo. Efficacy was evaluated using information entered by patients in a daily electronic headache diary throughout the treatment period.
Outcome measures
Headache severity and duration were evaluated by exploratory endpoints and post hoc analyses of the HALO CM, HALO EM, and FOCUS studies. Key severity and duration outcomes were percent change from baseline (28‐day pretreatment period) in the monthly average number of headache days of at least moderate severity during the 12‐week treatment period, the monthly average number of headache hours of any severity, and the monthly average number of headache hours of at least moderate severity during the 12‐week treatment period. Of note, the change from baseline for headache hours of at least moderate severity has been previously reported; however, the current analysis summarizes the percent change from baseline in headache hours of at least moderate severity across all three studies.
Additional post hoc endpoints evaluated in this study were the mean peak severity of headache days during the 12‐week treatment period and the mean headache hours per headache day (of any severity) during the 12‐week treatment period.
The monthly average number of days or hours of efficacy variables (days of headache with at least moderate severity, hours of headache with any severity, hours of headache with at least moderate severity) during the 12‐week period after the first dose of the study drug were derived and normalized to the 28‐day equivalent as follows: (sum of days or hours over the 12‐week period) divided by (sum of days with assessments recorded in the eDiary). The baseline value was calculated similarly using all data collected in the pretreatment period.
The mean peak severity was calculated by averaging the peak severity of headache days within each period, where 1 = mild, 2 = moderate, and 3 = severe. All endpoints compared baseline and the 12‐week treatment period after the first dose of the study drug, unless otherwise stated.
Statistical analyses
The sample size estimations for the HALO CM, HALO EM, and FOCUS studies have been described previously.8, 9, 10 The current analyses were based on available data from these studies.8, 9, 10 Baseline demographic and clinical variables were summarized using descriptive statistics (i.e., frequency, mean, standard deviation). Efficacy analyses were conducted in the full analysis set, which included randomized patients who received ≥1 dose of study drug and had ≥10 days of postbaseline efficacy assessments on the primary endpoint. The mean change from baseline for each endpoint during the 12‐week period was analyzed via analysis of covariance. The normality assumption was assessed using visual inspections of Q‐Q plots and histograms (HALO and FOCUS studies), as well as the Shapiro–Wilk test (HALO studies) for all efficacy outcomes using normal approximation theories. If the assumptions of normality and equal variances held true, parametric testing was used. Where the validity of the assumption was suspected, the nonparametric method was used for sensitivity analysis. As expected from the large‐sample normal approximation theory, the results from the sensitivity analyses and the primary analyses were consistent, demonstrating the robustness of study results using parametric tests. Therefore, in this study, we only conducted analyses and reported results based on the normality assumption. All statistical tests were two‐tailed at the 0.05 level of significance. All summaries and statistical analyses were generated using SAS® software (Version 9.4 or later of SAS Systems for Windows, SAS Institute Inc., Cary, NC, USA). Fixed effects included treatment, sex, region (US vs. non‐US), and baseline preventive migraine medication use. Covariates included baseline values and years since the onset of migraine. A mixed‐effects repeated‐measures analysis model (MRMM) was used to estimate the mean change from baseline for the monthly endpoints. The MRMMs used were prespecified models from each primary analysis in the parent studies. In the HALO studies, the MRMM included treatment, gender, region, baseline preventive migraine medication use (yes/no), month, and treatment* month as fixed effects and baseline number of headache days of at least moderate severity and years since the onset of migraine as covariates. In the FOCUS study, the MRMM included treatment, gender, region, special group of treatment failure (yes/no), migraine classification (CM/EM), month, treatment* migraine classification, treatment* month, and treatment* migraine classification* month as fixed effects and baseline number of headache days of at least moderate severity and years since the onset of migraine as covariates. In all three studies, patient was a random effect and an unconstructed covariance structure was used.
RESULTS
Patients
A total of 2843 patients were randomized with 2823 patients included in the full analysis sets for efficacy analyses across all three studies (HALO CM, N = 1121; HALO EM, N = 865; FOCUS, N = 837). Baseline demographics were similar between all treatment groups in all three studies (Table 1). Consistent with the clinical definitions of CM and EM, patients with CM enrolled in the HALO CM study had the highest baseline headache days of at least moderate severity, headache hours of any severity, and headache hours of at least moderate severity, whereas patients with EM enrolled in the HALO EM study had the lowest (Table 1). Patients with CM or EM and inadequate response to two to four prior migraine preventive medication classes enrolled in the FOCUS study were slightly older and had been diagnosed with migraine slightly longer than patients enrolled in the HALO studies (Table 1); patients who had inadequate response to ≥2 prior preventive medication clusters were excluded from HALO studies. Clinical characteristics were similar between treatment groups within each study (Table 1).
TABLE 1.
Baseline demographics and clinical characteristics
| HALO CM | HALO EM | FOCUS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fremanezumab | Placebo (n = 375) | Fremanezumab | Placebo (n = 294) | Fremanezumab | Placebo (n = 279) | ||||
| Quarterly (n = 376) | Monthly (n = 379) | Quarterly (n = 291) | Monthly (n = 290) | Quarterly (n = 276) | Monthly (n = 283) | ||||
| Patient demographics | |||||||||
| Age, mean ± SD, years | 42.0 ± 12.4 | 40.6 ± 12.0 | 41.4 ± 12.0 | 41.1 ± 11.4 | 42.9 ± 12.7 | 41.3 ± 12.0 | 45.8 ± 10.9 | 45.9 ± 11.1 | 46.8 ± 11.1 |
| Female sex, n (%) | 331 (88) | 330 (87) | 330 (88) | 251 (86) | 244 (84) | 247 (84) | 229 (83) | 238 (84) | 233 (84) |
| Disease history | |||||||||
| Years since initial migraine diagnosis, mean ± SD | 19.7 ± 12.8 | 20.1 ± 12.0 | 19.9 ± 12.9 | 20.0 ± 12.1 | 20.7 ± 12.9 | 19.9 (11.9) | 24.3 ± 12.8 | 24.0 ± 13.7 | 24.3 ± 13.6 |
| Disease characteristics during the 28‐day pretreatment period | |||||||||
| Headache days of at least moderate severity,a mean ± SD | 13.2 ± 5.5 | 12.8 ± 5.8 | 13.3 ± 5.8 | 7.2 ± 3.1 | 6.8 ± 2.9 | 6.9 ± 3.1 | 12.4 ± 5.8 | 12.7 ± 5.8 | 12.8 ± 5.9 |
| Headache hours of any severity, mean ± SD | 119.1 ± 73.2 | 129.0 ± 88.6 | 127.2 ± 86.0 | 57.1 ± 30.0 | 57.1 ± 30.0 | 55.7 ± 26.5 | 107.9 ± 85.3 | 113.1 ± 89.0 | 115.9 ± 96.4 |
| Headache hours of at least moderate severity, mean ± SD | 66.4 ± 58.8 | 68.0 ± 53.9 | 68.5 ± 57.0 | 33.3 ± 25.4 | 31.7 ± 23.7 | 31.6 ± 23.2 | 59.2 ± 54.7 | 64.3 ± 65.2 | 65.9 ± 70.2 |
| Peak severity of headache days, mean ± SD | 1.9 ± 0.4 | 1.9 ± 0.4 | 1.9 ± 0.4 | 1.9 ± 0.4 | 1.9 ± 0.4 | 1.9 ± 0.4 | 2.0 ± 0.4 | 2.0 ± 0.4 | 2.0 ± 0.4 |
| Headache hours per headache day, mean ± SD | 5.7 ± 2.8 | 6.2 ± 3.4 | 6.1 ± 3.3 | 5.1 ± 2.2 | 5.2 ± 2.4 | 5.0 ± 2.3 | 6.4 ± 3.3 | 6.5 ± 3.7 | 6.6 ± 3.6 |
Abbreviations: CM, chronic migraine; EM, episodic migraine; SD, standard deviation.
A calendar day in which the patient reported either a day with headache pain that lasted ≥4 h consecutively and meeting criteria for migraine, probable migraine, or a day when a headache of any duration was treated with migraine‐specific medications (triptans or ergot compounds).
Headache severity and duration
Patients with CM or EM experienced a significant least‐squares mean (LSM; 95% confidence interval) reduction in the proportion of headache days of at least moderate severity with both quarterly and monthly dosing during the 12‐week treatment period across all three trials compared with placebo, with similar reductions observed for both fremanezumab dosing groups: HALO CM, quarterly fremanezumab, 34.5% (−39.8, −29.2) and monthly fremanezumab, 36.2% (−41.4, −31.0) vs. placebo, 19.6% (−20.0, −14.3); HALO EM, quarterly fremanezumab, 40.7% (−47.8, −33.5) and monthly fremanezumab, 43.4% (−50.4, −36.3) vs. placebo, 17.9% (−24.9, −11.0); and FOCUS, quarterly fremanezumab, 36.5% (−41.9, −31.1) and monthly fremanezumab, 38.6% (−44.0, −33.3) vs. placebo, 3.5% (−8.9, 1.8); (all, p < 0.0001 vs. placebo; Figure 1).
FIGURE 1.
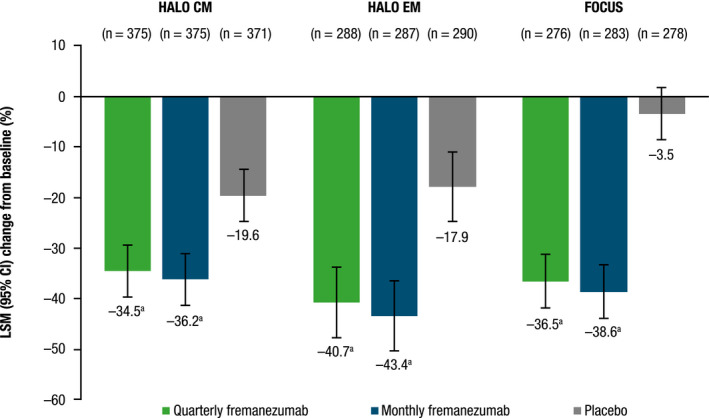
Percent change from baseline in headache days of at least moderate severity. CI, confidence interval; CM, chronic migraine; EM, episodic migraine; LSM, least‐squares mean. a p < 0.0001 versus placebo
The mean peak severity of headache days was also significantly reduced from baseline with both quarterly and monthly dosing compared with placebo during the 12‐week treatment period for patients with CM and EM (Figure 2; all, p < 0.0001 vs. placebo). Mean reductions were similar in all three studies in the fremanezumab treatment groups (0.3–0.2) and placebo groups (0.1 in all three studies; Figure 2).
FIGURE 2.
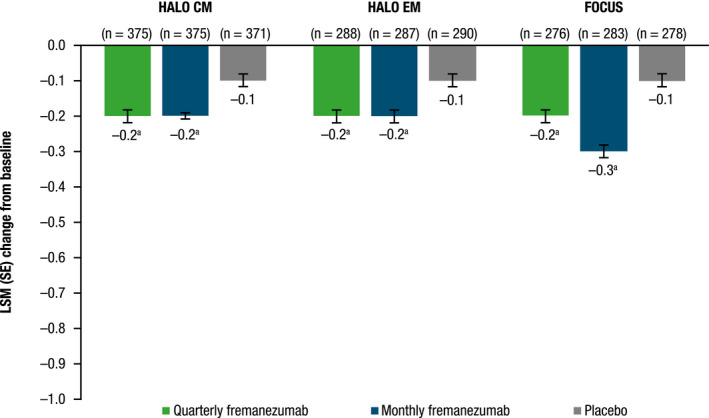
Change from baseline in mean peak severity of headache days during the 12‐week treatment period. CM, chronic migraine; EM, episodic migraine; LSM, least‐squares mean; SE, standard error. a p < 0.0001 versus placebo
Comparing the distributions of severity of headache days in the baseline and postbaseline periods, an overall reduction in the percentage of moderate and severe headache days and an increase in days rated as mild were apparent across all treatment groups (Figure 3). These changes in severity distributions were more pronounced in both fremanezumab quarterly and monthly dosing compared with placebo during the 12‐week treatment period (Figure 3). Greatest reductions in severe headache days were observed in the FOCUS study, with reductions of 12% with quarterly fremanezumab and 8% with monthly fremanezumab compared with 4% with placebo (Figure 3).
FIGURE 3.
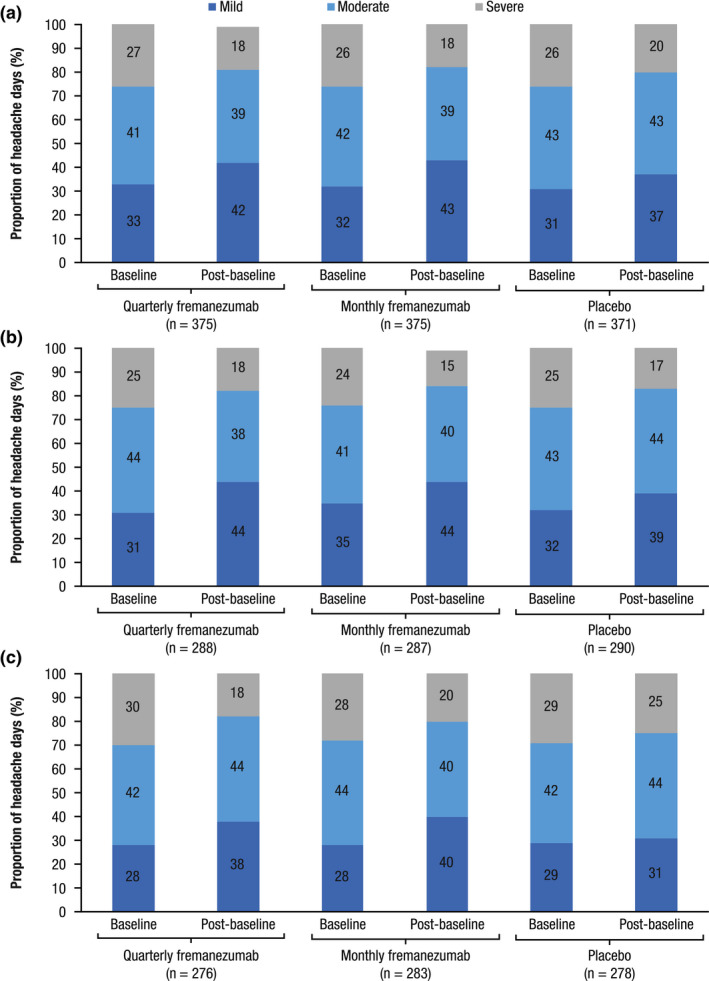
Distribution of mild, moderate, and severe headache days during the 12‐week treatment period for patients in (A) HALO CM, (B) HALO EM, and (C) FOCUS. CM, chronic migraine; EM, episodic migraine. Values are percentage of total headache days in each severity category
Across all three studies, there was a significant reduction in LSM (standard error [SE]) number of monthly headache hours of at least moderate severity with both quarterly and monthly dosing during the 12‐week treatment period compared with placebo: HALO CM, quarterly fremanezumab, 24.4 (2.5) and monthly fremanezumab, 26.4 (2.3) vs. placebo, 14.1 (2.5); HALO EM, quarterly fremanezumab, 14.5 (1.4) and monthly fremanezumab, 15.5 (1.3) vs. placebo, 8.1 (1.3); and FOCUS, quarterly fremanezumab, 16.8 (3.0) and monthly fremanezumab, 18.3 (3.0) vs. placebo, 2.3 (3.0); (all p < 0.001 vs. placebo; Figure 4). These reductions were higher in patients in HALO CM (LSM difference [LSMD] [SE] vs. placebo: quarterly fremanezumab, 10.3 [2.7]; monthly fremanezumab, 12.3 [2.7]) and FOCUS (quarterly fremanezumab, 14.5 [3.3]; monthly fremanezumab, 16.0 [3.3]) than in HALO EM (quarterly fremanezumab, 6.4 [1.5]; monthly fremanezumab, 7.4 [1.5]).
FIGURE 4.
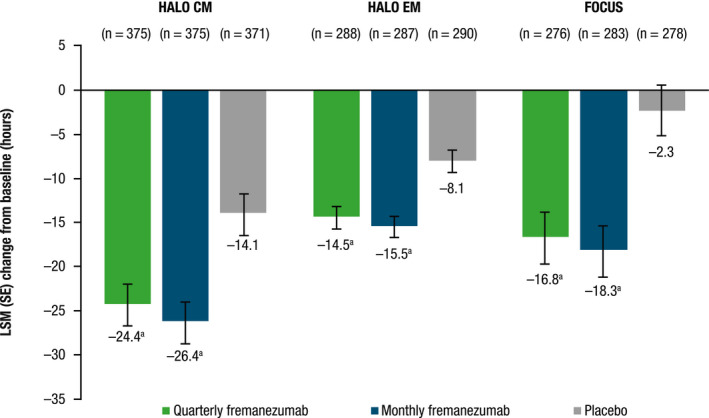
Change from baseline in monthly average number of headache hours of at least moderate severity during the 12‐week treatment period. CM, chronic migraine; EM, episodic migraine; LSM, least‐squares mean; SE, standard error. a p < 0.001 versus placebo
Across all three studies, similar reductions were seen in the monthly average number of headache hours of any severity with both quarterly and monthly fremanezumab dosing compared with placebo during the 12‐week treatment period (all p < 0.001 vs. placebo; Figure 5). Although patients in HALO CM had the largest LSM (SE) placebo response among the three studies (−24.0 [3.5] vs. −11.3 [2.3] and −3.9 [3.8] for HALO CM, HALO EM, and FOCUS, respectively), the LSMD versus placebo was still greater in HALO CM (quarterly fremanezumab, −14.0 [3.8]; monthly fremanezumab, −18.8 [3.8]) than in HALO EM (quarterly fremanezumab, −8.6 [2.5]; monthly fremanezumab, −12.7 [2.5]). Patients in the FOCUS study had the greatest LSMD (vs. placebo) in headache hours of any severity (quarterly fremanezumab, −21.4 [4.2]; monthly fremanezumab, −25.7 [4.2]).
FIGURE 5.
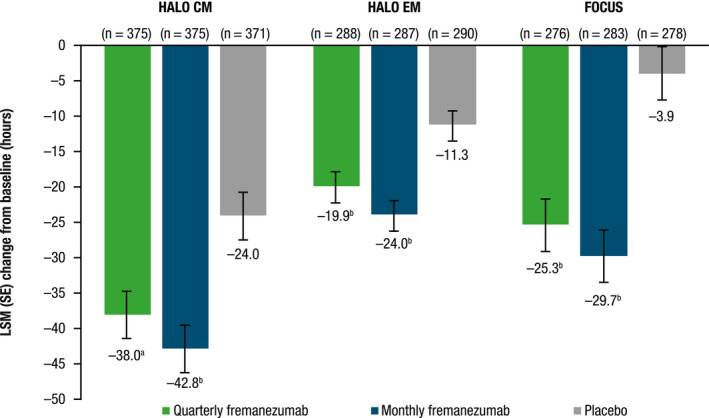
Change from baseline in monthly average number of headache hours of any severity during the 12‐week treatment period. CM, chronic migraine; EM, episodic migraine; LSM, least‐squares mean; SE, standard error. a p < 0.001 versus placebo. b p < 0.0001 versus placebo
Across all three studies, significant reductions in the average duration of headache in terms of mean headache hours per headache day of any severity were observed with both dosing regimens (all p < 0.001 vs. placebo; Figure 6). In the HALO CM study, reductions from baseline in mean headache hours per headache day (LSMD [SE] vs. placebo) were 0.6 (0.1) with quarterly fremanezumab and 0.7 (0.1) with monthly fremanezumab. Similarly, in both HALO EM and FOCUS studies, reductions from baseline in mean headache hours per headache day (LSMD vs. placebo) were 0.6 (0.2) with quarterly fremanezumab and 0.8 (0.2) with monthly fremanezumab.
FIGURE 6.
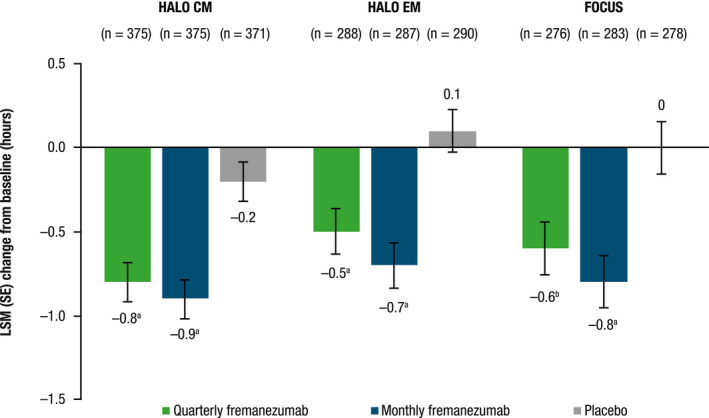
Change from baseline in average number of headache hours per headache day of any severity during the 12‐week treatment period for patients with CM and EM. CM, chronic migraine; EM, episodic migraine; LSM, least‐squares mean; SE, standard error. a p < 0.0001 versus placebo. b p < 0.001 versus placebo
DISCUSSION
In this study, analysis of fremanezumab efficacy data from three randomized controlled trials showed that both quarterly and monthly treatment with fremanezumab reduced headache severity and duration in patients with CM or EM.
Severity of headache has been shown to be directly proportional to headache‐related disability and impaired health‐related quality of life; increase in headache intensity is associated with higher levels of depression and emotional stress.11, 12, 13 Both headache severity and duration independently have been shown to be strong predictors for decreased workplace productivity.14, 15 As the magnitude of the impact of migraine is affected by the severity and duration of migraine, reducing headache severity and duration are important determinants of restoring functional ability and quality of life in patients with migraine.
In these analyses, both quarterly and monthly fremanezumab–treated patients across three randomized controlled trials experienced reductions from baseline in headache days of at least moderate severity. Fremanezumab also significantly reduced the mean peak severity of headache days from baseline during the 12‐week treatment period. At baseline, patients in all treatment groups across all three studies experienced similar distributions of migraine severity. Postbaseline, consistent distribution shifts were observed with fremanezumab treatment, with all patients experiencing a lower proportion of moderate and severe migraine attacks after study treatment. Of note, both patients with CM or EM were included in FOCUS, and the study population may have more difficult‐to‐treat migraine, as eligible patients had to have prior inadequate response to two to four classes of migraine preventive medications. Despite differences in study populations and disease characteristics across the three studies, fremanezumab offered consistent benefit in terms of reducing headache severity.
Treatment with fremanezumab also resulted in reductions in monthly headache hours of at least moderate severity, monthly headache hours of any severity, and headache hours per headache day of any severity, all of which were significant versus placebo. This also demonstrates a consistent benefit across all three studies of fremanezumab in reducing headache duration.
The substantial burden of disease that migraine imposes on individuals, their families, and global economies is well documented.16, 17, 18, 19 As previously mentioned, reductions in headache severity and duration are indicative of successful migraine preventive therapy.7, 20 Considering that fremanezumab has already proven efficacy providing significant reductions in migraine frequency compared with placebo, improving the severity and duration of remaining headaches may reduce overall disease burden. Migraine attacks that are less severe and of shorter duration may reduce the likelihood of overuse of acute medications and in turn decrease the risk of progression to CM. There is evidence that health‐related quality of life and disability worsens with increasing migraine severity; thus, assessing the impact of a migraine preventive medication on these outcomes is key to improving overall patient health and well‐being.13
Results from this analysis are in line with findings from the pivotal studies of HALO CM, HALO EM, and FOCUS, wherein both dosing regimens of fremanezumab demonstrated similar efficacy profiles in patients with CM or EM.8, 9, 10 Most patients with migraine place high value on treatment effectiveness and consider it as the most important factor when selecting a preventive therapy, regardless of dosing frequency.21 In a recent survey of adults with migraine (n = 417), the proportion of patients favoring monthly dosing (35%) was similar to the proportion favoring quarterly dosing (40%).22 Patients reported that they are more likely to fill the prescription and adhere with the treatment regimen when their preferred dosing regimen is available. Thus, dosing flexibility allows patients to choose their preferred therapy based on their individual needs.
The current study has a few limitations. Whereas some endpoints presented were prespecified exploratory endpoints, others were derived from post hoc analyses. Fremanezumab treatment effects were compared with placebo data within each study. The heterogeneity of study populations across studies precluded us from conducting a pooled data analysis. Nevertheless, results were consistent across the three studies, and the study populations included in the three studies are generalizable to the overall migraine patient population because both patients with CM and EM, including those with difficult‐to‐treat migraine, were included. Although significant improvements were demonstrated during the 12‐week treatment period of each study, this duration of follow‐up may be insufficient for understanding the prolonged benefits of fremanezumab treatment. However, long‐term efficacy and safety of fremanezumab have been evaluated in a separate 52‐week extension study.23 Results from this study could further our understanding of the long‐term benefits of fremanezumab.
CONCLUSION
These analyses demonstrate that, in addition to reducing the frequency of migraine and headache days, quarterly or monthly treatment with fremanezumab significantly reduced the headache severity and duration for the remaining migraine attacks in patients with CM or EM. These improvements were also seen in patients with documented inadequate response to two to four prior migraine preventive medication classes, which suggests that fremanezumab may also be efficacious in reducing severity and duration in individuals with difficult‐to‐treat migraine.
CLINICAL TRIALS REGISTRATION NUMBERS
HALO CM: NCT02621931; HALO EM: NCT02629861; FOCUS: NCT03308968 (ClinicalTrials.gov).
CONFLICT OF INTEREST
Dr. M. Ashina has received personal fees from AbbVie/Allergan, Amgen, Eli Lilly, Lundbeck, Novartis, and Teva Pharmaceuticals. He is the principal investigator for ongoing clinical trials for AbbVie/Allergan, Amgen, Eli Lilly, and Lundbeck. He has received research grants from the Lundbeck Foundation, Novo Nordisk Foundation, and Novartis. He has no ownership interest and does not own stocks of any pharmaceutical company. He serves as associate editor of Cephalalgia and The Journal of Headache and Pain. He is the President of the International Headache Society. J.M. Cohen, S.K. Gandhi, and E. Du are employees of Teva Pharmaceuticals.
AUTHOR CONTRIBUTIONS
Study concept and design: Messoud Ashina, Joshua M. Cohen, Sanjay K. Gandhi, Evelyn Du. Acquisition of data: Joshua M. Cohen, Sanjay K. Gandhi, Evelyn Du. Analysis and interpretation of data: Messoud Ashina, Joshua M. Cohen, Sanjay K. Gandhi, Evelyn Du. Drafting of the manuscript: Messoud Ashina, Joshua M. Cohen, Sanjay K. Gandhi, Evelyn Du. Revising it for intellectual content: Messoud Ashina, Joshua M. Cohen, Sanjay K. Gandhi, Evelyn Du. Final approval of the completed manuscript: Messoud Ashina, Joshua M. Cohen, Sanjay K. Gandhi, Evelyn Du.
ACKNOWLEDGMENTS
Editorial assistance was provided by Alyssa Nguyen, PharmD, of Cello Health Communications/MedErgy, which was in accordance with Good Publication Practice (GPP3) guidelines and funded by Teva Pharmaceuticals.
Funding information
This study was funded by Teva Pharmaceutical Industries Ltd., Petach Tikva, Israel
DATA AVAILABILITY STATEMENT
Anonymized data will be shared on request from any qualified investigator.
REFERENCES
- 1.Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343‐349. [DOI] [PubMed] [Google Scholar]
- 2.Garza I, Swanson JW. Prophylaxis of migraine. Neuropsychiatr Dis Treat. 2006;2:281‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silberstein SD. Preventive migraine treatment. Neurol Clin. 2009;27:429‐443. [DOI] [PubMed] [Google Scholar]
- 4.Herd CP, Tomlinson CL, Rick C, et al. Cochrane systematic review and meta‐analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9:e027953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta P, Singh S, Goyal V, Shukla G, Behari M. Low‐dose topiramate versus lamotrigine in migraine prophylaxis (the Lotolamp study). Headache. 2007;47:402‐412. [DOI] [PubMed] [Google Scholar]
- 6.Reuter U. A review of monoclonal antibody therapies and other preventative treatments in migraine. Headache. 2018;58(Suppl 1):48‐59. [DOI] [PubMed] [Google Scholar]
- 7.Silberstein SD. Preventive migraine treatment. Continuum (Minneap Minn). 2015;21:973‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377:2113‐2122. [DOI] [PubMed] [Google Scholar]
- 9.Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;319:1999‐2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari MD, Diener HC, Ning X, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double‐blind, placebo‐controlled, phase 3b trial. Lancet. 2019;394:1030‐1040. [DOI] [PubMed] [Google Scholar]
- 11.Stewart WF, Lipton RB. The economic and social impact of migraine. Eur Neurol. 1994;34(Suppl 2):12‐17. [DOI] [PubMed] [Google Scholar]
- 12.Magnusson JE, Becker WJ. Migraine frequency and intensity: relationship with disability and psychological factors. Headache. 2003;43:1049‐1059. [DOI] [PubMed] [Google Scholar]
- 13.Leonardi M, Raggi A, Bussone G, D'Amico D. Health‐related quality of life, disability and severity of disease in patients with migraine attending to a specialty headache center. Headache. 2010;50:1576‐1586. [DOI] [PubMed] [Google Scholar]
- 14.Serrano D, Manack AN, Reed ML, Buse DC, Varon SF, Lipton RB. Cost and predictors of lost productive time in chronic migraine and episodic migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Value Health. 2013;16:31‐38. [DOI] [PubMed] [Google Scholar]
- 15.Stewart WF, Lipton RB, Simon D. Work‐related disability: results from the American Migraine Study. Cephalalgia. 1996;16:231–238; discussion 215. [DOI] [PubMed] [Google Scholar]
- 16.Steiner TJ, Stovner LJ, Katsarava Z, et al. The impact of headache in Europe: principal results of the Eurolight project. J Headache Pain. 2014;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linde M, Gustavsson A, Stovner LJ, et al. The cost of headache disorders in Europe: the Eurolight project. Eur J Neurol. 2012;19:703‐711. [DOI] [PubMed] [Google Scholar]
- 18.Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81:479‐484. [DOI] [PubMed] [Google Scholar]
- 19.Gilligan AM, Foster SA, Sainski‐Nguyen A, Sedgley R, Smith D, Morrow P. Direct and indirect costs among United States commercially insured employees with migraine. J Occup Environ Med. 2018;60:1120‐1127. [DOI] [PubMed] [Google Scholar]
- 20.Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99:17‐24. [PubMed] [Google Scholar]
- 21.Peres MF, Silberstein S, Moreira F, et al. Patients’ preference for migraine preventive therapy. Headache. 2007;47:540‐545. [DOI] [PubMed] [Google Scholar]
- 22.Cowan R, Cohen JM, Rosenman E, Iyer R. Physician and patient preferences for dosing options in migraine prevention. J Headache Pain. 2019;20:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ClinicalTrials.gov . Efficacy and Safety of Subcutaneous Administration of Fremanezumab (TEV‐48125) for the Preventive Treatment of Migraine (HALO). https://clinicaltrials.gov/ct2/show/NCT02638103. Accessed on March 1, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared on request from any qualified investigator.


